Highlight
Non-contiguous clustering of small stomata in Begonia favours rapid stomatal closure by comparison with larger, solitary stomata, and enhances WUE under low light conditions.
Keywords: Begonia, gas exchange, photosynthesis, stomatal closure, WUE.
Abstract
Stomata are microscopic pores formed by specialized cells in the leaf epidermis and permit gaseous exchange between the interior of the leaf and the atmosphere. Stomata in most plants are separated by at least one epidermal pavement cell and, individually, overlay a single substomatal cavity within the leaf. This spacing is thought to enhance stomatal function. Yet, there are several genera naturally exhibiting stomata in clusters and therefore deviating from the one-cell spacing rule with multiple stomata overlaying a single substomatal cavity. We made use of two Begonia species to investigate whether clustering of stomata alters guard cell dynamics and gas exchange under different light and dark treatments. Begonia plebeja, which forms stomatal clusters, exhibited enhanced kinetics of stomatal conductance and CO2 assimilation upon light stimuli that in turn were translated into greater water use efficiency. Our findings emphasize the importance of spacing in stomatal clusters for gaseous exchange and plant performance under environmentally limited conditions.
Introduction
Stomata are pores found in the epidermis of most aerial parts of plants and are formed between a specialized pair of cells, the guard cells. Stomata facilitate the uptake of CO2 at the expense of water vapour release via transpiration (Hetherington and Woodward, 2003). Hence, stomata control the trade-off between transpirational water loss and carbon gain, and therefore they play a crucial role in water use efficiency (WUE). Regulation of gas exchange is achieved by dynamically controlling the stomatal pore relative to environmental changes including light quality and intensity, ambient CO2 concentration, and humidity (Aphalo and Jarvis, 1991; Hetherington and Woodward, 2003; Shimazaki et al., 2007). Stomatal movements are dependent on the transport and accumulation of osmotically active solutes (Blatt, 2000; Lawson and Blatt, 2014) as well as on the mechanistic properties of cells to allow lateral movements of guard cells (Franks and Farquhar, 2007).
Stomatal behaviour is also thought to be affected by the developmental pathway of stomatal lineage. The majority of plant species follow a ‘one-cell spacing rule’ during epidermal development that leads to the separation of stomata by at least one epidermal cell (Geisler et al., 2000; Peterson et al., 2010; Pillitteri and Dong, 2013). However, there are several genera that diverge from this rule, including members of the Begonia genus (Nebauer, 1967). Stomatal clustering in Begonia has been considered to be an adaptation for growth in ecological niches, imposing lower evaporative demand. For example, Begonia heracleifolia and Begonia nelumbifolia have been found to exhibit more stomatal clusters when growing on rocky surfaces near waterfalls rather than on well-watered soils (Hoover, 1986). In addition, Tang et al. (2002) reported that stomata in clusters share the same substomatal cavity and suggested a positive correlation between stomatal clustering and multiple layers of epidermis, which is regarded as a drought adaptation trait. No quantitative data are available confirming an advantage of species with stomatal clusters to grow in dry environments. To date, only studies with Arabidopsis transgenic lines have reported on the impact of stomatal clustering in leaf gas exchange and plant physiology (Dow et al., 2014; Lehmann and Or, 2015; Papanatsiou et al., 2016). Stomatal clustering in Arabidopsis mutants resulted in reduced stomatal conductance and assimilation of CO2 (Dow et al., 2014), and in compromised movements of stomatal pores that were related to the altered ion transport in stomata found in clusters (Papanatsiou et al., 2016).
We have revisited the physiological impact of stomatal clustering, by employing two species from the Begonia genus, B. coccinea and B. plebeja, the latter of which naturally forms stomatal clusters whereas the former does not (Burt-Utley, 1985). Stomatal clustering in Begonia differs from that of mutants of the model plant Arabidopsis thaliana. We find that stomatal clusters in B. plebeja are non-contiguous and therefore stomata are not in direct contact with each other despite occupying the same substomatal cavity. We also report on further morphological characteristics of stomata in Begonia species and their effects on stomatal behaviour. Our results emphasize the importance of spacing between stomata to allow plants to adjust gaseous exchange responses and enhance WUE in order to inhabit diverse niches.
Materials and methods
Plant material and growth conditions
Begonia coccinea and Begonia plebeja plants were obtained from Glasgow Botanic Gardens. Plants were grown under 70 μmol m–2 s–1 light in long-day conditions (16/8 h of light/dark), 22 °C/18 °C (light/dark) temperature, and 60%/70% (light/dark) relative humidity. Chemicals were reagent grade from Sigma-Aldrich.
Gas exchange
Gas exchange was carried out using the LI-COR 6400 XT Infrared Gas Analyser (LICOR Biosciences) standard leaf chamber. Measurements were carried out at 22 °C, 60% relative humidity, and at 390 ppm CO2. Gas exchange responses were measured using an external light source (LI-COR 6400-18) and after leaves were adapted to the dark for 1.5 h. The spectral profile of the light source was adjusted to that of the growth rooms where plants were grown. At least three plants per species were measured on different days at the same point of the diurnal cycle. Data were normalized to a stomatal ratio of 0 since stomata in the Begonia species investigated are only found in the abaxial surface of the leaf.
Stomatal assays
Stomatal patterns of Begonia plants were quantified by impressions taken from the abaxial area of mature young leaves. Xantopren VL Plus (silicon material, Heraeus, UK) and Activator (hardener, Heraeus, UK) were mixed in a ratio of 4:1, spread over the area of interest, and left to harden for at least 3 min before using a clear nail varnish to obtain the positive impression of the leaf epidermis.
Stomatal apertures were recorded from epidermal peels following pre-incubations in opening buffer (5 mM MES-NaOH pH 6.15, 60 mM KCl) for 2 h under 100 μmol m–2 s–1 light to open stomata fully. After imaging, epidermal peels were incubated for 5 min in depolarizing buffer supplemented with 20 μM fluorescein diacetate (FDA) and examined for fluorescence to confirm viability before the following further analysis as described before (Papanatsiou et al., 2015).
Maximum stomatal opening, stomatal size, and density were estimated as described before (Papanatsiou et al., 2016). Specifically, maximum stomatal aperture was estimated as
| (1) |
where Wa is the aperture width and La is the aperture length.
Stomatal size was obtained from
| (2) |
where Ws is the stoma width and Ls is the stoma length. The theoretical maximum stomatal conductance for water vapour (GWmax) was calculated as described by Franks et al. (2009)
| (3) |
where d is the diffusivity of water vapour in air (m2 s–1), v is the molar volume of air at 1 atm and 22 °C (m3 mol–1), SD is stomatal density (m–2), and l is the pore depth (m), estimated as the width of a fully inflated guard cell.
Stomatal closure was initiated after guard cells were fully open by superfusion with 10 mM MES-KCl, pH 6.1 supplemented with 6 mM CaCl2. Measurements were carried out on a cell-by-cell basis, and results are reported as means ±SE of n >80 stomata.
Both stomatal pattern and aperture were recorded by digital photomicrography using a Zeiss Axiovert200 microscope with Planapo ×20/0.80 objectives and an AxioCam HRc digital camera (Zeiss, Jena).
Data and statistical analysis
Data analysis and curve fitting were carried out using SigmaPlot 12 (Systat Software Inc.). Statistical significance was determined using Student’s t-test at P<0.05.
Results
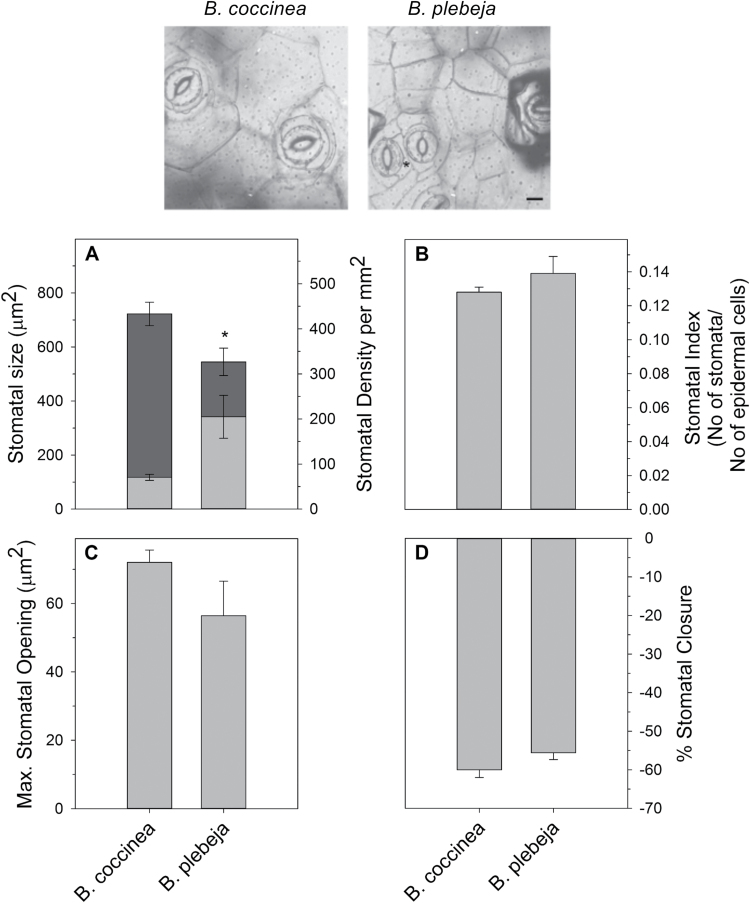
We employed two Begonia species with distinct stomatal patterning on B. coccinea and B. plebeja leaves (Fig. 1). Stomata in B. plebeja are found in clusters separated by a special type of non-stomatal cells (black asterisks; Fig. 1). In contrast, stomata in B. coccinea are solitary and surrounded by large epidermal cells. The mean stomatal density ranged from 70 to 200 stomata mm–2, with B. plebeja having significantly more stomata than B. coccinea. The stomatal size of B. plebeja was 24% smaller in comparison with B. coccinea (Fig. 1A). These data point to an inverse correlation between stomatal density and size that is in agreement with previous studies (Hunt and Gray, 2009; Doheny-Adams et al., 2012; Papanatsiou et al., 2015). However, when we calculated the stomatal index, which is the ratio of the number of stomata over the number of non-stomatal cells, no statistically significant differences between the two Begonia species were observed (Fig. 1B). The latter observation arises from the presence of the extra non-stomatal cells in stomatal clusters in B. plebeja.
Fig. 1.
Stomatal characteristics of two Begonia species. The upper panel displays representative micrographs from the abaxial side of B. coccinea and B. plebeja. Scale bar=20 μm. Stomatal patterning was determined from epidermal peels of B. coccinea and B. plebeja. Graphs represent (A) stomatal density (light grey) and stomatal size (dark grey), (B) stomatal stomatal index, (C) maximum stomatal opening, and (D) percentage of stomatal closure relative to the maximum for that species. Data are means ±SE of n >60 stomata. The asterisk indicates a statistical difference (P<0.05), as determined by two-tailed t-test.
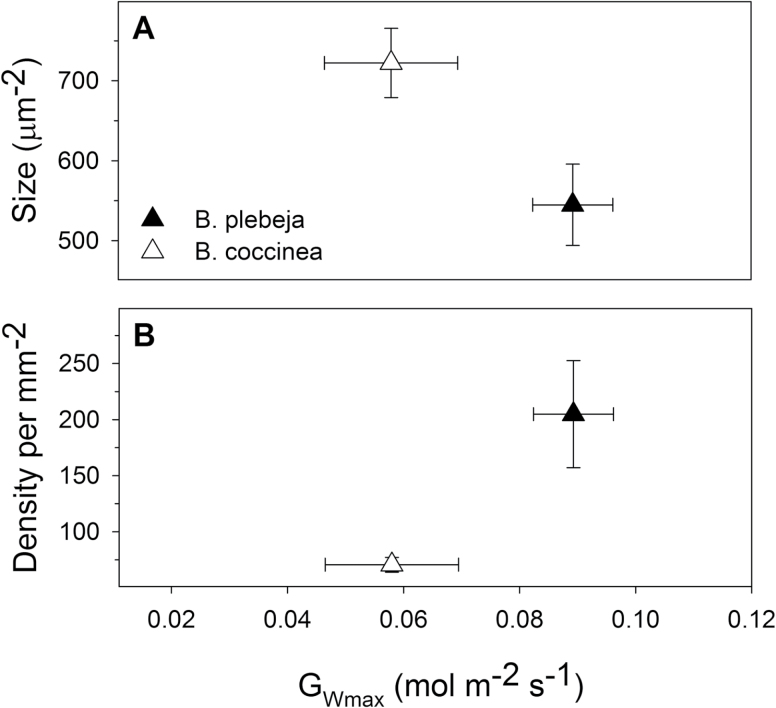
Stomatal clustering has been shown to impact stomatal movements negatively in Arabidopsis (Papanatsiou et al., 2016). We therefore examined stomatal opening in the two Begonia species by treating epidermal peels with opening buffer (60 mM KCl-MES, pH 6.1) under high light intensity for 2 h using standard protocols (Papanatsiou et al., 2015). Maximum opening of stomatal pores was measured as described before (Doheny-Adams et al., 2012; Papanatsiou et al., 2016). Stomata of B. plebeja opened 22% less compared with B. coccinea, albeit that this difference was not statistically significant (Fig 1C). Similarly, no significant differences were observed when we subjected the epidermal peels from leaves of both Begonia species to closing buffer (10 mM KCl-MES+6 mM CaCl2, pH 6.1) and darkness for 90 min (Fig. 1D). Based on measurements of maximum stomatal opening and the geometry of stomata, we calculated the anatomical conductance to water vapour (GWmax) of the two Begonia species according to Equation 1. GWmax describes the theoretical capacity of the leaf for gaseous exchange in relation to the total and maximum pore area (Franks and Beerling, 2009). The GWmax data agree with previous reports suggesting elevated GWmax in species having more stomata occupying the lead epidermis (Franks and Beerling, 2009). Indeed B. plebeja was estimated to have 34% greater GWmax when compared with B. coccinea plants (Fig. 2).
Fig. 2.
Relationship of maximum anatomical stomatal conductance to stomatal characteristics. Maximum anatomical stomatal conductance (GWmax) was determined from stomatal geometry and maximum stomatal opening for B. coccinea (open triangles) and B. plebeja (filled triangles). Graphs represent the relationship of (A) stomatal size and (B) stomatal density to maximum anatomical stomatal conductance (GWmax). Data are means ±SE of n >60 stomata.
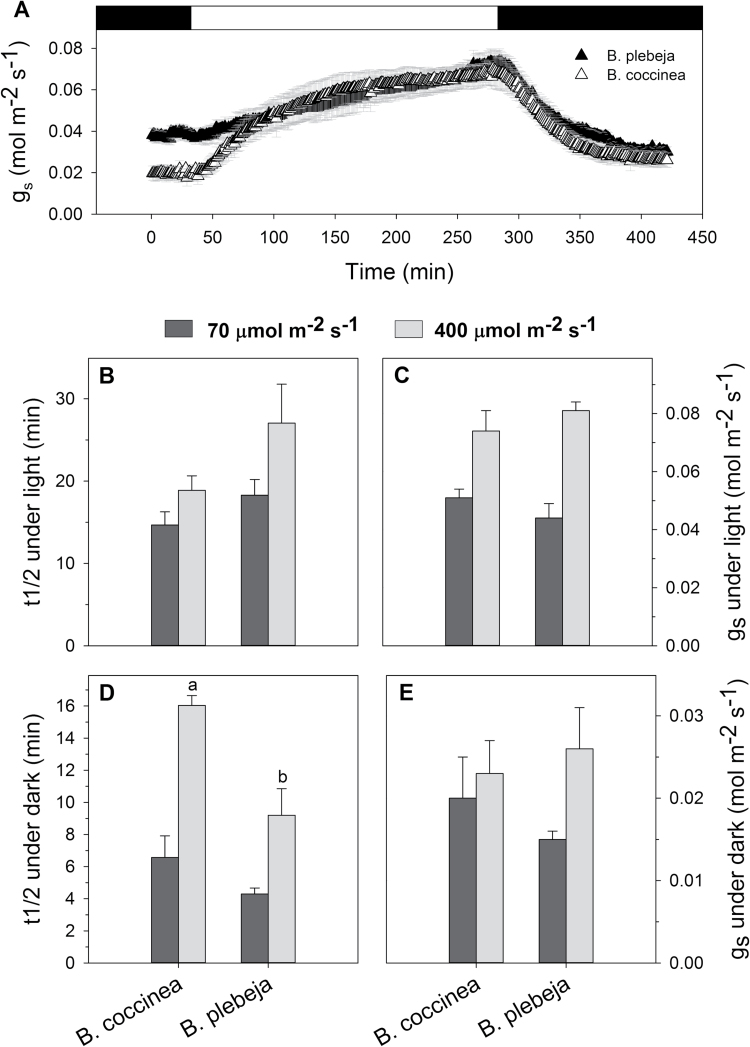
We also measured diffusive stomatal conductance (gs) from the Begonia species in response to light and dark treatments to investigate whether stomatal clustering of B. plebeja adversely influences gaseous exchange. gs was measured from leaves of B. coccinea and B. plebeja plants that were dark adapted before exposure to a light intensity of either 70 μmol m–2 s–1 or 400 μmol m–2 s–1 and subsequently when transferred back to darkness (Fig. 3A). We determined the kinetics of gs responses in light and dark treatments via non-linear curve fitting to either the opening or closing response (Fig. 3). The steady-state gs values in the dark and each of the light regimes were statistically indistinguishable between B. coccinea and B. plebeja (Fig. 3C, E). Stomata of B. coccinea and B. plebeja responded at a similar speed upon exposure to a light intensity of 70 μmol m–2 s–1 and on the subsequent transfer to darkness. Similarly, exposure of leaves to high light intensity did not result in any significant difference in the opening half-times of gs of B. coccinea and B. plebeja, with those being 19 ± 2 min and 27 ± 4 min, respectively. Interestingly, we noted that B. plebeja responded 42% faster in closure response when the leaves were transferred from high light back to darkness.
Fig. 3.
Stomatal patterning affects the gas exchange responses. (A) Representation of the experimental design measuring stomatal conductance (gs) response from dark-adapted leaves exposed to light and on subsequent transfer back to darkness. Graphs represent half-times (B and D) and steady-state rates (C and E) of gs upon exposure to a light intensity of 70 μmol m–2 s–1 (dark grey) and 400 μmol m–2 s–1 (light grey) and on transfer back to darkness. The kinetics of gas exchange responses were extracted by separately fitting exponential function to the opening and closing response. Data are means ±SE of n=3 plants per species. Lower case letters indicate statistical differences (P<0.05), as determined by two-tailed t-test.
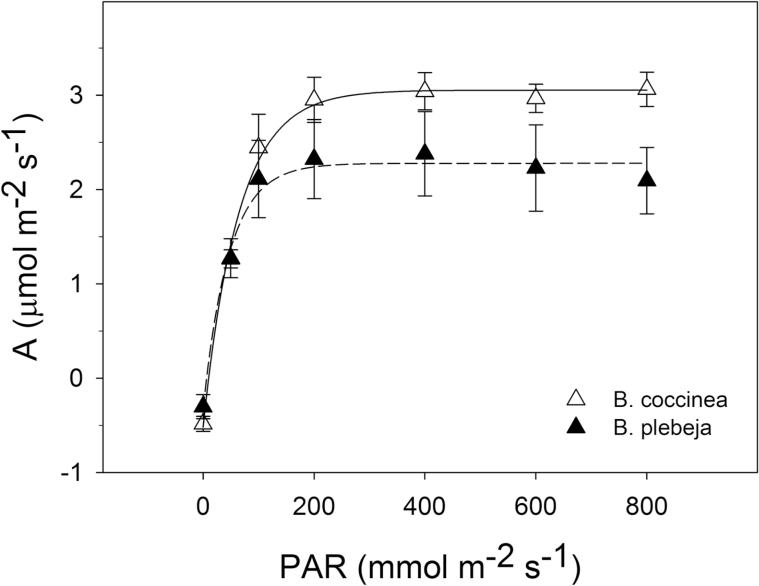
We carried out analysis of light responses to determine the steady-state assimilation rate of CO2 (A) over a range of distinct quantum flux densities (Fig. 4). Both B. coccinea and B. plebeja responded similarly to increasing photosynthetic active radiation. The CO2 assimilation response followed an exponential rise and reached a maximum rate after leaves were exposed to 200 μmol m–2 s–1 of light. The CO2 assimilation rates were undifferentiated between the two Begonia species at light intensities <400 μmol m–2 s–1. In contrast, B. plebeja showed smaller CO2 assimilation rates in comparison with those of B. coccinea at saturating light intensities.
Fig. 4.
Effect of photosynthetic active radiation on CO2 assimilation. Light curves from B. coccinea (open triangles) and B. plebeja (filled triangles) display the assimilation of CO2 over a series of quantum flux densities ranging from 0 to 800 μmol m–2 s–1. Data were jointly fitted to an exponential rise curve, and fits are shown for B. coccinea (solid line) and B. plebeja (dashed line). Data are means ±SE of n=3 plants per species.
We also performed A/Ci curves where A was estimated over a range of CO2 concentrations (see Supplementary Fig. S1 at JXB online). Fitting a non-linear regression model to the A/Ci curves allows us to distinguish among three distinct parameters contributing to photosynthetic machinery: the carboxylation rate of Rubisco (Vcmax); the electron transport (J); and the triose phosphate use (TPU) reactions (Sharkey et al., 2007). All three photosynthesis-related biochemical reactions were statistically indistinguishable between the two Begonia species at the lower light intensities.
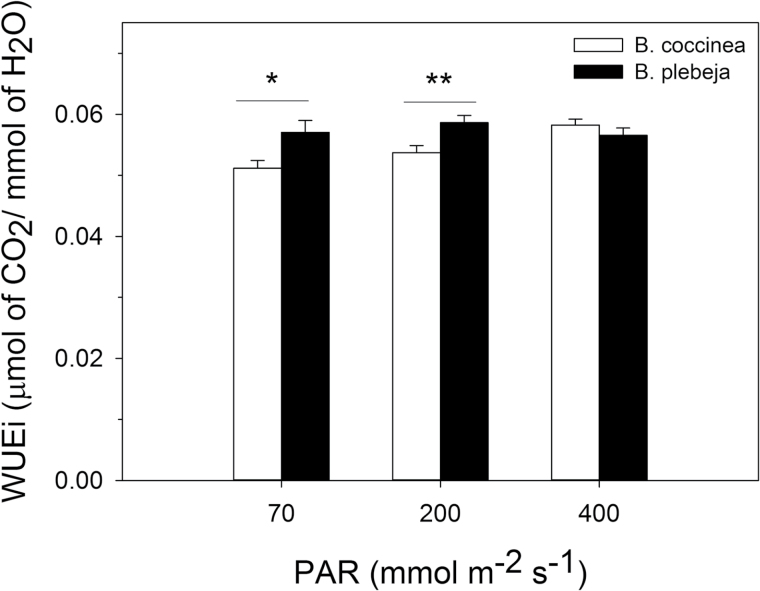
The above-mentioned differences in CO2 assimilation prompted us to investigate whether the distinct stomatal patterning and behaviour of the two Begonia species would influence WUE depending on the light regime. We therefore measured the intrinsic WUE (WUEi), calculated here as the ratio of the CO2 assimilation rate and stomatal conductance at 70, 200, and 400 μmol m–2 s–1 of light (Fig. 5). To our surprise, B. plebeja plants appear to show an increase in the WUEi of ~30% compared with B. coccinea plants at light intensities of 70 μmol m–2 s–1 and 200 μmol m–2 s–1. Yet, at the saturating light intensity, WUEi was statistically undifferentiated between the two species.
Fig. 5.
Intrinsic water use efficiency (WUEi) of Begonia plants under three light regimes. The WUEi of B. coccinea (white bars) and B. plebeja (black bars) was estimated as the ratio of the maximum CO2 assimilation rate over stomatal conductance at a light intensity of 70, 200, and 400 μmol m–2 s–1. Data are means ±SE of n=3 plant per species. Asterisks indicate statistically significant differences (P<0.05) between the two Begonia species at each light intensity, as determined by two-tailed t-test.
Discussion
The reduction in diffusive capacity of stomata found in clusters has recently been uncovered through studies employing mutants of the model organism Arabidopsis that show contiguous stomatal clusters [i.e. stomata are in direct contact (Dow et al., 2014; Lehmann and Or, 2015; Papanatsiou et al., 2016)]. Yet, species naturally having altered stomatal patterning, such as B. plebeja, display non-contiguous stomatal clusters (see Fig. 1). This unique morphological property of B. plebeja makes it a great tool to enrich further the selection of plants with different stomatal spatial arrangements, and therefore characterize how the mechanistic properties of stomata influence leaf gaseous exchange. We note, however, that B. coccinea and B. plebeja differ in many genetic and other morphological aspects and therefore we do not conclude that their difference in stomatal morphology is the sole driving force for their different adaptive strategies.
To the extent that B. plebeja shows non-contiguous stomatal clusters, it resembles Arabidopsis mutants with high numbers of small satellite and solitary stomata (Doheny-Adams et al., 2012; Dow et al., 2014; Hepworth et al., 2015). Reducing the size of the guard cells surrounding the stomatal pore has the effect of increasing the ratio of membrane surface area to guard cell volume. Provided the density of membrane transporters per unit surface area is nearly constant, a decrease in guard cell size can be expected to accelerate the solute flux per unit volume proportionally, thereby allowing for faster responses to environmental transients. This strategy is well documented in studies of Arabidopsis (Schlüter et al., 2003; Tanaka et al., 2013) and other plant species (Franks and Farquhar, 2007; Drake et al., 2013; Lawson and Blatt, 2014), and it is consistent with mathematical models that take into account the geometry of guard cells and the guard cell complex (Doheny-Adams et al., 2012; Dow et al., 2014, Lehman and Or, 2015) as well as membrane transport (Chen et al., 2012; Wang et al., 2012; Lawson and Blatt, 2014). Indeed, we observed a faster closing response of B. plebeja leaves, especially when this was commenced from a low light intensity (see Fig. 3).
Under lower light, gaseous exchange and therefore WUE is dependent on the light-limited photosynthetic reactions, whereas at high light intensities the WUE is mainly controlled by stomatal conductance (Lawson, 2009; Hummel et al., 2010; Eisenach et al., 2012; Lawson and Blatt, 2014). Thus, one can speculate that the enhanced dynamic movements of stomata together with the elevated WUE at limited photosynthetic conditions could be advantageous to the performance of the species. Reports on the habitat of B. plebeja suggest that this species is often found in rocks close to waterfalls, which limit water supply due to low water retention, and at the lower levels of forest canopy (Tebitt, 2005). In addition, Hoover (1986) suggested that stomatal clustering in Begonia species is an adaptive strategy to water-restricted environments. The altered stomatal patterning has previously been shown to affect gas exchange responses in Arabidopsis (Schlüter et al., 2003; Franks et al., 2009; Dow et al., 2014); high stomatal density resulted in an increase in CO2 assimilation (Tanaka et al., 2013), while the decrease in stomatal number resulted in the reduction of transpirational water loss (Yoo et al., 2010; Doheny-Adams et al., 2012). It is therefore suggested that stomatal patterning might provide a tool for fine-tuning the trade-off between these two processes, thus improving WUE (Drake et al., 2013; Lawson and Blatt, 2014).
Collectively, the data describe the difference in the spatial arrangement of stomata in the two Begonia species, and point to an unaltered dynamic range of movement of stomata found in clusters compared with those appearing solitary. Most importantly, we argue that the non-contiguous clustering of small stomata is a favourable trait over solitary large stomata by virtue of the faster stomatal closing response and the enhanced WUEi, especially under low light conditions. We speculate that high numbers of small stomata residing over the same substomatal cavity can confer an advantage to plants subjected to low water regimes. Undoubtedly, future comparative studies employing natural and experimental plant populations with distinct stomatal patterning should elucidate the above speculation and assess the impact of stomatal clustering in plant adaptation and performance.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. A/Ci curves of B. coccinea and B. plebeja plants under two light regimes.
Authors contribution
MP carried out the gas exchange measurements and stomatal assays; MP and MRB analysed the data; MP, AA, and MRB wrote the article.
Supplementary Material
Acknowledgements
We are grateful to Glasgow Botanic Gardens for supply of Begonia species. This work was supported by the M.L. MacIntyre Begonia Trust (PhD studentship to MP), the Biotechnology and Biological Sciences Research Council (grants BB/I024496/1, BB/K015893/1, BB/L001276/1, and BB/M01133X/1 to MRB), and EU grant NEURICE 678168 to MRB.
References
- Aphalo J, Jarvis PG. 1991. Do stomata respond to relative humidity? Plant, Cell and Environment 14, 127–132. [Google Scholar]
- Blatt MR. 2000. Cellular signaling and volume control in stomatal movements in plants. Annual Review of Cell and Developmental Biology 16, 221–241. [DOI] [PubMed] [Google Scholar]
- Burt-Utley K. 1985. A revision of Central American species of Begonia section Gireoudia (Begoniaceae). Tulane Studies in Zoology and Botany 25, 103–104. [Google Scholar]
- Chen ZH, Hills A, Bätz U, Amtmann A, Lew VL, Blatt MR. 2012. Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiology 159, 1235–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doheny-Adams T, Hunt L, Franks PJ, Beerling DJ, Gray JE. 2012. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow GJ, Berry JA, Bergmann DC. 2014. The physiological importance of developmental mechanisms that enforce proper stomatal spacing in Arabidopsis thaliana. New Phytologist 201, 1205–1217. [DOI] [PubMed] [Google Scholar]
- Drake PL, Froend RH, Franks PJ. 2013. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. Journal of Experimental Botany 64, 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach C, Chen ZH, Grefen C, Blatt MR. 2012. The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K+ channel activity with vegetative growth. The Plant Journal 69, 241–251. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Beerling DJ. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences, USA 106, 10343–10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Drake PL, Beerling DJ. 2009. Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: an analysis using Eucalyptus globulus. Plant, Cell and Environment 32, 1737–1748. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD. 2007. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiology 143, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Nadeau J, Sack FD. 2000. Oriented asymmetric divisions that generate the stomatal spacing pattern in arabidopsis are disrupted by the too many mouths mutation. The Plant Cell 12, 2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth C, Doheny-Adams T, Hunt L, Cameron DD, Gray JE. 2015. Manipulating stomatal density enhances drought tolerance without deleterious effect on nutrient uptake. New Phytologist 208, 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI. 2003. The role of stomata in sensing and driving environmental change. Nature 424, 901–908. [DOI] [PubMed] [Google Scholar]
- Hoover WS. 1986. Stomata and stomatal clusters in Begonia: ecological response in two Mexican species. Biotropica 18, 16–21. [Google Scholar]
- Hummel I, Pantin F, Sulpice R, et al. 2010. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: an integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiology 154, 357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L, Gray JE. 2009. The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development. Current Biology 19, 864–869. [DOI] [PubMed] [Google Scholar]
- Lawson T. 2009. Guard cell photosynthesis and stomatal function. New Phytologist 181, 13–34. [DOI] [PubMed] [Google Scholar]
- Lawson T, Blatt MR. 2014. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiology 164, 1556–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann P, Or D. 2015. Effects of stomata clustering on leaf gas exchange. New Phytologist 207, 1015–1025. [DOI] [PubMed] [Google Scholar]
- Nebauer SG. 1967. Bemerkungen uber den Bau der Begoniaceen. Berichte der Deutschen Botanischen Gesellschaft 80, 80–97. [Google Scholar]
- Papanatsiou M, Amtmann A, Blatt MR. 2016. Stomatal spacing safeguards stomatal dynamics by facilitating guard cell ion transport independent of the epidermal solute reservoir. Plant Physiology 172, 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanatsiou M, Scuffi D, Blatt MR, García-Mata C. 2015. Hydrogen sulfide regulates inward-rectifying K+ channels in conjunction with stomatal closure. Plant Physiology 168, 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson KM, Rychel AL, Torii KU. 2010. Out of the mouths of plants: the molecular basis of the evolution and diversity of stomatal development. The Plant Cell 22, 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Dong J. 2013. Stomatal development in Arabidopsis. The Arabidopsis Book 11, e0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter U, Muschak M, Berger D, Altmann T. 2003. Photosynthetic performance of an Arabidopsis mutant with elevated stomatal density (sdd1-1) under different light regimes. Journal of Experimental Botany 54, 867–874. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. 2007. Fitting photosynthetic carbon dioxide response curves for C(3) leaves. Plant, Cell and Environment 30, 1035–1040. [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T. 2007. Light regulation of stomatal movement. Annual Review of Plant Biology 58, 219–247. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sugano SS, Shimada T, Hara-Nishimura I. 2013. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytologist 198, 757–764. [DOI] [PubMed] [Google Scholar]
- Tang M, Hu YX, Lin JX, Jin XB. 2002. Developmental mechanism and distribution pattern of stomatal clusters in Begonia peltatifolia. Acta Botanica, 44, 384–389. [Google Scholar]
- Tebitt MC. 2005. Begonias: cultivation, identification and natural history. Portland, OR: Oregon Timber Press. [Google Scholar]
- Wang Y, Papanatsiou M, Eisenach C, Karnik R, Williams M, Hills A, Lew VL, Blatt MR. 2012. Systems dynamic modeling of a guard cell Cl– channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiology 160, 1956–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo CY, Pence HE, Jin JB, Miura K, Gosney MJ, Hasegawa PM, Mickelbart MV. 2010. The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. The Plant Cell 22, 4128–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.