Abstract
Background:
Methamphetamine (MA) was shown to have harmful effects on male reproductive system.
Objective:
To investigate probable effects of daily administration of MA on sperm parameters and chromatin/DNA integrity in mouse.
Material and Methods:
Thirty-five NMRI male mice were divided into five groups including low, medium, and high dosage groups which were injected intraperitoneally with 4, 8 and 15 mg/kg/day for 35 days, respectively. Normal saline was injected in sham group and no medications were used in control group. Then, the mice were killed and caudal epididymis of each animal was cut and placed in Ham’s F10 medium for sperm retrieval. To evaluate sperm chromatin abnormalities, the aniline blue, toluidine blue and chromomycine A3 were used. For sperm DNA integrity and apoptosis, the acridine orange, sperm chromatin dispersion, and TUNEL assay were applied. For sperm morphology, Papanicolaou staining was done
Results:
Normal morphology and progressive motility of spermatozoa decreased in medium and high dosage groups in comparison with the control group (p=0.035). There was a significant increase in rate of aniline blue, toluidine blue, and chromomycine A3 positive spermatozoa in high dosage group. In a similar manner, there was an increase in rates of acridine orange, TUNEL and sperm chromatin dispersion positive sperm cells in high dosage group with respect to others.
Conclusion:
MA abuse in a dose-dependent manner could have detrimental effects on male reproductive indices including sperm parameters and sperm chromatin/DNA integrity in mice.
Key Words: Methamphetamine, Apoptosis, Sperm, Sperm chromatin
Introduction
In recent years, methamphetamine (MA) has been attractive and road drug in several countries, because of its quite easy manufacture and low price versus to another drugs (1). MA is an illegal psycho stimulant drug profitable to amphetamine type (2). MA is a strongly addictive drug with a high probability of addition which is absorbed slowly for a long period of time, consequently (for 8-24 hr) (3). It is usually used by young and teenagers who are in the age of reproduction (4). In fact, young population experience MA for fun and improvement of sexual function at first times (5). Earlier, many studies proved negative effects of morphine and cocaine, but today consumption of synthetic drugs including amphetamines or MA is increased in developed and developing countries (6-8). The exact mechanism by which MA leads to male reproductive system dysfunctions is not completely understood. There are many studies indicating the deleterious effects of MA on reproductive organs (9). Experimental studies on rodents suggest some mechanisms of MA action on male fertility potential including altered hormonal profiles, oxidative stress, DNA damage of spermatozoa, and abnormal spermatogenesis (10, 11). It is also demonstrated that MA adversely affects on seminiferous epithelium including degeneration and apoptosis of germ cells (12). It is also suggested that MA affects male reproductive function at multiple levels due to its effects on the endocrine control of spermatogenesis (13, 14). Recently, it has been reported that MA decreases normal sperm morphology and count, as well as increase apoptotic cells in seminiferous tubules (15, 16). In a study conducted by Zuloaga et al, histopathological and histomorphometric alterations in seminiferous tubules have been reported in MA-treated animals (17).
Evaluation of sperm nuclear chromatin is a noticeable approach for male fertility investigations. During spermatogenesis, sperm chromatin is compacted more and more due to histones replacement at first by testis-specific nuclear proteins, then by transitional proteins and finally by protamines (18). Disulphide bonds of protamine molecules are crucial for sperm nuclear compaction and stabilization. It is believed that this kind of nuclear compaction protects sperm genome from damages including oxidative stress, elevated temperature and acid-induced DNA denaturation (19). Oxidative stress (OS) is considered as an important cause of male infertility leading to an increase in sperm DNA fragmentation. Imbalance between reactive oxygen species (ROS) production and semen antioxidant ability results OS (20). There is increasing confirmation that one mechanism of MA toxicity is the production of ROS (21). It is generally accepted that ROS affects sperm chromatin condensation and also may have harmful effects on sperm motility, morphology and fertilization ability (22).
To the best of our knowledge, there are no study that investigated the effects of MA on sperm chromatin condensation and DNA integrity. Therefore, we designed the present study to investigate the effects of different doses of MA on sperm count, motility, and morphology and sperm chromatin integrity in male mouse as an experimental model.
Materials and methods
In this experimental study, 12 wk old NMRI male mice (28±2 gr) were maintained in standard cages under controlled standard animal house conditions (room temperature 23±2oC, humidity 60±10% and 12 hr light/dark cycle) before and during experiments. They were fed with normal pellet diet and water adlibitum.
Drug administration
The MA suspension was prepared with the concentration of 4, 8 and 15 mg/kg in normal saline as low, medium and high doses respectively (23). After one week of acclimating, the mice were divided into five groups (n=7 for each group) including: low, medium and high dosage groups which were injected intraperitoneally with 4, 8 and 15 mg/kg/day MA for 35 days (more than a period of spermatogenesis of mice), respectively (24). Normal saline was injected daily in sham group, but, the control mice did not receive any medication. At the end of experiment, the animals were euthanized by cervical dislocation and the caudal part of right epididymis of each mouse was cut and transferred into a petri dish 60 mm (Falcon- 353037, USA) containing one ml of HamsʼF10 medium. Then, epididymis was disposed and spermatozoa suspension was incubated for 30 min in 37oC with 5% CO2 to let the spermatozoa swim out (25).
Spermatozoa parameters
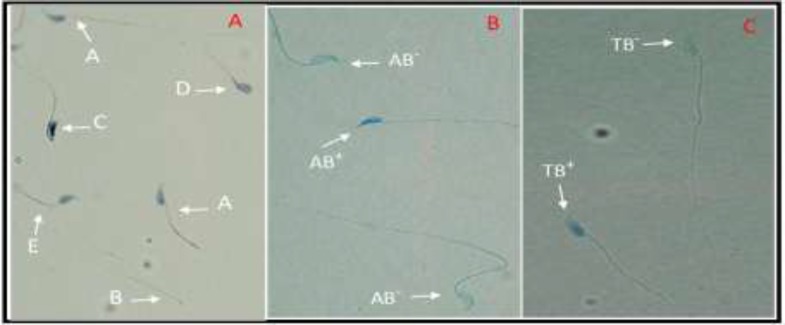
To get total sperm count, 10 µl of sperm suspension was loaded on the Makler counting chamber (Sefi medical instrument Ltd., Israel) and number of spermatozoa in a strip of 10 squares was multiplied to 106 which indicated spermatozoa concentration in millions/ml. The percentages of progressive (fast and slow movements), non-progressive and immotile spermatozoa were calculated (26). For assessment of sperm morphology, the Papanicolaou staining was done. Briefly, the smears were fixed by ethanol-ether (1:1) for 5 min and then they were stained with PAP staining solutions according to WHO guidelines (27). Different forms of sperm morphology were determined including normal, double head, pin head, amorphous head, coiled mid piece, coiled tail, bent tail, and cytoplasmic droplet (Figure 1-A).
Figure 1.
A) Papanicolaou staining: Normal (A), pin head (B), amorphous head (C), bent tail (D), coiled mid piece (E) forms. B) AB staining: Sperm head with immature nuclear chromatin is intense dark blue (AB+) and sperm head with mature nuclei is light blue (AB-). TB staining: sperm cells with normal chromatin are light blue (TB-) and abnormal chromatin is dark blue (TB+) (×1000 magnification).
Acrosome reaction (AR)
The AR was assessed by double staining method. In this assay, washed spermatozoa were fixed in glutaraldehyde 3% in PBS for 30 min. The smears were prepared after two times washing (2500 rpm, 2 min). The slides were stained with Bismarck brown (0.8% in deionized water, pH 1.8) for 10 min and then with Rose Bengal (0.8% in 0.1 M Tris buffer, pH 5.3) for 25 min. After washing, smears were dehydrated in ethanol series and rinsed in xylene (28). Red or pink staining of the acrosomal region determined as acrosome-intact spermatozoa.
Assessment of sperm chromatin/DNA integrity
Aniline blue staining (AB)
AB staining is a cytochemical assay for detection of remained histones in the process of sperm chromatin remodeling (29). The air-dried smears were fixed in a solution of 3% glutaraldehyde in 0.2M phosphate buffer, (14 ml of 10.2 M NaH2PO4 plus 36 ml of 0.2 M Na2HPO4, pH=7.2) for 30 min. Then, they were stained with solution of 5% AB in 4% acetic acid (pH=3.5) for 5 min (25). In this staining, the spermatozoa with unstained nucleus are considered as normal and spermatozoa with dark blue nuclei are counted as abnormal ones (Figure 1-B).
Toluidine blue (TB) staining
The air-dried smears were fixed in a solution of ethanol-acetone (1:1) at 4oC for 30 min. Hydrolysis of smears was performed by HCl (0.1 molar) for 5 min. Then, TB dye solution (0.05% TB in 50% Mcilvaine’s citrate phosphate buffer at pH 3.5) was used for 10 min. Finally, the slides were rinsed in distilled water and dehydrated with ethanol and xylene at room temperature for 3 min (30). Spermatozoa with normal chromatin are seen colorless but sperm cells with mild, medium and sever chromatin abnormality were seen in dark blue, violet and purple respectively (Figure 1-C).
Chromomycine A3 (CMA3) staining
CMA3 staining was used for indirect assessment of protamine deficiency. To do this assay, the smear of each sample was fixed in Carnoy’s solution (methanol and glacial acetic acid, 3:1) for 10 min. Then, they were treated with 100 µl of CMA3 solution for 10 min and washed with Mcilvaine buffer (7 ml of 0.1 M citric acid plus 32.9 ml of 0.2 M Na2HPO4×7H2O plus10 mM MgCl2), (PH= 7) (31). The prepared slides were evaluated under fluorescent microscope with 460-470 nm filter. The bright yellowish spermatozoa were considered as positive CMA3, while others without brightness were considered as negative CMA3 with normal protamine.
Terminal deoxynucleotidyltransferase mediated d-UTP nick end labeling assay (TUNEL)
The TUNEL kit was used to detect sperm DNA fragmentation according to manufacture protocol. Briefly, the slides were fixed with 4% paraformaldehyde for 1 hr at room temperature, and then they were washed three times with PBS, before treating with 3% H2O2 in methanol. In the next step, they were immersed in 0.1% triton X-100 in 0.1% sodium citrate for 5 min. After rinsing with PBS, the slides were treated with 5 µl enzyme solution plus 45 µl label solution and incubated for 1 hr and then evaluated by fluorescence microscope (Olympus BX51, Japan) (32).
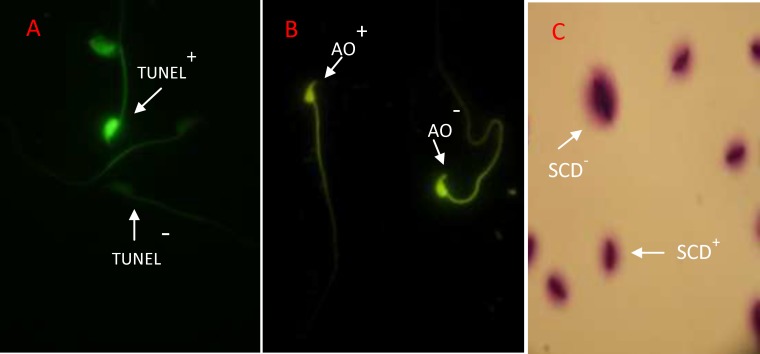
DNase I grade I (3 U/ml in 50 mM Tris-HCl, pH 7.5, 1 mg/ml BSA) was used to determine positive control. For negative controls, instead of the TUNEL reaction mixture, slides were incubated with 50 µl of label solution (without terminal transferase). The apoptotic cells with DNA fragmentation exhibited intensive and brilliant florescent green (TUNEL+ or apoptotic sperm), while the normal cells displayed pale and opaque green (TUNEL- or non-apoptotic sperm) (Figure 2-A). The apoptotic sperm cells were presented as percentage in each sample.
Figure 2.
A) TUNEL assay: Positive apoptosis cells is brilliant florescent green (TUNEL+) and negative apoptosis cells is pale and opaque green (TUNEL-). B) Acridine Orange test: positive AO cells are yellow (AO+) and negative cells with normal DNA exhibited in green color (AO-). C) Sperm chromatin dispersion (SCD) test: Big halo represents no DNA fragmentation (SCD-) and no halo and small halo show sperm with DNA fragmentation (SCD+) (×1000 magnification
Acridine orange test (AO)
This assay can differentiate the natural double strand DNA from denaturized single strand DNA in sperm nuclei. The air-dried smears were fixed by Carnoy’s solution (methanol-acetic acid, 3:1) overnight. After washing, they were treated with AO fluorescence solution (0.19 mg of AO in citrate phosphate buffer) for 10 min (30). In the evaluation of slides under fluorescence microscope (640 nm filter) the sperm cells with normal DNA were seen bright green, while abnormal spermatozoa with single stranded DNA were visualized in bright red or yellow color (Figure 2-B).
Sperm chromatin dispersion assay (SCD)
This assay is used for detection of sperm DNA damage. For SCD test, 30 µl of washed spermatozoa was diluted with 70 µl of 1% agarose and then 50 µl of the mixture was loaded on a slide which was coated by 0.65% agarose, covered with a coverslip and placed on a cold plate for 4 min. Then, coverslip was removed and slide was embedded in 0.08 NHCl solution at dark room. Each slide was immersed in lysis solutions 1 and 2 sequentially. The time of lysis solution 1 (0.4 M Tris, 2-Mercaptoethanol, 1% SDS, and 50 mM EDTA, pH 7.5) was 30 min, and the time of lysis solution 2 (0.4 M Tris, 2 M NaCl, and 1% SDS, pH 7.5) was 30 min.
Then, the slides were rinsed in Trisborate- EDTA buffer (0.09 M Tris-borate and 0.002 M EDTA, pH 7.5) for 27 min and then they were dehydrated in increasing concentrations of ethanol. Finally, each slide was rinsed in wright stain for 10 min. The small, medium and large halos around sperm heads were determined in comparison with core width of spermatozoa. The small halo showed high DNA fragmentation and the medium and large ones showed moderate and without DNA fragmentation in spermatozoa respectively (33) (Figure 2-C).
Ethical consideration
All protocols were performed according to the national institute of health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). The used protocols and animals’ ethics were approved by the research council of ethical committee of Yazd research and clinical center for infertility and Islamic Azad University of Ashkhezar, Yazd, Iran.
Statistical analysis
For statistical analysis, we used Statistical Package for the Social Sciences, version 19.0, SPSS Inc, Chicago, Illinois, USA (SPSS). ANOVA and Kruskall-Wallis tests were used for comparison of the data between different groups. The data have expressed as median (min-max) and the differences were statistically significant at p<0.05.
Results
Sperm parameters
According to table I, although, the sperm count showed a decreasing trend in medium and high dosage groups when compared to low dosage and control one, but it was not statistically significant. Injection of MA decreased the rate of progressive motility in medium and high dosage groups but not in low dosage group (p=0.867 and p=0.289, respectively; Table I). Also, non-progressive motility and immotile spermatozoa were significantly increased in medium and high dosage groups in comparison with control and low dosage groups (p>0.05, Table I). Normal morphology was significantly decreased in medium and high dosage groups compared to other groups.
Table I.
Effect of different doses of MA on sperm parameters and AR in mice
| Groups | Control | Low dosage | Medium dosage | High dosage | p-value |
|---|---|---|---|---|---|
| Sperm parameters | |||||
| Count (×106/ml) | 16 (15-37) | 16.5 (6-21) | 14 (3-19) | 13.50 (11-22) | 0.175 |
| Progressive motility (%) | 46 (30-61) | 31 (7-35) | 21 (18-40) | 20 (4-37) | 0.035 |
| Non-progressive motility (%) | 16 (15-37) | 24 (18-35) | 35 (25-42) | 39 (15-45) | 0.000 |
| Immotile sperm (%) | 38 (23-49) | 37 (25-67) | 39.50 (35-58) | 55.50 (28-67) | 0.009 |
| Normal morphology (%) | 85 (80-88) | 70 (66-72) | 66 (60-70) | 64 (62-66) | 0.003 |
| AR (%) | 91 (90-99) | 79 (9-89) | 76 (74-85) | 66.50 (50-75) | 0.017 |
Data are shown as median (min- max).
MA: Methamphetamine
AR: Acrosome reaction
Sperm chromatin and apoptosis evaluations
There was a significant increase in rate of AB+ and TB+ spermatozoa in high dosage group in comparison with others. In addition, the rate of CMA3+ sperm cells in medium and high dosage groups showed a significant increase when compared with control group. In similar case, a significant difference was observed between high dosage group and others in AO, TUNEL and SCD (p=0.000, p=0.001 and p=0.007 respectively) (Table II).
Table II.
Effect of different doses of MA on sperm nuclear integrity and apoptosis
|
Groups
Chromatin sperm assays |
Control | Low dosage | Medium dosage | High dosage | p-value |
|---|---|---|---|---|---|
| AB | 15 (12-18) | 15 (14-16) | 15.50 (13-18) | 20 (14-17) | 0.009 |
| TB | 13.50 (5-20) | 13.50 (12-16) | 13.50 (10-20) | 22.50 (14-24) | 0.007 |
| CMA3 | 23 (2-43) | 23.50 (19-36) | 29.50 (12-37) | 31.50 (12-34) | 0.000 |
| AO | 31 (27-43) | 33 (1-55) | 34 (28-55) | 40.50 (32-51) | 0.000 |
| TUNEL | 6.50 (2-17) | 5 (3-6) | 9 (7-11) | 14.50 (12-17) | 0.001 |
| SCD | 21 (16-33) | 22 (18-27) | 21 (16-25) | 26 (21-33) | 0.007 |
Data are shown as median (min- max) as percentage.
Discussion
Due to the high prevalence of MA abuse in young populations especially in men who are in reproductive period, the present study was planned to estimate the specific deleterious effects of this central nervous system stimulant as an addictive drug on sperm parameters and chromatin integrity in male mice. The results showed that MA decreases sperm concentration, progressive motility and increases abnormal sperm morphology in a dose-dependent manner.
Moreover, sperm chromatin packaging and apoptosis could be influenced by different dose of MA. The effects of MA on sperm chromatin also appear to be dose-depended as similar drugs which had more negative effects in high doses. Our findings were similar to the study by Alavi and colleagues which have demonstrated that frequent consumption of high dosage of MA may reduce the number of sperm in the epididymis tail and has negative effects on reproductive system in male rats (34). Additionally, several experimental studies showed that administration of different dose of MA caused decreasing in the sperm count and normal morphology (35, 36).
On the other hand, Lin and colleagues reported many defects in testicular tissue and spermatogenesis cell lines including the elevation of apoptotic cells and destructive changes in seminiferous tubules in male rats which were treated by MA. In addition, they presented that sperm count and motility were decreased in MA administration animals (37). It is generally accepted that in male fertility evaluations, the standard routine sperm analysis is not enough, because it does not show different forms of sperm chromatin and DNA damages. So, in the present study, we focused on molecular and cytochemically-based assays to determine the effects of MA on sperm chromatin condensation and apoptosis in mouse as a good experimental model. To the best our knowledge, this was the first study using AO, CMA3, AB, TB and SCD tests for the above mentioned aims. It is shown that there is a positive relationship between sperm chromatin defects and cell death or apoptosis (38). Similar to our study, Yamamoto and colleagues reported that administration of MA reduces the spermatogenesis and increases apoptosis in spermatogonial cell line in rat (36).
Additionally, Alavi and his colleagues demonstrated that repeated administration of MA in male rats enhances apoptosis of spermatogonia and primary spermatocytes in seminiferous tubules. In fact, they discussed that decreasing in sperm count and normal morphology and apoptotic cell increasing in seminiferous tubules may be due to the MA toxicity in sperm germ line and testicular tissue (34). Another recent study has reported a reduction of reproductive ability in animals that were administered by MA (39). Also, in a sex hormones assessment, it has been suggested that any alternation in serum testosterone during the MA treatment can affect the spermatogenesis and it has a main role in activation of apoptosis in spermatogonial cells (40).
Although in present study we can not report any evidence about the mechanism of harmful characteristics of MA on gonadal hormones and risky effect on spermatogenesis, but it could be mentioned that reduction in sperm chromatin quality was dependent on toxic effects of MA and hormonal profile defects. Recently, it was shown that MA can induce apoptosis in testicular germ cells and epididymal spermatozoa in laboratory animals (35).
AB and CMA3 are two main tests for identifying sperm chromatin packaging and protamine deficiency of spermatozoa (25). It is confirmed that the lower amounts of nuclear protamines affect the sperm chromatin packaging and as a result the sperm quality (41). Our results showed that the MA increases the rate of spermatozoa with excessive histones (AB+) and protamine deficiency (CMA3+) and these effects are dose-dependent.
Our findings also showed that the rate of AO+ spermatozoa was increased in high dose of MA administration. So, we can say that the MA may induce sperm apoptosis through DNA denaturation. In TB assay which is used for detection of sperm DNA fragmentation and chromatin packaging quality, we showed that the rate of TB+ spermatozoa was significantly higher in high dose MA administration than other groups (42).
In a same way, our data of TUNEL and SCD tests showed a significant increase in sperm apoptosis in high dose of MA administration. The present study was also the first study to the evaluation of the sperm acrosome in MA administration animals. There was a significant difference between the percentage of spermatozoa with intact acrosomes in experimental and control groups. To explain the probable mechanism of MA action on sperm parameters and chromatin/DNA integrity, we should note that there are some data indicating the increase of ROS in the sperm that exposed to ecstasy (43). On the other hand, studies suggested that ROS attack the integrity of sperm DNA causing base modifications, DNA strand breaks, and chromatin cross-linking (44, 45). In this view, we hypothesized that the main mechanism of sperm chromatin/DNA integrity disturbance regarding MA administration is oxidative stress and following increased ROS.
Conclusion
In conclusion, MA can increase abnormal spermatozoa with inadequate chromatin and DNA integrity in a dose-dependent manner and it is important to aware the young male people from deleterious effects of this drug on reproductive indices.
Acknowledgments
The authors would like to thank to Biotechnology group of Yazd Research and Clinical Center for Infertility. This study was funded by Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Note
This article extracted from M.Sc. thesis. (Mojdeh Sabour)
Conflict of interest
The authors have no financial or nonfinancial conflicts of interest.
References
- 1.Reuter PH. Can production and trafficking of illicit drugs be reduced or merely shifted? World Bank Policy Research Working Paper Series. 2008:1–35. [Google Scholar]
- 2.Schifano F, Corkery J, Cuffolo G. Smokable (ice, crystal meth) and non smokable amphetamine-type stimulants: clinical pharmacological and epidemiological issues, with special reference to the UK. Ann Ist Super Sanità. 2007;43:110–115. [PubMed] [Google Scholar]
- 3.Brick J, Erickson CK. Drugs, the brain, and behavior: The pharmacology of drug use disorders. 2nd Ed. Routledge; 2013. [Google Scholar]
- 4.Zhang X-D, Kelly-Hanku A, Chai J-J, Luo J, Temmerman M, Luchters S. Sexual and reproductive health risks amongst female adolescents who use amphetamine-type stimulants and sell sex: a qualitative inquiry in Yunnan, China. Harm Reduct J. 2015;34:1–12. doi: 10.1186/s12954-015-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly BC, LeClair A, Parsons JT. Methamphetamine use in club subcultures. Subst Use Misuse. 2013;48:1541–1552. doi: 10.3109/10826084.2013.808217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pal R, Megharaj M, Kirkbride KP, Naidu R. Illicit drugs and the environment-a review. Sci Total Environ. 2013;463:1079–1092. doi: 10.1016/j.scitotenv.2012.05.086. [DOI] [PubMed] [Google Scholar]
- 7.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 8.German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. 2014;97:2–8. doi: 10.1016/j.lfs.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spauwen MHF. The underlying biological mechanisms behind Helen Fisher's' Four primary temperament dimensions in the initial phase of mate choice' [Msc thesis] Utrecht University; 2013. [Google Scholar]
- 10.Urióstegui-Acosta M, Hernández-Ochoa I, Sánchez-Gutiérrez M, Piña-Guzmán B, Rafael-Vázquez L, Solís-Heredia M, et al. Methamidophos alters sperm function and DNA at different stages of spermatogenesis in mice. Toxicol Appl Pharmacol. 2014;279:391–400. doi: 10.1016/j.taap.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Singh P, Gupta R, Patidar D, Singh RK. Male infertility: causes and contributors. Int J Pharm Sci Res. 2014;5:2095. [Google Scholar]
- 12.Wang L, Qu G, Dong X, Huang K, Kumar M, Ji L, et al. Long-term effects of methamphetamine exposure in adolescent mice on the future ovarian reserve in adulthood. Toxicol Lett. 2016;242:1–8. doi: 10.1016/j.toxlet.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Fronczak CM, Kim ED, Barqawi AB. The insults of illicit drug use on male fertility. J Androl. 2012;33:515–528. doi: 10.2164/jandrol.110.011874. [DOI] [PubMed] [Google Scholar]
- 14.Safarinejad MR, Asgari SA, Farshi A, Ghaedi G, Kolahi AA, Iravani S, et al. The effects of opiate consumption on serum reproductive hormone levels, sperm parameters, seminal plasma antioxidant capacity and sperm DNA integrity. Reprod Toxicol. 2013;36:18–23. doi: 10.1016/j.reprotox.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Lin JF, Lin YH, Liao PC, Lin YC, Tsai TF, Chou KY, et al. Induction of testicular damage by daily methamphetamine administration in rats. Chin J Physiol. 2014;57:19–30. doi: 10.4077/CJP.2014.BAB155. [DOI] [PubMed] [Google Scholar]
- 16.Nudmamud-Thanoi S, Sueudom W, Tangsrisakda N, Thanoi S. Changes of sperm quality and hormone receptors in the rat testis after exposure to methamphetamine. Drug Chem Toxicol. 2016:39: 432–438. doi: 10.3109/01480545.2016.1141421. [DOI] [PubMed] [Google Scholar]
- 17.Zuloaga DG, Johnson LA, Agam M, Raber J. Sex differences in activation of the hypothalamic-pituitary-adrenal axis by methamphetamine. J Neurochem. 2014;129:495–508. doi: 10.1111/jnc.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talebi A, Vahidi S, Aflatoonian A, Ghasemi N, Ghasemzadeh J, Firoozabadi R, et al. Cytochemical evaluation of sperm chromatin and DNA integrity in couples with unexplained recurrent spontaneous abortions. Andrologia. 2012;44:462–470. doi: 10.1111/j.1439-0272.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- 19.Carrell DT, Emery BR, Hammoud S. Altered protamine expression and diminished spermatogenesis: what is the link? Hum Reprod Update. 2007;13:313–327. doi: 10.1093/humupd/dml057. [DOI] [PubMed] [Google Scholar]
- 20.Roychoudhury S, Sharma R, Sikka S, Agarwal A. Diagnostic application of total antioxidant capacity in seminal plasma to assess oxidative stress in male factor infertility. J Assist Reprod Genet. 2016;33:627–635. doi: 10.1007/s10815-016-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gharagozloo P, Gutiérrez-Adán A, Champroux A, Noblanc A, Kocer A, Calle A, et al. A novel antioxidant formulation designed to treat male infertility associated with oxidative stress: promising preclinical evidence from animal models. Hum Reprod. 2016;31:252–262. doi: 10.1093/humrep/dev302. [DOI] [PubMed] [Google Scholar]
- 22.Vessey W, McDonald C, Virmani A, Almeida P, Jayasena C, Ramsay J. Levels of reactive oxygen species (ROS) in the seminal plasma predicts the effectiveness of L-carnitine to improve sperm function in men with infertility. Endocrine Abstracts. 2016;44:232. [Google Scholar]
- 23.Green A, De Souza R, Williams J, Murray T, Cross A. The neurotoxic effects of methamphetamine on 5-hydroxytryptamine and dopamine in brain: evidence for the protective effect of chlormethiazole. Neuropharmacol. 1992;31:315–321. doi: 10.1016/0028-3908(92)90062-t. [DOI] [PubMed] [Google Scholar]
- 24.Petropoulos S, Matthews SG, Szyf M. Adult glucocorticoid exposure leads to transcriptional and DNA methylation changes in nuclear steroid receptors in the hippocampus and kidney of mouse male offspring. Biol Reprod. 2014;90:43. doi: 10.1095/biolreprod.113.115899. [DOI] [PubMed] [Google Scholar]
- 25.Talebi AR, Sarcheshmeh AA, Khalili MA, Tabibnejad N. Effects of ethanol consumption on chromatin condensation and DNA integrity of epididymal spermatozoa in rat. Alcohol. 2011;45:403–409. doi: 10.1016/j.alcohol.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Roodbari F, Abedi N, Talebi AR. Early and late effects of Ibuprofen on mouse sperm parameters, chromatin condensation, and DNA integrity in mice. Iran J Reprod Med. 2015;13:703. [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. WHO laboratory manual for the examination and processing of human semen. 5th Ed. Cambridge University Press; 2010. [Google Scholar]
- 28.Köhn F, Mack S, Schill W, Zaneveld L. Detection of human sperm acrosome reaction: comparison between methods using double staining, Pisum sativum agglutinin, concanavalin A and transmission electron microscopy. Hum Reprod. 1997;12:714–721. doi: 10.1093/humrep/12.4.714. [DOI] [PubMed] [Google Scholar]
- 29.Auger J, Mesbah M, Huber C, Dadoune J. Aniline blue staining as a marker of sperm chromatin defects associated with different semen characteristics discriminates between proven fertile and suspected infertile men. Int J Androl. 1990;13:452–462. doi: 10.1111/j.1365-2605.1990.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 30.Mangoli E, Talebi AR, Anvari M, Pourentezari M. Effects of experimentally-induced diabetes on sperm parameters and chromatin quality in mice. Iran J Reprod Med. 2013;11:53–60. [PMC free article] [PubMed] [Google Scholar]
- 31.Rahimipour M, Talebi AR, Anvari M, Sarcheshmeh AA, Omidi M. Saccharin consumption increases sperm DNA fragmentation and apoptosis in mice. Iran J Reprod Med. 2014;12:307–312. [PMC free article] [PubMed] [Google Scholar]
- 32.Khoradmehr A, Danafar A, Halvaei I, Golzadeh J, Hosseini M, Mirjalili T, et al. Effect of prenatal methamphetamine administration during gestational days on mice. Iran J Reprod Med. 2015;13:41–48. [PMC free article] [PubMed] [Google Scholar]
- 33.Nabi A, Khalili M, Halvaei I, Roodbari F. Prolonged incubation of processed human spermatozoa will increase DNA fragmentation. Andrologia. 2014;46:374–379. doi: 10.1111/and.12088. [DOI] [PubMed] [Google Scholar]
- 34.Alavi SH, Taghavi MM, Moallem SA. Evaluation of effects of methamphetamine repeated dosing on proliferation and apoptosis of rat germ cells. Syst Biol Reprod Med. 2008;54:85–91. doi: 10.1080/19396360801952078. [DOI] [PubMed] [Google Scholar]
- 35.Nudmamud‐Thanoi S, Thanoi S. Methamphetamine induces abnormal sperm morphology, low sperm concentration and apoptosis in the testis of male rats. Andrologia. 2011;43:278–282. doi: 10.1111/j.1439-0272.2010.01071.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto Y, Yamamoto K, Hayase T. Effect of methamphetamine on male mice fertility. J Obstet Gynaecol Res. 1999;25:353–358. doi: 10.1111/j.1447-0756.1999.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 37.Lin JF, Lin YH, Liao PC, Lin YC, Tsai TF, Chou KY, et al. Induction of testicular damage by daily methamphetamine administration in rats. Chin J Physiol. 2014;57:19–30. doi: 10.4077/CJP.2014.BAB155. [DOI] [PubMed] [Google Scholar]
- 38.Barroso G, Morshedi M, Oehninger S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod. 2000;15:1338–1344. doi: 10.1093/humrep/15.6.1338. [DOI] [PubMed] [Google Scholar]
- 39.Frohmader KS, Bateman KL, Lehman MN, Coolen LM. Effects of methamphetamine on sexual performance and compulsive sex behavior in male rats. Psychopharmacol. 2010;212:93–104. doi: 10.1007/s00213-010-1930-8. [DOI] [PubMed] [Google Scholar]
- 40.Lewis C, Dluzen DE. Testosterone enhances dopamine depletion by methamphetamine in male, but not female, mice. Neurosci Lett. 2008;448:130–133. doi: 10.1016/j.neulet.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Kazerooni T, Asadi N, Jadid L, Kazerooni M, Ghanadi A, Ghaffarpasand F, et al. Evaluation of sperm’s chromatin quality with acridine orange test, chromomycin A3 and aniline blue staining in couples with unexplained recurrent abortion. J Assist Reprod Genet. 2009;26:591–596. doi: 10.1007/s10815-009-9361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erenpreiss J, Jepson K, Giwercman A, Tsarev I, Erenpreisa J, Spano M. Toluidine blue cytometry test for sperm DNA conformation: comparison with the flow cytometric sperm chromatin structure and TUNEL assays. Hum Reprod. 2004;19:2277–2282. doi: 10.1093/humrep/deh417. [DOI] [PubMed] [Google Scholar]
- 43.Barenys M, Macia N, Camps L, de Lapuente J, Gomez-Catalan J, Gonzalez-Linares J, et al. Chronic exposure to MDMA (ecstasy) increases DNA damage in sperm and alters testes histopathology in male rats. Toxicol Lett. 2009;191:40–46. doi: 10.1016/j.toxlet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, Bianchi U. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4:31–37. doi: 10.1530/ror.0.0040031. [DOI] [PubMed] [Google Scholar]
- 45.Said TM, Agarwal A, Sharma RK, Thomas AJ, Sikka SC. Impact of sperm morphology on DNA damage caused by oxidative stress induced by β-nicotinamide adenine dinucleotide phosphate. Fertil Steril. 2005;83:95–103. doi: 10.1016/j.fertnstert.2004.06.056. [DOI] [PubMed] [Google Scholar]