Introduction
Cutaneous adnexal tumors are rare and can present a diagnostic and therapeutic dilemma. Distinguishing primary cutaneous tumors from metastatic carcinoma to the skin requires thorough diagnostic evaluation, including imaging, endoscopy, and histologic and molecular pathologic analysis. As with many rare cancers, there is no standard of care management.1 Here we present the diagnostic approach for a primary cutaneous adenocarcinoma of the scalp with lymph node metastasis and subsequent multimodal therapy.
Case report
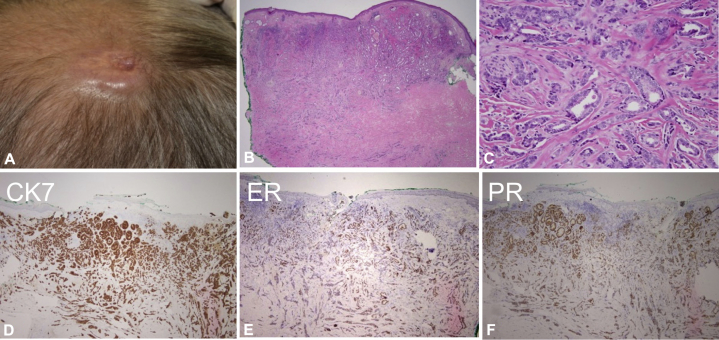
A 60-year-old man with history of an excised benign breast mass 20 years prior (gynecomastia, no adenocarcinoma identified) presented with a 2- × 2-cm fixed pink scalp nodule (Fig 1, A). The lesion was present for multiple years, and did not resolve with intralesional corticosteroids. On review of systems, he reported rectal bleeding but offered no other complaints. Family history was significant for a mother who died of gastric cancer in her 30s. A biopsy showed ductal adenocarcinoma with apocrine features (CK-7+, estrogen receptor [ER]+, and progesterone receptor [PR]+; focal staining for p53; HER2−, Pax8−, and D2-40−; Fig 1, B through F). Positron emission tomography (PET) and computed tomography of the chest, abdomen, and pelvis found no abnormalities. Diagnostic workup showed normal esophagoduodenoscopy and colonoscopy findings. MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets; targeted tumor sequencing test) was negative for somatic alterations in the clinically validated panel.2 Failure to identify a visceral malignancy supported the diagnosis of a primary cutaneous apocrine adenocarcinoma.
Fig 1.
A, Clinical lesion appearing as a pink plaque on the vertex scalp. B, Low-power histology shows ductal adenocarcinoma. C, High-power view of the glandular elements. D-F, Immunohistochemistry shows the tumor was CK-7+, ER+, and PR+.
Staged excision with rush permanent sections found invasive ductal adenocarcinoma in the dermis and subcutis, focally transected at the deep margin. Two days later, a second stage through the galea and periosteum showed persistent rare atypical cells at the calvarial outer table margin. Although computed tomography/PET found no abnormalities, physical examination 1 week after surgery found a firm, palpable postauricular lymph node. Ultrasound-guided biopsy was positive for adenocarcinoma. Staging magnetic resonance imaging of the head and neck showed 2 suspicious nodes but did not suggest tumor invasion of the dura. In the presence of lymph node metastasis, attempting definitive tumor margin clearance at the calvarium was foregone because of risk of bleeding, infection, and cerebrospinal fluid leak. Scalp repair with rotation flap and split-thickness skin graft occurred alongside selective neck dissection, which found adenocarcinoma in subcutaneous tissues with perineural invasion and 2 of 20 positive nodes with extranodal extension. Based on the high-risk features and regional lymph node metastases, intensive adjuvant therapy was recommended with weekly cisplatin and paclitaxel chemotherapy and concurrent image-guided intensity-modulated adjuvant radiation to the scalp and neck with total dose of 66 Gy in 33 fractions. Notably, persistent, biopsy-proven small-volume adenopathy was detected after the lymphadenectomy and required a boost to total dose 70 Gy in 35 fractions (Fig 2, A). He achieved complete response (Fig 2, B) and is 16 months posttreatment without clinical or radiographic evidence of disease. Fig 3 outlines the treatment course.
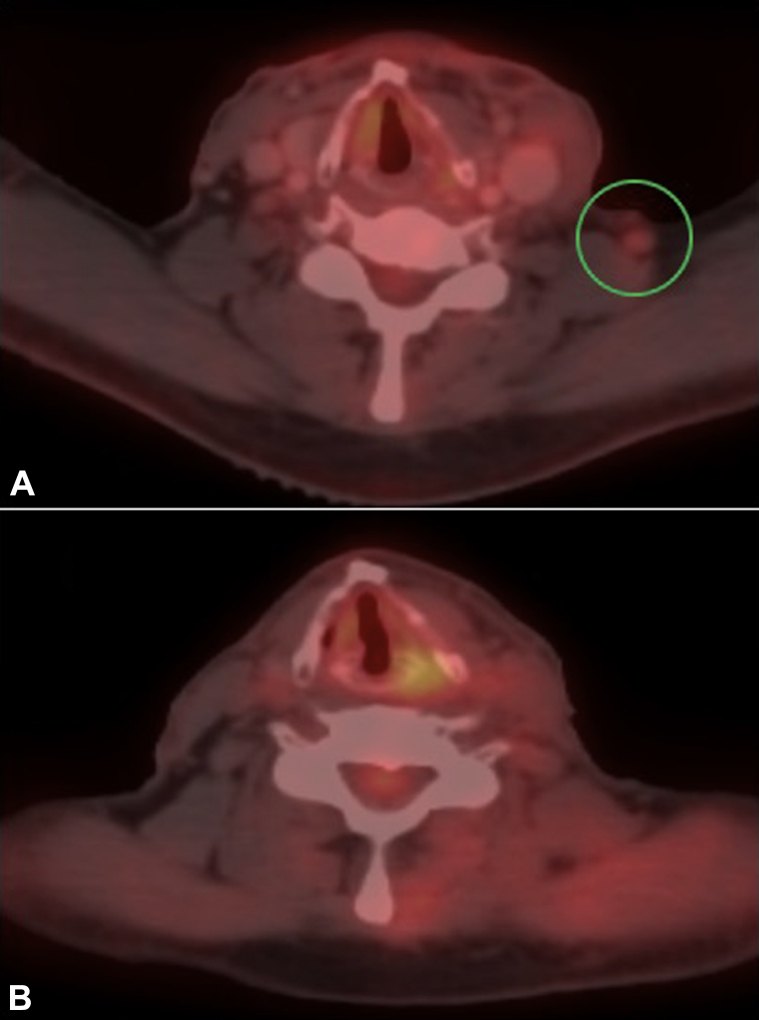
Fig 2.
A, PET after surgery, before radiation therapy, shows recurrent left cervical lymphadenopathy (green circle), proven to harbor carcinoma by fine-needle aspiration. B, PET 12 weeks after radiotherapy shows resolution of the left cervical lymphadenopathy after chemoradiotherapy (this lymph node was not excised).
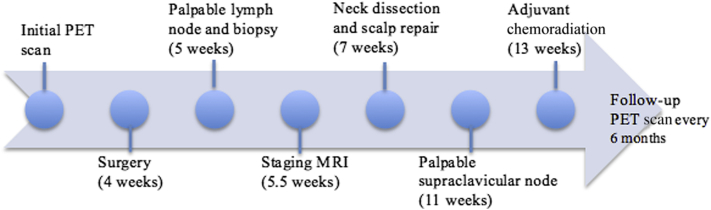
Fig 3.
Timeline of events. Time from initial PET scan is noted in parentheses.
Discussion
Cutaneous adnexal tumors are rare and can be a diagnostic and therapeutic challenge.3 Immunohistochemistry analysis is an important component in distinguishing cancer of unknown primary versus a rare primary tumor.4 However, markers for cancer of unknown primary are not uniformly site specific or sensitive. Adnexal carcinomas may be mistaken for metastatic adenocarcinoma, with immunohistochemistry staining and morphology mimicking breast and salivary gland cancers.5, 6 However, as in our case, when only 1 site of tumor is identified in a patient suspected of having cancer of unknown primary and metastatic workup is negative, one must consider an unusual primary cutaneous tumor mimicking metastatic disease.
A recent study of cutaneous and mammary apocrine carcinomas found that a panel including ER, PR, HER2, and other markers may be useful in distinguishing these diagnoses. Primary cutaneous apocrine carcinoma tends to be adipophilin negative, ER+, PR+/−, and HER2−, whereas mammary apocrine carcinoma is generally adipophilin positive, ER−, PR−, and HER2+.7 Our tumor was ER/PR+, and HER2−, supporting the diagnosis of cutaneous apocrine adenocarcinoma. Nevertheless, because breast carcinomas are exceedingly more common than apocrine carcinomas, a thorough exclusion of an extracutaneous primary is warranted in most cases.
With concern for metastatic disease, MSK-IMPACT testing was performed to hone in on a diagnosis or identify mutations for targeted oncologic drug therapies.2 Unfortunately, MSK-IMPACT testing failed to identify somatic mutations and did not assist in selecting a preferred chemotherapy regimen. However, its utility should not be undermined in the absence of detecting a targetable mutation in this case. Further sequencing of larger cohorts may identify mutations specific to primary cutaneous apocrine carcinomas, distinguishing them from metastatic disease, thus aiding in defining treatment regimens or dictating further workup to identify primary sites of malignancy.
Data for management of primary cutaneous apocrine carcinomas are limited. Although excision is standard treatment, the most important predictor of survival is lymph node status.8 For patients with positive lymph nodes, the median survival time is 33 months compared with 55 months in node-negative disease.8 As such, staging may help better define prognosis in these patients.
In squamous cell carcinoma of mucosal surfaces, adding chemotherapy to radiation is beneficial,9 but there is no definitive data for adding chemotherapy to radiation in cutaneous adenocarcinoma. Otsuka et al treated a metastatic cutaneous apocrine adenocarcinoma, which overexpressed HER2 using anti-HER2 monoclonal antibodies including combination pertuzumab and trastuzumab along with taxane chemotherapy according to treatment of HER2+ metastatic breast cancer.10 The patient also underwent wide local excision and lymph node dissection, along with radiotherapy showing complete response.
Similarly, with gross disease in the left supraclavicular node in our patient, chemotherapy was added to radiation, assuming it would improve the radiation as in squamous cell carcinoma types. ER/PR positivity was not deemed strong enough to merit targeting with hormone blocking (antiestrogen) therapy. Instead, cytotoxic chemotherapy used with regimens that cover breast cancer was chosen, including taxanes with a platinum compound (paclitaxel and cisplatin), with image-guided adjuvant radiation starting 1 week after the first chemotherapy dose.
Conclusion
This case shows the unique workup and therapeutic challenges of an exceedingly uncommon tumor. Initial radiologic evaluation may not show early lymph node metastasis, showing the need for ongoing clinical and radiographic screening. A multimodal treatment regimen may be needed for cutaneous apocrine carcinomas with aggressive behavior. Collaboration between many specialties is warranted for comprehensive management. Long-term follow-up is required to evaluate the outcome, and further reporting of additional cases may help define optimal treatment of these rare tumors.
Acknowledgments
The authors acknowledge the Memorial Sloan Kettering Cancer Center Multidisciplinary Skin Cancer Management Program along with the following clinicians who participated in the care of this patient: Klaus J. Busam, MD, Joseph J. Disa, MD, Snehal G. Patel, MD, Eric J. Sherman, MD, and David P. Kelsen, MD.
Footnotes
Funding sources: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflicts of interest: None declared.
References
- 1.Wang L.S., Handorf E.A., Wu H., Liu J.C., Perlis C.S., Galloway T.J. Surgery and adjuvant radiation for high-risk skin adnexal carcinoma of the head and neck. Am J Clin Oncol. 2015 doi: 10.1097/COC.0000000000000178. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng D.T., Mitchell T.N., Zehir A. Memorial Sloan Kettering-integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danialan R., Mutyambizi K., Aung P., Prieto V.G., Ivan D. Challenges in the diagnosis of cutaneous adnexal tumours. J Clin Pathol. 2015;68(12):992–1002. doi: 10.1136/jclinpath-2015-203228. [DOI] [PubMed] [Google Scholar]
- 4.Conner J.R., Hornick J.L. Metastatic carcinoma of unknown primary: diagnostic approach using immunohistochemistry. Adv Anat Pathol. 2015;22(3):149–167. doi: 10.1097/PAP.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 5.Wick M.R., Ockner D.M., Mills S.E., Ritter J.H., Swanson P.E. Homologous carcinomas of the breasts, skin, and salivary glands. A histologic and immunohistochemical comparison of ductal mammary carcinoma, ductal sweat gland carcinoma, and salivary duct carcinoma. Am J Clin Pathol. 1998;109(1):75–84. doi: 10.1093/ajcp/109.1.75. [DOI] [PubMed] [Google Scholar]
- 6.Mahalingam M., Nguyen L.P., Richards J.E., Muzikansky A., Hoang M.P. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod Pathol. 2010;23(5):713–719. doi: 10.1038/modpathol.2010.46. [DOI] [PubMed] [Google Scholar]
- 7.Piris A., Peng Y., Boussahmain C., Essary L.R., Gudewicz T.M., Hoang M.P. Cutaneous and mammary apocrine carcinomas have different immunoprofiles. Hum Pathol. 2014;45(2):320–326. doi: 10.1016/j.humpath.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Hollowell K.L., Agle S.C., Zervos E.E., Fitzgerald T.L. Cutaneous apocrine adenocarcinoma: defining epidemiology, outcomes, and optimal therapy for a rare neoplasm. J Surg Oncol. 2012;105(4):415–419. doi: 10.1002/jso.22023. [DOI] [PubMed] [Google Scholar]
- 9.Pignon J.P., le Maitre A., Maillard E., Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Otsuka M., Yamasaki O., Kaji T., Shien T., Iwatsuki K. Metastatic cutaneous apocrine adenocarcinoma treated with a combination of pertuzumab-based targeted therapy and taxane chemotherapy: a case report. JAMA Dermatol. 2016;152(1):111–113. doi: 10.1001/jamadermatol.2015.2507. [DOI] [PubMed] [Google Scholar]