Abstract
Introduction
Although four kinds of Alzheimer’s disease (AD) drugs are available at present there was only one drug until 2011 in Japan. This study aimed to elucidate prescription trends of these medications for AD in Japanese outpatients before and after the new drug releases in 2011.
Methods
This descriptive study of pharmacy claims databases analyzed outpatient prescription data from community pharmacies across Japan. The study patients were 20 years or older and first administered medications for AD (donepezil, memantine, rivastigmine, or galantamine) between January 2010 and September 2014. They were grouped on the basis of the year of their initial medications for AD administration into the 2010–2011 and 2012–2014 groups (1 and 2, respectively) and their characteristics and AD treatments were summarized by group. The subanalyses used a multivariable logistic regression model to examine the relationship between patient characteristics and discontinuation or change to combination therapy within a year.
Results
A total of 103,592 patients (group 1 and 2, 28,581 and 75,011, respectively) were prescribed medications for AD during the study period. The group 1 and 2 mean ± standard deviation (SD) ages were 79.6 ± 7.4 and 80.9 ± 7.3 years while female patients constituted 64.0% and 64.5%, respectively. Furthermore, in groups 1 and 2 patients, 99.0% and 94.3% received a medication for AD monotherapy, 92.3% and 59.6% were prescribed donepezil, and 40.5% and 41.5% discontinued treatment within a year, respectively. The subanalyses suggest that being at least 85 years old strongly correlated with treatment discontinuation and change to combination therapy within a year.
Conclusion
Although the prescription proportions of the various medications for AD have changed since 2011, no apparent changes occurred in the patient characteristics of those who initiated AD treatment between 2010–2011 and 2012–2014.
Keywords: Alzheimer’s disease, Japanese patients, Outpatient, Persistence, Population-based, Prescription trends, Japanese patients
Introduction
Dementia is one of the major causes of disability and dependency among older people worldwide. It is overwhelming for both the patients and their caregivers and families. Worldwide, 47.5 million people have dementia and 7.7 million new cases emerge yearly [1]. In Japan, the prevalence of dementia in people aged 65 years or older is an estimated 15% and is expected to increase as the Japanese society ages. For example, the prevalence of dementia in people aged 85 years or older is 47% and 59% in men and women, respectively [2]. Alzheimer’s disease (AD) is the most common cause of dementia and may contribute to 60–70% of the incidences [1, 3]. Although there are presently four kinds of drugs available for the treatment of AD in Japan, up until 2011 there was only one. The new medications for AD are memantine, galantamine, and rivastigmine. Memantine is the only N-methyl-d-aspartate receptor antagonist while the other three drugs are cholinesterase inhibitors. Rivastigmine is the only patch formulation while the other three are oral drug formulations.
The increase in medication for AD options may have drastically changed the course of AD treatment before and after 2011 in Japan. In Europe, galantamine was approved in 2000, memantine was approved in 2002, and rivastigmine (patch) was approved 2007. A database study in Ireland in 2006–2010 revealed that there was a relatively greater increase in the prescribing rate of donepezil and memantine compared with that of galantamine and rivastigmine, and the prescribing rate was persistently higher in women [4]. In France, the use of cholinesterase inhibitors slightly increased between 2004 and 2010 while the users aged 90 years or older increased and then subsequently decreased. After memantine was launched onto the market in France in 2003, the proportion of users was temporarily boosted and did not decrease in patients who were over 90 years old [5]. However, to the best of our knowledge, no studies have investigated the trends and prescription patterns of the four medications for AD currently used in Japan. Because most patients with AD are older people and are at risk of being subjected to polypharmacy and exhibiting low adherence, the availability of information on real-life prescription data is essential for the overall management of AD treatment.
Therefore, the aim of our study was to elucidate the trends in the prescription pattern of the four medications for AD currently used by Japanese outpatients. In the subanalyses, we examined the risk factor of change of treatment relative to discontinuation and combination therapy.
Methods
Data Source
We consulted a database of the prescriptions submitted at over 2000 [6–9] community pharmacies operated by four community pharmacy chains (Ain Holdings Inc., Hokkaido, Japan; Kraft Inc., Tokyo, Japan; Nihon Chouzai Co., Ltd., Tokyo, Japan; and Sogo Medical Co., Ltd., Fukuoka, Japan), which account for approximately 4% of the total dispensing pharmacies in Japan [10]. Most of the pharmacies were located in front of clinics, hospitals, or both. The database contains the identification number, age, sex, date of dispensing, name and code of the prescription drug, formulation, dosage, daily dose, and dosing days for individual patients [11]. The data were de-identified before being provided to the study group.
Cohort Definition
The total study population included patients aged 20 years or older who were first administered a medication for AD between January 2010 and September 2014. Each drug was identified as follows using the seven upper digits of the National Health Insurance Drug List of the Japanese Ministry of Health, Labor and Welfare: donepezil, 1,190,012; memantine, 1,190,018; galantamine, 1,190,019; and rivastigmine, 1,190,700. We excluded patients whom we did not consider to be established on any therapy because they had received only a single prescription of a medication for AD or their total prescription duration was 14 days or less. The patients included in the final cohort were divided into two groups, 2010–2011 and 2012–2014 (groups 1 and 2, respectively), based on the initial year of their prescriptions of medications for AD.
Prescription Proportion
We calculated the proportion of each prescription drug prescribed monthly out of the total prescriptions of medications for AD, and multiple prescriptions for the same drug in 1 month were counted once. In addition, prescriptions for more than 1 month were counted each month during the prescribed period. We adopted a similar method from a previous study to calculate the prescription proportion [12]. In summary, we calculated the prescription proportion by dividing the number of patients who received each medication for AD by the total number who received any medication for AD in that month. We also calculated the prescription proportion of donepezil by dividing the number of patients who received the branded or generic drug by those who received any donepezil formulation.
Supplementary Analysis
A patient who discontinued a treatment with medication for AD was defined as one who did not receive a refill for the index medication within 60 days after exhausting the previously prescribed and dispensed drug. A similar definition of discontinuation has been used in other studies of patient persistence with medications for AD [13, 14]. The duration of the treatment with medications for AD was defined as the period from the index date of the first administration to the date of discontinuation or the end date of the observation period (September 31, 2014). The durations were subsequently categorized as follows: within 1 month, at most 30 days; within 2 months, at most 60 days; within 6 months, at most 180 days; within 1 year, at most 365 days; within 2 years, at most 730 days; and over 2 years, more than 730 days. For patients who had received different kinds of medications for AD simultaneously with different prescription days, we considered the maximum prescription periods as the duration of treatment with a medication for AD. Moreover, we studied the rate of change from monotherapy to a combination therapy in the group 2 patients who received one medication for AD at the index date. A combination therapy was defined as simultaneous prescription of two or more medications for AD in a month. Furthermore, we calculated the duration from the index date to the date of administration of two or more medications for AD for the first time.
Statistical Analysis
For the statistical analyses of the data, we first summarized the patients’ characteristics at the index date for each group: age, sex, concomitant medications, and polypharmacy. The dates of birth of the patients were excluded in one of the four databases and, therefore, we imputed “1st” as their dates of birth. There were no other missing data in the variables we used. Concomitant drug administration was counted as follows, using the three upper digits of the National Health Insurance Drug List of the Japanese Ministry of Health, Labor and Welfare: antidepressants, 112; antipsychotics, 117; antihypertensives, 214; antihyperlipidemic, 218; and antidiabetics, 396. Polypharmacy was defined as the concomitant use of five or more non-AD drugs. Then, the proportion of the total prescriptions that each medication for AD represented was calculated. For the exploratory analysis, we used univariate logistic regression models and estimated the odds ratio (OR) and 95% confidence intervals (CIs). Additionally, multivariable logistic regression analyses were also performed to assess the factors related to the discontinuation or change from monotherapy to the combination therapy within 1 year. The multivariable model included variables with a univariate p < 0.05 and considered the different pharmacies as a random effect. In the multivariable analysis, we considered the effect of the interaction between being at least 85 years old and polypharmacy. All the statistical analyses were performed using the statistical analysis software (SAS) program version 9.4 (SAS Institute Inc., Cary, NC, USA).
Compliance with Ethics Guidelines
This study was exempt from requiring informed consent from individual patients according to the local institutional ethical guideline for epidemiological research because the data investigated were retrieved from automated electronic databases and de-identified before being provided to the study group. This study and the waiver of informed consent were approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee (Application No. R0190).
Results
Patient Characteristics
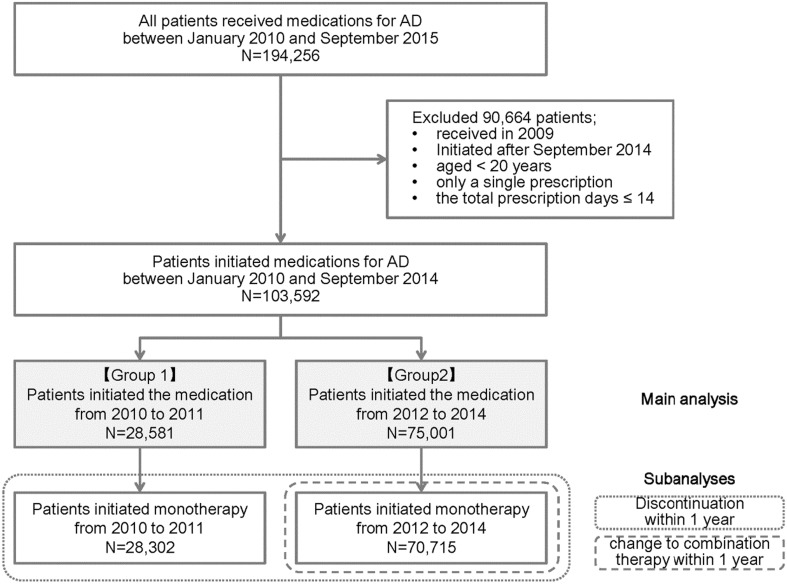
Between January 2010 and September 2014, 103,592 patients aged 20 years or older were identified from the combined pharmacy database as having initiated a medication for AD (Fig. 1). This cohort consisted of 28,581 and 75,011 patients who commenced the medication in 2010–2014 and 2012–2014 (groups 1 and 2, respectively). In groups 1 and 2, the mean ages ± standard deviation (SD) were 79.6 ± 7.4 and 80.9 ± 7.3 years, female patients comprised 64.0% and 64.5%, old patients (≥85 years) comprised 25.0% and 32.2%, and patients prescribed five or more non-AD drugs comprised 33.7% and 39.8%, respectively (Table 1).
Fig. 1.
Identification of patients from the pharmacy claims database for the main comparative analysis between both groups, subanalyses of discontinuation within 1 year, and change from monotherapy to combination therapy within 1 year
Table 1.
Characteristics of all patients who initiated medications for Alzheimer’s disease (AD) between January 2010 and December 2011 (group 1) and January 2012 and September 2014 (group 2)
| Characteristic | Group 1 (2010–2011) | Group 2 (2012–2014) | ||
|---|---|---|---|---|
| n = 28,581 | n = 75,011 | |||
| n | % | n | % | |
| Sex | ||||
| Female | 18,279 | 64.0 | 48,360 | 64.5 |
| Male | 10,302 | 36.0 | 26,651 | 35.5 |
| Age | ||||
| Mean (SD) | 79.6 | 7.4 | 80.9 | 7.3 |
| ≤64 | 1012 | 3.5 | 1965 | 2.6 |
| 65–74 | 4997 | 17.5 | 10,589 | 14.1 |
| 75–84 | 15,414 | 53.9 | 38,299 | 51.1 |
| ≥85 | 7158 | 25.0 | 24,158 | 32.2 |
| Co-prescribed drugs | ||||
| Antidepressants | 4872 | 17.0 | 13,544 | 18.1 |
| Antipsychotics | 5105 | 17.9 | 14,147 | 18.9 |
| Antihypertensives | 6223 | 21.8 | 19,525 | 26.0 |
| Antihyperlipidemics | 3488 | 12.2 | 10,960 | 14.6 |
| Antidiabetics | 1814 | 6.3 | 6140 | 8.2 |
| Polypharmacy | ||||
| 0–4 | 18,957 | 66.3 | 45,151 | 60.2 |
| ≥5 | 9624 | 33.7 | 29,860 | 39.8 |
Prescription Trends
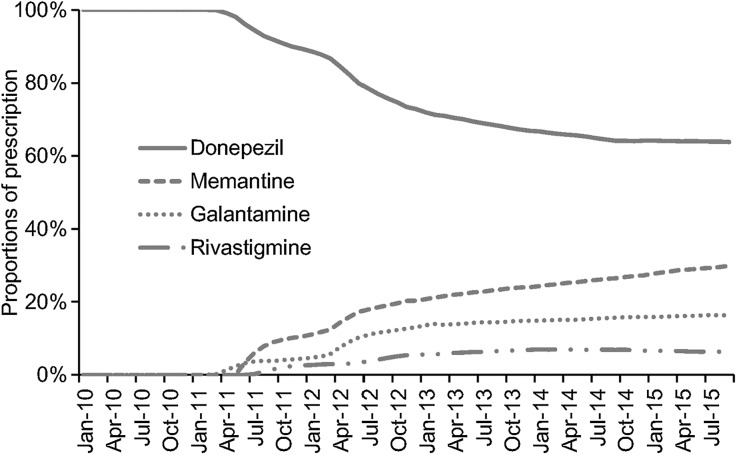
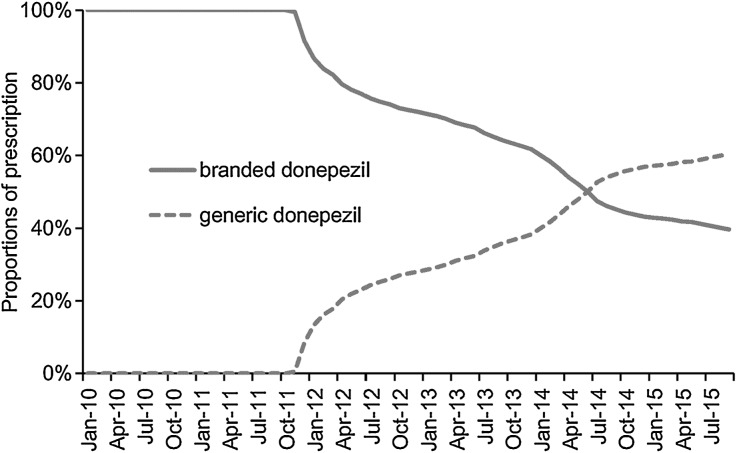
As illustrated in Fig. 2, the use of donepezil showed a decline while that of memantine and galantamine that were approved in March 2011 and rivastigmine, approved in July 2011, gradually increased from 2011. In September 2015, the proportion of the total prescriptions that were for donepezil, memantine, galantamine, and rivastigmine were 63.8%, 29.9%, 16.3%, and 6.3%. Furthermore, the use of generic donepezil, which was approved in November 2011, gradually increased after that until it was higher than that of the branded drug (Fig. 3). The proportion of prescriptions that constituted the generic and branded donepezil was 60% and 40%, respectively in September 2015. The first medication for AD is shown in Table 2. In addition, 99.0% and 94.3% of the patients received any medication for AD as a monotherapy while 92.3% and 59.6% received donepezil as a monotherapy in groups 1 and 2, respectively. Moreover, 5.7% of the group 2 patients received more than one AD drug, with donepezil and memantine being the most commonly prescribed combinations.
Fig. 2.
Proportions of prescription of four medications for Alzheimer’s disease (AD) per month in patients who initiated treatment between January 2010 and September 2014
Fig. 3.
Proportions of prescription of branded and generic donepezil per month in patients who received donepezil and initiated Alzheimer’s disease (AD) treatment between January 2010 and September 2014
Table 2.
Medications for Alzheimer’s disease (AD) administered to groups 1 and 2 patients with their first prescription
| Medications for AD | Group 1 (2010–2011) | Group 2 (2012–2014) | ||
|---|---|---|---|---|
| n = 28,581 | n = 75,011 | |||
| n | % | n | % | |
| Monotherapy | 28,302 | 99.0 | 70,715 | 94.3 |
| Donepezil | 26,378 | 92.3 | 44,741 | 59.6 |
| Generic of donepezil | 172 | 0.6 | 15,626 | 20.8 |
| Memantine | 799 | 2.8 | 10,034 | 13.4 |
| Galantamine | 791 | 2.8 | 10,588 | 14.1 |
| Rivastigmine | 334 | 1.2 | 5352 | 7.1 |
| Combination therapy | 279 | 1.0 | 4296 | 5.7 |
| Donepezil plus memantine | 235 | 0.8 | 3123 | 4.2 |
Discontinuation Within 1 Year
The patients who discontinued treatment within 1 year comprised 40.5% and 41.5% in groups 1 and 2, respectively. Patients who were at least 85 years old had the highest odds of discontinuing treatment within 1 year with an odds ratio (OR) of 1.42 and 95% confidence interval (CI) of 1.31–1.54 (Table 3). Patients administered memantine (OR, 1.34; 95% CI, 1.29–1.40) and rivastigmine (OR, 1.20; 95% CI, 1.13–1.26) also had higher odds of discontinuation but lower odds of receiving antihypertensive (OR, 0.81; 95% CI, 0.78–0.84) and antihyperlipidemic (OR, 0.78, 95% CI, 0.75–0.81) agents than those administered the other agents did. There were no significant associations between discontinuation and initiation year and no interaction effect between being at least 85 years old and polypharmacy.
Table 3.
Variables associated with treatment discontinuation within 1 year of all patients who initiated therapy with one medication for Alzheimer’s disease (AD) from 2010 to 2014
| Variable | Univariate analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| Sex | ||||||
| Male (vs. female) | 1.08 | 1.05–1.11 | <0.0001 | 1.09 | 1.06–1.12 | <0.0001 |
| Year | ||||||
| 65–74 (vs. ≤64) | 0.86 | 0.83–0.90 | <0.0001 | 1.04 | 0.96–1.13 | 0.319 |
| 75–84 (vs. ≤64) | 0.92 | 0.90–0.94 | <0.0001 | 1.17 | 1.08–1.26 | 0.0001 |
| ≥85 (vs. ≤64) | 1.23 | 1.19–1.26 | <0.0001 | 1.42 | 1.31–1.54 | <0.0001 |
| Medications for AD | ||||||
| Memantine (vs. donepezil) | 1.38 | 1.33–1.44 | <0.0001 | 1.34 | 1.29–1.40 | <0.0001 |
| Galantamine (vs. donepezil) | 1.01 | 0.97–1.05 | 0.587 | |||
| Rivastigmine (vs. donepezil) | 1.19 | 1.12–1.25 | <0.0001 | 1.20 | 1.13–1.26 | <0.0001 |
| Co–prescribed drugs | ||||||
| Antidepressants (vs. none) | 0.97 | 0.94–1.01 | 0.133 | |||
| Antipsychotics (vs. none) | 1.06 | 1.03–1.09 | 0.001 | 1.08 | 1.04–1.12 | <0.0001 |
| Antihypertensives (vs. none) | 0.77 | 0.75–0.80 | <0.0001 | 0.81 | 0.78–0.84 | <0.0001 |
| Antihyperlipidemic (vs. none) | 0.71 | 0.68–0.74 | <0.0001 | 0.78 | 0.75–0.81 | <0.0001 |
| Antidiabetics (vs. none) | 0.88 | 0.84–0.93 | <0.0001 | 1.03 | 0.98–1.09 | 0.228 |
| Polypharmacy | ||||||
| ≥5 (vs. 0–4 drugs) | 0.88 | 0.86–0.90 | <0.0001 | 0.96 | 0.93–0.99 | 0.007 |
| Year | ||||||
| 2012–2015 (vs. 2010–2011) | 1.10 | 1.06–1.13 | <0.0001 | 1.04 | 1.01–1.07 | 0.008 |
Change to Combination Therapy
In group 2, 70,715 patients (94.3%) initiated a medication for AD monotherapy (Table 2) and 4397 patients changed to a two or more combination therapy within 1 year. Furthermore, patients who were at least 85 years old showed the highest odds of changing to a combination therapy within 1 year (OR, 2.16; 95% CI, 1.68–2.77) (Table 4). Polypharmacy (OR, 1.68, 95% CI, 1.47–1.91) and memantine (OR, 1.26; 95% CI, 1.10–1.45) had higher odds than the other factors did. There was a significant interaction effect between patients aged at least 85 years old and polypharmacy with an OR of 0.71 (95% CI, 0.57–0.87).
Table 4.
Variables associated with change to combination therapy from monotherapy within 1 year of patients who initiated treatment with one medication for Alzheimer’s disease (AD) from 2012 to 2014
| Variable | Univariate analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| Sex | ||||||
| Male (vs. female) | 1.00 | 0.92–1.09 | 0.952 | |||
| Age | ||||||
| 65–74 (vs. ≤64) | 0.72 | 0.65–0.81 | <0.0001 | 1.04 | 0.82–1.32 | 0.767 |
| 75–84 (vs. ≤64) | 0.79 | 0.73–0.86 | <0.0001 | 1.19 | 0.95–1.49 | 0.140 |
| ≥85 (vs. ≤64) | 1.77 | 1.60–1.95 | <0.0001 | 2.16 | 1.68–2.77 | <0.0001 |
| Medications for AD | ||||||
| Memantine (vs. donepezil) | 1.45 | 1.27–1.66 | <0.0001 | 1.26 | 1.10–1.45 | 0.001 |
| Galantamine (vs. donepezil) | 0.68 | 0.62–0.76 | <0.0001 | 0.74 | 0.67–0.82 | <0.0001 |
| Rivastigmine (vs. donepezil) | 0.76 | 0.66–0.87 | 0.000 | 0.73 | 0.64–0.85 | <0.0001 |
| Co-prescribed drugs | ||||||
| Antidepressants (vs. none) | 1.52 | 1.35–1.72 | <0.0001 | 1.22 | 1.07–1.39 | 0.004 |
| Antipsychotics (vs. none) | 1.34 | 1.19–1.50 | <0.0001 | 1.08 | 0.96–1.23 | 0.202 |
| Antihypertensives (vs. none) | 1.22 | 1.11–1.35 | <0.0001 | 0.91 | 0.81–1.02 | 0.090 |
| Antihyperlipidemics (vs. none) | 1.15 | 1.01–1.29 | 0.030 | 0.92 | 0.81–1.05 | 0.237 |
| Antidiabetics (vs. none) | 1.32 | 1.12–1.56 | 0.001 | 1.10 | 0.92–1.32 | 0.271 |
| Polypharmacy | ||||||
| ≥5 (vs. 0–4 drugs) | 1.69 | 1.55–1.85 | <0.0001 | 1.68 | 1.47–1.91 | 0.004 |
| Interaction effect | ||||||
| Age (≥85) × polypharmacy (vs. none) | 0.71 | 0.57–0.87 | 0.001 | |||
Discussion
Our study provides the first insight into the prescription trends of four medications for AD in Japan. While there was an apparent change in the prescription proportion of the medications for AD, no apparent difference was observed in patient characteristics before and after 2011.
Main Findings
In Japan, dementia prevalence surveys were conducted in 2008 and 2010, which included over 100,000 residents aged 65 years or older in 10 areas, and the results estimated that the prevalence in this age group was 15% with a peak age range of 85–89 years for both male and female patients [15]. Furthermore, another study in Japan based on the data acquired in Hisayama town from 1985 to 2012 estimated the risk factors of the prevalence of dementia, and the relative risk for women was 1.25 (95% CI, 1.07–1.47) [2]. However, these studies lacked generalizability because only 1–10 areas were investigated in Japan and there was little information on the effects of the introduction of the novel drugs in 2011. In contrast, we included over 100,000 patients who were prescribed medications for AD from 2010 to 2014 across Japan, which enhanced our study generalizability. Our findings showed that male patients might take medications for AD less often than female patients do because there were approximately 1.8 times more female patients than there were male patients.
A prescription claims database study from Ireland examined the real-life dosing patterns and rates of persistence with AD medications in patients aged 70 years or older between 2006 and 2010 [4]. They reported that 77.4% of patient aged over 70 years received only one medication for AD: donepezil (65.5%), memantine (19.1%), galantamine (5.8%), and rivastigmine (9.5%) [4]; our results are similar to this previous study. The higher use of memantine than the other medication for AD suggests there were numerous patients with moderate to severe AD, who received their first prescriptions. According to the Japanese guideline [16], cholinesterase inhibitors are recommended for the treatment of mild to moderate AD; donepezil is also approved for severe AD while memantine is recommended for moderate or severe AD, either alone or in combination with cholinesterase inhibitors. These suggestions are very similar to the guidelines established by the European Federation of Neurological Societies (EFNS), the American Academy of Neurologists (AAN), and the National Institute of Clinical Excellence (NICE). In addition, Novartis and the US Food and Drug Administration (FDA) highlighted the fact that health care professionals and caregivers need to exercise caution in ensuring the proper use and application of the Exelon Patch (rivastigmine transdermal system) in 2010 [17, 18]. This warning might have had an effect on the prescribing habit of rivastigmine by doctors in Japan since 2011.
Treatment Discontinuation
An outpatient claims database study in Germany examined the continuous treatment with AD drugs in over 12,000 patients aged 45 years or older with a dementia diagnosis between 2003 and 2013 [19]. It showed that 60% of the patients continued with the therapy after 1 year. The patients on donepezil and memantine were less likely to discontinue treatment than those on rivastigmine were [19]. On the other hand, a study using a pharmacy claims database in the USA showed that newly treated patients with AD who received either rivastigmine or donepezil in a usual care setting had similar levels of treatment persistence [13]. Brewer et al. [4] reported that almost half of the patients investigated discontinued treatment within 1 year. The increase and decrease in discontinuing the use of rivastigmine and galantamine suggested that it may be related to the difference in the drug formulations. Moreover, this study showed that the patients who had commenced therapy more recently (2010) showed significantly higher rates of persistence with therapy at 6 months than those who had commenced therapy previously [4].
A Japanese retrospective study on the discontinuation of donepezil in a university hospital reported that 50% of the patients remained on the treatment after 1 year, and the main reason for discontinuing the drug was its perceived “ineffectiveness” by patients and their caregivers [20, 21]. In our cohort, almost 40% of patients discontinued treatment within 1 year. Interestingly, the proportion of patients who discontinued therapy within 1 year was similar in these different settings [4, 19, 20]. Our data suggests that memantine and rivastigmine had a risk of treatment discontinuation within 1 year that was higher than that of donepezil. Therefore, patients who received memantine or rivastigmine as their first prescription may have a high risk of discontinuation at that time. Since memantine is recommended for moderate and severe AD, the patients who were administered memantine might have been in a severe state of dementia at baseline. Also, as rivastigmine is the only patch formulation, the patients who were administered rivastigmine may have had difficulties with daily adherence to oral medications at baseline. Although our results did not show a difference in the risk of discontinuation in an initiation year, this may have resulted from the high accessibility of the health care system in Japan. Furthermore, we could not identify the reasons for drug therapy discontinuation from our data.
Our data suggests that patients who received antihypertensive and antihyperlipidemic agents had lower odds of discontinuation. These patients may have seen attending physicians more periodically and continuously than those who received the other drugs.
Patients Aged 85 Years or Older
Previous studies have shown that older people can be at high risk of early discontinuation of medications, and the concurrent cognitive decline significantly contributes to this risk [22, 23]. The patients who were aged 85 years or older in our cohort were more likely to discontinue treatment. However, there was a possibility that we might have overestimated the discontinuation because, in the database we used, we could not distinguish the discontinuation of treatment from lapses in data due patient relocation to a nursing home, death, or switching to other pharmacy chains or private pharmacies.
The subanalysis of the change from monotherapy to the combination therapy revealed that patients who were 85 years or older and subject to polypharmacy (≥5 drugs) had a higher risk of doing so, but the interactive effect of both variables exhibited a lower risk. This may reflect the aggressive treatment administered to patients, even the aged, and the need for enhanced treatment of Japanese outpatients with AD and associated comorbidities at baseline. Moreover, aged patients with other comorbidities have to visit general practices regularly, which may contribute to the maintenance of monotherapy.
Limitations
Our study had several limitations that are worth mentioning. First, the data were not derived from a random sampling of prescriptions because the pharmacies studied were heterogeneously located around Japan [11, 24]. Therefore, our data was affected by this unmeasured confounding factor, at least to some extent. The non-random sampling may have also induced a lack of generalizability. Second, our assumption of a diagnosis of AD was based only on prescription records from the study pharmacy database, and not diagnostic information. Therefore, our cohort may have included other types of dementia such as Lewy body or vascular forms. Third, the database only provided the prescriptions that were dispensed, and there was no information about whether the drugs had been taken or not. Moreover, the reasons for the discontinuation were not recorded as we mentioned earlier in the discussion. Therefore, we could not correctly analyze or describe the continuation or discontinuation of the treatment with medications for AD. Fourth, no valid information on the dementia stage or socioeconomic status, lifestyle, or caregiver risk factors, which likely affected the treatment choice and the treatment continuation, could be obtained. Furthermore, because we could not adjust the potential unmeasured confounders using regression models, there is a possibility that bias might have remained.
Conclusion
Our study demonstrated that the prescription proportions of the various medications for AD have changed since 2011, and that the proportion of donepezil monotherapy decreased after new drug releases. On the other hand, the monotherapy prescriptions were unchanged, and no apparent changes occurred in the patient characteristics of those who initiated AD treatment between 2010–2011 and 2012–2014.
Acknowledgements
We would like to acknowledge Mr. Yasuaki Takeda, Japan Medical Research Institute Co., Ltd., and Mr. Takahide Terada and Mr. Toshikazu Matsuo, Sogo Medical Co. Ltd., for the generous provision of the pharmacy claims data. Sponsorship for this research and the article processing charges were funded by the Keihanshin Consortium for Fostering the Next Generation of Global Leaders in Research (KCONNEX), established by Human Resource Development Program for Science and Technology, MEXT.
Disclosures
Koji Kawakami received a research fund from Novartis Pharmaceutical K.K.; honorarium from Eisai, Novartis Pharmaceutical K.K., Takeda Pharmaceutical Company Limited. Yuko Doi is an employee of Ain Holdings Inc. Yosuke Fujii is an employee of Ain Holdings Inc. Masaru Arai is an employee of Kraft Inc. Toshiyuki Matsunaga is an employee of Kraft Inc. Kimiko Kadohara and Izumi Sato have no commercial conflicts of interest that would affect the present paper. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Compliance with Ethics Guidelines
This study was exempt from requiring informed consent from individual patients according to the local institutional ethical guideline for epidemiological research because the data investigated were retrieved from automated electronic databases and de-identified before being provided to the study group. This study and the waiver of informed consent were approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee (Application No. R0190).
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/4327F06005B7FC2A.
References
- 1.World Health Organization. Dementia, Fact sheet. 2016. http://www.who.int/mediacentre/factsheets/fs362/en/. Accessed 4 May 2016.
- 2.Ninomiya T. The study of future estimation of elderly population with dementia in Japan. The Ministry of Health, Labour and Welfare grant system. 2014. https://mhlw-grants.niph.go.jp/niph/search/NIDD00.do?resrchNum=201405037A. Accessed 4 May 2016.
- 3.Ikejima C, Hisanaga A, Meguro K, et al. Multicentre population-based dementia prevalence survey in Japan: a preliminary report. Psychogeriatrics. 2012;12(2):120–123. doi: 10.1111/j.1479-8301.2012.00415.x. [DOI] [PubMed] [Google Scholar]
- 4.Brewer L, Bennett K, McGreevy C, Williams D. A population-based study of dosing and persistence with anti-dementia medications. Eur J Clin Pharmacol. 2013;69(7):1467–1475. doi: 10.1007/s00228-013-1483-y. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand M, Tzourio C, Alpérovitch A. Trends in recognition and treatment of dementia in France analysis of the 2004 to 2010 database of the national health insurance plan. Alzheimer Dis Assoc Disord. 2013;27(3):213–217. doi: 10.1097/WAD.0b013e3182695a3b. [DOI] [PubMed] [Google Scholar]
- 6.Ain Holdings Inc. http://www.ainj.co.jp/ir/finance/summary.html [In Japanese]. Accessed 26 Apr 2016.
- 7.Kraft Inc. http://www.kraft-net.co.jp/sakura/search/ [In Japanese]. Accessed 26 Apr 2016.
- 8.Nihon Chouzai Co. Ltd. http://www.nicho.co.jp/faq/article/6551/ [In Japanese]. Accessed 26 Apr 2016.
- 9.Sogo Medical Co. Ltd. http://www.sogo-medical.co.jp/information/summary.php [In Japanese]. Accessed 26 Apr 2016.
- 10.The Ministry of Health, Labour and Welfare. Report on Public Health Administration and Services. 2014. http://www.mhlw.go.jp/toukei/saikin/hw/eisei_houkoku/14/dl/kekka5.pdf [In Japanese]. Accessed 26 Apr 2016.
- 11.Urushihara H, Kobayashi S, Honjo Y, Kosugi S, Kawakami K. Utilization of antipsychotic drugs in elderly patients with Alzheimer’s disease seen in ambulatory practice in Japan. Sci Postscr. 2014;1(1):e00014. [Google Scholar]
- 12.Kohro T, Yamazaki T, Sato H, et al. Trends in antidiabetic prescription patterns in Japan from 2005 to 2011. Int Heart J. 2013;54(2):937. doi: 10.1536/ihj.54.93. [DOI] [PubMed] [Google Scholar]
- 13.Suh DC, Thomas SK, Valiyeva E, Arcona S, Vo L. Drug persistency of two cholinesterase inhibitors: rivastigmine versus donepezil in elderly patients with Alzheimer’s disease. Drugs Aging. 2005;22(8):695–707. doi: 10.2165/00002512-200522080-00006. [DOI] [PubMed] [Google Scholar]
- 14.Mauskopf JA, Paramore C, Lee WC, Snyder EH. Drug persistency patterns for patients treated with rivastigmine or donepezil in usual care settings. J Manag Care Pharm. 2005;11(3):231–251. doi: 10.18553/jmcp.2005.11.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asada T. The prevalence of dementia and the measures to functional disabilities in the urban areas. The Ministry of Health, Labour and Welfare grant system. 2012. https://mhlw-grants.niph.go.jp/niph/search/NIDD00.do?resrchNum=201218011A [In Japanese]. Accessed 1 May 2016.
- 16.Japanese Society of Neurology . Guideline for dementia management 2010 compact version. Tokyo: Igaku-shoin; 2012. [Google Scholar]
- 17.Ali TB, Schleret TR, Reilly BM, Chen WY, Abagyan R. Adverse effects of cholinesterase inhibitors in dementia, according to the pharmacovigilance databases of the United-States and Canada. Plos One. 2015;10(12):e0144337. doi: 10.1371/journal.pone.0144337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruber T, Jody D. Novartis Pharmaceutical Important Drug Warning Exelon. U.S. Food and Drug Administration. 2007. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/EnforcementActivitiesbyFDA/WarningLettersandNoticeofViolationLetterstoPharmaceuticalCompanies/ucm054180.pdf. Accessed 2 May 2016.
- 19.Bohlken J, Weber S, Rapp MA, Kostev K. Continuous treatment with antidementia drugs in Germany 2003–2013: a retrospective database analysis. Int Psychogeriatr. 2015;27(8):1335–1342. doi: 10.1017/S1041610215000654. [DOI] [PubMed] [Google Scholar]
- 20.Umegaki H, Itoh A, Suzuki Y, Nabeshima T. Discontinuation of donepezil for the treatment of Alzheimer’s disease in geriatric practice. Int Psychogeriatr. 2008;20(4):800–806. doi: 10.1017/S1041610208007011. [DOI] [PubMed] [Google Scholar]
- 21.Meguro K. Measures to prevent Alzheimer’s patients from discontinuing the use of cholinesterase inhibitors. Psychogeriatrics. 2014;14(3):210–211. doi: 10.1111/psyg.12058. [DOI] [PubMed] [Google Scholar]
- 22.Thiruchselvam T, Naglie G, Moineddin R, et al. Risk factors for medication nonadherence in older adults with cognitive impairment who live alone. Int J Geriatr Psychiatry. 2012;27(12):1275–1282. doi: 10.1002/gps.3778. [DOI] [PubMed] [Google Scholar]
- 23.Balkrishnan R. Predictors of medication adherence in the elderly. Clin Ther. 1998;20:764–771. doi: 10.1016/S0149-2918(98)80139-2. [DOI] [PubMed] [Google Scholar]
- 24.Urushihara H, Doi Y, Arai M, et al. Oseltamivir prescription and regulatory actions vis-a-vis abnormal behavior risk in Japan: drug utilization study using a nationwide pharmacy database. PLoS One. 2011;6:e28483. doi: 10.1371/journal.pone.0028483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.