Abstract
Introduction
Dimethyl fumarate (DMF) is a novel oral therapy used for the treatment of relapse-remitting multiple sclerosis (RRMS). In two 2-year pivotal Phase 3 trials in patients with RRMS, DMF significantly reduced disease activity based on both clinical and magnetic resonance imaging (MRI) findings and demonstrated an acceptable safety profile. However, there is currently a lack of comparative data which explore the relationship between work productivity and health-related quality of life (HRQoL) outcomes in RRMS and how these differ among RRMS therapies, including DMF.
Methods
We explored this relationship through patient-reported data from the EuroQol Five-Dimensions (EQ-5D) tool, Work Productivity and Activity Impairment Questionnaire (WPAI), and the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS) using the Adelphi MS DSP® dataset.
Results
Our data demonstrated that patients receiving DMF experienced better outcomes, relative to patients receiving beta (β)interferons or glatiramer acetate, in all WPAI subscales [overall; average treatment effect (ATE) −13.92, 95% confidence interval (CI) −18.87 to −7.08; p < 0.001], EQ-5D (ATE +0.075, 95% Cl 0.014–0.136; p = 0.016) and HAQUAMS [ATE −0.45, 95% Cl −0.61 to −0.29; p < 0.001]. The EQ-5D and HAQUAMS were used with WPAI to determine the relationship between HRQoL outcomes and work productivity. Multiple linear regression analyses were performed, adjusting for age, sex, body mass index, ethnicity and number of comorbid conditions.
Conclusions
These data demonstrate that therapy with DMF was associated with increased work productivity and HRQoL for patients with RRMS and that these outcomes were consistently improved compared to outcomes with interferon and glatiramer acetate therapies.
Keywords: Dimethyl fumarate, Relapse-remitting multiple sclerosis, Tecfidera, Health related quality of life, Work productivity
Introduction
Multiple sclerosis (MS) is a chronic, progressive demyelinating disease of the central nervous system [1] that affects approximately 2.1 million people worldwide (90 per 100,000 population in the USA [2]). A range of neurological symptoms are associated with MS, including spasticity, pain, ataxia, tremor, fatigue, weakness, cognitive effects, and depression [3]. The predominant clinical presentation of MS, diagnosed in 85% of patients, is the relapsing-remitting variant (RRMS), which is defined by periods of symptomatic flares interspersed by periods of relative stability [4]. Approximately 70% of patients with RRMS will develop progressive neurological decline described as secondary progressive MS [5]. Periods of symptomatic flares associated with RRMS patients may remit partially or completely following a relapse, and the various functional impairments associated with these flares have been shown to have a detrimental effect on a patient’s health-related quality of life (HRQoL) [6].
Currently available treatments include therapies with beta (β) interferons (including Avonex, Rebif, Betaseron, and Extavia) and glatiramer acetate (Copaxone), collectively referred to as ABCREs, newer oral agents, and monoclonal antibodies [7]. There is no cure for MS, and as a result of the typical onset of MS in young adults [8], patients often require life-long treatment, which can result in increasingly complex therapy decisions over the patient’s lifetime [9]. For example, approximately two-thirds of patients receiving ABCRE therapy have to switch to an alternative therapy due to the lack of efficacy [9], persistent disease activity, or occurrence of an adverse event [10]. However, the ABCRE therapies have the advantage of more than 20 years of safety data being available [7]. Since the approval of orally administered therapies, extensive uptake as both a first- and second-line option has been observed [11].
Dimethyl fumarate (DMF) (Tecfidera®) is a novel oral therapy for RRMS [12]. Its mechanism of action is believed to be through activation of the nuclear erythroid 2-related factor 2 (Nrf2) transcriptional pathway to exert neuroprotective effects [13, 14], thereby slowing disease progression and neuro-inflammation [15]. In addition, DMF has immunomodulatory effects which help reduce inflammation. Phase III clinical trials have demonstrated significant reductions in annual relapse rates of approximately 50% over 2 years [16] and a greater reduction of T2 lesions when compared to the existing therapy with glatiramer acetate [16]. Ad hoc analysis of the DEFINE/CONFIRM clinical trials showed increased efficacy in DMF patients compared to placebo patients across multiple end-points, including cognitive impairment [17], no evidence of disease activity [18], HRQoL outcomes [19], slower disability progression, and overall disease burden [20]. Evidence suggests that DMF has greater efficacy than ABCRE therapies [7], but how this efficacy translates into work productivity benefits has yet to be studied.
The DEFINE clinical trial demonstrated better HRQoL outcomes in patients in the DMF arm versus those in the placebo arm [19]. A significant correlation between presenteeism and reduced quality of life in MS patients has been demonstrated [21], but there remains a need for further comparisons of HRQoL and overall work productivity outcomes between patients on DMF and ABCRE therapy.
The aim of this study was to assess the impact of RRMS therapies on work productivity and HRQoL outcomes. To this end, we compared ABCRE therapies and DMF in terms of HRQoL and work productivity outcomes among RRMS patients. As work productivity remains an important factor for payers to consider, HRQoL outcomes were investigated as a predictor of work productivity.
Methods
Data were drawn from the Adelphi Real World MS IV Disease Specific Programme (DSP) (2014/2015). The Adelphi MS DSP® is a real-world, cross-sectional, survey of neurologists and their MS patients across the USA and Europe. The full methodology, including limitations, has been published [22]. The study is based on the completion of detailed patient record forms by physicians and a self-completed questionnaire by patients and caregivers.
Our study was based on previously conducted studies and did not involve any new studies of human or animal subjects performed by any of the authors. The research was conducted in full accordance with the U.S. Health Insurance Portability and Accountability Act 1996 (http://www.hhs.gov/ocr/privacy/). All data were collected through local fieldwork partners and fully de-identified prior to receipt by Adelphi.
Study Population
Participating physicians were asked to recruit their next eight consecutive consulting patients who met the eligibility criteria. All patients in the DSP patient sample required a formal diagnosis of RRMS and had to be >18 years of age. Of all patients captured in the database, RRMS patients on either DMF or another therapy, including ABCRE therapy, with a current treatment duration of >12 months were included in the analysis. A total of 828 RRMS patients on either DMF or ABCRE therapies were recruited, among whom 260 patients provided data for inclusion in the analysis on HRQoL outcomes (the HRQoL sample). Of these 260, 160 patients provided sufficient data for inclusion in the analysis on work productivity (the Work Productivity sample). Sample sizes for each patient-recorded outcome (PRO) and subscale can be found in Table 3.
Table 3.
Inverse-probability-weighted regression-adjustment analyses of patient-reported data from the EuroQol Five-Dimensions tool, Work Productivity and Activity Impairment Questionnaire, and the Hamburg Quality of Life Questionnaire in Multiple Sclerosis comparing ABCRE therapies and DMF therapy
| Outcome | Base (n) | ABCRE therapy | DMF average treatment effect | p value |
|---|---|---|---|---|
| WPAI: percentage work time missed (absenteeism) | 154 | 2.06 (0.46–3.67) | −2.06 (−3.67 to −0.46) | 0.012 |
| WPAI: percentage work time impaired (presenteeism) | 158 | 19.45 (16.26–22.64) | −12.97 (−18.87 to −7.08) | <0.001 |
| WPAI: percentage overall work impairment | 152 | 20.92 (17.38–24.46) | −13.92 (−19.86 to −7.98) | <0.001 |
| WPAI: percentage activity impairment | 243 | 25.31 (21.99–28.63) | −17.26 (−23.50 to −11.03) | <0.001 |
| EQ-5D | 255 | 0.823 (0.793–0.852) | 0.075 (0.014 to 0.136) | 0.016 |
| EQ-VAS | 252 | 74.55 (72.54–76.55) | 10.84 (6.15 to 15.53) | <0.001 |
| HAQUAMS fatigue/thinking subscale | 245 | 1.90 (1.77–2.03) | −0.47 (−0.75 to −0.19) | 0.001 |
| HAQUAMS mobility/lower limb subscale | 233 | 1.84 (1.72–1.95) | −0.43 (−0.75 to −0.11) | 0.009 |
| HAQUAMS mobility/upper limb subscale | 247 | 1.38 (1.30–1.46) | −0.15 (−0.32 to 0.02) | 0.075 |
| HAQUAMS social function subscale | 248 | 2.05 (1.96–2.15) | −0.54 (−0.77 to −0.31) | <0.001 |
| HAQUAMS mood subscale | 250 | 2.40 (2.28–2.53) | −0.45 (−0.64 to −0.26) | <0.001 |
| HAQUAMS total | 227 | 1.95 (1.86–2.04) | −0.45 (−0.61 to −0.29) | <0.001 |
EQ-5D, EuroQol Five-Dimensions tool; WPAI, Work Productivity and Activity Impairment Questionnaire; HAQUAMS, Hamburg Quality of Life Questionnaire in Multiple Sclerosis comparing ABCRE therapies and DMF therapy
Data Collection
Data were collected using patient self-completion forms (PSCs). These PSCs provided information on patient HRQoL outcomes, measured using the EuroQol Five-Dimensions (EQ-5D) health states and visual analog scale (VAS) [23, 24], and the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS) [25], and on work productivity, which was measured using the Work Productivity and Activity Impairment Questionnaire (WPAI) [26].
EQ-5D is a generic multi-attribute health-state classification system which assesses HRQoL in five dimensions, i.e., mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, using three levels, namely, no problems, some problems, and severe problems, respectively. Scores are reported on a 0 (dead) to 1 (full health) scale to represent preferences for a person’s health state. The EQ-5D also uses a VAS to rate an individual’s health state on a scale from 0 to 100, with 0 being the worst health state imaginable and 100 being the best health state imaginable. The EQ-5D scale ranges from 0 to 1, with a higher score indicating a better HRQoL outcome.
The WPAI consists of six items across four domains: absenteeism (work time missed), presenteeism (impairment at work/reduced on-the-job effectiveness), work productivity loss (overall work impairment/absenteeism + presenteeism), and activity impairment. Each domain is measured on a scale of 0–100% in terms of levels of impairment. The WPAI items include currently employed (Q1); hours missed due to health problems (Q2); hours missed for other reasons (Q3); hours actually worked (Q4); degree that health affected productivity while working (on a 0–10 VAS) (Q5); degree that health affected productivity in regular unpaid activities (0–10 VAS) (Q6). The recall period is 7 days. A lower score on the WPAI subscales indicates an improvement in work productivity.
The HAQUAMS is a 38-item, MS-specific quality of life self-assessment tool consisting of ten domains: sensory symptoms; fatigue/thinking; vision; mobility/lower extremities; mobility/upper extremities; bladder/bowel/sexuality; social function; mood; handicap; general health. Each domain is measured on a 5-point scale with 1 being ‘much better’ and 5 being ‘much worse’. Specific domains (i.e., not general health) have a recall period of 7 days. A low score in the HAQUAMS indicates better outcomes.
In addition to the PRO measures (t), patient demographic data, including age, sex, body mass index (BMI), ethnicity, gender, work status and type, and marital status, were collected. The Expanded Disability Status Scale (EDSS), a method used to quantify disability in MS and to monitor changes in disability levels over time, was also included [27]. No tests or investigations were required/conducted for a patient to be included in the study.
Statistical Analysis
Kruskal–Wallis, Fisher’s Exact, and Chi-squared tests were used to examine differences in demographics between patients receiving DMF therapy and those receiving ABCREs.
Propensity scores were generated using EDSS scores at initiation of current treatment, age, sex, BMI, duration of current treatment, line of therapy (by how many lines of therapy the patient has received), time since diagnosis, and number of comorbid conditions. Estimated average treatment effects (ATEs) of DMF therapy versus ABCRE therapies were generated by using the propensity score to weight regressions [inverse-probability-weighted regression-adjustment (IPWRA)]. Due to the observational nature of the data, any significant difference in an outcome (using a bivariate test) between the two groups may be due to confounding factors. To address this issue of confounding factors, we used IPWRA in an attempt to balance the pre-specified covariates (including EDSS at initiation) between the study group and control group through the use of inverse probability weights (inverse of the propensity score), estimated using a logistic regression model. The weighting serves to weight both the study group and control group up to the full sample. This balancing of pre-specified covariates is made so that differences in outcome will not be overestimated due to differences in the covariates among groups. IPWRA is a doubly-robust method, which means it will yield accurate treatment effect estimates if either the propensity score model or the outcome model is correctly specified.
To demonstrate the relationship between HRQoL outcomes and work productivity, we performed multiple linear regression analyses, adjusting for age, sex, BMI, ethnicity, and number of comorbid conditions.
Results
Patients who provided data on both HRQoL and WPAI outcomes were included in the regression analyses to explore the relationship between the two outcomes. The baseline demographics of the patient sample are shown in Table 1.
Table 1.
Patient demographics and clinical characteristics
| Characteristics | Work productivity sample | HRQoL sample | ||||
|---|---|---|---|---|---|---|
| DMF therapy (n = 23) | ABCRE therapy (n = 137) | p value | DMF therapy (n = 31) | ABCRE therapy (n = 229) | p value | |
| Age (years) | 36.83 ± 8.61 | 36.15 ± 8.49 | 0.686 | 36.97 ± 10.36 | 37.51 ± 9.74 | 0.791 |
| Years diagnosed | 4.44 ± 3.35 | 5.05 ± 3.65 | 0.263 | 5.03 ± 5.13 | 5.73 ± 4.47 | 0.108 |
| Gender | >0.999 | 0.307 | ||||
| Male | 7 (30.43) | 43 (31.39) | 12 (38.71) | 68 (29.69) | ||
| Female | 16 (69.57) | 94 (68.61) | 19 (61.29) | 161 (70.31) | ||
| Ethnicity | 0.001 | <0.001 | ||||
| White/Caucasian | 16 (69.57) | 124 (90.51) | 22 (70.97) | 208 (90.83) | ||
| African American/Afro-Caribbean | 5 (21.74) | 4 (2.92) | 7 (22.58) | 8 (3.49) | ||
| Hispanic/Latino | 0 (0) | 5 (3.65) | 0 (0) | 8 (3.49) | ||
| Other | 2 (8.70) | 4 (2.92) | 2 (6.45) | 5 (2.18) | ||
| Body mass index | 25.47 ± 4.31 | 24.39 ± 3.68 | 0.225 | 26.64 ± 5.06 | 25.19 ± 4.45 | 0.076 |
| Housing status | 0.324 | 0.097 | ||||
| Lives alone | 5 (21.74) | 15 (10.95) | 8 (25.81) | 27 (11.79) | ||
| Lives with partner/spouse | 16 (69.57) | 104 (75.91) | 19 (61.29) | 162 (70.74) | ||
| Lives with other family/friends | 2 (8.70) | 18 (13.14) | 4 (12.90) | 40 (17.47) | ||
| Dependents | 0.821 | >0.999 | ||||
| Dependents | 12 (52.17) | 78 (56.93) | 16 (51.61) | 118 (51.53) | ||
| No dependents | 11 (47.83) | 59 (43.07) | 15 (48.39) | 111 (48.47) | ||
| Relationship status | 0.536 | 0.304 | ||||
| Married/long term relationship | 17 (73.91) | 114 (83.21) | 20 (64.52) | 176 (76.86) | ||
| Divorced/separated | 1 (4.35) | 5 (3.65) | 2 (6.45) | 12 (5.24) | ||
| Single | 5 (21.74) | 18 (13.14) | 9 (29.03) | 41 (17.90) | ||
| EDSS at treatment initiation | 1.07 ± 0.98 | 1.66 ± 1.17 | 0.024 | 1.55 ± 1.68 | 1.94 ± 1.42 | 0.049 |
| Duration of current treatment (years) | 1.78 ± 0.79 | 3.82 ± 3.10 | <0.001 | 1.65 ± 0.73 | 4.22 ± 3.49 | <0.001 |
| Lines of therapy | 1.74 ± 0.75 | 1.24 ± 0.46 | <0.001 | 1.74 ± 0.77 | 1.25 ± 0.48 | <0.001 |
| Number of concomitant conditions | 0.17 ± 0.49 | 0.38 ± 1.00 | 0.466 | 0.23 ± 0.56 | 0.41 ± 0.99 | 0.370 |
Values in table are reported as the number with the percentage in parenthesis or as the mean ± standard deviation (SD)
DMF, Dimethyl fumarate; ABCRE, therapies for multiple sclerosis involving beta (β) interferons (including Avonex, Rebif, Betaseron, and Extavia) and glatiramer acetate (Copaxone); HRQoL, health-related quality of life; EDSS, Expanded Disability Status Scale
The total sample included almost twofold more female than male respondents, but this ratio not differ significantly between the two treatment groups (DMF therapy 30% male, 70% female vs. ABCRE therapies 31% male, 69% female) or the HRQoL and work productivity outcome groups (DMF therapy 39% male, 61% female vs. ABCRE therapies 30% male, 70% female). Compared with patients on DMF therapy, there was little difference in the mean age of patients receiving ABCREs (work productivity sample: 36.83 vs. 36.15 years, p = 0.686; HRQoL sample: 36.97 vs. 37.51 years, p = 0.791) or in mean years diagnosed (work productivity sample: 4.44 vs. 5.05 years, p = 0.263; HRQoL sample: 5.03 vs. 5.73 years, p = 0.108). There was a difference in the ethnicity profile between DMF patients and ABCRE patients (p < 0.001).
WPAI: Work Productivity Loss
Patients were largely in full-time employment across all comparator groups (work productivity sample: DMF therapy 91.30% vs. ABCRE therapies 75.18%, p = 0.622; HRQoL therapies: DMF 77.42% vs. ABCRE therapies 55.02%, p = 0.267) (Table 2), and no significant difference was observed between the comparator groups in the HRQoL or work productivity sample or the subgroups of patients receiving DMF versus ABCRE therapies. The most common type of work was professional or skilled (work productivity subscale: 78.26 vs. 79.67%, p > 0.999; HRQoL sample: 74.07 vs. 77.48%, p = 0.588) with no significant difference observed between the two patient groups (Table 2). Patients receiving ABCRE therapies were more likely to have retired or left work as a result of their MS than those receiving DMP therapy: 45.45% of all non-working ABCRE patients stated they retired/left work because of their MS compared to none of the DMF patients (work productivity sample) (Table 2).
Table 2.
Current work status
| Characteristics | Work productivity sample | HRQoL sample | ||||
|---|---|---|---|---|---|---|
| DMF therapy (n = 23) | ABCRE therapies (n = 137) | p value | DMF therapy (n = 31) | ABCRE therapies (n = 229) | p value | |
| Employment status | 0.622 | 0.267 | ||||
| Employed full time | 21 (91.30) | 103 (75.18) | 24 (77.42) | 126 (55.02) | ||
| Employed part time | 2 (8.69) | 20 (14.60) | 3 (9.68) | 25 (10.92) | ||
| Student | 0 (0) | 6 (4.38) | 2 (6.45) | 20 (8.73) | ||
| Homemaker | 0 (0) | 3 (2.19) | 0 (0) | 23 (10.04) | ||
| Unemployed | 0 (0) | 0 (0) | 2 (6.45) | 24 (10.48) | ||
| Retired | 0 (0) | 2 (1.46) | 0 (0) | 8 (3.49) | ||
| Other | 0 (0) | 3 (2.19) | 0 (0) | 3 (1.31) | ||
| DMF therapy (n = 23/23) | ABCRE therapies(n = 123/137) | p value | DMF therapy (n = 27/31) | ABCRE therapies (n = 151/229) | p value | |
|---|---|---|---|---|---|---|
| Type of work | >0.999 | 0.588 | ||||
| Professional or skilled | 18 (78.26) | 98 (79.67) | 20 (74.07) | 117 (77.48) | ||
| Manual work | 4 (17.39) | 21 (17.07) | 6 (22.22) | 26 (17.22) | ||
| Do not know | 1 (4.35) | 4 (3.25) | 1 (3.70) | 8 (5.30) |
| DMF therapy (n = 2/23) | ABCRE therapies (n = 22/137) | p value | DMF therapy (n = 5/31) | ABCRE therapies (n = 57/229) | p value | |
|---|---|---|---|---|---|---|
| Retired/unemployed/part-time because of their MS | 0.493 | >0.999 | ||||
| Yes | 0 (0) | 10 (45.45) | 3 (60) | 33 (57.89) | ||
| No | 2 (100) | 12 (54.55) | 2 (40) | 24 (42.11) |
Data are presented as a number with the percentage in parenthesis
WPAI: All subscales
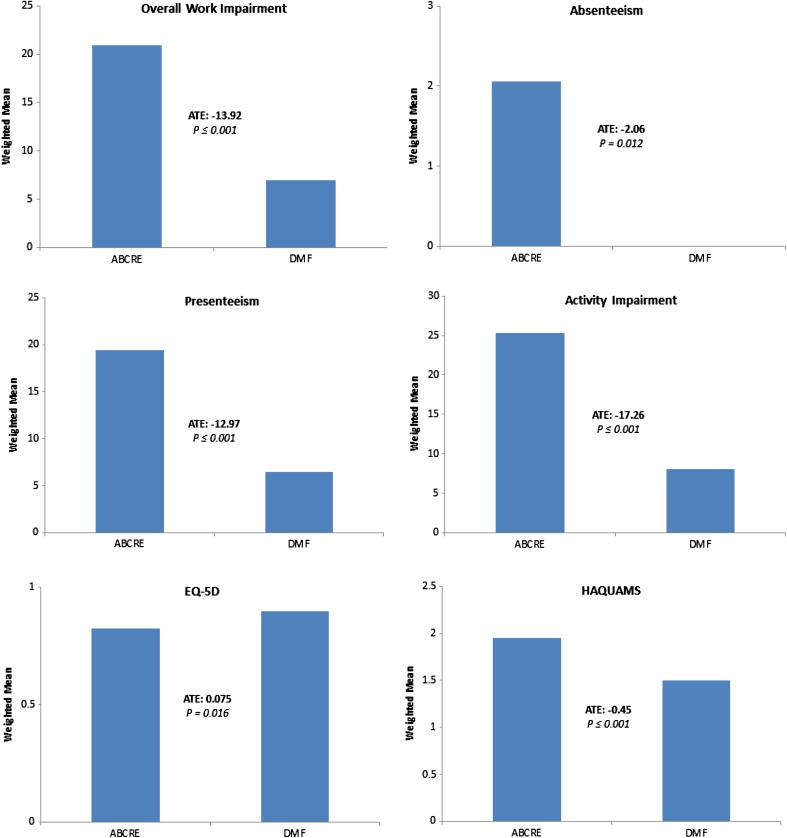
The ATEs for WPAI subscales were estimated in patients receiving DMF therapy versus those receiving ABCRE therapies. Patients receiving DMF therapy were associated with better WPAI scores with ATEs of −13.92 (p < 0.001) and −17.26 (p < 0.001) in overall work impairment and activity impairment subscales, respectively (Table 3; Fig. 1). Patients receiving DMF therapy were also associated with better absenteeism and presenteeism subscale scores than ABCRE patients (absenteeism: ATE = −2.06, p = 0.012; presenteeism −12.97, p < 0.001).
Fig. 1.
Graphical representation of data from the inverse-probability-weighted regression-adjustment analyses presented in Table 2 on patients with multiple sclerosis started on ABCRE therapies vs. DMF therapy. Data include scores for the Work Productivity and Activity Impairment Questionnaire and quality of life measures. ABCRE Therapy involving β interferons (including Avonex, Rebif, Betaseron, and Extavia) and glatiramer acetate (Copaxone), DMF dimethyl fumerate therapy, ATE average treatment effect
An ATE of 0.075 (p = 0.016) in the EQ-5D tool and 10.84 (p ≤ 0.001) in the EQ-VAS was observed in patients receiving DMF compared to ABCRE patients (Table 3; Fig. 1).
Relationship Between Overall Work Impairment Subscale and HRQoL
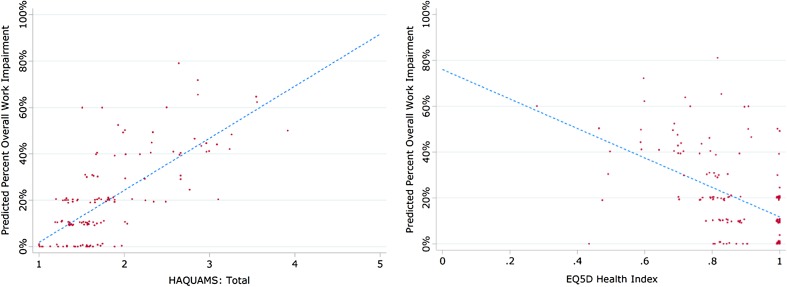
Regression analyses using the EQ-5D and HAQUAMS to predict the WPAI overall work impairment subscale were performed. Increasing EQ-5D scores were significantly associated with a decreasing score on the WPAI overall work impairment subscale (Table 4), demonstrating that for an improvement of 0.1 on the EQ-5D scale, an improvement of 6.42% (95% Cl −8.32 to −4.52; p < 0.001) can be predicted on the WPAI overall work impairment subscale scale. An improvement in the HAQUAMS score of −1.0 was similarly associated with an improvement in the WPAI overall work impairment subscale of 22.44% (95% Cl 18.11–26.77, p < 0.001) (Table 4). A lower HAQUAMS score indicates better HRQoL outcomes; therefore, the data demonstrate that a reduction of 1 point on the HAQUAMS scale leads to an reduction of WPAI overall work impairment by −22.44% on the WPAI overall work impairment subscale. The regression analysis using HAQUAMS and EQ-5D to predict WPAI was plotted to demonstrate that as HRQoL improves, so does work productivity (Fig. 2).
Table 4.
Regression relationship of Work Productivity and Activity Impairment Questionnaire scores with those of the EuroQol Five-Dimensions tool and Hamburg Quality of Life Questionnaire in Multiple Sclerosis questionnaire
| Independent variable | EQ-5D | p value | HAQUAMS | p value |
|---|---|---|---|---|
| EQ-5D | −64.25 (−83.23 to −45.27) | <0.001 | – | – |
| HAQUAMS | – | – | 22.44 (18.11 to 26.77) | <0.001 |
| Age | −0.26 (−0.56 to 0.04) | 0.083 | −0.15 (−0.41 to −0.11) | 0.255 |
| Male | 3.13 (−2.55 to 8.81) | 0.278 | 4.24 (−0.78 to 9.26) | 0.097 |
| Body mass index | 0.20 (−0.55 to 0.94) | 0.601 | 0.09 (−0.59 to 0.76) | 0.800 |
| Comorbidities | 3.02 (0.15 to 5.89) | 0.040 | 0.26 (−2.34 to 2.87) | 0.843 |
Regressions use the EQ-5D and HAQUAMS measures to predict overall work impairment by the Work Productivity and Activity Impairment Questionnaire, after adjusting for age, sex, body mass index and number of comorbid conditions
Fig. 2.
Regression of Work Productivity and Activity Impairment Questionnaire with EuroQol Five-Dimensions (EQ5D) and Hamburg Quality of Life Questionnaire in Multiple Sclerosis Questionnaire (HAQUAMS)
Discussion
The results of this real-world survey of MS patients demonstrate that those receiving DMF therapy experienced higher HRQoL and work productivity than those receiving ABCRE therapy. Among our patient cohort, those receiving DMF use was associated with better outcomes, including overall work impairment, absenteeism, presenteeism, activity impairment, EQ-5D, and HAQUAMS. Regression analyses of these outcomes were subsequently used to predict work productivity as a function of HRQoL [21]. Regression analysis of the EQ-5D and HAQUAMS scores demonstrated the relationship between HRQoL and work productivity outcomes, revealing that as Qol measured by HAQUAMS improved by a single point, the corresponding WPAI score also improved by 22.44% and that as EQ-5D increased by 0.1 (i.e., an improvement), WPAI again improved, in this case by 6.42%.
Full-time employment was lower in ABCRE patients for both the work productivity sample and the HRQoL sample (Table 2). Patients not in full-time employment were asked if this was a due to their MS, but no significant differences were observed in the responses between patients receiving DMF or ABCRE therapies. The ATE was used as a measure of how receiving DMF may impact various outcomes. Our research demonstrates that both absenteeism and presenteeism were improved in patients receiving DMF therapy compared to those receiving ABCRE therapies. This result highlights that not only were patients less likely to miss work as a result of receiving DMF (absenteeism) but that work impairment was reduced while at work (presenteeism). This finding was fortified by results indicating that both overall work impairment and activity impairment were significantly reduced in patients receiving DMF compared to those receiving ABCREs. ATEs for absenteeism and presenteeism in those receiving DMF were associated with improvements compared to those receiving ABCRE therapies, highlighting a better WPAI outcome for DMF patients.
DMF patients had significantly higher HRQoL scores for both the EQ-5D and HAQUAMS. These data are in line with results from published studies [7, 19, 28], but while these published results are from a clinical trial setting using a placebo control group, we have been able to demonstrate a comparison against ABCRE patients in the real-world setting. Further, we have been able to demonstrate, using regression analysis, how improvements in work productivity and activity impairment are associated with improved HRQoL outcomes (Table 4). When we considered specific components of the HAQUAMS subscale, such as fatigue, social function, mood, and mobility, we observed improvements across all subscales in DMF patients compared to ABCRE patients (Table 3). In addition to the outcomes collected, we have generated a WPAI overall work impairment predictor as a function of the HRQoL outcomes, EQ-5D and HAQUAMS (Table 4; Fig. 2). We observed that a better HRQoL score was significantly associated with a better WPAI overall work impairment score, and we have proved this association using two separate HRQoL measures. This is a significant finding as we have demonstrated this relationship using two independently validated HRQoL tools to highlight the relationship between HRQoL and the WPAI overall work impairment subscale in MS patients. A reduced HRQoL score was associated with an increase in work and activity impairment, highlighting that the costs associated with work and activity impairment are likely to be higher for those with poor HRQoL outcomes. This relationship is important to consider as HRQoL outcomes have been demonstrated to affect work and activity impairment outcomes.
The limitations of this study include the small population size. The respondent rate for patients receiving DMF therapy was lower than that of patients receiving ABCRE therapies. When patients were recruited to the study, they were not recruited based on the treatment they received (i.e., we did not recruit 50% of the sample on DMF and the other 50% on ABCREs); therefore, the difference in sample sizes between the two patient groups is more representative of the real-world setting from which they were recruited. A further general concern is that the propensity score is generated using specified variables, and hidden bias may remain in the analysis if important variables are omitted from the procedure. However, the results present a consistent picture of increased HRQoL and work productivity outcomes for DMF patients.
Conclusion
This study has highlighted the potential advantages of using DMF therapy to treat MS patients over ABCRE therapies (Avonex, Betaseron, Rebif, Extavia, and Copaxone). Higher HRQoL and work productivity outcomes were observed in DMF patients than in patients receiving an interferon or glatiramer acetate therapy. The HRQoL outcomes were used as a predictor of work productivity, which demonstrated a positive correlation with the EQ-5D and HAQUAMS measures, suggesting that as HRQoL outcomes improve so does productivity.
Acknowledgments
Sponsorship and article processing charges for this study was provided by Biogen. Medical writing support was provided by Adelphi Real World and funded by Biogen. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Eddie Jones is an employee of Adelphi Real World, the organization contracted by Biogen for the conduct of this study. James Pike is an employee of Adelphi Real World, the organization contracted by Biogen for the conduct of this study. John Waller is an employee of Adelphi Real World, the organization contracted by Biogen for the conduct of this study. Andrew Lee is an employee and stock holder of Biogen Inc. Michael Edwards is an employee and stock holder of Biogen Inc. Jenifer Petrillo is an employee and stock holder of Biogen Inc.
Compliance with Ethics Guidelines
This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors. The research was conducted in full accordance with the US Health Insurance Portability and Accountability Act 1996 (HIPAA; http://www.hhs.gov/ocr/privacy/) All data were collected through local fieldwork partners and fully de-identified prior to receipt by Adelphi.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/2D47F06060F2E04E.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Hersh C, Fox RJ. Multiple sclerosis. 2014. http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/neurology/multiple_sclerosis/. Accessed 29 Feb 2016.
- 3.Schapiro RT. Managing symptoms of multiple sclerosis. Neurol Clin. 2005;23:177–187. doi: 10.1016/j.ncl.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Dutta R, Trapp BD. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Curr Opin Neurol. 2014;27:271–278. doi: 10.1097/WCO.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scalfari A, Neuhaus A, Daumer M, Muraro PA, Ebers GC. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85:67–75. doi: 10.1136/jnnp-2012-304333. [DOI] [PubMed] [Google Scholar]
- 6.Jones KH, et al. How people with multiple sclerosis rate their quality of life: an EQ-5D survey via the UK MS register. PLoS One. 2013;8:e65640. doi: 10.1371/journal.pone.0065640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrithers MD. Update on disease-modifying treatments for multiple sclerosis. Clin Ther. 2014;36:1938–1945. doi: 10.1016/j.clinthera.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D. Early onset multiple sclerosis: a longitudinal study. Neurology. 2002;59:1006–1010. doi: 10.1212/WNL.59.7.1006. [DOI] [PubMed] [Google Scholar]
- 9.Pawate S, Bagnato F. Newer agents in the treatment of multiple sclerosis. Neurologist. 2015;19:104–117. doi: 10.1097/NRL.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 10.Gajofatto A, Bacchetti P, Grimes B, High A, Waubant E. Switching first-line disease-modifying therapy after failure: impact on the course of relapsing-remitting multiple sclerosis. Mult Scler. 2009;15:50–58. doi: 10.1177/1352458508096687. [DOI] [PubMed] [Google Scholar]
- 11.Racke M, Nicholas J, Boster A, Imitola J, O’Connell C. Design of oral agents for the management of multiple sclerosis: benefit and risk assessment for dimethyl fumarate. Drug Des Develop Ther. 2014 doi: 10.2147/DDDT.S50962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venci JV, Gandhi MA. Dimethyl fumarate (Tecfidera): a new oral agent for multiple sclerosis. Ann Pharmacother. 2013;47:1697–1702. doi: 10.1177/1060028013509232. [DOI] [PubMed] [Google Scholar]
- 13.Shen H, Zhou S, Wang J. The paradoxical role of Nrf2 in tumor biology. Crit Rev Eukaryot Gene Expr. 2013;23:37–47. doi: 10.1615/CritRevEukarGeneExpr.2013006288. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linker RA, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 16.Gold R, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 17.Giovannoni G, et al. Efficacy of delayed-release dimethyl fumarate in early multiple sclerosis: post hoc analysis of the phase 3 DEFINE and CONFIRM studies according to baseline cognitive function. Mult Scler J. 2015;21:252–253. doi: 10.1177/1352458514566261. [DOI] [Google Scholar]
- 18.Havrdova E, Gold R, Fox RJ, Giovannoni G, Xiao J, Edwards MR. Association between no evidence of disease activity (NEDA) and long-term clinical efficacy of delayed-release dimethyl fumarate (DMF) in patients with relapsing-remitting multiple sclerosis from the phase 3 study, ENDORSE. Mult Scler J. 2015;21:256–257. doi: 10.1177/1352458514546792. [DOI] [Google Scholar]
- 19.Kappos L, et al. Quality of life outcomes with BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: the DEFINE study. Mult Scler J. 2014;20:243–252. doi: 10.1177/1352458513507817. [DOI] [PubMed] [Google Scholar]
- 20.Gold R, et al. Efficacy of delayed-release dimethyl fumarate in early multiple sclerosis: post hoc analysis of the phase 3 DEFINE and CONFIRM studies according to baseline disability. Mult Scler J. 2015;21:263–264. doi: 10.1177/1352458514537013. [DOI] [Google Scholar]
- 21.Glanz BI, Dégano IR, Rintell DJ, Chitnis T, Weiner HL, Healy BC. Work productivity in relapsing multiple sclerosis: associations with disability, depression, fatigue, anxiety, cognition, and health-related quality of life. Value Health. 2012;15:1029–1035. doi: 10.1016/j.jval.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes—a means to understand. Curr Med Res Opin. 2008;24:3063–3072. doi: 10.1185/03007990802457040. [DOI] [PubMed] [Google Scholar]
- 23.Williams A. EuroQl—a new facility for the measurement of health-related quality-of-life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 24.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 25.Gold SM, et al. Disease specific quality of life instruments in multiple sclerosis: validation of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS) Mult Scler. 2001;7:119–130. doi: 10.1177/135245850100700208. [DOI] [PubMed] [Google Scholar]
- 26.Reilly M, Zbrozek A, Dukes E. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 27.Kurtzke J. Rating neurologic impairment in multiple-sclerosis—an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 28.Kita M, et al. Effects of BG-12 (dimethyl fumarate) on health-related quality of life in patients with relapsing-remitting multiple sclerosis: findings from the CONFIRM study. Mult Scler J. 2014;20:253–257. doi: 10.1177/1352458513507818. [DOI] [PubMed] [Google Scholar]