Summary
Exposure to pesticides may increase the generation of reactive oxygen species (ROS), leading to oxidation of cell membrane lipids and proteins. Although fruit bats are potentially exposed to pesticides during their entire lifespan, the impacts of this exposure are still poorly investigated. We examined the effects of low, commercially recommended concentrations (0, 1.05 and 2.1 g/l) of an organochlorine insecticide endosulfan (EDS) formulation on oxidative responses in the liver and kidneys of Neotropical fruit bats (Artibeus lituratus), as well as possible liver morphological alterations following a 35‐day oral exposure. Superoxide dismutase activity was significantly decreased upon exposure to 1.05 g/l of EDS in the liver and kidneys, catalase was decreased in the liver of 2.1 g/l EDS‐exposed bats, while glutathione S‐transferase was increased in the liver of 2.1 g/l EDS‐exposed bats. Protein carbonyls increased following the exposure to the highest EDS dose tested. Endosulfan‐induced morphological alterations in the liver included cell degeneration and cell death, with apparent cytoplasm lipid accumulation (steatosis) and pyknotic nuclei, karyolysis and deposit of collagen fibres. Our findings suggest that exposure to low concentrations of EDS induced a certain extent of oxidative damage in fruit bats, which may have led to liver morphological alterations.
Keywords: antioxidant enzymes, Chiroptera, toxicologic pathology, environmental contaminant
The organochlorine insecticide endosulfan (EDS) is a broad‐spectrum compound effective in a wide variety of agricultural crops, such as papaya, orange, strawberry, apple, soya beans, coffee, cocoa, rice and beans. Due to evidences of its negative effects in non‐target organisms, resulting in reproductive and developmental effects (Androustsopoulos et al. 2013; Bayat et al. 2014), EDS has been recently banned in more than 80 countries, including the United States, the European Union and Brazil (2012). However, this organochlorine is still extensively used in regions like China and India, and even in places where its use was discontinued, concentrations up to 8.42 ng/g can still be detected in soil samples (Kang et al. 2008).
In the Neotropics, fruit‐eating bats living in forest fragments near fruit crops are continuously exposed to pesticides, via dermal or gastrointestinal routes (Zocche et al. 2010; Lilley et al. 2013; Stechert et al. 2014; Dennis & Gartrell 2015). Cytotoxic effects of many pesticides in non‐target organisms include the production of reactive oxygen species (ROS), which induce cell damage to lipids, proteins and DNA (Abdollahi et al. 2004; Agrawal & Sharma 2010). Exposure to EDS in rats caused oxidative liver damage resulting from tissue generation of ROS (Hincal et al. 1995). Oscillations in antioxidative defence systems could be used as a tool to evaluate these effects, including the enzymatic (superoxide dismutase (SOD), catalase (CAT) and glutathione S‐transferase (GST) and non‐enzymatic (α‐tocopherol, ascorbic acid, carotenoids and glutathione) systems (Hermes‐Lima 2004). In bats, several studies emphasize the relationship between chronic exposure to pesticides and declines in their populations (Gerell & Gerell Lunderg 1993; Swanepoel et al. 1999; Bennett & Thies 2007). Previous studies from our laboratory showed histopathological alterations in the livers of fruit‐eating bats that have been exposed to environmentally relevant concentrations of the insecticide fenthion (Amaral et al. 2012a,b). As the liver and kidneys are the main organs involved with metabolism and excretion of xenobiotics and their residual metabolic by‐products (Astiz et al. 2009a,b), damage to these organs, including inflammation, apoptosis, hyperplasia or hypertrophy, would lead to impaired function (Naidoo et al. 2015). Although relevant, pesticide‐induced oxidative damage and morphological alterations in Neotropical bats tissues are yet to be evaluated. In the view of the above, this study aimed at evaluating the effects of a dietary exposure to environmentally relevant concentrations of endosulfan on antioxidant capacity of the liver and kidneys and liver histopathology of fruit‐eating bats (Artibeus lituratus).
Materials and methods
Chemicals
The commercial formulation Endosulfan 350 EC [1,2,3,4,7,7a‐hexacloro‐biciclo(2,2,1)hepteno‐2,5,6,bis(metileno)sulfito]; 350 g/l, 35% w/v], manufactured by Milenia Agro Ciências S.A., was obtained from the Laboratory of Integrated Pest Management of the Federal University of Viçosa (UFV), Minas Gerais, Brazil. The adhesive spreader Will Fix (dodecylbenzene sulphonic acid) (Charmon Destyl Industria Quimica LTDA) was added to the treatments at the concentration recommended by the manufacturer (30 g/l, 3% m/v), as our intention was to simulate the situation bats face in the field. The spreader is considered low toxic (ANVISA, 2013) and is regularly used to increase the efficiency of pesticide application. The other chemicals were purchased from Sigma‐Aldrich (São Paulo, Brazil).
Ethical approval statement
All procedures performed in the present study involving animals were in accordance with the ethical standards of the Animal Ethics Committee of the University of Viçosa (Process 69/2014) and Brazilian Government (SISBIO, Process no 25048), as well as applicable international guidelines for the care and use of animals.
Animals and experimental design
Adult male great fruit‐eating bats (Artibeus lituratus) (n = 18) were captured during spring and summer (September 2011–January 2012) using mist nets in Minas Gerais, south‐east Brazil (20°45′ S and 42°52′ W). To ensure adult status, only animals with complete ossification of the cartilaginous epiphyseal growth plates of the fourth metacarpal phalangeal joint were selected (Kunz & Anthony 1982). Bats were identified with the key for the identification of Brazilian bats (Vizzotto & Taddei 1973). Sex was visually determined, and reproductive status was assessed. Bats were transported, weighed and kept in individual steel cages (45 × 22 cm) under natural temperature and light cycles, in a fenced‐in bat house located at the University Zoology Museum backyard. Before the experiment, bats were fed papaya (Carica papaya) for 2 days, for their acclimation period. Bats were then randomly divided into three groups (n = 6 each): bats fed with untreated fruits (control); bats fed with fruits treated with endosulfan at 1.05 g/l and adhesive spreader at 0.015 g/l (EDS1); and bats fed with fruits treated with endosulfan at 2.01 g/l and adhesive spreader at 0.015 g/l (EDS2). Papaya fruits were chosen due to their high acceptance from bats in captivity (Amaral et al. 2012a,b). Fruits were dipped in one of the above‐mentioned solutions and left to dry in an adapted container to prevent contact and therefore losses of the insecticide in the outer layer. Fruits were then cut in half (approximately 150 g) and offered daily at 18 h to the animals in a Petri dish for 35 days, with the bark side up, to simulate the situation found by bats in nature. Water was available ad libitum. Food intake was measured daily by weighing the fruit offered and the leftovers in the following day. This commercial endosulfan formulation plus the addition of adhesive spreader were chosen to simulate the conditions in which bats are exposed in the field, where they are potentially exposed to commercial fruit crops grown with the use of pesticides while foraging. The concentrations were selected in accordance with those indicated by the manufacturer; therefore, they were considered environmentally relevant.
Chemical analysis
We performed limited chemical analysis (two replicates for each sample) to verify exposure differences. Results are provided in Table S1. Diet (4 g) samples were homogenized, transferred into a glass tube and added to 11 ml of extracting solution containing ethyl acetate and acetonitrile (3:8 v/v). The sample was thoroughly vortexed for 5 min and cooled at −20°C for 6 h. After this period, a two‐phase system was obtained, comprising the solid phase (frozen aqueous phase and matrix) and the liquid phase (supernatant). The supernatant was collected and filtered through a filter paper containing anhydrous sodium sulphate (ASS) (1 g) to eliminate the presence of water.
Tissue samples were obtained from animals after 35 days of exposure, from the liver (one replicate) or adipose tissue (two replicates) (1 g wet weight of each tissue), pooled from animals randomly taken from all groups. Samples were homogenized with extracting solution (3:8 v/v), stored at −20°C for 4 h and filtered. Endosulfan concentration was measured by the method of internal standardization. To these solutions and extract samples, we added 0.1 ml of methyl parathion (10 g/l) used as the internal standard. The chromatographic separation was carried out through a stationary phase capillary column (5% diphenyl, 95% dimethylsiloxane; 30 m × 0.25 mm of inner diameter and 0.1 μm in film thickness). The column temperature was set at 150°C (2 min), increasing the temperature in 20°C/min up to 190°C (3 min), followed by additional increases of 20°C/min up to the final temperature of 280°C (2 min). The total duration of the analysis was 13.5 min. Nitrogen was used to drag the gas flow rate of 1.2 ml/min. Endosulfan concentrations were identified by comparing the compound retention time to the pattern for this chemical. Standard curves were prepared with endosulfan at a known concentration, diluted in acetonitrile. Chromatography analysis was performed using a gas chromatograph (GC) (Shimadzu, Japan, GC–17A) with an electron capture detector (ECD) for diet samples and tissue samples.
Oxidative stress indexes: extraction and determination
Portions of the liver and kidneys were obtained, weighed (50 mg each), immediately frozen in liquid nitrogen and stored at −80°C until use. Samples were homogenized in Na phosphate buffer 50 mM pH 7.4 (500 μl) using a homogenizer (OMNI) and centrifuged (13,800 g at 4°C for 10 min). The supernatant was used for SOD, GST and malondialdehyde (MDA). For CAT activity determination, we used the supernatants from the tissues homogenized with Na phosphate buffer 50 mM pH 7.4 and triton X‐100 buffer (1%). Some preliminary enzyme determinations were performed to ensure appropriate maintenance of enzyme activity after freezing and thawing and to test the kinetic conditions. In addition, to ensure zero‐order kinetics, all enzyme activities were determined by duplicate using a spectrophotometer (UV‐Mini 1240, Shimadzu) or an ELISA reader (Thermo Scientific, Waltham, MA, USA).
Determination of malondialdehyde (MDA) was performed according to Buege and Aust (1978). Protein carbonyl content was measured using the 2,4‐dinitrophenylhydrazine (DNPH) procedure (Levine et al. 1994). The activity of SOD was determined by the method based on the reduction in the superoxide (O−2) and hydrogen peroxide, thereby decreasing the auto‐oxidation of pyrogallol, according to Dieterich et al. (2000). Catalase activity (CAT) was determined according to Aebi (1984). Glutathione S‐transferase activity (GST) was assayed according to Habig et al. (1974). Enzymatic activities were calculated in terms of sample protein content (Lowry et al. 1951) and expressed as U per milligram of protein.
Morphology and stereology of liver and kidneys
Liver fragments of all animals from each group were placed in Karnovsky's solution for 24 h, then dehydrated in ethanol and embedded in glycol methacrylate. Sections (3 μm thick) were cut with a Multicut 2045 rotary microtome (Reichert‐Jung, Germany), stained with Sirius red (Sirius red F3B, Mobay Chemical Co., Union, NJ, USA) for collagen fibre differentiation under polarizing microscopy and Toluidine blue for organ architecture analysis and mounted using Entellan mounting medium (Merck, Frankfurt, Germany). To avoid repeated analysis, sections were evaluated in semi‐series, using one in every five sections. Slides were visualized, images were captured using a light and polarizing microscope (Olympus BX‐60, Tokyo, Japan) connected to a digital camera (Olympus QColor‐3, Tokyo, Japan), and a total of 4.37 × 106 mm2 were analysed. For the stereological analysis, a test system of 216 points was used in a standard test area (At) of 73 × 103 mm2. In sections stained using the Toluidine blue method, volume density (Vv) was estimated by counting the points situated over cell degeneration by fat accumulation (steatosis/fat droplets), cell death (pyknotic nuclei, karyolysis and collagen fibres) and volumes of hepatocytes, sinusoid capillary and interstitial cells using the following formula: Vv = PP [structure]/PT, where PP is the number of points situated over the structure in question and PT is the total number of points in the test. All histomorphometric analyses were performed using the image pro‐plus 4.5 image analysis software (Media Cybernetics, Silver Spring, MD, USA). Brunt scales were used for the semiquantitative assessment of histopathological lesions. In this scoring system, histological lesions of steatosis and necro‐inflammation are introduced into a grading system. The system incorporates fatty changes, ballooning and inflammation into an overall grade (Gabriel et al. 2008).
Statistical analysis
Differences among groups were assessed with one‐way anova followed by Bonferroni multiple comparison test. All data were analysed by graphpad prism 6.0® statistical software (GraphPad Software, Inc., San Diego, CA, USA) and expressed as the mean ± SEM. Significance levels were set at P < 0.05.
Results
Body weight and food consumption
There were no significant effects of EDS on body weight and food consumption between control and 35‐day‐exposed bats (Table 1). Chemical analysis of EDS concentrations in the diet and bat tissues from different groups are also available (Table S1; Figures S1, S2 and S3).
Table 1.
Body weight and mean papaya consumption of Artibeus lituratus (n = 6) dietary exposed to endosulfan for 35 days
| Body weight (g) | Food consumption (g) | |
|---|---|---|
| Control | 76.76 ± 2.05 | 116.00 ± 4.97 |
| EDS1 | 74.01 ± 3.97 | 125.00 ± 3.08 |
| EDS2 | 68.49 ± 2.96 | 111.00 ± 4.09 |
Values are mean ± SEM.
Oxidative stress indexes
Lipid peroxidation (LPO) levels, measured as MDA content, did not change in the liver following EDS exposure (F 2 = 1.35, P = 0.29) at 1.05 g/l (post hoc Bonferroni test: P = 0.51) or 2.1 g/l EDS (post hoc Bonferroni test: P = 0.54) (Figure 1). In the same way, MDA values in the kidneys did not show any variation in both exposed groups (F 2 = 0.52, P = 0.60) (Table 2). Unlike MDA, carbonyl levels, which are another end product of oxidative stress damage, significantly increased (F 2 = 12.6, P = 0.0006) in bats exposure to the highest dose tested (post hoc Bonferroni test: P = 0.005) (Figure 1).
Figure 1.
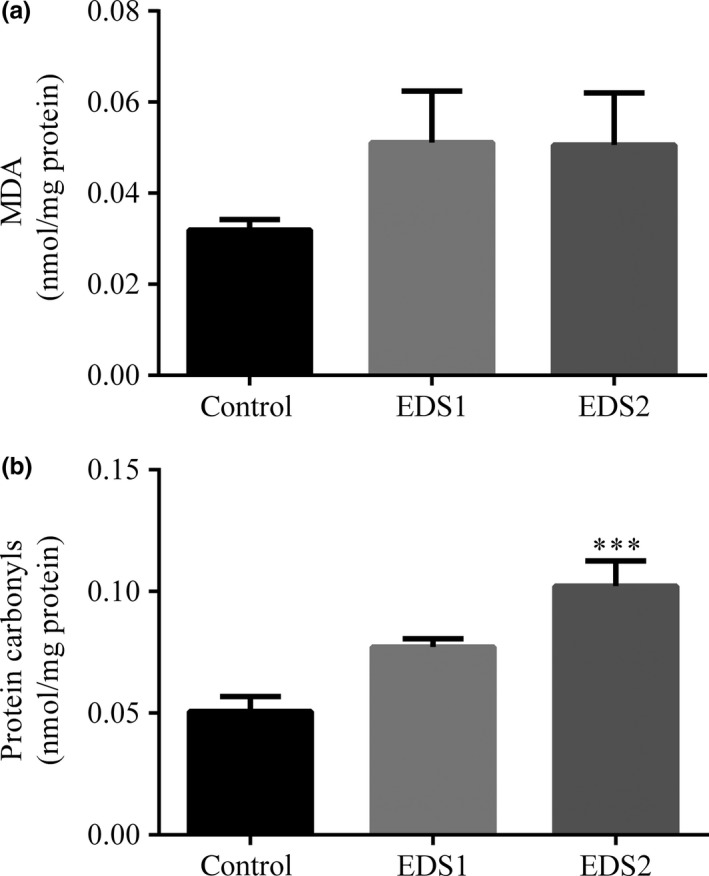
Products of lipid peroxidation: the malondialdehyde (MDA) (a) and protein carbonyl level (b) in the liver of the Artibeus lituratus (n = 6). Values are mean ± SEM. Values with superscript *** are significantly different from control (P ≤ 0.001).
Table 2.
Effect of exposure to endosulfan on the activity of catalase (CAT), superoxide dismutase (SOD), glutathione S‐transferase (GST) and malondialdehyde (MDA) in the kidneys of Artibeus lituratus (n = 6)
| MDA (nmol/mg protein) | CAT (U/mg protein) | SOD (U/mg protein) | GST (μmol/min/g) | |
|---|---|---|---|---|
| Control | 0.014 ± 0.002 | 0.456 ± 0.03 | 1.853 ± 0.05 | 5.252 ± 0.25 |
| EDS 1 | 0.015 ± 0.002 | 0.236 ± 0.03**** | 1.430 ± 0.12* | 3.240 ± 0.63 |
| EDS 2 | 0.013 ± 0.001 | 0.102 ± 0.008**** | 1.942 ± 0.09 | 5.469 ± 1.32 |
Values with superscript *P ≤ 0.05 and ****P ≤ 0.0001 are significantly different and compared to control.
Values are mean ± SEM.
SOD activity in the liver following EDS exposure was decreased (F 2= 63.70, P < 0.0001) in EDS1 (post hoc Bonferroni test: P < 0.0001) and EDS2 (post hoc Bonferroni test: P = 0.02) compared with the control (Figure 2). In the kidneys, SOD activity was also decreased (F 2 = 12.20, P = 0.0007) in EDS1 (post hoc Bonferroni test: P < 0.005) compared with control (Table 2). CAT activity in the liver (F 2 = 16.9, P = 0.0001) (Figure 2) and kidneys (F 2 = 45.2, P < 0.0001) (Table 2) was also lower (post hoc Bonferroni test: P ≤ 0.001) upon EDS1 and EDS2.
Figure 2.
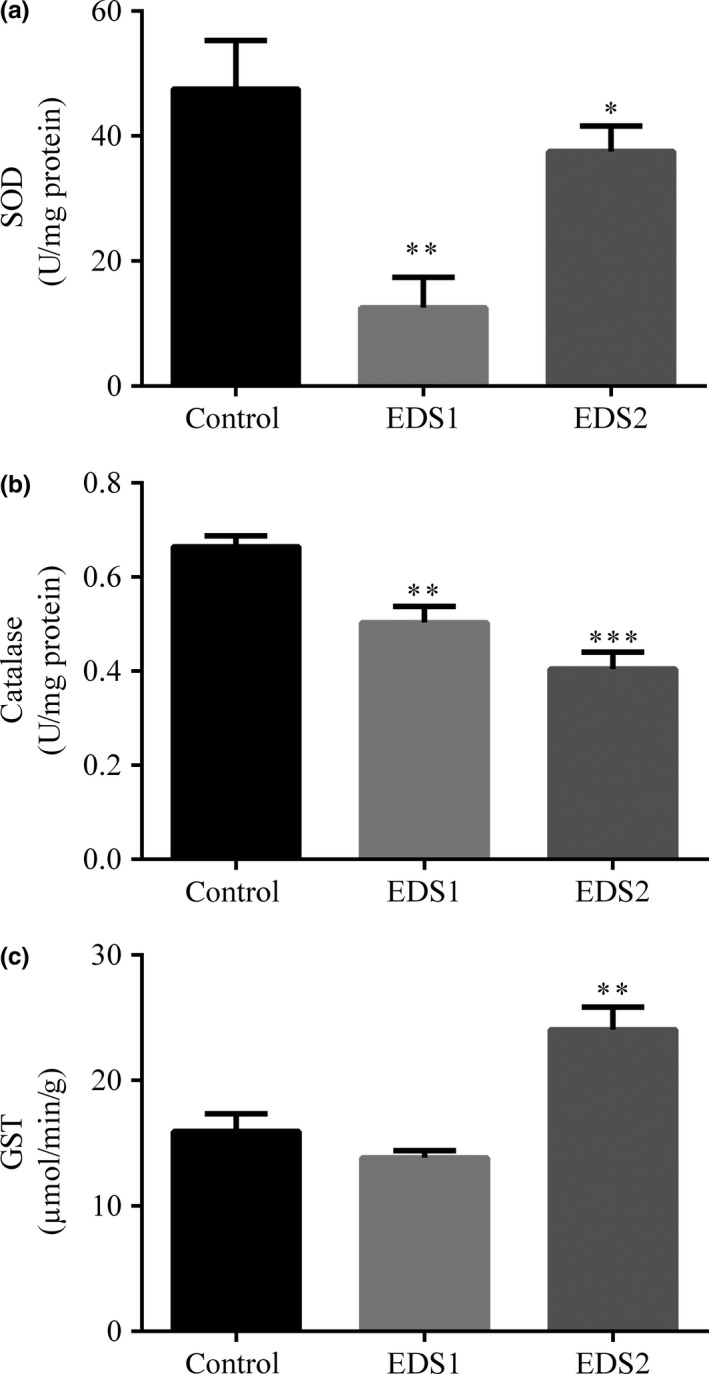
Effect of the exposure to endosulfan on SOD (a), CAT (b) and GST (c) activities in the liver of Artibeus lituratus (n = 6). Values are mean ± SEM. Values with superscript * are significantly different from control *P ≤ 0.05, **P ≤ 0.01.
GST activity was increased in the liver from EDS2 bats (F 2 = 15.8, P = 0.002) (post hoc Bonferroni test: P = 0.002) compared with control and EDS1 (Figure 2). In the kidneys, GST activity remained unaltered upon exposure (F 2 = 3.05, P = 0.07) (Table 2).
Liver morphology and stereology
Corroborating some of the biochemical alterations found, histological analysis also revealed abnormal patterns induced by EDS exposure. Histopathological alterations such as lipid droplet accumulation and cell death (pyknotic nuclei, karyolysis and collagen fibres) were found in EDS1 and EDS2 (F 2 = 1.040, P < 0.05) when compared to control (Figure 3). However, the volumes of hepatocytes (F 2 = 1.5, P = 0.66), sinusoid capillaries (F 2 = 0.70, P = 0.51) and interstitial cells (F 2 = 0.42, P = 0.51) were not different among groups. Semiquantitative analysis demonstrated grade three macrovesicular steatosis with inflammatory infiltrate and deposit of collagen fibres indicative of cell death and fibrosis (Figure 3).
Figure 3.
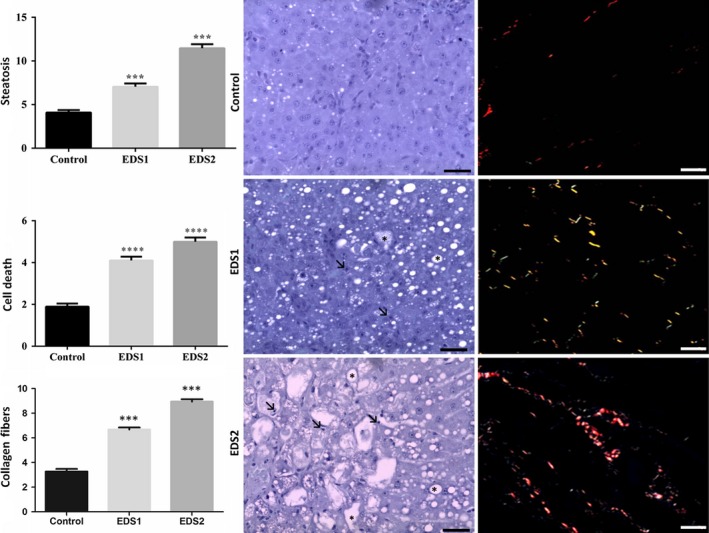
Effect of the exposure to endosulfan on liver morphology and stereological analysis of Artibeus lituratus (n = 5 in control and n = 6 EDS1 and EDS2). Values with superscript ***P ≤ 0.001 and ****P ≤ 0.0001 are significantly different from control. Photomicrographs: Toluidine blue and Sirius red. Black arrows in bold = necrosis; Black arrows = steatosis. Scale bars = 40 μm.
Discussion
This study investigated the effects of a dietary exposure of the insecticide endosulfan, at low concentrations, in Neotropical fruit bats, focusing on its effect on liver and kidneys, considering oxidative stress and morphological changes as toxicity markers. The lack of differences in body weight between the two exposed groups suggests that bats did not detect the presence of endosulfan in their diet and that they did not learn to avoid intoxicated fruit through potential postingestive effects. Chromatography analysis showed concentration of endosulfan accumulated in the liver and adipose tissue from EDS1 and EDS2 bats. Similar concentrations of endosulfan were found in the liver and adipose tissue of Rattus norvegicus collected in soya bean farm areas (Kuvarega & Taru 2007). These results show that even at low doses, EDS may accumulate in the tissue and is likely to cause injuries by changing the organ function (Raj et al. 2013).
Toxic effects of endosulfan have been attributed to increased generation of reactive species of oxygen (ROS). ROS leads to oxidation of cell components like DNA, lipids and protein, and the consequences of this process are the formation of radical lipoperoxyl (LOO−) and radical alkoxyl (RO−) that promote peroxidation chain of lipids and protein inside the cell. As by‐products of these reactions, malondialdehyde (MDA) and protein carbonyls are formed and accumulate. These two markers are important tissue stress signals (Limón‐Pacheco & Gonsebatt 2009; Amin & Hashem 2012). In this study, although MDA in tissues did not show significant changes in exposed groups, the higher endosulfan dose tested showed an increase in carbonyl proteins. This finding corroborates the results observed in rats acutely exposed to the insecticide malathion in the liver and kidney (Vijaya Padma et al. 2011) and also to what was observed in rats exposed to insecticide organochlorine lindane (Possamai et al. 2007). These results show that the exposure to endosulfan in a dose as low as 2.01 g/l caused oxidation of amino acids and led to the formation of aggregates in bat cells. These aggregates can accumulate and form insoluble fibrils, affecting protein function inside the cell (Gonçalves et al. 2016). Similar changes associated with the amount of carbonyl protein were found in the liver of fish exposed to deltamethrin, endosulfan and paraquat. Excessive production of this biomarker was considered a determining factor for oxidative stress in the detoxification organs of these animals (Parvez & Raisuddin 2005). Unlike other mammals, bats have a greater ability to maintain low levels of oxidative stress (Wilhelm Filho et al. 2007; Salmon et al. 2009). In addition, fruit bats exhibit the higher concentration of antioxidants in their fruit diet may also contribute to enhance their defence system (Schneeberger et al. 2014). Based on the characteristics described above, data presented in our study show that despite having potentially more effective antioxidants defence systems, fruit bats are also susceptible to the effects of the insecticide endosulfan. In the long run, this exposure might promote physiological changes in specific cells and pose a threat to their survival.
Endosulfan was proven to induce free radicals synthesis, such as superoxide radicals (O2 −) and subsequently generate hydrogen peroxide (H2O2) and hydroxyl radicals, highly reactive and harmful to cells (Shao et al. 2012). The excessive production of these compounds leads to infiltration and activation of macrophages, neutrophils and lymphocytes, increasing the presence of oxidizing agents such as nitric oxide (Fukushima et al. 2002). Infiltration of leucocytes caused by endosulfan is usually related to oxidative burst during of inflammation. This process is defined as a rapid and excessive cell release of reactive oxygen species (Gonçalves et al. 2016). These agents are known to amplify oxidative damage and induce tissue dysfunction (Limón‐Pacheco & Gonsebatt 2009; Amin & Hashem 2012). To protect the tissues against the action of ROS, there are different defensive enzymes, such as SOD and CAT. These are the first line of antioxidant response. SOD catalyses the conversion of superoxide (O2 −) into hydrogen peroxide (H2O2) and oxygen (O2). CAT performs cellular detoxification by converting the H2O2 into oxygen and water (Droge 2002; Ray et al. 2012). In our study, bats exposed to endosulfan at both concentrations showed decreased SOD and CAT activity in the liver. In the kidneys, bats exposed to the higher dose also showed decreased CAT activity, similar to that reported previously for rats exposed to sublethal concentrations of lindane for 30 days (Vijaya Padma et al. 2011; Salvo et al. 2012) and fish exposed to the sublethal concentration of endosulfan for 15 days (El‐Demerdasha et al. 2013). Usually when there is an intense cell production of ROS, antioxidant enzymes tend to increase their activity in an attempt to avoid possible damage to the body due to the action of these compounds. However, in this situation, it is also common to have a process known as enzyme depletion occurring, due to a metabolic overload of antioxidant enzymes, compromising their synthesis. In this case, existing proteins would no longer perform its function and show damage, as they also become a target of oxidative stress generated in the tissue. We believe that this metabolic condition reflects the decreases in SOD and CAT activities found in exposed bats. These results can be supported by protein damage, as observed in altered liver protein carbonyls, and the extensive morphological changes shown. Similar results were reported for rats exposed organochlorine lindane for 30 days and fish exposed to endosulfan for 28 days (Vijaya Padma et al. 2011; Salvo et al. 2012; Shao et al. 2012). Similarly, banana bats (N. nana) consuming wastewater‐contaminated food also showed decreased total antioxidant capacity (Naidoo et al. 2015).
Endosulfan‐induced changes in antioxidant enzymes of fruit bats also included GST activities, which increased in the liver from exposed bats. GST plays a role in phase II reactions, involved with the biotransformation of exogenous compounds and contributing to the body detoxification by adding a GSH group to xenobiotics or their metabolites, so they become more water soluble and thus can be excreted more easily (Hayes et al. 2004; Hermes‐Lima 2004). GST levels found here were similar to those observed in fish and toads after exposure to the same insecticide (Ezemonye & Tongo 2010; Dong et al. 2013). An increase in GST activity can be understood in view of the fact that pesticides consume GSH through a GST‐catalysed reaction as a major way of detoxification, and these chemicals are expected to induce an increase in the enzyme activity as a protection mechanism (Timur et al. 2003; El‐Shenawy 2010; Dong et al. 2013).
There is evidence of the relationship between oxidative stress markers and histopathological changes in the liver of animals exposed to pesticides (Novaes et al. 2012). These data can be supported by the results from our study, as the groups treated with the pesticide showed a significant increase in cell degeneration and cell death areas. We also showed a pesticide dose–response effect in the liver, especially regarding the association of morphological results with oxidative stress. The enzymatic exhaustion and high levels of protein carbonyls found might be directly related to the accumulation of glycogen, fat and also the presence of pyknotic nuclei and collagen fibre deposits in the tissue. These processes usually indicate cell degeneration and are followed by cell death (Abdollahi et al. 2004; Amaral et al. 2012a,b). It is known that the deposition of extracellular matrix (ECM) components depends on the activation of star cells, which turn into myofibroblasts to produce collagen (Krishnasamy et al. 2016). These cells are activated by free radicals and lipid peroxidation products such as malondialdehyde, generated by injured hepatocytes. Therefore, levels of tissue stress markers and morphological changes cannot be analysed separately. Oxidative stress markers are important to evaluate the oxidant and antioxidant balance of tissues, and there is evidence that cellular damage caused by excessive ROS in bats can range from immunosuppression to DNA damage (Zocche et al. 2010; Lilley et al. 2013; Naidoo et al. 2015). This results mostly in loss of function and behaviour abnormalities (Clark & Stafford 1981) – immunosuppression, for instance, can increase bats vulnerability to diseases like the white nose syndrome, which is known to cause massive population declines (Bennett & Thies 2007; Kannan et al. 2010). Bat population declines associated with pesticide accumulation already are also described for the species Tadarida brasiliensis mexicana in New Mexico, USA (Clark 2001), and for Mystacine tuberculata in New Zealand (Dennis & Gartrell 2015).
In conclusion, the endosulfan also caused an imbalance between the production of ROS and its detoxification by inhibiting the enzymatic defence systems and therefore generating irreversible morphologic lesions. Considering that Artibeus literatus play an important role on seed dispersal and forest regeneration and are potentially continuously exposed to pesticides, we believe that future research evaluating other physiological changes caused by pesticides exposure are needed to elucidate the extent of commitment of their populations in nature.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding source
This study was funded by grants from Brazilian agency CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais) for financial support.
Supporting information
Appendix S1. Result.
Table S1. Endosulfan concentraction (ng/g) found in the diet, and tissues of Artibeus lituratus dietary exposed for 35 days
Figure S1. Chromatograms of liver samples from bats exposed to endosulfan.(a) EDS1: 1.05 g/l, where tR = 6.456:parath. tRion = 7.629: α‐endosulfan e tR = 8.878: β‐endosulfan; (b) EDS2: 2.1 g/l; (c) Control group.
Figure S2. Chromatograms of adipose tissue samples of from bats exposed to endosulfan. (a) EDS1: 1.05 g/l, where tR = 6.456:parath. tRion = 7.629: α‐endosulfan e tR = 8.878: β‐endosulfan; (b) EDS2: 2.1 g/l; (c) Control group.
Figure S3. Chromatograms of fruits samples offered to bats. (a) fruit peels not contaminated with endosulfan, where α‐endosulfan e tR = 10.78: β‐endosulfan = 11.59. (b) fruit peels contaminated with endosulfan solution (1.05 g/l). (c) Fruit peels contaminated with endosulfan solution (2.01 g/l).
Acknowledgments
We thank to Neriamara Martins Dias, Dr. Damiana Dinis Rosa, Dr. Sérgio Luis Pinto da Mata for general assistance during this research.
References
- Abdollahi M., Ranjbar A., Shadnia S., Nikfar S. & Rezaiee A. (2004) Pesticides and oxidative stress: a review. Med. Sci. Monit. 10, RA141–A147. [PubMed] [Google Scholar]
- Aebi H. (1984) Catalase in vitro. Methods Enzymol. 105, 121–126. doi: 10.1016/S0076‐6879(84)05016‐3. [DOI] [PubMed] [Google Scholar]
- Agrawal A. & Sharma B. (2010) Pesticides induced oxidative stress in mammalian systems. Int. J. Biol. Med. Res. 3, 90–104. [Google Scholar]
- Amaral T., Carvalho T., Silva M. et al (2012a) Short‐term effects of a spinosyn's family insecticide on energy metabolism and liver morphology in frugivorous bats Artibeus lituratus (Olfers, 1818). Brazilian J. Biol. 72, 299–304. doi:10.1590/S1519‐69842012000200010. [DOI] [PubMed] [Google Scholar]
- Amaral T.S., Carvalho T.F., Silva M.C. et al (2012b) Metabolic and histopathological alterations in the fruit‐eating bat Artibeus lituratus induced by the organophosphorous pesticide fenthion. Acta Chiropter. 14, 225–232. doi:10.3161/150811012X654420. [Google Scholar]
- Amin K. & Hashem K.S. (2012) Deltamethrin‐induced oxidative stress and biochemical changes in tissues and blood of catfish (Clarias gariepinus): antioxidant defense and role of alpha‐tocopherol. BMC Vet. Res. 8, 45. doi:10.1186/1746‐6148‐8‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androustsopoulos V.P., Hernandez A.F., Liesivuori J. et al (2013) A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology 307, 89–94. doi:10.1016/j.tox.2012.09.011. [DOI] [PubMed] [Google Scholar]
- ANVISA [Agência Nacional de Vigilância Sanitária‐ Ministério da Saúde]. (2013) Nota Técnica: Reavaliação Toxicológica do Ingrediente Ativo endossulfam. <http://portal.anvisa.gov.br/wps/wcm/connect/d3f25b80474586588f6fdf3fbc4c6735/Nota+t%C3%A9cnica.pdf?MOD=AJPERES>. Accessed may 2013. [Google Scholar]
- Astiz M., de Alaniz M.J.T. & Marra C.A. (2009a) The impact of simultaneous intoxication with agrochemicals on the antioxidant defense system in rat. Pestic. Biochem. Physiol. 94, 93–99. doi:10.1016/j.pestbp.2009.03.005. [Google Scholar]
- Astiz M., de Alaniz M.J.T. & Marra C.A. (2009b) Antioxidant defense system in rats simultaneously intoxicated with agrochemicals. Environ. Toxicol. Pharmacol. 28, 465–473. doi:10.1016/j.etap.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Bayat S., Geiser F., Kristiansen P. & Wilson S.C. (2014) Organic contaminants in bats: trends and new issues. Environ. Int. 63, 40–52. doi:10.1016/j.envint.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Bennett B.S. & Thies M.L. (2007) Organochlorine pesticide residues in guano of Brazilian free‐tailed bats, Tadarida brasiliensis Saint‐Hilaire, from East Texas. Bull. Environ. Contam. Toxicol. 78, 191–194. doi:10.1007/s00128‐007‐9089‐7. [DOI] [PubMed] [Google Scholar]
- Buege J.A. & Aust A.D. (1978) Microsomal lipid peroxidation methods. Enzymology 52, 302–310. [DOI] [PubMed] [Google Scholar]
- Clark D.R. (2001) DDT and the decline of free‐tailed bats (Tadarida brasiliensis) at Carlsbad Cavern, New Mexico. Arch. Environ. Contam. Toxicol. 40, 537–543. doi:10.1007/s002440010207. [DOI] [PubMed] [Google Scholar]
- Clark D.R. & Stafford C.J. (1981) Effects of DDE and PCB (Aroclor 1260) on experimentally poisoned female little brown bats (Myotis lucifugus): lethal brain concentrations. JPN. J. Tox. Env. Health 7, 925–934. doi:10.1080/15287398109530035. [DOI] [PubMed] [Google Scholar]
- Dennis G.C. & Gartrell B.D. (2015) Nontarget mortality of New Zealand lesser short‐tailed bats (Mystacina tuberculata) caused by diphacinone. J. Wildl. Dis. 51, 177–186. doi:10.7589/2013‐07‐160. [DOI] [PubMed] [Google Scholar]
- Dieterich S., Bieligk U., Beulich K., Hasenfuss G. & Prestle J. (2000) Gene expression of antioxidative enzymes in the human heart : increased expression of catalase in the end‐stage failing heart. Circulation 101, 33–39. doi:10.1161/01.CIR.101.1.33. [DOI] [PubMed] [Google Scholar]
- Dong M., Zhu L., Shao B. et al (2013) The effects of endosulfan on cytochrome P450 enzymes and glutathione S‐transferases in zebrafish (Danio rerio) livers. Ecotoxicol. Environ. Saf. 92, 1–9. doi:10.1016/j.ecoenv.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Droge W. (2002) Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95. doi:10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- El‐Demerdasha F., Dewer Y., El‐Mazoudy R.H. & Attia A.A. (2013) Kidney antioxidant status, biochemical parameters and histopathological changes induced by methomyl in CD‐1 mice. Exp. Toxicol. Pathol. 65, 897–901. doi:10.1016/j.etp.2013.01.002. [DOI] [PubMed] [Google Scholar]
- El‐Shenawy N.S. (2010) Effects of insecticides fenitrothion, endosulfan and abamectin on antioxidant parameters of isolated rat hepatocytes. Toxicol. In Vitro 24, 1148–1157. doi:10.1016/j.tiv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Ezemonye L. & Tongo I. (2010) Sublethal effects of endosulfan and diazinon pesticides on glutathione‐S‐transferase (GST) in various tissues of adult amphibians (Bufo regularis). Chemosphere 81, 214–217. doi:10.1016/j.chemosphere.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Fukushima T., Tanaka K., Heejin L.I.M. & Moriyama M. (2002) Mechanism of cytotoxicity of paraquat. Environ. Health Prev. Med. 7, 89–94. doi:10.1265/ehpm.2002.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel E.A., Fragionato Locali R., Katsumi Matsuoka P. et al (2008) Lung perfusion during cardiac surgery with cardiopulmonary bypass: is it necessary? Interact Cardiovasc Thorac Surg. 7, 1089‐1095. doi:10.1510/icvts.2008.184275. [DOI] [PubMed] [Google Scholar]
- Gerell R. & Gerell Lunderg K. (1993) Decline of a bat Pipistrellus pipistrellus population in an industrialized area in south Sweden. Biol. Conserv. 65, 153–157. doi:10.1016/0006‐3207(93)90444‐6. [Google Scholar]
- Gonçalves R.V., Novaes R.D., Sarandy M.M. et al (2016) Schizocalyx cuspidatus (A. St.‐Hil.) Kainul. & B. Bremer extract improves antioxidant defenses and accelerates the regression of hepatic fibrosis after exposure to carbon tetrachloride in rats. Nat. Prod. Res. 00, 000–000. (in press) [DOI] [PubMed] [Google Scholar]
- Habig W.H., Pabst M.J. & Jakoby W.B. (1974) Glutathione S‐ Transferases: the First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 249, 7130–7139. [PubMed] [Google Scholar]
- Hayes J.D., Flanagan J.U. & Jowsey I.R. (2004) Glutathione S‐Transferases. Annu. Rev. Pharmacol. Toxicol. 45, 51–88. doi:10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Hermes‐Lima M.(2004) Oxygen in biology and biochemistry: role of free radicals In: Functional Metabolism: Regulation and Adaptation, pp. 319–368, Wiley‐Liss, E‐Publishing Inc., New York: John Wiley & Sons. [Google Scholar]
- Hincal F., Gürbay A. & Giray B. (1995) Induction of lipid peroxidation and alteration of glutathione redox status by endosulfan. Biol. Trace Elem. Res. 47, 321–326. doi:10.1007/BF02790133. [DOI] [PubMed] [Google Scholar]
- Kang J.H., Park H., Chang Y.S. & Choi J.W. (2008) Distribution of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in human serum from urban areas in Korea. Chemosphere 73, 1625–1631. doi:10.1016/j.chemosphere.2008.07.087. [DOI] [PubMed] [Google Scholar]
- Kannan K., Yun S.H., Rudd R.J. & Wadsworth M.B. (2010) High concentrations of persistent organic pollutants including PCBs, DDT, PBDEs and PFOS in little brown bats with white‐nose syndrome in New York, USA. Chemosphere 80, 613–618. doi:10.1016/j.chemosphere.2010.04.060. [DOI] [PubMed] [Google Scholar]
- Krishnasamy Y., Ramshesh V.K., Gooz M., Schnellmann R.G., Lemasters J.J. & Zhong Z. (2016) Ethanol and High Cholesterol Diet Causes Severe Steatohepatitis and early liver fibrosis in Mice. PLoS ONE 11, e0163342. doi:10.1371/journal.pone.0163342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz T.H. & Anthony E.L.P. (1982) Age estimation and post‐natal growth in the bat Myotis lucifugus . J. Mammal. 63, 23–32. doi:10.2307/1380667. [Google Scholar]
- Kuvarega A.T. & Taru P. (2007) Accumulation of endosulfan in wild rat, Rattus norvegious as a result of application to soya bean in mazoe (Zimbabwe). Environ. Monit. Assess. 125, 333–345. doi:10.1007/s10661‐006‐9526‐9. [DOI] [PubMed] [Google Scholar]
- Levine R.L., Willians J.A., Stadtman E.R. & Shacter E. (1994) Carbonyl Assays for determination of Oxidatively Modified Proteins. Methods Enzymol. 233, 346–357. doi:10.1016/S0076‐6879(94)33040‐9. [DOI] [PubMed] [Google Scholar]
- Lilley T.M., Ruokolainen L., Meierjohann A. et al (2013) Resistance to oxidative damage but not immunosuppression by organic tin compounds in natural populations of Daubenton's bats (Myotis daubentonii). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 157, 298–305. doi:10.1016/j.cbpc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Limón‐Pacheco J. & Gonsebatt M.E. (2009) The role of antioxidants and antioxidant‐related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 674, 137–147. doi:10.1016/j.mrgentox.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Lewis Farr A. & Randall R.J. (1951) Protein Measurement with the Folin Phenol Reagent. Readings 193, 265–275. doi:10.1016/0304‐3894(92)87011‐4. [PubMed] [Google Scholar]
- Naidoo S., Vosloo D. & Schoeman M.C. (2015) Pollutant exposure at wastewater treatment works affects the detoxification organs of an urban adapter, the Banana Bat. Environ. Pollut. 208, 830–839. doi:10.1016/j.envpol.2015.09.056. [DOI] [PubMed] [Google Scholar]
- Novaes R.D., Gonçalves R.V., Cupertino M.C. et al (2012) Bark extract of Bathysa cuspidata attenuates extra‐pulmonary acute lung injury induced by paraquat and reduces mortality in rats. Int. J. Exp. Pathol. 93, 225–233. doi:10.1111/j.1365‐2613.2012.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez S. & Raisuddin S. (2005) Protein carbonyls: novel biomarkers of exposure to oxidative stress‐inducing pesticides in freshwater fish Channa punctata (Bloch). Environ. Toxicol. Pharmacol. 20, 112–117. doi:10.1016/j.etap.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Possamai F.P., Fortunato J.J., Agostinho F.R. et al (2007) Oxidative stress after acute and sub‐chronic malathion intoxication in Wistar rats. Environ Toxicol Pharmacol. 23, 198‐204. doi:10.1016/j.etap.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Raj J., Chandra M., Dogra T.D., Pahuja M. & Raina A. (2013) Determination of median lethal dose of combination of endosulfan and cypermethrin in wistar rat. Toxicol. Int. 20, 1–5. doi:10.4103/0971‐6580.111531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P.D., Huang B.W. & Tsuji Y. (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24, 981–990. doi:10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon A.B., Leonard S., Masamsetti V. et al (2009) The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis. FASEB J. 23, 2317–2326. doi:10.1096/fj.08‐122523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvo L.M., Bainy A.C., Ventura E.C. et al (2012) Assessment of the sublethal toxicity of organochlorine pesticide endosulfan in juvenile common carp (Cyprinus carpio) . J. Environ. Sci. Heal. A. 47, 1652–1658. doi:10.1080/10934529.2012.687236. [DOI] [PubMed] [Google Scholar]
- Schneeberger K., Czirják G.A. & Voigt C.C. (2014) Frugivory is associated with low measures of plasma oxidative stress and high antioxidant concentration in free‐ranging bats. Naturwissenschaften 101, 285–290. doi:10.1007/s00114‐014‐1155‐5. [DOI] [PubMed] [Google Scholar]
- Shao B., Zhu L., Dong M. et al (2012) DNA damage and oxidative stress induced by endosulfan exposure in zebrafish (Danio rerio). Ecotoxicology 21, 1533–1540. doi:10.1007/s10646‐012‐0907‐2. [DOI] [PubMed] [Google Scholar]
- Stechert C., Kolb M., Bahadir M., Djossa B.A. & Fahr J. (2014) Insecticide residues in bats along a land use‐gradient dominated by cotton cultivation in northern Benin, West Africa. Environ. Sci. Pollut. Res. 21, 8812–8821. doi:10.1007/s11356‐014‐2817‐8. [DOI] [PubMed] [Google Scholar]
- Swanepoel R.E., Racey P.A., Shore R.F. & Speakman J.R. (1999) Energetic effects of sublethal exposure to lindane on pipistrelle bats (Pipistrellus pipistrellus). Environ. Pollut. 104, 169–177. doi:10.1016/S0269‐7491(98)00196‐1. [Google Scholar]
- Timur S., Onal S., Karabay N.U., Sayim F. & Zihnioglu F. (2003) In vivo effects of Malathion on Glutathione S‐transferase and acetylcholinesterase activities in various tissues of neonatal rats. Turk J. Zool. 27, 247–252. [Google Scholar]
- Vijaya Padma V., Sowmya P., Arun Felix T., Baskaran R. & Poornima P. (2011) Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem. Toxicol. 49, 991–998. doi:10.1016/j.fct.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Vizzotto L.D. & Taddei V.A. (1973) Chave para determinação de quirópteros brasileiros. São José do Rio Preto: Universidade Estadual Paulista. [Google Scholar]
- Wilhelm Filho D., Althoff S.L., Dafré A.L. & Boveris A. (2007) Antioxidant defenses, longevity and ecophysiology of South American bats. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 146, 214–220. doi:10.1016/j.cbpc.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Zocche J.J., Leffa D.D., Damiani A.P. et al (2010) Heavy metals and DNA damage in blood cells of insectivore bats in coal mining areas of Catarinense coal basin. Brazil. Environ. Res. 110, 684–691. doi:10.1016/j.envres.2010.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Result.
Table S1. Endosulfan concentraction (ng/g) found in the diet, and tissues of Artibeus lituratus dietary exposed for 35 days
Figure S1. Chromatograms of liver samples from bats exposed to endosulfan.(a) EDS1: 1.05 g/l, where tR = 6.456:parath. tRion = 7.629: α‐endosulfan e tR = 8.878: β‐endosulfan; (b) EDS2: 2.1 g/l; (c) Control group.
Figure S2. Chromatograms of adipose tissue samples of from bats exposed to endosulfan. (a) EDS1: 1.05 g/l, where tR = 6.456:parath. tRion = 7.629: α‐endosulfan e tR = 8.878: β‐endosulfan; (b) EDS2: 2.1 g/l; (c) Control group.
Figure S3. Chromatograms of fruits samples offered to bats. (a) fruit peels not contaminated with endosulfan, where α‐endosulfan e tR = 10.78: β‐endosulfan = 11.59. (b) fruit peels contaminated with endosulfan solution (1.05 g/l). (c) Fruit peels contaminated with endosulfan solution (2.01 g/l).


