Abstract
The AMP-activated protein kinase (AMPK) is a key regulator of cellular and whole body energy homeostasis, which acts to restore energy homoeostasis whenever cellular energy charge is depleted. Over the last two decades, it has become apparent that AMPK regulates a number of other cellular functions and has specific roles in cardiovascular tissues, acting to regulate cardiac metabolism and contractile function as well as promoting anti-contractile, anti-inflammatory and anti-atherogenic actions in blood vessels. In this review, we will discuss the role of AMPK in the cardiovascular system, including the molecular basis of mutations in AMPK that alter cardiac physiology and the proposed mechanisms by which AMPK regulates vascular function under physiological and pathophysiological conditions.
Keywords: AMPK, AMP-activated protein kinase, heart, heart defects (congenital), metabolism, vasculature
The AMP-activated protein kinase (AMPK) is the central component of a signalling pathway that regulates the switch between anabolism and catabolism, as well as many other aspects of cell function1–3. In this review we focus on the physiological roles of AMPK within the cardiovascular system, although we will start by discussing general features that apply in all mammalian cell types. Readers are also referred to two other recent reviews of the role of AMPK in cardiovascular disease 4, 5.
Structure and Regulation of AMPK
Occurrence of subunit isoforms
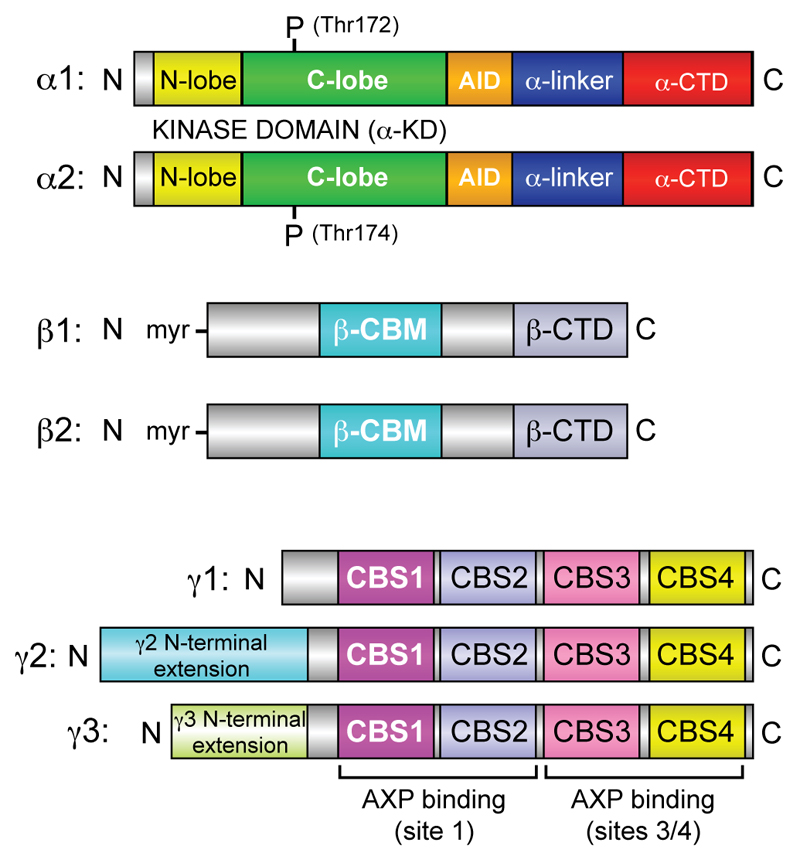
AMPK exists universally as heterotrimeric complexes containing catalytic α subunits and regulatory β and γ subunits, which occur as multiple isoforms (α1/α2; β1/β2; γ1/γ2/γ3) encoded by distinct genes. These could give rise in principle to twelve heterotrimeric combinations, although specific combinations appear to be favoured in specific cell types. For example, although skeletal muscle expresses mRNAs encoding all seven subunit isoforms, assays of immunoprecipitated isoforms suggest that AMPK activity in human skeletal muscle is accounted for by just three combinations: α1β2γ1, α2β2γ1 and α2β2γ36.
The domain organization of the seven AMPK subunit isoforms are shown in Fig. 1, and a representation of a crystal structure for the human α1β2γ1 heterotrimer7 is shown in Fig. 2. Similar structures of the α2β1γ18 and α1β1γ19 complexes are available.
Figure 1. Domain layouts of AMPK subunits and their isoforms.
The linear layout of domains is shown, approximately to scale and with similar color coding as in Fig. 2. Note that both β subunits are N-myristoylated, and that the γ2 and γ3 subunit isoforms have unrelated N-terminal extensions of unknown function, although both are also reported to exist as shorter, N-terminally truncated versions due to alternate start sites and/or splicing188.
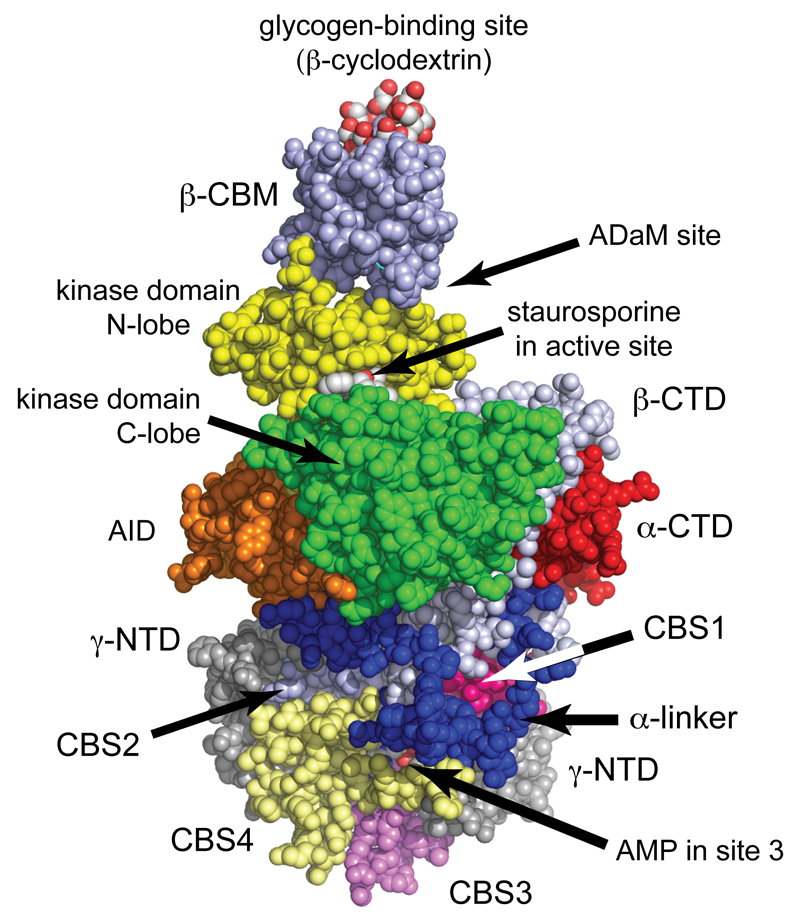
Figure 2. Structure of human AMPK (α1β2γ1 complex)7.
The model was created in spacefilling mode using PyMol v1.7.4.2 with the co-ordinates in PDB file 4RER, and with color coding similar to Fig. 1. The heterotrimer was crystallized in the presence of β-cyclodextrin, which occupies the glycogen-binding site, staurosporine, which occupies the active site, and AMP, which occupies sites 1, 3 and 4 on the γ subunit (sites 1 and 4 are round the back in this view). Although Thr172 was phosphorylated, it is not visible in this view but lies in the cleft between the α subunit C lobe and the β-CTD, just over the right-hand “shoulder” of the C-lobe.
The α subunits
The α subunits contain a kinase domain (α-KD) at their N-termini, with the small N-lobe (yellow in Figs.1 and 2) and larger C-lobe (green) typical of all protein kinases. The substrate Mg.ATP2- binds in a deep cleft between these lobes, occupied in the structure in Fig. 2 by the non-specific kinase inhibitor staurosporine. Isolated α-KDs are only significantly active after phosphorylation by upstream kinases at a conserved threonine, usually referred to as Thr172. Thr172 phosphorylation is a good marker for AMPK activity, and Western blotting using phosphospecific antibodies against this site is often used as a semi-quantitative measure of AMPK activity. However, this ignores the effects of allosteric activation of AMPK, a problem that can be overcome by also addressing the phosphorylation state of a downstream target such as acetyl-CoA carboxylase (ACC).
The α-KD is followed by a small globular domain termed the autoinhibitory domain (AID, orange) that, when AMP is not bound to the γ subunit, binds to both the N- and C-lobes of the KD and thus causes inhibition7. The AID is connected to the C-terminal domain (α-CTD, red) by the α-linker (blue), a region of extended polypeptide that interacts with the γ subunit when AMP is bound at site 3 (as is the case in Fig. 2), thus pulling the AID away from this inhibitory position.
The β subunits
Two well-conserved regions within the β subunits are the C-terminal domain (β-CTD, silver-grey), which forms the “core” of the heterotrimeric complex, and the central carbohydrate-binding module (β-CBM, silver-blue). The β-CBM is related to carbohydrate-binding domains usually occurring in enzymes that metabolize starch or glycogen, which localize those enzymes on their polysaccharide substrate. Consistent with this, the β-CBM causes a proportion of AMPK in cells to bind to the surface of glycogen particles10–12. In all three structures of mammalian heterotrimers, the β-CBM interacts with the N-lobe of the α subunit KD, with its glycogen-binding site (defined by binding of the oligosaccharide β-cyclodextrin) at the top in the view of Fig. 2.
What is the function of glycogen binding by AMPK? The muscle and liver isoforms of glycogen synthase, which are also bound to glycogen particles, are physiological targets for AMPK13, 14, and one function may be to co-localize AMPK with them. The β-CBM is also of interest because the cleft between it and the N-lobe of the α subunit forms the binding site for activators such as A769662 and 9918. This site has been termed the Allosteric Drug and Metabolite (ADaM) site15, and is discussed further below.
The γ subunits
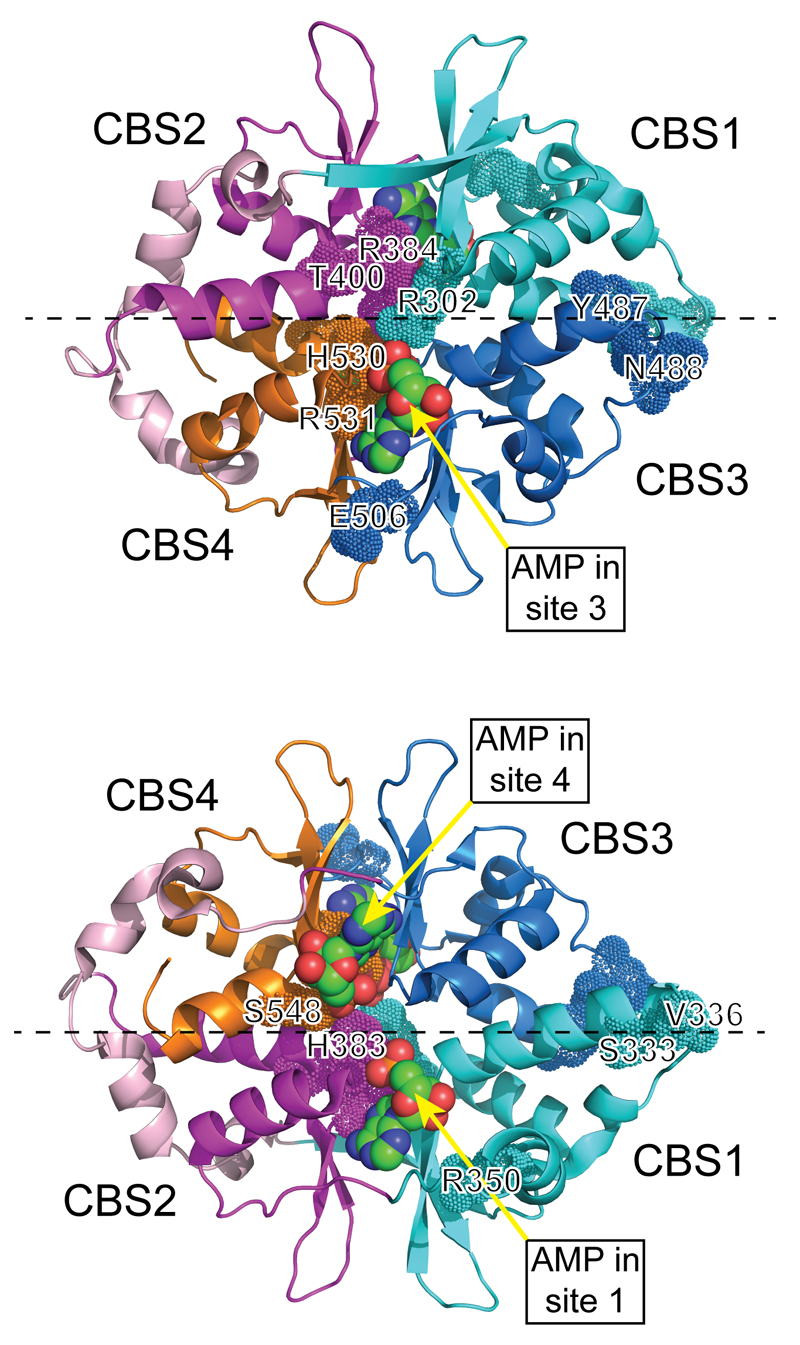
All γ subunits contain four tandem repeats of a sequence motif known as a CBS motif. In other proteins, pairs of CBS motifs often form binding sites for regulatory ligands containing adenosine16; in the AMPK-γ subunits they bind the regulatory nucleotides AMP, ADP or and ATP17. Two views of a structure for the γ1 subunit, containing three molecules of bound AMP18, are shown in Fig. 3. The two pairs of repeats (CBS1:CBS2 and CBS3:CBS4) assemble in a pseudosymmetrical “head-to-head” manner. The γ subunit thus forms a structure like a flattened disc (seen from different faces in Fig. 3) with one CBS repeat in each quadrant, generating four potential ligand-binding clefts in the centre. However, one of these appears to be unused, perhaps because conserved aspartate residues in CBS1, CBS3 and CBS4 that bind the ribose ring of adenine nucleotides in sites 1, 3 and 4 are absent from CBS218. Interestingly, mutation of any of these three aspartate residues interferes with multiple effects of AMP on kinase activity19, suggesting that occupancy of all three sites may be required for full activation.
Figure 3. Two views of the structure of the four CBS repeats of the γ1 subunit.
The model used the co-ordinates in PDB file 2V8Q18, and was rendered in PyMol v1.7.4.2 with the γ1 subunit in cartoon view. The two views are rotated 180° around the x axis (dashed line) with respect to each other, with the orientation of the top view being similar to that in Fig. 2. Note the pseudosymmetrical layout of the four CBS repeats, which are colored differently (and differently to Figs. 1 and 2). Residues equivalent to those mutated in γ2 are highlighted using the “dots” version of space-filling representation, and are numbered using the human γ2 numbering. The three molecules of bound AMP are labelled and are shown in standard space-filling view, with C atoms green, O red and N blue.
Identity of upstream kinases
Identifying the upstream kinases that phosphorylate Thr172 was a difficult challenge, eventually solved by genome-wide biochemical screens that identified three upstream kinases in budding yeast 20, 21. The mammalian kinases with catalytic domains most closely related to these were LKB1 and the Ca2+/calmodulin-dependent kinase CaMKK2 (CaMKKβ), and evidence was soon obtained that both could act as physiological upstream kinases in mammalian cells22–27. The discovery that LKB1 was an upstream kinase for AMPK was particularly interesting, because LKB1 had previously been identified to be a tumor suppressor28. Phosphorylation of Thr172 by CaMKK225–27 represents a mechanism by which hormones that increase cytosolic Ca2+ can activate AMPK in the absence of energy stress.
General Features of AMPK Regulation
Regulation by the canonical energy stress mechanism
It might be expected that a system that monitors cellular energy status would sense ATP and ADP but, interestingly, all metabolic enzymes known to directly monitor cellular energy charge (glycogen phosphorylase, 6-phosphofructo-1-kinase, fructose-1,6-bisphosphatase) primarily sense AMP and ATP, as does AMPK. The principal source of AMP in cells is thought to be the adenylate kinase reaction (2ADP ↔ ATP + AMP), which appears to operate close to equilibrium in most cells so that the AMP:ATP ratio will vary as the square of the ADP:ATP ratio29. The former is therefore a more sensitive indicator of falling energy status than the latter.
Binding of AMP activates AMPK by three complementary mechanisms, of which the first two are mimicked by ADP at higher concentration, while all three are antagonized by ATP: (i) inhibition of Thr172 dephosphorylation by protein phosphatases; (ii) promotion of Thr172 phosphorylation by LKB1; and (iii) allosteric activation30, 31. The structural model shown in Fig. 2 suggests a mechanism, for which there is now supporting evidence7, to explain mechanisms (i) and (iii). When AMP is bound at site 3, the α-linker interacts with the surface of the γ subunit containing that site (Fig. 2)7, 32. AMPK heterotrimers contain two rather distinct regions, the “catalytic module” (β-CBM, KD, AID, top/front section in Fig. 2) and the “nucleotide-binding module” (γ subunit, β-CTD, α-CTD, bottom/rear section in Fig. 2). The “hinge” connecting them is the α-linker, and release of the α-linker on binding of ATP rather than AMP at site 3 is envisaged to allow the two modules to move apart, causing the AID to rotate back into its inhibitory position behind the α-KD. This conformational change would also increase accessibility of Thr172 to protein phosphatases, which in the AMP-bound conformation of Fig. 2 is around the back, located in a deep cleft between the two modules. This model therefore explains not only how AMP binding at site 3 causes allosteric activation, but also why it protects against Thr172 dephosphorylation, with binding of ATP antagonizing both effects.
Multiple mechanisms of pharmacological activation of AMPK
A selection of compounds commonly used to activate AMPK experimentally are listed in Table 1. Those in Class 1, including the antidiabetic drug metformin and berberine (derived from traditional Chinese medicine) inhibit Complex I of the mitochondrial respiratory chain, while those in class 2, including 2-deoxyglucose, inhibit glycolysis. Both classes activate AMPK indirectly by increasing cellular AMP:ATP ratios33. These compounds are frequently used to activate AMPK experimentally, and studies utilizing them are described later in this review. However, because they work by depleting cellular ATP, they should not be regarded as specific AMPK activators and any results obtained with them should ideally be followed up using genetic approaches, such as the use of AMPK knockouts.
Table 1. List of pharmacological agents commonly used to activate AMPK in intact cells or in vivo, and their mechanisms of action.
| Class | Agent | Mechanism | Binding site used | Isoform-selective? | Ref. |
|---|---|---|---|---|---|
| 1 | metformin | mitochondrial inhibitor, AMP ↑ | γ subunit (binds AMP) | No | 33 |
| 1 | phenformin | mitochondrial inhibitor, AMP ↑ | γ subunit (binds AMP) | No | 33 |
| 1 | berberine | mitochondrial inhibitor, AMP ↑ | γ subunit (binds AMP) | No | 33 |
| 2 | 2-deoxyglucose | glycolytic inhibitor, AMP ↑ | γ subunit (binds AMP) | No | 33 |
| 3 | AICAR | pro-drug, converted to ZMP | γ subunit (binds ZMP) | No | 33, 34 |
| 4 | C13 | pro-drug, converted to C2 | γ subunit (binds C2) | α1-selective | 38, 40 |
| 5 | A769662 | direct activator | ADaM site | β1-selective | 189 |
| 5 | 991 | direct activator | ADaM site | β1>β2 | 8 |
| 5 | MT 63-78 | direct activator | ADaM site | β1-selective | 190 |
| 5 | PF-06409577 | direct activator | ADaM site | β1-selective | 191 |
| 5 | PF-249 | direct activator | ADaM site | β1-selective | 191 |
| 5 | salicylate | direct activator | ADaM site | β1-selective | 9, 44 |
The third class of activator includes the widely used compound 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), a nucleoside that is taken up into cells and converted to the equivalent nucleotide, ZMP. An important caveat here is that although ZMP is an AMP analog that mimics the effects of AMP to activate AMPK, it is about 50-fold less potent than AMP itself34. Because ZMP accumulates to millimolar concentrations inside cells AICAR does cause AMPK activation, but ZMP also has off-target effects. For example, it is known to mimic the effects of AMP on phosphorylase35 and fructose-1,6-bisphosphatase36 as well as AMPK. Another problem with AICAR is that it is an adenosine analogue and, while it does not appear to bind directly to adenosine receptors, in incubated cell systems it competes with endogenous adenosine for reuptake into cells by adenosine transporters, so can have adenosine-like effects 37.
A more specific activator that works via a related mechanism (class 4) is C13, which is taken up by cells and converted by cellular esterases to the AMP analog C2, a very potent AMPK activator38 although only for α1- and not α2-containing complexes39. Despite its selectivity for the α1 isoform, C2 binds to the γ subunit. Although the AMP and C2 binding sites overlap, they are not identical40.
The other molecules of choice for selective activation of AMPK are those binding at the ADaM site located between the β-CBM and the N-lobe on the α subunit, such as A769662 and 991 (class 5)8. All compounds binding this site are more potent activators of complexes containing AMPK-β1 rather than β2, and most are essentially β1-selective (Table 1). Using AMPK phosphorylated on Thr172, these activators cause a modest degree of allosteric activation (up to 4-fold) and, by inhibiting dephosphorylation, also promote net Thr172 phosphorylation41, 42. More remarkably, they cause a much larger allosteric activation (up to 65-fold) of AMPK that is not phosphorylated on Thr172, and this effect is synergistic with AMP43. At present, almost all of the activators known to bind at this site are synthetic compounds derived from high-throughput screens, although there is much speculation in the field that there is a naturally occurring ligand, hence the appearance of “metabolite” in its name15. However, no natural ligands occurring in animal cells that bind the ADaM site have yet been found. Salicylate, the natural plant product from which acetyl salicylic acid (ASA, aspirin) was derived, does activate AMPK by binding this site9, 44.
It should also be noted that there are currently no specific pharmacological inhibitors of AMPK; compound C (dorsomorphin) is sometimes claimed to be a specific inhibitor, but this is not the case45.
Targets and Pathways Downstream of AMPK
Catabolic effects switched on by AMPK
Once activated by energy stress, AMPK switches on catabolic pathways generating ATP, while switching off anabolic pathways and other processes consuming ATP, thus acting to restore energy homeostasis. This topic has been discussed in more detail in previous reviews1–3. Examples of catabolic processes acutely switched on include cellular glucose uptake mediated by GLUT1 and GLUT4. Activation of GLUT1 may occur via phosphorylation of the thioredoxin interacting protein TXNIP46, while enhanced GLUT4 translocation to the plasma membrane in muscle appears to occur, at least in part, by phosphorylation of TBC1D1, which modulates trafficking of GLUT4-containing vesicles47. AMPK activation also enhances GLUT4 expression48, in part via phosphorylation of the histone deacetylase HDAC5, promoting binding of 14-3-3 proteins and consequent retention of HDAC5, a transcriptional inhibitor, in the cytoplasm49, 50. AMPK can also cause a short-term activation of glycolysis via phosphorylation of PFKFB251 and PFKFB352, isoforms of the bifunctional enzyme that synthesizes and breaks down fructose-2,6-bisphosphate, a key allosteric activator of 6-phosphofructo-1-kinase and hence glycolysis. PFKFB2 is expressed in the heart, while PFKFB3 occurs as an “inducible” form whose expression in monocytes and macrophages is induced by inflammatory mediators such as lipopolysaccharide53. Phosphorylation of PFKFB2 or PFKFB3 at equivalent sites near their C-termini increases the synthesis of fructose-2,6-bisphosphate and hence promotes glycolysis during hypoxia in heart and in activated monocytes/macrophages, respectively. This may enhance survival of these cells during periods of hypoxia or ischemia.
Although AMPK can therefore activate glucose uptake and glycolysis in specific cell types, in the longer term it tends to promote instead the more glucose-sparing and energy-efficient oxidative metabolism. In skeletal muscle54, 55 and liver56, AMPK activates fatty acid oxidation by inhibiting the ACC1/ACC2 isoforms of acetyl-CoA carboxylase to reduce malonyl-CoA, an inhibitor of the uptake of fatty acids into mitochondria. AMPK promotes the expression of TCA cycle enzymes57, as well as mitochondrial biogenesis, which it achieves by increasing the expression/activity of the transcriptional co-activator PGC-1α, either by direct phosphorylation triggering a positive feedback effect on its own expression58, or by enhancing its deacetylation by SIRT159. PGC-1α acts as a co-activator for several transcription factors involved in mitochondrial biogenesis and oxidative metabolism, including myocyte enhancer factor-2 (MEF2), nuclear respiratory factors-1/-2 (NRF1/2), and PPAR-α and –δ60. Despite this evidence that AMPK promotes oxidative metabolism, in mice with a skeletal/cardiac muscle-specific knockout of both AMPK-β subunits, although the mice displayed evidence of dilated cardiomyopathy the rate of glucose and fatty acid oxidation in hearts perfused under normoxic conditions ex vivo was normal61.
A final catabolic pathway switched on by AMPK is autophagy, brought about via direct phosphorylation of the protein kinase that triggers that process, ULK162. By triggering digestion of cellular contents, autophagy may be critical in enhancing cell survival during periods of acute nutrient starvation, such as during ischemia in cardiac muscle. However, it has also been suggested that excessive autophagy might also contribute to cell damage during ischemia/reperfusion63.
Anabolic pathways switched off by AMPK
As well as its classical roles in inhibiting fatty acid and sterol synthesis34, AMPK inactivates key enzymes and regulatory proteins involved in triglyceride/phospholipid synthesis64, glycogen synthesis13, 14, rRNA synthesis65, and protein synthesis. The latter is inhibited both at the elongation step by phosphorylation and activation of elongation factor-2 (EF2) kinase66, and at the initiation step by inactivation of the mechanistic target-of-rapamycin complex-1 (mTORC1) by multiple mechanisms67, 68. By inhibiting mTORC1, AMPK would also be expected to oppose hypertrophy in organs such as the heart, which in most cases is considered to be a deleterious process.
Role of AMPK in the Heart
Role in the response to cardiac ischemia
It was reported in 1995 that AMPK was activated by no-flow ischemia in perfused rat hearts, an effect associated with high rates of fatty acid oxidation during reperfusion69. AMPK is also activated in the heart by increased workload70. The role of AMPK in cardiac ischemia has subsequently been addressed using mouse models where AMPK is down-regulated or absent. The first was a transgenic model where an inactive AMPK-α2 subunit was expressed in both skeletal and cardiac muscle. By competing with endogenous α subunits for available β and γ subunits, the over-expressed inactive α2 subunit down-regulates endogenous α1 and α2, and thus acts as a dominant negative mutant. There was no major phenotype under unstressed conditions, but during low-flow ischemia there was a failure to enhance glucose uptake and glycolysis, while during subsequent reperfusion there was less fatty acid oxidation, lower ATP levels and poorer recovery of contractile function71. The degree of damage to the myocardium was also greater, suggesting that AMPK exerts a cardioprotective effect overall, even though stimulation of fatty acid oxidation during reperfusion may be deleterious69. Using the same model, evidence was obtained that AMPK was required for increased autophagy during ischemia72.
Next to be studied were conditional knockouts of the upstream kinase LKB1 in both skeletal and cardiac muscle. The basal activity of AMPK-α2 complexes in the heart was completely abolished, and did not increase in response to no-flow ischemia or anoxia, correlating with a complete lack of ACC phosphorylation at the AMPK site. The AMP:ATP and ADP:ATP ratios were also elevated to a greater extent during no-flow ischemia than in control hearts, confirming that AMPK was protecting the cells against energetic stress. Surprisingly, the activity of AMPK-α1 complexes was almost unaffected by LKB1 knockout, and still increased in response to ischemia or anoxia73. Broadly similar results were obtained in studies of perfused hearts from mice with a whole body knockout of AMPK-α274. Interestingly, left ventricular hypertrophy induced by phenylephrine was greatly accentuated in the hearts from AMPK-α2 knockout mice; this may have been due to less restraint on the mTORC1 pathway, because the basal phosphorylation of p70S6K at the mTORC1 site was elevated, although it did not increase further upon phenylephrine treatment as in the controls75.
Mutations in γ2 and γ3 subunits causing heart disease and altered glycogen content
The only mutations in any of the seven genes encoding AMPK subunits clearly shown to cause human pathology are those in PRKAG2, encoding AMPK-γ2. These are associated with multiple cardiac disorders, specifically: (i) ventricular pre-excitation; (ii) excessive glycogen storage in cardiomyocytes; (iii) cardiac hypertrophy. Although rare, the PRKAG2 mutations are autosomal dominant in effect and therefore occur frequently in affected families. They are invariably missense mutations causing amino acid replacements, of which at least 14 have now been reported (Table 2). The affected residues are perfectly conserved between human γ1, γ2 and γ3, and occur in all four CBS repeats. The location of the residues affected in γ2, and of the three bound molecules of AMP, are mapped in Fig. 3 onto a structure for γ118. Interestingly, six of the mutations (R302Q, H383R, R384T, H530R, R531G and R531Q) affect basic residues whose side chains directly interact with the phosphate groups of one or more molecules of AMP or ATP, while two others also interact either directly (S548) or indirectly (T400) with AMP18. Other affected residues lie in more peripheral regions of the γ subunit, and it is less obvious why their replacement should affect function. In the context of bacterially expressed CBS repeats from AMPK-γ2, the R302Q, H383R, T400N, and R531G mutations all reduced the affinity for ATP as well as AMP, with the severity of the effects increasing in the order R302Q<H383R<T400N<R531G16. When expressed in mammalian cells as α1β1γ2 heterotrimers, these mutations also reduced allosteric activation by AMP, with the R531G mutation abolishing AMPK activation completely16, 76. The R384T and R531Q mutations were shown later to cause severe effects on AMPK function, similar to or even greater than R531G77, 78. In a comparison of the R531G and R531Q mutations expressed as α1β1γ2 heterotrimers in HEK-293 cells, these mutations not only abolished allosteric activation by AMP, but also caused significant increases in basal Thr172 phosphorylation and activity78. This was particularly clear in cells stably expressing the R531G mutant, when clones could be selected in which the level of expression of the WT and mutant was identical; the R531G mutant consistently had a 2-fold higher basal Thr172 phosphorylation and activity although, unlike the wild type, it was completely insensitive to further activation by agents that increase cellular AMP/ADP33.
Table 2. Amino acid replacements, generated by mutations in PRKAG2, that are associated with heart disorders.
Equivalent residues in the sequences of human γ1 and γ3 are shown in the second and third column and the CBS repeat affected in the fourth. V336 and R350 are located in the linker between CBS1 and CBS2.
| γ2 mutation | γ1 equivalent | γ3 equivalent | CBS repeat affected | Reference |
|---|---|---|---|---|
| R302Q | R70 | R225 | CBS1 | 192–194 |
| S333P | S101 | S333 | CBS1 | 79 |
| V336A | V104 | V259 | linker | 79 |
| L insert (after R350) | after R118 | after R273 | linker | 195 |
| H383R | H151 | H306 | CBS2 | 195 |
| R384T | R152 | R307 | CBS2 | 77 |
| T400N | T168 | T323 | CBS2 | 193 |
| Y487H | Y255 | Y410 | CBS3 | 196 |
| N488I | N256 | N411 | CBS3 | 193 |
| E506K | E274 | E429 | CBS4 | 79, 197 |
| H530R | H298 | H453 | CBS4 | 198 |
| R531G | R299 | R454 | CBS4 | 80 |
| R531Q | R299 | R454 | CBS4 | 78 |
| S548P | S316 | S471 | CBS4 | 199 |
Some of the mutations, such as R302Q, can cause relatively mild symptoms so that the patients may not present in the clinic until early adulthood79. By contrast, patients with the R531G mutation develop quite severe symptoms during childhood80, while the R384T77 and R531Q78 mutations were only detected post mortem in neonates who had died within weeks of birth, and appeared to be de novo mutations because neither parent was affected. Consistent with the idea that R531G, R531Q and R384T cause particularly severe forms of the disease, these were the mutations with the largest effects on binding of AMP and ATP to the γ subunit16, 77, 78. It is also interesting that R531 and R384 are involved in the binding of AMP and/or ATP at the critical site, site 3.
What causes the cardiac sequelae of the PRKAG2 mutations? The reduction of AMP binding to the γ subunit causes reduced activation by AMP and is a loss-of-function effect, whereas the reduction of ATP binding may be responsible for the increased basal activity of the R531G and R531Q mutants and is a gain-of-function effect. Since these mutations are dominant, it seems likely that it is the gain-of-function effect, increased basal activity, that causes most of the pathology; the loss-of-function effect may be compensated for by γ1, which is the major isoform expressed in heart, at least in rodents81. This is an important conclusion because pharmaceutical companies are developing AMPK activators to combat Type 2 diabetes, and it suggests that activation of γ2-containing complexes in the heart might have deleterious effects, as seen with these mutations.
Why does increased basal activity of γ2-containing complexes cause these cardiac phenotypes? AMPK is known to promote glucose uptake in cardiac muscle71, so increased basal activity would be expected to cause a high basal uptake, even in the absence of a real demand for glucose. There is good evidence for this scenario from studies of transgenic mice over-expressing the N488I mutation in the heart, which develop ventricular pre-excitation and cardiac hypertrophy similar to the human disorder82. By 50 days of age, they have a 20-fold increase in cardiac glycogen compared with controls, accompanied by higher rates of glucose uptake and glycogen synthesis but lower rates of lactate production, suggesting that increased glucose uptake is being directed into glycogen synthesis rather than glycolysis. Glycogen synthase was also much more highly phosphorylated in the transgenic mice (presumably due to the high basal AMPK activity), but cellular glucose-6-phosphate (G6P, an allosteric activator of glycogen synthase that over-rides the effects of phosphorylation) was also elevated >3-fold. Satisfying confirmation of this interpretation came when the N488I mice were crossed with knock-in mice carrying a mutation that renders glycogen synthase insensitive to G6P. The presence of the G6P-insensitive glycogen synthase reversed the high glycogen phenotype of the N488I mice and rescued the ventricular pre-excitation but not the cardiac hypertrophy, suggesting that the former but not the latter is secondary to increased glycogen content83. Supporting the idea that hyper-activation of the mTORC1 pathway was the cause of hypertrophy, mTORC1 targets such as p70S6K and 4EBP1 were more highly phosphorylated in the N488I mice, while treatment with rapamycin reduced, without completely preventing, the hypertrophy. Increased mTORC1 in the N488I mice is actually rather counter-intuitive, because AMPK is normally thought to inhibit that pathway67, 68. However, a recent study has suggested that AMPK can activate mTORC1 under certain circumstances by sustaining the supply of amino acids via autophagy84.
Why does abnormally high glycogen content lead to ventricular pre-excitation? During foetal development, the atria and the ventricles become separated by the growth of a fibrous layer called the annulus fibrosis, which ensures that the only electrical connection between the two chambers is via the atrioventricular node. In N488I mice the annulus fibrosis appeared to be thin and disrupted in places, and it was suggested that the presence of glycogen-containing vacuoles in myocytes disrupts its formation during fetal development, causing abnormal electrical connections between the atria and ventricles85.
Most mouse studies of γ2 mutations have involved transgenic mice over-expressing the mutations in human γ2. Although these mice do display the key clinical features of the human syndrome, and mice expressing wild type γ2 from the same promoters were used as controls, they are not perfect models because γ2 is being over-expressed. Recently, three knock-in mouse models, in which mutations in the mouse gene equivalent to human R302Q, N488I or R531G are expressed globally, were studied86, 87. There was evidence for increased basal AMPK activity in liver and/or muscle of all three strains. The N488I and R531G mice displayed ventricular pre-excitation and modest increases in heart weight, which was associated with large increases in glycogen content in hearts of R531G but not the N488I mice. Both N488I and R531G mice displayed resistance to obesity and hepatic steatosis induced by high-fat diet, but, unlike the N488I mice, the R531G mice also displayed impaired renal function associated with glycogen accumulation, cyst formation, and inflammation and apoptosis in the kidney, particularly when on a high-fat diet87, By contrast, in the R302Q mice there was no obvious cardiac phenotype, but the homozygotes developed marked obesity as they aged, with smaller effects in heterozygotes. Obesity appeared to be due mainly to increased food intake through enhanced action of ghrelin, whose effects are mediated by AMPK activation via the CaMKK2 pathway in the hypothalamus88, 89. The homozygotes were also hypoinsulemic, apparently due to reduced glucose-stimulated insulin secretion from the pancreas. Overall, the phenotypes of these mice with knock-in mutations in the PRKAG2 gene showed surprising variability. Interestingly, heterozygous human carriers of the R302Q mutation, who have a relatively mild cardiac phenotype, also display some evidence of increased obesity, as well as higher fasting glucose and glycated haemoglobin (HbA1c) and lower insulin levels than unaffected siblings86. These non-cardiac features of the R302Q mutation only became evident following studies of the mouse model.
Finally, all of the residues mutated in AMPK-γ2 are also conserved in γ1 and γ3 (Table 2). It is interesting that none of them have yet been reported to be mutated in the human γ1 gene. However, mutations equivalent to R302Q have been found in both pigs and humans in the γ3 isoform, which is predominantly expressed in skeletal muscle81. In human muscle, γ3 appears to be present exclusively as the α2β2γ3 complex, which is the only form of AMPK that is activated during exercise6. An R200Q mutation (equivalent to R302Q in human γ2) was found to be a relatively common dominantly acting genetic variant in Hampshire pigs, and was associated with a high glycogen content in skeletal muscle; although adversely affecting meat quality, it did not cause obvious clinical problems90. Interestingly, in a screen of around 1500 humans, an R225W mutation (R225 being the human equivalent of R302) was found in two apparently unrelated individuals, once again not associated with any obvious clinical defects; muscles from humans with this mutation had a higher basal AMPK activity, 2-fold higher glycogen content and lower triglyceride content91. In an in vitro study of myotubes from R225W carriers, they had 3-fold higher mitochondrial content and oxidative capacity, and 2-fold higher basal glucose uptake and glycogen synthesis rates than matched controls. The R225W subjects also had a remarkable resistance to fatigue during isometric contractions of the quadriceps92, suggesting that this mutation might confer an advantage during endurance exercise because of the high glycogen content.
Role of AMPK in the Vasculature
In addition to the heart, it has become clear that AMPK has an important role in regulating vascular function, with distinct roles in the functions of vascular endothelial cells (ECs), smooth muscle cells (VSMCs), adventitial cells and vascular immune cells.
Regulation of AMPK in endothelial and vascular smooth muscle cells
AMPK-α1 accounts for the majority of total AMPK activity in ECs93–95, yet specific down-regulation of AMPK-α2 still has marked effects96, 97. A wide variety of physiological stimuli have been reported to activate endothelial AMPK, including hypoxia, low glucose and shear stress98–100, adiponectin101, angiotensin II and ghrelin102, 103. In addition, CaMKK2 mediates AMPK activation by vasoactive molecules including thrombin, VEGF, sphingosine-1-phosphate, bradykinin and estrogen104–108, suggesting that any stimulus that increases Ca2+ in ECs will activate AMPK. Finally, a number of widely used hypoglycemic (metformin, thiazolidinediones, salicylate, DPP4 inhibitors and liraglutide)109–112 and hypocholesterolemic (statins and fenofibrate)113, 114 drugs activate AMPK in cultured ECs.
Physiological signals that inhibit AMPK in ECs include high nutrient concentrations115–117, yet the mechanisms by which this inhibition of AMPK in ECs occurs are unclear, although increased PP2A-mediated dephosphorylation of AMPK has been proposed115. Endothelial AMPK may therefore be suppressed by the high levels of nutrients associated with obesity and insulin resistance. As protein kinase C (PKC) activation is associated with over-nutrition, it is interesting that PKC-mediated inhibitory phosphorylation of Ser487 on AMPK-α1 has been recently reported in ECs118. Conversely, the very first study of AMPK function in ECs demonstrated that AICAR stimulated fatty acid oxidation119 and AMPK activation was subsequently found to normalise impaired fatty acid oxidation and insulin signaling due to high glucose120. Stimulation of fat oxidation may partly underlie the effect of AMPK to antagonize palmitate-mediated endothelial dysfunction, thus protecting against lipotoxicity.
As with ECs, AMPK-α1 accounts for the majority of AMPK activity in murine and human VSMCs121, 122, and low glucose, adiponectin, estradiol and metformin all activate AMPK123–126. Angiotensin II has also been reported to acutely activate AMPK127, although prolonged activation had no effect128. Similar to ECs, high glucose inhibits AMPK in VSMCs, and IGF-1 also inhibits AMPK, most likely via inhibitory phosphorylation of AMPK-α1 by Akt129. Several studies have demonstrated that culture in phosphate/β-glycerophosphate, used to examine VSMC calcification as described later, also inhibits AMPK130, 131.
AMPK and endothelial NO synthesis
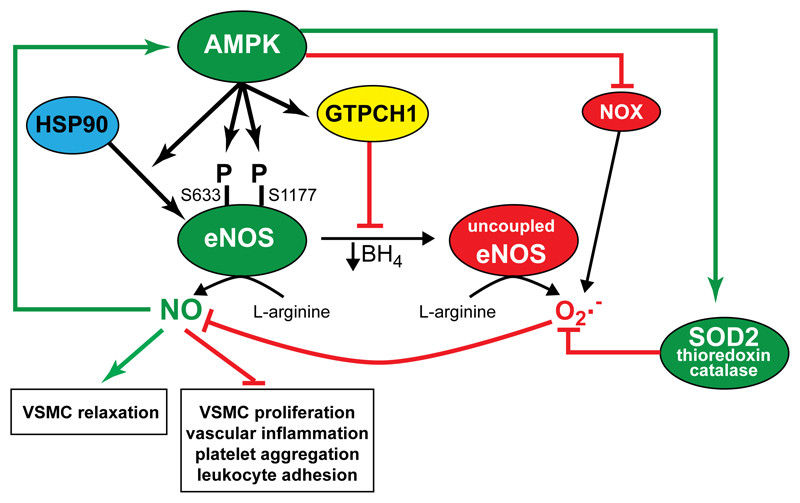
Endothelium-derived NO is a key regulator of vascular function, stimulating VSMC relaxation whilst inhibiting pro-inflammatory signaling, leukocyte adhesion, platelet aggregation, VSMC proliferation and migration associated with pathological vascular remodelling and atherosclerosis132. The first indication of a specific vascular role for AMPK came when AMPK was shown to phosphorylate at Ser1177 and activate endothelial NO synthase (eNOS), both in cell-free assays and in ECs93, 133. Multiple mechanisms regulate eNOS activity, including Ca2+/calmodulin binding, allosteric and protein:protein interactions, co-factor and substrate availability, phosphorylation, and subcellular localisation132. In addition to Ser1177, AMPK phosphorylates eNOS at Ser633, required for AICAR-stimulated NO synthesis in HEK293 cells134. NO synthesis requires dimerization of eNOS and sufficient tetrahydrobiopterin (BH4), in whose absence eNOS generates superoxide instead132. Another mechanism by which AMPK improves NO synthesis may be by enhancing BH4 levels, as AMPK prevents degradation of GTP cyclohydrolase I, which is the rate-limiting enzyme in BH4 synthesis135. AMPK can therefore act by multiple mechanisms to stimulate NO production (Fig. 4), although endothelial AMPK activation is not always associated with eNOS phosphorylation or NO synthesis94, 136. AMPK may also mediate some of the endothelial effects of NO, because NO donors activate AMPK in ECs96.
Figure 4. Regulation of endothelial NO and superoxide synthesis by AMPK.
AMPK activation stimulates NO synthesis via multiple mechanisms: (i) phosphorylation of eNOS at Ser633 and Ser1177; (ii) increasing Hsp90 association with eNOS; (iii) increasing BH4 concentrations via GTP cyclohydrolase I (GTPCH1); (iv) reducing superoxide synthesis via inhibition of Nox and increasing antioxidant protein (superoxide dismutase (SOD), thioredoxin and catalase) levels. NO itself is also reported to activate AMPK.
Endothelial AMPK and reactive oxygen species
Inappropriate levels of reactive oxygen species (ROS), in particular superoxide anions generated in response to high concentrations of glucose, lipids and proinflammatory cytokines by NAD(P)H oxidase (Nox), uncoupled eNOS or mitochondrial respiratory chain complexes, have been implicated in vascular disease97, 135, 137, 138. Superoxide reacts with NO to form peroxynitrite, reducing NO bioavailability132. AMPK activation in ECs has been widely demonstrated to inhibit ROS formation, increase antioxidant defences and promote mitochondrial biogenesis. Down-regulation of AMPK in ECs increased activity and expression of Nox97, whereas AMPK-dependent inhibition of Nox1/2 translocation to the plasma membrane has also been reported109. The mechanism by which AMPK inhibits Nox remains unclear, but may be secondary to reduced PKC-mediated Nox activation, or NFκB-mediated Nox transcription97, 109. AMPK-dependent inhibition of mitochondrial ROS formation has also been reported in ECs maintained in high glucose 137, whereas AMPK-mediated stimulation of BH4 synthesis, as described above, prevents uncoupling of eNOS and superoxide formation135. Several groups have reported that AMPK-mediated inhibition of ROS in ECs is associated with increased levels of the antioxidant enzymes superoxide dismutase-2 (SOD2), catalase and thioredoxin 137, 139, 140. In ECs, AMPK activation reduces ER stress141, which is tightly linked to oxidative stress and inflammation, whereas silencing of AMPK increases markers of ER stress95. Taken together, AMPK activation acts via multiple mechanisms to suppress chronic ROS synthesis in ECs, limiting their damaging actions as well as the sequestration of NO (Fig 4).
AMPK and vascular cell inflammation
The development of endothelial dysfunction and cardiovascular diseases is associated with elevated TNFα, IL-1β and IL-6142. TNFα and IL-1β stimulate activation of NFκB and the Jun N-terminal kinase (JNK) pathway, while IL-6 signals via Janus kinases (JAKs) leading to phosphorylation of signal transducer and activation of transcription (STAT) proteins143. The anti-inflammatory actions of AMPK in ECs were first described when AMPK activation was shown to suppress palmitate- or TNFα-stimulated NFκB activity117. Under basal conditions, NFκB is in an inactive form in the cytoplasm due to binding to IκB whereas, after cytokine stimulation, IκB phosphorylation by IκB kinase (IKK) targets it for degradation, allowing NFκB-mediated transcription of proinflammatory cytokines, adhesion molecules and chemokines143, 144. NO inhibits endothelial NFκB activity145, suggesting that AMPK-stimulated NO synthesis would inhibit NFκB, although NO donors do not effectively suppress NFκB in ECs with reduced AMPK activity96. AMPK-α2 has been reported to phosphorylate IKKβ in vitro, inhibiting IκB phosphorylation and NFκB activation, with reduced IL-1β-stimulated IKK phosphorylation observed in ECs lacking AMPK-α2 but not -α196. Alternatively, AMPK-mediated phosphorylation of the transcriptional co-activator p300 has been proposed to block acetylation and DNA binding by the p65 subunit of NFκB146. The idea that AMPK inhibits NFκB signalling is reinforced by functional studies demonstrating AMPK-dependent inhibition of NFκB-regulated expression of adhesion molecules and MCP-196, 144. Fewer studies have investigated the effect of AMPK on proinflammatory JNK and IL-6 signaling, although AICAR and metformin reduce JNK activity in ECs147, and increased JNK phosphorylation has been reported in ECs lacking AMPK-α295. The mechanism of JNK inhibition is uncertain, although AMPK-dependent inhibition of the upstream kinase MKK4 has been reported in other cells148. Recently, AMPK-dependent inhibition of IL-6-stimulated JAK-STAT signaling has been demonstrated in ECs, potentially via direct inhibitory phosphorylation of JAK1 by AMPK110. AMPK activation therefore appears to rapidly suppress multiple proinflammatory signaling pathways in ECs, by diverse mechanisms.
AMPK activation in VSMCs inhibits TNFα-stimulated NFκB activity and angiotensin II-stimulated STAT1 activity, as well as reducing expression of inducible NOS and cyclooxygenase-2, and secretion of IL-6 and MCP-1149, 150. Thus, AMPK has anti-inflammatory effects in VSMCs as well as ECs.
AMPK and angiogenesis
Hypoxia, VEGF and adiponectin all stimulate AMPK-dependent EC migration, although there are conflicting reports as to whether this is mediated by NO94, 98, 101, 105, 108. Conversely, down-regulation of AMPK attenuates angiogenesis caused by hypoxia, adiponectin, VEGF or statins, in either tube formation or matrigel plug assays94, 98, 101, 113. Mechanistically, increased UCP2 or SOD2 have been reported to increase angiogenesis in AMPK-deficient ECs151, 152, indicating that down-regulation of ROS may be critical. AMPK activation also stimulates VEGF expression, indicating that AMPK positively influences angiogenesis both by increasing VEGF levels and by increasing VEGF signaling153. As angiogenesis would also consume significant amounts of ATP, AMPK activation might also serve a permissive role, ensuring adequate generation of ATP to permit EC migration and proliferation.
AMPK and VSMC contraction
AMPK has direct anti-contractile effects on VSMCs, because AICAR relaxes aortic rings in an NO- and endothelium-independent manner, an effect lost in AMPK-α1 knockouts121. Furthermore, AMPK activation has been reported to affect VSMC contractile signalling, including dephosphorylation of myosin light chain (MLC) and/or myosin phosphatase targeting subunit 1 (MYPT1)125, 154. Mechanistically, MLC/MYPT1 dephosphorylation may be a consequence of AMPK-mediated inhibition and phosphorylation of RhoA at Ser188, causing subsequent inhibition of ROCK125. A769662 has also been reported to reduce intracellular Ca2+ in VSMCs by increasing sarco/endoplasmic Ca2+ ATPase (SERCA) activity, associated with increased phosphorylation of phospholamban on Thr17, which disinhibits SERCA and thereby may underlie SERCA activation and vessel relaxation155.
AMPK and proliferation, differentiation and migration of VSMCs
Unlike ECs, where AMPK up-regulates proliferation and migration thus supporting angiogenesis, in VSMCs AMPK inhibits proliferation in response to angiotensin II, PDGF and FCS, associated with stimulation of p53 Ser15 phosphorylation and reduced Rb phosphorylation128, 156. VSMCs from mice lacking AMPK-α2 exhibit increased proliferation, an effect mediated by increased degradation of the cyclin-dependent kinase inhibitor p27Kip1 triggered by the ubiquitin E3-ligase Skp2157. VSMCs retain significant plasticity in vivo and can exhibit a synthetic, proliferative phenotype rather than the quiescent, contractile phenotype during atherogenesis. AMPK not only suppresses VSMC proliferation, but also inhibits migration and maintains a pro-contractile phenotype158, 159. Inhibition of migration and proliferation are likely to be linked, because increased migration of VSMCs lacking AMPK-α2 is reported to be Skp2-dependent159. Furthermore, AICAR limits neointima formation after wire injury of rat femoral arteries, which is likely to reflect the anti-proliferative, anti-migratory actions of AMPK on VSMCs127.
AMPK and VSMC calcification
As mentioned above, culture in high phosphate/β-glycerophosphate concentrations is used experimentally to stimulate calcium deposition in VSMCs. In vivo, such vascular calcification is frequently associated with ageing, atherosclerosis and diabetes mellitus. AICAR, adiponectin and metformin all inhibit VSMC calcification in vitro130, 131, 160. In recent studies of atherosclerosis-prone ApoE-/- mice, deletion of AMPK-α1 but not AMPK-α2 caused greater calcification of atherogenic plaques, and levels of the osteogenic transcription factor Runx2160. Furthermore, metformin reduced atherosclerotic calcification and Runx2 expression in the mice, an effect that was absent in ApoE-/-mice lacking AMPK-α1. The authors of that study further demonstrated that VSMC-specific AMPK-α1 deletion in ApoE-/- mice phenocopied the increased calcification and Runx2 expression, whereas macrophage-specific AMPK-α1 deletion had no effect. The mechanism underlying the AMPK-mediated inhibition of Runx2 levels was further proposed to be mediated by phosphorylation of PIAS1 (protein inhibitor of activated STAT-1) at Ser510, which acts as a SUMO E3-ligase to trigger Runx2 SUMOylation and degradation160.
Role of AMPK in the Vasculature in vivo
As many of the actions of AMPK in ECs and VSMCs should have beneficial effects on vascular function (Fig 5), considerable efforts have been made to examine whether AMPK influences vascular tone, remodelling and atherogenesis in vivo. Under physiological conditions, vascular AMPK has been reported to be activated in vivo by exercise161 and estradiol125, and is suppressed by high fat and fructose diets in rodents162, 163. Furthermore, exercise stimulates aortic AMPK phosphorylation in both ECs and VSMCs as assessed by immunohistochemistry161.
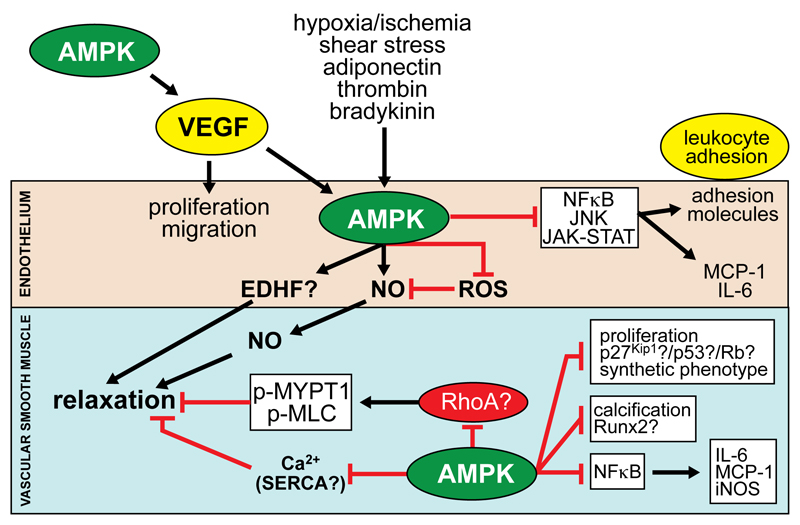
Figure 5. Actions of AMPK in vascular cells.
Physiological activators of AMPK in ECs include hypoxia/ischemia, shear stress, adiponectin, thrombin, bradykinin and VEGF (synthesis of which is stimulated by AMPK in other tissues). AMPK is reported to stimulate VSMC relaxation through: (i) increased NO synthesis and possibly endothelium-dependent hypopolarising factor (EDHF); (ii) inhibition of MYPT1/MLC phosphorylation and Ca2+ levels in VSMCs, reported to be mediated by reduced RhoA activity and increased sarco/endoplasmic Ca2+ ATPase (SERCA) activity respectively. AMPK activation in ECs stimulates proliferation and migration, whereas in VSMCs, proliferation and migration are inhibited, associated with p53 phosphorylation, Rb dephosphorylation and p27(Kip1) stabilisation. AMPK also inhibits VSMC calcification by reducing Runx2 and pro-inflammatory signalling pathways leading to leukocyte adhesion and cytokine/chemokine synthesis.
AMPK and vascular tone
As described above, AMPK can stimulate NO synthesis by ECs and independently inhibit contractile protein function in VSMCs in vitro. In intact arterial vessels, AICAR stimulates vasodilation in a diverse range of vascular beds from several species121, 164–167, an effect greatly attenuated in mice lacking AMPK-α1121. The mechanism of AICAR-stimulated vasodilation remains uncertain, and has been variously reported to be endothelium- and NO-dependent164, 167, partially NO- and endothelium-dependent165, 166 or NO- and endothelium-independent121. Using endothelium-specific knockouts it has been proposed that AMPK-α1 is important for endothelium-dependent hyperpolarisation-mediated relaxation of resistance arteries168. Interestingly, resistance arteries exhibited endothelium-independent dilation in response to A769662155, suggesting a VSMC-mediated effect. It is possible that ECs and VSMCs exhibit differential sensitivities to AICAR and A769662, or that some of these may be AMPK-independent effects. Despite studies indicating that AMPK-α2 has a less important role in regulation of vascular tone121, 168, AMPK-α2 knockout mice are hypertensive and exhibit increased contractile responses to phenylephrine in aortic rings169. Furthermore, impaired bradykinin-dependent vasodilation has been described in EC-specific AMPK-α2 knockout mice, thought to be due to increased bradykinin degradation by angiotensin-converting enzyme activity170. AICAR rapidly reduced blood pressure in spontaneously hypertensive rats, but was without effect in normotensive controls165, while prolonged administration of AICAR reduced systolic blood pressure in obese Zucker rats171. These data support a role for AMPK in the regulation of vascular tone in disease models, but cannot exclude systemic actions on the heart, kidney or other tissues. Surprisingly, whether systemic administration of more selective AMPK activators, such as A769662, 991 or C13, alters vascular tone has yet to be reported. More recently it has become clear that perivascular adipose tissue (PVAT), which is removed in most myography protocols, has a paracrine anti-contractile effect on the underlying vessel. AMPK activity in resistance arteries has been shown to alter the influence of PVAT-derived mediators172 and the anti-contractile action of PVAT is absent in mice lacking AMPK-α1, perhaps due to reduced adiponectin secretion173. AMPK in ECs, VSMCs and adventitial PVAT may therefore all contribute to the maintenance of vascular tone, but the cell type that mediates actions of AMPK on vascular tone may change, depending on the location of the vessel.
AMPK and atherosclerosis
The potential anti-atherogenic actions of AMPK have been investigated in vascular injury models and atherosclerosis-prone hypercholesterolemic mice. AICAR attenuates post-ischaemic leukocyte rolling and adhesion to the endothelium in vivo, an effect lost in AMPK-α1 or AMPK-α2 knockout mice174, and systemic administration of AICAR or metformin reduces atherosclerotic lesion size, macrophage accumulation and inflammation175–177. Similarly, berberine reduced the severity of atherosclerotic lesions in atherosclerosis-prone mice, an effect attenuated in AMPK-α2 knockouts178. AMPKα2-deficient mice also exhibited increased atherosclerosis95, although the global deficiency makes it difficult to assess whether this was due to a direct effect on vascular tissues. With respect to plaque stability and progression, recent studies have yielded exciting results. Metformin reduced plaque calcification, and mice with a SMC-specific knockout of AMPK-α1 had exacerbated calcification of atherosclerotic plaques in brachiocephalic arteries, phenocopying the increased calcification observed in global AMPK-α1 but not AMPK-α2 knockouts160. As the clinical use of metformin is associated with reduced macrovascular morbidity and mortality independently of glycemia179, these results may help to define the pathways involved. Using a ligation model for studying injury-induced neointima stability in brachiocephalic arteries, mice with deletion of AMPK-α1 but not AMPK-α2 exhibited more occlusive lesions, with lower collagen and higher macrophage content, indicative of plaque instability180. Similarly, in a high fat diet model, SMC-specific AMPK-α2 knockouts exhibited features of unstable plaques, including phenotype switching of VSMCs158. These data therefore indicate that AMPK activation may not only inhibit atherogenesis, but also inhibit the generation of vulnerable, calcified plaques. Repair of damaged endothelium is also considered important to prevent endothelial dysfunction after injury, and EC-specific expression of constitutively active AMPK has also been reported to promote re-endothelialization in a wire injury model, attributable in part to increased mobilization and incorporation of endothelial progenitor cells181.
Immune cells are involved in all stages of atherosclerosis, and AMPK suppresses inflammatory signalling, monocyte-to-macrophage differentiation and foam cell formation175, 177, 182. Indeed, myeloid-specific AMPK-α1 knockout mice fed an atherogenic diet on a LDL receptor knockout background have recently been shown to exhibit exacerbated atherosclerosis, with increased plaque macrophage content and inflammatory gene expression182. All of these studies suggest that AMPK activation limits atherosclerosis, although all of the results are from rodent models, rather than humans. One intriguing question is how, despite only contributing a small fraction of total AMPK activity in vascular cells, specific down-regulation of AMPK-α2 has such marked effects on atheroma development in mice 95, 158, 174, 178, similar to the specific effects noted with respect to vascular tone169, 170. It remains to be determined whether there are isoform-specific substrates or specific subcellular localisations that contribute to the vascular function of AMPK-α2.
AMPK and pulmonary vascular remodelling
Several studies have identified a critical role for AMPK in the pulmonary vascular remodelling and perivascular inflammation that characterizes pulmonary arterial hypertension (PAH). Metformin reduces pulmonary artery VSMC proliferation in an AMPK-dependent manner183, and inhibits the vascular remodelling of pulmonary arteries in rodent models of PAH184, 185 . Mice with an EC-specific lack of AMPK also exhibit accelerated pulmonary remodeling185. Whether the beneficial action of metformin in PAH is mediated by AMPK remains to be determined. By contrast, AMPK activation by hypoxia may contribute to the development of PAH by promoting pulmonary VSMC survival186, while AMPK-mediated inhibition of the voltage-gated K+ channel Kv1.5 may underlie the acute detrimental effects of hypoxia on PAH187.
Conclusions and Perspectives
AMPK exists as heterotrimeric complexes consisting of catalytic α subunits and regulatory β and γ subunits. AMPK complexes sense the energy status of cells by sensing increases in the cellular AMP:ATP and/or ADP:ATP ratios. AMP, ADP and ATP bind at up to three sites on the γ subunits; binding of AMP and/or ADP causes activation by promoting net phosphorylation at Thr172 within the activation loop on the α subunit, while binding of AMP only causes further allosteric activation. Once activated by energy stress, AMPK switches on catabolic pathways that generate ATP, while switching off cell growth and proliferation and other processes that consume ATP.
AMPK appears to exert a protective effect in rodent heart during ischemic episodes. Inherited and/or de novo mutations in the PRKAG2 gene (encoding AMPK-γ2) in humans cause heart disease of varying severity characterized by ventricular pre-excitation, excessive cardiac glycogen content, and hypertrophy. The mutations cause an increase in basal AMPK activity, leading to increased glucose uptake that accounts for the first two abnormalities. They also cause a failure of γ2-containing complexes to be further activated by AMP, which might explain the hypertrophy through unrestrained activity of the mTORC1 pathway.
In blood vessels, AMPK inactivation is associated with anti-contractile, anti-inflammatory and anti-atherogenic actions on both the vascular endothelium and smooth muscle. Exciting recent studies link AMPK activation to increased stability and reduced calcification of atherosclerotic plaques as well as highlighting a potential role for AMPK in pulmonary arterial hypertension. Given the critical role of AMPK in the regulation of nutrient metabolism, therapies that activate AMPK may not only normalise metabolic dysfunction but also reduce the burden of cardiovascular complications in obesity and type 2 diabetes. Recent studies using vascular tissue-specific deletion of AMPK-α isoforms are beginning to elucidate specific roles for AMPK-α isoforms, yet despite the wealth of research demonstrating the functional cardiovascular consequences of AMPK activation, the AMPK substrates involved and underlying mechanisms in several cases remain poorly defined. Emerging technologies such as phosphoproteomics may prove beneficial in understanding such mechanisms after AMPK up- or down-regulation in cardiovascular tissues in different disease settings and disease models.
Sources of Funding
Recent studies in the DGH laboratory have been supported by a Senior Investigator Award from the Wellcome Trust (097726) and a Programme Grant from Cancer Research UK (C37030/A15101). Recent studies in the IPS laboratory have been supported by Project Grants from the British Heart Foundation (PG/12/1/29276 and PG/13/82/30483) and Diabetes UK (BDA09/0003904 and BDA12/0004489).
References
- 1.Hardie DG, Schaffer BE, Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology. 2014;29:99–107. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daskalopoulos EP, Dufeys C, Beauloye C, Bertrand L, Horman S. AMPK in cardiovascular diseases. EXS. 2016;107:179–201. doi: 10.1007/978-3-319-43589-3_8. [DOI] [PubMed] [Google Scholar]
- 5.Daskalopoulos EP, Dufeys C, Bertrand L, Beauloye C, Horman S. AMPK in cardiac fibrosis and repair: Actions beyond metabolic regulation. J Mol Cell Cardiol. 2016;91:188–200. doi: 10.1016/j.yjmcc.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Birk JB, Wojtaszewski JF. Predominant alpha-1/beta-2/gamma-3 AMPK activation during exercise in human skeletal muscle. J Physiol. 2006;577:1021–1032. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Wang L, Zhou XE, Ke J, de Waal PW, Gu X, Tan MH, Wang D, Wu D, Xu HE, Melcher K. Structural basis of AMPK regulation by adenine nucleotides and glycogen. Cell Res. 2015;25:50–66. doi: 10.1038/cr.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao B, Sanders MJ, Carmena D, Bright NJ, Haire LF, Underwood E, Patel BR, Heath RB, Walker PA, Hallen S, Giordanetto F, et al. Structural basis of AMPK regulation by small molecule activators. Nature Commun. 2013;4:3017. doi: 10.1038/ncomms4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabrese MF, Rajamohan F, Harris MS, Caspers NL, Magyar R, Withka JM, Wang H, Borzilleri KA, Sahasrabudhe PV, Hoth LR, Geoghegan KF, et al. Structural basis for AMPK activation: natural and synthetic ligands regulate kinase activity from opposite poles by different molecular mechanisms. Structure. 2014;22:1161–1172. doi: 10.1016/j.str.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 11.Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, et al. AMPK b-Subunit targets metabolic stress-sensing to glycogen. Current Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 12.Bendayan M, Londono I, Kemp BE, Hardie GD, Ruderman N, Prentki M. Association of AMP-activated protein kinase subunits with glycogen particles as revealed in situ by immunoelectron microscopy. J Histochem Cytochem. 2009;57:963–971. doi: 10.1369/jhc.2009.954016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, et al. The a2-5'AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004;53:3074–3081. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
- 14.Bultot L, Guigas B, Von Wilamowitz-Moellendorff A, Maisin L, Vertommen D, Hussain N, Beullens M, Guinovart JJ, Foretz M, Viollet B, Sakamoto K, et al. AMP-activated protein kinase phosphorylates and inactivates liver glycogen synthase. Biochem J. 2012;443:193–203. doi: 10.1042/BJ20112026. [DOI] [PubMed] [Google Scholar]
- 15.Langendorf CG, Kemp BE. Choreography of AMPK activation. Cell Res. 2015;25:5–6. doi: 10.1038/cr.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townley R, Shapiro L. Crystal structures of the adenylate sensor from fission yeast AMP-activated protein kinase. Science. 2007;315:1726–1729. doi: 10.1126/science.1137503. [DOI] [PubMed] [Google Scholar]
- 18.Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, Martin SR, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 19.Oakhill JS, Chen ZP, Scott JW, Steel R, Castelli LA, Ling N, Macaulay SL, Kemp BE. beta-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK) Proc Natl Acad Sci USA. 2010;107:19237–19241. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci U S A. 2003;100:8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutherland CM, Hawley SA, McCartney RR, Leech A, Stark MJ, Schmidt MC, Hardie DG. Elm1p is one of three upstream kinases for the Saccharomyces cerevisiae SNF1 complex. Curr Biol. 2003;13:1299–1305. doi: 10.1016/s0960-9822(03)00459-7. [DOI] [PubMed] [Google Scholar]
- 22.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADa/b and MO25a/b are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmoldulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 28.Alessi DR, Sakamoto K, Bayascas JR. Lkb1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 29.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. BioEssays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- 30.Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–566. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross FA, Jensen TE, Hardie DG. Differential regulation by AMP and ADP of AMPK complexes containing different gamma subunit isoforms. Biochem J. 2016;473:189–199. doi: 10.1042/BJ20150910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 35.Longnus SL, Wambolt RB, Parsons HL, Brownsey RW, Allard MF. 5-Aminoimidazole-4-carboxamide 1-beta -D-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms. Am J Physiol. 2003;284:R936–R944. doi: 10.1152/ajpregu.00319.2002. [DOI] [PubMed] [Google Scholar]
- 36.Vincent MF, Marangos PJ, Gruber HE, Van den Berghe G. Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes. 1991;40:1259–1266. doi: 10.2337/diab.40.10.1259. [DOI] [PubMed] [Google Scholar]
- 37.Gadalla AE, Pearson T, Currie AJ, Dale N, Hawley SA, Sheehan M, Hirst W, Michel AD, Randall A, Hardie DG, Frenguelli BG. AICA riboside both activates AMP-activated protein kinase and competes with adenosine for the nucleoside transporter in the CA1 region of the rat hippocampus. J Neurochem. 2004;88:1272–1282. doi: 10.1046/j.1471-4159.2003.02253.x. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Galeno JE, Dang Q, Nguyen TH, Boyer SH, Grote MP, Sun Z, Chen M, Craigo WA, van Poelje PD, MacKenna DA, Cable EE, et al. A potent and selective AMPK activator that inhibits de novo lipogenesis. ACS Med Chem Lett. 2010;1:478–482. doi: 10.1021/ml100143q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter RW, Foretz M, Bultot L, Fullerton MD, Deak M, Ross FA, Hawley SA, Shpiro N, Viollet B, Barron D, Kemp BE, et al. Mechanism of action of Compound-13: an alpha1-selective small molecule activator of AMPK. Chem Biol. 2014;21:866–879. doi: 10.1016/j.chembiol.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langendorf CG, Ngoei KR, Scott JW, Ling NX, Issa SM, Gorman MA, Parker MW, Sakamoto K, Oakhill JS, Kemp BE. Structural basis of allosteric and synergistic activation of AMPK by furan-2-phosphonic derivative C2 binding. Nat Commun. 2016;7:10912. doi: 10.1038/ncomms10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem. 2007;282:32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- 42.Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282:32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott JW, Ling N, Issa SM, Dite TA, O'Brien MT, Chen ZP, Galic S, Langendorf CG, Steinberg GR, Kemp BE, Oakhill JS. Small molecule drug A-769662 and AMP synergistically activate naive AMPK independent of upstream kinase signaling. Chem Biol. 2014;21:619–627. doi: 10.1016/j.chembiol.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, Kemp BE, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu N, Zheng B, Shaywitz A, Dagon Y, Tower C, Bellinger G, Shen CH, Wen J, Asara J, McGraw TE, Kahn BB, et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell. 2013;49:1167–1175. doi: 10.1016/j.molcel.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Q, Xie B, Zhu S, Rong P, Sheng Y, Ducommun S, Chen L, Quan C, Li M, Sakamoto K, MacKintosh C, et al. A Tbc1d1 Ser231Ala-knockin mutation partially impairs AICAR- but not exercise-induced muscle glucose uptake in mice. Diabetologia. 2017;60:336–345. doi: 10.1007/s00125-016-4151-9. [DOI] [PubMed] [Google Scholar]
- 48.Zheng D, MacLean PS, Pohnert SC, Knight JB, Olson AL, Winder WW, Dohm GL. Regulation of muscle GLUT-4 transcription by AMP-activated protein kinase. J Appl Physiol. 2001;91:1073–1083. doi: 10.1152/jappl.2001.91.3.1073. [DOI] [PubMed] [Google Scholar]
- 49.McGee SL, van Denderen BJ, Howlett KF, Mollica J, Schertzer JD, Kemp BE, Hargreaves M. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes. 2008;57:860–867. doi: 10.2337/db07-0843. [DOI] [PubMed] [Google Scholar]
- 50.Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Current Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 52.Marsin AS, Bouzin C, Bertrand L, Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem. 2002;277:30778–30783. doi: 10.1074/jbc.M205213200. [DOI] [PubMed] [Google Scholar]
- 53.Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem J. 2004;381:561–579. doi: 10.1042/BJ20040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Neill HM, Lally JS, Galic S, Thomas M, Azizi PD, Fullerton MD, Smith BK, Pulinilkunnil T, Chen Z, Samaan MC, Jorgensen SB, et al. AMPK phosphorylation of ACC2 is required for skeletal muscle fatty acid oxidation and insulin sensitivity in mice. Diabetologia. 2014;57:1693–1702. doi: 10.1007/s00125-014-3273-1. [DOI] [PubMed] [Google Scholar]
- 55.Merrill GM, Kurth E, Hardie DG, Winder WW. AICAR decreases malonyl-CoA and increases fatty acid oxidation in skeletal muscle of the rat. Am J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 56.Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O'Neill HM, Ford RJ, Palanivel R, O'Brien M, Hardie DG, et al. Single phosphorylation sites in ACC1 and ACC2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 58.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1{alpha} Proc Natl Acad Sci USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD(+) metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Sung MM, Zordoky BN, Bujak AL, Lally JS, Fung D, Young ME, Horman S, Miller EJ, Light PE, Kemp BE, Steinberg GR, et al. AMPK deficiency in cardiac muscle results in dilated cardiomyopathy in the absence of changes in energy metabolism. Cardiovasc Res. 2015;107:235–245. doi: 10.1093/cvr/cvv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang S, Wang C, Yan F, Wang T, He Y, Li H, Xia Z, Zhang Z. N-Acetylcysteine attenuates diabetic myocardial ischemia reperfusion injury through inhibiting excessive autophagy. Mediators Inflamm. 2017;2017:9257291. doi: 10.1155/2017/9257291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol- 3-phosphate acyltransferase is a novel target. Biochem J. 1999;338:783–791. [PMC free article] [PubMed] [Google Scholar]
- 65.Hoppe S, Bierhoff H, Cado I, Weber A, Tiebe M, Grummt I, Voit R. AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply. Proc Natl Acad Sci USA. 2009;106:17781–17786. doi: 10.1073/pnas.0909873106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- 67.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 68.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5'-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 70.Coven DL, Hu X, Cong L, Bergeron R, Shulman GI, Hardie DG, Young LH. Physiologic role of AMP-activated protein kinase (AMPK) in the heart: graded activation during exercise. Am J Physiol. 2003;285:E629–E636. doi: 10.1152/ajpendo.00171.2003. [DOI] [PubMed] [Google Scholar]
- 71.Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 73.Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, Vanoverschelde JL, Ashworth A, Jovanovic A, Alessi DR, Bertrand L. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1. Am J Physiol Endocrinol Metab. 2006;290:E780–E788. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zarrinpashneh E, Carjaval K, Beauloye C, Ginion A, Mateo P, Pouleur AC, Horman S, Vaulont S, Hoerter J, Viollet B, Hue L, et al. Role of the alpha2-isoform of AMP-activated protein kinase in the metabolic response of the heart to no-flow ischemia. Am J Physiol Heart Circ Physiol. 2006;291:H2875–H2883. doi: 10.1152/ajpheart.01032.2005. [DOI] [PubMed] [Google Scholar]
- 75.Zarrinpashneh E, Beauloye C, Ginion A, Pouleur AC, Havaux X, Hue L, Viollet B, Vanoverschelde JL, Bertrand L. AMPK-alpha2 counteracts the development of cardiac hypertrophy induced by isoproterenol. Biochem Biophys Res Commun. 2008;376:677–681. doi: 10.1016/j.bbrc.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 76.Daniel T, Carling D. Functional analysis of mutations in the gamma 2 subunit of AMP-activated protein kinase associated with cardiac hypertrophy and Wolff-Parkinson-White syndrome. J Biol Chem. 2002;277:51017–51024. doi: 10.1074/jbc.M207093200. [DOI] [PubMed] [Google Scholar]
- 77.Akman HO, Sampayo JN, Ross FA, Scott JW, Wilson G, Benson L, Bruno C, Shanske S, Hardie DG, Dimauro S. Fatal infantile cardiac glycogenosis with phosphorylase kinase deficiency and a mutation in the gamma-2 subunit of AMP-activated protein kinase. Pediatr Res. 2007;62:499–504. doi: 10.1203/PDR.0b013e3181462b86. [DOI] [PubMed] [Google Scholar]
- 78.Burwinkel B, Scott JW, Buhrer C, van Landeghem FK, Cox GF, Wilson CJ, Hardie DG, Kilimann MW. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the g2 subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am J Hum Genet. 2005;76:1034–1049. doi: 10.1086/430840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thevenon J, Laurent G, Ader F, Laforet P, Klug D, Duva Pentiah A, Gouya L, Maurage CA, Kacet S, Eicher JC, Albuisson J, et al. High prevalence of arrhythmic and myocardial complications in patients with cardiac glycogenosis due to PRKAG2 mutations. Europace. 2016 doi: 10.1093/europace/euw067. [DOI] [PubMed] [Google Scholar]
- 80.Gollob MH, Seger JJ, Gollob TN, Tapscott T, Gonzales O, Bachinski L, Roberts R. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation. 2001;104:3030–3033. doi: 10.1161/hc5001.102111. [DOI] [PubMed] [Google Scholar]
- 81.Cheung PCF, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase g subunit isoforms and their role in AMP binding. Biochem J. 2000;346:659–669. [PMC free article] [PubMed] [Google Scholar]
- 82.Luptak I, Shen M, He H, Hirshman MF, Musi N, Goodyear LJ, Yan J, Wakimoto H, Morita H, Arad M, Seidman CE, et al. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest. 2007;117:1432–1439. doi: 10.1172/JCI30658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim M, Hunter RW, Garcia-Menendez L, Gong G, Yang YY, Kolwicz SC, Jr, Xu J, Sakamoto K, Wang W, Tian R. Mutation in the gamma2-subunit of AMP-activated protein kinase stimulates cardiomyocyte proliferation and hypertrophy independent of glycogen storage. Circ Res. 2014;114:966–975. doi: 10.1161/CIRCRESAHA.114.302364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dalle Pezze P, Ruf S, Sonntag AG, Langelaar-Makkinje M, Hall P, Heberle AM, Razquin Navas P, van Eunen K, Tolle RC, Schwarz JJ, Wiese H, et al. A systems study reveals concurrent activation of AMPK and mTOR by amino acids. Nat Commun. 2016;7:13254. doi: 10.1038/ncomms13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arad M, Moskowitz IP, Patel VV, Ahmad F, Perez-Atayde AR, Sawyer DB, Walter M, Li GH, Burgon PG, Maguire CT, Stapleton D, et al. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation. 2003;107:2850–2856. doi: 10.1161/01.CIR.0000075270.13497.2B. [DOI] [PubMed] [Google Scholar]
- 86.Yavari A, Stocker CJ, Ghaffari S, Wargent ET, Steeples V, Czibik G, Pinter K, Bellahcene M, Woods A, Martinez de Morentin PB, Cansell C, et al. Chronic activation of gamma2 AMPK induces obesity and reduces beta cell function. Cell Metab. 2016;23:821–836. doi: 10.1016/j.cmet.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang X, Mudgett J, Bou-About G, Champy MF, Jacobs H, Monassier L, Pavlovic G, Sorg T, Herault Y, Petit-Demouliere B, Lu K, et al. Physiological expression of AMPKgamma2RG mutation causes Wolff-Parkinson-White syndrome and induces kidney injury in mice. J Biol Chem. 2016;291:23428–23439. doi: 10.1074/jbc.M116.738591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 90.Milan D, Jeon JT, Looft C, Amarger V, Robic A, Thelander M, Rogel-Gaillard C, Paul S, Iannuccelli N, Rask L, Ronne H, et al. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science. 2000;288:1248–1251. doi: 10.1126/science.288.5469.1248. [DOI] [PubMed] [Google Scholar]
- 91.Costford SR, Kavaslar N, Ahituv N, Chaudhry SN, Schackwitz WS, Dent R, Pennacchio LA, McPherson R, Harper ME. Gain-of-function R225W mutation in human AMPKgamma3 causing increased glycogen and decreased triglyceride in skeletal muscle. PLoS ONE. 2007;2:e903. doi: 10.1371/journal.pone.0000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crawford SA, Costford SR, Aguer C, Thomas SC, deKemp RA, DaSilva JN, Lafontaine D, Kendall M, Dent R, Beanlands RS, McPherson R, et al. Naturally occurring R225W mutation of the gene encoding AMP-activated protein kinase (AMPK)gamma(3) results in increased oxidative capacity and glucose uptake in human primary myotubes. Diabetologia. 2010;53:1986–1997. doi: 10.1007/s00125-010-1788-7. [DOI] [PubMed] [Google Scholar]
- 93.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 94.Stahmann N, Woods A, Spengler K, Heslegrave A, Bauer R, Krause S, Viollet B, Carling D, Heller R. Activation of AMP-activated protein kinase by vascular endothelial growth factor mediates endothelial angiogenesis independently of nitric-oxide synthase. J Biol Chem. 2010;285:10638–10652. doi: 10.1074/jbc.M110.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, Choi HC, Zou MH. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 2010;121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bess E, Fisslthaler B, Fromel T, Fleming I. Nitric oxide-induced activation of the AMP-activated protein kinase alpha2 subunit attenuates IkappaB kinase activity and inflammatory responses in endothelial cells. PLoS One. 2011;6:e20848. doi: 10.1371/journal.pone.0020848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPK{alpha}2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo. Role of 26S proteasomes. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 99.Wang J, Alexanian A, Ying R, Kizhakekuttu TJ, Dharmashankar K, Vasquez-Vivar J, Gutterman DD, Widlansky ME. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arteriosclerosis Thromb Vasc Biol. 2012;32:712–720. doi: 10.1161/ATVBAHA.111.227389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 101.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Day RM, Lee YH, Han L, Kim YC, Feng YH. Angiotensin II activates AMPK for execution of apoptosis through energy-dependent and -independent mechanisms. Am J Physiol Lung Cell Mol Physiol. 2011;301:L772–L781. doi: 10.1152/ajplung.00072.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu X, Jhun BS, Ha CH, Jin ZG. Molecular mechanisms of ghrelin-mediated endothelial nitric oxide synthase activation. Endocrinology. 2008;149:4183–4192. doi: 10.1210/en.2008-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26:5933–5945. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J Biol Chem. 2007;282:20351–20364. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 106.Mount PF, Lane N, Venkatesan S, Steinberg GR, Fraser SA, Kemp BE, Power DA. Bradykinin stimulates endothelial cell fatty acid oxidation by CaMKK-dependent activation of AMPK. Atherosclerosis. 2008;200:28–36. doi: 10.1016/j.atherosclerosis.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 107.Yang S, Wang J. Estrogen activates AMP-activated protein kinase in human endothelial cells via ERbeta/Ca/calmodulin-dependent protein kinase kinase beta pathway. Cell biochemistry and biophysics. 2015;72:701–707. doi: 10.1007/s12013-015-0521-z. [DOI] [PubMed] [Google Scholar]
- 108.Reihill JA, Ewart MA, Salt IP. The role of AMP-activated protein kinase in the functional effects of vascular endothelial growth factor-A and -B in human aortic endothelial cells. Vasc Cell. 2011;3:9. doi: 10.1186/2045-824X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A, Avogaro A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27:2627–2633. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- 110.Rutherford C, Speirs C, Williams JJ, Ewart MA, Mancini SJ, Hawley SA, Delles C, Viollet B, Costa-Pereira AP, Baillie GS, Salt IP, et al. Phosphorylation of Janus kinase 1 (JAK1) by AMP-activated protein kinase (AMPK) links energy sensing to anti-inflammatory signaling. Sci Signal. 2016;9:ra109. doi: 10.1126/scisignal.aaf8566. [DOI] [PubMed] [Google Scholar]
- 111.Hwang HJ, Chung HS, Jung TW, Ryu JY, Hong HC, Seo JA, Kim SG, Kim NH, Choi KM, Choi DS, Baik SH, et al. The dipeptidyl peptidase-IV inhibitor inhibits the expression of vascular adhesion molecules and inflammatory cytokines in HUVECs via Akt- and AMPK-dependent mechanisms. Mol Cell Endocrinol. 2015;405:25–34. doi: 10.1016/j.mce.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 112.Krasner NM, Ido Y, Ruderman NB, Cacicedo JM. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS One. 2014;9:e97554. doi: 10.1371/journal.pone.0097554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun W, Lee TS, Zhu M, Gu C, Wang Y, Zhu Y, Shyy JY. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 114.Murakami H, Murakami R, Kambe F, Cao X, Takahashi R, Asai T, Hirai T, Numaguchi Y, Okumura K, Seo H, Murohara T. Fenofibrate activates AMPK and increases eNOS phosphorylation in HUVEC. Biochem Biophys Res Commun. 2006;341:973–978. doi: 10.1016/j.bbrc.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 115.Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- 116.Weikel KA, Cacicedo JM, Ruderman NB, Ido Y. Glucose and palmitate uncouple AMPK from autophagy in human aortic endothelial cells. Am J Physiol Cell Physiol. 2015;308:C249–C263. doi: 10.1152/ajpcell.00265.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 118.Heathcote HR, Mancini SJ, Strembitska A, Jamal K, Reihill JA, Palmer TM, Gould GW, Salt IP. Protein kinase C phosphorylates AMP-activated protein kinase alpha1 Ser487. Biochem J. 2016;473:4681–4697. doi: 10.1042/BCJ20160211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dagher Z, Ruderman N, Tornheim K, Ido Y. The effect of AMP-activated protein kinase and its activator AICAR on the metabolism of human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;265:112–115. doi: 10.1006/bbrc.1999.1635. [DOI] [PubMed] [Google Scholar]
- 120.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 121.Goirand F, Solar M, Athea Y, Viollet B, Mateo P, Fortin D, Leclerc J, Hoerter J, Ventura-Clapier R, Garnier A. Activation of AMP kinase alpha1 subunit induces aortic vasorelaxation in mice. J Physiol. 2007;581:1163–1171. doi: 10.1113/jphysiol.2007.132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song P, Wang S, He C, Liang B, Viollet B, Zou MH. AMPK-alpha2 deletion exacerbates neointima formation by upregulating Skp2 in vascular smooth muscle cells. Circ Res. 2011;109:1230–1239. doi: 10.1161/CIRCRESAHA.111.250423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Motobayashi Y, Izawa-Ishizawa Y, Ishizawa K, Orino S, Yamaguchi K, Kawazoe K, Hamano S, Tsuchiya K, Tomita S, Tamaki T. Adiponectin inhibits insulin-like growth factor-1-induced cell migration by the suppression of extracellular signal-regulated kinase 1/2 activation, but not Akt in vascular smooth muscle cells. Hypertens Res. 2009;32:188–193. doi: 10.1038/hr.2008.19. [DOI] [PubMed] [Google Scholar]
- 124.Ning J, Clemmons DR. AMP-activated protein kinase inhibits IGF-I signaling and protein synthesis in vascular smooth muscle cells via stimulation of insulin receptor substrate 1 S794 and tuberous sclerosis 2 S1345 phosphorylation. Mol Endocrinol. 2010;24:1218–1229. doi: 10.1210/me.2009-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gayard M, Guilluy C, Rousselle A, Viollet B, Henrion D, Pacaud P, Loirand G, Rolli-Derkinderen M. AMPK alpha 1-induced RhoA phosphorylation mediates vasoprotective effect of estradiol. Arterioscler Thromb Vasc Biol. 2011;31:2634–2642. doi: 10.1161/ATVBAHA.111.228304. [DOI] [PubMed] [Google Scholar]
- 126.Pyla R, Pichavaram P, Fairaq A, Park MA, Kozak M, Kamath V, Patel VS, Segar L. Altered energy state reversibly controls smooth muscle contractile function in human saphenous vein during acute hypoxia-reoxygenation: Role of glycogen, AMP-activated protein kinase, and insulin-independent glucose uptake. Biochem Pharmacol. 2015;97:77–88. doi: 10.1016/j.bcp.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110:444–451. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- 128.Kim JE, Choi HC. Losartan Inhibits vascular smooth muscle cell proliferation through activation of AMP-activated protein kinase. The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2010;14:299–304. doi: 10.4196/kjpp.2010.14.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ning J, Xi G, Clemmons DR. Suppression of AMPK activation via S485 phosphorylation by IGF-I during hyperglycemia is mediated by AKT activation in vascular smooth muscle cells. Endocrinology. 2011;152:3143–3154. doi: 10.1210/en.2011-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Son BK, Akishita M, Iijima K, Kozaki K, Maemura K, Eto M, Ouchi Y. Adiponectin antagonizes stimulatory effect of tumor necrosis factor-alpha on vascular smooth muscle cell calcification: regulation of growth arrest-specific gene 6-mediated survival pathway by adenosine 5'-monophosphate-activated protein kinase. Endocrinology. 2008;149:1646–1653. doi: 10.1210/en.2007-1021. [DOI] [PubMed] [Google Scholar]
- 131.Cao X, Li H, Tao H, Wu N, Yu L, Zhang D, Lu X, Zhu J, Lu Z, Zhu Q. Metformin inhibits vascular calcification in female rat aortic smooth muscle cells via the AMPK-eNOS-NO pathway. Endocrinology. 2013;154:3680–3689. doi: 10.1210/en.2013-1002. [DOI] [PubMed] [Google Scholar]
- 132.Siragusa M, Fleming I. The eNOS signalosome and its link to endothelial dysfunction. Pflugers Arch. 2016;468:1125–1137. doi: 10.1007/s00424-016-1839-0. [DOI] [PubMed] [Google Scholar]
- 133.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 134.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, Defea K, Pan S, Tsai MD, Shyy JY. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang S, Xu J, Song P, Viollet B, Zou MH. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 2009;58:1893–1901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mount PF, Hill RE, Fraser SA, Levidiotis V, Katsis F, Kemp BE, Power DA. Acute renal ischemia rapidly activates the energy sensor AMPK but does not increase phosphorylation of eNOS-Ser1177. Am J Physiol Renal Physiol. 2005;289:F1103–F1115. doi: 10.1152/ajprenal.00458.2004. [DOI] [PubMed] [Google Scholar]
- 137.Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120–127. [PubMed] [Google Scholar]
- 138.Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118:1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Colombo SL, Moncada S. AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J. 2009;421:163–169. doi: 10.1042/BJ20090613. [DOI] [PubMed] [Google Scholar]
- 140.Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, Shen YH. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun. 2010;396:199–205. doi: 10.1016/j.bbrc.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 141.Dong Y, Zhang M, Wang S, Liang B, Zhao Z, Liu C, Wu M, Choi HC, Lyons TJ, Zou MH. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes. 2010;59:1386–1396. doi: 10.2337/db09-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35:1782–1791. doi: 10.1093/eurheartj/ehu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Salt IP, Palmer TM. Exploiting the anti-inflammatory effects of AMP-activated protein kinase activation. Expert Opin Investig Drugs. 2012;21:1155–1167. doi: 10.1517/13543784.2012.696609. [DOI] [PubMed] [Google Scholar]
- 144.Ewart MA, Kohlhaas CF, Salt IP. Inhibition of tumor necrosis factor alpha-stimulated monocyte adhesion to human aortic endothelial cells by AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2008;28:2255–2257. doi: 10.1161/ATVBAHA.108.175919. [DOI] [PubMed] [Google Scholar]
- 145.Spiecker M, Peng HB, Liao JK. Inhibition of endothelial vascular cell adhesion molecule-1 expression by nitric oxide involves the induction and nuclear translocation of IkappaBalpha. J Biol Chem. 1997;272:30969–30974. doi: 10.1074/jbc.272.49.30969. [DOI] [PubMed] [Google Scholar]
- 146.Zhang Y, Qiu J, Wang X, Xia M. AMP-activated protein kinase suppresses endothelial cell inflammation through phosphorylation of transcriptional coactivator p300. Arterioscler Thromb Vasc Biol. 2011;31:2897–2908. doi: 10.1161/ATVBAHA.111.237453. [DOI] [PubMed] [Google Scholar]
- 147.Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, Wenzel P, Munzel T, Keaney JF., Jr Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118:1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mancini SJ, White AD, Bijland S, Rutherford C, Graham D, Richter EA, Viollet B, Touyz RM, Palmer TM, Salt IP. Activation of AMP-activated protein kinase rapidly suppresses multiple pro-inflammatory pathways in adipocytes including IL-1 receptor-associated kinase-4 phosphorylation. Mol Cell Endocrinol. 2017;440:44–56. doi: 10.1016/j.mce.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim SA, Choi HC. Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells. Biochem Biophys Res Commun. 2012;425:866–872. doi: 10.1016/j.bbrc.2012.07.165. [DOI] [PubMed] [Google Scholar]
- 150.He C, Li H, Viollet B, Zou MH, Xie Z. AMPK suppresses vascular inflammation in vivo by inhibiting Signal Transducer and Activator of Transcription-1. Diabetes. 2015;64:4285–4297. doi: 10.2337/db15-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zippel N, Malik RA, Fromel T, Popp R, Bess E, Strilic B, Wettschureck N, Fleming I, Fisslthaler B. Transforming growth factor-beta-activated kinase 1 regulates angiogenesis via AMP-activated protein kinase-alpha1 and redox balance in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:2792–2799. doi: 10.1161/ATVBAHA.113.301848. [DOI] [PubMed] [Google Scholar]
- 152.Xu MJ, Song P, Shirwany N, Liang B, Xing J, Viollet B, Wang X, Zhu Y, Zou MH. Impaired expression of uncoupling protein 2 causes defective postischemic angiogenesis in mice deficient in AMP-activated protein kinase alpha subunits. Arterioscler Thromb Vasc Biol. 2011;31:1757–1765. doi: 10.1161/ATVBAHA.111.227991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 154.Wang S, Liang B, Viollet B, Zou MH. Inhibition of the AMP-activated protein kinase-alpha2 accentuates agonist-induced vascular smooth muscle contraction and high blood pressure in mice. Hypertension. 2011;57:1010–1017. doi: 10.1161/HYPERTENSIONAHA.110.168906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schneider H, Schubert KM, Blodow S, Kreutz CP, Erdogmus S, Wiedenmann M, Qiu J, Fey T, Ruth P, Lubomirov LT, Pfitzer G, et al. AMPK dilates resistance arteries via activation of SERCA and BKCa channels in smooth muscle. Hypertension. 2015;66:108–116. doi: 10.1161/HYPERTENSIONAHA.115.05514. [DOI] [PubMed] [Google Scholar]
- 156.Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, et al. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res. 2005;97:837–844. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- 157.Song P, Wang S, He C, Liang B, Viollet B, Zou MH. AMPKalpha2 deletion exacerbates neointima formation by upregulating Skp2 in vascular smooth muscle cells. Circ Res. 2011;109:1230–1239. doi: 10.1161/CIRCRESAHA.111.250423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 158.Ding Y, Zhang M, Zhang W, Lu Q, Cai Z, Song P, Okon IS, Xiao L, Zou MH. AMP-activated protein kinase alpha 2 deletion induces VSMC phenotypic switching and reduces features of atherosclerotic plaque stability. Circ Res. 2016;119:718–730. doi: 10.1161/CIRCRESAHA.116.308689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Song P, Zhou Y, Coughlan KA, Dai X, Xu H, Viollet B, Zou MH. Adenosine monophosphate-activated protein kinase-alpha2 deficiency promotes vascular smooth muscle cell migration via S-phase kinase-associated protein 2 upregulation and E-cadherin downregulation. Arterioscler Thromb Vasc Biol. 2013;33:2800–2809. doi: 10.1161/ATVBAHA.113.301869. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 160.Cai Z, Ding Y, Zhang M, Lu Q, Wu S, Zhu H, Song P, Zou MH. Ablation of adenosine monophosphate-activated protein kinase alpha1 in vascular smooth muscle cells promotes diet-Induced atherosclerotic calcification in vivo. Circ Res. 2016;119:422–433. doi: 10.1161/CIRCRESAHA.116.308301. [DOI] [PubMed] [Google Scholar]
- 161.Cacicedo JM, Gauthier MS, Lebrasseur NK, Jasuja R, Ruderman NB, Ido Y. Acute exercise activates AMPK and eNOS in the mouse aorta. Am J Physiol Heart Circ Physiol. 2011;301:H1255–H1265. doi: 10.1152/ajpheart.01279.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ma L, Ma S, He H, Yang D, Chen X, Luo Z, Liu D, Zhu Z. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens Res. doi: 10.1038/hr.2010.11. [DOI] [PubMed] [Google Scholar]
- 163.Zhao CX, Xu X, Cui Y, Wang P, Wei X, Yang S, Edin ML, Zeldin DC, Wang DW. Increased endothelial nitric-oxide synthase expression reduces hypertension and hyperinsulinemia in fructose-treated rats. J Pharmacol Exp Ther. 2009;328:610–620. doi: 10.1124/jpet.108.143396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bradley EA, Eringa EC, Stehouwer CD, Korstjens I, van Nieuw Amerongen GP, Musters R, Sipkema P, Clark MG, Rattigan S. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside in the muscle microcirculation increases nitric oxide synthesis and microvascular perfusion. Arterioscler Thromb Vasc Biol. 2010;30:1137–1142. doi: 10.1161/ATVBAHA.110.204404. [DOI] [PubMed] [Google Scholar]
- 165.Ford RJ, Teschke SR, Reid EB, Durham KK, Kroetsch JT, Rush JW. AMP-activated protein kinase activator AICAR acutely lowers blood pressure and relaxes isolated resistance arteries of hypertensive rats. J Hypertens. 2012;30:725–733. doi: 10.1097/HJH.0b013e32835050ca. [DOI] [PubMed] [Google Scholar]
- 166.Ford RJ, Rush JW. Endothelium-dependent vasorelaxation to the AMPK activator AICAR is enhanced in aorta from hypertensive rats and is NO and EDCF dependent. Am J Physiol Heart Circ Physiol. 2011;300:H64–H75. doi: 10.1152/ajpheart.00597.2010. [DOI] [PubMed] [Google Scholar]
- 167.Bosselaar M, Boon H, van Loon LJ, van den Broek PH, Smits P, Tack CJ. Intra-arterial AICA-riboside administration induces NO-dependent vasodilation in vivo in human skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E759–E766. doi: 10.1152/ajpendo.00141.2009. [DOI] [PubMed] [Google Scholar]
- 168.Enkhjargal B, Godo S, Sawada A, Suvd N, Saito H, Noda K, Satoh K, Shimokawa H. Endothelial AMP-activated protein kinase regulates blood pressure and coronary flow responses through hyperpolarization mechanism in mice. Arterioscler Thromb Vasc Biol. 2014;34:1505–1513. doi: 10.1161/ATVBAHA.114.303735. [DOI] [PubMed] [Google Scholar]
- 169.Liang B, Wang S, Wang Q, Zhang W, Viollet B, Zhu Y, Zou MH. Aberrant endoplasmic reticulum stress in vascular smooth muscle increases vascular contractility and blood pressure in mice deficient of AMP-activated protein kinase-alpha2 in vivo. Arterioscler Thromb Vasc Biol. 2013;33:595–604. doi: 10.1161/ATVBAHA.112.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kohlstedt K, Trouvain C, Boettger T, Shi L, Fisslthaler B, Fleming I. AMP-activated protein kinase regulates endothelial cell angiotensin-converting enzyme expression via p53 and the post-transcriptional regulation of microRNA-143/145. Circ Res. 2013;112:1150–1158. doi: 10.1161/CIRCRESAHA.113.301282. [DOI] [PubMed] [Google Scholar]
- 171.Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51:2199–2206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- 172.Meijer RI, Bakker W, Alta CL, Sipkema P, Yudkin JS, Viollet B, Richter EA, Smulders YM, van Hinsbergh VW, Serne EH, Eringa EC. Perivascular adipose tissue control of insulin-induced vasoreactivity in muscle is impaired in db/db mice. Diabetes. 2013;62:590–598. doi: 10.2337/db11-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Almabrouk TA, Ugusman AB, Katwan OJ, Salt IP, Kennedy S. Deletion of AMPKα1 attenuates the anticontractile effect of perivascular adipose tissue (PVAT) and reduces adiponectin release. Br J Pharmacol. 2017 doi: 10.1111/bph.13633. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gaskin FS, Kamada K, Zuidema MY, Jones AW, Rubin LJ, Korthuis RJ. Isoform-selective 5'-AMP-activated protein kinase-dependent preconditioning mechanisms to prevent postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol. 2011;300:H1352–H1360. doi: 10.1152/ajpheart.00944.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li D, Wang D, Wang Y, Ling W, Feng X, Xia M. Adenosine monophosphate-activated protein kinase induces cholesterol efflux from macrophage-derived foam cells and alleviates atherosclerosis in apolipoprotein E-deficient mice. J Biol Chem. 2010;285:33499–33509. doi: 10.1074/jbc.M110.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen B, Li J, Zhu H. AMP-activated protein kinase attenuates oxLDL uptake in macrophages through PP2A/NF-kappaB/LOX-1 pathway. Vascul Pharmacol. 2016;85:1–10. doi: 10.1016/j.vph.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 177.Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 Activation: potential role in atherosclerosis. Diabetes. 2015;64:2028–2041. doi: 10.2337/db14-1225. [DOI] [PubMed] [Google Scholar]
- 178.Wang Q, Zhang M, Liang B, Shirwany N, Zhu Y, Zou MH. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PLoS One. 2011;6:e25436. doi: 10.1371/journal.pone.0025436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.UKPDS. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 180.Dai X, Ding Y, Liu Z, Zhang W, Zou MH. Phosphorylation of CHOP (C/EBP Homologous Protein) by the AMP-activated protein kinase alpha 1 in macrophages promotes CHOP degradation and reduces injury-induced neointimal disruption in vivo. Circ Res. 2016;119:1089–1100. doi: 10.1161/CIRCRESAHA.116.309463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li FY, Lam KS, Tse HF, Chen C, Wang Y, Vanhoutte PM, Xu A. Endothelium-selective activation of AMP-activated protein kinase prevents diabetes mellitus-induced impairment in vascular function and reendothelialization via induction of heme oxygenase-1 in mice. Circulation. 2012;126:1267–1277. doi: 10.1161/CIRCULATIONAHA.112.108159. [DOI] [PubMed] [Google Scholar]
- 182.Cao Q, Cui X, Wu R, Zha L, Wang X, Parks JS, Yu L, Shi H, Xue B. Myeloid deletion of alpha1AMPK exacerbates atherosclerosis in LDL receptor knockout (LDLRKO) mice. Diabetes. 2016;65:1565–1576. doi: 10.2337/db15-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Song Y, Wu Y, Su X, Zhu Y, Liu L, Pan Y, Zhu B, Yang L, Gao L, Li M. Activation of AMPK inhibits PDGF-induced pulmonary arterial smooth muscle cells proliferation and its potential mechanisms. Pharmacol Res. 2016;107:117–124. doi: 10.1016/j.phrs.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 184.Dean A, Nilsen M, Loughlin L, Salt IP, MacLean MR. Metformin reverses development of pulmonary hypertension via aromatase inhibition. Hypertension. 2016;68:446–454. doi: 10.1161/HYPERTENSIONAHA.116.07353. [DOI] [PubMed] [Google Scholar]
- 185.Omura J, Satoh K, Kikuchi N, Satoh T, Kurosawa R, Nogi M, Otsuki T, Kozu K, Numano K, Suzuki K, Sunamura S, et al. Protective roles of endothelial AMP-activated protein kinase against hypoxia-induced pulmonary hypertension in mice. Circ Res. 2016;119:197–209. doi: 10.1161/CIRCRESAHA.115.308178. [DOI] [PubMed] [Google Scholar]
- 186.Ibe JC, Zhou Q, Chen T, Tang H, Yuan JX, Raj JU, Zhou G. Adenosine monophosphate-activated protein kinase is required for pulmonary artery smooth muscle cell survival and the development of hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol. 2013;49:609–618. doi: 10.1165/rcmb.2012-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moral-Sanz J, Mahmoud AD, Ross FA, Eldstrom J, Fedida D, Hardie DG, Evans AM. AMP-activated protein kinase inhibits Kv 1.5 channel currents of pulmonary arterial myocytes in response to hypoxia and inhibition of mitochondrial oxidative phosphorylation. J Physiol. 2016;594:4901–4915. doi: 10.1113/JP272032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pinter K, Grignani RT, Czibik G, Farza H, Watkins H, Redwood C. Embryonic expression of AMPK gamma subunits and the identification of a novel gamma2 transcript variant in adult heart. J Mol Cell Cardiol. 2012;53:342–349. doi: 10.1016/j.yjmcc.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 190.Zadra G, Photopoulos C, Tyekucheva S, Heidari P, Weng QP, Fedele G, Liu H, Scaglia N, Priolo C, Sicinska E, Mahmood U, et al. A novel direct activator of AMPK inhibits prostate cancer growth by blocking lipogenesis. EMBO Mol Med. 2014;6:519–538. doi: 10.1002/emmm.201302734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Salatto CT, Miller RA, Cameron KO, Cokorinos E, Reyes A, Ward J, Calabrese M, Kurumbail R, Rajamohan F, Kalgutkar AS, Tess DA, et al. Selective Activation of AMPK b1-containing Isoforms Improves Kidney Function in a Rat Model of Diabetic Nephropathy. J Pharmacol Exp Ther. 2017 doi: 10.1124/jpet.116.237925. [DOI] [PubMed] [Google Scholar]
- 192.Gollob MH, Green MS, Tang AS, Gollob T, Karibe A, Ali Hassan AS, Ahmad F, Lozado R, Shah G, Fananapazir L, Bachinski LL, et al. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med. 2001;344:1823–1831. doi: 10.1056/NEJM200106143442403. [DOI] [PubMed] [Google Scholar]
- 193.Arad M, B DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Charron P, Genest M, Richard P, Komajda M, Pochmalicki G. A familial form of conduction defect related to a mutation in the PRKAG2 gene. Europace. 2007;9:597–600. doi: 10.1093/europace/eum071. [DOI] [PubMed] [Google Scholar]
- 195.Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, Salmon A, Ostman-Smith I, Watkins H. Mutations in the gamma-2 subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10:1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 196.Arad M, Maron BJ, Gorham JM, Johnson WH, Jr, Saul JP, Perez-Atayde AR, Spirito P, Wright GB, Kanter RJ, Seidman CE, Seidman JG. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med. 2005;352:362–372. doi: 10.1056/NEJMoa033349. [DOI] [PubMed] [Google Scholar]
- 197.Bayrak F, Komurcu-Bayrak E, Mutlu B, Kahveci G, Basaran Y, Erginel-Unaltuna N. Ventricular pre-excitation and cardiac hypertrophy mimicking hypertrophic cardiomyopathy in a Turkish family with a novel PRKAG2 mutation. Eur J Heart Fail. 2006;8:712–715. doi: 10.1016/j.ejheart.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 198.Xie C, Zhang YP, Song L, Luo J, Qi W, Hu J, Lu D, Yang Z, Zhang J, Xiao J, Zhou B, et al. Genome editing with CRISPR/Cas9 in postnatal mice corrects PRKAG2 cardiac syndrome. Cell Res. 2016;26:1099–1111. doi: 10.1038/cr.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Laforet P, Richard P, Said MA, Romero NB, Lacene E, Leroy JP, Baussan C, Hogrel JY, Lavergne T, Wahbi K, Hainque B, et al. A new mutation in PRKAG2 gene causing hypertrophic cardiomyopathy with conduction system disease and muscular glycogenosis. Neuromuscul Disord. 2006;16:178–182. doi: 10.1016/j.nmd.2005.12.004. [DOI] [PubMed] [Google Scholar]