Abstract
Objective
To evaluate texture data of the torn supraspinatus tendon (SST) on preoperative T2-weighted magnetic resonance arthrography (MRA) using the gray-level co-occurrence matrix (GLCM) for prediction of post-operative tendon state.
Materials and Methods
Fifty patients who underwent arthroscopic rotator cuff repair for full-thickness tears of the SST were included in this retrospective study. Based on 1-year follow-up, magnetic resonance imaging showed that 30 patients had intact SSTs, and 20 had rotator cuff retears. Using GLCM, two radiologists measured independantly the highest signal intensity area of the distal end of the torn SST on preoperative T2-weighted MRA, which were compared between two groups.The relationships with other well-known prognostic factors, including age, tear size (anteroposterior dimension), retraction size (mediolateral tear length), grade of fatty degeneration of the SST and infraspinatus tendon, and arthroscopic fixation technique (single or double row), also were evaluated.
Results
Of all the GLCM features, the retear group showed significantly higher entropy (p < 0.001 and p = 0.001), variance (p = 0.030 and 0.011), and contrast (p = 0.033 and 0.012), but lower angular second moment (p < 0.001 and p = 0.002) and inverse difference moment (p = 0.027 and 0.027), as well as larger tear size (p = 0.001) and retraction size (p = 0.002) than the intact group. Retraction size (odds ratio [OR] = 3.053) and entropy (OR = 17.095) were significant predictors.
Conclusion
Texture analysis of torn SSTs on preoperative T2-weighted MRA using the GLCM may be helpful to predict postoperative tendon state after rotator cuff repair.
Keywords: Rotator cuff, Shoulder joint, Magnetic resonance imaging, Texture analysis, Statistical data analyses
INTRODUCTION
While the number of arthroscopic rotator cuff repairs has increased, complications, including tendon retear, still occur. Recent estimates suggest that retear rates are approximately 11% to 94% (1,2,3). There is no established management for tendon retear, and not all patients receive revision surgery. Some authors have reported that function can be maintained despite retear following rotator cuff repair (4,5). However, if patients experience persistent pain, revision surgery is considered, though the outcome of revision surgery is worse than that of primary repair (6). Moreover, apart from concerns regarding pain and function, persistent morphologic disruption of tendon integrity related to a retear may induce or aggravate osteoarthritis of the shoulder joint (7).
The cause of a tendon retear is thought to be multifactorial and remains controversial. Intrinsic factors may include initial tear size, advanced age, fatty degeneration of muscle, and tendon quality while operating time, repair technique, and overaggressive postoperative rehabilitation is thought to be extrinsic factors contributing to retear (3,8,9,10,11,12,13). Prior studies have revealed that poor quality of the torn rotator cuff tendon is a risk factor for retear, and have made efforts to assess the preoperative integrity of the tendon (3,9,12). Wu et al. (12) and Le et al. (3) have made intraoperative biomechanical evaluations of tendon quality based on thickness and evaluated surgical sutures using four criteria: fair, good, very good, and excellent. Another study evaluated tendon quality arthroscopically based on tissue thickness and mobilization during surgery (9). In consideration of these previous studies, if torn tendon quality can be assessed objectively preoperatively, orthopedic surgeons may be able to predict postoperative outcome better.
Texture analysis has been emerging in the field of medical imaging for the purpose of quantifying spatial heterogeneity to describe tumor characteristics, classify different types of tissue, and automatically segment organs of interest (14,15,16,17). The gray-level co-occurrence matrix (GLCM) is a second-order texture calculation that relies on mathematical methods to describe relationships between the gray level of pixels (usually neighboring) and their spatial information (18).
To the best of our knowledge, there has been no study on imaging-based preoperative assessments of torn tendon quality. Therefore, in this study, we evaluated texture data of torn supraspinatus tendon (SST) on preoperative magnetic resonance arthrography (MRA) using the GLCM to document the relationship between preoperative texture characteristics and postoperative tendon state after rotator cuff repair.
MATERIALS AND METHODS
Study Population
This retrospective study was approved by our hospital's Institutional Review Board, and the requirement to obtain informed consent was waived. Inclusion criteria were as follows: 1) chronic stage of a full-thickness tear of the SST seen on preoperative MRA, 2) full-thickness tear of the SST seen intraoperatively during arthroscopic rotator cuff repair at our institution, and 3) underwent magnetic resonance imaging (MRI) at 1 year postoperatively. Exclusion criteria were as follows: 1) poor image quality (n = 9), or 2) underwent preoperative MRA at an outside hospital (n = 28). We graded the tendon status at the 1-year postoperative follow-up MRI using the classification system of Sugaya et al. (19): type I, repaired cuff appeared to have sufficient thickness compared with a normal cuff with homogeneously low intensity on each image; type II, sufficient thickness compared with normal cuff associated with a partial high-intensity area; type III, insufficient thickness with less than half the thickness when compared with normal cuff, but without discontinuity, suggesting a partial-thickness delaminated tear; type IV, presence of a minor discontinuity in only 1 or 2 slices on both oblique coronal and sagittal images, suggesting a small, full-thickness tear; and type V, presence of a major discontinuity observed in more than 2 slices of both oblique coronal and sagittal images, suggesting a medium or large full-thickness tear. Retear was defined as Sugaya type V and intact tendon was defined as Sugaya type I and II, with the two radiologists' (with 11 years of experience and with 2 years of experience) consent. We also excluded patients who showed Sugaya type III and IV on the follow-up MRI. From January to December 2013, 50 consecutive patients were enrolled in this study. Based on 1-year follow-up MRI, 30 patients had an intact SST and 20 had a retear.
MRA/MRI Acquisition
Both preoperative MRA and postoperative MRI were performed using a 1.5T MR system (Intera, Philips Medical Systems, Best, the Netherlands) with a dedicated shoulder coil. All patients underwent preoperative MRA after injection of 10 to 15 mL gadobutrol (Gadovist, Bayer Healthcare, Berlin, Germany) and a normal saline mixture (1:200, 20 mL) into the glenohumeral joint space via an anterior approach under fluoroscopic guidance. Two-dimensional fast-spin echo images were obtained in the axial, oblique coronal, and oblique sagittal planes. Oblique coronal sequences were obtained perpendicular to the glenohumeral joint, while oblique sagittal sequences were obtained parallel to the glenoid and medially up to the scapular Y view. Axial, oblique coronal, and oblique sagittal T1-weighted fat-suppressed images, and oblique coronal and oblique sagittal T2-weighted images were obtained. Oblique coronal and oblique sagittal T2-weighted images were acquired using the following parameters: repetition time, 2900 to 3500 ms; echo time, 80 to 100 ms; echo train length, 17; flip angle, 90°; number of signal averages, 3; field of view, 200 × 170 mm; and imaging matrix, 256 × 256. Twenty-five slices were obtained, resulting in a slice thickness of 3 mm. Postoperative MRI included two sequences of oblique coronal and oblique sagittal T2-weighted images using the same parameters described above.
Image/Statistical Analysis
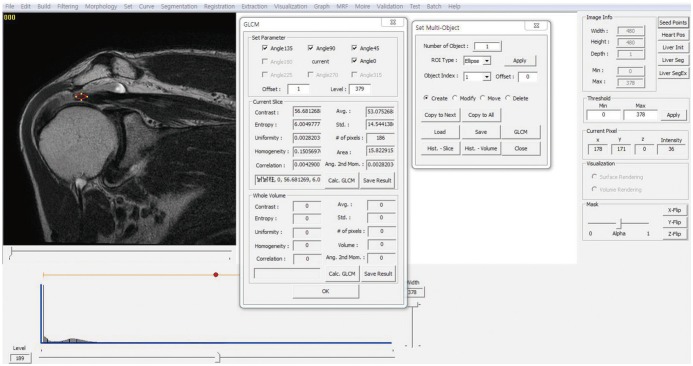
Texture analysis was performed using a homemade GLCM software package made by a specialist (with 18 years of experience in image processing) as follows. Two radiologists, under the agreement, captured and saved a single slice that best presented the full-thickness SST tear on preoperative T2-weighted coronal images as a DICOM file, which the GLCM program processed for texture analysis. One region of interest (ROI) was chosen manually with the GLCM program, which corresponded to the highest signal-intensity area of the distal end of the torn SST. Drawing of the ROI was done under the following guidelines: 1) should be an eclipse shape; 2) should be as large as possible (medial margin: musculotendinous junction, lateral margin: end of the torn tendon); and 3) should include the tendon only, with joint fluid, bony cortex, fat, and other material excluded (Fig. 1). Each radiologist completed this process independently with the 50 DICOM files, and a total of 2 datasets were obtained.
Fig. 1. GLCM program.
Texture analysis of T2-weighted coronal images using gray-level co-occurrence matrix software package. GLCM = graylevel co-occurrence matrix
The GLCM is a second-order texture calculation of how often different combinations of gray levels co-occur in an image or image section (20,21). The following texture parameters were derived: angular second moment (ASM), contrast, entropy, variance, correlation, inverse difference moment (IDM), sum average, sum variance, sum entropy, difference variance, difference entropy, information measures of correlation, and maximal correlation coefficient. In this study, we used ten popular features, including 6 GLCM features (ASM, IDM, contrast, entropy, variance, and correlation) and 4 first-order statistics (mean, standard deviation, skewness, and kurtosis).
Angular second moment, also known as uniformity or energy, is high when the image has very good homogeneity or when the pixels are very similar. IDM represents local homogeneity and is high when the local gray level is uniform, and the inverse GLCM is high. Contrast is the local gray level variation in the GLCM, and can be thought of as the linear dependency of gray levels of neighboring pixels; if the neighboring pixels have very similar gray level values, then the contrast is very low. Entropy is a measure of spatial disorder, which gives us information about which type of texture can be considered statistically more chaotic. Variance measures dispersion around the average value, which becomes larger in coarse images. Correlation measures linear dependency of the gray levels of neighboring pixels. Skewness and kurtosis are numeric measurements of the shape of the data. Skewness is a measure of the asymmetry of the distribution, while kurtosis is a measure of whether the data are peaked or flat about a normal Gaussian distribution. Large ASM, IDM, and correlation values represent homogeneity of the image, while large contrast, entropy, and variance values indicate heterogeneity of the image (20,21).
The relationships with other well-known prognostic factors, including patient age, tear size (anteroposterior dimension), retraction size (mediolateral tear length), grade of fatty degeneration of the SST and infraspinatus tendon (IST), and arthroscopic fixation technique (single or double row), were also evaluated in this study. Tear size was measured at the lateral edge of the footprint, while retraction size was estimated as the distance from the lateral edge of the tear to the footprint of the humerus head. Fatty degeneration of the SST and IST were evaluated according to criteria established by Goutallier et al. (22). Tear size, retraction size, and fatty degeneration were evaluated with agreements of the two radiologists.
All statistical analyses were performed using PASW Statistics version 23 (SPSS Inc., Chicago, IL, USA). Independent t test was performed to statistically assess the age, tear size, retraction size, and GLCM features. Chi-square test was used for the comparison of the fatty grade and fixation technique. The interobserver variability of ROI measurement was evaluated by using intraclass correlation coefficients (ICCs). Binary logistic regression using forward stepwise (conditional) method was performed to determine independent predictors of the variables in contributing retear. In all analysis, statistical significance was defined as p < 0.05.
RESULTS
From January to December 2013, 50 patients (intact group, n = 30; retear group, n = 20) were included in this study. The mean age was 63.4 years (range, 53–81 years) in the intact group and 64.2 years (range, 50–80 years) in the retear group.
The analysis of radiologist 1 revealed significant differences between groups: ASM (p < 0.001), contrast (p = 0.033), entropy (p < 0.001), variance (p = 0.030), correlation (p = 0.009), IDM (p = 0.027), and skewness (p = 0.025) (Table 1). The analysis of radiologist 2 showed significant differences for all texture parameters between groups: ASM (p = 0.002), contrast (p = 0.012), entropy (p = 0.001), variance (p = 0.017), correlation (p = 0.011), IDM (p = 0.027), kurtosis (p = 0.005), and skewness (p = 0.002) (Table 1). These results represent significantly higher entropy, variance, and contrast, but lower ASM and IDM in the retear group compared with the intact group among six GLCM features.
Table 1. Evaluation of Intact and Retear Groups Using Gray-Level Co-Occurrence Matrix.
| Characteristic | Radiologist 1 | Radiologist 2 | ||||
|---|---|---|---|---|---|---|
| Mean | P | Mean | P | |||
| Retear | Intact | Retear | Intact | |||
| First order texture | ||||||
| Mean* | 97.107 | 62.550 | 0.175 | 96.394 | 61.208 | 0.092 |
| Standard deviation* | 41.806 | 19.757 | 0.053 | 48.683 | 23.876 | 0.021 |
| Skewness | 0.737 | 0.264 | 0.025 | 1.182 | 0.523 | 0.002 |
| Kurtosis | 1.212 | 0.316 | 0.133 | 2.841 | 0.260 | 0.005 |
| Second order texture | ||||||
| ASM | 0.002 | 0.003 | < 0.001 | 0.001 | 0.002 | 0.002 |
| Contrast | 862.554 | 190.023 | 0.033 | 645.346 | 202.201 | 0.012 |
| Entropy | 6.617 | 6.141 | < 0.001 | 7.069 | 6.711 | 0.001 |
| Variance | 3661.725 | 530.089 | 0.030 | 3906.174 | 750.414 | 0.017 |
| Correlation† | 0.002 | 0.005 | 0.009 | 0.001 | 0.003 | 0.011 |
| IDM | 0.109 | 0.146 | 0.027 | 0.103 | 0.136 | 0.027 |
*Unit: × 10−6 mm2/sec, †Unit: × 10−3. ASM = angular second moment, IDM = inverse difference moment
The ICCs for the texture parameter measurements made by the two radiologists were more than 0.75 in most parameters, except kurtosis (0.74) (Table 2).
Table 2. Interobserver Agreement for Measurement of Texture Parameters.
| Intraclass Correlation Coefficeints | 95% CI | |
|---|---|---|
| First order texture | ||
| Mean | 0.98 | 0.96–0.99 |
| Standard deviation | 0.96 | 0.93–0.98 |
| Skewness | 0.76 | 0.62–0.85 |
| Kurtosis | 0.74 | 0.59–0.84 |
| Second order texture | ||
| ASM | 0.76 | 0.63–0.86 |
| Contrast | 0.94 | 0.90–0.96 |
| Entropy | 0.82 | 0.72–0.89 |
| Variance | 0.96 | 0.92–0.98 |
| Correlation | 0.82 | 0.72–0.89 |
| IDM | 0.97 | 0.95–0.99 |
ASM = angular second moment, CI = confidence interval, IDM = inverse difference moment
In evaluation of the relationships between other contributing factors and postoperative SST state, only tear size (p = 0.001) and retraction size (p = 0.002) were significantly different between groups (Table 3). Fatty degeneration of the SST (p = 0.094) and IST (p = 0.183) and fixation technique (p = 0.818) showed no significant correlation with retear (Table 3). In multivariate analysis using binary logistic regression, retraction size (odds ratio [OR] = 3.053; 95% confidence interval [CI] = 1.336–6.981; p = 0.008) and entropy (OR = 17.095; 95% CI = 2.298–127.193; p = 0.006) were significant predictors in the trial of radiologist 1 (Fig. 2). However, only retraction size (OR = 3.331; 95% CI = 1.527–7.263; p = 0.002) was a significant factor in the trial of radiologist 2.
Table 3. Clinical Characteristics of Intact and Retear Groups.
| Characteristic | Intact Group (n = 30) | Retear Group (n = 20) | P |
|---|---|---|---|
| Age, years | 63.4 | 64.2 | 0.107 |
| Tear size, cm | 1.4 | 2.3 | 0.001 |
| Retraction size, cm | 1.4 | 2.6 | 0.002 |
| Fatty grade of SST* | 1.9 | 2.5 | 0.094 |
| Fatty grade of IST* | 1.1 | 1.5 | 0.183 |
| Fixation technique, S/D, n | 10/20 | 7/13 | 0.818 |
All data are presented as means unless otherwise noted. *Fatty grade was evaluated using criteria established by Goutallier et al.(22) on the basis of fatty streaks within the muscle belly.
D = double rows, IST = infraspinatus tendon, S = single row, SST = supraspinatus tendon
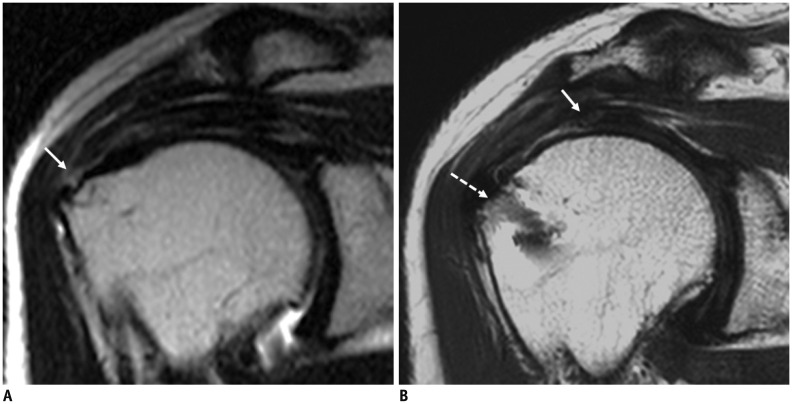
Fig. 2. 70-year-old female with retear shows high entropy value measured by GLCM.
A. Small-sized rim-rent tear of supraspinatus tendon is identified at greater tuberosity in 70-year-old woman on T2-weighted coronal image of right shoulder MRI (arrow), which has high entropy value measured by GLCM. B. One year later, she has retear of supraspinatus tendon (arrow) with large retraction from anchor of rotator cuff repair (dashed arrow) on postoperative MRI. GLCM = gray-level co-occurrence matrix
DISCUSSION
Rotator cuff retearsare common clinically important conditions in older adults and can affect shoulder joint functions, thus leading to a deteriorating quality of life for each affected individual. However, all patietns are not eligible for revision surgery for numerous reasons e.g., the ability to tolerate pain, relatively maintained good function, or the risk inherent in a second operation. Consequently, surgeons need to know which preoperative factors may determine the prognosis following the surgical repair, that is re-tear or not. This study was carried out based on the assumption that preoperative tendon integrity may be related to postoperative tendon state after rotator cuff repair. Assessments were made based on preoperative T2-weighted MRA using the GLCM. Also, some well-known prognostic factors, such as patient age and degree of fatty degeneration of the rotator cuff, might affect the tendon integrity. Our results indicated that patients with tendon retears demonstrated increased textural heterogeneities on preoperative images, including higher entropy, variance, and contrast, and lower ASM and IDM compared with patients with an intact tendon. Thus, increased preoperative heterogeneity may be related to retear of the SST at one year after rotator cuff repair. In contrast, there were no significant differences between groups about other possible contributing factors, except for tear size and retraction size.
Multivariate analysis using binary logistic regression including tear size, retraction size, and six GLCM features, retraction size and entropy may be significant predictors for retear.
Although many researchers have studied the possible factors contributing to rotator cuff retear, they remain unclear. To the best of our knowledge, there have been few reports on the relationship between preoperative tendon integrity and rotator cuff retear (3,12) among the numerous reports on the variable prognostic factors affecting retear, such as the patients' age, tear size, retraction size, and repair technique (2,3,9,11,13,23,24). According to Cummins and Murrell (25), the most common mechanism for a retear might be the tendon pulling through the sutures, i.e., a weak link in the tendon-suture interface. This indicates that the tendon integrity itself, which is an intrinsic quality, may play a critical role in predicting postoperative tendon state. Le et al. (3) found that lower surgeon-rated tissue quality and lower tendon mobility during arthroscopic surgery were significantly related to retear which occurred in 17% of patients. They subjectively rated rotator cuff quality using a 4-point scale (fair, good, very good, or excellent) and reported that patients whose long head of the biceps tendons had completely ruptured were significantly more likely to experience retears. However, this subjective intraoperative tendon evaluation is not entirely conclusive. Wu et al. (12) reported that surgeon-ranked intraoperative assessments were not significant predictors of tendon states after repair compared with tear sizes and the age of patients. Our study tries to demonstrate objective measurement of the tendon quality and supports those of Le et al. (3) in that the GLCM parameters in the retear group showed increased heterogeneity, which is presumed to be due to lower tissue quality. The notable point is that our study implemented preoperative image-based quantitative analysis using the GLCM, which can be reproducible.
Texture analysis is a mathematical technique for quantification of complex structures according to different combinations of gray levels among neighboring pixels. Many studies utilizing the GLCM have been emerging in the field of medical imaging (14,15,16,17,26,27). Researchers targeting tumors using enhanced images have reported that locally increased heterogeneity of the tumor was related to increased vascularity and distortion of normal vascular structures (15,17). In our study, the presumed reason for the textural heterogeneity of retorn tendons could be related to change in collagen composition due to degeneration (28), fraying of the tendon, or inevitably measured interposing joint fluid in the fraying tendon. Further study about GLCM features of SST in young and healthy patients will be helpful to evaluate the influence on the degeneration of the torn tendon.
Our results also support the significant relationships between the rotator cuff retear, the initial tear size (anteroposterior dimension) and retraction size (mediolateral tear length). Le et al. (3) also emphasized that the preoperative tear size (dimension, area, and thickness) was a strong predictor of retear, while Wu et al. (12) reported that the tear size (area) was the best intraoperative predictor of repair integrity after rotator cuff repair.
Recently, there has been controversy about whether advancing age or repair technique could be independent predicting factors for retears. Most previous studies have reported that advancing age is related to retear for such reasons as poor tissue perfusion and decreased healing potential (1,3,9,12,23,29). Oh et al. (10) reported that age was a significant predicting factor for postoperative cuff integrity according to univariate analysis; however, multivariate regression analysis revealed that age was not an independent determinant of the anatomical or functional outcome, whereas tear retraction and fatty degeneration of the IST were independent factors for repair integrity. Our results also confirmed that age was not a determinant for retear, though our study was limited to univariate analysis, which is not free of confounding factors. However, like our study, tendon heterogeneity related to degeneration by aging may be evaluated indirectly using GLCM features.
Nozaki et al. (30) found that MR imaging quantification of preoperative fat fractions by using a two-point Dixon sequence within the rotator cuff muscles may play a significant role in predicting postoperative retear. The preoperative fat fractions in the supraspinatus muscle were significantly higher in the failed repair group than in the intact-repair group. Interestingly, our study did not find a significant difference in the degree of fatty degeneration of the rotator cuff between the retear and intact groups, which may have been due to the small number of cases in our study.
We also investigated the possible role of surgical technique, which was divided into single row and double row. We found no significant difference in surgical technique between groups, which is in-line with the results of Chung et al. (11).
A notable point of our study is that we suggested a simple method for prediction of postoperative tendon state that utilizes preoperative T2-weighted images. To the best of our knowledge, there has been no report on the association between preoperative tendon integrity, and postoperative rotator cuff retears based on MRA.
A major limitation of this study is that only 50 cases were included, which is a relatively small number compared with previous studies. Second, for each case, we used only one image slice that best presented the full-thickness SST tear on coronal T2-weighted images. Third, we did not acquire surgical confirmation for the retear. However, our study only included Sugaya type V, which showed major discontinuity of a tendon. According to a recent study (31), repaired SSTs exhibited high signal intensity in 90% of clinically improving patients on the early postoperative MRI, and decreased signal intensity on the later MRI. The only high signal of repaired tendon probably means a gradual healing process rather than a retear. Furthermore, this study was intended to show preoperative GLCM characteristics of signal intensity in overt retear group, and we did not include the cases with Sugaya type III or IV, because these types may not be clinically significant retear patterns affecting the functional outcome or need for re-operation (32). Lastly, as this was a retrospective study, we could not perform multifactorial analysis regarding other possible prognostic factors, such as postoperative care/rehabilitation and degree of patient activity.
In conclusion, based on objective texture analysis using the GLCM, preoperative tendon integrity on T2-weighted MRA may be related to postoperative tendon state at one year after rotator cuff repair. Therefore, texture analysis using the GLCM may serve as a complementary tool for prediction of tendon retear.
Footnotes
This work was supported by the grant No. 09-2014-005 from the SNUBH Research Fund.
References
- 1.Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229–1240. doi: 10.2106/JBJS.D.02035. [DOI] [PubMed] [Google Scholar]
- 2.Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89:1248–1257. doi: 10.2106/JBJS.E.00743. [DOI] [PubMed] [Google Scholar]
- 3.Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42:1134–1142. doi: 10.1177/0363546514525336. [DOI] [PubMed] [Google Scholar]
- 4.Jost B, Zumstein M, Pfirrmann CW, Gerber C. Long-term outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am. 2006;88:472–479. doi: 10.2106/JBJS.E.00003. [DOI] [PubMed] [Google Scholar]
- 5.Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73:982–989. [PubMed] [Google Scholar]
- 6.Denard PJ, Burkhart SS. Arthroscopic revision rotator cuff repair. J Am Acad Orthop Surg. 2011;19:657–666. doi: 10.5435/00124635-201111000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Jost B, Pfirrmann CW, Gerber C, Switzerland Z. Clinical outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am. 2000;82:304–314. doi: 10.2106/00004623-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35:719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 9.Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18:13–20. doi: 10.1016/j.jse.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 10.Oh JH, Kim SH, Kang JY, Oh CH, Gong HS. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010;38:672–678. doi: 10.1177/0363546509352460. [DOI] [PubMed] [Google Scholar]
- 11.Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39:2099–2107. doi: 10.1177/0363546511415659. [DOI] [PubMed] [Google Scholar]
- 12.Wu XL, Briggs L, Murrell GA. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771–2776. doi: 10.1177/0363546512462677. [DOI] [PubMed] [Google Scholar]
- 13.Maqdes A, Abarca J, Moraiti C, Boughebri O, Dib C, Leclère FM, et al. Does preoperative subscapularis fatty muscle infiltration really matter in anterosuperior rotator cuff tears repair outcomes? A prospective multicentric study. Orthop Traumatol Surg Res. 2014;100:485–488. doi: 10.1016/j.otsr.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging. 2012;3:573–589. doi: 10.1007/s13244-012-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun BL, Cho N, Li M, Jang MH, Park SY, Kang HC, et al. Intratumoral heterogeneity of breast cancer xenograft models: texture analysis of diffusion-weighted MR imaging. Korean J Radiol. 2014;15:591–604. doi: 10.3348/kjr.2014.15.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loizou CP, Petroudi S, Seimenis I, Pantziaris M, Pattichis CS. Quantitative texture analysis of brain white matter lesions derived from T2-weighted MR images in MS patients with clinically isolated syndrome. J Neuroradiol. 2015;42:99–114. doi: 10.1016/j.neurad.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Raja JV, Khan M, Ramachandra VK, Al-Kadi O. Texture analysis of CT images in the characterization of oral cancers involving buccal mucosa. Dentomaxillofac Radiol. 2012;41:475–480. doi: 10.1259/dmfr/83345935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haralick RM, Shanmugam K. Textural features for image classification. IEEE Trans Syst Man Cybern Syst. 1973;3:610–621. [Google Scholar]
- 19.Sugaya H, Maeda K, Matsuki K, Moriishi J. Functional and structural outcome after arthroscopic full-thickness rotator cuff repair: single-row versus dual-row fixation. Arthroscopy. 2005;21:1307–1316. doi: 10.1016/j.arthro.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Gebejes A, Huertas R. Texture characterization based on grey-level co-occurrence matrix. Conference of Informatics and Management Sciences. Zilina: EDIS-Publishing Institution of the University of Zilina; 2013. pp. 375–378. [Google Scholar]
- 21.Mohanaiah P, Sathyanarayana P, GuruKumar L. Image texture feature extraction using GLCM approach. Int J Sci Res Publ. 2013;3:1–5. [Google Scholar]
- 22.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78–83. [PubMed] [Google Scholar]
- 23.Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33:1861–1868. doi: 10.1177/0363546505279575. [DOI] [PubMed] [Google Scholar]
- 24.Shen C, Tang ZH, Hu JZ, Zou GY, Xiao RC. Incidence of retear with double-row versus single-row rotator cuff repair. Orthopedics. 2014;37:e1006–e1013. doi: 10.3928/01477447-20141023-58. [DOI] [PubMed] [Google Scholar]
- 25.Cummins CA, Murrell GA. Mode of failure for rotator cuff repair with suture anchors identified at revision surgery. J Shoulder Elbow Surg. 2003;12:128–133. doi: 10.1067/mse.2003.21. [DOI] [PubMed] [Google Scholar]
- 26.Yip C, Landau D, Kozarski R, Ganeshan B, Thomas R, Michaelidou A, et al. Primary esophageal cancer: heterogeneity as potential prognostic biomarker in patients treated with definitive chemotherapy and radiation therapy. Radiology. 2014;270:141–148. doi: 10.1148/radiol.13122869. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Tong L, Wang L, Li N. Texture analysis of multiple sclerosis: a comparative study. Magn Reson Imaging. 2008;26:1160–1166. doi: 10.1016/j.mri.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994;53:359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saridakis P, Jones G. Outcomes of single-row and double-row arthroscopic rotator cuff repair: a systematic review. J Bone Joint Surg Am. 2010;92:732–742. doi: 10.2106/JBJS.I.01295. [DOI] [PubMed] [Google Scholar]
- 30.Nozaki T, Tasaki A, Horiuchi S, Ochi J, Starkey J, Hara T, et al. Predicting Retear after repair of full-thickness rotator cuff tear: two-point dixon MR imaging quantification of fatty muscle degeneration-initial experience with 1-year follow-up. Radiology. 2016;280:500–509. doi: 10.1148/radiol.2016151789. [DOI] [PubMed] [Google Scholar]
- 31.Lee JE, Park JS, Ryu KN, Rhee YG, Yoon SH, Park SY, et al. Repaired supraspinatus tendons in clinically improving patients: early postoperative findings and interval changes on MRI. Korean J Radiol. 2015;16:363–371. doi: 10.3348/kjr.2015.16.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89:953–960. doi: 10.2106/JBJS.F.00512. [DOI] [PubMed] [Google Scholar]