Abstract
Purpose
To evaluate the ability of the new food supplement, Body Lipid (BL), containing red yeast rice, berberine, coenzyme Q10 and hydroxytyrosol, to lower the LDL-C in patients with mild-to-moderate hypercholesterolemia and to assess the overall safety profile of the product.
Methods
In this multicenter, randomized, double-blind, placebo and active comparator (the marketed Armolipid Plus® [AM]) controlled study, 158 hypercholesterolemic patients were randomized following a 4-week dietary run-in period. After 4 weeks of treatment with a daily oral dose of the new food supplement BL, AM or placebo, plus diet, the main outcome was the decrease of LDL-C, total cholesterol (TC), and triglyceride levels.
Findings
The absolute changes of LDL-C and TC levels from baseline, at week 4 were: −39.1 mg/dL ±17.76 and −45.9 mg/dL ±21.54, respectively in the BL group; 5.7 mg/dL ±14.98 and 2.4 mg/dL ±18.43, respectively in the placebo group. Results were statistically significant. In terms of mean percentage, BL was shown to be more effective in lowering LDL-C levels as compared to placebo and the active comparator (AM), with a reduction of −26.3%, +4.2%, −18.3%, respectively. Five adverse events (AEs) were reported by five patients after the initiation of the study treatment: two in the BL group (influence and insomnia), two in the AM group (ear pain and rash), and one in the placebo group (back pain). All AEs were mild in intensity, except for back pain (severe). The case of insomnia in the BL group and the case of rash in the AM group were judged as treatment related. The safety review of the laboratory (blood and urine) analyses, vital signs and physical findings did not show any clinical effect of the study products on any of the parameters.
Implications
BL showed a good efficacy and safety profile and, for this reason, it can be considered an alternative to pharmacological treatment, for patients with mild-to-moderate hypercholesterolemia.
Keywords: lipid profile, red yeast rice, berberine, coenzyme Q10, hydroxytyrosol
Introduction
Cholesterol is a lipid belonging to the family of sterols, present in the body both in free and esterified form. It is a fundamental constituent of cell membranes, the precursor of several hormones, bile acids, and vitamin D. Lipid disorders most often imply hypercholesterolemia. There is a large body of epidemiological evidence demonstrating a strong correlation and causal relationship between serum cholesterol level, particularly serum low-density lipoprotein cholesterol (LDL-C), and the risk of coronary heart disease (CHD). Other clinical manifestations of atherosclerosis also appear to be linked to plasma LDL-C levels such as cerebrovascular disease (ie, stroke) or peripheral vascular disease. In addition, clinical trials have shown that LDL-lowering therapy is able to reduce the risk of CHD.1
Statins are effective for treating cardiovascular diseases in primary and secondary prevention.2 However, discontinuation rates are high and almost one-third of statin users discontinue therapy within 1 year due to poor tolerability and adverse events (AEs).3 The most commonly reported AEs are: elevated hepatic enzyme levels, gastrointestinal symptoms and statin-associated myalgias, which include muscle pain and weakness. Myositis (elevated creatinine phosphokinase [CPK] level) and rhabdomyolysis are more serious, but rare complications of therapy.4 For this reason, the use of statins to reduce cardiovascular risk in clinical practice is rarely encouraged for primary prevention.
Although statins represent an effective treatment for hypercholesterolemia, there is a medical need to use alternative products that could link a safer tolerability profile to the effectiveness. For this reason, nutraceuticals with lipid-lowering characteristics have been studied as a viable alternative to conventional therapy for patients with mild-to-moderate hypercholesterolemia, with the aim to reduce the main atherosclerotic risk factors and promote cardiovascular health.5–8 In particular, lipid-lowering effects have been claimed for nutraceuticals containing berberine, red yeast rice (RYR) and coenzyme Q10.9
In this context, the clinical use of a new food supplement, Body Lipid (BL; Farmaceutici Procemsa, Torino, Italy), a nutraceutical combination containing a mixture of RYR (monacolin K 10 mg), berberine (500 mg), coenzyme Q10 (2 mg) and hydroxytyrosol (5 mg), could represent an effective approach for mild-to-moderate hypercholesterolemic patients’ management.
The interest in a nutraceutical approach to metabolic disorders is growing because patients with metabolic conditions suitable for a nutraceutical approach seem to appreciate a therapeutic management that does not involve drug treatment.10
Both monacolin K and berberine and have been shown to exert a positive effect on lipid control.6–8,10–13
Monacolin K is a statin-like molecule which is derived from Chinese RYR, a dietary supplement made by fermenting the yeast, Monascus purpureus, over rice. It acts by inhibiting the 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA), which in turn inhibits the enzyme HMG-CoA reductase that is a key enzyme in cholesterol biosynthesis. Since plasma levels of cholesterol depend mainly on the biosynthetic pathway and, to a lesser extent, on diet, integration of the diet with RYR has been shown to be effective in improving lipid pattern.7 The approval granted by EFSA (European Food Safety Authority) in 2011 for RYR reads as follows: “Red yeast rice contributes to the maintenance of normal blood cholesterol concentrations” and it refers to the administration of 10 mg daily of monacolin K from fermented RYR preparations.14
Berberine is an alkaloid isoquinoline ring with cholesterol lowering properties, that is extracted from plants of the genus Berberis. It acts by upregulating the LDL-receptor (LDL-R) expression, dependent on extracellular signal-regulated kinases (ERK) and c-Jun N-terminal kinase (JNK) activation.11 Moreover, berberine is able to reduce hepatic total cholesterol (TC) and triglycerides’ (Tg) synthesis through the activation of adenosine monophosphate-activated protein kinase that leads to the inactivation of HMG-CoA and acetyl-CoA carboxylase enzymes.
Combinations of monacolin K and berberine are available on the market and are approved for the management of hyperlipidemia in different countries.10
The positive effect of berberine and monacolin K on lipid profile has been studied in mono- and combination therapy, demonstrating approximately a 20% reduction of LDL-C.14 The aim of this study was to evaluate the ability of BL to lower the LDL-C in patients with mild-to-moderate hypercholesterolemia. A placebo and an active comparator (the marketed food supplement Armolipid Plus® [AM]; MEDA-Rottapharm S.p.A., Monza, Italy), containing 3 mg of monacolin K, were employed as controls.
Material and methods
Study design
This was a randomized, double-blind, parallel group, three arm including placebo and active comparator study (AM containing Berberis aristata d.e. 588 mg [equivalent to Berberine chloride 500 mg], RYR 200 mg [equivalent to monacolin K 3 mg], policosanol 10 mg, folic acid 0.2 mg, coenzyme Q10 2.0 mg and astaxanthin 0.5 mg).
The primary endpoint was the evaluation of the ability of BL to decrease the LDL-C level in comparison to placebo, in terms of change from baseline, at week 4. Secondary endpoints were: i) to establish the sensitivity of the study by comparing AM to placebo, in terms of change from baseline at week 4 in LDL-C decrease, ii) to evaluate the effect of BL and AM in comparison to placebo on TC, high-density lipoprotein (HDL), Tg, and serum safety markers (aspartate aminotransferase [AST], alanine aminotransferase [ALT], CPK, urea), in terms of change from baseline at week 4, iii) to evaluate the safety profile by monitoring AEs, vital signs, body weight, body mass index (BMI), waist circumference, physical examination and laboratory tests. Additional explorative comparisons between BL and AM were performed on lipid change and serum safety markers. The trial was conducted at four experimental sites: the Department of Medical and Surgical science, S. Orsola Malpighi Hospital, University of Bologna, Bologna, Italy; the Department of Internal Medicine and Therapeutics, University of Pavia, Pavia, Italy; the Dyslipidemia Center, Hospital Niguarda Ca’ Granda, Milan, Italy; the Department of Internal Medicine, Azienda Ospedaliera-Polo Universitario Ospedale Luigi Sacco, Milan, Italy.
The study protocol was approved by each Institutional Review Board (Ethical Committee of S. Orsola Malpighi Hospital, Bologna, Italy; Ethical Committee of Azienda Ospedaliera-Polo Universitario Ospedale Luigi Sacco, Milan, Italy; Ethical Committee of Hospital Niguarda Ca’ Granda, Milan, Italy; Ethical Committee of University of Pavia, Pavia, Italy), and was conducted in accordance with the Declaration of Helsinki and its amendments. Suitable patients, identified from review of case notes and/or computerized clinic registers were contacted personally or by telephone. All eligible candidates had to provide signed informed consent before enrolling in the study.
Study population
A total of 275 adults, of both sex, 18–75 years of age clinically diagnosed with mild-to-moderate hypercholesterolemia, were enrolled. At the screening visit, patients had to have the following laboratory values:
LDL-C level >3.0 mmol/L or 115 mg/dL
TC level >5.2 mmol/L or 200 mg/dL
Tg level <2.8 mmol/L or 250 mg/dL.
In order to select a population with mild-to-moderate hypercholesterolemia, at randomization, the patients had to fulfill the following laboratory values:
LDL-C level between 3.0 and 4.7 mmol/L or 115 and 180 mg/dL
TC level between 5.2 and 6.8 mmol/L or 200–260 mg/dL
Tg level <2.8 mmol/L or 250 mg/dL.
Patients were excluded if they were treated with lipid lowering products (drugs or other compounds) or medications that may interfere with the metabolism of lipids in the 6 weeks before screening. In addition, they must have been off all dietary supplements containing any of the constituents of the products administered in the study. Exclusion criteria included also: patients with history of previous allergy or sensitivity to any component of any formulation; pregnant women or women planning to conceive and breastfeeding women; patients participating in another trial involving any investigational drug during the past 3 months; patients with clinically significant abnormalities at the screening visit. Patients with previous myocardial infarction, history of angina, stroke, history of abnormal stress test consistent with ischemia were also excluded. Patients treated for secondary prevention of cardiovascular disease, or patients with a 10-year coronary risk estimation >20%, according to the recommendations of the Task Force for the prevention of cardiovascular diseases, could not be enrolled. Patients with diabetes, peripheral vascular disease, history of hepatitis, concomitant thyroid disease, if their clinical condition and treatment were unstable during the previous 3 months, were not enrolled. Even patients taking medications of the following types or closely related medications were not enrolled: cyclosporins, azole antifungals, macrolide antibiotics, anti-arrhythmic medications, nefazodone, protease inhibitors, warfarin, seizure medications. Finally, patients with inability to provide their signed informed consent could not participate in the study.
Study procedures
The study procedures consisted of three visits for each patient:
– visit 1 – screening;
– visit 2, divided into visit 2a – baseline (4 weeks ±3 days after visit 1) for laboratory analyses and visit 2b – randomization (±4 days after Visit 2a), performed after the availability of laboratory tests;
– visit 3 – follow-up (4 weeks ±3 days after visit 2).
At screening, patients were instructed to follow a normocaloric and hypolipidemic diet for 4 weeks (run-in period). In particular, they were asked to:
– keep constant meal times (3 meals, 2 snacks between meals);
– avoid large meals and feeling of fullness in the stomach;
– not eat the following during the same meal: bread and pasta/rice, bread and potatoes, pasta/rice and potatoes;
– prefer the intake of pasta/rice for lunch and meat/fish in the evening;
– when possible, start the meal with vegetables, preferably raw (no quantity limits), seasoned with olive oil, lemon and vinegar;
– not lie down immediately after a meal.
Following the run-in period, patients were asked to return to the investigational site in fasting conditions. Patients who confirmed to fulfil all inclusion and exclusion criteria were randomized to receive BL, AM or placebo in a single daily oral dose, every evening, together or immediately after dinner. The study products were dispensed according to the randomization list and patients had to continue the diet until the end of the study. The treatments were supplied as identical, opaque, grey capsules, in labeled bottles, to ensure the blind status of the study.
At screening, visit 2 and visit 3, the study patients were evaluated by medical history, vital sign recording (blood pressure and heart rate measurements) and physical examination, including body weight, BMI, and waist circumference. Blood samples were collected for routine blood tests, measurement of serum lipid levels (LDL-C, TC and HDL-C), Tg, and serum safety markers (AST, ALT, CPK and urea). Laboratory analyses were performed at the centralized laboratory Centro Diagnostico Italiano (CDI), Milan (Italy). In order to evaluate the safety and tolerability of the study products, all AEs were monitored throughout the study.
Statistical analysis
A sample of 50 patients per group was enough to detect a statistically significant difference of 0.65 mmol/L (corresponding to 25 mg/dL) with a standard deviation of 1 mmol/L (corresponding to 39 mg/dL) between test (BL) and placebo in LDL cholesterol level change from baseline at week 4, at a significance level of 5%, a power ≥80% and considering a drop-out rate of 20%.
The following populations were defined for analysis: the modified intent-to-treat (m-ITT) population, the per protocol (PP) population, and the Safety population (SP). The m-ITT, defined as all randomized patients who received at least 80% of the study treatment and who had at least one LDL evaluation following baseline, represented the primary population used to evaluate the primary outcome. Shapiro-Wilk test was applied on the primary outcome in order to verify the normality, consequently the analysis of covariance (ANCOVA) was applied. The other efficacy endpoints were analyzed by ANCOVA or an analysis of variance (ANOVA). Statistical analyses were performed using SAS For Windows Version 9.2.
All differences were evaluated at an α level of 0.05 (two-sided). No adjustments for multiple comparisons were applied for secondary endpoints and explorative comparisons, due to the explorative nature of the testing.
The SP, defined as all randomized patients who received at least one dose of the study treatments, was used for the analyses of safety parameters (AEs incidence, change from baseline in laboratory variables, physical examination, and vital sign recording).
Results
A total of 275 patients were enrolled in this study. Of these, 158 were randomized.
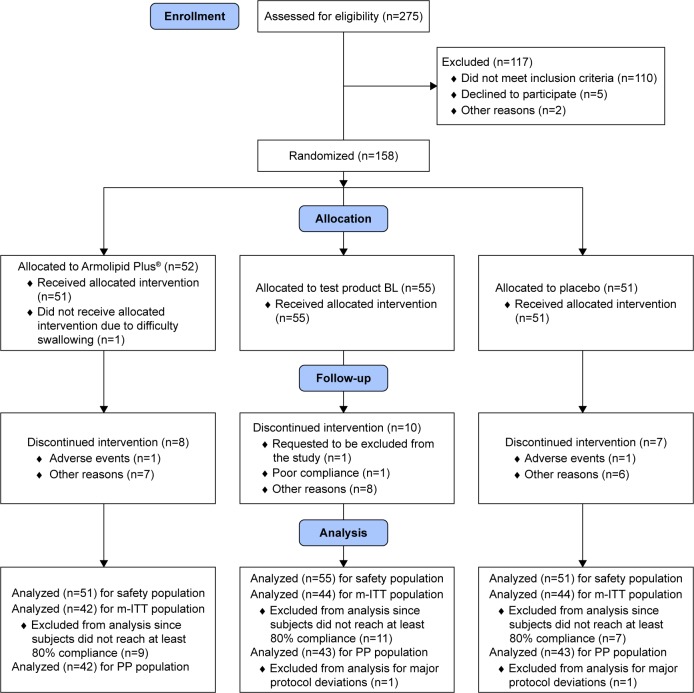
The m-ITT population included 130 patients, the SP included 157 patients. Results hereinafter presented refer to the m-ITT population, unless otherwise stated. The disposition of patients is reported in Figure 1.
Figure 1.
Consort flow diagram.
Abbreviations: BL, Body Lipid; m-ITT, modified intent-to-treat; PP, per protocol.
At baseline, patients randomized in each arm were comparable for sex, age, body weight, and BMI characteristics (Table 1). The percentages of women with menopause were 54/97 (55.7%), 43/81 (53.1%) and 42/79 (53.2%) in the safety, m-ITT and PP populations respectively.
Table 1.
Baseline characteristics in the m-ITT population
| Characteristics | BL (n=44) | AM (n=42) | Placebo (n=44) |
|---|---|---|---|
| Sex, M/F | 15/29 | 17/25 | 17/27 |
| Age (years), mean ± SD | 52.8±12.4 | 53.7±11.6 | 49.7±12.3 |
| Smokers, % | 25.0 | 21.4 | 25.0 |
| BMI (kg/m2), mean ± SD | 25.9±4.1 | 24.0±4.0 | 24.9±4.6 |
Abbreviations: BL, Body Lipid; m-ITT, modified intent-to-treat; BMI, body mass index; SD, standard deviation; AM, Armolipid Plus®.
After 4 weeks of treatment plus diet, BL treatment significantly decreased (P<0.001) LDL-C levels compared to placebo (Table 2), in both the m-ITT and PP populations. For the m-ITT population, in the BL group, mean ± standard deviation LDL cholesterol level was 146.4±18.1 mg/dL at baseline and 107.3±17.2 mg/dL at week 4. In the placebo group, LDL cholesterol level was 143.6±15.0 mg/dL at baseline and 149.3±19.5 mg/dL at week 4.
Table 2.
Efficacy results in the m-ITT population
| Parameters | BL (n=44) | AM (n=42) | Placebo (n =44) | BL vs placebo | AM vs placebo | BL vs AM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Baseline | End of study | Δ from baseline | Baseline | End of study | Δ from baseline | Baseline | End of study | Δ from baseline | P-value difference | (CI 95%) | ||
| LDL-C (mg/dL) | 146.4±18.1 | 107.3±17.2 | −39.1±17.8 | 147.5±16.3 | 120.4±18.8 | −27.0±14.6 | 143.6±15.0 | 149.3±19.5 | 5.7±15.0 | <0.001 −43.8 (−50.1; −37.5) |
<0.001 −31.1 (−37.7; −24.9) |
0.000 −12.5 (−18.9; −6.1) |
| TC (mg/dL) | 237.0±21.3 | 191.2±20.2 | −45.9±21.5 | 234.6±18.0 | 204.9±22.2 | −29.7±18.0 | 235.6±17.9 | 238.0±21.5 | 2.4±18.4 | <0.001 −47.7 (−55.2; −40.1) |
<0.001 −32.3 (−39.9; −24.6) |
0.000 −15.4 (−23.1; −7.7) |
| HDL-C (mg/dL) | 66.8±17.2 | 62.2±17.0 | −4.6±8.6 | 65.1±13.3 | 63.4±13.6 | −1.7±6.3 | 70.0±16.2 | 68.1±14.9 | −1.9±7.9 | 0.044 −3.2 (−6.3; −0.1) |
0.831 −0.3 (−3.5; 2.8) |
0.075 −2.8 (−6.0; 0.3) |
| Tg (mg/dL) | 118.9±51.6 | 108.5±46.5 | −10.4±38.2 | 110.8±41.5 | 105.7±37.0 | −5.1±29.7 | 110.5±41.9 | 103.5±40.3 | −7.0±33.5 | 0.953 −0.4 (−13.2; 12.4) |
0.789 1.8 (−11.2; 14.7) |
0.744 −2.1 (−15.1; 10.8) |
Notes: Data are expressed as mean ± SD. A statistically (P<0.05) but not clinically significant reduction in HDL cholesterol was found in the test product BL group, but not in the Armolipid Plus® group compared to placebo.
Abbreviations: BL, Body Lipid; AM, Armolipid Plus®; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Tg, triglycerides; SD, standard deviation; CI, confidence interval; m-ITT, modified intent-to-treat.
The absolute change in LDL cholesterol level from baseline was −39.1±17.76 mg/dL corresponding to 1 mmol/L, in the BL group and 5.7±14.98 mg/dL corresponding to 0.15 mmol/L in the placebo group. A statistically significant difference (P<0.001) in the mean changes from baseline in LDL cholesterol level was observed between the two groups.
Similarly, LDL-C levels significantly decreased (P<0.001) in the AM group vs the placebo group: −27.0±14.55 mg/dL, corresponding to 0.7 mmol/L, demonstrating the sensitivity of the study.
In terms of mean percentage LDL-C change, BL, AM and placebo produced, in the m-ITT population, a variation of −26.3%, −18.3%, and +4.2%, was observed respectively.
Moreover, at week 4, both BL and AM significantly (P<0.001) reduced TC concentrations compared to placebo, in the m-ITT and PP populations.
In particular, the absolute change in TC level from baseline was −45.9±21.54 mg/dL corresponding to 1.19 mmol/L in the BL group, −29.7±17.95 mg/dL corresponding to 0.77 mmol/L in the AM group, and 2.4±18.43 mg/dL corresponding to 0.06 mmol/L in the placebo group.
No statistically significant (P>0.05) difference in Tg was detected between treatment groups.
Safety and tolerability results
No statistically significant (P>0.05) difference in the change from baseline of serum safety markers (AST, ALT, CPK, and urea) was detected between treatment groups (Table 3).
Table 3.
Safety markers in the m-ITT population
| Parameters | BL (n=44) | AM (n=42) | Placebo (n=44) | BL vs placebo | AM vs placebo | BL vs AM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Baseline | End of study | Δ from baseline | Baseline | End of study | Δ from baseline | Baseline | End of study | Δ from baseline | P-value difference | (CI 95%) | ||
| AST (UL/L) | 21.6±11.0 | 22.6±9.8 | 1.0±13.8 | 23.3±10.7 | 23.6±6.3 | 0.3±7.6 | 22.8±5.2 | 22.2±4.5 | −0.6±4.0 | 0.433 1.6 (−2.4; 5.5) |
0.608 1.0 (−3.0; 5.1) |
0.794 0.5 (−3.5; 4.6) |
| ALT (UL/L) | 22.3±13.7 | 24.3±17.4 | 2.0±17.3 | 22.2±10.7 | 23.5±10.4 | 1.3±5.9 | 22.5±9.3 | 21.0±8.4 | −1.5±5.8 | 0.133 3.3 (−1.0; 7.6) |
0.200 2.8 (−1.5; 7.2) |
0.838 0.4 (−3.9; 4.8) |
| CPK (UL/L) | 95.6±41.7 | 97.6±49.7 | 2.0±39.3 | 114.8±63.5 | 124.7±65.9 | 9.9±31.8 | 109.2±69.9 | 115.9±66.3 | 6.7±70.9 | 0.709 −4.4 (−27.4; 18.7) |
0.781 3.3 (−20.1; 26.7) |
0.511 −7.6 (−30.6; 15.3) |
| Urea (mg/dL) | 35.3±8.7 | 36.2±8.5 | 0.9±6.9 | 35.8±7.4 | 35.1±7.1 | −0.7±5.7 | 35.7±8.8 | 35.6±9.1 | −0.1±6.7 | 0.434 1.0 (−1.5; 3.5) |
0.647 −0.6 (−3.1; 1.9) |
0.220 1.6 (−0.9; 4.1) |
Notes: Data are expressed as mean ± SD. Analyses on CPK were performed excluding subject number 470 who had a clinically significant abnormal CPK value (4,205 U/L) due to hard physical activity at baseline. CPK laboratory tests were not performed for some subjects (n=5 for BL, n=5 for AM, n=8 for placebo) due to technical problems at the central laboratory.
Abbreviations: BL, Body Lipid; AM, Armolipid Plus®; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CPK, creatinine phosphokinase; SD, standard deviation; CI, confidence interval; m-ITT, modified intent-to-treat.
Five AEs were reported by five patients after the initiation of the study treatment: two in the BL group (influence and insomnia), two in the AM group (ear pain and rash), and one in the placebo group (back pain). All AEs were mild in intensity, except for back pain (severe). The case of insomnia in the BL group and the case of rash in the AM group were judged as treatment related. The safety review of the laboratory (blood and urine) analyses, vital signs and physical findings did not show any clinical effect of the study products on any of the parameters.
Musculoskeletal disorders were not reported in any treatment group, despite BL containing higher concentrations of monacolin K (10 mg) in comparison to AM (3 mg).
Moreover, no clinically significant alterations in hematology, serum chemistry, and urinalysis were observed. There were neither significant differences in the baseline lipid variables values between treatment groups nor significant differences in the baseline safety markers values.
On the whole, a good safety and tolerability profile of the test product BL was shown in this study.
Discussion
The present study demonstrates, in a large study population, the good efficacy and safety of BL, a new nutraceutical formulation containing RYR, berberine, coenzyme Q10 and hydroxytyrosol, in improving lipid profile in patients with mild-to-moderate hypercholesterolemia.
In recent years, nutraceuticals and functional foods have attracted much interest as possible alternative therapies for lowering plasma TC and LDL-C in mild-to-moderate hypercholesterolemic patients or in patients for whom the prescription of cholesterol-lowering medications is not indicated.
After 4 weeks of treatment plus diet, BL was more effective than placebo in reducing the LDL cholesterol levels (−26.3% vs 4.2%, respectively). Moreover, in an explorative analysis, BL was more effective than the comparator, the marketed AM, in reducing the LDL cholesterol levels (−26.3% vs −18.3%, respectively).
Although the greater efficacy of BL compared to AM was likely due to a higher monacolin K concentration, the safety profile of the two products was comparable.
Notably, BL induced a reduction of LDL-C comparable with that usually obtained with low-density statin therapies, such as simvastatin 10 mg/day and pravastatin 10–20 mg/day (LDL-C reduction of approximately 30%) and very close to the lower range of the moderate-intensity statin therapies, such as atorvastatin 10 mg/day and simvastatin 20–40 mg/day (LDL-C reduction of approximately 30% to <50%).15
The particular merit of RYR is that, when consumed in doses providing 10 mg of monacolins daily, it is usually well tolerated by patients who previously have been intolerant to prescription statins (RYR rarely causes serious adverse effects), as reported in several clinical trials.16–18
A regimen combining RYR and berberine could work in complementary ways to upregulate the expression of LDL receptors in hepatocytes, likely achieving a reduction in LDL cholesterol comparable with that seen with moderate-dose prescription statin therapy. Studies with RYR suggest that monacolin K would be far less likely than prescription statins to induce myopathy or hepatic damage, and it might represent an effective alternative for patients who are statin intolerant. Moreover, berberine tends to decrease Tg levels and promote insulin sensitivity and glycemic control.6 Hence, by favorably influencing both LDL cholesterol and improving glycemic control, the combination of monacolin K and berberine might be effective in reducing the cardiovascular risk.9,10,19
The above considerations suggest that combined administration of monacolin K and berberine could provide similar protection from cardiovascular diseases compared to prescription statin therapy, with potentially lower risks for adverse effects.
Conclusion
This is the first clinical trial carried out with such a large number of hypercholesterolemic patients.
The recruited patients were screened twice, before and after the run-in period in order to obtain a homogeneous population showing LDL-C levels that clearly define patients with mild-to-moderate hypercholesterolemia. In addition, the new food supplement BL was compared vs placebo, and vs the market leader, AM, with the aim to test the study sensitivity.
The absolute change in LDL-C level from baseline was −39.1±17.76 mg/dL corresponding to 1 mmol/L, in the BL group and 5.7±14.98 mg/dL corresponding to 0.15 mmol/L in the placebo group.
Despite the positive results, the study has the limitation of a short treatment period; prolonged therapy could have provided additional information about the effect of the combination and about the long-term safety and tolerability.
In conclusion, the new food supplement BL could be considered a very satisfactory treatment in terms of efficacy, safety and tolerability, for patients with mild-to-moderate hypercholesterolemia. This nutraceutical supplement treatment could be a valid alternative for patients who are statin intolerant or for those who prefer alternative treatments.
Acknowledgments
The test product BL was provided as capsules of berberine, monacolin, hydroxytyrosol, and coenzyme Q10 manufactured by Farmaceutici Procemsa. Farmaceutici Procemsa performed the analysis of all the batches of the study products used in the study. The analytical certificates were provided to the sponsor.
Footnotes
Disclosure
F Focanti, F Pelacchi, E Salvatori, G Di Loreto, and A Comandini are employed by ACRAF S.p.A. Angelini Research Center RR&D. The authors report no other conflicts of interest in this work.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 3.Kamal-Bahl SJ, Burke T, Watson D, Wentworth C. Discontinuation of lipid modifying drugs among commercially insured United States patients in recent clinical practice. Am J Cardiol. 2007;99(4):530–534. doi: 10.1016/j.amjcard.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 4.Bays H. Statin safety: an overview and assessment of the data – 2005. Am J Cardiol. 2006;97(8A):6C–26C. doi: 10.1016/j.amjcard.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Derosa G, Romano D, D’Angelo A, Maffioli P. Berberis aristata combined with Silybum marianum on lipid profile in patients not tolerating statins at high doses. Atherosclerosis. 2015;239(1):87–92. doi: 10.1016/j.atherosclerosis.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 6.Derosa G, Bonaventura A, Bianchi L, et al. Berberis aristata/Silybum marianum fixed combination on lipid profile and insulin secretion in dyslipidemic patients. Expert Opin Biol Ther. 2013;13(11):1495–1506. doi: 10.1517/14712598.2013.832751. [DOI] [PubMed] [Google Scholar]
- 7.Cicero AF, Derosa G, Parini A, et al. Red yeast rice improves lipid pattern, high-sensitivity C-reactive protein, and vascular remodeling parameters in moderately hypercholesterolemic Italian subjects. Nutr Res. 2013;33(8):622–628. doi: 10.1016/j.nutres.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Derosa G, Bonaventura A, Bianchi L, et al. A randomized, placebo-controlled study on the effects of a nutraceutical combination of red yeast rice, silybum marianum and octasonol on lipid profile, endothelial and inflammatory parameters. J Biol Regul Homeost Agents. 2014;28(2):317–324. [PubMed] [Google Scholar]
- 9.Derosa G, Maffioli P. Nutraceuticals for the treatment of metabolic diseases: evidence from clinical practice. Expert Rev Endocrinol Metab. 2015;10(3):297–304. doi: 10.1586/17446651.2015.995630. [DOI] [PubMed] [Google Scholar]
- 10.Ruscica M, Gomaraschi M, Mombelli G, et al. Nutraceutical approach to moderate cardiometabolic risk: results of a randomized, double-blind and crossover study with Armolipid Plus. J Clin Lipidol. 2014;8(1):61–68. doi: 10.1016/j.jacl.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10(12):1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 12.Brusq JM, Ancellin N, Grondin P, et al. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006;47(6):1281–1288. doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Derosa G, D’Angelo A, Bonaventura A, Bianchi L, Romano D, Maffioli P. Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert Opin Biol Ther. 2013;13(4):475–482. doi: 10.1517/14712598.2013.776037. [DOI] [PubMed] [Google Scholar]
- 14.EFSA Panel on Dietetic Products, Nutrition and Allergies Scientific Opinion on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL-cholesterol concentrations (ID 1648, 1700) pursuant to Article 13(1) of Regulation (EC) No 1924/20061. EFSA Journal. 2011;9(7):2304. [Google Scholar]
- 15.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 16.Becker DJ, Gordon RY, Halbert SC, French B, Morris PB, Rader DJ. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150(12):830–839. doi: 10.7326/0003-4819-150-12-200906160-00006. [DOI] [PubMed] [Google Scholar]
- 17.Venero CV, Venero JV, Wortham DC, Thompson PD. Lipid-lowering efficacy of red yeast rice in a population intolerant to statins. Am J Cardiol. 2010;105(5):664–666. doi: 10.1016/j.amjcard.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 18.Borden WB. Red yeast rice for dyslipidemia in statin-intolerant patients. Curr Atheroscler Rep. 2010;12(1):11–13. doi: 10.1007/s11883-009-0084-9. [DOI] [PubMed] [Google Scholar]
- 19.McCarty MF, O’Keefe JH, DiNicolantonio JJ. Red Yeast Rice Plus Berberine: Practical Strategy for Promoting Vascular and Metabolic Health. Altern Ther Health Med. 2015;21(Suppl 2):40–45. [PubMed] [Google Scholar]