Abstract
Background
Platelet-rich plasma (PRP) contains high concentrations of growth factors involved in wound healing. Hydrogel is a 3-dimensional, hydrophilic, high-molecular, reticular substance generally used as a dressing formulation to accelerate wound healing, and also used as a bio-applicable scaffold or vehicle. This study aimed to investigate the effects of PRP and hydrogel on wound healing, in combination and separately, in an animal wound model.
Methods
A total of 64 wounds, with 2 wounds on the back of each nude mouse, were classified into 4 groups: a control group, a hydrogel-only group, a PRP-only group, and a combined-treatment group. All mice were assessed for changes in wound size and photographed on scheduled dates. The number of blood vessels was measured in all specimens. Immunohistochemical staining was used for the analysis of vascular endothelial growth factor (VEGF) expression.
Results
Differences in the decrease and change in wound size in the combined-treatment group were more significant than those in the single-treatment groups on days 3, 5, 7, and 10. Analysis of the number of blood vessels through histological examination showed a pattern of increase over time that occurred in all groups, but the combined-treatment group exhibited the greatest increase on days 7 and 14. Immunohistochemical staining showed that VEGF expression in the combined-treatment group exhibited its highest value on day 7.
Conclusions
This experiment demonstrated improved wound healing using a PRP–hydrogel combined treatment compared to either treatment individually, resulting in a decrease in wound size and a shortening of the healing period.
Keywords: Platelet-rich plasma, Hydrogel, Tissue scaffold, Wound healing
INTRODUCTION
The components of blood platelets include glycoproteins, α-granules, and δ-granules. Among these, α-granules contain at least 7 growth factors, and these growth factors help accelerate the normal wound healing process [1]. Platelet-rich plasma (PRP) is a plasma from which blood cells are removed by centrifugation of the whole blood of normal persons. The platelets are thus sequestered and contain high concentrations of self-growth factors [2,3]. Previous studies have reported that the application of PRP can promote the healing process of a wound, a fracture surface, or a bone defect. As a result, there have been attempts to apply PRP clinically to accelerate the healing of diabetic foot ulcers or engraftment during bone grafting. However, the efficacy of PRP is still controversial [1,2,4]. Recently, studies have attempted to produce and apply single recombinant growth factors to wound healing, but such studies are limited by cost and a short half-life [5,6].
Hydrogel generally refers to a 3-dimensional, hydrophilic, high-molecular, reticular substance that easily expands on account of its considerable degree of moisture. Hydrogel has been used as a dressing formulation. Due to its high water content, hydrogel provides a humid environment for a wound, promotes cell migration and epithelialization, and accelerates wound healing by dead-tissue removal. Additionally, it is characterized as having superior biocompatibility and adequate mechanical traits, its biodegradation can be controlled, and it shows cell adhesion. In particular, due to its high biocompatibility, it can be used as a soft-form bio-applicable scaffold or vehicle [7].
On this basis, an experiment was carried out using an animal model under the assumption that in a combined treatment of hydrogel and PRP, hydrogel would act as an effective scaffold for growth factors and help in their maintenance and expression, thereby accelerating wound healing.
METHODS
Creation of the animal experimental model
For this experiment, 32 9-week-old female BALB/c nude mice with a weight of 20–30 g (26 g on average) and without a thymus gland (Orientbio Inc., Sungnam, South Korea) were used. They underwent a 1-week adaptation period while being bred, fed, and given water at the laboratory. For anesthesia, 40 mg/kg of tiletamine and zolazepam (Zoletil50, Virbac, France) and 5 mg/kg of xylazine (Rompun, Bayer, Germany) were injected into the peritoneal cavity. After the removal of the hair in the dorsum of each mouse using an epilator, the dorsum was disinfected with chlorhexidine, and circular wounds were drawn in the head and tail sides at a 10-mm distance from one another, using a sterilized punch biopsy tool with an 8-mm diameter in the center. With a no. 15 blade and fine scissors, a full-thickness skin defect wound reaching the panniculus carnosus was made (Fig. 1). To inhibit the building up of the wound and to accurately measure epithelialization, a 0.5-mm-thick donut-shaped silicone sheet 1 mm larger than the diameter of the wound was placed and fixed using no. 5 nylon (Ethicon, Cornelia, GA, USA). The 32 mice were divided into 2 groups: a group of 16 mice to which PRP was applied, and a group of 16 mice to which PRP was not applied. In all mice, hydrogel was applied to the wound on the tail side, while hydrogel was not applied to the wound on the head side. Therefore, a total of 64 wounds (n=16 per group) were created. They were divided into 4 treatment method groups: the control group, to which neither PRP nor hydrogel was applied, the hydrogel-only group, the PRP-only group, and the combined-treatment group, to which both hydrogel and PRP treatment were applied. For each wound, occlusive film dressing was applied using Opsite (Smith & Nephew, London, UK) and Surgifix (Panamedic, Seoul, Korea). The dressing was replaced daily, and the wounds were photographed on days 3, 5, 7, 10, and 14 to assess their size.
Fig. 1. Skin defects in the nude mouse model.
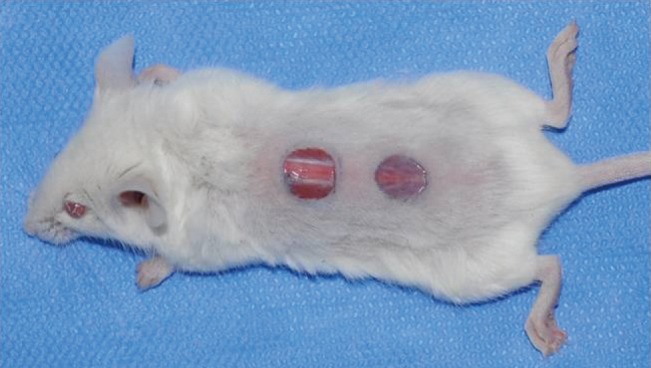
Circular full-thickness (8-mm-diameter) skin defects were made on the back of a nude mouse.
Production of PRP
The production of PRP required the collection of 54 mL of blood from the vein of a donor, a 30-year-old adult male. The collected blood was mixed with 6 mL of anticoagulant citrate dextrose solution. Using the double-spin method, centrifugation was performed for 10 minutes at 3,000 rpm at room temperature, and after the removal of the underlying blood cell layer, the rest of the blood was divided into 2 test tubes. Centrifugation was repeated on the 2 test tubes for 5 minutes at 5,000 rpm. The precipitated PRP layer was carefully separated with a micropipette, and 6.5 mL of PRP was produced (Fig. 2). An automated hematology system (XE-2100, Sysmex, Kobe, Japan) was used to measure the average platelet count of the normal blood of the donors (as 320×109/L), and the average platelet count of the manufactured PRP as 1.08×1012/L, indicating that the process resulted in an approximately 3.375-fold higher platelet concentration.
Fig. 2. Preparation of platelet-rich plasma.
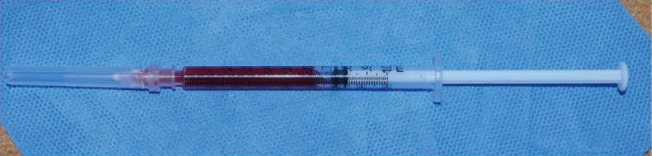
Platelet-rich plasma was prepared with an injectable syringe.
PRP application and treatment
Purified bovine thrombin (Sigma-Aldrich, Munich, Germany) was diluted at 100 units per mL in 10% calcium chloride and was then coagulated and activated by mixing with PRP. The hydrogel that was used in this experiment was Purilon gel (Coloplast, Humlebæk, Denmark), which has an amorphous property. For the hydrogel-only group, a 0.1-mL saline solution was injected into the base of each wound with a 1-mL syringe and a 27-gauge needle in place of PRP. In the PRP-only and combined-treatment groups, 0.1 mL of activated PRP was injected into the base of each wound using a 1-mL syringe and a 27-gauge needle. For the wound on the tail side, about 1 mL of hydrogel was applied locally. Each wound was dressed using Opsite (Smith & Nephew, London, UK), and the dressing was fixed with Surgifix (Panamedic). Each mouse was placed in a separate cage and observed for 2 weeks.
Visual examination and measurement of wound size
After the creation of the wound, the dressing was replaced daily, and photos of the wound were taken on days 3, 5, 7, 10, and 14 at the same resolution and distance using a digital single-lens reflex camera (Nikon D90, macro-lens 50 mm, Tokyo, Japan), to reduce errors. The photos were taken with a ruler placed next to the wound to show the wound size. The quantitative assessment of the wound size was based on the edge of the epithelialized portion. The area was measured at the pixel level using the ImageJ software program (National Institutes of Health, Bethesda, MD, USA).
Measurement of the average number of blood vessels
On days 7 and 14, 16 mice were sacrificed, respectively, and 8 specimens were collected from each group. The samples, including about 1 mm of normal skin from the edge of the wound (including the epithelialized portion), were resected with a no. 15 blade. After resection of the center of the wound, hematoxylin and eosin (H&E) staining was applied to 1 fragment and immunohistochemical analysis was applied to another. The collected samples were stored by freezing them at −70℃ and fixed in 10% formalin for 1 day in order to produce histopathology slides. Thereafter, paraffin embedding was performed, the specimens were sliced into 4-µm-thick parts, and H&E staining was applied to produce tissue sample slides. To determine the number of blood vessels, structures with the shape of the inner diameter of a blood vessel were observed with a microscope in each of the different fields of 3 sites for each slide, at ×100 magnification.
Analysis of vascular endothelial growth factor expression
Immunohistochemistry was performed to analyze vascular endothelial growth factor (VEGF) expression. The VEGF signal protein (1:1,000; Santa Cruz Biotechnology, Dallas, TX, USA) was used as a mouse monoclonal antibody, with beta-actin (1:1,000, Abcam, Cambridge, UK) used as a reference value antibody. After dissolving the tissue samples in a sodium dodecyl sulfate (SDS) sample and buffer solution, they were passed through 10% polyacrylamide gel. Thereafter, they were passed through a nitrocellulose membrane, and the first and second antibodies were reacted. Immune protein analysis was performed through chemical fluorescence analysis of the X-ray film, and the size of the expressed band was determined by measuring the area using the software program ImageJ. The values compared to the reference value of beta-actin expression were used for relative comparison of the groups.
Statistical analysis
To compare the wound sizes, change rates, and numbers of blood vessels at the different time points per group, 2-way repeated-measures analysis of variance (ANOVA) was conducted. To determine if the difference between the groups at each time point was significant, ANOVA tests were performed at each time point, and post hoc tests were conducted using the Tukey honest significant difference test. All statistical analysis was conducted using SPSS ver. 14.0KO (SPSS Inc., Chicago, IL, USA) and R 3.1.3 (R Foundation for Statistical Computing, Vienna, Austria), and the significance level was set at P<0.05.
RESULTS
Quantitative analysis of wound size
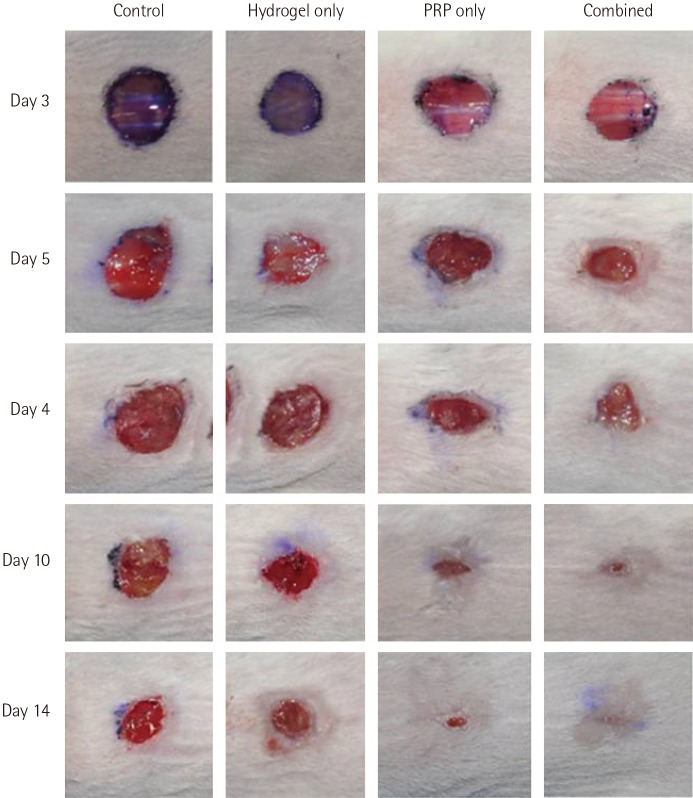
The initial wound sizes were similar across all groups. The wounds of the mice in the combined-treatment group on day 3 were smaller than those of the mice in the PRP-only, hydrogel-only, and control groups. On days 3, 5, and 10, the same results were obtained, and on day 14, wound healing was complete in the combined-treatment group, while the wounds of the mice in the other groups remained (Fig. 3). In the quantitative analysis, the differences in wound size between the combined-treatment group and the 3 other groups on days 3, 5, 7, and 10 were all significant (P<0.05). The differences in the changes in the wound size were also significant (P<0.05) (Table 1). For the PRP- and hydrogel-only groups, the differences and changes in the wound size and were significant on days 3, 5, and 7 compared to the control group, but they were not significant after day 10.
Fig. 3. Gross appearance of the wounds.
Time course of the gross appearance of the wounds: control, hydrogel-only, PRP-only, and combined-treatment groups. PRP, platelet-rich plasma.
Table 1. Comparison of wound sizes in all groups with time progression.
| Category | Measurement | Comparison (P-value) | |||||
|---|---|---|---|---|---|---|---|
| At baseline | Day 3 | Day 5 | Day 7 | Day 10 | Day 14 | ||
| Wound size (pixel) | Group, < 0.001 Time, < 0.001 Interaction, < 0.001 |
||||||
| Control | 129 ± 0.8 | 104.2 ± 1.3a,b,c) | 89 ± 1.7a,b,c) | 70.1 ± 3.2a,b,c) | 46.9 ± 6.0a) | 24.6 ± 3.2a) | |
| Hydrogel-only | 127.5 ± 0.8 | 91.4 ± 2.0a) | 75.2 ± 1.9a) | 55.1 ± 3.4a) | 38 ± 4.5a) | 15.9 ± 2.6a) | |
| PRP-only | 128.4 ± 0.7 | 86.5 ± 1.5a) | 68.1 ± 1.7a) | 45.9 ± 2.0a) | 31.5 ± 3.0a) | 16.1 ± 2.4a) | |
| Combined | 128.4 ± 0.7 | 69.5 ± 3.0 | 40.9 ± 3.8 | 24.5 ± 2.3 | 6.6 ± 1.7 | 1.5 ± 0.5 | |
These results were derived through 2-way repeated-measures ANOVA.
Values are presented as mean±standard deviation.
PRP, platelet-rich plasma; SE, standard error; ANOVA, analysis of variance; HSD, honest significant differences.
At each time point, a post-hoc comparison was made using the Tukey HSD test.
a)Significant difference with the combined treatment; b)Significant difference with PRP-only; c)Significant difference with hydrogel-only.
Measurement of the average number of blood vessels
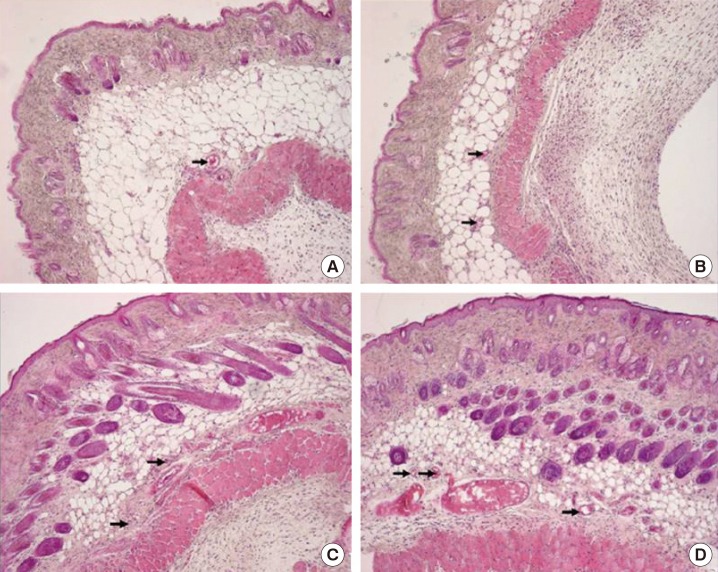
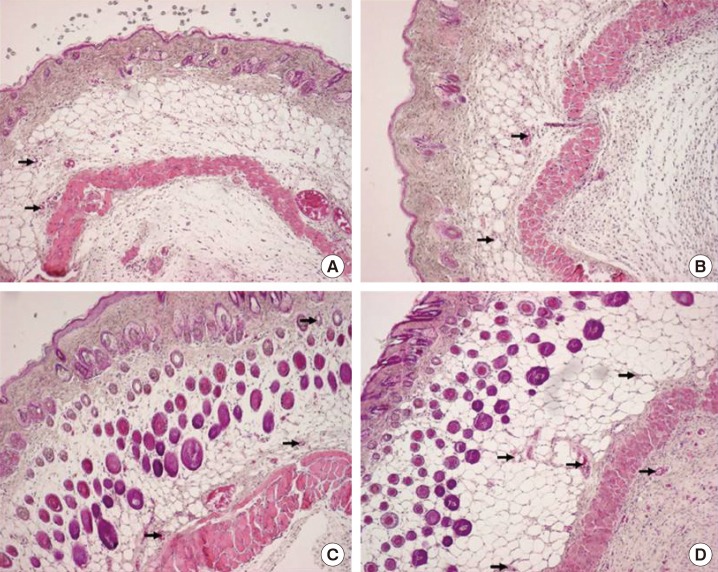
The number of blood vessels was determined using an optical microscope, and the changes were analyzed (Figs. 4, 5). After treatment, the differences in the average number of blood vessels in the 4 groups were found to be significant (P<0.05), and the increase in the number of blood vessels over time was found to be significant in all groups (P<0.05) (Table 2). The increase patterns were the same with regard to the number of blood vessels in the 4 groups, but there was no difference due to the interaction effect (P=0.747). In a comparison of the average numbers of blood vessels per area, the combined-treatment group showed significant differences on days 7 and 14 compared to the hydrogel-only and control groups, but showed no significant difference compared to the PRP-only group. Compared to the hydrogel-only and control groups, the PRP-only group showed a significant difference on day 7, but only showed a significant difference on day 14 compared to the control group.
Fig. 4. Histologic findings on day 7.
H&E, ×100. (A) Control group. (B) Hydrogel-only group. (C) PRP-only group. (D) Combined-treatment group. PRP, platelet-rich plasma. The black arrows indicate the staining of the blood vessels.
Fig. 5. Histologic findings on day 14.
H&E, ×100. (A) Control group. (B) Hydrogel-only group. (C) PRP-only group. (D) Combined-treatment group. PRP, platelet-rich plasma. The black arrows indicate the staining of the blood vessels.
Table 2. Comparison of the average numbers of blood vessels per unit area.
| Category | Measurement | Comparison (P-value) | |
|---|---|---|---|
| Day 7 | Day 14 | ||
| No. of blood vessels | Group, <0.001 Time, <0.001 Interaction, =0.747 |
||
| Control | 4.7±0.2a,b) | 5.2±0.1a,b) | |
| Hydrogel-only | 5.1±0.1a,b) | 5.6±0.2a) | |
| PRP-only | 5.6±0.1 | 6.3±0.2 | |
| Combined | 5.9±0.1 | 6.6±0.2 | |
These results were derived through 2-way repeated-measures ANOVA.
At each time point, a post-hoc comparison was made using the Tukey HSD test.
Values are presented as mean±standard error.
PRP, platelet-rich plasma; ANOVA, analysis of variance; HSD, honest significant differences.
a)Significant difference with the combined treatment; b)Significant difference with PRP-only.
Analysis of VEGF expression
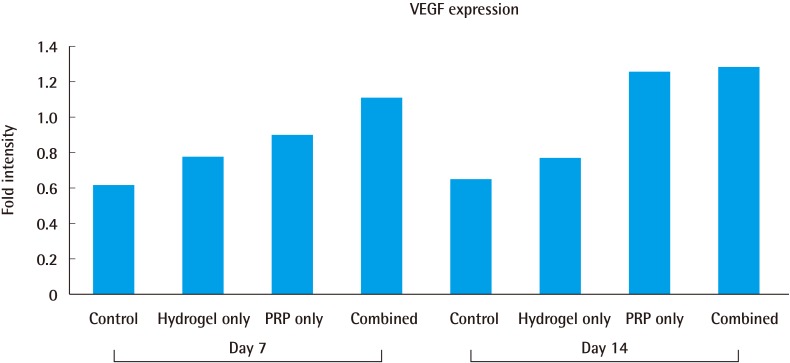
The VEGF expression values were compared by measuring the size of the band expressed through the projection of an X-ray film using ImageJ. On day 7, the combined-treatment group showed the highest value, which represented a 1.79-fold increase compared to that of the control group. On day 14, a 1.97-fold increase was also observed. The PRP-only group showed a 1.46-fold increase on day 7 compared to the control group. On day 14, a 1.93-fold increase was also observed, which did not differ significantly from that of the combined-treatment group (Fig. 6).
Fig. 6. Vascular endothelial growth factor expression.
Vascular endothelial growth factor expression was measured using a computer planimetry software program. The 4 graphs on the left pertain to day 7, while the 4 graphs on the right pertain to day 14. VEGF, vascular endothelial growth factor; PRP, platelet-rich plasma.
DISCUSSION
The α-granules of PRP contain 7 major growth factors, including 3 isomers of platelet-derived growth factor (PDGF-αα, PDGF-αβ, and PDGF-ββ), 2 isomers of transforming growth factor-β (TGF-β1 and TGF-β2), VEGF, and epithelial growth factor (EGF) [2]. They are secreted by activated platelets in the process of wound healing, and each serves to promote wound healing as a growth factor or cytokine [8]. PRP can be injected into the base of the wound or applied directly to the wound. It can promote activation of the physiological functions by supplying a greater than normal amount of growth factors to the damaged site, and can reduce the chance of infection, as well as pain and blood loss [9].
Some clinical trials have reported that PRP is effective for wound healing, but no evidence of its efficacy has been clearly established [10]. A study reported that due to its short half-life, the effect of PRP is weak, and it should be used within a certain period of time after its production [11]. However, another study reported that PRP has a sufficient effect if it is repeatedly applied [12]. In such cases, repeated application of PRP imposes an inconvenience, and there is a disadvantage in that the quality of PRP (i.e., the concentration) may not be consistent. To overcome this limitation, a study was conducted on increasing the action time and effect by utilizing a scaffold or vehicle along with a bioactive substance such as PRP [13]; in particular, this study aimed to investigate the effect by using hydrogel as a scaffold.
Hydrogel generally refers to a 3-dimensional, hydrophilic, high-molecular, reticular substance that can easily expand on account of its considerable degree of moisture. Hydrogel has received much attention and has been extensively discussed in the literature as a dressing formulation. It is in current use in clinics. It comes in various types, including amorphous hydrogel, hydrogel-impregnated gauze dressing, and sheet hydrogel. Hydrogel dressing acts as a humectant, prevents the formation of a scab by maintaining the water content of the wound, and promotes wound healing in the center.
Hydrogel has a 3-dimensional structure, absorbs secretions and foreign materials, and secures a space by dilating the polymer chain cross-linking structure. Additionally, hydrogel does not strongly adhere to the wound surface and does not cause irritation. It can also be easily removed, without pain. The biodegradation and cell adhesion of hydrogel can be controlled, with superior biocompatibility, and it is mechanically adequate for use as a soft-form bio-applicable scaffold. One study has reported on its use as a combination treatment with biological materials [14].
Since hydrogel has dual properties as a scaffold or vehicle due to its 3-dimensional structure, previous studies have noted its use for promoting the healing of soft tissue and bone defects by the addition of other bioactive substances. The added substances have varied and include traditional antibiotic substances such as povidone-iodine, collagen or antibiotics, PRP, polydeoxyribonucleotide, and stem cells [14]. The hydrogel that was used in this experiment was Purilon gel (Coloplast), an amorphous gel consisting of natural materials with high biocompatibility, such as purified water, sodium carboxymethylcellulose, and calcium alginate; it is applicable to any type of wound.
The increased size and number of blood vessels are among the factors important for wound healing, because blood vessels supply oxygen and nutrients to the tissues undergoing regeneration. Various factors are known to induce angiogenesis [15], and among them, VEGF is the most powerful angiogenic cytokine secreted from platelets, macrophages, and fibroblasts. It diffuses the vascular endothelial cells and increases vascular permeability [16]. Thus, the abundant supply and maintenance of these factors can be helpful for wound healing, and a study has reported that VEGF concentration alone induced accelerated in wound healing [17].
This study showed that wound healing was significantly accelerated in the combined-treatment (PRP and hydrogel) group compared to the PRP- and hydrogel-only groups. The degree of wound healing acceleration in the PRP-only group was significant compared to the control group, but there was no significant difference between the PRP- and hydrogel-only groups in this regard. Additionally, in the analysis of the number of blood vessels per unit area, the differences and changes in the number of blood vessels were significant in the combined-treatment group compared to the control and hydrogel-only groups on days 7 and 14. In the PRP-only group, significant differences were observed on days 7 and 14 compared to the control group, and on day 7 compared to the hydrogel-only group. Therefore, it can be said that the application of PRP accelerated angiogenesis. Moreover, while there was no significant difference in the hydrogel-only group compared to the control group, an increase in the overall number of blood vessels was observed. Thus, it is thought that angiogenesis was promoted by the cell migration due to the humid environment of the hydrogel. The effective results in the combined-treatment group are thought to have been due to the fact that hydrogel served as a scaffold to increase maintenance and expression, promoted angiogenesis, and further accelerated wound healing. In addition, in the analysis of the VEGF expression on the wound surface using immunohistochemistry, the most greatly increased values were observed on days 7 and 14 in the combined-treatment group. Since hydrogel acts as a scaffold for PRP, it increased the expression of growth factors and helped in the maintenance and acceleration of wound healing.
There are limitations to this study. Since the wounds were located in 2 different areas (in the head and tail of each mouse, respectively) the wound locations did not exactly match for each experimental group. This introduces a potential bias due to the fact that differences in the movement of the head and tail at different wound locations can affect the healing of the wound. However, the distance between these 2 areas was within 10 mm, with wounds largely located in the dorsum and central portions, and we believe this limitation is unlikely to have significantly affected the results. Further work including quantitative analysis and measurement is required to accurately assess the effects of VEGF expression. Although H&E staining was used in our study, another important way of identifying the number of blood vessels generated would be through the use of CD31 staining, which would provide a potential area for further research.
In this study, the application of PRP–hydrogel combination therapy resulted in more effective wound healing acceleration. It is thought that hydrogel acted as a scaffold for the growth factor, which led to an increase of the expression of growth factors and angiogenesis.
Footnotes
This work is supported by Soonchunhyang University Research Fund.
No potential conflict of interest relevant to this article was reported.
References
- 1.Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:229–237. doi: 10.1097/00006534-200101000-00037. [DOI] [PubMed] [Google Scholar]
- 2.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Bhanot S, Alex JC. Current applications of platelet gels in facial plastic surgery. Facial Plast Surg. 2002;18:27–33. doi: 10.1055/s-2002-19824. [DOI] [PubMed] [Google Scholar]
- 4.Abegao KG, Bracale BN, Delfim IG, et al. Effects of heterologous platelet-rich plasma gel on standardized dermal wound healing in rabbits. Acta Cir Bras. 2015;30:209–215. doi: 10.1590/S0102-865020150030000008. [DOI] [PubMed] [Google Scholar]
- 5.Ito R, Morimoto N, Pham LH, et al. Efficacy of the controlled release of concentrated platelet lysate from a collagen/gelatin scaffold for dermis-like tissue regeneration. Tissue Eng Part A. 2013;19:1398–1405. doi: 10.1089/ten.TEA.2012.0375. [DOI] [PubMed] [Google Scholar]
- 6.Morimoto N, Yoshimura K, Niimi M, et al. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: clinical trial for chronic skin ulcers. Tissue Eng Part A. 2013;19:1931–1940. doi: 10.1089/ten.tea.2012.0634. [DOI] [PubMed] [Google Scholar]
- 7.Montoya TI, Acevedo JF, Smith B, et al. Myogenic stem cell-laden hydrogel scaffold in wound healing of the disrupted external anal sphincter. Int Urogynecol J. 2015;26:893–904. doi: 10.1007/s00192-014-2620-6. [DOI] [PubMed] [Google Scholar]
- 8.Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118:147e–159e. doi: 10.1097/01.prs.0000239606.92676.cf. [DOI] [PubMed] [Google Scholar]
- 9.Hahn HM, Jeon YR, Rha DK, et al. Acceleration of wound healing using adipose-derived stem cell therapy with platelet concentrates: platelet-rich plasma (PRP) vs. Platelet-rich Fibrin (PRF) J Korean Soc Plast Reconstr Surg. 2011;38:345–350. [Google Scholar]
- 10.Martinez-Zapata MJ, Marti-Carvajal AJ, Sola I, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2012;10:CD006899. doi: 10.1002/14651858.CD006899.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Sugimori E, Shintani S, Ishikawa K, et al. Effects of apatite foam combined with platelet-rich plasma on regeneration of bone defects. Dent Mater J. 2006;25:591–596. doi: 10.4012/dmj.25.591. [DOI] [PubMed] [Google Scholar]
- 12.Serra R, Buffone G, Dominijanni A, et al. Application of platelet-rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients. Int Wound J. 2013;10:612–615. doi: 10.1111/iwj.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyoshi M, Kawazoe T, Igawa HH, et al. Effects of bFGF incorporated into a gelatin sheet on wound healing. J Biomater Sci Polym Ed. 2005;16:893–907. doi: 10.1163/1568562054255709. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Varshney RR, Wang DA. Therapeutic cell delivery and fate control in hydrogels and hydrogel hybrids. Adv Drug Deliv Rev. 2010;62:699–710. doi: 10.1016/j.addr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Schilling JA, Favata BV, Radakovich M. Studies of fibroplasia in wound healing. Surg Gynecol Obstet. 1953;96:143–149. [PubMed] [Google Scholar]
- 16.Banks RE, Forbes MA, Kinsey SE, et al. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer. 1998;77:956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birkenhauer E, Neethirajan S. A double-edged sword: the role of VEGF in wound repair and chemoattraction of opportunist pathogens. Int J Mol Sci. 2015;16:7159–7172. doi: 10.3390/ijms16047159. [DOI] [PMC free article] [PubMed] [Google Scholar]