Abstract
Ancylostoma caninum, a blood feeding nematode parasite (Family: Ancylostomatidae, Superfamily: Ancylostomatoidea) can cause anaemia, dark reddish-brown to black haemorrhagic diarrhoea, dehydration, wasting and deaths due to heavy blood loss. Adult hook worm parasites recovered from the intestine of a stray dog at the time of necropsy were identified as A. caninum based on morphological characters and morphometric observations involving scanning electron microscopy (SEM). Different developmental stages of hookworm eggs viz. 8 cell stage, morula, gastrula and vermiform were observed during the culture process of faecal sample. High quality SEM photographs showed teeth of dimensions 52.5, 42.3 and 23.5 μm on one side and 55.4, 43.8 and 21.0 μm on the other side along with the presence of characteristic transverse cuticular striations on body surface of A. caninum parasites.
Keywords: Ancylostoma caninum, Dog, Faecal samples, Morphology, Scanning electron microscopy (SEM)
Introduction
Among various nematode parasites of dogs, the hookworms belonging to family ancylostomatidae of superfamily ancylostomatoidea are of great importance due to their blood sucking activities, subsequent pathogenesis. These parasites are further divided into two subfamilies ancylostominae and necatorinae. The buccal capsule of these worms is sub-globular, lips and leaf crown are absent, oral opening is unarmed or with teeth and cutting plates (Bhatia et al. 2010). The severe manifestation mainly depends upon the worm burden, strain and age of the animals (Kaur et al. 2004). Among the most common hookworms, A. caninum (dogs) and A. tubaeforme (cats) are species-specific, whereas A. braziliense, A. ceylanicum and Uncinaria stenocephala are being harboured by both the pet animal species (Anderson 2000). These worms are voracious blood suckers and results in severe pathogenic effects on their hosts. Hookworm infection routinely diagnosed by coprological examination. As Ancylostoma eggs are morphologically indistinguishable from that of other species, the differentiation of different genera and species of hook worms is not feasible through faecal examination, thus complicating epidemiological studies. Unidentified hookworm species were also commonly prevalent in different parts of the India as 50.5 % in Miraj ((Joshi and Sabne 1977), 73 % in Bangalore ((Malaki 1966), 71 % in Uttar Pradesh (Sahai 1969) and 91–99 % in Calcutta (Mapplestone and Bhaduri 1940). The morphological identification of the adult parasites is the supportive aid for the confirmation of positive faecal sample. Further, Scanning electron microscopy (SEM) is beneficial to study the ultrastructure as it provides the detailed three-dimensional and topographical imaging and the versatile information garnered from parasites. Present study deals with morphometric observations coupled with SEM for identification of A. caninum parasites.
Materials and methods
Nematode parasites recovered from a stray nondiscript lethargic, weak, diarroheic dog met with an accident were included in present study. At the time of presentation, on clinical examination the dog emaciated and had fever. Dog died later on and was confirmed positive for rabies by indirect florescent antibody test in brain smear. At the time of necropsy, adult hook worm parasites were recovered from the intestine of the dog. Faecal sample was also collected from rectum of dog and examined by conventional faecal flotation technique for presence of helminth eggs. Quantitative examination of faeces with McMaster egg counting technique was done (Soulsby 2005). Remaining faecal sample was cultured to study the different developmental stages of eggs. Daily faecal floatation from the faeces was carried out to study the changes in ova development. Morphometric measurements were done for specific identification of eggs/parasites by using DPZ-BSW (OLYMPUS) software and conventional stage and occular micrometry.
Further, SEM studies were conducted for exploring minute morphological features of A. caninum parasites. Specimen preparation was done at Electron Microscopy and Nanoscience Laboratory, Punjab Agricultural University (PAU), Ludhiana, Punjab as per the specific protocol. Adult worms were kept overnight in 2.5 % glutaraldehyde immediately after collection. Specimens were washed thrice with 0.2 M rinsing buffer at 4 °C for 15 min each. Rinsing buffer was drained off and specimens were kept in osmium tetraoxide for 2–4 h at 4 °C. Then specimens were again washed thrice with 0.2 M rinsing buffer at 4 °C for 15 min each and drained off with rinsing buffer. Specimens were kept in different concentrations of ethanol viz; 30, 50, 70 and 90 % for 15–20 min each at 4 °C followed by three changes in absolute alcohol for 20 min. Finally, after complete decanting of the sampling container, specimens were placed in desiccators for overnight. Stubbing was done for SEM analysis. High quality SEM pictures of adult parasites were obtained depicting different morphological features.
Results and discussion
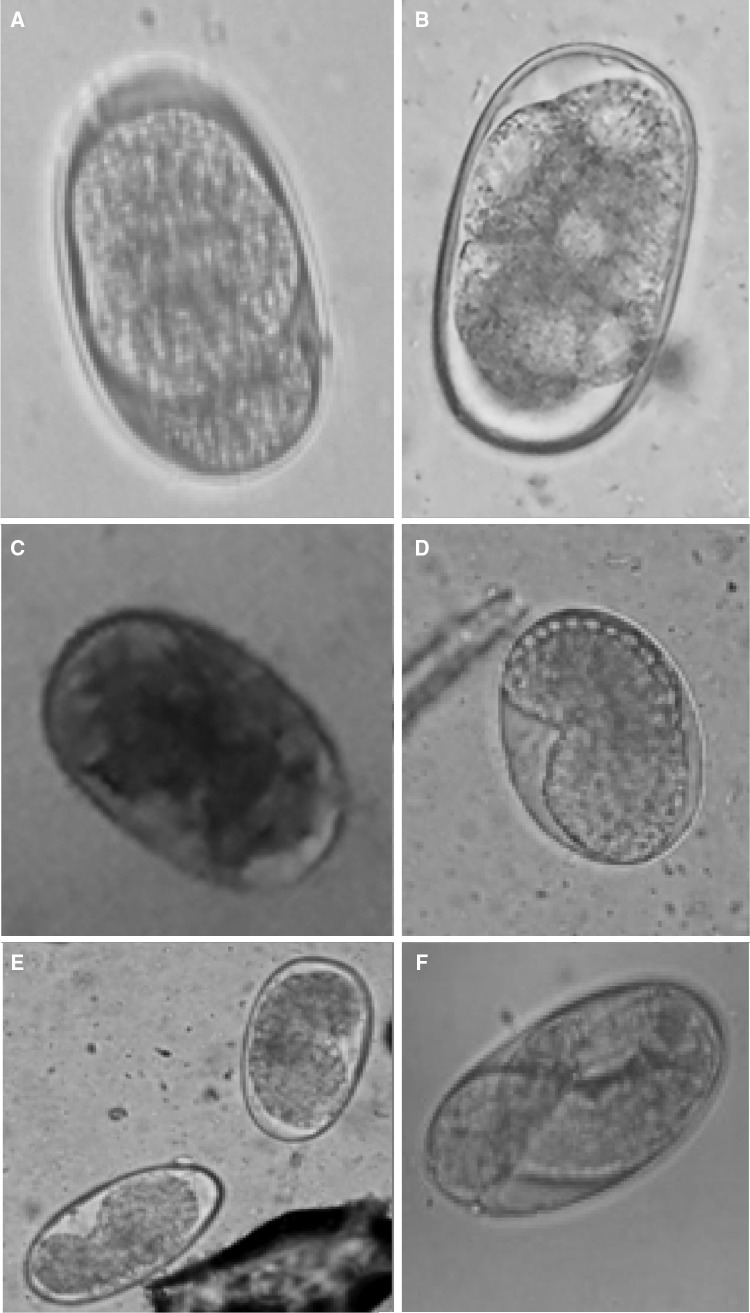
Qualitative analysis by feacal floatation showed presence of hook worm eggs with an average six eggs per field. Quantitative examination revealed 2700 eggs per gam of faeces. Average measurements of the recovered oval eggs [60.6 ± 3.57 µm × 38 ± 2.54 µm (length × breadth)] were relating to Ancylosoma spp. eggs (Soulsby 2005). Hookworms eggs isolated from faecal culture showed different developmental stages viz; 8 cell stage, morula, gastula and vermiform (Fig. 1). Hookworm eggs in the early cleavage stages (2–8 cells) were seen in fresh faecaes (Fig. 1a, b), after 12–18 h morula stage was developed (Fig. 1c), after 36 h morula stage turned into gastrula (Fig. 1d, e) and by 48 h the larvated eggs were found (Fig. 1f).
Fig. 1.
Different developmental stages of hookworm eggs. a, b 2–8 cell stage, c morula stage, d, e gastrula stage, f vermiform stage (larvated egg)
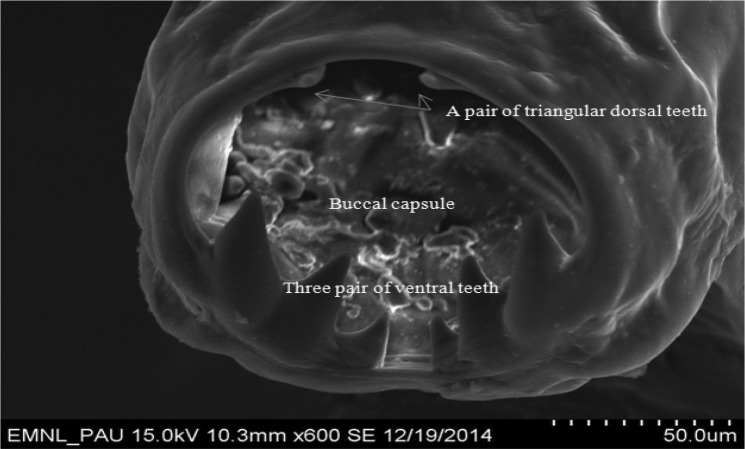
Adult hook worm parasites recovered from the intestine of dog at the time of necropsy were identified as A. caninum based on morphological characters. The worms were fairy rigid and reddish in colour. The anterior end was bent dorsally. Buccal capsule was deep, which contain three pairs of teeth on either side of ventral margin (Figs. 2, 3). In the depth of the capsule there was a pair of triangular dorasal teeth and a pair of centrolateral teeth. Females were larger than males. Males were 10–12 mm long, however, length of female worms was found to be 14–16 mm. The male bursa was well developed. In females, vulva was well developed and was located at the boundary of second and final thirds of the body (Fig. 4). Haemorrhages and congestion were observed in the intestine at the site of worm attachment. Most of the morphological features of A. caninum resembled that of A. tubaeforme. For many years it was presumed that A. caninum occurs in cats, however later studies demonstrated A. tubaeformae to be a valid distinct hook worm species of cats. Mouth capsule of A. tubaeformae is similar to A. caninum, whereas teeth on the ventral margin are slightly larger. Another species prevalent in the tropical area is A. braziliense, which differ from A. caninum, being small in size and ventral teeth consisits of large and small teeth on either sides (Soulsby 2005).
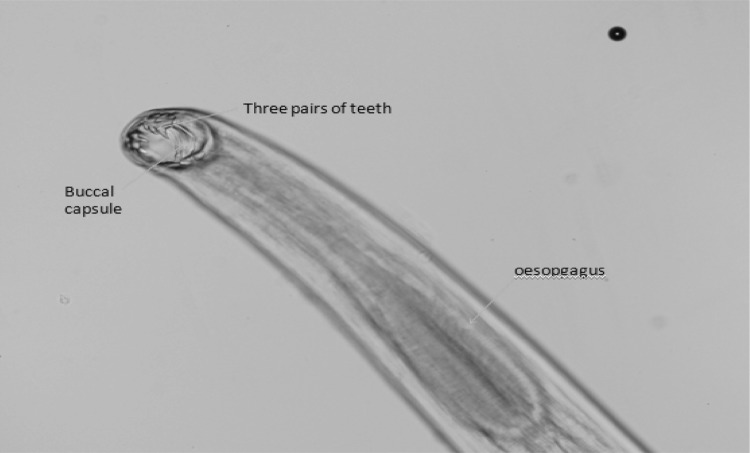
Fig. 2.
Anterior end of Ancylostoma canium showing three pairs of ventral teeth and oesophagus (×10)
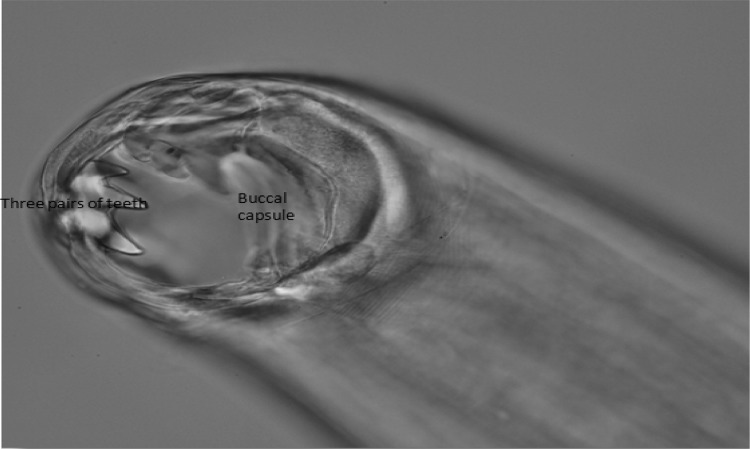
Fig. 3.
Anterior end of Ancylostoma canium three pairs of ventral teeth (Enlarged view ×40)
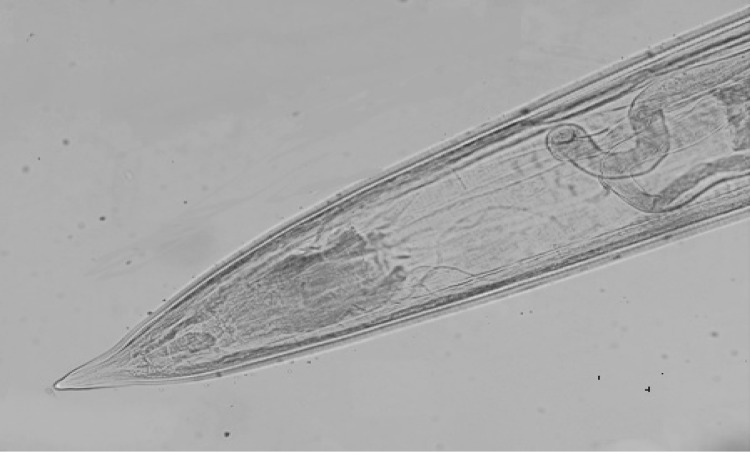
Fig. 4.
Posterior end of Ancylostoma caninum female (×10)
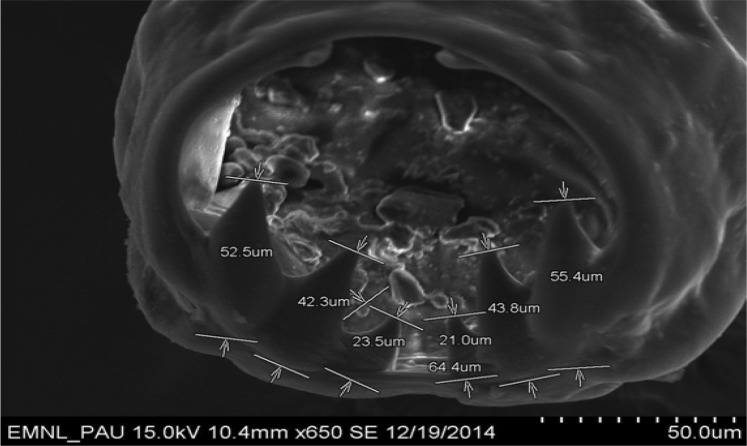
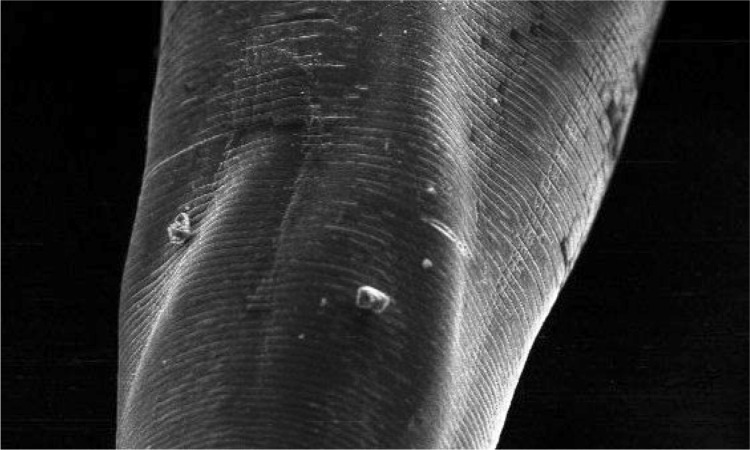
High quality SEM showed deep buccal capsule with three pairs of teeth on either side of ventral margin along with a pair of triangular dorsal teeth (Fig. 5). Measurements of different teeth revealed that all the three pairs of ventral teeth are of different sizes. Dimensions of teeth on one side were measured as 52.5, 42.3 and 23.5 µm, whereas on other side were 55.4, 43.8 and 21.0 µm (Fig. 6). SEM microphotographs also revealed presence of characteristic transverse cuticular striations on body surface (Fig. 7). Carroll et al. (1985) also observed transverse cuticular striations on body surface of Ancylostoma ceylanicum by SEM and observed the distance between adjacent transverse cuticular striations as 5.2 μm. Maciel et al. (2009) observed the capturing of Ancylostoma species larvae by nematophagous fungus by taking SEM photographs at different time intervals. Malan et al. (1986) and Mahannop et al. (1997) employed SEM to study surface morphology of Bunostomum phlebotomum (hook worm of calf) and Necator americanus (hookworm of man), respectively collected from the intestine.
Fig. 5.
Scanning electron microphotograph of Ancylostoma caninum showing three pairs of ventral teeth and a pair of triangular teeth
Fig. 6.
Scanning electron microphotograph of Ancylostoma caninum showing dimensions of ventral and dorsal teeth
Fig. 7.
Scanning electron microphotograph of Ancylostoma caninum showing transverse cuticular striations on body surface
Overall, typical morphometric features confirmed the nematode parasites as A. caninum by conventional microscopy. Further, SEM studies helped in exploring minute morphological details of parasite viz; dimensions of teeth and transverse cuticular striations, which was not feasible by conventional microscopy.
Acknowledgments
Authors are highly thankful to Incharge Electron Microscopy and Nanoscience (EMN) Laboratory, PAU, Ludhiana, Punjab for providing facilities for scanning electron microscopy of parasitic specimens.
References
- Anderson RC. Nematode parasites of vertebrates in their development and transmission. 2. Wallingford, Oxon (UK): CABI Publishing; 2000. [Google Scholar]
- Bhatia BB, Pathak KML, Juyal PD. A text book of veterinary parasitology. New Delhi: Kalyani Publishers; 2010. [Google Scholar]
- Carroll SM, Robertson TA, Papadimitriou JM, Grove DI. Scanning electron microscopy of Ancylostoma ceylanicum and its site of attachment to the small intestinal mucosa of the dog. Z Parasitenkd. 1985;71(1):79–85. doi: 10.1007/BF00932921. [DOI] [PubMed] [Google Scholar]
- Joshi BN, Sabne SS. Incidence of Toxocara canis infection in stray dogs in Miraj area. Indian J Pathol Microbiol. 1977;20:239–242. [PubMed] [Google Scholar]
- Kaur P, Kaur K, Singla LD (2004) Clinicotherapeutic observations on ancylostomosis in dogs. In: Third global meet on parasitic diseases, Bangalore, p 105
- Maciel AS, Araújo JV, Campos AK, Benjamin LA, Freitas LG. Scanning electron microscopy of Ancylostoma spp. dog infective larvae captured and destroyed by the nematophagous fungus Duddingtonia flagrans. Micron. 2009;40(4):463–470. doi: 10.1016/j.micron.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Mahannop P, Chunnoo V, Prathepasaen R. Scanning Electron Microscopy of Human Hookworm, Necator americanus, an Isolate from Thailand. J Health Sci. 1997;6(3):570–574. [Google Scholar]
- Malaki A. A survey of gastro-intestinal parasites of dogs in Bangalore with notes on some interesting observations. Indian Vet J. 1966;43:409–412. [PubMed] [Google Scholar]
- Malan FS, de Kock M, Els HJ. Scanning electron microscopy of Bunostomum phlebotomum (Railliet, 1900) J South Afr Vet Assoc. 1986;57(4):227–230. [PubMed] [Google Scholar]
- Mapplestone FA, Bhaduri NV. The helminth parasites of dogs in Calcutta and their bearing on human parasitology. Indian J Med Res. 1940;28:595. [Google Scholar]
- Sahai BN. A survey of the helminth parasites of stray dogs in and around Bareilly, Uttar Pradesh. Indian Vet J. 1969;46:734. [PubMed] [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7. New Delhi, India: Elsevier India Pvt. Limited; 2005. [Google Scholar]