Highlight
We model the impact of improving photosynthesis on rice productivity. We consider all major photosynthesis-enhancing approaches under one umbrella and scale them from leaf photosynthesis to crop production.
Keywords: Crop modelling, crop productivity, GECROS, genetic transformation, photosynthesis, radiation use efficiency, simulation, water use efficiency, yield potential.
Abstract
Various genetic engineering routes to enhance C3 leaf photosynthesis have been proposed to improve crop productivity. However, their potential contribution to crop productivity needs to be assessed under realistic field conditions. Using 31 year weather data, we ran the crop model GECROS for rice in tropical, subtropical, and temperate environments, to evaluate the following routes: (1) improving mesophyll conductance (gm); (2) improving Rubisco specificity (Sc/o); (3) improving both gm and Sc/o; (4) introducing C4 biochemistry; (5) introducing C4 Kranz anatomy that effectively minimizes CO2 leakage; (6) engineering the complete C4 mechanism; (7) engineering cyanobacterial bicarbonate transporters; (8) engineering a more elaborate cyanobacterial CO2-concentrating mechanism (CCM) with the carboxysome in the chloroplast; and (9) a mechanism that combines the low ATP cost of the cyanobacterial CCM and the high photosynthetic capacity per unit leaf nitrogen. All routes improved crop mass production, but benefits from Routes 1, 2, and 7 were ≤10%. Benefits were higher in the presence than in the absence of drought, and under the present climate than for the climate predicted for 2050. Simulated crop mass differences resulted not only from the increased canopy photosynthesis competence but also from changes in traits such as light interception and crop senescence. The route combinations gave larger effects than the sum of the effects of the single routes, but only Route 9 could bring an advantage of ≥50% under any environmental conditions. To supercharge crop productivity, exploring a combination of routes in improving the CCM, photosynthetic capacity, and quantum efficiency is required.
Introduction
Yields of major crops have increased steadily during the last decades. Fischer et al., (2014) claimed that, in order to ensure food and energy security for a growing and increasingly demanding population, staple crop production will need to grow by 60% from 2010 to 2050, with the greatest increases in the next 20 years. They further stressed that higher rates of increase than the current rate should be aimed for to drive faster reductions in world hunger and to guard against unanticipated negative contingencies, for example as a result of increasing frequencies of extreme weather under global climate change.
Crop yield per area of land is the production of mass per unit area multiplied by harvest index. Yield gains associated with the first Green Revolution in cereal crops such as wheat and rice were mainly due to increased harvest index by introducing (semi-)dwarfing genes (e.g. Miflin, 2000; Sadras and Lawson, 2011). For further progress to be made, improvement in crop mass production via increasing leaf and canopy photosynthetic capacity and efficiency should be explored (Long et al., 2006; Murchie et al., 2009; Parry et al., 2011; Ort et al., 2015). Evidence suggests that genetic variation in leaf photosynthesis has not been exploited to be incorporated into crop cultivars (e.g. Driever et al., 2014), except for recent releases in which yield gains were accompanied, to some extent, by traits related to increased leaf photosynthesis (Fischer et al., 2014).
Arguably, exploiting natural genetic variation is still the most feasible approach to improve yield traits including photosynthesis (Flood et al., 2011). For example, exploring natural variation in mesophyll conductance for CO2 diffusion (gm) may improve photosynthesis (Gu et al., 2012; Flexas et al., 2013; Chen et al., 2014). However, very often there is little correlation between leaf photosynthesis and crop productivity across germplasm (Driever et al., 2014; Koester et al., 2016) or across individual lines of a segregating population (e.g. Gu et al., 2014a, b), partly because natural variation in leaf photosynthesis and underlying traits is generally small (Driever et al., 2014). To enhance crop productivity at a greater pace, genetic engineering and synthetic biology approaches to improving leaf photosynthesis should be explored (Long et al., 2006, 2015; Singh et al., 2014; Ort et al., 2015; Kromdijk and Long 2016).
Major crops such as rice follow the pathway of C3 photosynthesis. Compared with C4 crops such as maize, C3 crops have lower photosynthetic productivity primarily because ~20–35% of the carbohydrate is lost through photorespiration (Long et al., 2006; Walker et al., 2016), resulting from the oxygenation of ribulose-1,5-biphosphate by Rubisco, the primary enzyme for CO2 fixation. Various genetic engineering routes to enhance C3 leaf photosynthesis have been proposed to suppress photorespiration, thereby improving crop productivity. These include: replacing Rubisco with foreign forms with higher specificity for CO2 relative to O2 (Sc/o) (Whitney et al., 2001; Zhu et al., 2004; Parry et al., 2011), designing a photorespiratory bypass (Kebeish et al., 2007), and transforming the C4 CO2-concentrating mechanism (CCM) (e.g. von Caemmerer et al., 2012) or the cyanobacterial bicarbonate-based CCM (Price et al., 2011, 2013; Lin et al., 2014) into main C3 crops.
Some of the engineering approaches have already made progress and were evaluated experimentally for mass production using model plants, such as Arabidopsis thaliana engineered with a photorespiratory bypass (Kebeish et al., 2007) and tobacco with a cyanobacterial bicarbonate transporter (Pengelly et al., 2014) and with accelerated recovery from photoprotection (Kromdijk et al., 2016). Any progress for major crops may not be expected in the near future, and modelling should be considered as an important tool to assess the potential of yield improvement by these photosynthesis-enhancing routes. Many researchers (e.g. Zhu et al., 2004; Song et al., 2013; Kromdijk and Long, 2016) have published modelling studies to assess the potential benefit of using various routes in improving photosynthesis; but most of their analyses were based on the simulation of canopy photosynthesis at a fixed leaf area index (LAI) for a given environmental condition.
During the growth cycle of annual field crops, LAI expands initially, reaches its maximum size, and then senesces, and complex interactions and feedback mechanisms can occur between photosynthesis and other physiological components (Yin and Struik, 2008). These complexities should be considered when scaling up from instantaneous leaf assimilation to daily canopy photosynthesis and to total mass production over the growing season (Boote et al., 2013). To that end, Gu et al., (2014a) ran numerical simulations using a full crop growth model, GECROS (Yin and van Laar, 2005), and examined the potential of exploiting the natural genetic variation in leaf photosynthesis within a single segregating population for contributing to crop productivity in rice (Oryza sativa L.) under field conditions.
Here we ran the crop model GECROS to quantify the extent to which improved leaf photosynthesis, predominantly from genetic engineering to suppress photorespiration, can result in an expected increase in crop mass production under well-watered, as well as water-limited, field conditions, using rice as an example.
Materials and methods
Model algorithms and approach in applying GECROS (v4.0) for this study are outlined below. Model parameters, if not defined in the text, are given in Table 1.
Table 1.
Input parameter values for various parts of biochemical leaf photosynthesis models
| Category | Symbol | Definition (unit) | C3 | C4 | ||
|---|---|---|---|---|---|---|
| Value | Reference | Value | Reference | |||
| e− transport | Φ2LL | Quantum efficiency of PSII e− transport under limiting light (mol mol−1) at Topt | 0.78 | Yin et al., (2014) | 0.78 | Assumed to be the same as for C3 |
| r 2/1 | Ratio of Φ2LL to quantum efficiency of PSI e− transport under limiting light (–) | 0.85 | Genty and Harbinson (1996) | 0.85 | Assumed to be the same as for C3 | |
| θ | Convexity of irradiance response of PSII e− transport rate (–) | 0.8 | Yin et al., (2009) | 0.8 | Assumed to be the same as for C3 | |
| f cyc | Fraction of total PSI e− flux that follows cyclic e− transport (–) | 0.05 | Yin et al., (2006) | 0.45a | Yin and Struik (2012) | |
| f pseudo | Fraction of total PSI e− flux that follows pseudocyclic e− transport (–) | 0.10 | Yin et al., (2006) | 0.05 | Yin and Struik (2012) | |
| f Q | Fraction of total plastoquinone e− flux that follows the Q-cycle (–) | NU | NU | 1 | Furbank et al., (1990) | |
| h | H+ required per ATP production (mol mol−1) | NU | NU | 4 | Yin and Struik (2012) | |
| α | Fraction of O2 evolution in bundle-sheath cells (–) | NA | NA | 0.1 | Standard value for C4 species such as maize | |
| x | Fraction of ATP used for CCM (–) | NA | NA | 0.4a | von Caemmerer and Furbank (1999) | |
| φ | Extra ATP required for the CCM per CO2 fixed (mol mol−1) | NA | NA | 2a | von Caemmerer and Furbank (1999) | |
| T opt | Optimum temperature for Φ2LL (°C) | 23 | Data of Yin et al., (2014) | 34 | Data of Yin et al., (2016) | |
| Ω | Difference between Topt and the temperature at which Φ2LL falls to e−1 of its maximum (°C) | 36.8 | Data of Yin et al., (2014) | 38.4 | Data of Yin et al., (2016) | |
| Enzyme kinetics and activity | S c/o25 | Relative CO2/O2 specificity of Rubisco at 25 °C (mol mol−1) | 3022 | Cousins et al., (2010) | 2862 | Cousins et al., (2010) |
| γ*25 | Half the reciprocal of Sc/o25 (mol mol−1) | 0.5/Sc/o25 | By definition | 0.5/Sc/o25 | By definition | |
| K mC25 | Michaelis–Menten constant of Rubisco for CO2 at 25 °C (μmol mol−1) | 291 | Cousins et al., (2010) | 485 | Cousins et al., (2010) | |
| K mO25 | Michaelis–Menten constant of Rubisco for O2 at 25 °C (mmol mol−1) | 194 | Cousins et al., (2010) | 146 | Cousins et al., (2010) | |
| χVcmax25 | Linear slope of maximum Rubisco activity at 25°C (Vcmax25) versus (n–nb)b (μmol s−1 g−1) | 75 | Derived from data of Yin et al., (2009) | 93 | 1.24 times that for C3 (Cousins et al., 2010; Perdomo et al., 2015) | |
| χJmax25 | Linear slope of maximum PSII e− transport rate at 25 °C (Jmax25) versus (n–nb) (μmol s−1 g−1) | 100 | Harley et al., (1992); Yin et al., (2009) | 200 | Derived from data of Yin et al., (2011) | |
| χεp25 | Linear slope of PEP carboxylation efficiency at 25 °C (εp25) versus (n–nb) (mol s−1 g−1) | NA | NA | 0.791 | Derived from data of Yin et al., (2011) | |
| Leaf respiration | R d25 | Day respiration at 25 °C (μmol m−2 s−1) | 0.01Vcmax25 | Common assumption | 0.01Vcmax25 | Assumed to be the same as for C3 |
| R m | Respiration rate occurring in mesophyll cells (μmol m−2 s−1) | NA | NA | 0.5Rda | von Caemmerer and Furbank (1999) | |
| CO2 diffusion | g 0 | Empirical residual stomatal conductance if light approaches zero (mol m−2 s−1) | 0.01 | Leuning (1995) | 0.01 | Assumed to be the same as for C3 |
| a 1 | Empirical constant for gs response to VPD (–) | 0.9 | Derived from Morison and Gifford (1983) | 0.9 | Set the same as for C3 cropsc | |
| b 1 | Empirical constant for gs response to VPD (kPa−1) | 0.15 | Derived from Morison and Gifford (1983) | 0.15 | Set the same as for C3 cropsc | |
| χgm25 | Linear slope of mesophyll conductance at 25 °C (gm25) versus (n–nb) (mol s−1 g−1) | 0.125 | Derived from data of Yin et al., (2009); Gu et al., (2012) | NU | NU | |
| χgbs25 | Linear slope of bundle-sheath conductance at 25 °C (gbs25) versus (n–nb) (mol s−1 g−1) | NA | NA | 0.007a | Yin et al., (2011) | |
| u oc25 | Coefficient lumping diffusivities and solubilities of CO2 and O2 in H2O at 25 °C | NA | NA | 0.047 | von Caemmerer and Furbank (1999) | |
| Temperature response | Eγ* | Activation energy for γ* (J mol−1) | 24 460 | Bernacchi et al., (2002) | 27 417 | Yin et al., (2016) |
| E Vcmax | Activation energy for Vcmax (J mol−1) | 65 330 | Bernacchi et al., (2001) | 53 400 | Yin et al., (2016) | |
| E KmC | Activation energy for KmC (J mol−1) | 80 990 | Bernacchi et al., (2002) | 35 600 | Perdomo et al., (2015) | |
| E KmO | Activation energy for KmO (J mol−1) | 23 720 | Bernacchi et al., (2002) | 15 100 | Yin et al., (2016) | |
| E Rd | Activation energy for Rd (J mol−1) | 46 390 | Bernacchi et al., (2001) | 41 853 | Yin et al., (2016) | |
| E Jmax | Activation energy for Jmax (J mol−1) | 88 380d | Yin and van Laar (2005) | 116 439 | Yin et al., (2016) | |
| D Jmax | Deactivation energy for Jmax (J mol−1) | 200 000 | Harley et al., (1992) | 135 982 | Yin et al., (2016) | |
| S Jmax | Entropy term for Jmax (J K−1 mol−1) | 650 | Harley et al., (1992) | 458.7 | Yin et al., (2016) | |
| Eεp | Activation energy for εp (J mol−1) | NA | NA | 51 029 | Data of Yin et al., (2016) | |
| Dεp | Deactivation energy for εp (J mol−1) | NA | NA | 130 363 | Data of Yin et al., (2016) | |
| Sεp | Entropy term for εp (J K−1 mol−1) | NA | NA | 425.6 | Data of Yin et al., (2016) | |
| E gm | Activation energy for gm (J mol−1) | 49 600 | Bernacchi et al., (2001) | NU | NU | |
| D gm | Deactivation energy for gm (J mol−1) | 437 400 | Bernacchi et al., (2002) | NU | NU | |
| S gm | Entropy term for gm (J K−1 mol−1) | 1400 | Bernacchi et al., (2002) | NU | NU | |
| E gbs | Activation energy for gbs (J mol−1) | NA | NA | 116 767 | Yin et al., (2016) | |
| D gbs | Deactivation energy for gbs (J mol−1) | NA | NA | 264 604 | Yin et al., (2016) | |
| S gbs | Entropy term for gbs (J K−1 mol−1) | NA | NA | 860 | Yin et al., (2016) | |
| E uoc | Activation energy for uoc (J mol−1) | NA | NA | –1630 | Yin et al., (2016) | |
| Base leaf N | n b | Base leaf nitrogen, at and below which leaf photosynthesis is zero (g m−2) | 0.3 | Sinclair and Horie (1989) | 0.3 | Assumed to be the same as for C3 |
NA, not applicable; NU, not used by the model presented herein.
a These parameter values need to be adjusted if the C4 model is used for simulating the cyanobacterial CCM (see the text and Table 2).
b Where n is leaf nitrogen (g N m−2); and nb is the base leaf nitrogen, below which no leaf photosynthesis is observed.
c Data of Morison and Gifford (1983) showed that stomatal sensitivity to VPD could differ between C3 and C4; such a difference can be mimicked by our stomatal conductance model, Equation 2 for C3 and Equation 11 for C4 leaves, when using the same values of a1 and b1.
d Parameter set in GECROS to be dependent on crop species; the value 88 380 was set as default for rice (Yin and van Laar, 2005).
C3 photosynthesis model
The model of Farquhar et al., (1980; the FvCB model hereafter) calculates net CO2 assimilation rate (A) as the minimum of the Rubisco-limited (Ac) and e− transport-limited (Aj) rates. The two limiting rates can be expressed collectively as:
| (1) |
where for Ac, x1 = Vcmax and x2 = KmC(1 + O/KmO); for Aj, x1 = [1–fpseudo/(1–fcyc)]J2/4 and x2 = 2O γ *, where x1 is written according to the FvCB model extended by Yin et al. (2004) to be compatible with a C4 model for which accounting for fcyc is required (see later). In the model, Cc and O are the CO2 and O2 level, respectively, at the carboxylation sites of Rubisco, J2 is the total PSII e− transport rate, and Γ *, defined as O γ * (where γ * is half of the inverse of Sc/o) is the CO2 compensation point in the absence of day respiration (Rd).
The submodel for stomatal conductance for CO2 transfer (gs) is:
| (2) |
where g0 is the residual value of gs when irradiance approaches zero, Ci* is the intercellular CO2 level (Ci) at which A + Rd = 0, and fvpd is the relative effect of leaf-to-air vapour difference (VPD) on gs (see later).
CO2 transfer from Ca (the ambient CO2 level) to Cc can be written as (Flexas et al., 2013):
| (3) |
| (4) |
Combining Equations 1–4 gives a standard cubic equation, as shown in Supplementary Text 1 at JXB online.
C4 photosynthesis model
The C4 model of von Caemmerer and Furbank (1999), as modified by Yin and Struik (2009, 2012), is used here. In C4 plants, CO2 is fixed initially in the mesophyll by phosphoenolpyruvate (PEP) carboxylase into C4 acids that are then decarboxylated to supply CO2 to Rubisco, which is localized in the bundle-sheath chloroplasts. The co-ordinated functioning of the ‘Kranz’ anatomy and C4 biochemistry enables an effective CCM. The extra ATP consumption for sustaining the CCM requires a higher fcyc in C4 than in C3 photosynthesis (Yin and Struik, 2012; Nakamura et al., 2013). The rate of PEP carboxylation (Vp) could be limited either by the PEP carboxylase or by the rate of e− transport (Yin and Struik, 2009):
| (5) |
where εp is the initial carboxylation efficiency of the PEP carboxylase, φ is the extra ATP required for the CCM per CO2 fixed, and z is the conversion factor of J2 into the ATP production rate: z = (2 + fQ–fcyc)/[h(1–fcyc)] (here h is the H+:ATP ratio; Yin et al., 2004; Yin and Struik, 2012), and x represents the fraction of ATP partitioned to the reactions associated with the operation of Vp. In the standard C4 model for malic-enzyme subtypes such as crop plants maize and sorghum, x was set to 0.4, arising from φ/(3 + φ), where φ = 2, and 3 is mol ATP required for the Calvin cycle to fix 1 mol CO2.
An effective CCM requires a small bundle-sheath conductance (gbs) as gbs determines the CO2 leakage from the bundle sheath to the mesophyll (L) that affects CO2 assimilation (von Caemmerer and Furbank, 1999):
| (6) |
| (7) |
Equations 5–7 can be combined to result in:
| (8) |
where a = 1 + εp/gbs and b = 0 if Vp is PEP carboxylase limited, and a = 1 and b = xJ2z/φ if Vp is e− transport limited (Yin and Struik, 2009).
The rate of CO2 fixation by Rubisco is modelled in the same way as for C3 photosynthesis:
| (9) |
where x1 = Vcmax, x2 = KmC/KmO, x3 = KmC for the enzyme (Rubisco)-limited rate, and x1 = [1–fpseudo/(1–fcyc)]J2/4, x2 = 2γ*, and x3 = 0 for the e− transport-limited rate. This form of the e− transport-limited rate implies that it is the NADPH supply that causes the e− transport limitation in C4 photosynthesis, in comparison with the standard C4 model in which the ATP supply was assumed to cause the e− transport limitation (von Caemmerer and Furbank, 1999). Yin and Struik (2012) discussed the rationale that either the ATP- or the NADPH-limited form can be used for modelling C4 photosynthesis provided that fcyc and fpseudo are set as appropriate. We prefer to use the NADPH-limited form here because the ATP-limited form gives x1 = (1–x)zJ2/3 (Yin et al., 2011), which would predict a monotonic increase in ATP production rate, thus in an e− transport-limited carboxylation rate, with increasing fcyc. This does not agree with the more efficient CCM in terms of ATP use (e.g. cyanobacterial CCM; Price et al., 2011). Using the NADPH-limited form allows a revised C4 model to simulate photosynthesis of other CCM systems (see below) and to be consistent with the C3 photosynthesis modelling where the NADPH-limited form is predominantly used.
A relationship for O2 partial pressure between the intercellular air space (Oi) and the sites around Rubisco in bundle-sheath cells (O) is described as (von Caemmerer and Furbank, 1999):
| (10) |
A model for gs of C4 leaves was formulated in a way that slightly differed from Equation 2 of the C3 counterpart, to solve analytically for A in C4 photosynthesis (Yin and Struik, 2009):
| (11) |
where Cs is the CO2 level at leaf surface, and Cs* is the Cs-based CO2 compensation point in the absence of Rd and can be calculated as [gbsγ*Oi–(1 + γ*α/uoc)Rd + Rm]/(gbs + εp) (Yin and Struik, 2009). Equation 11 for C4 and Equation 2 for C3, although both empirical, can reproduce experimentally observed linear relationships between A and gs across various levels of irradiance and nutrients (e.g. Wong et al., 1985) (see Supplementary Fig. S1).
Equation 3 also applies to C4 photosynthesis. Combining Equations 3 and 8–11 can yield the standard cubic equation that gives the prediction of A (Supplementary Text 1).
Algorithms common to C3 and C4 photosynthesis
Some common algorithms were used for C3 and C4 models. First, J2 is described as a function of absorbed irradiance Iabs as (Yin et al., 2006; Yin and Struik, 2012):
| (12) |
Equation 12 differs from the equation used in the standard FvCB model, in that fcyc, Φ2LL, and r2/1 are introduced. We consider Equation 12 as a better choice as it accounts for the decrease of the overall noncyclic e− transport efficiency (α2LL) with increasing cyclic e− transport, which runs at a higher rate in cases involving the CCM.
Secondly, in the gs model, fvpd is the function for the effect of VPD, which may be described phenomenologically as (Yin and Struik, 2009):
| (13) |
where a1 and b1 represent the Ci:Ca ratio in water vapour-saturated air and the slope of the decrease of this ratio with increasing VPD, respectively, if g0 in Equation 2 or 11 approaches nil.
Thirdly, a number of parameters are related to leaf temperature (Tl), and some of these can be described by the Arrhenius equation normalized with respect to 25 °C:
| (14) |
where R is the universal gas constant (8.314 J K−1 mol−1). Equation 14 applies to Rd, γ*, Vcmax, KmC, KmO, and uoc. The temperature response of Jmax, εp, gm, and gbs is described by the modified Arrhenius equation:
| (15) |
Fourthly, the values at 25 °C of parameters Vcmax, Jmax, εp, gm, and gbs can be further quantified as a linear function of leaf nitrogen (N) content (n) above a certain base value (nb):
| (16) |
where χ has different values for different parameters (e.g. Harley et al., 1992; Yin et al., 2011). We estimated χVcmax25 for C3 leaves from existing data and then projected to C4 leaves (Table 1), based on the reported higher catalytic turnover rate of C4 Rubisco than that of C3 Rubisco (Seemann et al., 1984; Sage, 2002; Cousins et al., 2010; Perdomo et al., 2015). There is less information about the difference in χJmax25 between C3 and C4 types, but our χJmax25 estimates (Table 1) are in line with Makino et al., (2003), who reported a considerably higher photosynthetic N use efficiency under saturated CO2 conditions in C4 than in C3 leaves.
Fifthly, experimental evidence suggests that Φ2LL responds to temperature (Bernacchi et al., 2003; Yin et al., 2014). Due to the lack of understanding of this response, we empirically express the factor for the temperature effect, using a normal distribution alike equation (June et al., 2004):
| (17) |
Finally, Equations 14, 15, and 17 require Tl, and Tl is solved from coupled modelling of leaf photosynthesis and transpiration: the algorithms in Supplementary Text 1 solve A and gs simultaneously; the obtained gs is used as input to the Penman–Monteith equation (Monteith, 1973; Goudriaan and van Laar, 1994) to solve leaf transpiration and Tl. This procedure involves iterations, in which Tl is initially set to be the same as the air temperature and then the solved Tl is used for re-calculating A, gs, and leaf transpiration (Yin and van Laar, 2005).
Revising the C4 model for simulating the cyanobacterial CCM
The single-cell C4 photosynthesis model of von Caemmerer and Furbank (2003; see also Supplementary Text 2) can be used for simulating cyanobacterial photosynthesis (Price et al., 2011). However, this model is hard to solve once it is coupled to a gs model (Equation 2 or 11). We therefore revise the above C4 model to simulate the cyanobacterial CCM, based on the model concept of Price et al. (2011). These revisions are: (i) set gbs to a high value to mimic gch (conductance of the chloroplast envelope to CO2); (ii) set Vp as if it stands for the combined rate of cyanobacterial bicarbonate transporters; (iii) set Rm = Rd; and (iv) re-estimate fcyc and x, in view of the fact that extra ATP required for the cyanobacterial CCM also comes from the cyclic e− pathway (Shikanai, 2007). The ATP cost of bicarbonate transport may be lower than that of the C4 CCM (Price et al., 2013; Furbank et al., 2015). Two single-gene transporters (BicA and SbtA) that have been well characterized in cyanobacteria are considered here, and Price et al. (2011) estimated that the two transporters require 0.25 and 0.50 ATP per transport event, respectively (so, φ in Equation 5 is 0.75). We re-estimated x as 0.2 and fcyc as 0.18 (Table 1), where 0.2 arises from 0.75/(3 + 0.75), and 0.18 arises from the C4 model of Yin and Struik (2009) for the balanced NADPH:ATP ratio assuming h = 4. This revised C4 model gives simulated rates of A virtually identical to the model of Price et al., (2011) using the same set of parameter values (Supplementary Text 2) under normal and elevated [CO2] conditions.
Setting scenarios of improved leaf photosynthesis for simulation
Major routes in enhancing photosynthesis will be examined, except for the photorespiratory bypass. Modelling this bypass would require more complicated algorithms and parameters (von Caemmerer, 2013), which cannot be straightforwardly implemented to simulate field environments where modelling of gs is also needed. We examined the impact of improving gm (Tholen et al., 2012; Flexas et al., 2013), improving Sc/o (Zhu et al., 2004; Parry et al., 2011), introducing the C4 mechanisms into C3 crops (von Caemmerer et al., 2012), and using cyanobacterial bicarbonate transporters and the CCM (Price et al., 2011, 2013). Given that efforts to engineer these routes, especially the latter two, into new crops will most probably make progress step-wise, we propose the following nine routes (Table 2):
Table 2.
Nine photosynthesis-enhancing routes, the corresponding photosynthesis models, and parameter sets used for simulation in this study
| Route | Description | Model | Parameter set |
|---|---|---|---|
| 1 | Improved mesophyll conductance gm | C3 | All C3 default parameters in Table 1 but χgm25 = 0.375 |
| 2 | Improved Rubisco specificity Sc/oa | C3 | All C3 default parameters in Table 1 but Sc/o = 4427 |
| 3 | Improved value for both gm and Sc/o | C3 | All C3 default parameters in Table 1 but χgm25 = 0.375 and Sc/o = 4427 |
| 4 | C4 biochemistry introduced | C4 | All C4 parameters (including χVcmax25 and χJmax25b) in Table 1, but χbs25 = 0.125 |
| 5 | C4 Kranz anatomy introduced effectively to minimize CO2 leakage | C4 | All default C3 enzymatic parameters plus necessary C4 parameters to run C4 model in Table 1, but χbs25 = 0.007 |
| 6 | Complete C4 mechanism engineered | C4 | All C4 parameters in Table 1, including low χbs25 (= 0.007) |
| 7 | Only cyanobacterial bicarbonate transporters engineered | C4 | All C3 default parameters plus necessary C4 parameters to run C4 model in Table 1, but χgbs25 = 0.125, φ = 0.75, x = 0.2, fcyc = 0.18, and Rm = Rd |
| 8 | More elaborate cyanobacterial CCM added | C4 | The same as Route 7, but χgbs25 = 0.007 |
| 9 | Complete cyanobacterial CCM engineered | C4 | The same as Route 8, but with χVcmax25 = 93 and χJmax25 = 200c |
a This route assumes that crop plants are engineered to have a high Sc/o25 of the non-green alga Griffithsia monilis while maintaining a similar Rubisco turnover rate (Whitney et al., 2001); any effect of the trade-off between Rubisco Sc/o and carboxylase turnover rate was not quantified here, and readers are suggested to refer to Zhu et al., (2014) on this effect.
b Based on measurements on existing maize and wheat plants, parameters χVcmax25 and χJmax25 have higher values in C4 than in C3 leaves (Table 1), probably reflecting the acclimation of C4 enzymatic activities to high a CO2 environment within the bundle-sheath compartment. While strictly speaking these higher values cannot be guaranteed for hypothetical C4 plants of Route 4 which is not yet incorporated with the full Kranz anatomy, high values of χVcmax25 and χJmax25 for maize plants (Table 1) were used here for simulation of Route 4 in order to represent the full package of the C4 biochemistry components.
c Cyanobacterial Rubisco has a higher carboxylation rate than C3 Rubisco (Hanson et al., 2016), allowing a higher investment of nitrogen in other photosynthetic protein components. However, we are not aware of the N cost for e− transport protein components in cyanobacteria for estimating χJmax25. For simplicity, χVcmax25 and χJmax25 for maize plants (Table 1) are used for this route, based on the expectation of engineering cyanobacterial CCM that approaches typical C4 photosynthetic capacities (Price et al., 2013).
(1) Improving gm, where the slope of gm25 versus leaf N (χgm25; see Equation 16) is set from its default value 0.125 (Table 1) to be three times higher (i.e. 0.375).
(2) Improving Sc/o, where Sc/o25 is set from its C3 default value 3022 (Table 1) to 4427, the observed Sc/o25 for the non-green alga Griffithsia monilis (Whitney et al., 2001).
(3) Improving gm as well as Sc/o, where χgm25 of 0.375 and Sc/o25 of 4427 are combined.
(4) Introducing C4 biochemistry, where the C4 photosynthesis model is used with C4 kinetic constants (Table 1) while setting gbs as high as the C3 default gm (i.e. setting the slope of gbs25 versus leaf N; χgbs25; see Equation 16) to 0.125.
(5) Making C4 Kranz anatomy function effectively to minimize CO2 leakage, where the low χgbs25 (0.007) is combined with C3 enzyme kinetic constants (Table 1).
(6) Engineering the complete C4 mechanism, where C4 kinetic constants (Table 1) combined with a low χgbs25 (0.007) is used in the C4 photosynthesis model.
(7) Engineering cyanobacterial single-subunit bicarbonate transporters (BicA and SbtA), where the above revised C4 model is combined with the default C3 parameters with χgbs25 = 0.125 and the revised values for φ, x, Rm, and fcyc (Table 2).
(8) Adding a more elaborate cyanobacterial CCM, whereby the carboxysome shell proteins are expressed in chloroplasts to enrich the CO2 level around Rubisco similar to the level in the C4 bundle-sheath compartment. This route assumes that the chloroplastic C3 Rubisco can be reorganized into effective carboxysome structures and other requirements for carboxysome to function are optimized (Price et al., 2011). So, the same model and parameter values as for Route 7 are used, except for χgbs25 which is now set to a lower value of 0.007 as for C4 bundle-sheath conductance.
(9) A complete cyanobacterial CCM installed. The complete cyanobacterial CCM will require replacement of the C3 Rubisco with a cyanobacterial Rubisco in order to take advantage of better kinetic properties in a high-CO2 carboxysome (Long et al., 2016). Based on the expectation of engineering the cyanobacterial CCM that approaches photosynthetic capacities typical of C4 plants (Price et al., 2013), we used χVcmax25 and χJmax25 of C4 photosynthesis (Table 1) for this route. So, this route has the low ATP cost of the cyanobacterial CCM as well as a high enzymatic capacity per unit N to mimic the complete cyanobacterial CCM.
Simulation results of all nine routes will be compared with those of the default in which the C3 photosynthesis model with the C3 parameter values in Table 1 is used.
Modelling daily canopy photosynthesis and transpiration
In GECROS, instantaneous canopy photosynthesis and transpiration were calculated using the sun/shade model of de Pury and Farquhar (1997), in which the sunlit and shaded portions of the canopy each are considered as a big leaf, and the above leaf-level model is applied. Assuming an exponential profile of leaf N, total photosynthetically active N for each portion was calculated, and N-dependent photosynthetic parameters Vcmax, Jmax, gm, gbs, and εp (see Equation 16) were then scaled up accordingly to each portion of the canopy. Instantaneous rates were scaled up to daily total, using the Gaussian integration (Goudriaan, 1986) to account for any asymmetric diurnal courses of radiation and temperature. These approaches for spatial and temporal extensions apply to the case in the absence of water limitation.
In the presence of water limitation, the available water is partitioned between sunlit and shaded leaves according to the relative share of their potential transpiration (Ep) to obtain their actual transpiration (Ea). The diurnal course of available water is assumed to follow that of radiation. Based on the Penman–Monteith equation, the actual transpiration is transformed into the actual level of stomatal resistance to water vapour (rsw,a) (Yin and van Laar, 2005):
| (18) |
where rsw,p is the stomatal resistance to water vapour in the absence of water limitation [ = 1/(1.6gs), where gs is solved from the algorithm in Supplementary Text 1]; rbh and rbw are the boundary-layer resistance to heat and to water vapour, respectively; γ is the psychrometric constant; and s is the slope of the saturated vapour pressure as a function of temperature (kPa °C−1). The actual rsw,a was converted into the actual gs, which can be used as input to the analytical quadratic model (see Supplementary Text 3) to estimate the instantaneous actual photosynthesis of the sunlit and shaded leaves. The Gaussian integration was again used to obtain the daily total of the actual photosynthesis. Equation 18 suggests that the impact of water deficit is mainly via stomatal conductance; any non-stomatal effect of water deficit is not modelled in GECROS, except when accounting for changes in Tl under drought.
Crop simulation approaches
Simulations were conducted for three sites, Los Baños (14°6'N, 121°9'E; the Philippines), Nanjing (32°56'N, 118°59'E; China), and Shizukuishi (39°41'N, 140°57'E; Japan), representing tropical, subtropical, and temperate rice-growing conditions, respectively, using 31 year (1980–2010) baseline weather data and the present atmospheric [CO2] of 400 μmol mol−1. We also ran the model under the climate scenario for 2050, at which the expected [CO2] is ~550 μmol mol−1 and air temperature is 2 °C higher than the baseline (Li et al., 2015). As we only examined the impact of changed leaf photosynthesis on crop productivity, we decoupled the GECROS soil module and used only the crop module for simulation to avoid any confounding effects from uncertainties in simulating soil processes. Potential production was simulated by setting the daily water supply to the crop as non-limiting. Water-limited production was simulated by setting the daily available water for evapotranspiration to no more than 50% of seasonal average daily transpiration simulated for the potential production, which was 1.97, 1.63, and 1.34 mm H2O d−1 for Los Baños, Nanjing, and Shizukuishi, respectively. Daily N supply was set in such a way that the accumulated N uptake by the crop followed the sigmoid curve of Yin et al. (2003) and that the total uptake at maturity reached 20 g N m−2, equivalent to the N uptake in high-yielding rice experiments (Setter et al., 1994).
Model parameters for phenology were calibrated (Table 3) so that simulated baseline crop duration was in line with that of the standard cultivar at each site (Li et al., 2015). Crop models are less accurate in predicting spikelet number and therefore harvest index than in predicting crop mass (Boote et al., 2013; Li et al., 2015). To minimize the impact of this uncertainty, we used the simulated total shoot mass (excluding dead leaves) at maturity as the proxy for crop productivity. Input parameters were set as the default values of GECROS for rice (Yin and van Laar, 2005) and those relevant to our study are given in Table 3. As C4 enzyme kinetic parameters, especially their temperature responses, are less certain than the C3 counterparts (Boyd et al., 2015), additional analysis was conducted for C4 simulation (Supplementary Text 4). Similarly, because the exact ATP cost of bicarbonate transport is uncertain (Fridlyand et al., 1996; McGrath and Long, 2014), sensitivity analysis was conducted for Route 9 with regard to this cost (Supplementary Text 5). Further details about GECROS are given in Supplementary Text 6, and source codes of the full GECROS model can be obtained upon request.
Table 3.
Values of some input parameters of the GECROS crop model relevant to this study
| Parameter | Definition (unit) | Value |
|---|---|---|
| S la | Specific leaf area constant for newly emerging leaves (m2 g−1) | 0.03 |
| n Rmin | Base value of root nitrogen concentration (g g−1) | 0.005 |
| n Smin | Base value of stem nitrogen concentration (g g−1) | 0.005 |
| n RV | Nitrogen concentration in plant reserves (g g−1) | 0.0015 |
| S W | Potential weight of a single grain (g) | 0.025 |
| n SO | Potential nitrogen concentration in grains (g g−1) | 0.0145 |
| H max | Maximum final plant height (m) | 1.0 |
| TC S | Time constant for senescence (d) | 2 |
| T b | Base temperature for phenology (°C) | 8 |
| T o | Optimum temperature for phenology (°C) | 30 |
| T c | Ceiling temperature for phenology (°C) | 42 |
| m V | Minimum number of days for pre-flowering period (thermal daya) | 70, 85, 48b |
| m R | Minimum number of days for post-flowering period (thermal day) | 28, 32, 22b |
| STTIME | Starting time of simulation, equivalent to day number (from 1 January) for seedling emergence | 10, 145, 125b |
a One thermal day is equivalent to one calendar day if the temperature at each moment of the day is always at the optimum.
b Values used for Los Baños (the Philippines), Nanjing (China), and Shizukuishi (Japan), respectively. The STTIME value for Los Baños is for the dry season there (which is the season with the high yield potential), and that for Nanjing is for single-cropping rice (that is predominant in the region, compared with the double-cropping rice where rice is planted twice per year).
Results and Discussion
Simulated leaf photosynthesis
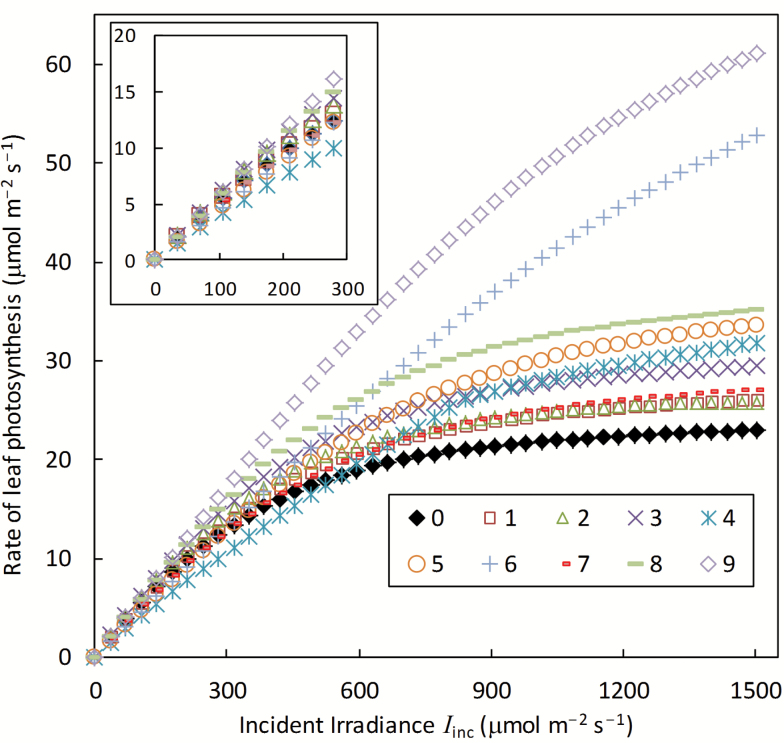
All routes could increase A in the light-saturated region, especially Routes 6 and 9 (Fig. 1). In the light-limited region, the impact of the routes was smaller, and Routes 4, 5, and 6 in fact had a negative effect (see the inset of Fig. 1). This negative impact is associated with the two extra ATPs required for PEP regeneration in the C4 cycle (von Caemmerer and Furbank, 1999), for which a high fcyc is required (Yin and Struik, 2012; Table 1). In Route 4 where this high ATP cost was not compensated by an effective CCM to suppress photorespiration, the negative effect was particularly high. Because these routes act differently for the light-saturated and limited regions, the curvature in the light response curve was diverse (Fig. 1) despite the same curvature factor θ (0.8; Table 1) used for Equation 12 describing the light response of PSII e− transport rate for all these curves.
Fig. 1.
Calculated leaf photosynthesis of the default C3 (0) and nine (1–9) photosynthesis-enhancing routes in response to incident irradiance (Iinc). The calculation was made using the model described in the text, based on parameter values listed in Tables 1 and 2 and the following input conditions: Ca = 400 μmol mol−1, Tl = 25 °C, leaf nitrogen content n = 2.3 g m−2, nb = 0.3 g m−2, VPD = 2.0 kPa, and leaf photosynthetic absorptance = 0.85. The inset is for the same response curves when Iinc is <300 μmol m−2 s−1.
Simulated canopy photosynthesis
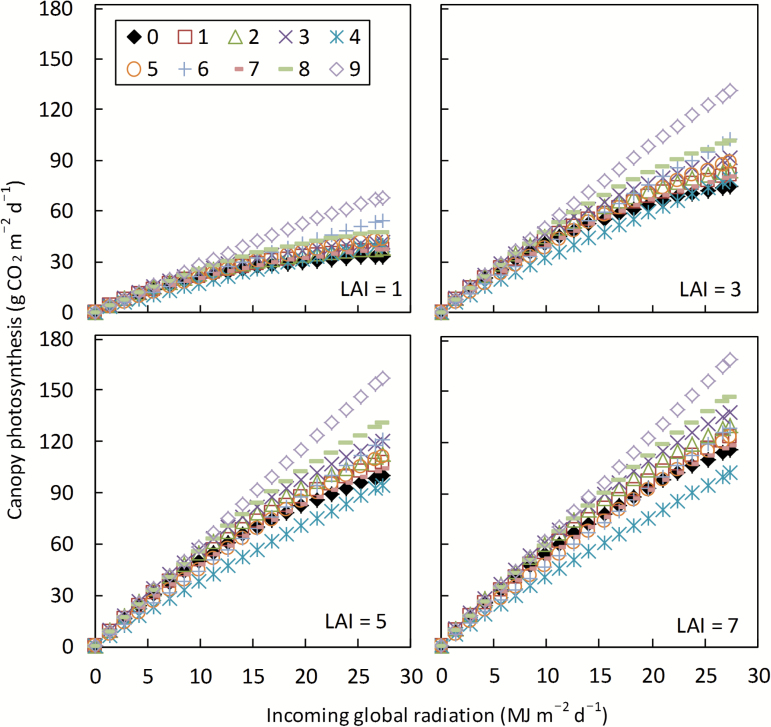
Not surprisingly, the calculated daily canopy photosynthesis (Acanopy,d) increased with increasing LAI (Fig. 2), due to a higher interception of photosynthetically active radiation (PAR) at higher LAI. Also, the light response curve of Acanopy,d became increasingly linear with increasing LAI, because at high LAI, leaves in the canopy are predominantly light limited, and within the light-limited range leaf photosynthesis increases almost linearly with light level (Fig. 1). Because the difference in leaf photosynthesis among the routes was mainly recognized in the light-saturated region (Fig. 1), the ratio of Acanopy,d of photosynthesis-enhancing routes to that of the default C3 route increased with increasing radiation level, and decreased with increasing LAI (Fig. 2). Acanopy,d of Route 4, compared with the default C3 route, was notably lower, regardless of the radiation level, when LAI was ≥3 (Fig. 2).
Fig. 2.
Calculated daily canopy photosynthesis of the default C3 (0) and nine (1–9) photosynthesis-enhancing routes in response to daily incoming global solar radiation, for four different sizes of canopy [leaf area index (LAI) = 1, 3, 5, and 7, respectively]. The calculation was made using the model described in the text, based on parameter values listed in Tables 1 and 2 and the following input conditions: Ca = 400 μmol mol−1, Tl = 25 °C, canopy average leaf nitrogen = 2.3 g m−2, nb = 0.3 g m−2, VPD = 2.0 kPa, daylength = 13 h d−1, fraction of diffuse irradiance = 0.2, and canopy average leaf angle (from horizontal) = 65 °. Light extinction coefficient and nitrogen extinction coefficient required for the canopy photosynthesis model were calculated using the formulae as in GECROS with leaf scattering coefficient of 0.2 for PAR.
Default simulation for crop durations and mass production
Using GECROS, we simulated crop duration and mass production. A 2 °C warming for 2050, relative to the present climate, was simulated to shorten crop duration by ~5, 10, and 20 d, for tropical, subtropical, and temperate environments, respectively (Table 4). This different effect across the environments is due to the fact that temperature during the growing season in the tropics is around the optimum value, at which warming is expected to have a smaller effect than at the other sites where the growing season temperature is mostly in the range where development rate increases greatly with warming.
Table 4.
Days from seedling emergence to flowering and to maturity, aboveground mass at maturity, and season-long canopy photosynthesis and canopy transpiration of rice crop simulated under the default scenario (SDs of the mean of 31 years in parentheses) for the present climate and the 2050 climate, under either potential or water-limited environments, in three representative sites
| Site | Los Baños (tropics) | Nanjing (subtropics) | Shizukuishi (temperate) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Production level | Potential | Water limited | Potential | Water limited | Potential | Water limited | ||||||
| Climate | Present | 2050 | Present | 2050 | Present | 2050 | Present | 2050 | Present | 2050 | Present | 2050 |
| Days to flowering | 80.6 (1.8) | 76.2 (1.2) | – | – | 98.3 (2.2) | 95.7 (1.0) | – | – | 98.7 (5.8) | 87.0 (4.4) | – | – |
| Days to maturity | 110.7 (2.0) | 105.2 (1.3) | – | – | 143.6 (7.6) | 133.0 (2.2) | – | – | 133.4 (14.8) | 113.8 (5.8) | – | – |
| Crop mass (g DM m−2) | 1979.9 (55.4) | 2141.7 (59.0) | 1538.2 (60.4) | 1644.8 (69.0) | 2246.1 (93.1) | 2420.3 (109.5) | 1662.4 (108.4) | 1782.9 (104.7) | 1694.3 (65.5) | 1803.4 (73.4) | 1193.5 (104.6) | 1272.6 (74.9) |
| Canopy photosynthesis (g CO2 m−2) | 5225.8 (145.7) | 5598.9 (157.2) | 4408.3 (130.5) | 4600.7 (147.2) | 5914.8 (231.7) | 6330.9 (286.6) | 4793.3 (185.9) | 5011.2 (169.8) | 4510.6 (153.1) | 4702.0 (152.8) | 3543.9 (218.3) | 3668.0 (142.9) |
| Canopy transpiration (mm H2O) | 435.7 (25.4) | 413.8 (18.9) | 177.8 (3.6) | 168.1 (3.1) | 468.5 (37.3) | 470.6 (33.4) | 167.1 (4.3) | 167.2 (3.2) | 357.2 (20.0) | 328.2 (19.8) | 121.7 (10.3) | 109.3 (5.6) |
| WUEb (g m−2 mm−1) | 5.1 | 6.0 | 9.7 | 11.0 | 5.6 | 6.1 | 11.2 | 11.9 | 5.3 | 6.1 | 11.4 | 13.4 |
–, simulations assumed that drought had no impact on phenological development, so the predicted phenology was the same under water-limited as under the potential production level.
a Water use efficiency, defined as total crop mass production divided by the amount of water transpired during the growth season.
Despite the shorter duration, simulated aboveground mass at crop maturity increased for 2050 compared with the present climate (Table 4), largely due to CO2 elevation from 400 μmol mol−1 to 550 μmol mol−1. This is because we implicitly assumed that future breeding can develop rice cultivars capable of coping with any effect of warming on spikelet sterility, so the effect of CO2 elevation was dominant. However, a recent FACE (free-air CO2 enrichment) study (Cai et al., 2016) using a present cultivar showed that yields of rice were decreased by 17–35% under the combination of elevated CO2 and temperature, compared with the ambient condition, due to fewer filled grains at the elevated temperature. As expected, water limitation decreased mass production, but increased water use efficiency (WUE) (Table 4). The WUE differed little among the three sites, but was higher for 2050 than for the present climate, partly due to increased Acanopy,d and partly due to generally decreased canopy transpiration under the 2050 climate (Table 4). The reduced canopy transpiration for the 2050 climate was largely a result of partial stomatal closure induced by higher [CO2] (e.g. Wong et al., 1985).
Impact of photosynthesis-enhancing routes on crop mass production
Compared with the default C3 photosynthesis, all routes increased aboveground mass production, except for three cases for the potential production–2050 climate combination where the benefit from Route 5 was virtually nil or slightly negative (Table 5). In general, the benefit from Routes 1, 2, and 7 was ≤10%. All routes resulted in higher benefits in the presence of drought than in the absence of drought, and under the present climate than for 2050. This could be explained by the shape of a diminishing return for A–Ci curves, because drought and the present climate both result in a lower Ci compared with the potential production level and the 2050 climate, respectively.
Table 5.
The percentage increase of the 31 year average aboveground mass by nine photosynthesis-enhancing routes, relative to that shown in Table 4 for the default route, in rice crop simulated for the present climate and the 2050 climate, under either potential or water stress environments, in three representative sites
| Site | Los Baños (tropics) | Nanjing (subtropics) | Shizukuishi (temperate) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Production level | Potential | Water limited | Potential | Water limited | Potential | Water limited | |||||||
| Climate | Present | 2050 | Present | 2050 | Present | 2050 | Present | 2050 | Present | 2050 | Present | 2050 | |
| Routea | 1 | 4.3 | 2.5 | 4.8 | 3.1 | 4.2 | 2.6 | 4.5 | 4.1 | 4.3 | 2.7 | 4.5 | 4.1 |
| 2 | 8.8 | 8.0 | 7.5 | 6.8 | 9.3 | 8.5 | 11.7 | 9.7 | 9.2 | 8.1 | 11.0 | 9.2 | |
| 3 | 12.9 | 9.9 | 13.6 | 12.5 | 14.0 | 10.8 | 16.8 | 13.8 | 13.5 | 10.2 | 15.5 | 14.0 | |
| 4 | 10.4 | 4.1 | 12.4 | 6.4 | 8.0 | 3.9 | 11.8 | 6.2 | 14.8 | 8.3 | 19.2 | 10.4 | |
| 5 | 7.6 | –0.8 | 26.6 | 13.6 | 5.0 | –2.4 | 24.5 | 11.6 | 7.0 | –0.7 | 26.6 | 14.9 | |
| 6 | 38.0 | 23.1 | 51.2 | 33.8 | 33.0 | 21.9 | 50.5 | 34.1 | 39.8 | 25.4 | 54.5 | 36.0 | |
| 7 | 5.4 | 1.6 | 9.1 | 5.2 | 4.5 | 0.8 | 10.6 | 6.0 | 5.5 | 2.1 | 11.3 | 7.7 | |
| 8 | 17.9 | 10.5 | 39.7 | 28.7 | 18.1 | 10.7 | 39.9 | 27.9 | 19.1 | 11.3 | 38.7 | 28.1 | |
| 9 | 70.1 | 57.5 | 78.5 | 61.2 | 63.2 | 51.3 | 74.8 | 57.9 | 60.8 | 49.0 | 73.8 | 57.4 | |
a Route numbers correspond to those defined in Table 2.
Route combinations had an equal effect to, or a larger effect than, the sum of the routes acting alone. For example, the benefit from Route 3 (improving both gm and Sc/o) was about the sum of the benefits from Route 1 (improving gm) and Route 2 (improving Sc/o) for the potential production and was higher than the sum of the two for the water-limited condition (Table 5).
The benefit from Route 6 (the complete C4 mechanism) was considerably higher than the sum of the benefits from Route 4 (C4 biochemistry components) and Route 5 (Kranz anatomy components for low gbs) for any condition (Table 5). This result suggests that the ongoing programme of installing C4 photosynthesis into C3 crops (von Caemmerer et al., 2012), if successful, needs to engineer the complete C4 mechanism. The benefit of a partial engineering is only marginal or even counter-productive under future high-CO2 environments (see Route 5 in Table 5), because of the high ATP cost for operating the C4 cycle. In an earlier preliminary simulation analysis (Yin and Struik, 2008), we showed that a low gbs alone would increase rice yield in the tropics by ~25%. However, that analysis used an arbitrarily low gbs and a version of the C4 model that assumes an H+:ATP ratio of 3, whereas a recent analysis suggested that this ratio is most probably 4 (Yin and Struik, 2012), suggesting that the model version Yin and Struik (2008) used may have underestimated quantum requirement for the C4 CCM. From our present analysis, even with the complete C4 mechanism, its advantage over the C3 default was simulated to be >50% only under the combination of water limitation and the present climate; for other conditions, its advantage ranged between 22% and 40% (Table 5).
As engineering for the complete Kranz anatomy is challenging, the CCM in cyanobacteria, which is probably less expensive energetically, has been suggested as an obvious alternative to engineer (Price et al., 2011, 2013; Furbank et al., 2015). Our simulation showed that the simplest form for the cyanobacterial CCM with bicarbonate transporters, Route 7, had a marginal advantage (Table 5). Pengelly et al., (2014) showed that tobacco plants transformed with the BicA transporter had no discernible effect on CO2 assimilation rates, suggesting that BicA was either not located or not activated correctly. Our simulation (Table 5) showed that a more elaborate cyanobacterial CCM, where the carboxysome shell proteins were expressed to enrich the CO2 level around Rubisco in chloroplasts (Route 8), had a higher advantage than the equivalent C4 CCM (Route 5), largely because of a lower ATP cost assumed for the cyanobacterial CCM. However, its benefit was lower than from the complete C4 mechanism, namely Route 6, which includes the additional mechanism that C4 plants have a considerably high carboxylation rate per unit leaf N (Evans and von Caemmerer, 2000; Makino et al., 2003). The complete cyanobacterial mechanism (Route 9), which has the low energy cost for the CCM as well as the high carboxylation and e– transport capacity per unit N (presumably as high as for C4 plants), was the only route that could bring an advantage of ≥50% under any environmental conditions (Table 5). However, as the exact ATP cost for the cyanobacterial CCM is uncertain, the simulated benefits of Routes 7–9 should be considered as tentative and their real benefits might be lower (Supplementary Text 5).
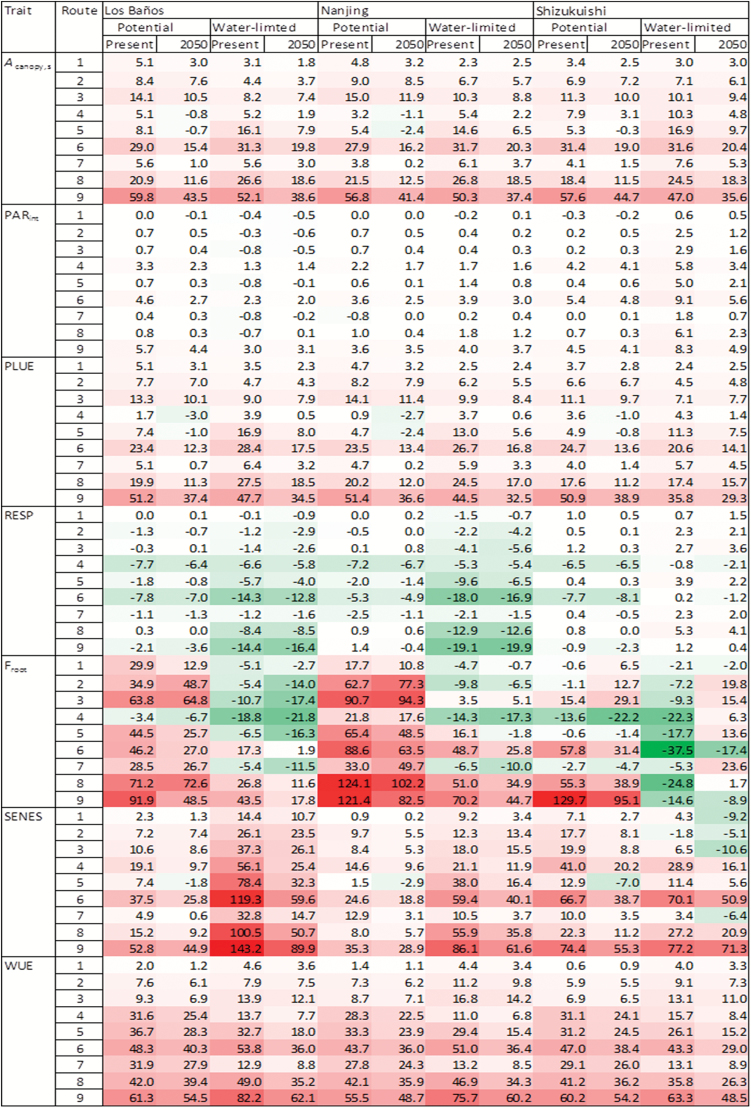
Effects of enhanced leaf photosynthesis on some other crop traits
The benefit from all routes for simulated season-long canopy photosynthesis (Acanopy,s), shown in the upper rows of Fig. 3, differed from that shown in Table 5 for aboveground mass. This difference suggests that other traits were also affected by altered photosynthesis. Aboveground mass can be calculated as:
Fig. 3.
Heat map for the percentage change (%) of the 31 year average trait value for each of the nine photosynthesis-enhancing routes (route numbers defined in Table 2), relative to that for the default route, in a rice crop simulated for the present climate and the 2050 climate, under either potential or water-limited environments, at three representative sites. Traits shown are: Acanopy,s, season-long canopy photosynthesis; PARint, season-long intercepted PAR; PLUE, overall photosynthetic light use efficiency defined as Acanopy,s divided by PARint; RESP, season-long crop respiration; Froot, fraction of mass for roots; SENES, shoot mass lost due to leaf senescence; and WUE, water use efficiency. (Colours: white for no change, green for decrease, red for increase, and colour intensity for the magnitude of decrease or increase.)
where PARint is the season-long intercepted PAR by the crop (MJ m−2), PLUE is the overall photosynthetic light use efficiency (= Acanopy,s/PARint, g CO2 MJ−1 PAR), RESP is season-long crop respiration (g CO2 m−2), Froot is fraction of mass for roots, and SENES is aboveground mass lost after leaf senescence (g DM m−2). We obtained the data for these five traits from the GECROS output, and calculated the percentage change of each of these traits for each route relative to the C3 default (Fig. 3).
The enhanced leaf photosynthesis from most routes also resulted in increased PARint, although to a small extent only, up to a maximum of 8.3% (Fig. 3). As a result, the percentage increase in PLUE by the routes was generally slightly lower than that in Acanopy,s (Fig. 3). The increased PARint stemmed from an increased LAI in the early growth phase (results not shown), in line with the recent result of Kromdijk et al., (2016) showing that increased leaf photosynthesis also resulted in increased leaf area. The increased canopy photosynthesis, on the one hand, increased the component of growth respiration; on the other hand, it decreased the component of maintenance respiration which is modelled in GECROS dependent on crop N status. The net result is that most routes decreased RESP (Fig. 3). The simulated Froot generally became higher with photosynthesis-enhancing routes (Fig. 3), as expected from the classical functional equilibrium theory (Brouwer, 1983). However, in the presence of water limitation, Froot could be lower compared with the default values, probably because the crop maintained a comparatively high N status under drought. Because a higher photosynthesis resulted in a lower N:C ratio in the crop and GECROS modelled leaf senescence depending on the relative magnitude of N- and C-determined LAI, the most effective enhancing routes, such as Routes 6 and 9, resulted in increased leaf senescence (Fig. 3). This simulation result is in analogy to the faster senescence and reduced LAI in later stages of development for C3 crops grown under elevated [CO2] in FACE experiments (e.g. Kim et al., 2003). Sinclair et al., (2004) simulated that a 33% increase in leaf photosynthesis may translate into only a 5% increase in soybean grain yield, or a –6% change in grain yield in the absence of additional N, presumably associated with more leaf senescence. Long et al., (2006) reported experimentally a lower than expected crop yield stimulation with rising [CO2].
Next, we evaluated the extent to which these secondary effects also contributed to differences in the simulated aboveground mass. This was done from the difference in the significance level of individual terms of multiple linear regression of aboveground mass versus PARint, PLUE, RESP, Froot, and SENES (Table 6). Although the decreasing effect of SENES on mass could not be identified (because of the collinearity between mass and SENES), the significant effect of the other four terms (PLUE, PARint, RESP, and Froot) was well estimated. While the primary PLUE had the strongest effect under both potential and water-limited conditions, the secondary PARint always had the second strongest effect. These results on the importance of the secondary effects on crop-level traits suggest that most existing simulation studies on assessing the impact of engineering photosynthetic targets are incomplete, because computation was only done for leaf photosynthesis (e.g. McGrath and Long, 2014) or for canopy photosynthesis (Zhu et al., 2004; Song et al., 2013).
Table 6.
The coefficients (with probability of significance in parentheses) of linear regression of 31 year average simulated aboveground mass against the simulated values of five component traits, for either potential or water-limited environments, or using the pooled data for the two environments
The five component traits are: PARint, season-long intercepted PAR; PLUE, overall photosynthetic light use efficiency defined as season-long canopy photosynthesis divided by PARint; RESP, season-long crop respiration; Froot, fraction of mass for roots; SENES, aboveground mass lost due to leaf senescence. Linear regression is given as: Y = b0 + b1∙PARint + b2∙PLUE + b3∙RESP + b4∙Froot + b5∙SENES
| Coefficient (unit) | Potential | Water-limited | Pooled data |
|---|---|---|---|
| b 0 (g DM m−2) | –3435.24 (5.92 × 10–18) | –1907.22 (5.34 × 10–32) | –1751.43 (1.57 × 10–34) |
| b 1 (g DM MJ−1 PAR) | 4.495 (2.00 × 10–24) | 3.064 (7.19 × 10–40) | 3.342 (9.92 × 10–42) |
| b 2 [g DM m−2 (g CO2 MJ−1 PAR)−1] | 415.65 (9.36 × 10–35) | 339.49 (3.47 × 10–48) | 328.83 (1.32 × 10–70) |
| b 3 [g DM (g CO2)−1] | –0.2635 (0.045) | –0.5735 (6.53 × 10–22) | -0.7445 (1.22 × 10–23) |
| b 4 (g DM m−2) | –3967.04 (5.05 × 10–13) | –539.44 (0.001) | -1245.07 (2.92 × 10–8) |
| b 5 (g DM g−1 DM) | –1.1938 (0.153) | 1.6387 (6.84 × 10–6) | 1.9219 (0.001) |
| R 2 | 0.992 | 0.999 | 0.993 |
| Data points | 60 | 60 | 120 |
Assessing the importance of individual biochemical targets
While secondary traits were affected, after all one would assess how the primary PLUE is affected by individual biochemical targets or parameters of photosynthesis. We regressed PLUE against individual photosynthetic parameters, with site included as covariate in the regression to remove any effect of possible site differences.
The regression analysis based on simulation results using the C3 model (Routes 1–3 plus the default) indicated that manipulating Sc/o affected PLUE more than manipulating gm (results not shown), consistent with the result that the percentage change in PLUE by Route 2 was higher than that by Route 1 (Fig. 3). However, the relative impact of manipulating Sc/o and gm depends on the extent to which they could actually be changed. Furthermore, an improvement in Sc/o may be at the cost of decreasing Vcmax, because of the often observed negative correlation between Rubisco Sc/o and carboxylase turnover rate (e.g. Kubien et al., 2008; Perdomo et al., 2015). This negative correlation was not considered here, in view of the fact that the non-green alga G. monilis has a high Sc/o25 while maintaining a Rubisco turnover rate similar to C3 plants (Whitney et al., 2001). The impact of the trade-off between Rubisco Sc/o and carboxylase turnover on canopy photosynthesis was analysed by Zhu et al., (2004).
Our simulations using the C4 model (Routes 4–9) involved changes in values of a set of parameters. The most important ones are χgbs25 (which determines the effectiveness of the CCM), φ (extra ATP requirement for the CCM, which determines the required fcyc and, therefore, light-limited photosynthetic efficiency), and χVcmax25 or χJmax25 (which determine light-saturated photosynthetic capacity). Other parameters (e.g. some C3 parameters used for Route 5) had little impact on the shape and values of light response curves (results not shown). We therefore conducted the analysis of regressing PLUE versus χgbs25, 3 + φ (total ATP requirement per mol CO2 assimilated, ATPreq), and χJmax25 (Table 7). There was little effect of sites on PLUE. All three parameters were important for any production level–climate combination. Comparatively, the CCM parameter χgbs25 became most important for water-limited production, because a more effective CCM to elevate the CO2 level around Rubisco can more effectively overcome the negative effect of low Ci under drought. Under the potential production, especially combined with high [CO2] of the 2050 climate, the photosynthetic capacity parameter χJmax25 and quantum efficiency parameter ATPreq were comparatively more important (Table 7). These results suggest that photosynthetic capacity, quantum efficiency, and CCM strength all need improving in order to turbocharge canopy photosynthesis. Based on natural variation of leaf photosynthesis, Gu et al., (2014a) showed that quantum efficiency parameters had even higher effects than capacity parameters on rice productivity.
Table 7.
The coefficients with their probability of significance of linear regression of 31 year average simulated PLUE (overall photosynthetic light-use efficiency as defined in Table 6) against three biochemical parameters (χgbs25, ATPreq, and χJmax25, representing the strength of the CCM, quantum requirement, and photosynthetic capacity, respectively) used in the C4 photosynthesis model, for four cases where potential or water-limited environments were combined with present or 2050 climate conditions
| Potential level | Water-limited level | |||||||
|---|---|---|---|---|---|---|---|---|
| Present climate | 2050 climate | Present climate | 2050 climate | |||||
| Coefficient | Probability | Coefficient | Probability | Coefficient | Probability | Coefficient | Probability | |
| Intercept | 11.674 | 1.45 × 10–11 | 12.108 | 2.67 × 10–12 | 9.019 | 2.31 × 10–12 | 9.385 | 1.49 × 10–14 |
| Nanjinga | 0.097 | 0.56 | 0.102 | 0.50 | –0.078 | 0.48 | –0.112 | 0.15 |
| Shizukuishia | 0.167 | 0.32 | 0.415 | 0.01 | –0.200 | 0.09 | –0.051 | 0.49 |
| χgbs25 | –12.751 | 1.64 × 10–7 | –10.574 | 3.91 × 10–7 | –10.262 | 1.84 × 10–8 | –8.406 | 2.23 × 10–9 |
| ATPreqb | –1.237 | 1.22 × 10–7 | –1.244 | 3.46 × 10–8 | –0.659 | 1.24 × 10–6 | –0.676 | 1.40 × 10–8 |
| χJmax25 | 0.0162 | 7.37 × 10–8 | 0.0150 | 5.12 × 10–8 | 0.0083 | 1.22 × 10–6 | 0.0072 | 7.76 × 10–8 |
| R 2 | 0.963 | 0.965 | 0.961 | 0.976 | ||||
| Data points | 18 | 18 | 18 | 18 | ||||
a Site was included as the covariate in regression, with Los Baños as the reference having a coefficient of zero.
b Total ATP requirement per CO2 assimilated (= 3 + φ), i.e. 5 for C4 photosynthesis and 3.75 for cyanobacterial photosynthesis (see the text).
Concluding remarks
We simulated the likely impact of major routes in ongoing programmes using transgenic technology to improve photosynthesis (Table 2). Our analysis showed that improving leaf photosynthesis can result in an increased rice mass production to a different extent (Table 5), thereby also resulting in different improvements in resource use efficiency such as WUE (Fig. 3). However, to supercharge photosynthesis significantly, engineering for a single improvement route can hardly be effective. Some single routes may be counter-productive at the canopy level. For example, installing C4 biochemistry, if not combined with an effective CCM, is only beneficial for upper leaves of the canopy, while it has no or even a negative impact for lower shaded leaves because such a mechanism requires extra ATP for the C4 cycle. Note that the standard C4 model of von Caemmerer and Furbank (1999) for e− transport limitation does not explicitly consider the increased cyclic e− transport due to the extra ATP costs relative to C3 photosynthesis, and, therefore, cannot recognize the little advantage of C4 photosynthesis under shade. Similarly, the simulation by McGrath and Long (2014) in assessing the potential of cyanobacterial CCM took no account of the extra ATP required by bicarbonate transporters. Our simulation also showed that manipulating photosynthesis may result in unwanted secondary effects on some traits at crop level (e.g. inducing faster senescence if nutrient uptake is not increased). Therefore, the beneficial effect of the single route for high photosynthesis on increasing crop productivity may have previously been overestimated. To supercharge crop productivity, combined routes for improved CCM, photosynthetic capacity, and quantum efficiency are required.
Supplementary Data
Supplementary data are available at JXB online.
Fig. S1. Simulated versus observed relationships between A and gs.
Text 1. Analytical solution to the cubic equation as a result of combined stomatal conductance, CO2 diffusion, and biochemical leaf photosynthesis models.
Text 2. Revising the C4 photosynthesis model for simulating the cyanobacterial CCM.
Text 3. Analytical solution to the quadratic equation as a result of combined CO2
Text 4. Analysis with respect to temperature response parameters of C4 enzyme kinetics.
Text 5. Sensitivity analysis with respect to ATP cost for the cyanobacterial CCM.
Text 6. Description of the crop model GECROS (version 4.0).
Supplementary Material
Acknowledgements
This research is financed in part by the BioSolar Cells open innovation consortium, supported by the Dutch Ministry of Economic Affairs, Agriculture and Innovation. We thank the AgMIP-Rice team for the weather data used in this study.
References
- Bernacchi CJ, Pimentel C, Long SP. 2003. In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant, Cell and Environment 26, 1419–1430. [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. 2002. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiology 130, 1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Jr, Long SP. 2001. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell and Environment 24, 253–259. [Google Scholar]
- Boote KJ, Jones JW, White JW, Asseng S, Lizaso JI. 2013. Putting mechanisms into crop production models. Plant, Cell and Environment 36, 1658–1672. [DOI] [PubMed] [Google Scholar]
- Boyd RA, Gandin A, Cousins AB. 2015. Temperature responses of C4 photosynthesis: biochemical analysis of rubisco, phosphoenolpyruvate carboxylase, and carbonic anhydrase in Setaria viridis. Plant Physiology 169, 1850–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer R. 1983. Functional equilibrium: sense or nonsense? Netherlands Journal of Agricultural Science 31, 335–348. [Google Scholar]
- Cai C, Yin X, He S, et al. 2016. Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Global Change Biology 22, 856–874. [DOI] [PubMed] [Google Scholar]
- Chen CP, Sakai H, Tokida T, Usui Y, Nakamura H, Hasegawa T. 2014. Do the rich always become richer? Characterizing the leaf physiological response of the high-yielding rice cultivar Takanari to free-air CO2 enrichment. Plant and Cell Physiology 55, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins AB, Ghannoum O, von Caemmerer S, Badger MR. 2010. Simultaneous determination of Rubisco carboxylase and oxygenase kinetic parameters in Triticum aestivum and Zea mays using membrane inlet mass spectrometry. Plant, Cell and Environment 33, 444–452. [DOI] [PubMed] [Google Scholar]
- de Pury DGG, Farquhar GD. 1997. Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant, Cell and Environment 20, 537–557. [Google Scholar]
- Driever SM, Lawson T, Andralojc PJ, Raines CA, Parry MA. 2014. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. Journal of Experimental Botany 65, 4959–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S. 2000. Would C4 rice produce more biomass than C3 rice? In: Sheehy JE, Mitchell PL, Hardy B, eds. Redesigning rice photosynthesis to increase yield. Los Baños, the Philippines: International Rice Research Institute, 53–71. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. [DOI] [PubMed] [Google Scholar]
- Fischer RA, Byerlee D, Edmeades GO. 2014. Crop yields and global food security: will yield increase continue to feed the world? ACIAR Monograph No. 158. Canberra: Australian Centre for International Agricultural Research. [Google Scholar]
- Flexas J, Niinemets U, Gallé A, et al. 2013. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynthesis Research 117, 45–59. [DOI] [PubMed] [Google Scholar]
- Flood PJ, Harbinson J, Aarts MG. 2011. Natural genetic variation in plant photosynthesis. Trends in Plant Science 16, 327–335. [DOI] [PubMed] [Google Scholar]
- Fridlyand L, Kaplan A, Reinhold L. 1996. Quantitative evaluation of the role of a putative CO2-scavenging entity in the cyanobacterial CO2-concentrating mechanism. Bio Systems 37, 229–238. [DOI] [PubMed] [Google Scholar]
- Furbank RT, Jenkins CLD, Hatch MD. 1990. C4 photosynthesis: quantum requirement, C4 acid overcycling and Q-cycle involvement. Australian Journal of Plant Physiology 17, 1–7. [Google Scholar]
- Furbank RT, Quick WP, Sirault XRR. 2015. Improving photosynthesis and yield potential in cereal crops by targeted genetic manipulation: prospects, progress and challenges. Field Crops Research 182, 19–29. [Google Scholar]
- Genty B, Harbinson J. 1996. Regulation of light utilization for photosynthetic electron transport. In: Baker NR, ed. Photosynthesis and the environment. Dordrecht, The Netherlands: Kluwer Academic Publishers, 67–99. [Google Scholar]
- Goudriaan J. 1986. A simple and fast numerical method for the computation of daily totals of crop photosynthesis. Agricultural and Forest Meteorology 38, 249–254. [Google Scholar]
- Goudriaan J, van Laar HH. 1994. Modelling potential crop growth processes. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Gu J, Yin X, Stomph TJ, Struik PC. 2014a Can exploiting natural genetic variation in leaf photosynthesis contribute to increasing rice productivity? A simulation analysis. Plant, Cell and Environment 37, 22–34. [DOI] [PubMed] [Google Scholar]
- Gu J, Yin X, Stomph TJ, Wang H, Struik PC. 2012. Physiological basis of genetic variation in leaf photosynthesis among rice (Oryza sativa L.) introgression lines under drought and well-watered conditions. Journal of Experimental Botany 63, 5137–5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Yin X, Zhang C, Wang H, Struik PC. 2014b Linking ecophysiological modelling with quantitative genetics to support marker-assisted crop design for improved yields of rice (Oryza sativa) under drought stress. Annals of Botany 114, 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MR, Lin MT, Carmo-Silva AE, Parry MA. 2016. Towards engineering carboxysomes into C3 plants. The Plant Journal 87, 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley PC, Thomas RB, Reynolds JF, Strain BR. 1992. Modelling photosynthesis of cotton grown in elevated CO2. Plant, Cell and Environment 15, 271–282. [Google Scholar]
- June T, Evans JR, Farquhar GD. 2004. A simple new equation for the reversible temperature dependence of photosynthetic electron transport: a study on soybean leaf. Functional Plant Biology 31, 275–283. [DOI] [PubMed] [Google Scholar]
- Kebeish R, Niessen M, Thiruveedhi K, Bari R, Hirsch HJ, Rosenkranz R, Stäbler N, Schönfeld B, Kreuzaler F, Peterhänsel C. 2007. Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nature Biotechnology 25, 593–599. [DOI] [PubMed] [Google Scholar]
- Kim HY, Lieffering M, Kobayashi K, Okada M, Miura S. 2003. Seasonal changes in the effects of elevated CO2 on rice at three levels of nitrogen supply: a free air CO2 enrichment (FACE) experiment. Global Change Biology 9, 826–837. [Google Scholar]
- Koester RP, Nohl BM, Diers BW, Ainsworth EA. 2016. Has photosynthetic capacity increased with 80 years of soybean breeding? An examination of historical soybean cultivars. Plant, Cell and Environment 39, 1058–1067. [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Long SP. 2016. One crop breeding cycle from starvation? How engineering crop photosynthesis for rising CO2 and temperature could be one important route to alleviation. Proceedings of the Royal Society B: Biological Sciences 283, 20152578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP. 2016. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861. [DOI] [PubMed] [Google Scholar]
- Kubien DS, Whitney SM, Moore PV, Jesson LK. 2008. The biochemistry of Rubisco in Flaveria. Journal of Experimental Botany 59, 1767–1777. [DOI] [PubMed] [Google Scholar]
- Leuning R. 1995. A critical appraisal of a combined stomatal–photosynthesis model for C3 plants. Plant, Cell and Environment 18, 339–355. [Google Scholar]
- Li T, Hasegawa T, Yin X, et al. 2015. Uncertainties in predicting rice yield by current crop models under a wide range of climatic conditions. Global Change Biology 21, 1328–1341. [DOI] [PubMed] [Google Scholar]
- Lin MT, Occhialini A, Andralojc PJ, Devonshire J, Hines KM, Parry MA, Hanson MR. 2014. β-Carboxysomal proteins assemble into highly organized structures in Nicotiana chloroplasts. The Plant Journal 79, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long BM, Rae BD, Rolland V, Förster B, Price GD. 2016. Cyanobacterial CO2-concentrating mechanism components: function and prospects for plant metabolic engineering. Current Opinion in Plant Biology 31, 1–8. [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Leakey AD, Nösberger J, Ort DR. 2006. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312, 1918–1921. [DOI] [PubMed] [Google Scholar]
- Long SP, Marshall-Colon A, Zhu XG. 2015. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56–66. [DOI] [PubMed] [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR. 2006. Can improvement in photosynthesis increase crop yields? Plant, Cell and Environment 29, 315–330. [DOI] [PubMed] [Google Scholar]
- Makino A, Sakuma H, Sudo E, Mae T. 2003. Differences between maize and rice in N-use efficiency for photosynthesis and protein allocation. Plant and Cell Physiology 44, 952–956. [DOI] [PubMed] [Google Scholar]
- McGrath JM, Long SP. 2014. Can the cyanobacterial carbon-concentrating mechanism increase photosynthesis in crop species? A theoretical analysis. Plant Physiology 164, 2247–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith JL. 1973. Principles of environmental physics. London: Edward Arnold. [Google Scholar]
- Miflin B. 2000. Crop improvement in the 21st century. Journal of Experimental Botany 51, 1–8. [PubMed] [Google Scholar]
- Morison JI, Gifford RM. 1983. Stomatal sensitivity to carbon dioxide and humidity: a comparison of two C3 and two C4 grass species. Plant Physiology 71, 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie EH, Pinto M, Horton P. 2009. Agriculture and the new challenges for photosynthesis research. New Phytologist 181, 532–552. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Iwano M, Havaux M, Yokota A, Munekage YN. 2013. Promotion of cyclic electron transport around photosystem I during the evolution of NADP-malic enzyme-type C4 photosynthesis in the genus Flaveria. New Phytologist 199, 832–842. [DOI] [PubMed] [Google Scholar]
- Ort DR, Merchant SS, Alric J., et al. 2015. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proceedings of the National Academy of Sciences, USA 112, 8529–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry MA, Reynolds M, Salvucci ME, Raines C, Andralojc PJ, Zhu XG, Price GD, Condon AG, Furbank RT. 2011. Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. Journal of Experimental Botany 62, 453–467. [DOI] [PubMed] [Google Scholar]
- Pengelly JJ, Förster B, von Caemmerer S, Badger MR, Price GD, Whitney SM. 2014. Transplastomic integration of a cyanobacterial bicarbonate transporter into tobacco chloroplasts. Journal of Experimental Botany 65, 3071–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdomo JA, Cavanagh AP, Kubien DS, Galmés J. 2015. Temperature dependence of in vitro Rubisco kinetics in species of Flaveria with different photosynthetic mechanisms. Photosynthesis Research 124, 67–75. [DOI] [PubMed] [Google Scholar]
- Price GD, Badger MR, von Caemmerer S. 2011. The prospect of using cyanobacterial bicarbonate transporters to improve leaf photosynthesis in C3 crop plants. Plant Physiology 155, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Pengelly JJ, Forster B, Du J, Whitney SM, von Caemmerer S, Badger MR, Howitt SM, Evans JR. 2013. The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. Journal of Experimental Botany 64, 753–768. [DOI] [PubMed] [Google Scholar]
- Sadras VO, Lawson C. 2011. Genetic gain in yield and associated changes in phenotype, trait plasticity and competitive ability of South Australian wheat varieties released between 1958 and 2007. Crop and Pasture Science 62, 533–549. [Google Scholar]
- Sage RF. 2002. Variation in the kcat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. Journal of Experimental Botany 53, 609–620. [DOI] [PubMed] [Google Scholar]
- Seemann JR, Badger MR, Berry JA. 1984. Variations in the specific activity of ribulose-1,5-bisphosphate carboxylase between species utilizing differing photosynthetic pathways. Plant Physiology 74, 791–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter TL, Peng S, Kirk GJD, Virmani SS, Kropff MJ, Cassman KG. 1994. Physiological considerations and hybrid rice. In: Cassman KG, ed. Breaking the yield barrier. Proceedings of a workshop on rice yield potential in favorable environments. Los Baños, Philippines: IRRI, 39–62. [Google Scholar]
- Shikanai T. 2007. Cyclic electron transport around photosystem I: genetic approaches. Annual Review of Plant Biology 58, 199–217. [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Horie T. 1989. Leaf nitrogen, photosynthesis, and crop radiation use efficiency: a review. Crop Science 29, 90–98. [Google Scholar]
- Sinclair TR, Purcell LC, Sneller CH. 2004. Crop transformation and the challenge to increase yield potential. Trends in Plant Science 9, 70–75. [DOI] [PubMed] [Google Scholar]
- Singh J, Pandey P, James D, Chandrasekhar K, Achary VM, Kaul T, Tripathy BC, Reddy MK. 2014. Enhancing C3 photosynthesis: an outlook on feasible interventions for crop improvement. Plant Biotechnology Journal 12, 1217–1230. [DOI] [PubMed] [Google Scholar]
- Song Q, Zhang G, Zhu X-G. 2013. Optimal crop canopy architecture to maximise canopy photosynthetic CO2 uptake under elevated CO2—a theoretical study using a mechanistic model of canopy photosynthesis. Functional Plant Biology 40, 109–124. [DOI] [PubMed] [Google Scholar]
- Tholen D, Boom C, Zhu XG. 2012. Opinion: prospects for improving photosynthesis by altering leaf anatomy. Plant Science 197, 92–101. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. 2013. Steady-state models of photosynthesis. Plant, Cell and Environment 36, 1617–1630. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Furbank RT. 1999. Modeling C4 photosynthesis. In: Sage RF, Monson RK, eds. C4 plant biology. Toronto: Academic Press, 173–211. [Google Scholar]
- von Caemmerer S, Furbank RT. 2003. The C4 pathway: an efficient CO2 pump. Photosynthesis Research 77, 191–207. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Quick WP, Furbank RT. 2012. The development of C4 rice: current progress and future challenges. Science 336, 1671–1672. [DOI] [PubMed] [Google Scholar]
- Walker BJ, VanLoocke A, Bernacchi CJ, Ort DR. 2016. The costs of photorespiration to food production now and in the future. Annual Review of Plant Biology 67, 107–129. [DOI] [PubMed] [Google Scholar]
- Whitney SM, Baldet P, Hudson GS, Andrews TJ. 2001. Form I Rubiscos from non-green algae are expressed abundantly but not assembled in tobacco chloroplasts. The Plant Journal 26, 535–547. [DOI] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. 1985. Leaf conductance in relation to rate of CO2 assimilation: I. Influence of nitrogen nutrition, phosphorus nutrition, photon flux density, and ambient partial pressure of CO2 during ontogeny. Plant Physiology 78, 821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Belay DW, van der Putten PE, Struik PC. 2014. Accounting for the decrease of photosystem photochemical efficiency with increasing irradiance to estimate quantum yield of leaf photosynthesis. Photosynthesis Research 122, 323–335. [DOI] [PubMed] [Google Scholar]
- Yin X, Goudriaan J, Lantinga EA, Vos J, Spiertz JHJ. 2003. A flexible sigmoid function of determinate growth. Annals of Botany 91, 361–371 (erratum in Annals of Botany 2003. 91, 753). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Harbinson J, Struik PC. 2006. Mathematical review of literature to assess alternative electron transports and interphotosystem excitation partitioning of steady-state C3 photosynthesis under limiting light. Plant, Cell and Environment 29, 1771–1782. [DOI] [PubMed] [Google Scholar]
- Yin X, Struik PC. 2008. Applying modelling experiences from the past to shape crop systems biology: the need to converge crop physiology and functional genomics. New Phytologist 179, 629–642. [DOI] [PubMed] [Google Scholar]
- Yin X, Struik PC. 2009. C3 and C4 photosynthesis models: an overview from the perspective of crop modelling. NJAS-Wageningen Journal of Life Sciences 57, 27–38. [Google Scholar]
- Yin X, Struik PC. 2012. Mathematical review of the energy transduction stoichiometries of C4 leaf photosynthesis under limiting light. Plant, Cell and Environment 35, 1299–1312. [DOI] [PubMed] [Google Scholar]
- Yin X, Struik PC, Romero P, Harbinson J, Evers JB, van der Putten PE, Vos J. 2009. Using combined measurements of gas exchange and chlorophyll fluorescence to estimate parameters of a biochemical C3 photosynthesis model: a critical appraisal and a new integrated approach applied to leaves in a wheat (Triticum aestivum) canopy. Plant, Cell and Environment 32, 448–464. [DOI] [PubMed] [Google Scholar]
- Yin X, Sun Z, Struik PC, Van der Putten PE, Van Ieperen W, Harbinson J. 2011. Using a biochemical C4 photosynthesis model and combined gas exchange and chlorophyll fluorescence measurements to estimate bundle-sheath conductance of maize leaves differing in age and nitrogen content. Plant, Cell and Environment 34, 2183–2199. [DOI] [PubMed] [Google Scholar]
- Yin X, van der Putten PE, Driever SM, Struik PC. 2016. Temperature response of bundle-sheath conductance in maize leaves. Journal of Experimental Botany 67, 2699–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, van Laar HH. 2005. Crop systems dynamics: an ecophysiological simulation model for genotype-by-environment interactions. Wageningen, The Netherlands: Wageningen Academic Publishers. [Google Scholar]
- Yin X, van Oijen M, Schapendonk AHCM. 2004. Extension of a biochemical model for the generalized stoichiometry of electron transport limited C3 photosynthesis. Plant, Cell and Environment 27, 1211–1222. [Google Scholar]
- Zhu X-G, Portis AR, Jr, Long SP. 2004. Would transformation of C3 crop plants with foreign Rubisco increase productivity? A computational analysis extrapolating from kinetic properties to canopy photosynthesis. Plant, Cell and Environment 27, 55–165. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.