Abstract
Background
Dysnatremia is a risk factor for poor outcomes. We aimed to describe the prevalence and outcomes of various dysnatremia in hospitalized patients. High-risk patients must be identified to improve the prognosis of dysnatremia.
Material/Methods
This prospective study included all adult patients admitted consecutively to a university hospital between October 1, 2014 and September 30, 2015.
Result
All 90 889 patients were included in this study. According to the serum sodium levels during hospitalization, the incidence of hyponatremia and hypernatremia was 16.8% and 1.9%, respectively. Mixed dysnatremia, which was defined when both hyponatremia and hypernatremia happened in the same patient during hospitalization, took place in 0.3% of patients. The incidence of dysnatremia was different in various underlying diseases. Multiple logistic regression analyses showed that all kinds of dysnatremia were independently associated with hospital mortality. The following dysnatremias were strong predictors of hospital mortality: mixed dysnatremia (OR 22.344, 95% CI 15.709–31.783, P=0.000), hypernatremia (OR 13.387, 95% CI 10.642–16.840, P=0.000), and especially hospital-acquired (OR 16.216, 95% CI 12.588–20.888, P=0.000) and persistent (OR 22.983, 95% CI 17.554–30.092, P=0.000) hypernatremia. Hyponatremia was also a risk factor for hospital mortality (OR 2.225, 95% CI 1.857–2.667). However, the OR increased to 56.884 (95% CI 35.098–92.193) if hyponatremia was over-corrected to hypernatremia.
Conclusions
Dysnatremia was independently associated with poor outcomes. Hospital-acquired and persistent hypernatremia were strong risk factors for hospital mortality. Effective prevention and proper correction of dysnatremia in high-risk patients may reduce the hospital mortality.
MeSH Keywords: Acute Kidney Injury; Adolescent, Hospitalized; Hypernatremia; Hyponatremia; Mortality
Background
Dysnatremia, a disorder of sodium concentration, is common not only in critically ill patients but also hospitalized patients, and it confers increased risk for adverse outcomes [1]. The prevalence of dysnatremia in the intensive care unit (ICU) ranges from 6.9 to 17.7%, and varies according to the time of onset, the threshold for diagnosis, and the population being assessed [2]. Except for hyponatremia in heart failure [3,4] and cirrhosis [5], there have been rare reports of dysnatremia in hospitalized patients. Substantial additional work is still required to determine the actual occurrence of dysnatremia in clinical settings.
Dysnatremia at admission, including hyponatremia [6] and hypernatremia [7], is an independent risk factor for mortality. Even slightly abnormal sodium levels are independently associated with poor outcomes [2,8]. However, hospital-acquired (HA) dysnatremia may be more lethal than community-acquired (CA) dysnatremia [9], and there may be significant differences in the clinical characteristics, outcomes, and economic burdens between them [10,11]. If iatrogenic pathogenic factors are avoided, and high-risk patients are identified and treated properly, the incidence of hospital-acquired dysnatremia can be decreased.
Although a recent study [12] showed that the impact of sodium correction on mortality was not significant in the presence of multiple comorbidities or severe diseases, the correction of dysnatremia is another important factor influencing the prognosis [13]. About 3.6 to 6.4% of ICU patients developed both hypo- and hypernatremia during hospitalization [14]; this mixed dysnatremia may be induced by improper correction and is associated with mortality rates of up to 42% [15]. Persistent hyponatremia was an independent predictor of death in heart failure patients, but whether persistent dysnatremia predicts higher mortality than improved ones has not been investigated in hospitalized patients.
This study aimed to describe the prevalence of dysnatremia in various underlying diseases, to evaluate the impact of different dysnatremia on outcomes, and to identify high-risk patients who may benefit from effective prevention and proper correction.
Material and Methods
Study design and data collection
This study prospectively enrolled all adult patients admitted consecutively to Zhongshan Hospital, Fudan University in Shanghai, China, between October 1, 2014 and September 30, 2015. All the data were collected from the electronic medical record database of this university hospital. The data included demographics, categories of underlying diseases, and laboratory values, including electrolyte status at admission and during hospitalization. The primary outcome was hospital mortality, while the incidence of acute kidney injury (AKI), the length of hospital stay, and hospital costs were also recorded as secondary outcomes. This study was approved by the Institutional Review Board of the Ethics Committee, Zhongshan Hospital, Fudan University, Shanghai China. As an observational survey, the requirement for informed consent was waived. The patient records and information were anonymized and de-identified before analysis.
Definitions and calculation
Hyponatremia and hypernatremia were defined according to the reference range provided by the Central Laboratory (normal range: 137~147mmol/L). Mixed dysnatremia was defined as both hyponatremia and hypernatremia during hospitalization in the same patient [16]. It was defined as “hypo- to hyper-” mixed dysnatremia if hyponatremia occurred before hypernatremia, and “hyper- to hypo-” if not. CA-dysnatremia was diagnosed when dysnatremia happened at admission, HA-dysnatremia was defined when dysnatremia occurred after 24 h of hospitalization [11], persistent dysnatremia was any degree of dysnatremia which persisted until discharge or death, and if not, improved dysnatremia was defined. We defined acute kidney injury (AKI) according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria [17]: an increase in serum creatinine (SCr) ≥26.5 μmol/L (0.3 mg/dl) within 48 h or an increase in SCr to ≥1.5 times baseline known or presumed to have occurred within the prior 7 days. We did not consider the urine output for unavailable data. All samples were analyzed in our Central Laboratory. The anion gap (AG) was calculated by the standard formula [18]: AG=[Na+]–[Cl−]–[HCO3−], with an elevated AG defined as greater than or equal to 16 mmol/L. Calculated osmolality was=2×([Na+]+[K+])+[glucose]+[urea], with the normal range from 280 to 310 mOsm/L. Hyperkalemia, hypokalemia, hyperchloremia, hypochloremia, hypercalcemia, hypocalcemia, hyperphosphatemia, hypophosphatemia, hypermagnesemia, hypomagnesemia, hyperuricemia, and hypouricemia were defined according to the reference ranges provided by the Central Laboratory.
Statistical methods
The data were analyzed using SPSS version 24.0 software (SPSS, Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation (SD) if they were statistically normally distributed and categorical variables as numbers and percentages. For the continuous variables, data were analyzed using the t test, analysis of variance (ANOVA) (post hoc analysis of LSD between 2 groups), Mann-Whitney test, or Kruskal–Wallis test, depending on their distribution and number of variables. The chi-squared (χ2) test was used to compare categorical variables. Kaplan-Meier survival curves stratified by dysnatremia categories were plotted and compared using a signed log-rank test. Multiple binary logistic regression models with the Wald forward stepwise method were used to assess the independent risk factors for hospital mortality, and the results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). Three multiple logistic regression models were used according to different classification methods of dysnatremia, and further subgroup analyses were conducted. We also used multinomial logistic regression analysis to identify risk factors for developing HA-dysnatremia and persistent dysnatremia. Normonatremia was the reference of HA-dysnatremia and improved dysnatremia as the reference of persistent dysnatremia. A P-value <0.05 was deemed to indicate statistical significance.
Results
Demographic and baseline clinical characteristics
The study flowchart is presented in Figure 1. We included 90 889 patients in this study after the screening. The median age was 59 years old (inter-quartile range [IQR]: 49–67), and 60.9% of the included patients were males. The underlying diseases were cardiovascular (17.8%), cancer (14.7%), general surgery (11.9%), digestive (11.1%), urinary and renal (7.6%), cardiothoracic surgery (7.3%), pulmonary (6.8%), orthopedic surgery (3.8%), endocrine (3.0%), hematological (3.0%), neurological (2.9%), obstetrics and gynecology (2.3%), and others (7.9%). According to the serum sodium levels during hospitalization, the included subjects were divided into 4 groups: hyponatremia (16.8%), normonatremia (81.1%), hypernatremia (1.9%), and mixed dysnatremia (0.3%). In the hyponatremia group, 7059 patients (46.3%) developed hyponatremia at admission and 8180 patients (53.7%) after 24 h of hospitalization, while 5214 patients (34.2%) improved and 10 025 patients (65.8%) remained persistent. In the hypernatremia group, 1687 patients were divided into community-acquired (n=850, 50.4%) and hospital-acquired (n=837, 49.6%) hypernatremia, while 859 patients (50.9%) had improved hypernatremia and 828 patients (49.1%) had persistent hypernatremia. Mixed dysnatremia patients were also divided into “hypo- to hyper-” group (n=102) and “hyper- to hypo-” group (n=183) according to the sequence of dysnatremia.
Figure 1.
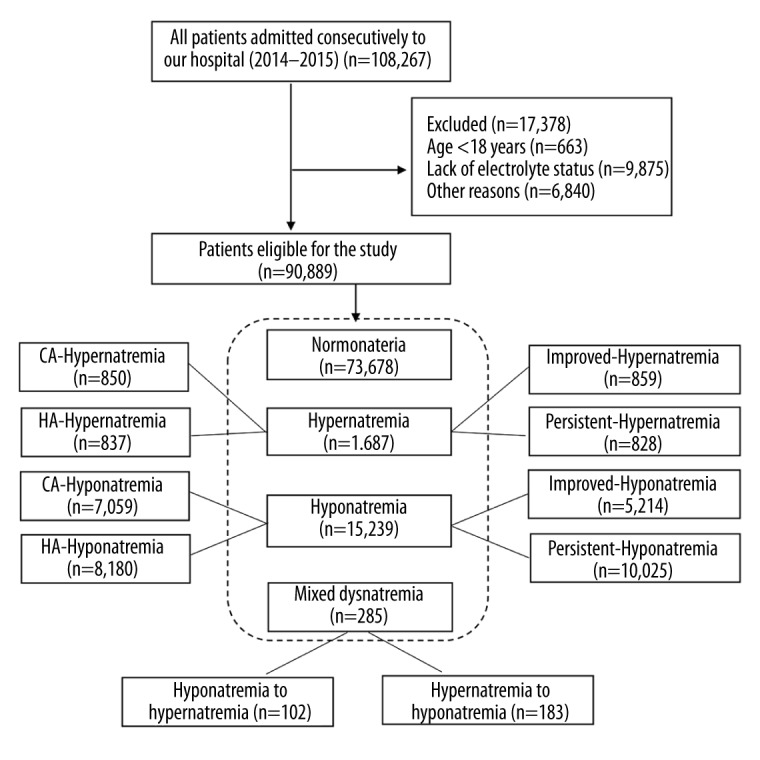
Flowchart of the study.
CA – community-acquired;
HA – hospital-acquired.
Characteristics of the study subjects are listed in Tables 1–3. As described in Table 1, patients in the 3 dysnatremia groups were older, had higher white blood cell counts (WBC), blood urea nitrogen (BUN), serum uric acid (SUA), and AG, had lower albumin, hemoglobin, and carbon dioxide combining power (CO2CP), and had more severe liver injury at admission than patients in the normonatremia group. Table 2 shows that patients in the HA-hyponatremia were younger, had a higher body mass index (BMI), hemoglobin, albumin, SCr, and SUA, had lower WBC and glucose, and had better liver function than patients in the CA-hyponatremia group. HA-hypernatremia patients were younger, had higher WBC, lower hemoglobin, albumin, and CO2CP, and had more severe liver injuries at admission than CA-hypernatremia patients. Table 3 shows that the patients with persistent hyponatremia were older, more were males, and more had higher aspartate aminotransferase (AST) and total bilirubin (TBIL) and lower WBC, SCr, serum sodium, and osmolality at admission than the patients with improved hyponatremia. Compared with the improved hypernatremia group, patients in the persistent hypernatremia group had higher serum sodium, chlorine, magnesium, calcium, phosphorus, and osmolality, and had lower AST at admission. Patients in the “hypo- to hyper-” mixed dysnatremia group were older, had higher WBC and K+, and had lower hemoglobin, albumin, SUA, Na+, Cl−, osmolality, and CO2CP at admission than the patients in the “hyper- to hypo-” group.
Table 1.
Baseline characteristics of patients according to dysnatremia during hospitalization.
| Variable | All (n=90,889) | Normonatremia (n=73,678) | Hyponatremia (n=15,239) | Hypernatremia (n=1,687) | Mixed dysnatremia (n=285) | P value |
|---|---|---|---|---|---|---|
| Age, year | 59.0 (49.0–67.0) | 59.0 (48.0–67.0) | 61.0 (51.0–70.0)# | 64.0 (54.0–74.0)# | 66.0 (56.0–74.0)# | 0.000 |
| >65, n (%) | 27,680 (30.5) | 21,135 (28.7) | 5,649 (37.1) | 751 (44.5) | 145 (50.9) | 0.000 |
| Male sex, n (%) | 55,383 (60.9) | 43,966 (59.7) | 10,267 (67.4) | 968 (57.4) | 182 (63.9) | 0.000 |
| BMI, kg/m2 | 23.2 (20.8–25.6) | 23.4 (21.0–25.7) | 22.7 (20.5–25.1)# | 23.6 (21.4–25.9) | 22.5 (20.7–25.5) | 0.000 |
| Underlying diseases, n (%)§ | 0.000 | |||||
| Cancer | 13,324 (100.0) | 8,926 (67.0) | 4,177 (31.3) | 188 (1.4) | 33 (0.2) | |
| Pulmonary | 6,164 (100.0) | 4,890 (79.3) | 1,151 (18.7) | 108 (1.8) | 15 (0.2) | |
| Cardiovascular | 16,139 (100.0) | 14,427 (89.4) | 1,445 (9.0) | 245 (1.5) | 22 (0.1) | |
| Digestive | 10,091 (100.0) | 8,614 (85.4) | 1,363 (13.5) | 100 (1.0) | 14 (0.1) | |
| Endocrine | 2,707 (100.0) | 2,426 (89.6) | 215 (7.9) | 66 (2.4) | 0 (0.0) | |
| Hematological | 2,753 (100.0) | 2,319 (84.2) | 380 (13.8) | 45 (1.6) | 9 (0.3) | |
| Neurological | 2,681 (100.0) | 2,113 (78.8) | 393 (14.7) | 149 (5.6) | 26 (1.0) | |
| Urinary and renal | 6,934 (100.0) | 5,961 (86.0) | 810 (11.7) | 146 (2.1) | 17 (0.2) | |
| Cardiothoracic surgery | 6,633 (100.0) | 4,075 (61.4) | 2,156 (32.5) | 304 (4.6) | 98 (1.5) | |
| General surgery | 10,857 (100.0) | 8,609 (79.3) | 2,045 (18.8) | 172 (1.6) | 31 (0.3) | |
| Orthopedic surgery | 3,411 (100.0) | 3,091 (90.6) | 277 (8.1) | 39 (1.1) | 4 (0.1) | |
| Gynecological | 2,056 (100.0) | 1,731 (84.2) | 302 (14.7) | 23 (1.1) | 0 (0.0) | |
| Others | 7,139 (100.0) | 6,496 (91.0) | 525 (7.4) | 102 (1.4) | 16 (0.2) | |
| Clinical data at admission | ||||||
| SBP, mmHg | 122.0 (115.0–135.0) | 122.0 (116.0–135.0) | 120.0 (110.0–134.0)# | 130.0 (119.0–140.0)# | 128.0 (110.8–140.0) | 0.000 |
| DBP, mmHg | 79.0 (70.0–80.0) | 80.0 (70.0–80.0) | 77.0 (70.0–80.0)# | 80.0 (70.0–82.0) | 75.0 (66.0–80.0)** | 0.000 |
| WBC, 109/L | 6.0 (4.8–7.5) | 5.9 (4.8–7.3) | 6.4 (4.9–8.6)# | 6.4 (5.0–8.6)# | 7.0 (5.2–10.3)# | 0.000 |
| Hemoglobin, g/L | 129.0 (116.0–141.0) | 130.0 (118.0–142.0) | 123.0 (106.0–137.0)# | 125.0 (108.0–138.0)# | 123.0 (100.5–135.5)# | 0.000 |
| AST, U/L | 20.0 (16.0–28.0) | 20.0 (16.0–26.0) | 24.0 (17.0–42.0)# | 21.0 (16.0–30.0)# | 25.0 (18.0–44.0)# | 0.000 |
| ALT, U/L | 19.0 (13.0–30.0) | 18.0 (13.0–29.0) | 21.0 (13.0–38.0)# | 18.0 (12.0–29.0) | 21.0 (13.0–34.0)# | 0.000 |
| TBIL, μmol/L | 9.2 (6.6–12.9) | 9.0 (6.6–12.5) | 10.2 (7.0–15.9)# | 9.7 (6.8–14.1)# | 11.4 (7.5–19.2)# | 0.000 |
| Albumin, g/L | 40.0 (37.0–43.0) | 40.0 (37.0–43.0) | 38.0 (33.0–41.0)# | 38.0 (34.0–41.0)# | 36.0 (30.0–40.0)# | 0.000 |
| Glucose, mmol/L | 5.2 (4.7–6.2) | 5.2 (4.7–6.2) | 5.4 (4.8–6.8)# | 5.2 (4.7–6.3) | 5.5 (4.7–7.7)# | 0.000 |
| SCr, μmol/L | 72.0 (61.0–86.0) | 72.0 (61.0–86.0) | 72.0 (60.0–88.0) | 80.0 (65.0–105.0)# | 78.0 (61.0–101.0)# | 0.000 |
| BUN, mmol/L | 5.0 (4.0–6.2) | 4.9 (4.0–6.1) | 5.1 (3.9–6.6)# | 5.9 (4.5–8.5)# | 6.5 (4.9–10.4)# | 0.000 |
| SUA, mmol/L | 304.0 (244.0–371.0) | 306.0 (249.0–371.0) | 286.0 (219.0–364.0)# | 326.0 (255.0–415.0)# | 317.0 (230.0–406.0) | 0.000 |
| Na, mmol/L | 141.0 (139.0–143.0) | 141.0 (140.0–143.0) | 137.0 (135.0–141.0)# | 148.0 (143.0–148.0)# | 141.0 (138.0–145.0) | 0.000 |
| K, mmol/L | 4.0 (3.8–4.3) | 4.0 (3.8–4.3) | 4.1 (3.8–4.4)# | 4.0 (3.6–4.3)# | 4.0 (3.7–4.4) | 0.000 |
| Cl, mmol/L | 103.0 (101.0–105.0) | 103.0 (101.0–105.0) | 100.0 (97.0–103.0)# | 107.0 (104.0–110.0)# | 103.0 (99.0–107.0) | 0.000 |
| Mg, mmol/L | 0.91 (0.85–0.97) | 0.92 (0.86–0.97) | 0.90 (0.83–0.96)# | 0.93 (0.86–1.00)# | 0.91 (0.83–0.99) | 0.000 |
| Ca, mmol/L | 2.31 (2.22–2.39) | 2.32 (2.23–2.40) | 2.26 (2.14–2.36)# | 2.27 (2.14–2.36)# | 2.24 (2.11–2.34)# | 0.000 |
| P, mmol/L | 1.13 (0.99–1.26) | 1.13 (1.00–1.27) | 1.09 (0.93–1.24)# | 1.12 (0.93–1.27)# | 1.08 (0.88–1.26)# | 0.000 |
| Osmolality, mOsm/L | 301.6 (297.7–305.4) | 302.0 (298.6–305.6) | 297.0 (290.2–302.5)# | 313.1 (305.1–317.2)# | 303.9 (298.6–313.1)# | 0.000 |
| CO2CP, mmol/L | 24.0 (23.0–26.0) | 25.0 (23.0–26.0) | 24.0 (22.0–26.0)# | 24.0 (22.0–26.0)** | 23.0 (21.0–26.0)# | 0.000 |
| AG, mmol/L | 14.0 (12.0–15.0) | 14.0 (12.0–15.0) | 14.0 (12.0–16.0)# | 15.0 (13.0–17.0)# | 15.0 (13.0–17.0)# | 0.000 |
P<0.05;
P<0.01;
P<0.001;
Row N%.
The normonatremia group served as the reference when comparing between two groups. BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; WBC – white blood cell; ALT – alanine aminotransferase; AST – aspartate aminotransferase; TBIL – total bilirubin; SCr – serum creatinine; BUN – blood urea nitrogen; SUA – serum uric acid; CO2CP – carbon dioxide combining power; AG – anion gap.
Table 2.
Characteristics of patients with community-acquired or hospital-acquired dysnatremia.
| Variable | Hyponatremia | Hypernatremia | ||||
|---|---|---|---|---|---|---|
| CA (n=7,059) | HA (n=8,180) | P | CA (n=850) | HA (n=837) | P | |
| Age, year | 63.0 (52.0–72.0) | 60.0 (50.0–68.0) | 0.000 | 64.0 (54.0–74.3) | 63.0 (54.0–73.5) | 0.515 |
| >65, n (%) | 2,969 (42.1) | 2,680 (32.8) | 0.000 | 386 (45.4) | 365 (43.6) | 0.456 |
| Male sex, n (%) | 4,845 (68.6) | 5,422 (66.3) | 0.002 | 496 (58.4) | 472 (56.4) | 0.415 |
| BMI, kg/m2 | 22.4 (20.0–24.9) | 22.8 (20.7–25.3) | 0.010 | 23.4 (20.6–25.7) | 23.6 (21.8–26.3) | 0.731 |
| Underlying diseases, n(%)§ | 0.000 | 0.000 | ||||
| Cancer | 1,577 (37.8) | 2,600 (62.2) | 0.000 | 73 (38.8) | 115 (61.2) | 0.001 |
| Pulmonary | 956 (83.1) | 195 (16.9) | 0.000 | 66 (61.1) | 42 (38.9) | 0.021 |
| Cardiovascular | 802 (55.5) | 643 (44.5) | 0.000 | 180 (73.5) | 65 (26.5) | 0.000 |
| Digestive | 1,016 (74.5) | 347 (25.5) | 0.000 | 71 (71.0) | 29 (29.0) | 0.000 |
| Endocrine | 151 (70.2) | 64 (29.8) | 0.000 | 37 (56.1) | 29 (43.9) | 0.347 |
| Hematological | 211 (55.5) | 169 (44.5) | 0.000 | 28 (62.2) | 17 (37.8) | 0.107 |
| Neurological | 189 (48.1) | 204 (51.9) | 0.476 | 67 (45.0) | 82 (55.0) | 0.166 |
| Urinary and renal | 458 (56.5) | 352 (43.5) | 0.000 | 67 (45.9) | 79 (54.1) | 0.256 |
| Cardiothoracic surgery | 299 (13.9) | 1,857 (86.1) | 0.000 | 81 (26.6) | 223 (73.4) | 0.000 |
| General surgery | 728 (35.6) | 1,317 (64.4) | 0.000 | 74 (43.0) | 98 (57.0) | 0.042 |
| Orthopedic surgery | 137 (49.5) | 140 (50.5) | 0.291 | 22 (56.4) | 17 (43.6) | 0.446 |
| Gynecological | 139 (46.0) | 163 (54.0) | 0.917 | 13 (56.5) | 10 (43.5) | 0.553 |
| Others | 396 (75.4) | 129 (24.6) | 0.000 | 71 (69.6) | 31 (30.4) | 0.000 |
| Clinical data at admission | ||||||
| SBP, mmHg | 120.0 (110.0–135.0) | 120.0 (111.0–132.0) | 0.596 | 130.0 (120.0–140.0) | 130.0 (118.0–140.0) | 0.825 |
| DBP, mmHg | 76.0 (70.0–80.0) | 78.0 (70.0–80.0) | 0.000 | 80.0 (70.0–81.0) | 80.0 (69.0–82.0) | 0.109 |
| WBC, 109/L | 7.1 (5.2–9.8) | 6.0 (4.7–7.6) | 0.000 | 6.3 (4.8–8.2) | 6.6 (5.2–8.9) | 0.002 |
| Hemoglobin, g/L | 117.0 (99.0–132.0) | 128.0 (113.0–140.0) | 0.000 | 126.0 (111.0–139.0) | 123.0 (106.0–138.0) | 0.025 |
| AST, U/L | 26.0 (17.0–49.0) | 23.0 (17.0–37.0) | 0.000 | 20.0 (16.0–29.0) | 21.0 (16.0–31.0) | 0.296 |
| ALT, U/L | 22.0 (13.0–42.0) | 21.0 (13.0–36.0) | 0.000 | 18.0 (13.0–29.0) | 18.0 (12.0–30.0) | 0.421 |
| TBIL, μmol/L | 10.3 (6.6–18.0) | 10.1 (7.2–14.7) | 0.000 | 9.4 (6.9–13.1) | 10.2 (6.8–15.2) | 0.009 |
| Albumin, g/L | 36.0 (31.0–40.0) | 39.0 (35.0–42.0) | 0.000 | 38.0 (34.0–41.0) | 37.0 (33.0–40.0) | 0.002 |
| Glucose, mmol/L | 5.9 (5.0–7.9) | 5.2 (4.7–6.2) | 0.000 | 5.2 (4.7–6.2) | 5.2 (4.7–6.4) | 0.277 |
| SCr, μmol/L | 71.0 (58.0–90.0) | 73.0 (61.0–87.0) | 0.000 | 80.0 (65.0–101.0) | 79.0 (65.0–109.0) | 0.526 |
| BUN, mmol/L | 5.0 (3.8–7.0) | 5.1 (4.0–6.4) | 0.455 | 5.6 (4.4–8.3) | 6.1 (4.6–8.8) | 0.081 |
| SUA, mmol/L | 267.0 (195.0–356.0) | 298.0 (239.0–369.0) | 0.000 | 328.0 (256.0–412.0) | 323.5 (255.0–417.3) | 0.748 |
| Na, mmol/L | 135.0 (133.0–136.0) | 140.0 (139.0–142.0) | 0.000 | 148.0 (148.0–150.0) | 143.0 (141.0–144.0) | 0.000 |
| K, mmol/L | 4.1 (3.8–4.4) | 4.1 (3.8–4.3) | 0.000 | 4.0 (3.7–4.3) | 3.9 (3.6–4.2) | 0.018 |
| Cl, mmol/L | 97.0 (94.0–99.0) | 102.0 (100.0–104.0) | 0.000 | 109.0 (107.0–111.0) | 104.0 (102.0–106.0) | 0.000 |
| Mg, mmol/L | 0.89 (0.82–0.96) | 0.90 (0.84–0.97) | 0.000 | 0.94 (0.87–1.01) | 0.92 (0.85–0.99) | 0.000 |
| Ca, mmol/L | 2.23 (2.11–2.35) | 2.28 (2.17–2.37) | 0.000 | 2.28 (2.18–2.37) | 2.26 (2.11–2.36) | 0.001 |
| P, mmol/L | 1.05 (0.88–1.22) | 1.12 (0.97–1.26) | 0.000 | 1.12 (0.95–1.26) | 1.11 (0.92–1.29) | 0.880 |
| Osmolality, mOsm/L | 289.0 (285.2–293.4) | 300.7 (297.0–304.4) | 0.000 | 316.2 (314.1–321.1) | 305.2 (301.3–309.7) | 0.000 |
| CO2CP, mmol/L | 23.0 (21.0–25.0) | 24.0 (22.0–26.0) | 0.000 | 25.0 (23.0–27.0) | 24.0 (22.0–26.0) | 0.000 |
| AG, mmol/L | 14.0 (12.0–16.0) | 14.0 (12.0–16.0) | 0.000 | 15.0 (13.0–16.0) | 15.0 (13.0–17.0) | 0.866 |
Row N%.
CA – community-acquired; HA – hospital-acquired; BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; WBC – white blood cell; ALT – alanine aminotransferase; AST – aspartate aminotransferase; TBIL – total bilirubin; SCr – serum creatinine; BUN – blood urea nitrogen; SUA – serum uric acid; CO2CP – carbon dioxide combining power; AG – anion gap.
Table 3.
Characteristics of selected patients with improved, persistent and mixed dysnatremia.
| Variable | Hyponatremia | Hypernatremia | Mixed dysnatremia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Improved (n=5,214) | Persistent (n=10,025) | P | Improved (n=859) | Persistent (n=828) | P | Hypo to hyper (n=102) | Hyper to hypo (n=183) | P | |
| Age, year | 61.0 (50.0–70.0) | 61.0 (52.0–70.0) | 0.028 | 63.3±15.1 | 63.4±15.5 | 0.910 | 70.4±15.6 | 61.8±14.8 | 0.000 |
| >65, n (%) | 1,918 (36.8) | 3,731 (37.2) | 0.601 | 375 (43.7) | 376 (45.4) | 0.468 | 67 (65.7) | 78 (42.6) | 0.000 |
| Male sex, n (%) | 3,348 (64.2) | 6,919 (69.0) | 0.000 | 476 (55.4) | 492 (59.4) | 0.096 | 65 (63.7) | 117 (63.9) | 0.972 |
| BMI, kg/m2 | 22.9±3.7 | 22.9±3.9 | 0.743 | 23.3±4.4 | 24.1±3.7 | 0.214 | 21.8±3.9 | 23.6±3.5 | 0.249 |
| Underlying diseases, n (%)§ | 0.000 | 0.000 | 0.000 | ||||||
| Cancer | 1,391 (33.3) | 2,786 (66.7) | 0.144 | 124 (66.0) | 64 (34.0) | 0.000 | 12 (36.4) | 21 (63.6) | 0.942 |
| Pulmonary | 292 (25.4) | 859 (74.6) | 0.000 | 41 (38.0) | 67 (62.0) | 0.005 | 6 (40.0) | 9 (60.0) | 0.727 |
| Cardiovascular | 495 (34.3) | 950 (65.7) | 0.972 | 99 (40.4) | 146 (59.6) | 0.000 | 5 (22.7) | 17 (77.3) | 0.183 |
| Digestive | 324 (23.8) | 1039 (76.2) | 0.000 | 27 (27.0) | 73 (73.0) | 0.000 | 7 (50.0) | 7 (50.0) | 0.255 |
| Endocrine | 113 (52.6) | 102 (47.4) | 0.000 | 38 (57.6) | 28 (42.4) | 0.270 | 0 | 0 | – |
| Hematological | 146 (38.4) | 234 (61.6) | 0.080 | 14 (31.1) | 31 (68.9) | 0.007 | 5 (55.6) | 4 (44.4) | 0.209 |
| Neurological | 165 (42.0) | 228 (58.0) | 0.001 | 80 (53.7) | 69 (46.3) | 0.478 | 14 (53.8) | 12 (46.2) | 0.044 |
| Urinary and renal | 325 (40.1) | 485 (59.9) | 0.000 | 49 (33.6) | 97 (66.4) | 0.000 | 11 (64.7) | 6 (35.3) | 0.010 |
| Cardiothoracic surgery | 700 (32.5) | 1,456 (67.5) | 0.065 | 222 (73.0) | 82 (27.0) | 0.000 | 17 (17.3) | 81 (82.7) | 0.000 |
| General surgery | 885 (43.3) | 1,160 (56.7) | 0.000 | 113 (65.7) | 59 (34.3) | 0.000 | 11 (35.5) | 20 (64.5) | 0.970 |
| Orthopedic surgery | 101 (36.5) | 176 (63.5) | 0.426 | 11 (28.2) | 28 (71.8) | 0.004 | 3 (75.0) | 1 (25.0) | 0.099 |
| Gynecological | 84 (27.8) | 218 (72.2) | 0.018 | 15 (65.2) | 8 (34.8) | 0.167 | 0 | 0 | – |
| Others | 193 (36.8) | 332 (63.2) | 0.211 | 26 (25.5) | 76 (74.5) | 0.000 | 11 (68.8) | 5 (31.2) | 0.005 |
| Clinical data at admission | |||||||||
| SBP, mmHg | 124.3±17.8 | 124.2±17.4 | 0.719 | 129.0±20.1 | 129.4±19.5 | 0.701 | 127.3±19.3 | 129.9±25.2 | 0.503 |
| DBP, mmHg | 75.3±11.0 | 75.9±12.4 | 0.004 | 75.7±11.9 | 78.5±32.7 | 0.033 | 74.1±12.1 | 75.0±13.6 | 0.624 |
| WBC, 109/L | 6.5 (5.0–8.9) | 6.3 (4.8–8.4) | 0.000 | 8.0±11.1 | 7.6±4.6 | 0.325 | 8.4 (5.8–12.4) | 6.4 (5.0–9.1) | 0.001 |
| Hemoglobin, g/L | 120.6±24.3 | 120.2±23.8 | 0.260 | 121.2±25.1 | 120.5±26.0 | 0.553 | 108.8±27.2 | 121.4±27.2 | 0.000 |
| AST, U/L | 23.0 (17.0–39.0) | 24.0 (17.0–43.0) | 0.000 | 21.0 (16.0–31.0) | 20.0 (16.0–29.0) | 0.035 | 118.1±575.9 | 65.5±241.1 | 0.283 |
| ALT, U/L | 21.0 (13.0–37.3) | 22.0 (13.0–39.0) | 0.008 | 47.9±334.6 | 36.1±84.4 | 0.321 | 46.3±83.6 | 39.8±77.1 | 0.512 |
| TBIL, μmol/L | 10.1 (6.9–15.6) | 10.3 (7.0–16.1) | 0.045 | 14.9±27.9 | 15.3±41.8 | 0.795 | 10.5 (6.9–18.9) | 11.7 (8.3–19.5) | 0.427 |
| Albumin, g/L | 38.0 (33.0–41.0) | 38.0 (33.0–41.0) | 0.204 | 37.0 (34.0–40.0) | 38.0 (34.0–41.0) | 0.106 | 32.9±6.2 | 36.3±6.1 | 0.000 |
| Glucose, mmol/L | 6.5±3.4 | 6.5±3.2 | 0.277 | 6.1±2.8 | 6.1±2.7 | 0.978 | 7.0±3.3 | 6.6±3.3 | 0.418 |
| SCr, μmol/L | 73.0 (60.0–89.0) | 72.0 (60.0–88.0) | 0.006 | 79.0 (65.0–106.0) | 80.0 (65.0–104.0) | 0.641 | 75.5 (58.2–110.3) | 79.0 (62.0–100.0) | 0.509 |
| BUN, mmol/L | 5.0 (3.8–6.7) | 5.1 (3.9–6.6) | 0.873 | 5.9 (4.5–8.6) | 5.8 (4.5–8.5) | 0.629 | 9.8±9.1 | 8.6±7.2 | 0.222 |
| SUA, mmol/L | 285.0 (217.0–366.0) | 286.0 (220.0–363.0) | 0.664 | 346.1±137.1 | 351.2±145.5 | 0.468 | 307.3±166.1 | 348.1±147.8 | 0.034 |
| Na, mmol/L | 138.0±4.2 | 137.1±4.5 | 0.000 | 145.5±4.9 | 146.8±5.0 | 0.000 | 135.9±4.6 | 144.4±4.6 | 0.000 |
| K, mmol/L | 4.1 (3.8–4.3) | 4.1 (3.8–4.4) | 0.000 | 3.9±0.6 | 4.0±0.6 | 0.028 | 4.2±0.7 | 4.0±0.5 | 0.005 |
| Cl, mmol/L | 101.0 (97.0–103.0) | 100.0 (96.0–103.0) | 0.000 | 106.4±5.7 | 107.4±5.7 | 0.000 | 98.4±5.4 | 105.7±5.5 | 0.000 |
| Mg, mmol/L | 0.90 (0.83–0.97) | 0.90 (0.83–0.96) | 0.354 | 0.93±0.14 | 0.95±0.15 | 0.009 | 0.92 (0.83–1.00) | 0.90 (0.83–0.98) | 0.418 |
| Ca, mmol/L | 2.24 (2.12–2.35) | 2.27 (2.16–2.37) | 0.000 | 2.22±0.20 | 2.27±0.22 | 0.000 | 2.20±0.19 | 2.22±0.18 | 0.548 |
| P, mmol/L | 1.08 (0.89–1.24) | 1.10 (0.94–1.24) | 0.000 | 1.10±0.34 | 1.15±0.42 | 0.029 | 1.07±0.39 | 1.11±0.38 | 0.383 |
| Osmolality, mOsm/L | 297.5±9.4 | 295.7±9.6 | 0.000 | 312.3±13.4 | 315.4±13.7 | 0.000 | 297.0 (290.9–300.7) | 308.0 (302.1–316.9) | 0.000 |
| CO2CP, mmol/L | 24.0 (22.0–26.0) | 24.0 (22.0–26.0) | 0.000 | 23.1±5.5 | 22.6±6.2 | 0.179 | 22.5±4.3 | 23.7±4.0 | 0.019 |
| AG, mmol/L | 14.0 (12.0–16.0) | 14.0 (12.0–16.0) | 0.000 | 14.9±3.8 | 15.1±4.1 | 0.187 | 15.2±4.1 | 15.2±3.5 | 0.995 |
Row N%.
BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; WBC – white blood cell; ALT – alanine aminotransferase; AST – aspartate aminotransferase; TBIL – total bilirubin; SCr – serum creatinine; BUN – blood urea nitrogen; SUA – serum uric acid; CO2CP – carbon dioxide combining power; AG – anion gap.
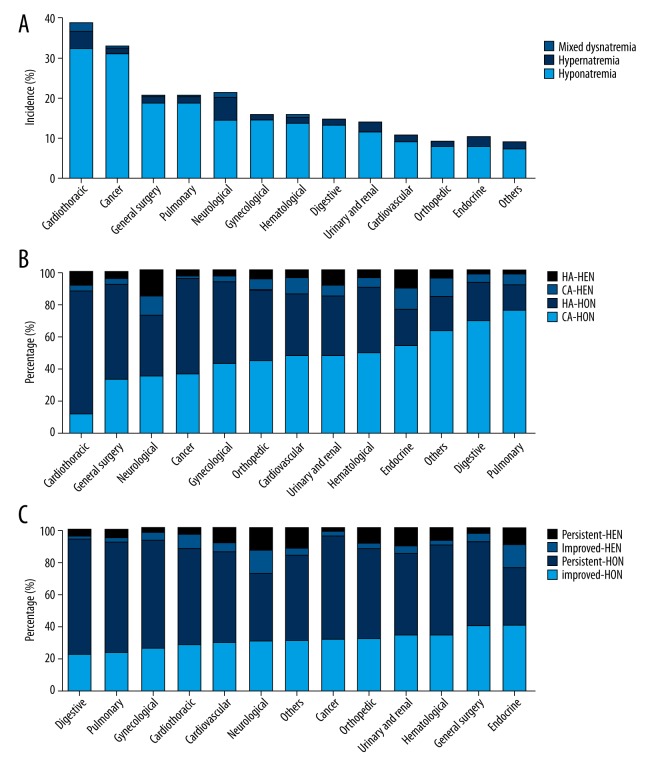
Prevalence of dysnatremia in various underlying diseases
The prevalence of dysnatremia in different underlying diseases is shown in Figure 2, and detailed information is provided in Tables 1–3. The top 4 underlying diseases of hyponatremia were cardiothoracic surgery (32.5%), cancer (31.3%), general surgery (18.8%), and pulmonary diseases (18.7%), while the top 4 ones of hypernatremia were neurological (5.6%), cardiothoracic surgery (4.6%), endocrine (2.4%), and urinary and renal diseases (2.1%). Mixed dysnatremia most often occured in patients with cardiothoracic surgery (1.5%) and neurological diseases (1.0%). The incidence rates of HA-hyponatremia and HA-hypernatremia were higher than the rates of corresponding CA-dysnatremia in patients with cardiothoracic surgery, general surgery, and cancer, while the rates were lower in patients with pulmonary, digestive, and cardiovascular diseases. There were fewer patients with HA-hyponatremia than CA-hyponatremia in endocrine, hematological, and urinary and renal diseases. Hyponatremia was more likely to be persistent than become improved in patients with digestive, pulmonary, gynecological, neurological, general surgery, and urinary and renal diseases, while hypernatremia was more likely to be persistent than become improved in patients with urinary and renal, orthopedic surgery, cardiovascular, hematological, pulmonary, and digestive diseases. All the above differences were statistically significant. “Hypo- to hyper-” mixed dysnatremia was more likely to occur in neurological and urinary and renal diseases than “hyper- to hypo-” mixed dysnatremia, and the reverse was true in cardiothoracic surgery.
Figure 2.
Incidence or percentage of dysnatremia in various underlying diseases. (A) The incidence of hyponatremia, hypernatremia, and mixed dysnatremia; (B) Percentage of community-acquired or hospital-acquired dysnatremia; (C) Percentage of improved or persistent dysnatremia. CA – community-acquired; HA – hospital-acquired; HON – hyponatremia; HEN – hypernatremia.
Outcomes associated with dysnatremia
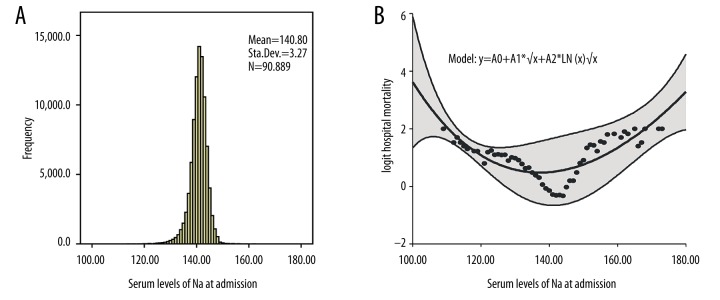
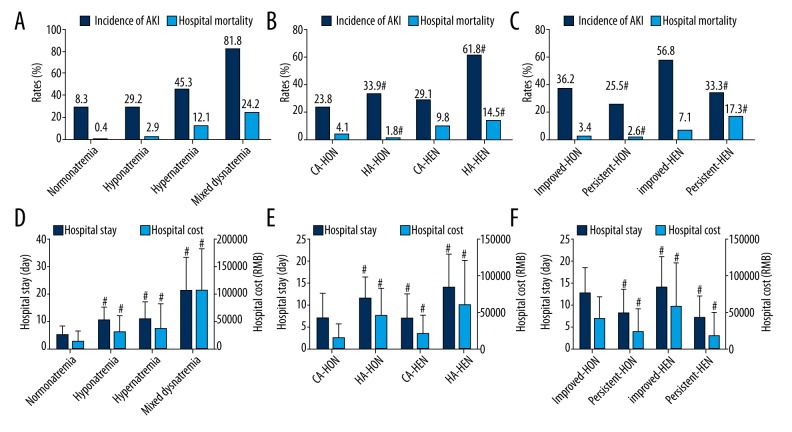
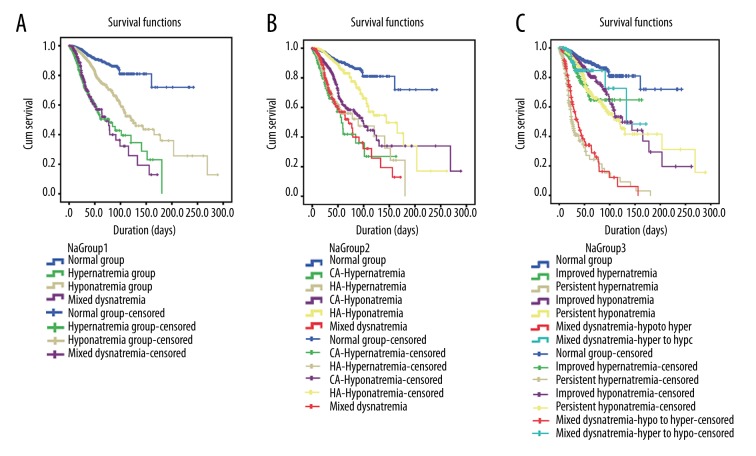
The frequency of sodium levels at admission and its relation to hospital mortality are shown in Figure 3. The graph also shows the U-shaped relationship, the fitting curve, and equation. As demonstrated in Figure 4 and Supplementary Table 1, hyponatremia, hypernatremia, and mixed dysnatremia increased hospital mortality, the incidence of AKI, the length of hospital stay, and hospital costs when compared with the normonatremia group. Also, hospital mortalities and AKI were higher, the length of hospital stay was longer, and hospital costs were higher in HA-dysnatremia groups than in the corresponding CA-dysnatremia groups, except that hospital mortality in the HA-hyponatremia group was lower than in the CA-hyponatremia group. Hospital mortalities and AKI were lower, the length of hospital stay was shorter, and hospital costs were lower in persistent dysnatremia groups than in the corresponding improved dysnatremia groups, except that hospital mortality in the persistent hypernatremia group was higher than in the improved hypernatremia group. In addition, the hospital mortality in the “hypo- to hyper-” mixed dysnatremia group was greater than in the “hyper- to hypo-” group (79.7% vs. 20.3%, respectively, P=0.000). All differences were statistically significant. Survival analysis (Figure 5) shows the differences in hospital survival.
Figure 3.
The histogram of the serum sodium levels at admission and its relation to hospital mortality. (A) The histogram of serum sodium levels at admission; (B) Non-linear relationship between baseline serum sodium levels and the predicted probability of hospital mortality. The range area indicates 95% confidence intervals.
Figure 4.
Outcomes of hospitalized patients with dysnatremia. (A–C) The incidence of acute kidney injury and hospital mortality; (D–F) The length of hospital stay and hospital cost. AKI – acute kidney injury; CA – community-acquired; HA – hospital-acquired; HON – hyponatremia; HEN – hypernatremia; RMB – Ren Min Bi. # P<0.01 compared with normonatremia (D), CA-dysnatremia (B, E) or improved dysnatremia (C, F).
Figure 5.
Kaplan-Meier survival curves stratified by dysnatremia categories. (A) Survival curve of hyponatremia, normonatremia, hypernatremia and mixed dysnatremia; (B) Survival curve of community-acquired vs. hospital-acquired dysnatremia; (C) Survival curve of improved vs. persistent dysnatremia, “hypo to hyper” vs. “hyper to hypo” mixed dysnatremia. The vertical lines represent censored subjects. The follow-up duration is different for each subject because it is censored at the end of hospitalization. CA – community-acquired; HA – hospital-acquired.
Multiple logistic regression analyses of risk factors for hospital mortality
We further conducted multiple logistic regression analyses to identify dysnatremia and other independent risk factors for hospital mortality in 3 models, and the results are shown in Tables 4 and 5. In all 3 models, mixed dysnatremia was identified as the leading independent risk factor for hospital mortality (OR: 22.344 to 22.387, P=0.000). The results from Model 1 revealed that hypernatremia was a stronger predictor of hospital mortality than hyponatremia (OR [95% CI]: 13.387 [10.642–16.840] vs. 2.225 [1.857–2.667]). The OR of HA-hypernatremia for mortality was nearly twice that of CA-hypernatremia (OR [95% CI]: 16.216 [12.588–20.888] vs. 8.827 [6.141–12.689]), while the OR of HA-hyponatremia for mortality was slightly higher than that of CA-hyponatremia (OR [95% CI]: 16.216 [12.588–20.888] vs. 8.827 [6.141–12.689]). The OR of persistent hypernatremia for mortality was 3 times higher than that of improved hypernatremia (OR [95% CI]: 22.983 [17.554–30.092] vs. 6.830 [4.903–9.516]). However, the OR of persistent hyponatremia for mortality was lower than that of improved hyponatremia (OR [95% CI]: 2.023 [1.653–2.476] vs. 2.563 [2.062–3.186]). In addition, the OR of “hypo- to hyper-” mixed dysnatremia for mortality was higher than that of the “hyper- to hypo-” group (OR [95% CI]: 56.884 [35.098–92.193] vs. 6.629 [3.499–12.557]).
Table 4.
Final multiple logistic regression analysis of dysnatremia as independent risk factors for hospital mortality.
| Variable | Multiple logistic analysis | |
|---|---|---|
| OR (95%CI) | P value | |
| Model 1§ | ||
| Hyponatremia | 2.225 (1.857–2.667) | 0.000 |
| Hypernatremia | 13.387 (10.642–16.840) | 0.000 |
| Mixed dysnatremia | 22.344 (15.709–31.783) | 0.000 |
| Model 2§ | ||
| CA-hyponatremia | 1.950 (1.552–2.451) | 0.000 |
| HA-hyponatremia | 2.567 (2.087–3.156) | 0.000 |
| CA-hypernatremia | 8.827 (6.141–12.689) | 0.000 |
| HA-hypernatremia | 16.216 (12.588–20.888) | 0.000 |
| Mixed dysnatremia | 21.387 (14.992–30.510) | 0.000 |
| Model 3§ | ||
| Improved hyponatremia | 2.561 (2.062–3.182) | 0.000 |
| Persistent hyponatremia | 2.016 (1.649–2.465) | 0.000 |
| Improved hypernatremia | 6.755 (4.847–9.413) | 0.000 |
| Persistent hypernatremia | 22.292 (17.041–29.162) | 0.000 |
| “Hypo to hyper” mixed dysnatremia | 56.884 (35.098–92.193) | 0.000 |
| “Hyper to hypo” mixed dysnatremia | 6.629 (3.499–12.557) | 0.000 |
Normonatremia as the reference.
CA – community acquired; HA – hospital acquired; OR – odds ratio; CI – confidence interval.
Table 5.
Final multiple logistic regression analysis of other independent risk factors for hospital mortality.
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, per year increase | 1.048 (1.043–1.054) | 0.000 | 1.049 (1.043–1.054) | 0.000 | 1.048 (1.043–1.054) | 0.000 |
| Male sex | 1.197 (1.035–1.384) | 0.015 | 1.197 (1.035–1.384) | 0.015 | 1.199 (1.037–1.388) | 0.015 |
| BMI <18.5 kg/m2§ | 3.155 (1.966–5.063) | 0.000 | 3.076 (1.912–4.949) | 0.000 | 3.240 (2.018–5.201) | 0.000 |
| DBP >90 mmHg§ | 1.470 (1.120–1.930) | 0.006 | 1.473 (1.121–1.934) | 0.005 | 1.468 (1.118–1.927) | 0.006 |
| Hypochloridemia§ | 1.998 (1.668–2.393) | 0.000 | 2.179 (1.785–2.659) | 0.000 | 2.003 (1.671–2.402) | 0.000 |
| CO2CP >29 mmol/L§ | 1.902 (1.452–2.491) | 0.000 | 1.927 (1.467–2.533) | 0.000 | 1.898 (1.447–2.490) | 0.000 |
| AG >16 mmol/L§ | 1.717 (1.430–2.062) | 0.000 | 1.698 (1.412–2.043) | 0.000 | 1.720 (1.432–2.067) | 0.000 |
| ALT, per 50U/L increase | 1.030 (1.015–1.046) | 0.000 | 1.030 (1.014–1.046) | 0.000 | 1.031 (1.015–1.047) | 0.000 |
| TBIL, per 20 μmol/L increase | 1.038 (1.015–1.046) | 0.001 | 1.037 (1.014–1.046) | 0.001 | 1.039 (1.016–1.063) | 0.001 |
| Albumin, per 5 g/L decrease | 1.418 (1.328–1.514) | 0.000 | 1.424 (1.334–1.521) | 0.000 | 1.426 (1.335–1.523) | 0.000 |
| Hemoglobin (90–119 g/L)§ | 1.451 (1.228–1.715) | 0.000 | 1.454 (1.230–1.719) | 0.000 | 1.446 (1.222–1.710) | 0.000 |
| Hemoglobin (60–89 g/L)§ | 1.871 (1.491–2.348) | 0.000 | 1.893 (1.508–2.376) | 0.000 | 1.865 (1.484–2.343) | 0.000 |
| Hemoglobin (<60 g/L)§ | 4.174 (2.961–5.883) | 0.000 | 4.264 (3.027–6.007) | 0.000 | 4.099 (2.902–5.791) | 0.000 |
| WBC >12.0×109/L§ | 2.252 (1.874–2.705) | 0.000 | 2.257 (1.878–2.712) | 0.000 | 2.246 (1.868–2.701) | 0.000 |
| BUN, per 10 mg/dl increase | 1.281 (1.215–1.351) | 0.000 | 1.277 (1.211–1.347) | 0.000 | 1.282 (1.067–1.540) | 0.000 |
| SCr, per 0.5 mg/dl increase | 0.935 (0.909–0.961) | 0.000 | 0.932 (0.906–0.959) | 0.000 | 0.932 (0.906–0.959) | 0.000 |
| Hypouricemia§ | 1.296 (1.079–1.555) | 0.005 | 1.299 (1.082–1.560) | 0.005 | 1.282 (1.067–1.540) | 0.008 |
| Hyperuricemia§ | 1.536 (1.279–1.846) | 0.000 | 1.523 (1.267–1.830) | 0.000 | 1.546 (1.285–1.859) | 0.000 |
Normal range as the reference.
BMI – body mass index; DBP – diastolic blood pressure; CO2CP – carbon dioxide combining power; AG – anion gap; ALT – alanine aminotransferase; TBIL – total bilirubin; WBC – white blood cell; BUN – blood urea nitrogen; SCr – serum creatinine; OR – odds ratio; CI – confidence interval.
In addition to dysnatremia, 16 risk factors for hospital mortality were also identified, including older age, male sex, BMI <18.5 kg/m2, DBP at admission >90 mmHg, and laboratory variables at admission (hypochloremia, CO2CP >29 mmol/L, AG >16 mmol/L, increased ALT and TBIL, decreased albumin and hemoglobin, WBC>12×109/L, increased BUN, lower SCr, hypouricemia, and hyperuricemia).
Subgroup analyses
To identify the high-risk patients with poor outcomes, we also conducted subgroup analyses of dysnatremia as risk factors for hospital mortality. Supplementary Table 2 shows that age ≤65 years, BMI <18.5 kg/m2, the incidence of AKI, and length of hospital stay ≤30 days increased the ORs of hypernatremia for mortality compared to other subgroups. Different ORs of dysnatremia for mortality were also observed across various underlying diseases. The top 3 ORs of hypernatremia were from cardiothoracic surgery (OR 27.029, 95% CI 12.388–58.976, P=0.000), urinary and renal (OR 17.257, 95% CI 8.551–34.826, P=0.000), and neurological diseases (OR 16.821, 95%CI 8.191–34.544, P=0.000). Supplementary Table 3 shows that the ORs of HA-hypernatremia were higher in these patients with age ≤65 years, male sex, BMI <18.5 kg/m2, AKI, and length of hospital stay ≤30 days than in other corresponding subgroups. The different ORs of CA- and HA-dysnatremia for mortality across various underlying diseases are also displayed, and both HA-hyponatremia and HA-hypernatremia increased the risk of death in patients with digestive diseases compared to other diseases according to the ORs (21.924 [4.875–98.609] vs. 55.185 [13.645–223.184], respectively). Supplementary Table 4 shows that age ≤65 years, BMI <18.5 kg/m2, presence of AKI, and length of hospital stay ≤30 days elevated the ORs of persistent hyponatremia for mortality compared to the corresponding subgroups. BMI <18.5 kg/m2, and length of hospital stay ≤30 days also increased the ORs of persistent hypernatremia for mortality. The top 3 ORs of persistent hyponatremia were from hematological diseases, cancer, and cardiothoracic surgery (OR: 29.114, 11.343, and 10.885, respectively), while the top 3 ORs of persistent hypernatremia were from digestive, hematological, and urinary and renal diseases (OR: 50.959, 37.481, and 32.258, respectively).
Risk factors for developing HA-dysnatremia and persistent dysnatremia
As before, if HA-dysnatremia is prevented and dysnatremia is corrected in a timely and appropriate manner, in-hospital mortality can be reduced significantly. Therefore, we further conducted multinomial logistic regression analysis. Patients with cancer, cardiothoracic surgery, general surgery, and neurological diseases were prone to develop both HA-hyponatremia and HA-hypernatremia, while patients with cardiovascular, hematological, and gynecological diseases and orthopedic surgery were more likely to develop HA-hyponatremia, and patients with endocrine diseases were high-risk patients for HA-hypernatremia. Hyponatremia was prone to continue until discharge or death in patients with cardiovascular surgery and pulmonary, digestive, and gynecological diseases. Other risk factors were also identified and are shown in Supplementary Table 5.
Discussion
In this observational study, we found that dysnatremia was common in hospitalized patients and was independently associated with poor outcomes, including higher hospital mortality and incidence of AKI, longer length of hospital stay, and higher hospital costs than in patients with normonatremia. Nearly half of dysnatremia happened during hospitalization, while half of hypernatremia and one-third of hyponatremia continued until discharge or death. Multiple logistic regression revealed that all kinds of dysnatremia were independently associated with in-hospital mortality, and mixed dysnatremia (especially “hypo- to hyper-” ones), hypernatremia (especially HA- and persistent hypernatremia), were strong predictors of mortality. Further subgroup analyses proved that the effects of dysnatremia on in-hospital mortality were influenced by age, sex, BMI, AKI, length of hospital stay, and underlying diseases. Patients with cardiothoracic surgery, general surgery, cancer, and neurological diseases were more likely to develop HA-dysnatremia. Other independent risk factors for in-hospital mortality and predictors of HA- and persistent dysnatremia were also identified.
Hyponatremia is the most common electrolyte disorder encountered in clinical practice [19]. The reported prevalence of hyponatremia is determined by various factors, including the definition of hyponatremia, the time of onset, the clinical setting, and the patient population. The incidence of hyponatremia has been reported to be 14.5% on initial measurement in hospitalized patients [20]. Our study found that the total incidence of hyponatremia was 16.8% in hospitalized patients, 46.3% had hyponatremia at admission and 53.7% had it after 24 h of hospitalization, indicating that the incidence of HA-hyponatremia may be underestimated. Timely correction of hyponatremia may be another factor affecting outcomes. Therefore, hyponatremia patients were also divided into improved and persistent groups according to the serum sodium levels before discharge or at death. We found that 34.2% had improved hyponatremia, and 65.8% had persistent hyponatremia. A recent study based on the general population [21] reported that hyponatremia was more common in patients with hypertension, diabetes, coronary artery disease, stroke, chronic obstructive pulmonary disease, and cancer, and was less common in those with none of these comorbidities. In our study, the top 4 underlying conditions associated hyponatremia were cardiothoracic surgery (32.5%), cancer (31.3%), general surgery (18.8%), and pulmonary diseases (18.7%), and the incidence of hyponatremia was 14.7% and 9.0% in cardiovascular and neurological diseases, respectively.
Hypernatremia is another common electrolyte disturbance and is always a reflection of water loss rather than sodium gain. However, it can also be associated with concomitant loss of sodium via hypotonic fluids or adding hypertonic fluids. Research in 2 large Dutch cohorts [22] reported a marked shift in the incidence of dysnatremia from hyponatremia to hypernatremia over 2 decades in ICU patients, which may be related to the increased use of sodium-containing infusions, diuretics, and hydrocortisone. Therefore, iatrogenic factors play a major role in the occurrence and development of hypernatremia. In critically ill patients, the incidence of hypernatremia at admission was 6.9% [2], and 7.7% of the patients with normonatremia at admission developed hypernatremia during ICU hospitalization [23]. Our study found that the total incidence of hypernatremia was 1.9% in hospitalized patients: 50.4% developed hypernatremia at admission and 49.6% after 24 h of hospitalization; 50.9% had improved hypernatremia; and 49.1% had persistent hypernatremia. The top 4 underlying conditions associated with hypernatremia were neurological diseases (5.6%), cardiothoracic surgery (4.6%), endocrine diseases (2.4%), and urinary and renal diseases (2.1%).
Extensive studies have proved the association between dysnatremia and poor outcomes. In this study, we further showed that hypernatremia (OR 13.387, 95% CI 10.642–16.840, P=0.000) and mixed dysnatremia (OR 22.344, 95% CI 15.709–31.783, P=0.000) were stronger predictors of mortality than was hyponatremia (OR 2.225, 95% CI 1.857–2.667, P=0.000). Improper correction of dysnatremia can lead to mixed dysnatremia. One survey [16] proved that fluctuations in serum sodium levels were independently associated with an increased risk of in-hospital mortality. The mortality rate of patients with mixed dysnatremia in ICU may be up to 42% [15], and we reported that the mortality was 24.2% in hospitalized patients. The OR of mortality was elevated if hyponatremia was over-corrected and changed to hypernatremia (OR 56.884, 95% CI 35.098–92.193, P=0.000). However, not only a too quick, but also a too slow correction can increase the risk of death regardless of initial dysnatremia [24]. The ORs of HA- and persistent hypernatremia for in-hospital mortality were obviously higher than the ORs of CA- and improved hypernatremia. These differences of ORs did not exist in hyponatremia. All data indicated that hypernatremia was a strong predictor of in-hospital death, and needed to be corrected in a timely and appropriate manner, while hyponatremia required careful correction.
The effect of dysnatremia on in-hospital mortality may vary substantially across different clinical settings and patient population. Therefore, we further tried to identify the patients at high risk of in-hospital death when dysnatremia occurs. Limited studies have reported the risk factors that increase the ORs of dysnatremia for mortality. Several risk factors were identified in our study. Although older age (>65 years) increased the risk of death in patients with persistent hypernatremia and mixed dysnatremia, the risk of mortality in younger patients was higher in other kinds of dysnatremia than in older patients. This difference have occurred because dysnatremia developed in younger patients only when the underlying diseases were more severe. Low BMI (<18.5 kg/m2) also increased the risk of mortality in patients with HA-hypernatremia, improved and persistent hypernatremia, mixed dysnatremia, and persistent hyponatremia compared to normal and high BMI patients.
Since dysnatremia was independently associated with poor outcomes, we can improve the prognosis of these patients if HA-dysnatremia is prevented and dysnatremia is corrected in a timely and appropriate manner. The main finding in this respect was that there are different risks of dysnatremia in various underlying diseases. Patients with cancer, cardiothoracic surgery, general surgery, and neurological diseases were prone to develop both HA-hyponatremia and HA-hypernatremia. Age, sex, and BMI had not only a great impact on the risk of death, but also on the incidence of dysnatremia. We also identified several risk factors from laboratory data at admission for HA-dysnatremia and persistent dysnatremia.
The present study has several limitations. First, it was designed as an observational and retrospective study and was open to selection bias. Second, we defined persistent dysnatremia when dysnatremia continued until discharge or death, and if not, improved dysnatremia was defined, which had not been reported and needed further evaluation. Although we tried to include many clinical risk factors, some relevant data, including fluid administration, were unavailable in this study.
Conclusions
Dysnatremia was independently associated with poor outcomes. Hospital-acquired and persistent hypernatremia were strong risk factors for in-hospital mortality. Effective prevention and proper correction of dysnatremia in high-risk patients may reduce the in-hospital mortality, but hyponatremia should be carefully corrected.
Supplementary Tables
Supplementary Table 1.
Outcomes of included patients with dysnatremia.
| Group | AKI, n (%) | Death, n (%) | Length of hospital stay, days | Hospital cost, RMB |
|---|---|---|---|---|
| Model 1 | ||||
| Normonatremia | 6,149 (8.3) | 317 (0.4) | 5.0 (2.5–8.0) | 13,869.1 (7,669.2–32,798.7) |
| Hyponatremia | 4,446 (29.2) | 439 (2.9) | 10.0 (5.5–15.0)# | 29,533.5 (13,362.4–60,616.8)# |
| Hypernatremia | 764 (45.3) | 204 (12.1) | 10.5 (6.0–17.0)# | 37,104.3 (14,016.3–80,466.7)# |
| Mixed dysnatremia | 233 (81.8) | 69 (24.2) | 21.0 (13.5–32.5)# | 106,510.3 (46,623.5–180,214.8)# |
| P value (χ2 or ANOVA) | 0.000 | 0.000 | 0.000 | 0.000 |
| Model 2 | ||||
| CA-hyponatremia | 1,677 (23.8) | 291 (4.1) | 7.0 (4.0–12.5) | 16,176.1 (9,060.1–33,854.8) |
| HA-hyponatremia | 2,769 (33.9) | 148 (1.8) | 11.5 (8.0–16.5) | 46,061.3 (23,207.6–83,343.8) |
| P value | 0.000 | 0.000 | 0.000 | 0.000 |
| CA-hypernatremia | 247 (29.1) | 83 (9.8) | 7.0 (3.5–12.5) | 20,248.1 (9,198.2–46,825.4) |
| HA-hypernatremia | 517 (61.8) | 121 (14.5) | 14.0 (9.5–21.5) | 60,351.1 (29,892.6–121,096.3) |
| P value | 0.000 | 0.003 | 0.000 | 0.000 |
| Model 3 | ||||
| Improved hyponatremia | 1,888 (36.2) | 176 (3.4) | 12.5 (8.5–18.5) | 41,283.8 (20,389.7–71,168.3) |
| Persistent hyponatremia | 2,558 (25.5) | 263 (2.6) | 8.0 (4.5–13.5) | 22,206.9 (11,558.2–54,202.7) |
| P value | 0.000 | 0.008 | 0.000 | 0.000 |
| Improved hypernatremia | 488 (56.8) | 61 (7.1) | 14.0 (9.5–21.0) | 57,157.7 (30,575.9–116,872.8) |
| Persistent hypernatremia | 276 (33.3) | 143 (17.3) | 7.0 (3.5–12.0) | 17,773.6 (8,782.9–49,237.5) |
| P value | 0.000 | 0.000 | 0.000 | 0.000 |
| “Hypo to hyper” mixed dysnatremia | 82 (35.2) | 55 (79.7) | 21.8 (13.4–38.5) | 89,939.4 (29,995.7–180,373.3) |
| “Hyper to hypo” mixed dysnatremia | 151 (64.8) | 14 (20.3) | 20.5 (14.0–30.5) | 113,523 (52,375.1–181,003.8) |
| P value | 0.657 | 0.000 | 0.603 | 0.050 |
P<0.001 vs. normonatremia group.
AKI – acute kidney injury; RMB – Ren Min Bi; CA – community-acquired; HA – hospital-acquired.
Supplementary Table 2.
Subgroup multiple logistic regression analysis of dysnatremia as independent risk factors for hospital mortality in model 1.
| Subgroup | Hyponatremia§ | Hypernatremia§ | Mixed dysnatremia§ | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, year | ||||||
| ≤65 | 2.722 (2.034–3.643) | 0.000 | 18.350 (12.853–26.198) | 0.000 | 21.886 (11.753–40.755) | 0.000 |
| <65 | 2.075 (1.653–2.606) | 0.000 | 11.112 (8.440–14.631) | 0.000 | 24.623 (16.162–37.513) | 0.000 |
| Gender | ||||||
| Male | 1.963 (1.575–2.447) | 0.000 | 14.659 (11.014–19.512) | 0.000 | 21.475 (13.748–33.544) | 0.000 |
| Female | 2.860 (2.090–3.913) | 0.000 | 11.952 (8.231–17.353) | 0.000 | 27.276 (15.320–48.564) | 0.000 |
| BMI, kg/m2 | ||||||
| <18.5 | 1.534 (0.453–5.198) | 0.492 | 71.139 (12.345–409.950) | 0.000 | 1233.153 (38.420–39580.284) | 0.000 |
| 18.5–24.99 | 2.297 (1.906–2.769) | 0.000 | 13.003 (10.241–16.509) | 0.000 | 20.950 (14.578–30.105) | 0.000 |
| ≥25 | 1.689 (0.632–4.513) | 0.296 | 7.195 (2.006–25.806) | 0.002 | 32.104 (2.952–349.133) | 0.004 |
| Underlying diseases | ||||||
| Cancer | 1.635 (1.069–2.502) | 0.023 | 11.942 (6.105–23.359) | 0.000 | 26.998 (9.615–75.806) | 0.000 |
| Pulmonary | 3.062 (2.010–4.633) | 0.000 | 6.266 (3.353–11.710) | 0.000 | 25.139 (7.850–80.503) | 0.000 |
| Cardiovascular | 4.863 (3.203–7.385) | 0.000 | 9.117 (5.037–16.502) | 0.000 | 9.550 (2.855–31.946) | 0.000 |
| Digestive | 1.445 (0.726–2.877) | 0.295 | 11.231 (3.914–32.232) | 0.000 | 53.383 (13.800–206.493) | 0.000 |
| Hematological | 3.398 (2.103–5.490) | 0.000 | 8.980 (3.723–21.660) | 0.000 | 48.642 (8.840–267.664) | 0.000 |
| Neurological | 2.199 (1.038–4.658) | 0.040 | 16.821 (8.191–34.544) | 0.000 | 23.310 (7.966–68.207) | 0.000 |
| Urinary and renal | 1.390 (0.746–2.588) | 0.300 | 17.257 (8.551–34.826) | 0.000 | 33.917 (7.819–147.114) | 0.000 |
| Cardiothoracic surgery | 2.385 (1.065–5.340) | 0.035 | 27.029 (12.388–58.976) | 0.000 | 18.501 (6.336–54.021) | 0.000 |
| General surgery | 2.282 (1.056–4.931) | 0.036 | 6.568 (2.299–18.763) | 0.000 | 8.285 (1.500–45.761) | 0.003 |
| Others | 2.321 (10.22–5.273) | 0.044 | 11.917 (4.531–31.341) | 0.000 | 77.078 (16.156–367.723) | 0.000 |
| AKI | ||||||
| Yes | 1.810 (1.411–2.322) | 0.000 | 10.784 (8.161–14.252) | 0.000 | 11.053 (7.502–16.285) | 0.000 |
| No | 1.526 (1.157–2.013) | 0.003 | 4.596 (2.817–7.499) | 0.000 | 16.996 (6.574–43.944) | 0.000 |
| Length of hospital stay, day | ||||||
| ≤30 | 1.627 (1.323–2.001) | 0.000 | 14.168 (11.042–18.179) | 0.000 | 18.563 (11.980–28.763) | 0.000 |
| >30 | 2.928 (1.879–4.561) | 0.000 | 5.233 (2.926–9.360) | 0.000 | 9.789 (5.116–18.730) | 0.000 |
Normonatremia as the reference.
BMI – body mass index; AKI – acute kidney injury; OR – odds ratio; CI – confidence interval.
Supplementary Table 3.
Subgroup multiple logistic regression analysis of dysnatremia as independent risk factors for hospital mortality in model 2.
| Subgroup | CA-hyponatremia§ | HA-hyponatremia§ | CA-hypernatremia§ | HA-hypernatremia§ | Mixed dysnatremia§ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Age, year | ||||||||||
| ≤65 | 2.433 (1.694–3.495) | 0.000 | 3.077 (2.216–4.273) | 0.000 | 9.953 (5.545–17.865) | 0.000 | 22.295 (14.862–33.445) | 0.000 | 18.570 (9.827–35.092) | 0.000 |
| >65 | 1.878 (1.414–2.494) | 0.000 | 2.329 (1.786–3.037) | 0.000 | 7.767 (5.146–11.724) | 0.000 | 13.665 (9.957–18.754) | 0.000 | 23.599 (15.462–36.017) | 0.000 |
| Gender | ||||||||||
| Male | 1.755 (1.335–2.308) | 0.000 | 2.219 (1.713–2.875) | 0.000 | 9.094 (5.740–14.406) | 0.000 | 18.348 (13.365–25.190) | 0.000 | 20.081 (12.820–31.454) | 0.000 |
| Female | 2.307 (1.561–3.410) | 0.000 | 3.387 (2.389–4.803) | 0.000 | 8.502 (4.900–14.752) | 0.000 | 13.847 (9.030–21.232) | 0.000 | 25.346 (14.148–45.406) | 0.000 |
| BMI, kg/m2 | ||||||||||
| <18.5 | 2.063 (0.531–8.018) | 0.296 | 0.961 (0.168–5.515) | 0.965 | – | – | 74.385 (12.840–430.910) | 0.000 | 1112.828 (35.443–34940.099) | 0.000 |
| 18.5–24.99 | 1.951 (1.543–2.4680) | 0.000 | 2.696 (2.182–3.332) | 0.000 | 8.762 (6.066–12.655) | 0.000 | 15.957 (12.260–20769) | 0.000 | 19.960 (13.854–28.758) | 0.000 |
| ≥25 | 3.595 (1.223–10.570) | 0.020 | 0.455 (0.058–3.565) | 0.454 | 9.688 (1.099–85.409) | 0.041 | 7.266 (1.712–30.840) | 0.007 | 34.163 (3.095–377.072) | 0.004 |
| Underlying diseases | ||||||||||
| Cancer | 1.368 (0.804–2.328) | 0.248 | 0.036 (0.001–1.2) | 0.063 | 19.477 (9.896–38.334) | 0.000 | 33.15 (12.192–90.129) | 0.000 | 1.368 (0.804–2.328) | 0.248 |
| Pulmonary | 2.886 (1.546–5.389) | 0.001 | 3.527 (1.571–7.919) | 0.002 | 11.744 (5.31–25.971) | 0.000 | 24.852 (7.745–79.748) | 0.000 | 2.886 (1.546–5.389) | 0.001 |
| Cardiovascular | 4.514 (2.7–7.548) | 0.000 | 6.997 (3.207–15.263) | 0.000 | 12.403 (5.67–27.133) | 0.000 | 9.718 (2.904–32.513) | 0.000 | 4.514 (2.7–7.548) | 0.000 |
| Digestive | 1.991 (0.855–4.635) | 0.110 | 21.924 (4.875–98.609) | 0.000 | 7.671 (1.914–30.755) | 0.004 | 55.185 (13.645–223.184) | 0.000 | 1.991 (0.855–4.635) | 0.110 |
| Hematological | 3.627 (1.909–6.89) | 0.000 | 4.458 (1.212–16.404) | 0.025 | 17.19 (4.915–60.119) | 0.000 | 28.916 (5.208–160.535) | 0.000 | 3.627 (1.909–6.89) | 0.000 |
| Neurological | 2.671 (1.112–6.42) | 0.028 | 8.124 (2.671–24.713) | 0.000 | 24.574 (11.16–54.11) | 0.000 | 23.028 (7.879–67.307) | 0.000 | 2.671 (1.112–6.42) | 0.028 |
| Urinary and renal | 2.579 (1.175–5.661) | 0.018 | 13.427 (4.347–41.475) | 0.000 | 20.734 (9.183–46.811) | 0.000 | 36.159 (7.867–166.184) | 0.000 | 2.579 (1.175–5.661) | 0.018 |
| Cardiothoracic surgery | 2.09 (0.875–4.994) | 0.097 | 20.563 (6.3–67.125) | 0.000 | 29.047 (12.987–64.968) | 0.000 | 18.291 (6.262–53.425) | 0.000 | 2.09 (0.875–4.994) | 0.097 |
| General surgery | 2.559 (1.084–6.039) | 0.032 | 5.917 (1.332–26.276) | 0.019 | 5.545 (1.547–19.872) | 0.009 | 6.703 (1.179–38.101) | 0.032 | 2.559 (1.084–6.039) | 0.032 |
| Others | 3.039 (1.109–8.327) | 0.031 | 11.19 (2.581–48.514) | 0.001 | 12.402 (3.918–39.254) | 0.000 | 71.597 (14.877–344.574) | 0.000 | 3.039 (1.109–8.327) | 0.031 |
| AKI | ||||||||||
| Yes | 1.666 (1.224–2.266) | 0.001 | 1.921 (1.455–2.537) | 0.000 | 10.749 (7.000–16.507) | 0.000 | 10.869 (8.026–14.720) | 0.000 | 10.983 (7.443–16.206) | 0.000 |
| No | 1.558 (1.110–2.188) | 0.010 | 1.541 (1.080–2.199) | 0.017 | 2.844 (1.391–5.817) | 0.004 | 7.200 (3.939–13.163) | 0.000 | 17.113 (6.638–44.117) | 0.000 |
| Length of hospital stay, day | ||||||||||
| ≤30 | 1.599 (1.241–2.061) | 0.000 | 1.781 (1.381–2.296) | 0.000 | 8.593 (5.808–12.712) | 0.000 | 17.738 (13.487–23.329) | 0.000 | 17.934 (11.561–27.822) | 0.000 |
| >30 | 4.548 (2.809–7.363) | 0.000 | 2.718 (1.701–4.342) | 0.000 | 5.579 (2.488–12.509) | 0.000 | 4.680 (2.461–8.902) | 0.000 | 10866 (5.760–20.499) | 0.000 |
Normonatremia as the reference.
CA – community-acquired; HA – hospital-acquired; BMI – body mass index; AKI – acute kidney injury; OR – odds ratio; CI – confidence interval.
Supplementary Table 4.
Subgroup multiple logistic regression analysis of dysnatremia as independent risk factors for hospital mortality in model 3.
| Subgroup | Improved hyponatremia | Persistent hyponatremia | Improved hypernatremia | Persistent hypernatremia | ||||
|---|---|---|---|---|---|---|---|---|
| OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | |
| Age, year | ||||||||
| ≤65 | 2.887 (2.113–3.945) | 0.000 | 12.639 (7.705–20.731) | 0.000 | 25.364 (16.541–38.893) | 0.000 | 21.923 11.773–40.825) | 0.000 |
| >65 | 1.644 (1.264–2.138) | 0.000 | 4.827 (3.166–7.359) | 0.000 | 20.010 (14.552–27.516) | 0.000 | 24.275 (15.899–37.066) | 0.000 |
| Gender | ||||||||
| Male | 1.779 (1.394–2.271) | 0.000 | 7.318 (4.819–11.113) | 0.000 | 26.151 (18.611–36.745) | 0.000 | 21.742 (13.914–33.974) | 0.000 |
| Female | 2.555 (1.794–3.639) | 0.000 | 6.448 (3.781–10.996) | 0.000 | 19.409 (12.602–29.891) | 0.000 | 27.078 (15.178–48.307) | 0.000 |
| BMI, kg/m2 | ||||||||
| <18.5 | 1.931 (0.472–7.894) | 0.360 | 20.986 (14.599–30.168) | 0.000 | 55.895 (8.888–351.535) | 0.000 | 1209.328 (37.744–38746.89) | 0.000 |
| 18.5–24.99 | 2.093 (1.701–2.576) | 0.000 | 6.28 (4.43–8.902) | 0.000 | 22.766 (17.252–30.042) | 0.000 | 20.986 (14.599–30.168) | 0.000 |
| ≥25 | 1.777 (0.559–5.648) | 0.330 | 5.025 (0.587–42.991) | 0.140 | 8.816 (1.948–39.891) | 0.005 | 32.095 (2.959–348.159) | 0.004 |
| Underlying diseases | ||||||||
| Cancer | 1.184 (0.731–1.916) | 0.492 | 11.343 (5.09–25.277) | 0.000 | 13.494 (4.684–38.876) | 0.000 | 25.983 (9.194–73.434) | 0.000 |
| Pulmonary | 3.416 (2.139–5.456) | 0.000 | 0.962 (0.288–3.214) | 0.949 | 16.5 (8.103–33.597) | 0.000 | 25.592 (7.866–83.263) | 0.000 |
| Cardiovascular | 4.409 (2.724–7.137) | 0.000 | 5.893 (2.519–13.789) | 0.000 | 13.128 (6.396–26.943) | 0.000 | 9.585 (2.866–32.059) | 0.000 |
| Digestive | 1.44 (0.699–2.963) | 0.323 | 3.019 (0.329–27.677) | 0.328 | 16.053 (5.374–47.952) | 0.000 | 50.959 (13.174–197.116) | 0.000 |
| Hematological | 2.78 (1.571–4.918) | 0.000 | 29.114 (7.417–114.275) | 0.000 | 3.738 (1.076–12.978) | 0.038 | 37.481 (7.029–199.851) | 0.000 |
| Neurological | 1.492 (0.598–3.722) | 0.391 | 8.002 (3.076–20.817) | 0.000 | 47.75 (20.678–110.263) | 0.000 | 28.754 (9.403–87.927) | 0.000 |
| Urinary and renal | 1.67 (0.829–3.364) | 0.152 | 4.526 (0.977–20.973) | 0.054 | 24.442 (11.571–51.631) | 0.000 | 32.258 (7.501–138.731) | 0.000 |
| Cardiothoracic surgery | 0.936 (0.304–2.882) | 0.909 | 10.885 (4.104–28.867) | 0.000 | 79.21 (32.91–190.65) | 0.000 | 18.084 (6.162–53.07) | 0.000 |
| General surgery | 2.279 (0.966–5.377) | 0.060 | 3.499 (0.86–14.233) | 0.080 | 15.866 (4.211–59.776) | 0.000 | 8.242 (1.487–45.693) | 0.016 |
| Others | 3.978 (1.902–8.317) | 0.000 | 2.31 (0.425–12.545) | 0.332 | 29.003 (10.267–81.93) | 0.000 | 122.536 (27.544–545.138) | 0.000 |
| AKI | ||||||||
| Yes | 1.915 (1.457–2.518) | 0.000 | 4.893 (3.387–7.068) | 0.000 | 25.307 (18.021–35.538) | 0.000 | 10.927 (7.422–16.087) | 0.000 |
| No | 1.247 (0.911–1.706) | 0.168 | 1.656 (0.591–4.643) | 0.337 | 7.438 (4.331–12.774) | 0.000 | 17.028 (6.573–44.108) | 0.000 |
| Length of hospital stay, day | ||||||||
| ≤30 | 1.507 (1.197–1.898) | 0.000 | 7.109 (4.89–10.337) | 0.000 | 22.685 (16.984–30.299) | 0.000 | 18.657 (12.039–28.914) | 0.000 |
| >30 | 3.514 (2.166–5.703) | 0.000 | 2.823 (1.411–5.651) | 0.003 | 25.407 (10.435–61.859) | 0.000 | 9.913 (5.18–18.973) | 0.000 |
Normonatremia as the reference.
BMI – body mass index; AKI – acute kidney injury; OR – odds ratio; CI – confidence interval.
Supplementary Table 5.
Multinomial logistic regression analysis of independent risk factors for developing of hospital-acquired and persistent dysnatremia.
| Variable | HA-hyponatremia | HA-hypernatremia | Persistent hyponatremia | Persistent hypernatremia | ||||
|---|---|---|---|---|---|---|---|---|
| OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | |
| Age, per year increase | 1.008 (1.006–1.010) | 0.000 | 1.019 (1.013–1.024) | 0.000 | 1.004 (1.002–1.006) | 0.001 | ||
| Male sex | 1.182 (1.117–1.252) | 0.000 | 0.692 (0.596–0.804) | 0.000 | 1.258 (1.164–1.360) | 0.000 | 1.274 (1.023–1.586) | 0.030 |
| BMI <18.5 kg/m2§ | 1.811 (1.396–2.350) | 0.000 | ||||||
| BMI 25–27.99 kg/m2§ | 1.297 (1.102–1.527) | 0.002 | ||||||
| BMI 28–32 kg/m2§ | 1.269 (0.959–1.680) | 0.096 | ||||||
| Cancer | 8.344 (7.640–9.113) | 0.000 | 2.768 (2.173–3.525) | 0.000 | ||||
| Pulmonary | 1.449 (1.249–1.681) | 0.000 | ||||||
| Cardiovascular | 1.279 (1.145–1.428) | 0.000 | ||||||
| Cardiothoracic surgery | 13.330 (12.125–14.654) | 0.000 | 10.989 (9.009–13.403) | 0.000 | 1.416 (1.270–1.580) | 0.000 | ||
| Digestive | 1.651 (1.432–1.903) | 0.000 | ||||||
| Endocrine | 2.187 (1.465–3.263) | 0.000 | ||||||
| General surgery | 4.042 (3.670–4.452) | 0.000 | 1.843 (1.429–2.376) | 0.000 | ||||
| Hematological | 1.532 (1.280–1.834) | 0.000 | ||||||
| Neurological | 2.575 (2.185–3.035) | 0.000 | 6.007 (4.586–7.868) | 0.000 | ||||
| Gynecology | 3.084 (2.565–3.708) | 0.000 | 1.741 (1.330–2.279) | 0.000 | ||||
| Orthopedic surgery | 1.360 (1.128–1.640) | 0.001 | ||||||
| SBP>140 mmHg | 1.359 (1.111–1.661) | 0.003 | ||||||
| DBP <60 mmHg§ | 1.546 (1.304–1.834) | 0.000 | 2.330 (1.657–3.276) | 0.000 | ||||
| Glucose 5.7–10 mmol/L§ | 1.133 (1.071–1.198) | 0.000 | ||||||
| Glucose >10 mmol/L§ | 1.319 (1.163–1.496) | 0.000 | ||||||
| Hypokalemia | 2.169 (1.769–2.659) | 0.000 | ||||||
| Hypochloremia§ | 2.064 (1.874–2.273) | 0.000 | 1.420 (1.308–1.540) | 0.000 | ||||
| Hyperchloremia | 1.868 (1.234–2.826) | 0.003 | ||||||
| Hypocalcemia | 1.460 (1.344–1.586) | 0.000 | 1.650 (1.347–2.021) | 0.000 | ||||
| Hypomagnesemia | 1.917 (1.492–2.464) | 0.000 | 1.929 (1.107–3.362) | 0.020 | 1.579 (1.124–2.217) | 0.008 | ||
| Hypermagnesemia | 1.504 (1.174–1.928) | 0.001 | ||||||
| Hypophosphatemia | 1.142 (1.052–1.240) | 0.002 | 1.818 (1.484–2.228) | 0.000 | ||||
| Hyperphosphatemia | 1.242 (1.137–1.357) | 0.000 | ||||||
| CO2CP <23 mmol/L§ | 1.230 (1.155–1.310) | 0.000 | ||||||
| AG >16 mmol/L§ | 1.293 (1.199–1.394) | 0.000 | 1.558 (1.298–1.870) | 0.000 | 1.370 (1.069–1.755) | 0.013 | ||
| ALT, per 50 U/L increase | 1.015 (1.001–1.030) | 0.041 | ||||||
| TBIL, per 20 μmol/L increase | 1.087 (1.066–1.108) | 0.000 | 1.102 (1.059–1.147) | 0.000 | 1.031 (1.013–1.050) | 0.001 | ||
| Albumin, per 5g/L decrease | 1.275 (1.234–1.318) | 0.000 | 1.458 (1.347–1.578) | 0.000 | ||||
| Hemoglobin (90–119 g/L)§ | 1.161 (1.090–1.237) | 0.000 | 1.179 (1.087–1.280) | 0.000 | ||||
| Hemoglobin (60–89 g/L)§ | 1.471 (1.307–1.656) | 0.000 | 1.277 (1.119–1.457) | 0.000 | ||||
| Hemoglobin (<60 g/L)§ | 1.713 (1.303–2.252) | 0.000 | 1.233 (0.929–1.638) | 0.147 | ||||
| WBC <4.0×109/L§ | 1.142 (1.025–1.273) | 0.016 | ||||||
| WBC >12.0×109/L§ | 1.556 (1.378–1.758) | 0.000 | 2.322 (1.805–2.987) | 0.000 | ||||
| BUN, per 10 mg/dl increase | 1.107 (1.066–1.149) | 0.000 | 1.238 (1.149–1.334) | 0.000 | ||||
| SCr, per 0.5 mg/dl increase | 0.958 (0.926–0.990) | 0.011 | ||||||
| Hypouricemia§ | 1.084 (1.003–1.170) | 0.041 | ||||||
| Hyperuricemia§ | 1.135 (1.046–1.231) | 0.002 | 1.481 (1.212–1.810) | 0.000 | 1.132 (1.012–1.267) | 0.031 | ||
Normonatremia served as the reference for HA-dysnatremia and improved dysnatremia as the reference for corresponding persistent dysnatremia. For underlying diseases, the rest of the other patients served as the references.
Normal range as the reference.
BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; CO2CP – carbon dioxide combining power; AG – anion gap; ALT – alanine aminotransferase; TBIL – total bilirubin; WBC – white blood cell; BUN – blood urea nitrogen; SCr – serum creatinine; OR – odds ratio; CI – confidence interval.
Footnotes
Competing interests
None of the authors have any financial or non-financial competing interests.
Source of support: This work was supported by grants from the Shanghai Key Discipline Construction Project on the Fourth Round of Three-year Action Plan for Public Health Systems: Subject of Dialysis and Body Fluids (15GWZK0502), Chinese Ministry of Health 2013 and the Shanghai Science and Technology Committee Foundation: Shanghai Key Laboratory of Kidney and Blood Purification (14DZ2260200)
References
- 1.Pokaharel M, Block CA. Dysnatremia in the ICU. Curr Opin Crit Care. 2011;17(6):581–93. doi: 10.1097/MCC.0b013e32834cd388. [DOI] [PubMed] [Google Scholar]
- 2.Funk GC, Lindner G, Druml W, et al. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. 2010;36(2):304–11. doi: 10.1007/s00134-009-1692-0. [DOI] [PubMed] [Google Scholar]
- 3.Nankabirwa H, Kalyesubula R, Ssinabulya I, et al. A cross-sectional study of hyponatraemia among elderly patients with heart failure in Uganda. BMJ Open. 2016;6(5):e009775. doi: 10.1136/bmjopen-2015-009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saepudin S, Ball PA, Morrissey H. Hyponatremia during hospitalization and in-hospital mortality in patients hospitalized from heart failure. BMC Cardiovasc Disord. 2015;15:88. doi: 10.1186/s12872-015-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreau R. Hyponatremia in cirrhosis. Pathophysiology, prevalence, prognostic value, treatment. Acta Gastroenterol Belg. 2008;71(4):379–85. [PubMed] [Google Scholar]
- 6.Bennani SL, Abouqal R, Zeggwagh AA, et al. Incidence, causes and prognostic factors of hyponatremia in intensive care. Rev Med Interne. 2003;24(4):224–29. doi: 10.1016/s0248-8663(02)00811-1. [DOI] [PubMed] [Google Scholar]
- 7.Lindner G, Funk GC, Schwarz C, et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis. 2007;50(6):952–57. doi: 10.1053/j.ajkd.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Darmon M, Diconne E, Souweine B, et al. Prognostic consequences of borderline dysnatremia: pay attention to minimal serum sodium change. Crit Care. 2013;17(1):R12. doi: 10.1186/cc11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darmon M, Timsit J-F, Francais A, et al. Association between hypernatraemia acquired in the ICU and mortality: A cohort study. Nephrol Dial Transplant. 2010;25(8):2510–15. doi: 10.1093/ndt/gfq067. [DOI] [PubMed] [Google Scholar]
- 10.Turgutalp K, Ozhan O, Oguz EG, et al. Community-acquired hypernatremia in elderly and very elderly patients admitted to the hospital: Clinical characteristics and outcomes. Med Sci Monit. 2012;18(12):CR729–34. doi: 10.12659/MSM.883600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varun S, Bhaskar E, Abraham G, et al. Risk factors for hospital-acquired hypernatremia among critically ill medical patients in a setting utilizing a preventive free water protocol: Do we need to do more? Indian J Crit Care Med. 2013;17(1):28–33. doi: 10.4103/0972-5229.112157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han SS, Bae E, Kim DK, et al. Dysnatremia, its correction, and mortality in patients undergoing continuous renal replacement therapy: A prospective observational study. BMC Nephrol. 2016;17:2. doi: 10.1186/s12882-015-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soupart A, Ngassa M, Decaux G. Therapeutic relowering of the serum sodium in a patient after excessive correction of hyponatremia. Clin Nephrol. 1999;51(6):383–86. [PubMed] [Google Scholar]
- 14.Stelfox HT, Ahmed SB, Khandwala F, et al. The epidemiology of intensive care unit-acquired hyponatraemia and hypernatraemia in medical-surgical intensive care units. Crit Care. 2008;12(6):R162. doi: 10.1186/cc7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neithercut WD, Spooner RJ. Nosocomial dysnatremia. Clin Chem. 1988;34(11):2239–40. [PubMed] [Google Scholar]
- 16.Sakr Y, Rother S, Ferreira AMP, et al. Fluctuations in serum sodium level are associated with an increased risk of death in surgical ICU patients. Crit Care Med. 2013;41(1):133–42. doi: 10.1097/CCM.0b013e318265f576. [DOI] [PubMed] [Google Scholar]
- 17.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 18.Engle JE. Clinical physiology of acid-base and electrolyte disorders. JAMA. 1990;263(17):2375–76. [Google Scholar]
- 19.Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119:S30–35. doi: 10.1016/j.amjmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122(9):857–65. doi: 10.1016/j.amjmed.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: Results from NHANES. Am J Med. 2013;126(12):1127–37. doi: 10.1016/j.amjmed.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oude Lansink-Hartgring A, Hessels L, Weigel J, et al. Long-term changes in dysnatremia incidence in the ICU: A shift from hyponatremia to hypernatremia. Ann Intensive Care. 2016;6(1):22. doi: 10.1186/s13613-016-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donoghue SD, Dulhunty JM, Bandeshe HK, et al. Acquired hypernatraemia is an independent predictor of mortality in critically ill patients. Anaesthesia. 2009;64(5):514–20. doi: 10.1111/j.1365-2044.2008.05857.x. [DOI] [PubMed] [Google Scholar]
- 24.Bataille S, Baralla C, Torro D, et al. Undercorrection of hypernatremia is frequent and associated with mortality. BMC Nephrol. 2014;15:37. doi: 10.1186/1471-2369-15-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Outcomes of included patients with dysnatremia.
| Group | AKI, n (%) | Death, n (%) | Length of hospital stay, days | Hospital cost, RMB |
|---|---|---|---|---|
| Model 1 | ||||
| Normonatremia | 6,149 (8.3) | 317 (0.4) | 5.0 (2.5–8.0) | 13,869.1 (7,669.2–32,798.7) |
| Hyponatremia | 4,446 (29.2) | 439 (2.9) | 10.0 (5.5–15.0)# | 29,533.5 (13,362.4–60,616.8)# |
| Hypernatremia | 764 (45.3) | 204 (12.1) | 10.5 (6.0–17.0)# | 37,104.3 (14,016.3–80,466.7)# |
| Mixed dysnatremia | 233 (81.8) | 69 (24.2) | 21.0 (13.5–32.5)# | 106,510.3 (46,623.5–180,214.8)# |
| P value (χ2 or ANOVA) | 0.000 | 0.000 | 0.000 | 0.000 |
| Model 2 | ||||
| CA-hyponatremia | 1,677 (23.8) | 291 (4.1) | 7.0 (4.0–12.5) | 16,176.1 (9,060.1–33,854.8) |
| HA-hyponatremia | 2,769 (33.9) | 148 (1.8) | 11.5 (8.0–16.5) | 46,061.3 (23,207.6–83,343.8) |
| P value | 0.000 | 0.000 | 0.000 | 0.000 |
| CA-hypernatremia | 247 (29.1) | 83 (9.8) | 7.0 (3.5–12.5) | 20,248.1 (9,198.2–46,825.4) |
| HA-hypernatremia | 517 (61.8) | 121 (14.5) | 14.0 (9.5–21.5) | 60,351.1 (29,892.6–121,096.3) |
| P value | 0.000 | 0.003 | 0.000 | 0.000 |
| Model 3 | ||||
| Improved hyponatremia | 1,888 (36.2) | 176 (3.4) | 12.5 (8.5–18.5) | 41,283.8 (20,389.7–71,168.3) |
| Persistent hyponatremia | 2,558 (25.5) | 263 (2.6) | 8.0 (4.5–13.5) | 22,206.9 (11,558.2–54,202.7) |
| P value | 0.000 | 0.008 | 0.000 | 0.000 |
| Improved hypernatremia | 488 (56.8) | 61 (7.1) | 14.0 (9.5–21.0) | 57,157.7 (30,575.9–116,872.8) |
| Persistent hypernatremia | 276 (33.3) | 143 (17.3) | 7.0 (3.5–12.0) | 17,773.6 (8,782.9–49,237.5) |
| P value | 0.000 | 0.000 | 0.000 | 0.000 |
| “Hypo to hyper” mixed dysnatremia | 82 (35.2) | 55 (79.7) | 21.8 (13.4–38.5) | 89,939.4 (29,995.7–180,373.3) |
| “Hyper to hypo” mixed dysnatremia | 151 (64.8) | 14 (20.3) | 20.5 (14.0–30.5) | 113,523 (52,375.1–181,003.8) |
| P value | 0.657 | 0.000 | 0.603 | 0.050 |
P<0.001 vs. normonatremia group.
AKI – acute kidney injury; RMB – Ren Min Bi; CA – community-acquired; HA – hospital-acquired.
Supplementary Table 2.
Subgroup multiple logistic regression analysis of dysnatremia as independent risk factors for hospital mortality in model 1.
| Subgroup | Hyponatremia§ | Hypernatremia§ | Mixed dysnatremia§ | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, year | ||||||
| ≤65 | 2.722 (2.034–3.643) | 0.000 | 18.350 (12.853–26.198) | 0.000 | 21.886 (11.753–40.755) | 0.000 |
| <65 | 2.075 (1.653–2.606) | 0.000 | 11.112 (8.440–14.631) | 0.000 | 24.623 (16.162–37.513) | 0.000 |
| Gender | ||||||
| Male | 1.963 (1.575–2.447) | 0.000 | 14.659 (11.014–19.512) | 0.000 | 21.475 (13.748–33.544) | 0.000 |
| Female | 2.860 (2.090–3.913) | 0.000 | 11.952 (8.231–17.353) | 0.000 | 27.276 (15.320–48.564) | 0.000 |
| BMI, kg/m2 | ||||||
| <18.5 | 1.534 (0.453–5.198) | 0.492 | 71.139 (12.345–409.950) | 0.000 | 1233.153 (38.420–39580.284) | 0.000 |
| 18.5–24.99 | 2.297 (1.906–2.769) | 0.000 | 13.003 (10.241–16.509) | 0.000 | 20.950 (14.578–30.105) | 0.000 |
| ≥25 | 1.689 (0.632–4.513) | 0.296 | 7.195 (2.006–25.806) | 0.002 | 32.104 (2.952–349.133) | 0.004 |
| Underlying diseases | ||||||
| Cancer | 1.635 (1.069–2.502) | 0.023 | 11.942 (6.105–23.359) | 0.000 | 26.998 (9.615–75.806) | 0.000 |
| Pulmonary | 3.062 (2.010–4.633) | 0.000 | 6.266 (3.353–11.710) | 0.000 | 25.139 (7.850–80.503) | 0.000 |
| Cardiovascular | 4.863 (3.203–7.385) | 0.000 | 9.117 (5.037–16.502) | 0.000 | 9.550 (2.855–31.946) | 0.000 |
| Digestive | 1.445 (0.726–2.877) | 0.295 | 11.231 (3.914–32.232) | 0.000 | 53.383 (13.800–206.493) | 0.000 |
| Hematological | 3.398 (2.103–5.490) | 0.000 | 8.980 (3.723–21.660) | 0.000 | 48.642 (8.840–267.664) | 0.000 |
| Neurological | 2.199 (1.038–4.658) | 0.040 | 16.821 (8.191–34.544) | 0.000 | 23.310 (7.966–68.207) | 0.000 |
| Urinary and renal | 1.390 (0.746–2.588) | 0.300 | 17.257 (8.551–34.826) | 0.000 | 33.917 (7.819–147.114) | 0.000 |
| Cardiothoracic surgery | 2.385 (1.065–5.340) | 0.035 | 27.029 (12.388–58.976) | 0.000 | 18.501 (6.336–54.021) | 0.000 |
| General surgery | 2.282 (1.056–4.931) | 0.036 | 6.568 (2.299–18.763) | 0.000 | 8.285 (1.500–45.761) | 0.003 |
| Others | 2.321 (10.22–5.273) | 0.044 | 11.917 (4.531–31.341) | 0.000 | 77.078 (16.156–367.723) | 0.000 |
| AKI | ||||||
| Yes | 1.810 (1.411–2.322) | 0.000 | 10.784 (8.161–14.252) | 0.000 | 11.053 (7.502–16.285) | 0.000 |
| No | 1.526 (1.157–2.013) | 0.003 | 4.596 (2.817–7.499) | 0.000 | 16.996 (6.574–43.944) | 0.000 |
| Length of hospital stay, day | ||||||
| ≤30 | 1.627 (1.323–2.001) | 0.000 | 14.168 (11.042–18.179) | 0.000 | 18.563 (11.980–28.763) | 0.000 |
| >30 | 2.928 (1.879–4.561) | 0.000 | 5.233 (2.926–9.360) | 0.000 | 9.789 (5.116–18.730) | 0.000 |
Normonatremia as the reference.
BMI – body mass index; AKI – acute kidney injury; OR – odds ratio; CI – confidence interval.
Supplementary Table 3.
Subgroup multiple logistic regression analysis of dysnatremia as independent risk factors for hospital mortality in model 2.
| Subgroup | CA-hyponatremia§ | HA-hyponatremia§ | CA-hypernatremia§ | HA-hypernatremia§ | Mixed dysnatremia§ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Age, year | ||||||||||
| ≤65 | 2.433 (1.694–3.495) | 0.000 | 3.077 (2.216–4.273) | 0.000 | 9.953 (5.545–17.865) | 0.000 | 22.295 (14.862–33.445) | 0.000 | 18.570 (9.827–35.092) | 0.000 |
| >65 | 1.878 (1.414–2.494) | 0.000 | 2.329 (1.786–3.037) | 0.000 | 7.767 (5.146–11.724) | 0.000 | 13.665 (9.957–18.754) | 0.000 | 23.599 (15.462–36.017) | 0.000 |
| Gender | ||||||||||
| Male | 1.755 (1.335–2.308) | 0.000 | 2.219 (1.713–2.875) | 0.000 | 9.094 (5.740–14.406) | 0.000 | 18.348 (13.365–25.190) | 0.000 | 20.081 (12.820–31.454) | 0.000 |
| Female | 2.307 (1.561–3.410) | 0.000 | 3.387 (2.389–4.803) | 0.000 | 8.502 (4.900–14.752) | 0.000 | 13.847 (9.030–21.232) | 0.000 | 25.346 (14.148–45.406) | 0.000 |
| BMI, kg/m2 | ||||||||||
| <18.5 | 2.063 (0.531–8.018) | 0.296 | 0.961 (0.168–5.515) | 0.965 | – | – | 74.385 (12.840–430.910) | 0.000 | 1112.828 (35.443–34940.099) | 0.000 |
| 18.5–24.99 | 1.951 (1.543–2.4680) | 0.000 | 2.696 (2.182–3.332) | 0.000 | 8.762 (6.066–12.655) | 0.000 | 15.957 (12.260–20769) | 0.000 | 19.960 (13.854–28.758) | 0.000 |
| ≥25 | 3.595 (1.223–10.570) | 0.020 | 0.455 (0.058–3.565) | 0.454 | 9.688 (1.099–85.409) | 0.041 | 7.266 (1.712–30.840) | 0.007 | 34.163 (3.095–377.072) | 0.004 |
| Underlying diseases | ||||||||||
| Cancer | 1.368 (0.804–2.328) | 0.248 | 0.036 (0.001–1.2) | 0.063 | 19.477 (9.896–38.334) | 0.000 | 33.15 (12.192–90.129) | 0.000 | 1.368 (0.804–2.328) | 0.248 |
| Pulmonary | 2.886 (1.546–5.389) | 0.001 | 3.527 (1.571–7.919) | 0.002 | 11.744 (5.31–25.971) | 0.000 | 24.852 (7.745–79.748) | 0.000 | 2.886 (1.546–5.389) | 0.001 |
| Cardiovascular | 4.514 (2.7–7.548) | 0.000 | 6.997 (3.207–15.263) | 0.000 | 12.403 (5.67–27.133) | 0.000 | 9.718 (2.904–32.513) | 0.000 | 4.514 (2.7–7.548) | 0.000 |
| Digestive | 1.991 (0.855–4.635) | 0.110 | 21.924 (4.875–98.609) | 0.000 | 7.671 (1.914–30.755) | 0.004 | 55.185 (13.645–223.184) | 0.000 | 1.991 (0.855–4.635) | 0.110 |
| Hematological | 3.627 (1.909–6.89) | 0.000 | 4.458 (1.212–16.404) | 0.025 | 17.19 (4.915–60.119) | 0.000 | 28.916 (5.208–160.535) | 0.000 | 3.627 (1.909–6.89) | 0.000 |
| Neurological | 2.671 (1.112–6.42) | 0.028 | 8.124 (2.671–24.713) | 0.000 | 24.574 (11.16–54.11) | 0.000 | 23.028 (7.879–67.307) | 0.000 | 2.671 (1.112–6.42) | 0.028 |
| Urinary and renal | 2.579 (1.175–5.661) | 0.018 | 13.427 (4.347–41.475) | 0.000 | 20.734 (9.183–46.811) | 0.000 | 36.159 (7.867–166.184) | 0.000 | 2.579 (1.175–5.661) | 0.018 |
| Cardiothoracic surgery | 2.09 (0.875–4.994) | 0.097 | 20.563 (6.3–67.125) | 0.000 | 29.047 (12.987–64.968) | 0.000 | 18.291 (6.262–53.425) | 0.000 | 2.09 (0.875–4.994) | 0.097 |
| General surgery | 2.559 (1.084–6.039) | 0.032 | 5.917 (1.332–26.276) | 0.019 | 5.545 (1.547–19.872) | 0.009 | 6.703 (1.179–38.101) | 0.032 | 2.559 (1.084–6.039) | 0.032 |
| Others | 3.039 (1.109–8.327) | 0.031 | 11.19 (2.581–48.514) | 0.001 | 12.402 (3.918–39.254) | 0.000 | 71.597 (14.877–344.574) | 0.000 | 3.039 (1.109–8.327) | 0.031 |
| AKI | ||||||||||
| Yes | 1.666 (1.224–2.266) | 0.001 | 1.921 (1.455–2.537) | 0.000 | 10.749 (7.000–16.507) | 0.000 | 10.869 (8.026–14.720) | 0.000 | 10.983 (7.443–16.206) | 0.000 |
| No | 1.558 (1.110–2.188) | 0.010 | 1.541 (1.080–2.199) | 0.017 | 2.844 (1.391–5.817) | 0.004 | 7.200 (3.939–13.163) | 0.000 | 17.113 (6.638–44.117) | 0.000 |
| Length of hospital stay, day | ||||||||||
| ≤30 | 1.599 (1.241–2.061) | 0.000 | 1.781 (1.381–2.296) | 0.000 | 8.593 (5.808–12.712) | 0.000 | 17.738 (13.487–23.329) | 0.000 | 17.934 (11.561–27.822) | 0.000 |
| >30 | 4.548 (2.809–7.363) | 0.000 | 2.718 (1.701–4.342) | 0.000 | 5.579 (2.488–12.509) | 0.000 | 4.680 (2.461–8.902) | 0.000 | 10866 (5.760–20.499) | 0.000 |
Normonatremia as the reference.
CA – community-acquired; HA – hospital-acquired; BMI – body mass index; AKI – acute kidney injury; OR – odds ratio; CI – confidence interval.
Supplementary Table 4.
Subgroup multiple logistic regression analysis of dysnatremia as independent risk factors for hospital mortality in model 3.
| Subgroup | Improved hyponatremia | Persistent hyponatremia | Improved hypernatremia | Persistent hypernatremia | ||||
|---|---|---|---|---|---|---|---|---|
| OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | |
| Age, year | ||||||||
| ≤65 | 2.887 (2.113–3.945) | 0.000 | 12.639 (7.705–20.731) | 0.000 | 25.364 (16.541–38.893) | 0.000 | 21.923 11.773–40.825) | 0.000 |
| >65 | 1.644 (1.264–2.138) | 0.000 | 4.827 (3.166–7.359) | 0.000 | 20.010 (14.552–27.516) | 0.000 | 24.275 (15.899–37.066) | 0.000 |
| Gender | ||||||||
| Male | 1.779 (1.394–2.271) | 0.000 | 7.318 (4.819–11.113) | 0.000 | 26.151 (18.611–36.745) | 0.000 | 21.742 (13.914–33.974) | 0.000 |
| Female | 2.555 (1.794–3.639) | 0.000 | 6.448 (3.781–10.996) | 0.000 | 19.409 (12.602–29.891) | 0.000 | 27.078 (15.178–48.307) | 0.000 |
| BMI, kg/m2 | ||||||||
| <18.5 | 1.931 (0.472–7.894) | 0.360 | 20.986 (14.599–30.168) | 0.000 | 55.895 (8.888–351.535) | 0.000 | 1209.328 (37.744–38746.89) | 0.000 |
| 18.5–24.99 | 2.093 (1.701–2.576) | 0.000 | 6.28 (4.43–8.902) | 0.000 | 22.766 (17.252–30.042) | 0.000 | 20.986 (14.599–30.168) | 0.000 |
| ≥25 | 1.777 (0.559–5.648) | 0.330 | 5.025 (0.587–42.991) | 0.140 | 8.816 (1.948–39.891) | 0.005 | 32.095 (2.959–348.159) | 0.004 |
| Underlying diseases | ||||||||
| Cancer | 1.184 (0.731–1.916) | 0.492 | 11.343 (5.09–25.277) | 0.000 | 13.494 (4.684–38.876) | 0.000 | 25.983 (9.194–73.434) | 0.000 |
| Pulmonary | 3.416 (2.139–5.456) | 0.000 | 0.962 (0.288–3.214) | 0.949 | 16.5 (8.103–33.597) | 0.000 | 25.592 (7.866–83.263) | 0.000 |
| Cardiovascular | 4.409 (2.724–7.137) | 0.000 | 5.893 (2.519–13.789) | 0.000 | 13.128 (6.396–26.943) | 0.000 | 9.585 (2.866–32.059) | 0.000 |
| Digestive | 1.44 (0.699–2.963) | 0.323 | 3.019 (0.329–27.677) | 0.328 | 16.053 (5.374–47.952) | 0.000 | 50.959 (13.174–197.116) | 0.000 |
| Hematological | 2.78 (1.571–4.918) | 0.000 | 29.114 (7.417–114.275) | 0.000 | 3.738 (1.076–12.978) | 0.038 | 37.481 (7.029–199.851) | 0.000 |
| Neurological | 1.492 (0.598–3.722) | 0.391 | 8.002 (3.076–20.817) | 0.000 | 47.75 (20.678–110.263) | 0.000 | 28.754 (9.403–87.927) | 0.000 |
| Urinary and renal | 1.67 (0.829–3.364) | 0.152 | 4.526 (0.977–20.973) | 0.054 | 24.442 (11.571–51.631) | 0.000 | 32.258 (7.501–138.731) | 0.000 |
| Cardiothoracic surgery | 0.936 (0.304–2.882) | 0.909 | 10.885 (4.104–28.867) | 0.000 | 79.21 (32.91–190.65) | 0.000 | 18.084 (6.162–53.07) | 0.000 |
| General surgery | 2.279 (0.966–5.377) | 0.060 | 3.499 (0.86–14.233) | 0.080 | 15.866 (4.211–59.776) | 0.000 | 8.242 (1.487–45.693) | 0.016 |
| Others | 3.978 (1.902–8.317) | 0.000 | 2.31 (0.425–12.545) | 0.332 | 29.003 (10.267–81.93) | 0.000 | 122.536 (27.544–545.138) | 0.000 |
| AKI | ||||||||
| Yes | 1.915 (1.457–2.518) | 0.000 | 4.893 (3.387–7.068) | 0.000 | 25.307 (18.021–35.538) | 0.000 | 10.927 (7.422–16.087) | 0.000 |
| No | 1.247 (0.911–1.706) | 0.168 | 1.656 (0.591–4.643) | 0.337 | 7.438 (4.331–12.774) | 0.000 | 17.028 (6.573–44.108) | 0.000 |
| Length of hospital stay, day | ||||||||
| ≤30 | 1.507 (1.197–1.898) | 0.000 | 7.109 (4.89–10.337) | 0.000 | 22.685 (16.984–30.299) | 0.000 | 18.657 (12.039–28.914) | 0.000 |
| >30 | 3.514 (2.166–5.703) | 0.000 | 2.823 (1.411–5.651) | 0.003 | 25.407 (10.435–61.859) | 0.000 | 9.913 (5.18–18.973) | 0.000 |
Normonatremia as the reference.
BMI – body mass index; AKI – acute kidney injury; OR – odds ratio; CI – confidence interval.
Supplementary Table 5.
Multinomial logistic regression analysis of independent risk factors for developing of hospital-acquired and persistent dysnatremia.
| Variable | HA-hyponatremia | HA-hypernatremia | Persistent hyponatremia | Persistent hypernatremia | ||||
|---|---|---|---|---|---|---|---|---|
| OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | |
| Age, per year increase | 1.008 (1.006–1.010) | 0.000 | 1.019 (1.013–1.024) | 0.000 | 1.004 (1.002–1.006) | 0.001 | ||
| Male sex | 1.182 (1.117–1.252) | 0.000 | 0.692 (0.596–0.804) | 0.000 | 1.258 (1.164–1.360) | 0.000 | 1.274 (1.023–1.586) | 0.030 |
| BMI <18.5 kg/m2§ | 1.811 (1.396–2.350) | 0.000 | ||||||
| BMI 25–27.99 kg/m2§ | 1.297 (1.102–1.527) | 0.002 | ||||||
| BMI 28–32 kg/m2§ | 1.269 (0.959–1.680) | 0.096 | ||||||
| Cancer | 8.344 (7.640–9.113) | 0.000 | 2.768 (2.173–3.525) | 0.000 | ||||
| Pulmonary | 1.449 (1.249–1.681) | 0.000 | ||||||
| Cardiovascular | 1.279 (1.145–1.428) | 0.000 | ||||||
| Cardiothoracic surgery | 13.330 (12.125–14.654) | 0.000 | 10.989 (9.009–13.403) | 0.000 | 1.416 (1.270–1.580) | 0.000 | ||
| Digestive | 1.651 (1.432–1.903) | 0.000 | ||||||
| Endocrine | 2.187 (1.465–3.263) | 0.000 | ||||||
| General surgery | 4.042 (3.670–4.452) | 0.000 | 1.843 (1.429–2.376) | 0.000 | ||||
| Hematological | 1.532 (1.280–1.834) | 0.000 | ||||||
| Neurological | 2.575 (2.185–3.035) | 0.000 | 6.007 (4.586–7.868) | 0.000 | ||||
| Gynecology | 3.084 (2.565–3.708) | 0.000 | 1.741 (1.330–2.279) | 0.000 | ||||
| Orthopedic surgery | 1.360 (1.128–1.640) | 0.001 | ||||||
| SBP>140 mmHg | 1.359 (1.111–1.661) | 0.003 | ||||||
| DBP <60 mmHg§ | 1.546 (1.304–1.834) | 0.000 | 2.330 (1.657–3.276) | 0.000 | ||||
| Glucose 5.7–10 mmol/L§ | 1.133 (1.071–1.198) | 0.000 | ||||||
| Glucose >10 mmol/L§ | 1.319 (1.163–1.496) | 0.000 | ||||||
| Hypokalemia | 2.169 (1.769–2.659) | 0.000 | ||||||
| Hypochloremia§ | 2.064 (1.874–2.273) | 0.000 | 1.420 (1.308–1.540) | 0.000 | ||||
| Hyperchloremia | 1.868 (1.234–2.826) | 0.003 | ||||||
| Hypocalcemia | 1.460 (1.344–1.586) | 0.000 | 1.650 (1.347–2.021) | 0.000 | ||||
| Hypomagnesemia | 1.917 (1.492–2.464) | 0.000 | 1.929 (1.107–3.362) | 0.020 | 1.579 (1.124–2.217) | 0.008 | ||
| Hypermagnesemia | 1.504 (1.174–1.928) | 0.001 | ||||||
| Hypophosphatemia | 1.142 (1.052–1.240) | 0.002 | 1.818 (1.484–2.228) | 0.000 | ||||
| Hyperphosphatemia | 1.242 (1.137–1.357) | 0.000 | ||||||
| CO2CP <23 mmol/L§ | 1.230 (1.155–1.310) | 0.000 | ||||||
| AG >16 mmol/L§ | 1.293 (1.199–1.394) | 0.000 | 1.558 (1.298–1.870) | 0.000 | 1.370 (1.069–1.755) | 0.013 | ||
| ALT, per 50 U/L increase | 1.015 (1.001–1.030) | 0.041 | ||||||
| TBIL, per 20 μmol/L increase | 1.087 (1.066–1.108) | 0.000 | 1.102 (1.059–1.147) | 0.000 | 1.031 (1.013–1.050) | 0.001 | ||
| Albumin, per 5g/L decrease | 1.275 (1.234–1.318) | 0.000 | 1.458 (1.347–1.578) | 0.000 | ||||
| Hemoglobin (90–119 g/L)§ | 1.161 (1.090–1.237) | 0.000 | 1.179 (1.087–1.280) | 0.000 | ||||
| Hemoglobin (60–89 g/L)§ | 1.471 (1.307–1.656) | 0.000 | 1.277 (1.119–1.457) | 0.000 | ||||
| Hemoglobin (<60 g/L)§ | 1.713 (1.303–2.252) | 0.000 | 1.233 (0.929–1.638) | 0.147 | ||||
| WBC <4.0×109/L§ | 1.142 (1.025–1.273) | 0.016 | ||||||
| WBC >12.0×109/L§ | 1.556 (1.378–1.758) | 0.000 | 2.322 (1.805–2.987) | 0.000 | ||||
| BUN, per 10 mg/dl increase | 1.107 (1.066–1.149) | 0.000 | 1.238 (1.149–1.334) | 0.000 | ||||
| SCr, per 0.5 mg/dl increase | 0.958 (0.926–0.990) | 0.011 | ||||||
| Hypouricemia§ | 1.084 (1.003–1.170) | 0.041 | ||||||
| Hyperuricemia§ | 1.135 (1.046–1.231) | 0.002 | 1.481 (1.212–1.810) | 0.000 | 1.132 (1.012–1.267) | 0.031 | ||
Normonatremia served as the reference for HA-dysnatremia and improved dysnatremia as the reference for corresponding persistent dysnatremia. For underlying diseases, the rest of the other patients served as the references.
Normal range as the reference.
BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; CO2CP – carbon dioxide combining power; AG – anion gap; ALT – alanine aminotransferase; TBIL – total bilirubin; WBC – white blood cell; BUN – blood urea nitrogen; SCr – serum creatinine; OR – odds ratio; CI – confidence interval.