Abstract
The diagnotic criteria for disorder of the executive function of the brain (DEFB) as a syndrome of sequela were administratively established (ad-DEFB) in Japan in 2006 to support disabled patients whose impairment, limited to cognition (memory, attention, execution, and behavior), emerges after organic brain injuries regardless of physical deficits. However, some patients suffering from traumatic brain injury (TBI) have been excluded from receiving medico-social services. In particular, this tendency is more prominent in patients with mild TBI because no lesions are apparent on conventional computed tomography (CT) or magnetic resonance imaging (MRI) in the chronic phase. Recent development of new MRI neuroimaging modalities and positron emission tomography (PET) imaging makes it possible to detect regions of minute organic lesions and metabolic dysfunction in the brain where organic lesions may be absent or cannot be detected on conventional CT or MRI. In this review, we discuss diagnostic criteria for mild TBI and ad-DEFB, the relationship between the two disorders, characteristic neuroimaging [(MRI and 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET)] of diffuse brain injury including cerebral concussion, which is the principal cause of mild TBI, and suggested pathological mechanisms of ad-DEFB in DBI.
Keywords: executive function of the brain, mild TBI, DBI, MRI, FDG-PET
Introduction
After suffering traumatic brain injury (TBI), there may be a substantial number of disabled people whose impairment is not recognized socially because their disabilities are limited to cognitive impairment without obvious physical deficits. Since 2006, the Japan Ministry of Health, Labour and Welfare (MHLW) has provided medico-social services for such patients diagnosed with an administratively established syndrome of disorder of executive function of the brain (ad-DEFB) as sequela.1–3) One of the diagnostic criteria for ad-DEFB is the confirmation of organic brain lesions (Table 1).2,3) Consequently, some TBI patients have been excluded from receiving services because no lesions were apparent on conventional computed tomography (CT) or magnetic resonance imaging (MRI) in the chronic phase. In particular, this tendency is more prominent in patients with mild TBI. Recent development of new MRI neuroimaging modalities and positron emission tomography (PET) imaging makes it possible to detect regions of minute organic lesions and metabolic dysfunction where organic lesions may be absent or have not been detected on conventional CT or MRI. Another issue that may prevent adequate support of patients with mild TBI is a poor understanding and confusion of diagnostic criteria for ad-DEFB and mild TBI, as well as the relationship between the two disorders. In this review, we discuss the diagnostic criteria of mild TBI and ad-DEFB, the relationship between the two disorders, characteristic neuroimaging (MRI and 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET)) of diffuse brain injury (DBI) including cerebral concussion, which is the principal cause of mild TBI, and suggested pathological mechanisms of ad-DEFB in DBI.
Table 1.
Diagnostic criteria for disorder of the executive function of the brain administratively defined by the Department of Health and Welfare for Persons with Disabilities, The Japan Ministry of Health, Labour and Welfare, and National Rehabilitation Center for Persons with Disabilities2,3)
|
Head Injury and Traumatic Brain Injury
Up to now, injury of the head due to trauma has been referred to as “head injury”. This medical term is generally recognized to mean organic or non-organic (functional) damage of the intra- and extra-cranial organs including the brain, scalp, and skull, and partly includes damage of the nerves, vessels, bone, and soft tissues of the neck due to a direct impact to the head.
On the other hand, there is another medical term “TBI”. These similar terms are often used interchangeably in the medical literature. However, there are some nuances between these terms. TBI refers to organic or non-organic (functional) damage of the brain regardless of direct or indirect impact to the head. These include injuries of the brain caused by indirect impact to the head; for example, impacts to the body or neck including whiplash injury and blast injury can also be included in TBI. However, simple injuries of the scalp, skull, extracranial vessels, nerves, and soft tissues are excluded in this category even if these are related to direct head impact.
For both categories of injury, evaluation of brain damage induced by impact is an important clinical goal for patient care. From this point of view, “TBI” is thought to be a more appropriate designation to be utilized than “head injury”. Advancement and propagation of CT and MRI have led the recent rapid progress toward more precise evaluation of acute brain pathology due to trauma. However, the clinical evaluation of the severity of brain damage still depends on an examination of the neurological status and the consciousness level of the patients.
Internationally, clinical severity in the acute phase of TBI generally can be divided into the following three grades according to the Glasgow Coma Scale (GCS)4); mild TBI (GCS score: 13–15 points), moderate TBI (GCS score: 9–12 points), and severe TBI (GCS score: 3–8 points).5–7)
Diagnostic criteria of mild TBI were first advocated by the TBI Committee of the American Congress of Rehabilitation Medicine (ACRM) in 1993 (Table 2).8) Eleven years after the ACRM definition was published, the World Health Organization (WHO) Collaborative Center Task Force on Mild TBI published a comprehensive review of definitions utilized in research studies and identified significant definition discrepancies.9) According to these publications, a GCS score of 13–15 points is necessary but not sufficient for a mild TBI diagnosis. We do not know what diagnosis should be made for cases with a GCS score of 13–15 points, but with no loss of consciousness, loss of memory for events immediately before or after accident, alteration in mental state at the time of the accident, nor focal neurologic deficits that may or may not be transient (this may be called “super mild TBI”). We should note that “mild TBI” is just a medical term used as a clinical diagnosis made in the acute phase, but does not depend on whether brain damage is organic or functional, and additionally whether symptomatic sequelae are present or absent afterward.
Table 2.
Diagnostic criteria for mild TBI by the American Congress of Rehabilitation Medicine Special Interest Group on Mild Traumatic Brain Injury8)
A traumatically induced physiological disruption of brain function, as manifested by at least one of the following:
But where the severity of the injury does not exceed the following: loss of consciousness of approximately 30 min or less after 30 min, an initial Glasgow Coma Scale score of 13–15, and post-traumatic amnesia not greater than 24 hr This definition includes:
It excludes stroke anoxia, tumor, encephalitis, etc. CT, MRI, electroencephalogram, or routine neurological evaluations may be normal. Due to the lack of medical emergency, or the realities of certain medical systems, some patients may not have the above factors medically documented in the acute stage. In such cases, it is appropriate to consider symptomatology that, when linked to a traumatic head injury, can suggest the existence of a mild TBI. |
In the United States of America (USA), about 1,700,000 patients are diagnosed with TBI every year, about 75% of which correspond to mild TBI, while about 30% of mild TBI patients are suffering symptomatic sequelae for several months to years in the chronic phase.10)
In the USA, the number of patients with disorders of cognition, attention, emotion, motivation, and behavior as sequelae following mild TBI caused by a war injury including blast injury and sports (for example, football, wrestling, and boxing) injury have increased in recent years, leading to social problems that cannot be overlooked. In terms of war-related injury, the US government budgeted $300 million in 2008 for investigations into the pathophysiology of TBI disorders, as well as treatment and care of patients.11) In Japan, no epidemiological investigation of mild TBI has been performed, and the number of affected patients is not known precisely, although injuries related to traffic accidents and sports are likely to predominate in mild TBI cases.
Diagnosis of Disorder of Executive Function of the Brain
In 2006, the Japan MHLW launched a comprehensive administration support system project for patients with ad-DEFB who have four major cognitive symptoms including memory, attention, execution, and behavior dysfunction due to organic brain damage types such as head injury, cerebrovascular disease, hypoxic encephalopathy, and radiation injury.1) Based on this support project, patients with ad-DEFB are issued a Certificate for Mental Disability and receive medico-social services (life/medical care, reduction/exemption from taxes and medical fees, and job training) to achieve independence in Japanese society.1,2)
The Japan MHLW and National Rehabilitation Center for Persons with Disabilities administratively established a diagnostic criteria of ad-DEFB to execute this support system by regarding ad-DEFB as a syndrome of sequela (Table 1).2,3) To use this criteria, the following two important points should be noted.
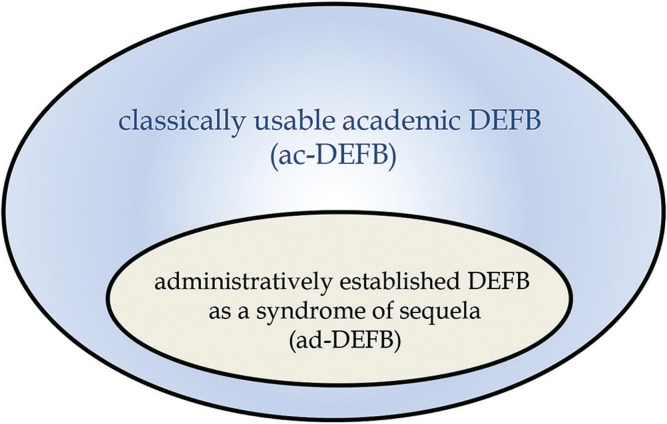
First, the term ad-DEFB is different from a classically recognized academic term, DEFB (ac-DEFB) (Fig. 1). The major distinctions of ad-DEFB from ac-DEFB are that the diagnosis of ad-DEFB should be made 6 months or more after brain injury with a necessity of the existence of organic brain damage demonstrated by neuroimaging, and that patients with pathologies caused by congenital disease, perinatal brain damages, developmental disorders, or progressive diseases have to be eliminated in the diagnostic criteria.2,3) Thus, there are double standard definitions of DEFB in Japan at the present time, and we should pay attention to the differences at the time of discussion of DEFB to avoid confusion.
Fig. 1.
The relationship between classically usable academic disorder of executive function of the brain (DEFB) (ac-DEFB) and administratively established DEFB as a syndrome of sequela (ad-DEFB) by The Japan Ministry of Health, Labour and Welfare and National Rehabilitation Center for Persons with Disabilities.
Second, clinical symptoms of DBI have been intentionally incorporated into these diagnostic criteria. So far, clinical symptoms due to higher cortical dysfunction associated with local brain injury (LBI) including cerebral contusion, such as aphasia, agnosia, apraxia, amnesia, disorientation, poor judgement, character change, poor abstract thought, apathy, and disconnection syndromes, have been predominantly taken into consideration for diagnosis of ac-DEFB. On the other hand, DBI symptoms, in which the pathological essence is axonal damage, causing disruptions in information transmission among the cerebral neocortex, basal ganglia, and limbic system, have been underestimated. These symptoms include not only cognitive symptoms like an impairment of memory, but also disorders of attention, execution, emotion, and behavior that cannot be easily explained as the brain focal signs mentioned above. The diagnostic criteria of ad-DEFB make it feasible to attend to symptomatic patients with DBI but without focal traumatic lesions on conventional neuroradiology, who have been overlooked medically and socially so far.
Ad-DEFB in Mild TBI
Regardless of organic or non-organic brain damage, chronic sequelae that are related to mild TBI include disturbances in motor/sensory function, taste, smell, swallowing, and urination, in addition to nausea, tinnitus, dizziness, vertigo, headache, fatigue, sleeplessness, photophobia, apathy, depression, anxiety, euphoria, restlessness, and cognitive dysfunction. Symptoms related to memory, attention, executive function, and behavioral dysfunction that are defined as major symptoms of ad-DEFB in Japan are also included.
In terms of the administrative support for patients with ad-DEFB launched by The Japan MHLW, any patients with TBI (including mild TBI) who fulfill the diagnostic criteria for ad-DEFB are candidates. However, even after the enforcement of this support project, a substantial number of patients with mild TBI may have been overlooked and may have not been diagnosed with ad-DEFB, due to the following reasons. Symptoms in patients with mild TBI are relatively mild in the acute phase. This is a reason why no medical examinations using CT and/or MRI may have been performed in the acute phase. Another reason is anosognosia, often associated with cognitive dysfunction, which may impede patients from visiting hospitals. Finally, this is the most likely reason, some patients may have been treated as suffering from posttraumatic stress disorder, posttraumatic depression, or other psychiatric disorders in the chronic phase in case of no observable lesion on conventional CT and/or MRI.
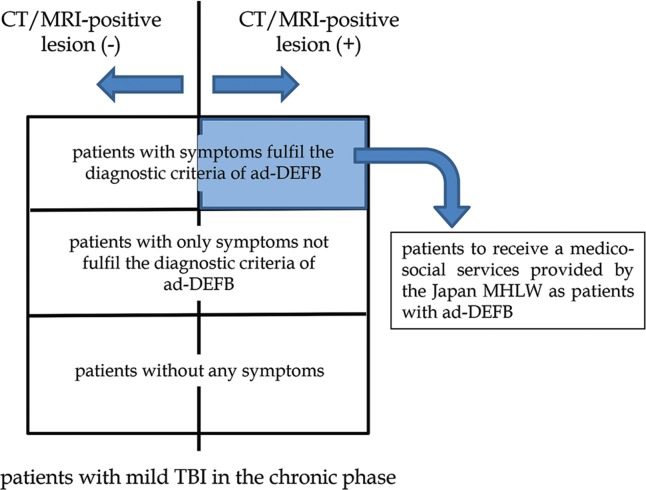
It is our mission to improve administrative support for patients suffering from mild TBI who may have missed prior support because no diagnosis of ad-DEFB has been made. To carry out this mission, first, we should correctly evaluate the clinical symptoms of patients and recognize characteristic pathologies responsible for damages observed on neuroimaging in patients with organic brain lesions due to TBI. Correct diagnosis of ad-DEFB based on these clinical and neuro-radiological findings is needed (Fig. 2).
Fig. 2.
Classification of patients with mild traumatic brain injury (TBI) in the chronic phase. ad-DEFB = administratively established disorder of executive function of the brain as a syndrome of sequela; MHLW = Ministry of Health, Labour and Welfare.
Organic Brain Lesions in Mild TBI
Concussion-related pathophysiology is evident in the acute phase of the great majority of patients with mild TBI, and the clinical symptoms seen in the chronic phase often correspond to post-concussion syndrome.12) TBI is commonly classified into two categories based on the localization and pathogenic mechanism of injury, which are LBI and DBI. Cerebral concussion used to be attributed to brain stem damage, while now it is thought caused by DBI. At present, cerebral concussion is categorized as a mild type of DBI, which is described by a loss of consciousness less than 6 hours.13) Cerebral concussion is defined as a clinical syndrome characterized by immediate and transient post-traumatic impairment of neural functions without any organic brain lesions.5) However, it has long been known that there may be a pathology (termed punch drunk) with diffuse brain atrophy caused by repeated cerebral concussion, in which cognitive impairment emerges during the chronic phase.14)
In 2011, Omalu et al.15) investigated 17 autopsied brains (9 accident, 5 suicide, and 3 natural death) and verified tau-positive neurofibrillary changes corresponding to chronic traumatic encephalopathy (CTE) in 10 (71%) of 14 professional athletes (8 American football, 4 wrestling, 1 boxing, and 1 martial arts athlete) and 1 (33%) of 3 American high school football players who had very likely received repetitive impact to the brain and had shown cognitive, behavioral, and emotional impairments while they were alive. Additionally, Omalu et al.16) reported a case of a 27-year-old veteran of CTE with tau-positive neurofibrillary changes in the autopsied brain, who had received a repetitive blast injury in a war, but no history of direct head injury. He had been suffering memory, behavioral, and emotional impairments after an honorable discharge from the US Marine Corps, and committed suicide 8 months after retirement.
At present, CTE is considered to be a progressive tauopathy that occurs as a consequence of repetitive mild TBI. However, there has been no pathological evidence as to whether organic brain lesions including CTE can emerge after a single mild TBI like a cerebral concussion caused by direct or non-direct head injury (such as whiplash injury). In CTE, it is unclear how much head trauma is causative, what type, and how frequent, in addition to the age when patients are most susceptible, and whether some individuals are genetically more prone than others. CTE may be regarded as a spectrum of disease; if so, even a single small cerebral concussion may produce CTE.17)
The presence of axonal retraction balls, axonal swelling, micro-bleeding, and aggregation of microglia can often be observed histopathologically in the cerebral white matter, subcortical zone, corpus callosum, cerebellum, cerebellar peduncle, and brain stem during the acute/subacute phases of diffuse axonal injury (DAI), which is defined as a severe type of DBI.18) Furthermore, degeneration of long tracts, such as the pyramidal tract and spinothalamic tract, can also be seen in the chronic phase.18) The pathological mechanism of cerebral concussion, a mild type of DBI, is regarded as a shearing stress of the brain due to roll acceleration impact similar to DAI, and if so, there may exist organic brain lesions, axonopathy, in cerebral concussions in the same brain site as DAI.19) Normally, tau protein is most abundant in the axons. Although the pathological relationship between DAI and CTE is still not fully elucidated, DAI can be a leading source candidate for CTE. Axonopathy following cerebral concussion may lead to the aberrant production and/or aggregation of tau.20)
To elucidate minute organic brain lesions due to TBI in living patients, meticulous studies using incisive neuroimaging are needed. First, the characteristics of organic brain lesions (where and how originated) in patients with DAI in the chronic phase should be evaluated to identify organic brain lesions in mild TBI patients with cerebral concussion in the chronic phase. In the following, we summarize recent knowledge and characteristic findings using diagnostic neuroimaging in patients with DAI in the chronic phase, presenting neuro-radiological findings obtained at our institute using MRI and FDG-PET.
Neuroimaging of DBI
Structural MRI
We can evaluate organic brain lesions and the related morphological changes caused by TBI using T1 weighted imaging (T1WI), T2 weighted imaging (T2WI), and fluid attenuated inversion recovery (FLAIR) imaging taken in widespread standard MRI in the chronic phase. The most common neuro-radiological finding due to DBI in the chronic phase is diffuse brain atrophy. Opening of the sulcus and dilatation of the ventricles can suggest the presence of diffuse brain atrophy. Generally, diffuse brain atrophy is prominent according to the severity of DBI. Local marked atrophy of the corpus callosum, fornix, and brain stem, which are predisposed to be injured in DBI, is another characteristic finding in this clinical entity. However, severity of brain atrophy is strongly affected by age and past pathological histories of patients, and, furthermore, there can be individual differences within the same age category. For a correct evaluation of brain atrophy caused by DBI, it is important to compare CT/MRI obtained in the chronic phase with those obtained in the acute phase in the same patients.
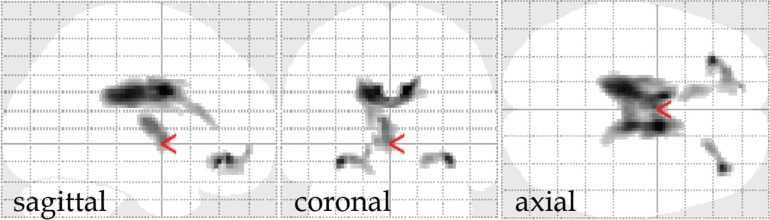
It is difficult to detect minute intracranial hemorrhages that have been seen in the acute phase of head injury on ordinary CT/MRI in the chronic phase. However, T2* weighted imaging (T2*WI) and susceptibility weighted imaging (SWI) can visualize those remnant hemorrhages as abnormal low signal intensity areas by sensitively visualizing hemosiderin in the chronic phase.21,22) Hemosiderin can often be seen in the corpus callosum, basal ganglia, and deep white matter in the cerebral hemisphere in cases of DBI (Fig. 3). Geurts et al.23) compared the sensitivity of visualizing hemosiderin on imaging among T2WI, FLAIR, T2*WI, and SWI in 56 patients with TBI in the chronic phase. In the results of the study, the sensitivity of T2WI was the same as FLAIR. However, the sensitivity of T2*WI was five times superior to that of FLAIR, and, furthermore, the sensitivity of SWI was 2 times superior to that of T2*WI.
Fig. 3.
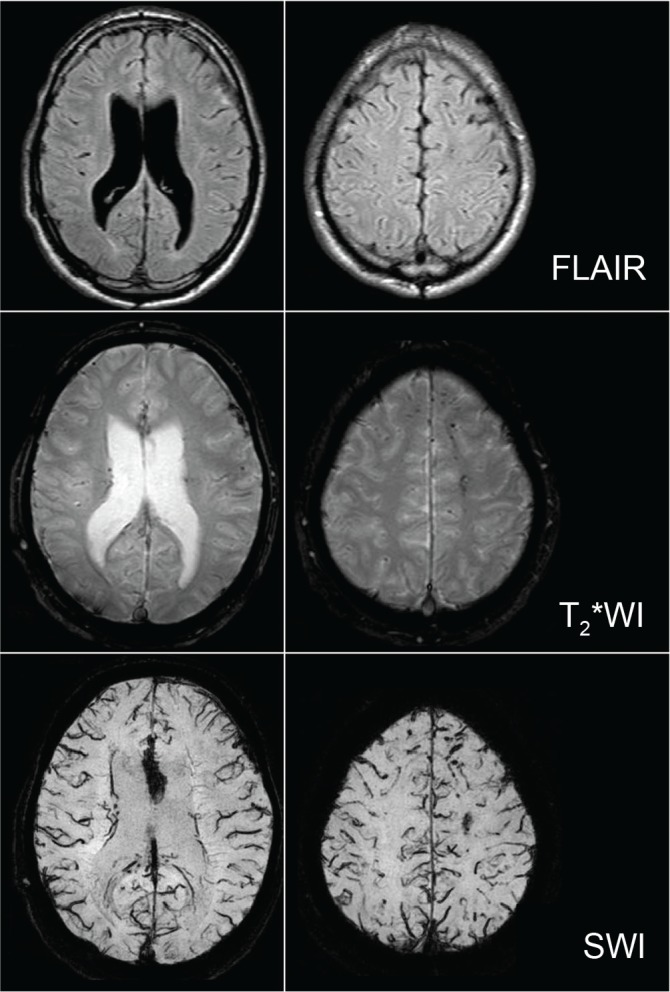
MRI of a patient with severe diffuse brain injury in the chronic phase. Hemosiderin can be well visualized on T2* weighted imaging (T2*WI) and susceptibility weighted imaging (SWI) compared to fluid attenuated inversion recovery (FLAIR) imaging. Especially, hemosiderin in the corpus callosum and cerebral white matter can be more conspicuously seen on SWI.
Diffusion-tensor imaging (DTI)
Diffusion-tensor imaging (DTI) is an MRI technique that uses anisotropic diffusion of water coexisting along the axonal fibers in the brain. The axonal organization of the brain can thus be estimated by DTI. Based on fractional anisotropy (FA) value, which shows the degree of anisotropy of the coexisting water, an FA map can be produced from DTI. Regions with decreased FA, which are regarded as those with organic lesions of injured axons, can be detectable in the brain by calculating FA values in the region of interest (ROI) on an FA map. A significant decrease in FA values can commonly be seen in the corpus callosum, fornix, coronal radiation, centrum semiovale, and cingulum in cases of DBI.24) Decreases in FA values in the corticospinal tract were reported to positively correlate with decreases in motor evoked potentials caused by transcranial magnetic stimulation. Such evidence shows that FA values may be useful for the evaluation of not only morphological changes, but also functional changes in the brain.25)
Fiber tractography (FT), which is an imaging obtained by using a 3D reconstruction technique to access neural tracts on FA maps on top of data collected by DTI, can make it easy to evaluate axonal disruption anatomically in the ROI. The fibers of the corpus callosum, cingulum, superior longitudinal fasciculus, corticospinal tract, and optic radiation can be easily depicted on FT. Injured tracts can be elucidated in terms of sparsity or disruption, and evaluated in terms of damage by visually comparing to a normal control (or normal hemisphere) in patients (Fig. 4). However, in such an image analysis, there are shortcomings in terms of possible arbitrary determination of ROI and threshold of FA value, and unavailability of data from regions other than the ROI. To make up for these shortcomings, statistical image analysis using statistical parametric mapping (SPM) to account for the whole brain in comparison with FA values from a normal control is performed. From this, the corpus callosum and cingulum were elucidated to be the most common regions with significantly decreased FA values in patients with DBI in a group analysis of DTI compared with normal controls.23) This result shows that these anatomical regions are the most vulnerable in the brain of patients with DBI. Recently, regions with decreased FA values can be depicted in each patient by individual analyses (FA-SPM imaging).26) Even minute regions with significantly decreased FA values in patients with mild/moderate TBI, in whom lesions have been missed on T2*WI, can be detectable. FA-SPM imaging is often considered for evaluating brain lesions in DBI cases (Fig. 5).
Fig. 4.
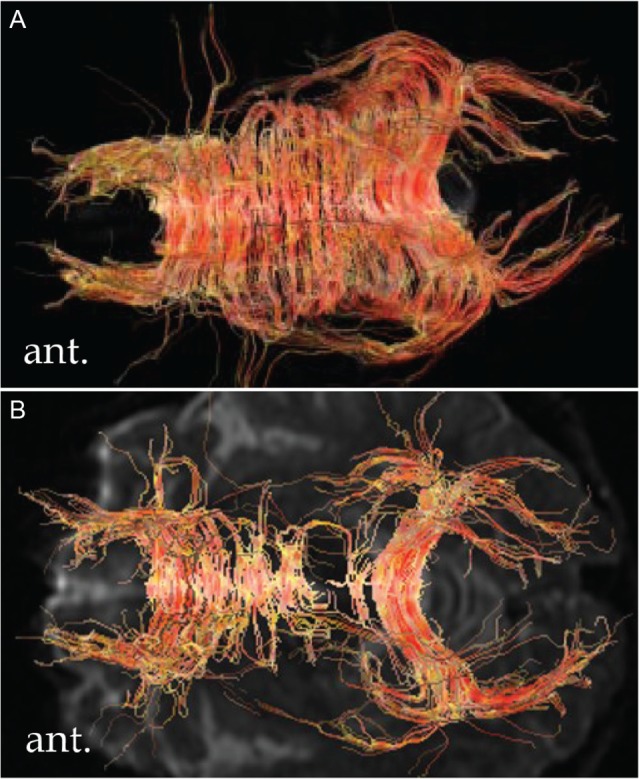
Fiber tractography of the corpus callosum. The number of neuronal fibers in the body of the corpus callosum is decreased in a patient with diffuse brain injury in the chronic phase (B) compared to a normal control (A).
Fig. 5.
FA-SPM imaging of a patients with diffuse axonal injury in the chronic phase. Regions with significantly decreased fractional anisotropy (FA) values (black shadows in the brain, p < 0.001) compared to the normal control can be seen in the corpus callosum, bilateral frontal lobe base, and right medial thalamus.
Another parameter of axonal damage on DTI is mean diffusivity (MD), which indicates the degree of diffusion of water around the axonal fibers. Even if axons are partly injured, bundles of axons still likely remain and may maintain isotropy in some cases of TBI. In these cases, FA values may not be affected; however, increases in water diffusion caused by partial axonal injury can be sensitively shown as an increase in MD value.27) MD value changes due to minute axonal injury in patients with post-concussion syndrome are reported to be more sensitive than FA value changes.28)
We present three clinical papers with high evidence levels published over the last 5 years, which describe radiological changes in the brain using DTI in patients with mild TBI. MacDonald et al.29) reported FA value abnormalities using DTI performed within 90 days after mild TBI due to blast injury in 63 personnel engaged in battles in Iraq and Afghanistan. In their report, significantly decreased FA values could be seen in the bilateral middle cerebellar peduncle, bilateral cingulum, and right pars orbitals white matter in the inferior frontal gyrus compared with 21 personnel who had suffered blast injuries but had not been diagnosed with mild TBI due to the absence of symptoms after injury. Additionally, in 47 of the 63 personnel with mild TBI who could undergo DTI, a significantly decreased FA value could be observed in the same anatomical regions 6–12 months later. These results show that even an indirect head injury such as mild TBI due to blast injury may be responsible for axonal injury, although it is still unclear whether the pathological mechanism of blast injury is related to a shearing stress of the brain due to roll acceleration impact. A meta-analysis including 10 ROI studies and 10 whole brain studies on DTI of the brain in patients with mild TBI published between 1980 and 2012 was performed by Aoki et al.30) They concluded that significantly decreased FA values could be seen in the splenium of the corpus callosum in patients with mild TBI compared with normal controls from the results of ROI studies. However, there were no significant differences in FA values in any brain regions between patients with mild TBI and normal controls in the results of whole brain studies. In a systematic review by Gardner et al.31) including 8 clinical papers on DTI of the brain in patients with cerebral concussion due to sports-related injury published between 2003 and 2012, 7 papers concluded that some kind of abnormal signal intensity lesions could be verified in various sites of the brain in those patients.
Recently, DTI studies using tract-based spatial statistics (TBSS), which is a statistical image analysis with a potential of more sophisticated normalization of white matter fibers compared with SPM, have been presented. TBSS is considered to be more reliable in the evaluation of axonal fibers versus SPM. In 3 of 4 papers using group analysis of whole brain DTI using TBSS, significantly decreased FA values or significantly increased AD values could be seen in the brains of patients with mild TBI compared with normal controls.27,28,32,33) These results from DTI studies conclude that some patients with mild TBI may have organic brain lesions due to impacts to the brain.
FDG-PET
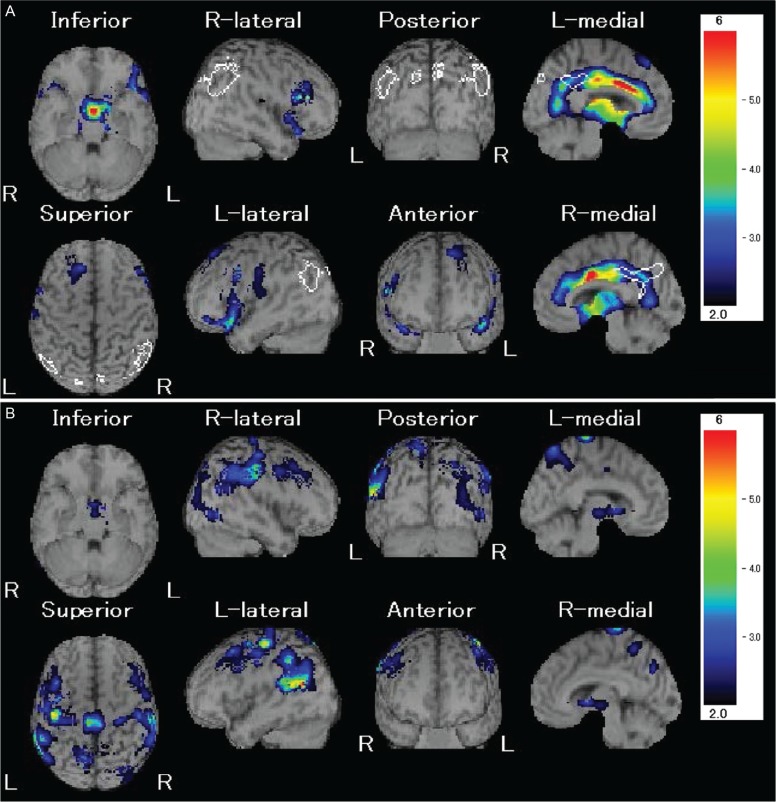
Application of SPM analysis on PET imaging made it easy in that brain regions with significantly decreased FDG metabolism in patients with severe TBI compared with normal controls could be depicted via PET. The results of group analysis of FDG-PET using SPM reported by Nakayama et al.34) demonstrated that significantly decreased FDG metabolism could be seen in the cingulum, medial prefrontal regions, frontal base, and thalamus bilaterally in patients with DBI in the chronic phase compared with normal controls. Additionally, this study verified that these are the brain regions commonly impaired in FDG metabolism in multiple groups of patients suffering from vegetative state, minimally consciousness state, and ad-DEFB due to severe TBI, and that the areas of decreased FDG metabolism are extended according to clinical severity (Fig. 6).3) These results show that the cingulum, medial prefrontal regions, frontal base, and thalamus are, characteristically, the most vulnerable regions in DBI.
Fig. 6.
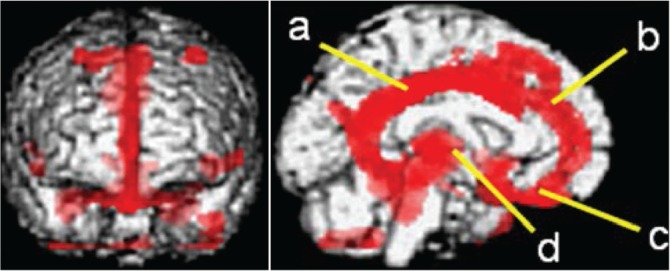
FDG-PET of a patient with diffuse axonal injury in the chronic phase (group analysis using SPM). Significant decrease of FDG metabolism (red areas, p < 0.001) can be seen in the cingulum (a), medial prefrontal region (b), frontal base (c), and thalamus (d) bilaterally compared to the normal control.
In a clinical setting, statistical image analysis using the easy Z score imaging system (eZIS) has been widely used in PET analyses to evaluate individual brain FDG metabolism in patients with brain disorders in Japan. This image analysis is useful to judge whether each DBI-suggestable patient in the chronic phase has the same metabolic impairment pattern on FDG-PET as the typical pattern seen in patients with DBI, in which hypometabolism is characteristically represented in the cingulum, medial prefrontal regions, frontal base, and thalamus.
One study aimed to determine the differences in brain glucose metabolism between groups of mild/moderate TBI (m/m TBI) patients with and without visible traumatic brain lesions based on MRI, in either of which group scores of cognitive tests were lower than those of the normal control group, although there were no significant score differences between the patient groups. FDG uptake on PET was significantly lower in the cingulum and the medial thalamus in the group of patients with visible lesions compared with the group of patients without visible lesions, and lower in the medial thalamus compared with the control group. However, there was no significant difference in FDG metabolism between the group of patients without visible lesions and normal controls.35) These results show that, even in m/m TBI, in patients with MRI-positive TBI lesions, brain regions with FDG hypometabolism are almost the same as the typical regions characteristically seen in patients with severe TBI, and also suggest that different pathological mechanisms of cognitive impairment may be present in m/m TBI patients with and without MRI-positive TBI lesions (Fig. 7).
Fig. 7.
Differences of decreased FDG metabolism on FDG-PET between a patient with diffuse brain injury who has MRI-positive lesions in the brain (A) and a patient who has no MRI-positive lesions in the brain (B) (eZIS analysis). Relative decrease of FDG metabolism (colored area) compared to the normal control can be seen bilaterally in the cingulum, medial prefrontal, frontal base, and thalamus in a patient with MRI-positive lesions. However, that (colored area) can be seen mainly in the convexity of the parietal and occipital lobes in a patient without MRI-positive lesions.
Pathological Mechanism of DEFB in DBI
Ordinary structural MRI and DTI demonstrate that organic brain lesions associated with DBI are consistently represented in the corpus callosum, fornix, basal ganglia, and deep white matter in the cerebral hemisphere. Axonal injury in these regions is strongly suggested to cause disruptions in a mutual network for information transmission among the cerebral neocortex, basal ganglia, and limbic system and to result in ad-DEFB. On the other hand, functional neuroimaging techniques like FDG-PET consistently demonstrate a hypometabolism in the cingulum, medial prefrontal regions, frontal base, and thalamus in patients with DBI. These regions may represent secondary lesions with functional damage affected by dysfunction of information transmission originating with axons injured in the primary structural lesions mentioned above rather than as primary lesions.
Conclusions
There are highly likely a substantial number of patients with mild TBI who have still not been given any administrative welfare and medical services because of an absence of ad-DEFB diagnosis in Japan. The presence of organic lesions in the brain remains a crucial factor for such diagnosis in Japan. In TBI, MRI-positive lesions always represent organic lesions; however, organic lesions may not be necessarily represented as MRI-positive lesions. Among cases without visible lesions in ordinary MRI, there can be cases with some detectable minute lesions observable via DTI. Furthermore, in cases without positive lesions in ordinary MRI or DTI, there may be strongly suggestive cases of ad-DEFB if their FDG-PET imaging show a typical hypometabolic pattern characteristic to those seen in FDG-PET imaging in cases of DAI. In these cases, some organic minute lesions may be represented in the brain, which could not be seen in MRI. Advancement of these new neuroimaging technologies will hopefully be advantageous for the correct diagnosis of ad-DEFB in patients with mild TBI.
Footnotes
Conflicts of Interest Disclosure
The authors have no conflicts of interest with regard to this manuscript. All authors, who are members of The Japan Neurosurgical Society (JNS), have registered Self-reported COI Disclosure Statement Forms online through the website for JNS members.
References
- 1). Nakajima Y: Construction of support network system for persons with disorders of executive function of the brain in regional society in Japan. Report of research summary in 2006–2008. Research Project of Mental Health and Science Financially Supported by The Japan Ministry of Health, Labour and Welfare. 2009. (Japanese) [Google Scholar]
- 2). Nakajima Y: Diagnosis of higher brain dysfunction after traumatic brain injury. No Shinkei Geka 39: 731– 742, 2011. (Japanese) [PubMed] [Google Scholar]
- 3). Department of Health and Welfare for Persons with Disabilities. The Japan Ministry of Health, Labour and Welfare. National Rehabilitation Center for Persons with Disabilities : Handbook for support of persons with disorders of executive function of the brain, 2nd ed., 2008. (Japanese) [Google Scholar]
- 4). Teasdale G, Jennett B: Assessment of coma and impaired consciousness. A practical scale. Lancet 2: 81– 84, 1974. [DOI] [PubMed] [Google Scholar]
- 5). Gabriel EM, Turner DA: Minor head injury Management and outcome. In Wilkins RH , Rengachary SS , ed., Neurosurgery Vol. 3 2nd ed., New York, McGraw-Hill, 1996, pp 2723– 2726 [Google Scholar]
- 6). Jennett B: Epidemiology of head injury. Arch Dis Child 78: 403– 406, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Parikh S, Koch M, Narayan RK: Traumatic brain injury. Int Anesthesiol Clin 45: 119– 135, 2007. [DOI] [PubMed] [Google Scholar]
- 8). The Mild Traumatic Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine : Definition of mild traumatic brain injury. J Head Traum Rehabil 8: 86– 87, 1993. [Google Scholar]
- 9). Caroll LJ, Cassidy JD, Holm L, Kraus J, Coronado VG; WHO collaborating centre task force on mild traumatic brain injury: methodological issue and research recommendations for mild traumatic brain injury: the WHO collaborating centre task force on mild traumatic brain injury. J Rehabili Med 43 Suppl, 113– 125, 2004. [DOI] [PubMed] [Google Scholar]
- 10). Newswise of Albert Einstein College of Medicine of Yeshiva University : Researchers find evidence that brain compensates after traumatic injury. http://www.newswise.com/articles/view/596393 , 2012. Access 2012.11.26
- 11). Zoroya G: Pentagon spends $300M to study troop’ stress, trauma. http://abcnews.go.com/Health/MindMoodNews/story?id=5517802&page=1 , 2008. Access 2008.8.5
- 12). Dean PJ, O’Neill D, Sterr A: Post-concussion syndrome: prevalence after mild traumatic brain injury in comparison with a sample without head injury. Brain Inj 26: 14– 26, 2012. [DOI] [PubMed] [Google Scholar]
- 13). Le Roux PD, Choudhri H, Andrews BT: Cerebral concussion and diffuse brain injury. In Cooper PR, Golfinos JG, ed., Head Injury 4th ed., New York, McGraw–Hill, 2000, pp 175– 199 [Google Scholar]
- 14). Martland HS: Punch drunk. JAMA 91: 1103– 1107, 1928. [Google Scholar]
- 15). Omalu B, Bailes J, Hamilton RL, Kamboh MI, Hammers J, Case M, Fitzsimmons R: Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery 69: 173– 183, 2011. [DOI] [PubMed] [Google Scholar]
- 16). Omalu B, Hammers J, Bailes J, Hamilton RL, Kamboh MI, Webster G, Fitzsimmons RP: Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg Focus 31: E3, 2011. [DOI] [PubMed] [Google Scholar]
- 17). McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, Lee HS, Wojtowicz SM, Hall G, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC: The spectrum of disease in chronic traumatic encephalopathy. Brain 136: 43– 64, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Teasdale G, Mathew P: Mechanisms of cerebral concussion, contusion, and other effects of head injury. In Youmans JR , ed., Neurological Surgery 4th ed., Philadelphia, Saunders Company, 1996, pp 1533– 1548 [Google Scholar]
- 19). Levin H, Smith D: Traumatic brain injury: networks and neuropathology. Lancet Neurol 12: 15– 16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Smith DH: Neuromechanics and pathophysiology of diffuse axonal injury in concussion. Bridge 46: 79– 84, 2016. [PMC free article] [PubMed] [Google Scholar]
- 21). Metting Z, Rödinger LA, De Keyser J, De Keyser J, van der Naalt J: Structural and functional neuroimaging in mild–to–moderate head injury. Lancet Neurol 6: 699– 710, 2007. [DOI] [PubMed] [Google Scholar]
- 22). Van Boven RW, Harrington GS, Hackney DB, Ebel A, Gauger G, Bremner JD, D’Esposito M, Detre JA, Haacke EM, Jack CR, Jr, Jagust WJ, Le Bihan D, Mathis CA, Mueller S, Mukherjee P, Schuff N, Chen A, Weiner MW: Advances in neuroimaging of traumatic brain injury and posttraumatic stress disorder. J Rehabil Res Dev 46: 717– 757, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Geurts BH, Andriessen TM, Goraj BM, Vos PE: The reliability of magnetic resonance imaging in traumatic brain injury lesion detection. Brain Inj 26: 1439– 1450, 2012. [DOI] [PubMed] [Google Scholar]
- 24). Nakayama N, Okumura A, Shinoda J, Yasokawa YT, Miwa K, Yoshimura SI, Iwama T: Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry 77: 850– 855, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Yasokawa Y, Shinoda J, Okumura A, Nakayama N, Miwa K, Iwama T: Correlation between diffusion-Tensor magnetic resonance imaging and motor-evoked potential in chronic severe diffuse axonal injury. J Neurotrauma 24: 163– 173, 2007. [DOI] [PubMed] [Google Scholar]
- 26). Asano Y, Shinoda J, Okumura A, Aki T, Takenaka S, Miwa K, Yamada M, Ito T, Yokoyama K: Utility of fractional anisotropy imaging analyzed by statistical parametric mapping for detecting minute brain lesions in chronic-stage patients who had mild or moderate traumatic brain injury. Neurol Med Chir (Tokyo) 52: 31– 40, 2012. [DOI] [PubMed] [Google Scholar]
- 27). Cubon VA, Putukian M, Boyer C, Dettwiler A: A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma 28: 189– 201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Messé A, Caplain S, Pélégrini-Issac M, Blancho S, Montreuil M, Lévy R, Lehéricy S, Benali H: Structural integrity and postconcussion syndrome in mild traumatic brain injury patients. Brain Imaging Behav 6: 283– 292, 2012. [DOI] [PubMed] [Google Scholar]
- 29). Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R, Flaherty SF, Brody DL: Detection of blast-related traumatic brain injury in US military personnel. N Eng J Med 364: 2091– 2100, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Aoki Y, Inokuchi R, Gunshin M, Yahagi N, Suwa H: Diffusion tensor imaging studies of mild traumatic brain injury: a meta-analysis. J Neurol Neurosurg Psychiatry 83: 870– 876, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Gardner A, Kay-Lambkin F, Stanwell P, Donnelly J, Williams WH, Hiles A, Schofield P, Levi C, Jones DK: A systematic review of diffusion tensor imaging findings in sports-related concussion. J Neurotrauma 29: 2521– 2538, 2012. [DOI] [PubMed] [Google Scholar]
- 32). Wada T, Asano Y, Shinoda J: Decreased fractional anisotropy evaluated using tract-based spatial statistics and correlated with cognitive dysfunction in patients with mild traumatic brain injury in the chronic stage. AJNR Am J Neuroradiol 33: 2117– 2122, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Zhang K, Johnson B, Penell D, Ray W, Sebastianelli W, Slobounov S: Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Exp Brain Res 204: 57– 70, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Nakayama N, Okumura A, Shinoda J, Nakashima T, Iwama T: Relationship between regional cerebral metabolism and consciousness disturbance in traumatic diffuse brain injury without large focal lesions: an FDG-PET study with statistical parametric mapping analysis. J Neurol Neurosurg Psychiatry 77: 856– 862, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Ito K, Asano Y, Ikegame Y, Shinoda J. Differences in brain metabolic impairment between chronic mild/moderate TBI patients with and without visible brain lesions based on MRI. Biomed Res Int 2016; 2016:3794029 [DOI] [PMC free article] [PubMed] [Google Scholar]