Abstract
Background and Purpose
Visit-to-visit variability in systolic blood pressure (SBP) is associated with an increased risk of stroke and was reduced in randomised trials by calcium channel blockers (CCBs) and diuretics but not by renin-angiotensin system inhibitors (RASi). However, time of day effects could not be determined. Day-to-day variability on home readings (HBPM) predicts stroke risk and potentially offers a practical method of monitoring response to variability-directed treatment.
Methods
SBP mean, maximum and variability (CV=SD/mean) were determined in 500 consecutive TIA or minor stroke patients on one-month HBPM (3 BPs, 3 times daily). Hypertension was treated to a standard protocol. Differences in SBP variability from 3-10 days before to 8-15 days after starting or increasing CCBs/diuretics vs RASi vs both were compared by general linear models, adjusted for risk factors and baseline BP.
Results
Among 288 eligible interventions, variability in SBP was reduced following increased treatment with CCBs/diuretics vs both vs RASi (-4.0 vs 6.9 vs 7.8%, p=0.015), due primarily to effects on maximum SBP (-4.6 vs -1.0 vs -1.0%, p=0.001), with no differences in effect on mean SBP. Class differences were greatest for early morning SBP variability (3.6 vs 17.0 vs 38.3 p=0.002) and maximum (-4.8 vs -2.0 vs -0.7, p=0.001), with no effect on mid-morning (p=0.29), evening (p=0.65) or diurnal variability (p=0.92).
Conclusions
After TIA or minor stroke, CCBs and diuretics reduced variability and maximum home SBP, due primarily to effects on morning readings. HBPM enables monitoring of response to SBP variability directed treatment in patients with recent cerebrovascular events.
Introduction
Episodic hypertension, maximum SBP and visit-to-visit variability in SBP between clinic appointments1–2 were strong predictors of incident and recurrent cardiovascular events in 5 large cohorts. Calcium channel blockers and thiazide diuretics reduced maximum SBP and SBP variability in the ASCOT-BPLA and MRC-2 studies compared to renin-angiotensin system inhibitors (RASi) or beta-blockers,3 explaining differences in stroke risk between treatment groups, with similar effects in meta-analyses of all published studies.4–6 Drug effects on SBP variability were seen in a wide-range of patients4 and persisted when used in combination.7 However, clinic readings are impractical for prospectively assessing the effects of drug changes on SBP variability and maximum SBP, particularly in secondary prevention of acute cerebrovascular events when rapid control of BP variability may be desirable.8 Furthermore, clinic readings in these randomised trials were performed during office hours with no consistent time of measurement. Therefore, clinic readings from RCTs can not be used to determine differences in drug class effects on BP variability at different times of day.
Day-to-day SBP variability on home BP monitoring (HBPM) is also significantly associated with the risk of stroke and cardiovascular events in both primary prevention9–10 and cerebrovascular disease,11 with particularly strong associations on day-to-day readings in the early morning, prior to the time of clinic readings used in estimating visit-to-visit BP variability.9–10 This may reflect the association between the morning surge in blood pressure and risk of cardiovascular events, with episodic morning surges potentially being responsible for the association between high maximum SBP and stroke risk,1,11 as well as a parallel relationship between diurnal variability in blood pressure and stroke risk.12 Home BP monitoring (HBPM) potentially offers a practical method of assessing the effects of antihypertensive treatment on SBP variability in clinical practice, and could also determine whether drug class effects on BP variability are greater in the early morning, when day-to-day variability has the greatest prognostic significance, but which can not be assessed in large RCTs.
In an observational study of home blood pressure monitoring after TIA or minor stroke, we investigated whether drug class effects on visit-to-visit variability in SBP demonstrated in large randomised controlled trials can be identified on day-to-day home SBP variability and whether drug class effects on day-to-day variability are different at different times of day.
Methods
Study Population
Consecutive patients were recruited between April 2008 and January 2012 from the Oxford Vascular Study’s (OXVASC)13 TIA and minor stroke clinic, usually within twenty-four hours of referral.14 The OXVASC population consists of 92,728 individuals registered with 100 primary-care physicians in nine practices in Oxfordshire, UK, with very high ascertainment of cardiovascular events through multiple overlapping methods of ascertainment.13 All patients requiring treatment for probable TIA or stroke for whom consent was given underwent a standardised medical history and examination, ECG and routine blood tests, with follow up at 1,3, 6, 12, 24 and 60 months, usually face-to-face. The majority of patients underwent a stroke protocol MRI brain and contrast-enhanced MRA of the extracranial brain-supplying arteries, with the remaining patients having a CT-brain and either a carotid Doppler ultrasound or CT-angiogram. The large majority of patients also routinely underwent transcranial Doppler ultrasound, echocardiography and 5 days of ambulatory cardiac monitoring.
Procedures
Clinic BP was measured at ascertainment and the one month follow-up visit in the non-dominant arm, by trained personnel, in the sitting position after five minutes of rest, with two measurements made 5 minutes apart. From the ascertainment visit, or the earliest opportunity after discharge, all patients performed sets of three home BP readings (HBPM), three times daily (on waking, mid-morning and before sleep) with a Bluetooth-enabled, regularly-calibrated, telemetric BP monitor, either an IEM Stabil-o-Graph or an A&D UA-767 BT.15 Patients were instructed to relax in a chair for 5 minutes before performing readings in the non-dominant arm, or the arm with the higher reading if the mean BP differed by >20mmHg between arms, and were assessed at doing so at ascertainment. Anonymised measures were transmitted by Bluetooth radio to a mobile phone, for secure transmission to a server hosting a password-protected website for review and download of readings (t+ Medical, Abingdon, UK). Patients continued home monitoring until at least the one month follow-up appointment, if tolerated, but could continue to achieve adequate BP control. Mean BP was treated to a target of <130/80 on home monitoring, except in the minority of patients with a haemodynamically significant stenosis (bilateral carotid stenosis >70% or severe end-artery stenosis) when targets were determined on an individual basis. Patients could be treated at the treating physician’s discretion, but were most commonly treated according to a standardised protocol: a combination of perindopril 5mg and indapamide 1.25mg followed by addition of amlodipine 5mg, then amlodipine 10mg or indapamide 2.5mg, with dose increases or the addition of other agents as required. Treatment was started in clinic if necessary, or after at least 1 week of home BP monitoring. The choice of drug followed this standardised protocol regardless of BP level, BP variability or demographic factors, but could be altered on the basis of absolute or relative contraindications, such as a previous reaction or heart failure (in the case of CCBs).
Analysis
Analyses were performed comparing baseline home readings acquired from 3-10 days prior to starting or increasing the dose of a drug to follow-up readings acquired from 8-15 days after the intervention. For each time period, the mean, minimum and maximum SBP and DBP were derived from the average of the last two readings of each cluster of three readings at a specific time of day. Diurnal variability in SBP was measured as the coefficient of variation of the cluster averages for each day (standard deviation of three timepoints / mean). Day-to-day variability in SBP and DBP was measured as the residual CV about a moving average over 5 days (to remove the influence of both mean BP and any underlying trend in BP) for the mean of all clusters and the mean of clusters at each time of day.16 All follow-up measures were then expressed as a percentage change compared to the baseline period. Eligible medication changes were identified as any initiation or dose increase occurring after day 7 with at least 7 days of BP monitoring data prior to the drug change, and a minimum of 10 days after, excluding changes in which another drug was altered within these time limits. Changes were classified as increases in treatment (starting or increasing dose) of a drug associated with low variability in SBP in RCTs (CCBs/diuretics) or high variability in SBP (RASi or beta-blockers), or treatment with a combination of a drug from each class, usually a RASi and a diuretic. Secondly, changes were classified as increased treatment with a CCB, diuretic, RASi or combination of RASi and diuretic.
Statistical analysis
Unadjusted differences in BP indices between patient groups were assessed by t-tests or ANOVA. Univariate correlations with continuous demographic indices were measured by linear regression and differences between groups in frequency of discrete variables were compared by chi-squared tests. Multivariate general linear models (SPSS sum of squares type IV) were derived for each SBP or DBP index with independent variables including drug class allocation, age, gender, atrial fibrillation (premorbid or diagnosed within 6 weeks of the event), creatinine, current smoking and a history of hypertension, diabetes, hyperlipidaemia, or family history of stroke. A second model included these variables plus baseline mean SBP and baseline CV.
All analyses were performed with Matlab R2012a, Microsoft Excel 2010 and IBM SPSS 20. The study received ethical approval from the Oxfordshire Research Ethics Committee, and all participants or their relatives provided informed consent to perform home monitoring.
Results
500 of 536 patients had adequate home readings (1 died, 8 non-cerebrovascular diagnoses, 27 inadequate recordings, Figure 1 in the online-only Data Supplement), with a median of 15 premorbid BP measurements (IQR 6.4-30.5) and 83.6 BP clusters on home monitoring (IQR 56-174) over 30.8 days (IQR 21.1-64.5), with 2.9 BPs per cluster (SD 0.4). Of 462 drug changes made after day 7, 112 were made within 10 days of stopping monitoring and 62 were made too close to another drug change. Of the remaining 288 eligible changes, there were 131 with CCBs (69 drug initiation, 62 dose increases), 55 with RASi (35 drug initiation, 20 dose increase), 47 with diuretics (33 drug initiation, 14 dose increases), 5 with beta-blockers (4 drug initiation, 1 drug increase) and 50 with a combination of a RASi and a diuretic (12 drug initiation, 38 dose increase). Of these eligible interventions 100 were with amlodipine, 52 with perindopril (39 in combination with indapamide), 25 with ramipril and 32 with indapamide alone. Baseline demographic characteristics were well-matched between intervention groups (table 1), and there were no significant differences between groups in baseline SBP or DBP variability in day-to-day BP variability (table 2 and table I in the online-only Data Supplement), but baseline mean and maximum SBP were slightly higher in the patients treated with low variability drugs.
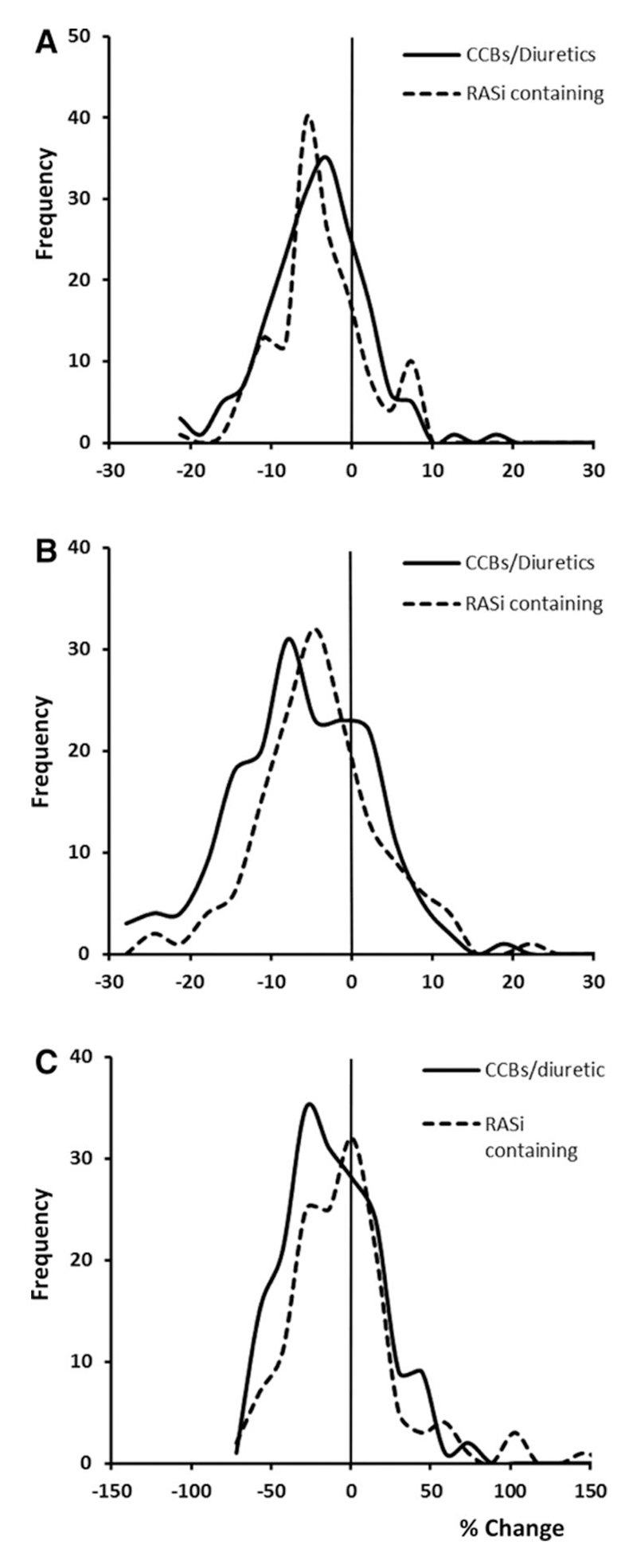
Figure 1. Distribution of changes in mean, maximum and variability in SBP after intervention with CCBs/diuretics versus RAS inhibitors.
Distributions are presented as frequency of interventions resulting in a % change in mean SBP (A), max SBP (B) and rCV (C), comparing initiation or dose increases of CCBs or diuretics versus RAS inhibitors.
Table 1. Characteristics of individuals starting a drug or increasing the dose for each drug class.
CCB= calcium channel blocker alone; RASi=renin-angiotensin system inhibitor, with or without a diuretic; Diuretic=diuretic alone. Frequencies (%) are given, compared by chi-squared tests, means by ANOVA. AF = atrial fibrillation; BMI = body mass index; TSH = thyroid stimulating hormone; CCB = calcium channel blocker; RASi = renin-angiotensin system inhibitor; D = diuretic.
| CCB (n=131) | RASi (n=55) | Diuretic (n=47) | RASi +D (n=50) | p-value | |
|---|---|---|---|---|---|
| Male | 55 (42) | 27 (49) | 21 (45) | 21 (42) | 0.83 |
| Hypertension | 84 (64) | 31 (56) | 27 (57) | 28 (56) | 0.64 |
| Family History | 31 (24) | 18 (33) | 9 (19) | 14 (28) | 0.41 |
| Hyperlipidaemia | 56 (43) | 21 (38) | 17 (36) | 20 (40) | 0.86 |
| Diabetes | 23 (18) | 9 (16) | 3 (6) | 9 (18) | 0.30 |
| Heart Failure | 11 (8) | 5 (9) | 2 (4) | 2 (4) | 0.59 |
| AF | 18 (14) | 2 (4) | 8 (17) | 6 (12) | 0.16 |
| Smoker | 19 (15) | 6 (11) | 10 (21) | 7 (14) | 0.52 |
| Stroke vs TIA | 76 (58) | 32 (58) | 24 (51) | 26 (52) | 0.75 |
| Myocardial Infarction | 3 (2) | 1 (2) | 4 (9) | 3 (6) | 0.19 |
| Age | 69.8 | 69.7 | 68.6 | 69.1 | 0.96 |
| BMI | 27.4 | 27.8 | 28.3 | 27.2 | 0.70 |
| Creatinine | 92.2 | 93.3 | 83.3 | 94.2 | 0.12 |
| Cholesterol | 5.1 | 5.4 | 5.3 | 5.1 | 0.59 |
| TSH | 2.3 | 2.2 | 2.1 | 2.2 | 0.96 |
Table 2. Differences in baseline systolic BP and SBP variability and percentage change in each measure 8 days after starting or increasing the dose of each class of antihypertensive drug.
Treatment groups are defined as low variability drugs (CCB or diuretic), high variability drugs (RASi or beta-blockers) or a combination of a RASi and a diuretic. SD=standard deviation; CV=coefficient of variation; rCV=residual CV about a 5 day moving average. Mean values are compared by ANOVA. *p<0.05; ** p<0.01; *** p<0.001.
| 3-10 days before intervention | % change >8 days after intervention | |||||||
|---|---|---|---|---|---|---|---|---|
| High | Combined | Low | High | Combined | Low | |||
| 62 | 50 | 178 | p-val | 62 | 49 | 178 | P-val | |
| All measures | ||||||||
| Mean | 129.5 | 127.7 | 132.4 | 0.038* | -1.9 | -2.4 | -3 | 0.39 |
| Minimum | 110.2 | 109.4 | 112.3 | 0.26 | -1.6 | -3.6 | -1.3 | 0.26 |
| Maximum | 149.4 | 147.2 | 154.2 | 0.017* | -1.0 | -1.0 | -4.6 | 0.001** |
| rCV | 7.9 | 8 | 7.9 | 0.98 | 7.8 | 6.9 | -4 | 0.015* |
| Morning | ||||||||
| Mean | 131.9 | 129.9 | 134.3 | 0.13 | -1.6 | -3 | -3.7 | 0.09 |
| Minimum | 120.4 | 117.5 | 122.1 | 0.09 | -1.9 | -1.7 | -2.5 | 0.76 |
| Maximum | 143.7 | 142.1 | 147.2 | 0.13 | -0.7 | -2.0 | -4.8 | 0.001** |
| rCV | 5.3 | 5.9 | 5.6 | 0.62 | 38.3 | 7 | 3.6 | 0.002** |
| Diurnal SD | 9.8 | 9.1 | 10.4 | 0.23 | -0.6 | 0.6 | -1.9 | 0.92 |
| Diurnal CV | 7.5 | 7.4 | 7.8 | 0.64 | 2.0 | 3.8 | 1.0 | 0.92 |
There was a significant reduction in variability in home SBP following treatment with CCBs or diuretics compared to RASi, with an intermediate effect of combinations containing both (table 2) and no difference between CCBs and diuretics in effects on SBP variability (table I in the online-only Data Supplement). There was no difference in change in global variability in DBP between drug groups (table II in the online-only Data Supplement). Differences in change in SBP variability persisted after adjustment for age, gender and cardiovascular risk factors and baseline mean and variability in SBP (CCB/diuretic vs combination vs RASi p=0.005, post-hoc: CCB/diuretic vs RASi p=0.008, CCB/diuretic vs combination p=001) and for models including CCBs vs diuretics vs RASi vs combinations (p=0.004). Differences between drug groups were consistent at different levels of baseline SBP variability (figure 1). Differences between drug classes in variability in SBP were greatest immediately after the intervention, due partly to an increase in BP variability with RASi, but the differences persisted beyond day 8 (figure II in the online-only Data Supplement).
Differences in drug-class effects on SBP variability reflected greater differences in effects on maximum SBP than mean or minimum SBP (table 2, figure 2), with a similar distribution of effects at each level of maximum SBP. The effect of treatment on maximum SBP with a combination of a low and a high variability drug was intermediate between the two monotherapy interventions. Similar effects were found when treatment groups were defined as CCBs vs other drugs or as CCBs vs diuretics vs any regimen containing a RASi. There was no significant difference between groups in change in mean, minimum or maximum DBP (table I in the online-only Data Supplement).
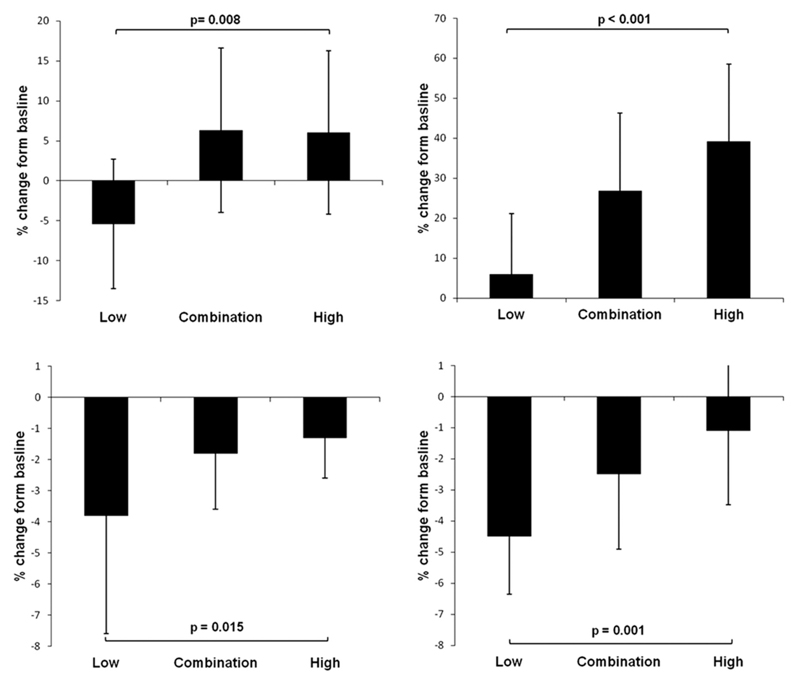
Figure 2. Differences between change in variability or maximum SBP after initiation of drugs associated with lower SBP variability, higher SBP variability or a combination of both.
Results presented are mean percentage change in maximum SBP or residual coefficient of variation (RCV) in SBP for days 8-15 after starting or increasing each treatment compared to 3-10 days before the intervention, adjusted for age, gender, diabetes, history of hypertension, current smoking, family history of stroke, dyslipidaemia, atrial fibrillation, creatinine baseline mean SBP and baseline maximum SBP or SBP-rCV. P-values and confidence intervals are derived from general linear models, comparing drugs associated with lower SBP variability (“low”) with drugs associated with higher SBP variability (“high”).
The reduction in day-to-day variability in SBP and maximum SBP with low variability drugs was greatest for day-to-day early morning SBP readings, with a significant increase in patients treated with RASi, no significant change in patients treated with CCBs or diuretics and an intermediate effect of treatment with a combination of the two classes (table 2). There was no significant difference between groups in change in day-to-day variability in SBP in the middle of the morning (p=0.79) or in the evening (p=0.72), and no difference between groups in change in diurnal variability (table 2). The differences between drug groups in day-to-day SBP variability immediately after waking persisted after adjustment for age, gender, cardiovascular risk factors, baseline mean SBP and baseline variability in SBP (figure 2; table IV in the online-only Data Supplement). Again drug class differences in early morning SBP variability primarily reflected greater reductions in maximum SBP with CCBs or diuretics (table 2) with no difference in change in mean or minimum SBP between groups.
Discussion
Calcium channel blockers and diuretics reduced variability in SBP and maximum SBP on home BP monitoring compared to RAS inhibitors. These drug-class effects persisted with combinations of diuretics and RASi and were particularly evident with day-to-day SBP variability on early morning readings. Effects on day-to-day variability were primarily due to a significantly greater reduction in maximum SBP with CCBs or diuretics in the morning with no differences between drugs in reduction of mean SBP at any time of day.
This is the first study to demonstrate the effect of antihypertensive medications on day-to-day home SBP variability, with CCBs and diuretics reducing SBP variability compared to RASi, due primarily to effects on maximum SBP, with no significant drug-class differences in effects on mean SBP. These differences were similar for maximum and variability in SBP across the population. Although the 4% reduction in BPV with low variability agents appears small, this is significantly different to the 7.8% increase with high variability agents and is likely to reflect greater reductions in some patients. This effect is consistent with the effect of CCBs and diuretics on visit-to-visit SBP variability and maximum SBP in large RCTs1–6 and may explain the reduction in the subsequent risk of stroke, although an appropriate choice of antihypertensives also needs to consider other demographic factors such as ethnicity and comorbidities. Nonetheless, this study suggests that HBPM offers a potential practical method of monitoring the change in BP variability in response to antihypertensive treatment, with the potential to reduce BP variability-associated cardiovascular risk.
The effect of CCBs and diuretics on day-to-day SBP variability was greatest immediately after waking compared to other times of day, with a similar effect on maximum SBP but with no difference in effects on mean or minimum SBP after waking. This implies that the most important effect of CCBs or diuretics on SBP variability results from limiting episodic peaks in SBP after waking, which is consistent with the greater prognostic significance of morning day-to-day variability in SBP compared to the evening.10 This may be due to a reduction in day-to-day variability in the morning surge in blood pressure, which is predictive of future cardiovascular events and the timing of which matches the increased risk of stroke in the morning compared to later in the day.12 These effects are not due to consistent changes in the magnitude of the morning surge every day as there was no difference in mean diurnal variability, but are likely to result from limiting episodic surges. Therefore, the shorter half-life of RASi is unlikely to explain the difference with CCBs and diuretics, as this would cause a consistent difference in diurnal CV.
There were too few drugs used in this study from each class to determine if there were differences between agents within drug classes. In fact, amlodipine, perindopril and indapamide were the most commonly used drugs, and it is possible that the differences found in this study are driven by specific differences between these drugs rather than drug classes. However, such differences within drug classes were not found in previous meta-analyses of drug class effects on other forms of BP variability.4 This study also demonstrated that the combination of a RASi and indapamide resulted in an intermediate effect on day-to-day SBP variability compared to RASi or diuretics alone. This is consistent with a previous meta-analysis of RCTs7 which used inter-individual SBP variability as an indirect measure of intra-individual SBP variability. Therefore, in patients with increased SBP variability on a RASi the addition of a diuretic will limit variability in SBP.
There were some limitations to this study. Firstly, patients received antihypertensive drugs at the non-randomised discretion of the treating physician, guided by a standard protocol. However the primary aim of this study was to investigate the effects of antihypertensive treatment on home day-to-day SBP variability at different times of day, rather than prove the existence of any effect on SBP variability as this has already been demonstrated in analyses of large RCTs.1–4 Secondly, the study was performed in patients with acute TIA or minor stroke limiting its generalisability but this is the optimal group of patients to investigate as they are at the highest risk of recurrent stroke, and currently there is only limited evidence of the effect of antihypertensives on SBP variability in secondary prevention.1–4 Thirdly, diurnal variability on HBPM was only measured at three timepoints in each day rather than using ambulatory blood pressure monitoring. However, repeated ABPM would be impractical for the assessment of treatment responses and multiple HBPM readings on different days produce a more reliable estimate than can be obtained with a single day of ambulatory readings.17–18 Fourthly, patients receiving CCBs had higher premorbid BPs and there were more dose-adjustments in this group, which could potentially confound between-group comparisons. However, as we used within-individual change in BPV, and as the results are highly consistent with previous findings of RCTs, it is unlikely that there is significant confounding. Fifthly, there was no direct measure of medication compliance, but as blood pressure was monitored daily any clinically significant lack of compliance resulted in contact with the patient. Sixth, relatively few patients received eligible drug changes as not all patients required treatment or else required treatment changes within the first seven days of the study, or close to stopping monitoring, and therefore change in BPV could not be reliably analysed. Nonetheless, there were adequate interventions within the major drug classes to enable analysis. Finally, all readings were taken in a relaxed sitting position at home, which may not accurately reflect real-life blood pressure behaviour. However, this does not necessarily reduce its prognostic significance and is likely to increase reproducibility and accuracy by limiting artefactual surges in measured blood pressure.19
In summary, calcium channel blockers and diuretics reduced self-measured, home blood pressure variability in the secondary prevention of cerebrovascular events compared to RASi, due largely to a reduction in day-to-day variability and maximum SBP after waking. These effects persisted when used in combinations. This supports the use of these drugs in the secondary prevention of patients with cerebrovascular disease, particularly in patients with increased BP variability or excessive morning surges in SBP, and raises the potential for the use of home BP monitoring as a method of monitoring the response to SBP-variability directed treatment.
Supplementary Material
Acknowledgments
We acknowledge the invaluable support from the facilities provided by the Oxford Acute Vascular Imaging Centre.
Funding
OXVASC has been funded by the Wellcome Trust, Wolfson Foundation, UK Stroke Association, British Heart Foundation, Dunhill Medical Trust, National Institute of Health Research (NIHR), Medical Research Council, and the NIHR Oxford Biomedical Research Centre. AJSW is in receipt of an MRC Clinical Training Research Fellowship.
Footnotes
Disclosures
None
References
- 1.Rothwell PM. Limitations of the usual BP hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, et al. Prognostic significance of visit-to-visit variability, maximum systolic BP, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JR, Dahlof B, et al. Effects of β blockers and calcium-channel blockers on within-individual variability in BP and risk of stroke. Lancet Neurology. 2010;9:469–480. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 4.Webb AJS, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in BP and risk of stroke. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 5.Webb AJ, Fischer U, Rothwell PM. Effects of beta-blocker selectivity on blood pressure variability and stroke: a systematic review. Neurology. 2011;77:731–737. doi: 10.1212/WNL.0b013e31822b007a. [DOI] [PubMed] [Google Scholar]
- 6.Webb AJS, Rothwell PM. Blood pressure variability and the risk of new-onset atrial fibrillation: A systematic review. Stroke. 2010;41:2091–2093. doi: 10.1161/STROKEAHA.110.589531. [DOI] [PubMed] [Google Scholar]
- 7.Webb AJS, Rothwell PM. Effect of dose and combinations of antihypertensives on interindividual blood pressure variability: a systematic review. Stroke. 2011;42:2860–2865. doi: 10.1161/STROKEAHA.110.611566. [DOI] [PubMed] [Google Scholar]
- 8.Robinson TG, James M, Youde J, Panerai R, Potter J. Cardiac baroreceptor sensitivity is impaired after acute stroke. Stroke. 1997;28:1671–1676. doi: 10.1161/01.str.28.9.1671. [DOI] [PubMed] [Google Scholar]
- 9.Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, et al. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52:1045–1050. doi: 10.1161/HYPERTENSIONAHA.107.104620. [DOI] [PubMed] [Google Scholar]
- 10.Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home-measured blood pressure and heart rate:The Finn-Home Study. Hypertension. 2012;59:212–218. doi: 10.1161/HYPERTENSIONAHA.111.178657. [DOI] [PubMed] [Google Scholar]
- 11.Webb AJS, Wilson M, Paul NL, Fischer U, Rothwell PM. Clinical and physiological validity of maximum blood pressure on multi-day home measurement versus single-day ambulatory monitoring: frequency versus duration? Cer Dis. 2013;35(suppl 3):124. [Google Scholar]
- 12.Kario K, Shimada K, Pickering TG. Clinical implication of morning blood pressure surge in hypertension. J Cardiovasc Pharmacol. 2003;42(suppl 1):S87–91. doi: 10.1097/00005344-200312001-00019. [DOI] [PubMed] [Google Scholar]
- 13.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–1933. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study) Lancet. 2007;370:1432–1442. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]
- 15.Webb AJS, Wilson M, Paul NL, Fischer U, Lovett NG, Li L, et al. Identification of missed hypertension and hypertensive arteriopathy with home versus ambulatory blood pressure measurement in patients with TIA or minor stroke. Cer Dis. 2013;35(suppl 3):72. [Google Scholar]
- 16.Webb AJS, Rothwell PM. Physiological correlates of beat-to-beat, ambulatory and day-to-day home blood pressure variability after transient ischemic attack or minor stroke. Stroke. 2014;45:533–535. doi: 10.1161/STROKEAHA.113.003321. [DOI] [PubMed] [Google Scholar]
- 17.García-García Á, García-Ortiz L, Recio-Rodríguez JI, Patino-Alonso MC, Agudo-Conde C, Rodriguez-Sanchez E, et al. Relationship of 24-h blood pressure variability with vascular structure and function in hypertensive patients. Blood Press Monit. 2013;18:101–6. doi: 10.1097/MBP.0b013e32835ebc58. [DOI] [PubMed] [Google Scholar]
- 18.Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Björklund-Bodegård K, et al. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–57. doi: 10.1161/HYPERTENSIONAHA.109.140798. [DOI] [PubMed] [Google Scholar]
- 19.Calvo C, Hermida RC, Ayala DE, Lopez JE, Fernandez JR, Dominguez MJ, et al. The ‘ABPM effect’ gradually decreases but does not disappear in successive sessions of ambulatory monitoring. J Hypertens. 2003;21:2265–73. doi: 10.1097/01.hjh.0000084799.73547.45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.