Abstract
Background.
Mycobacterium tuberculosis (Mtb) contributes to the pathogenesis of childhood acute community-acquired pneumonia in settings with a high tuberculosis burden. The incremental value of a repeated induced sputum (IS) sample, compared with a single IS or gastric aspirate (GA) sample, is not well known.
Methods.
Two IS samples were obtained for Mtb culture from children enrolled as cases in the Pneumonia Etiology Research for Child Health (PERCH) study in South Africa. Nonstudy attending physicians requested GA if pulmonary tuberculosis was clinically suspected. We compared the Mtb yield of 2 IS samples to that of 1 IS sample and GA samples.
Results
. Twenty-seven (3.0%) culture-confirmed pulmonary tuberculosis cases were identified among 906 children investigated with IS and GA samples for Mtb. Results from 2 IS samples were available for 719 children (79.4%). Of 12 culture-confirmed pulmonary tuberculosis cases identified among children with ≥2 IS samples, 4 (33.3%) were negative at the first IS sample. In head-to-head comparisons among children with both GA and IS samples collected, the yield of 1 GA sample (8 of 427; 1.9%) was similar to that of 1 IS sample (5 of 427, 1.2%), and the yield of 2 GA samples (10 of 300; 3.3%) was similar to that of 2 IS samples (5 of 300; 1.7%). IS samples identified 8 (42.1%) of the 19 culture-confirmed pulmonary tuberculosis cases that were identified through submission of IS and GA samples.
Conclusions.
A single IS sample underestimated the presence of Mtb in children hospitalized with severe or very severe pneumonia. Detection of Mtb is enhanced by combining 2 IS with GA sample collections in young children with acute severe pneumonia.
Keywords: child, tuberculosis, induced sputum, gastric aspirate, yield.
The optimal approach to diagnosis of childhood pulmonary tuberculosis remains to be determined, as existing methods lack sensitivity [1]. In settings with a high tuberculosis burden, up to 15% of incident tuberculosis cases occur in children <14 years of age. Also, children <5 years of age are at higher risk of developing disease soon after infection with Mycobacterium tuberculosis (Mtb), most often manifest as pulmonary tuberculosis [2].
The microbiological diagnosis of pulmonary tuberculosis depends on detection of Mtb in respiratory samples obtained from a suspected case. Because children <5 years of age swallow rather than expectorate their sputum, the recommended approach to obtain respiratory secretions is by gastric aspirate (GA) or induced sputum (IS) sample collection [3]. The acidic environment in the stomach, however, may impair the viability of Mtb in GA samples, leading to lower yields compared with other respiratory samples [4]. Because primary pulmonary tuberculosis in children is generally noncavitating, results of microscopy of respiratory samples for acid-fast bacilli are often negative, and the sensitivity of culture of respiratory secretion samples among clinically diagnosed pulmonary tuberculosis cases is 7%–53% [5–8].
The feasibility of using IS sampling as an alternate to GA sampling for culturing Mtb was demonstrated in South African children <5 years of age hospitalized with clinically suspected pulmonary tuberculosis [9]. The same study reported that Mtb culture positivity from a single IS sample was similar to that from 3 GA samples collected on consecutive days [9]. A more recent systematic literature review and meta-analysis estimated that 1–3 IS samples may identify 79% (95% confidence interval [CI], 62%–92%) of culture-confirmed pediatric pulmonary tuberculosis cases identified through submission of comparator samples, including GA, pleural/lymph node culture, and nasopharyngeal aspirate samples [10].
Whereas pulmonary tuberculosis is generally considered a subacute or chronic illness [11–13], studies from high-burden countries have demonstrated that up to 8% of children hospitalized for acute community-acquired pneumonia also have culture-confirmed tuberculosis [14–16]. It remains unclear, however, whether pulmonary tuberculosis diagnosed in young children with severe acute community-acquired pneumonia represents Mtb as a cause of the pneumonia, or whether the pulmonary tuberculosis is an underlying immunosuppressive condition predisposing children to acute bacterial or viral pneumonia [16].
The objective of the current study was to compare the yield of a single IS sample with that of 2 IS samples collected on separate days for culture of Mtb in children <5 years of age hospitalized with severe or very severe community-acquired pneumonia, in a setting with a high tuberculosis and human immunodeficiency virus (HIV) burden. In addition, we compared the Mtb culture yield of 1 or 2 IS samples to the yield from 1, 2, or 3 GA samples obtained as part of standard care in these children.
METHODS
Cases
Children between 1 and 59 months of age hospitalized with World Health Organization (WHO)–defined severe or very severe pneumonia were recruited as part of the Pneumonia Etiology Research for Child Health (PERCH) study at the South African site, based at Chris Hani Baragwanath Academic Hospital, Soweto, Gauteng Province from 17 August 2011 to 31 August 2013. Exclusion criteria for cases were hospitalization within the previous 14 days, discharge as a PERCH case within the past 30 days, residence outside the study catchment area, or resolution of lower chest wall indrawing after bronchodilator therapy for those with wheezing. Further details about case identification and case definitions are provided elsewhere [17, 18].
IS samples were obtained for standard microbiological and Mtb culture in the children enrolled into the PERCH study, as well as polymerase chain reaction testing for multiple respiratory pathogens [19]. Whereas all the PERCH sites planned on collecting at least a single IS sample from the cases, it was decided a priori at the South African site to collect 2 IS samples on separate days to compare the yield of Mtb culture between 1 and 2 IS samples. The second IS sample was obtained for study purposes only, and not as part of the standard of care. In 2011 and 2012, the incidence (per 100 000 population) of a hospital-based diagnosis of culture-confirmed pulmonary tuberculosis in children <5 years of age resident in Soweto was 33 (95% CI, 26–41), and 185 (114–282) in HIV-positive children [20]. Because of the high tuberculosis burden at the South African site [20–23], clinicians admitting children with pneumonia frequently send 2 or 3 GA samples on separate days for Mtb culture. The vertical transmission prevalence of HIV infection from mother to child at the community level has been <3% in Gauteng Province since 2010, and the prevalence of HIV infection among children hospitalized at Chris Hani Baragwanath Academic Hospital was 19% in 2010/2011 [24, 25].
Procedures
Collection of IS samples was undertaken according to a standardized operating procedure used by all PERCH sites [26]. Trained study nurses obtained most of the IS samples, including those collected from children admitted to the high-care ward, where the procedure was performed under supervision of the study clinician. Whenever possible, the first IS sample was obtained on the morning after enrollment, and the second obtained 24–72 hours after the initial sample. All IS sample collections were undertaken at least 2–3 hours after the child’s most recent meal or feeding. Temporary contraindications to IS sample collection have been described elsewhere [27].
IS samples were transported in a cooler box to the research laboratory for processing. The first sample was apportioned into 3 aliquots, 1 each for molecular testing, standard microbiological culture, and Mtb culture. The second IS sample was processed only for Mtb culture.
GA samples were collected after an overnight fast of ≥4 hours in a subset of PERCH cases with suspected Mtb infection, at the discretion of treating clinicians. Hospital procedure is to collect 3 GA samples on 3 consecutive days, whenever possible, to evaluate young children with suspected pulmonary tuberculosis. GA samples were collected into sterile containers without sodium bicarbonate buffer.
IS aliquots (designated for Mtb culture) and GA samples were sent to the National Health Laboratory Service Mycobacteriology Referral Laboratory, Braamfontein, Johannesburg. Samples were processed according to current guidelines by decontamination and digestion with sodium hydroxide/N-acetyl-cysteine [28]. Fluorescent microscopy for mycobacteria was conducted by auramine O staining of a portion of the resuspended sputum/aspirate pellet. Samples were inoculated into mycobacterial growth indicator vials (MGIT; Becton-Dickinson) primed with growth supplement and antibiotics (PANTA™; Becton-Dickinson), which were incubated for a maximum of 42 days. Positive cultures were examined microscopically for acid-fast bacilli staining positive with Ziehl-Neelsen stain, which were identified using a commercially available line probe assay (HAIN GenoType MtbDRplus, HAIN Lifescience). Flag-positive MGIT vials that stained negative for acid-fast bacilli and lacked contaminants were reincubated. Flag-positive MGIT vials that on staining demonstrated the presence of contaminants only were not processed further.
Children clinically suspected of having pulmonary tuberculosis were tested using tuberculin skin tests, administered according to the Mantoux method by nonresearch clinicians [29]. Tuberculin skin tests were interpreted 48–72 hours after intradermal inoculation of 0.5 mL of Tuberculin PPD RT 23 (Statens Serum Institut), according to WHO guidelines [3].
Participant discharge diagnoses were abstracted from health records, and the hospital-based tuberculosis registry was reviewed to determine whether pulmonary tuberculosis had been diagnosed clinically by the attending clinician during the hospitalization. A case with clinically diagnosed tuberculosis was defined as a child who, regardless of tuberculin skin test response, had antituberculosis treatment started and was registered as having pulmonary tuberculosis during the PERCH hospitalization episode but had negative Mtb cultures. Children were designated as having culture-confirmed pulmonary tuberculosis if the IS, GA or other samples (endotracheal tube aspirates and mycobacterial blood cultures) were culture positive for Mtb. Children with nonrespiratory specimens (eg, Mtb cultured on blood) were deemed to have pulmonary tuberculosis if chest radiographic findings suggested pulmonary infection.
Analysis
We hypothesized that comprehensiveness of respiratory specimen sampling to establish a diagnosis of pulmonary tuberculosis in acutely ill children may be influenced by the severity of illness and the degree of clinical suspicion of pulmonary tuberculosis. We conducted univariate and multivariate analyses, using forward stepwise logistic regression, to determine which factors were associated with collection of 1 rather than 2 IS samples, as well as factors associated with the treating clinician’s decision to request GA sample collection. Factors associated with collection of 2 IS samples and those associated with GA sample collections were incorporated into the multivariate models if 2-sided P values were ≤0.20 at univariate analysis.
The yield of culture-confirmed Mtb was assessed in 7 comparisons: 1 versus 2 IS samples, 1 IS versus 1 GA sample, 1 IS versus 2 GA samples, 1 IS versus 3 GA samples, 2 IS versus 1 GA sample, 2 IS versus 2 GA samples, and 2 IS versus 3 GA samples. McNemar χ2 testing was performed to test the difference in yield between paired samples. Children who did not have IS or GA samples available for pairwise comparison according to the schema set out for comparison analyses outlined above, were excluded as appropriate. Occasionally, IS and GA samples were submitted for auramine O staining for mycobacteria and were not processed further for mycobacterial culture. Only IS or GA samples with available mycobacterial culture results were included in these analyses. Children with smear-positive, culture-negative samples were not considered confirmed pulmonary tuberculosis cases [30].
Contamination of IS and GA samples with oropharyngeal bacterial or fungal organisms may inadvertently occur during sample collection or processing and thus represents an actual operational scenario [31, 32]. Focusing our analysis only on instances where contaminated specimens did not occur would tend to overestimate the utility of IS and GA in pediatric pulmonary tuberculosis diagnosis (by decreasing sample denominators while maintaining the numerator). Our analysis therefore included contaminated specimens in the denominators of IS and GA submitted to identify the culture-confirmed pulmonary tuberculosis cases. All analyses were performed using Stata version 13.0 (StataCorp, College Station, TX). Permissions to conduct the study, nested within the overarching PERCH study, were obtained from the institutional review boards of the University of the Witwatersrand and the Johns Hopkins Bloomberg School of Public Health.
RESULTS
Baseline Characteristics
Of 920 children enrolled as cases with WHO-defined severe (n = 622; 67.6%) or very severe (n = 298; 32.4%) pneumonia at the South African PERCH site, 906 (98.5%) had at least one IS or GA collected: 840 (92.7%) of these had at least one IS sample collected, with a second sample obtained in 785 (86.6%). Furthermore, 399 (44.0%) cases had ≥2 GA samples collected, on consecutive days in 228 (57.1%) of the 399, >1 day apart in 122 (30.6%), and on the same day in 49 (12.3%). Of the 2782 samples submitted to the laboratory, 157 (5.6%) did not undergo Mtb culture for varying reasons (75 culture not requested, 34 lost in transit to the laboratory, 22 leaked, 22 unlabeled, 4 not processed owing to laboratory accidents). The first IS sample result was available in 799 children, of whom 328 (41.1%) had 1 IS and ≥2 GA results, and 300 (37.5%) had 2 IS and ≥2 GA results (see Supplementary Figure 1).
Indicators of increasing pneumonia severity (central cyanosis at baseline and in-hospital death) were the 2 factors that, at multivariate analysis, were associated with omission of a second IS sample collection among children who had 1 IS sample submitted (data not shown). There were significant differences in baseline characteristics between children with and those without a GA sample collected. At multivariate analysis, GA sample collection was positively associated with increasing age, duration of difficulty breathing, and positive tuberculin skin test result and negatively associated with nasal flaring and administration of antituberculosis treatment on the day of enrollment (Supplementary Table 1).
Of the 920 cases with pneumonia, 146 (15.9%) were identified either clinically or by culture confirmation as having pulmonary tuberculosis, 119 (12.9%) were discharged with a clinical diagnosis of pulmonary tuberculosis without culture confirmation, and 27 (2.9%) had culture-confirmed tuberculosis, including 1 with a diagnosis based on mycobacterial blood culture whose chest radiograph was abnormal and whose 2 IS cultures were negative (case 27 in Table 1). Twenty-five (92.6%) of the 27 children with culture-confirmed pulmonary tuberculosis were subjected to IS sample collections, and 19 (70.4%) had GA samples submitted. Similarly, 114 (95.8%) of the 119 children with clinically diagnosed pulmonary tuberculosis had IS samples submitted, and 97 (81.5%) underwent GA sample collections. The crude yields of Mtb culture positivity in IS and GA samples among the children with clinically diagnosed and/or culture-confirmed pulmonary tuberculosis were 9% (13 of 139) and 14% (16 of 116), respectively. Forty-eight (32.9%) of the 146 children with pulmonary tuberculosis were HIV positive, 3 with culture-confirmed and 45 with clinically diagnosed pulmonary tuberculosis. HIV-positive children had a 6-fold greater odds (95% CI, 3.96–10.11) of having clinically diagnosed pulmonary tuberculosis but were just as likely to have culture-confirmed pulmonary tuberculosis as those who were HIV negative (odds ratio, 0.87; 95% CI, .17–2.94).
Table 1.
Culture-Confirmed Pulmonary Tuberculosis Cases in the South African PERCH Cohorta
| Case | HIV Status | Pneumonia Severity | CR | IS Sample | GA Sample | ETT Sample | Mtb Blood Culture | Samples With Pos Mtb Culture | Basis for Diagnosis | Added Value Analysisb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st |
2nd |
1st | 2nd | 3rd | 1st | 2nd | GA Only (n = 27) | IS Only (n = 27) | GA + IS (n = 27) | Other Sample (n = 27) | IS in Cases With GA Samples (n = 19) | |||||||
| 1 | Neg | Very severe | AC | Neg c | Neg | Pos | Neg | N/R1 | GA1 | ✓ | 1–3, 5, 6 | |||||||
| 2 | Neg | Severe | Uninterp | Neg | Neg | Pos | Neg | Neg | GA1 | ✓ | 1–7 | |||||||
| 3 | Neg | Severe | Normal | Neg | Neg | Pos | Neg | Neg | GA1 | ✓ | 1–7 | |||||||
| 4 | Neg | Severe | AC | Neg | Neg | Pos | Neg | N/R2 | GA1 | ✓ | 1–3, 5, 6 | |||||||
| 5 | Neg | Very severe | Normal | Neg | Neg | Pos | GA1 | ✓ | 1, 2, 5 | |||||||||
| 6 | Neg | Severe | AC | Neg | Neg | Neg | Pos | Neg | GA2 | ✓ | 1–7 | |||||||
| 7 | Pos | Very severe | AC | Neg | Neg | Neg | Pos | GA2 | ✓ | 1–3, 5, 6 | ||||||||
| 8 | Neg | Severe | Normal | Neg | Neg | Neg | Pos | Neg | GA2 | ✓ | 1–7 | |||||||
| 9 | Neg | Severe | AC | N/R2 | Neg | Neg | Pos | Pos | GA2, GA3 | ✓ | Excludedd | |||||||
| 10 | Neg | Severe | AC | Neg | Neg | N/R3 | Neg | Pos | GA3 | ✓ | 1 | |||||||
| 11 | Pos | Severe | AC | Neg | Neg | Neg | N/R2 | Pos | GA3 | ✓ | 1, 2, 5 | |||||||
| 12 | Neg | Severe | Normal | Pos | Neg | Neg | Neg | Neg | IS1 | ✓ | ✓ | 1–7 | ||||||
| 13 | Neg | Very severe | AC | Pos | Neg | IS1 | ✓ | 1 | ||||||||||
| 14 | Neg | Severe | AC | Pos | Neg | Neg | Neg | IS1 | ✓ | ✓ | 1–3, 5, 6 | |||||||
| 15 | Neg | Very severe | Normal | Pos | Neg | IS1 | ✓ | 1 | ||||||||||
| 16 | Neg | Very severe | AC | Pos | Pos | Neg | IS1, IS2 | ✓ | ✓ | 1, 2, 5 | ||||||||
| 17 | Neg | Severe | AC | Neg | Pos | IS2 | ✓ | 1 | ||||||||||
| 18 | Neg | Severe | Normal | Neg | Pos | IS2 | ✓ | 1 | ||||||||||
| 19 | Neg | Severe | Normal | Neg | Pos | IS2 | ✓ | 1 | ||||||||||
| 20 | Neg | Very severe | AC | Pos | Neg | Pos | Neg | IS1, GA1 | ✓ | ✓ | 1–3, 5, 6 | |||||||
| 21 | Neg | Severe | AC | Pos | N/R4 | N/R4 | Pos | IS1, GA2 | ✓ | ✓ | Excludedd | |||||||
| 22 | Neg | Severe | AC | Pos | Neg | N/R1 | Pos | Pos | IS1, GA2, GA3 | ✓ | ✓ | 1 | ||||||
| 23 | Neg | Severe | AC | Pos | Pos | Pos | Pos | IS1, IS2, GA1, GA2 | ✓ | ✓ | 1–3, 5, 6 | |||||||
| 24 | Neg | Severe | AC | Neg | Pos | Pos | Neg | Neg | Neg | IS2, GA1 | ✓ | ✓ | 1–7 | |||||
| 25 | Neg | Very severe | AC | Pos | Neg | ETT1 | ✓ | |||||||||||
| 26 | Pos | Very severe | AC | Neg | Pos | ETT2 | ✓ | |||||||||||
| 27 | Neg | Severe | AC | Neg | Neg | Pos | Mtb blood culture | ✓ | 1 | |||||||||
| Total, No. (%) | 11 (40.7) | 8 (29.6) |
5 (18.5) |
3 (11.1) |
8 (42.1) |
|||||||||||||
Abbreviation AC, alveolar consolidation; CR, chest radiograph; ETT, endotracheal tube sputum sample; GA, gastric aspirate; HIV, human immunodeficiency virus; IS, induced sputum; Mtb, Mycobacterium tuberculosis; Neg, negative; N/R, not resulted; N/R1, specimen leaked; N/R2, culture not requested; N/R3, unlabeled specimen; N/R4, smear positive, culture not requested; PERCH, Pneumonia Etiology Research for Child Health; Pos, positive; Uninterp, uninterpretable.
aChildren were designated as having culture-confirmed pulmonary tuberculosis if they had a specimen positive for Mtb at mycobacterial culture, in the presence of an abnormal chest radiograph. All but 1 of the 27 cases with culture-confirmed pulmonary tuberculosis had their illness confirmed through culture of respiratory specimens (GA, IS, or ETT). One child had a positive Mtb blood culture in the presence of an abnormal chest radiograph. The table is sorted by culture-confirmed pulmonary tuberculosis cases, according to type of specimen that was positive for Mtb on culture, listing GA before IS samples, then GA- and IS-positive cases, followed by cases culture positive for Mtb from specimens other than GA and IS.
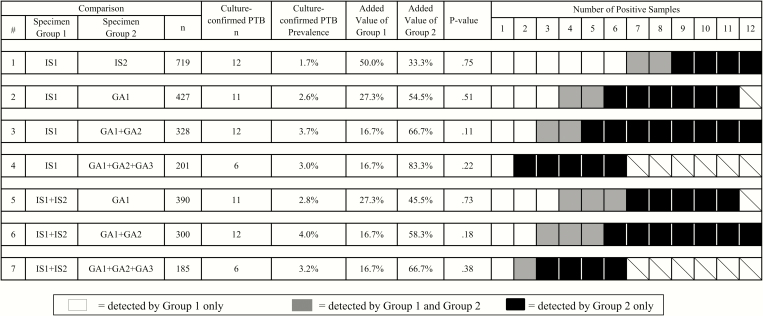
bSee Figure 1
cBolded cells highlight the following: in the 2-IS and 2-GA sample comparison in which Mtb culture results were available, the first IS sample identified 4 (33.3%) of the 12 positive pulmonary tuberculosis cases, and the GA samples with or without the second IS sample identified the remaining 8 (66.7%).
dChildren were excluded from the added yield analyses if mycobacterial culture results were unavailable for the first IS sample (case 9) or the first GA and second IS samples (case 21).
Auramine O staining was performed on samples from 896 (98.9%) of 906 children with samples submitted for mycobacterial culture. Eight (0.9%) had microscopic findings positive for mycobacteria, including 2 with culture-confirmed Mtb, 1 with clinically diagnosed pulmonary tuberculosis without culture confirmation, and 5 in whom a clinical diagnosis of pulmonary tuberculosis was not made.
Contamination of IS samples occurred in 47 (5.9%) and 64 (8.6%) of first and second IS samples, respectively, with higher contamination rates for the second IS samples (odds ratio, 1.50; 95% CI, 1.00–2.27). Contamination rates ranged from 5.8% to 8.7% in GA samples, with no significant difference in these rates between specimens.
Yield of 2 Versus 1 IS Sample in Mtb Culture
Twelve (1.7%) culture-confirmed pulmonary tuberculosis cases were identified among 719 children who had ≥2 IS samples with available culture results. Of the 12 Mtb culture-confirmed cases based on IS samples, 2 (16.7%) had positive cultures from both the first and second IS samples, 6 (50.0%) were detected only from the first sample, and 4 (33.3%) only from the second sample. The first IS sample identified 66.7% (95% CI, 34.9%–90.1%) of all culture-confirmed pulmonary tuberculosis cases detected with any IS sample, and the second IS sample identified 50.0% (95% CI, 21.1%–78.9%) (comparison 1 in Figure 1).
Figure 1.
Added value of induced sputum (IS) and gastric aspirate (GA) specimens in the detection of Mycobacterium tuberculosis (Mtb). Added value represents the number of cases positive for Mtb that were detected by submission of specimens/specimen groups and not by the comparator specimens/specimen groups. P values were determined with exact McNemar tests. Boxes with diagonal lines represent categories not applicable for comparison.
Yield of IS Versus GA Samples in Mtb Culture
IS samples identified 8 (42.1%) of the 19 culture-confirmed pulmonary tuberculosis cases identified among patients who had both ≥1 IS and ≥1 GA sample collected (Table 1). In head-to-head comparisons of single IS and GA samples and double IS and GA samples, GA samples alone identified a consistently greater proportion (6 of 11 [54.5%] for one GA sample, and 7 of 12 [58.3%] for two GA samples) of culture-confirmed pulmonary tuberculosis cases than did IS samples alone (3 of 11 [27.3%] for one IS sample, and 2 of 12 [16.7%] for two IS samples) (comparisons 2 and 6 in Figure 1), although these findings were not statistically significant. In the comparison of cases with 2 IS and 2 GA samples, among whom there were 12 with culture-confirmed pulmonary tuberculosis (bolded cells in Table 1), the first IS sample identified 4 cases (33.3%), and the 8 remaining cases (66.7%) were identified through GA sample culture, with or without culture of the second IS sample. The Mtb yields were greatest (3.7% and 4.0%, respectively) when 2 GA samples were combined with 1 or 2 IS samples (comparisons 3 and 6 in Figure 1).
Culture-Confirmed Tuberculosis Identified in Children With No GA Samples Collected
Eight (29.6%) of the 27 culture-confirmed pulmonary tuberculosis cases occurred among children in whom no GA samples were collected, and who were therefore not included in the yield analyses involving GA samples, described above. Five children had diagnoses based on IS samples alone, 2 had positive endotracheal endotracheal tube aspirate samples, and 1 had a positive Mtb blood culture (Table 1).
DISCUSSION
In this study, conducted in a high-burden tuberculosis setting with high HIV prevalence, we demonstrate that a second IS sample was able to identify 50% more pulmonary tuberculosis cases than would have been identified if relying on the submission of a single IS sample (comparison 1 in Figure 1). Among a subset of children who were investigated for pulmonary tuberculosis by ward clinicians through submission of GA samples, we found that GA samples had a higher yield of Mtb by culture than IS samples for diagnosing culture-confirmed pulmonary tuberculosis in children <5 years of age hospitalized with WHO severe or very severe pneumonia. This contrasts with the findings of others that IS samples provide a higher yield of Mtb culture positivity in children with suspected pulmonary tuberculosis [9]. Furthermore, we showed that a single IS sample underestimates the prevalence of culture-confirmed pulmonary tuberculosis by 67%, compared with the combination of 2 IS and ≥2 GA samples.
The yield of a single IS sample was similar to that of the second IS sample in our study (67% and 50%, respectively; comparison 1 in Figure 1), but both yields were greater than those observed in a large survey of the utility of IS sampling in the diagnosis of pediatric pulmonary tuberculosis in the ambulatory setting; in that study, the yields of the first and second IS samples were 38% and 27%, respectively [30].
Our study demonstrates a 3% prevalence of culture-confirmed Mtb in children hospitalized with WHO-defined severe or very severe pneumonia. This is lower than in previous South African studies, in which up to 8% of children hospitalized with acute severe community-acquired pneumonia, with or without hypoxia, had culture-confirmed pulmonary tuberculosis [14, 15]. Significant advancements have been made in child healthcare policy in South Africa since the mid-1990s, which may explain why culture-confirmed Mtb is now less prevalent in young children with community-acquired pneumonia in our setting. Advances in prevention of mother-to-child transmission of HIV [33, 34] as well as earlier detection of pediatric HIV infection and scale-up of pediatric antiretroviral therapy [35], have contributed to declines in culture-confirmed Mtb in South Africa [21, 36]. Furthermore, there might be a lower force of infection of Mtb in South African children, with the ongoing decline in incidence of tuberculosis among adults since 2009 [36].
One or 2 IS specimens and 1–3 GA specimens submitted for Mtb culture were positive in 9% and 14% of the children with a clinical or culture-confirmed tuberculosis diagnosis in our study, which is toward the lower estimates of test positivity (7%–43%) in previous reports of children with clinically suspected pulmonary tuberculosis [6, 37]. The extent of lung disease in children with classic pulmonary tuberculosis symptoms may be greater than that occurring in children with primary tuberculosis and concomitant bacterial pneumonia presenting with acute symptoms, making it difficult to draw direct comparisons about sample yields for pulmonary tuberculosis between studies. However, limited ability to establish a culture-confirmed diagnosis of pulmonary tuberculosis in young children reemphasizes the pressing need for more accurate diagnostic assays for childhood tuberculosis, which would also decrease the potential for misdiagnosing the condition and subjecting children to an unnecessary course of antituberculosis treatment.
Limitations of our study include the small number of culture-confirmed pulmonary tuberculosis cases in our cohort, the different manners in which the first and second IS samples were handled in the study, and the selective collection of GA samples. First IS samples were aliquoted for other microbiologic testing in addition to culture for Mtb, but the second IS samples were submitted for Mtb culture only. This could have reduced the sensitivity of the first IS sample in detecting Mtb because of the lower sample volume for Mtb culture, although the utility of the second sample in detecting Mtb may have been compromised by the increased contamination rates observed in the second IS samples. Children from whom GA samples were collected were significantly more likely to have clinical characteristics associated with tuberculosis disease. If GA samples were routinely collected during the PERCH study, rather than just at the discretion of attending clinicians, the relative contributions of GA and IS samples to culture-confirmed pulmonary tuberculosis diagnosis in the cohort could have been more fully evaluated. Nevertheless, when limiting the comparison of GA and IS samples only to children in whom both specimen types were collected, the yield of Mtb culture positivity from GA samples was higher than that from IS samples.
In high-burdened settings of tuberculosis and HIV, children 1–59 months of age presenting with acute community-acquired pneumonia have an appreciable burden of culture-confirmed pulmonary tuberculosis that can be diagnosed through submission of GA and IS samples. Considering that the sensitivity of culture for childhood pulmonary tuberculosis is only 30%–40% across studies [38], it is possible that up to 10% of children hospitalized with acute pneumonia in our setting have underlying pulmonary tuberculosis. The GA and IS sampling techniques complement one another, and a strategy in which 1 or 2 IS and 2 GA samples are submitted on separate days may improve the diagnosis of pulmonary tuberculosis in such patients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. D. P. M. performed analyses and drafted manuscripts. M. M. H. assisted with analyses and drafting of the manuscript. L. L. H., M. D .K., D. R. F., D. R. M., K. L. O., and S. A. M. provided guidance on analysis and manuscript. S. A. M. was the principal investigator of the South Africa PERCH study site. P. D. S., V. L. B., and P. V. A. performed laboratory testing. A. M. performed data collection throughout the study. C. P. and A. N. D. assisted with study coordination and manuscript development.
Acknowledgments. We acknowledge the work of the PERCH Study Group and contributors, the PERCH Expert Group, the Pneumonia Methods Working Group, and the PERCH Chest Radiograph Reading Panel. See Supplemental Data for the list of members in each group. We offer sincere thanks to the patients and families who participated in this study. This article is published with the permission of the Director of the Kenya Medical Research Institute.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the US Department of Health and Human Services, or the US government.
Financial support. This work is supported by the Bill & Melinda Gates Foundation (grant 48968 to the International Vaccine Access Center, Department of International Health, Johns Hopkins Bloomberg School of Public Health).
Supplement sponsorship. This article appears as part of the supplement “Pneumonia Etiology Research for Child Health (PERCH): Foundational Basis for the Primary Etiology Results,” sponsored by a grant from the Bill & Melinda Gates Foundation to the PERCH study of Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Potential conflict of interest. M. D. K. has received funding for consultancies from Merck, Pfizer, and Novartis, and grant funding from Merck. L. L. H. has received grant funding from Pfizer and GlaxoSmithKline (GSK). S. A. M. has received honoraria for advisory board participation from Bill & Melinda Gates Foundation (BMGF), Pfizer, Medimmune, and Novartis and institutional grants from GSK, Novartis, Pfizer, Minervax, and BMGF and has served on the speakers bureau for Sanofi Pasteur and GSK. K. L. O. has received grant funding from GSK and Pfizer and participates on technical advisory boards for Merck, Sanofi Pasteur, PATH, Affinivax, and ClearPath. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Nicol MP, Zar HJ. New specimens and laboratory diagnostics for childhood pulmonary TB: progress and prospects. Paediatr Respir Rev 2011; 12:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med 2012; 367:348–61. [DOI] [PubMed] [Google Scholar]
- 3. Stop TB Partnership Childhood TB Subgroup. Chapter 1: introduction and diagnosis of tuberculosis in children. Int J Tuberc Lung Dis 2006; 10: 1091–7. [PubMed] [Google Scholar]
- 4. Sprick MG, Towey JW. Isolation of Mycobacterium tuberculosis from gastric contents neutralized after varying periods. Public Health Rep 1946; 61:648–51. [PubMed] [Google Scholar]
- 5. Lighter J, Rigaud M. Diagnosing childhood tuberculosis: traditional and innovative modalities. Curr Probl Pediatr Adolesc Health Care 2009; 39:61–88. [DOI] [PubMed] [Google Scholar]
- 6. Maciel EL, Brotto LD, Sales CM, Zandonade E, Sant’anna CC. Gastric lavage in the diagnosis of pulmonary tuberculosis in children: a systematic review. Rev Saude Publica 2010; 44:735–42. [DOI] [PubMed] [Google Scholar]
- 7. Fiebig L, Hauer B, Brodhun B, Balabanova Y, Haas W. Bacteriological confirmation of pulmonary tuberculosis in children with gastric aspirates in Germany, 2002–2010. Int J Tuberc Lung Dis 2014; 18: 925–30. [DOI] [PubMed] [Google Scholar]
- 8. Kordy F, Richardson SE, Stephens D, Lam R, Jamieson F, Kitai I. Utility of gastric aspirates for diagnosing tuberculosis in children in a low prevalence area: predictors of positive cultures and significance of non-tuberculous mycobacteria. Pediatr Infect Dis J 2015; 34:91–3. [DOI] [PubMed] [Google Scholar]
- 9. Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet 2005; 365:130–4. [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez-Angulo Y, Wiysonge CS, Geldenhuys H, et al. Sputum induction for the diagnosis of pulmonary tuberculosis: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 2012; 31:1619–30. [DOI] [PubMed] [Google Scholar]
- 11. Kendig EL., Jr Pulmonary and pleural tuberculosis seminar. Semin Pediatr Infect Dis 1993; 4: 241–49. [Google Scholar]
- 12. Hesseling AC, Schaaf HS, Gie RP, Starke JR, Beyers N. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. Int J Tuberc Lung Dis 2002; 6:1038–45. [PubMed] [Google Scholar]
- 13. Marais BJ, Gie RP, Hesseling AC, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics 2006; 118:e1350–9. [DOI] [PubMed] [Google Scholar]
- 14. Madhi SA, Petersen K, Madhi A, Khoosal M, Klugman KP. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis 2000; 31:170–6. [DOI] [PubMed] [Google Scholar]
- 15. Zar HJ, Hanslo D, Tannenbaum E, et al. Aetiology and outcome of pneumonia in human immunodeficiency virus-infected children hospitalized in South Africa. Acta Paediatr 2001; 90:119–25. [PubMed] [Google Scholar]
- 16. Oliwa JN, Karumbi JM, Marais BJ, Madhi SA, Graham SM. Tuberculosis as a cause or comorbidity of childhood pneumonia in tuberculosis-endemic areas: a systematic review. Lancet Respir Med 2015; 3:235–43. [DOI] [PubMed] [Google Scholar]
- 17. Deloria-Knoll M, Feikin DR, Scott JA, et al. ; Pneumonia Methods Working Group Identification and selection of cases and controls in the Pneumonia Etiology Research for Child Health project. Clin Infect Dis 2012; 54(suppl 2:S117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scott JA, Wonodi C, Moïsi JC, et al. ; Pneumonia Methods Working Group The definition of pneumonia, the assessment of severity, and clinical standardization in the Pneumonia Etiology Research for Child Health study. Clin Infect Dis 2012; 54suppl 2:S109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murdoch DR, O’Brien KL, Driscoll AJ, Karron RA, Bhat N; Pneumonia Methods Working Group; PERCH Core Team Laboratory methods for determining pneumonia etiology in children. Clin Infect Dis 2012; 54suppl 2:S146–52. [DOI] [PubMed] [Google Scholar]
- 20. Mammen VG, Dangor Z, Moore DP, Izu A, Beylis N, Madhi SA. Hospitalization for culture-confirmed pulmonary tuberculosis in the era of childhood pneumococcal conjugate vaccine immunization. Pediatr Infect Dis J 2017; 36:e14–21. [DOI] [PubMed] [Google Scholar]
- 21. Dangor Z, Izu A, Hillier K, et al. Impact of the antiretroviral treatment program on the burden of hospitalization for culture-confirmed tuberculosis in South African children: a time-series analysis. Pediatr Infect Dis J 2013; 32:972–7. [DOI] [PubMed] [Google Scholar]
- 22. Fairlie L, Muchiri E, Beylis CN, Meyers T, Moultrie H. Microbiological investigation for tuberculosis among HIV-infected children in Soweto, South Africa. Int J Tuberc Lung Dis 2014; 18:676–81. [DOI] [PubMed] [Google Scholar]
- 23. Karstaedt AS, Bolhaar M. Tuberculosis in older adults in Soweto, South Africa. Int J Tuberc Lung Dis 2014; 18:1220–2. [DOI] [PubMed] [Google Scholar]
- 24.Goga AE, Dinh TH, Jackson DJ, et al. Population-level effectiveness of PMTCT option A on early mother-to-child (MTCT) transmission of HIV in South Africa: implications for eliminating MTCT. J Glob Health 2016; 6:020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meyers T, Dramowski A, Schneider H, Gardiner N, Kuhn L, Moore D. Changes in paediatric HIV-related hospital admissions and mortality in Soweto, South Africa 1996–2011: light at the end of the tunnel? J Acquir Immune Defic Syndr 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murdoch DR, Morpeth SC, Hammitt LL, et al. Microscopic analysis and quality assessment of induced sputum from children with pneumonia in the PERCH Study. Clin Infect Dis 2017; 64(suppl 3):S271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DeLuca AN, Hammitt LL, Kim J, et al. Safety of induced sputum collection in children hospitalized with severe or very severe pneumonia. Clin Infect Dis 2017; 64(suppl 3):S301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stop TB Partnership. Mycobacteriology laboratory manual. Geneva: World Health Organization, 2014. [Google Scholar]
- 29. Dacso CC. Skin testing for tuberculosis. In: Walker HK, Hall WD, Hurst JW, eds. Clinical methods: the history, physical, and laboratory examinations. Boston, MA: Butterworths Publishers, a division of Reed Publishing, 1990. [PubMed] [Google Scholar]
- 30. Hatherill M, Hawkridge T, Zar HJ, et al. Induced sputum or gastric lavage for community-based diagnosis of childhood pulmonary tuberculosis? Arch Dis Child 2009; 94:195–201. [DOI] [PubMed] [Google Scholar]
- 31. Suitters BT, Brogger SA. Some aspects of laboratory investigations in a mass campaign against tuberculosis. Bull World Health Organ 1967; 36:837–45. [PMC free article] [PubMed] [Google Scholar]
- 32. Kalema N, Boon SD, Cattamanchi A, et al. Oral antimicrobial rinse to reduce mycobacterial culture contamination among tuberculosis suspects in Uganda: a prospective study. PLoS One 2012; 7:e38888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barron P, Pillay Y, Doherty T, et al. Eliminating mother-to-child HIV transmission in South Africa. Bull World Health Organ 2013; 91:70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kerber KJ, Lawn JE, Johnson LF, et al. South African child deaths 1990–2011: have HIV services reversed the trend enough to meet Millennium Development Goal 4? AIDS 2013; 27: 2637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davies MA, Phiri S, Wood R, et al. ; IeDEA Southern Africa Steering Group Temporal trends in the characteristics of children at antiretroviral therapy initiation in southern Africa: the IeDEA-SA Collaboration. PLoS One 2013; 8:e81037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nanoo A, Izu A, Ismail NA, et al. Nationwide and regional incidence of microbiologically confirmed pulmonary tuberculosis in South Africa, 2004-12: a time series analysis. Lancet Infect Dis 2015; 15:1066–76. [DOI] [PubMed] [Google Scholar]
- 37. Stockdale AJ, Duke T, Graham S, Kelly J. Evidence behind the WHO guidelines: hospital care for children: what is the diagnostic accuracy of gastric aspiration for the diagnosis of tuberculosis in children? J Trop Pediatr 2010; 56:291–8. [DOI] [PubMed] [Google Scholar]
- 38. Marais BJ, Pai M. Recent advances in the diagnosis of childhood tuberculosis. Arch Dis Child 2007; 92:446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.