Highlight
Genotypic variation in both average stomatal conductance operating levels and photosynthesis rates for given conductance levels make important contributions to the variation in intrinsic transpiration efficiency in diverse sugarcane-related germplasm.
Keywords: Breeding, genotypic variation, photosynthesis, sugarcane, transpiration efficiency.
Abstract
Sugarcane, derived from the hybridization of Saccharum officinarum×Saccharum spontaneum, is a vegetative crop in which the final yield is highly driven by culm biomass production. Cane yield under irrigated or rain-fed conditions could be improved by developing genotypes with leaves that have high intrinsic transpiration efficiency, TEi (CO2 assimilation/stomatal conductance), provided this is not offset by negative impacts from reduced conductance and growth rates. This study was conducted to partition genotypic variation in TEi among a sample of diverse clones from the Chinese collection of sugarcane-related germplasm into that due to variation in stomatal conductance versus that due to variation in photosynthetic capacity. A secondary goal was to define protocols for optimized larger-scale screening of germplasm collections. Genotypic variation in TEi was attributed to significant variation in both stomatal and photosynthetic components. A number of genotypes were found to possess high TEi as a result of high photosynthetic capacity. This trait combination is expected to be of significant breeding value. It was determined that a small number of observations (16) is sufficient for efficiently screening TEi in larger populations of sugarcane genotypes The research methodology and results reported are encouraging in supporting a larger-scale screening and introgression of high transpiration efficiency in sugarcane breeding. However, further research is required to quantify narrow sense heritability as well as the leaf-to-field translational potential of genotypic variation in transpiration efficiency-related traits observed in this study.
Introduction
Sugarcane is a major tropical and subtropical crop derived from interspecific hybridization of Saccharum officinarum×Saccharum spontaneum (Roach, 1989). Sugarcane is harvested worldwide in a greater quantity than any other crop, with about 1.9 billion tonnes harvested in 2014 (FAO, 2015). It is the main source of sugar production worldwide and increasingly important in bio-energy production (Cardona et al., 2010). Production areas of sugarcane mostly lie in rain-fed regions, but there is also a significant area that is irrigated. Water stress is a major limitation to growth in many rain-fed production regions (Passioura and Angus, 2010). Given that irrigation costs and competing demands for water in sugarcane production regions are predicted to grow, the development of sugarcane cultivars with enhanced water-use efficiency is becoming increasingly desirable.
Crop yield in any water-limited environment can be expressed in terms of the following components:
| (1) |
This expression has been useful for researchers to conceptualize and interpret causes of variation in yield in water-limited environments (e.g. Passioura, 1977; Condon and Richards, 1993; Richards et al., 2002). In sugarcane, harvest index can be defined as the proportion of above-ground biomass present as millable cane, and typically is approximately 0.8 at harvest (Robertson et al., 1996). Genotypic improvement for biomass yield can potentially arise only through greater water use (transpiration), or greater biomass per amount of water transpired (TE). In environments where available soil water is used prior to harvest, increases in yield through genetic improvements can arise only by improving water-use efficiency or harvest index (Ghannoum, 2016).
Leaf CO2 assimilation (A) and H2O vapour transpiration (E) rates can be expressed as:
| (2) |
and
| (3) |
where Ci and Ca are the leaf intercellular and ambient CO2 partial pressures, and ei and ea the H2O vapour pressures inside the leaf and in the surrounding air, respectively. In addition, gs_H2O = 1.6gs_CO2, where gs_CO2 and gs_H2O refer to the stomatal conductance (gs) for CO2 and H2O vapour, respectively, and 1.6 is the ratio of binary diffusivity of H2O vapour to that of CO2 in air (Farquhar et al., 1989). Accordingly, leaf-level transpiration efficiency (TEL) is given by:
| (4) |
Whereas assimilation rates depend on the CO2 supply (stomatal conductance) and demand (photosynthetic capacity) functions, transpiration rates depend on stomatal and boundary layer conductance as well as the leaf-to-air vapour pressure difference, which in turn depends on leaf temperature and the relative humidity of the surrounding air. Another expression that excludes the direct effects of vapour pressure in surrounding air and is commonly used in comparing genotypes is termed intrinsic transpiration efficiency, TEi, given by (Farquhar et al., 1989):
| (5) |
Reduced gs leads to lower Ci and Ci/Ca, which represents an integrative parameter of TEi, reflecting changes in both A and gs (equation 5).
Recently, Jackson et al. (2016) reported significant genetic variations in leaf and whole-plant TE in sugarcane and related germplasm, and observed that Ci had a negative genotypic correlation with whole-plant TE, at least at mid-range gs levels.
While there is usually a close positive relationship between A and gs, it has been shown in many species that this relationship is not linear, such that the slope of A versus gs decreases as gs becomes larger (Gilbert et al., 2011). Thus, in examining variation within any population of genotypes, relatively high TEi in any particular genotype may arise because of low conductance, or because of greater photosynthetic capacity compared with other genotypes for a given conductance (Gilbert et al., 2011). In addition, while enhancing TE may be a worthwhile goal in crop improvement programmes, a potential problem arises because a negative covariance is frequently observed between transpiration and TE (Condon et al., 2004; Blum, 2005, 2009; Sinclair, 2012; Vadez et al., 2014). For example, if high TEi arises mainly due to reduced gs, this may be associated with reduced water use and productivity. By contrast, increased TEi due to a higher rate of photosynthesis at any given level of conductance (and leading to lower Ci) may be expected to be of more general agronomic value (Ghannoum, 2016).
Sugarcane is a crop in which the final yield is highly driven by total biomass production, unlike most grain crops, for which environmental conditions impacting the timing of flowering and grain filling complicate the relationship between TE, water use, and crop yield. Hence, in sugarcane, photosynthetic capacity is likely to be more directly related to crop yield than is the case with grain crops.
The aim of this study was to partition genotypic variation in leaf-level TEi in a sample of diverse sugarcane-related genotypes into that attributable to the variation in photosynthetic capacity (TEpc) and that attributable to the variation in stomatal conductance (TEgs), following the concepts advocated by Gilbert et al. (2011). The genotypes examined were sampled from the extensive Chinese sugarcane-related germplasm collection. The genera and species contained in this collection are from the so-called Saccharum complex (Mukherjee, 1957), which are believed to be involved in the evolution of sugarcane and are able to be crossed with sugarcane (Berding and Roach, 1987). Previous genetic diversity studies using DNA markers indicated some relationships between and within species in this collection (Cai et al., 2005) and were used to support the sampling methodology in this study. A stratified random sample of genotypes was taken that represents a range of species and a major portion of the genotypic diversity contained within this germplasm collection. The results from this study are discussed in relation to whether an introgression breeding programme targeting improvement of TEi would be of value, and to help define methodology that could be used for efficiently screening larger populations of sugarcane genotypes in any future introgression breeding programmes.
Materials and methods
Overview of the experiment and germplasm collection
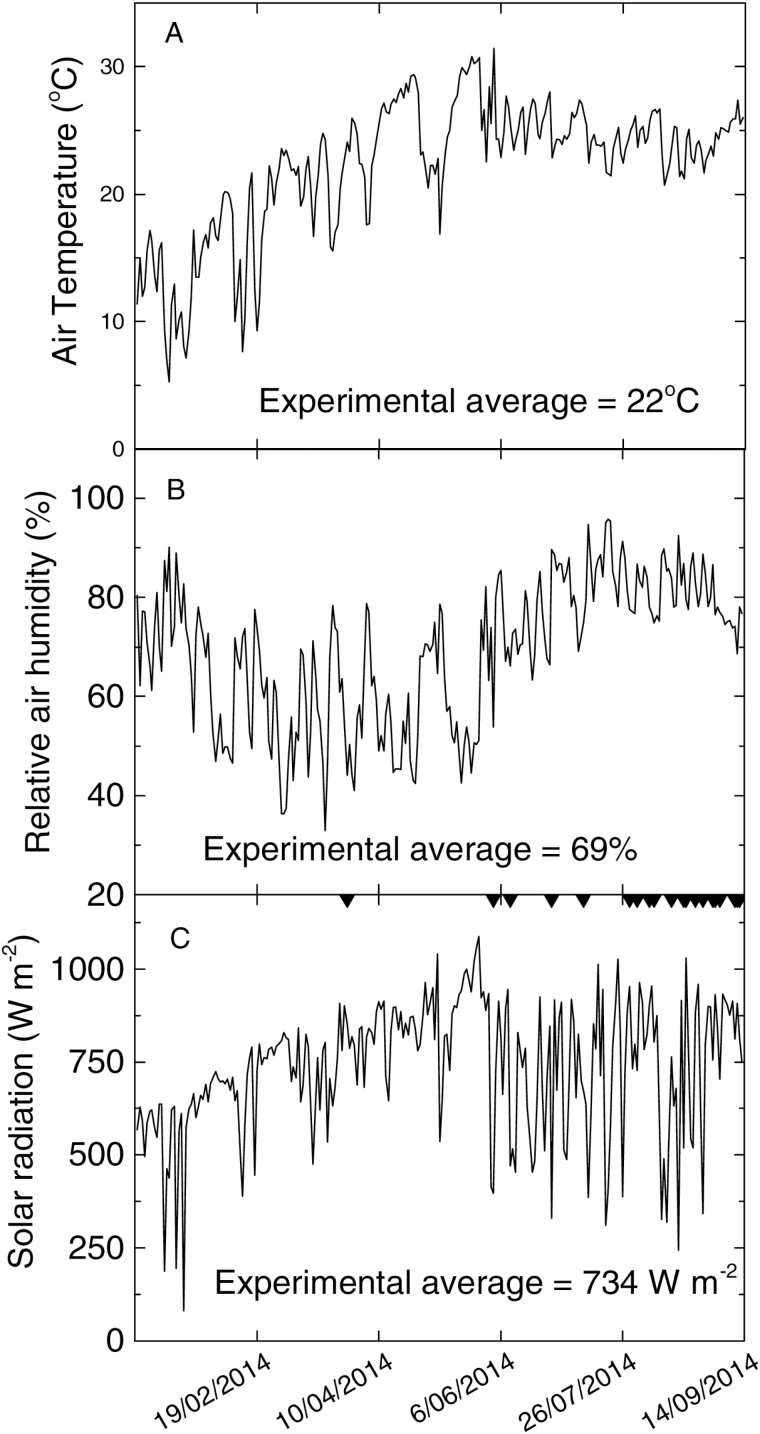
Twenty clones of sugarcane-related germplasm (Table 1) were grown under well-watered conditions at the National Germplasm Repository of Sugarcane in Kaiyuan (NGRS-KY), located within a commercial sugarcane-growing region in Yunnan Province, Southwestern China (103.25°E, 23.70°N, altitude 1052 m). Weather conditions during the experiment are shown in Fig. 1. The NGRS-KY is the major field gene bank for conservation of sugarcane and related germplasm clones in China. The repository, which was established in 1995 and expanded in 2003, seeks to explore, collect, characterize, and provide materials for use in sugarcane research and breeding programmes, as well as to exchange germplasm with institutes and organizations around the world. Currently, the repository is 2.3 ha in area and contains 2664 accessions across 15 species in five genera. Most of the germplasm clones were domestically collected from 14 provinces in southern China. Foreign clones, received through exchange pathways, are also included (http://www.yngzs.net/news_detailkjcx/newsId=139.html). The 20 clones sampled in this experiment (Table 1) were selected to represent most of the species curated in the collection, including replication of clones from some of the largest species groups. The selected clones included a set of wild species and a number of commercial cultivars selected from breeding programmes and bred for high sugar content and cane yield.
Table 1.
Summary of leaf gas exchange characteristics for 20 sugarcane-related clones measured in the germplasm screening experiment
| Clone | Species |
A
(μmol m−2 s−1) |
gs (mol m−2 s−1) |
Ci (μL L−1) |
TEi (A/gs) (μmol mol−1) |
|---|---|---|---|---|---|
| 51NG63 | Saccharum robustum | 23.1 | 0.169 | 122 | 142 |
| Guangxi87-20 | Saccharum spontaneum | 20.2 | 0.146 | 128 | 141 |
| Hainanlingshui4 | Saccharum spontaneum | 20.8 | 0.154 | 132 | 138 |
| India2 | Saccharum spontaneum | 24.9 | 0.185 | 124 | 139 |
| Yunnan2009-2 | Saccharum spontaneum | 23.3 | 0.183 | 137 | 132 |
| Uba | Saccharum sinense | 13.9 | 0.095 | 123 | 150 |
| 96NG16 | Saccharum officinarum | 23.9 | 0.176 | 124 | 140 |
| Pansahi | Saccharum barberi | 16.8 | 0.13 | 143 | 135 |
| Guangdong64# | Narenga porphyrocoma | 17.6 | 0.127 | 129 | 143 |
| Yunnan95-35# | Miscanthus sinensis | 10 | 0.076 | 156 | 133 |
| Guangxi79-8# | Miscanthus floridulus | 10.3 | 0.07 | 129 | 150 |
| Guangdong2010-102# | Imperata cylindrica | 12.6 | 0.09 | 133 | 145 |
| Yunnan95-20# | Erianthus rockii | 15.8 | 0.112 | 121 | 149 |
| Yunnan97-4# | Erianthus fulvus | 12 | 0.093 | 159 | 129 |
| Hainan92-84# | Erianthus arundinaceus | 14.1 | 0.088 | 102 | 164 |
| KQ01-1390 | Commercial cultivara | 17.9 | 0.131 | 134 | 139 |
| Q208 | Commercial cultivara | 16.8 | 0.13 | 146 | 133 |
| ROC22 | Commercial cultivarb | 20.4 | 0.157 | 142 | 132 |
| Yuetang93-159 | Commercial cultivarb | 16.8 | 0.126 | 136 | 139 |
| Yunzhe03-194 | Commercial cultivarb | 22.3 | 0.174 | 132 | 136 |
| Mean | 17.4 | 0.128 | 133 | 141 | |
| Least significant difference (P < 0.05) | 2.1 | 0.018 | 12.3 | 7.7 | |
The clones were grown outdoors in pots or in the field. Plants were well watered and fertilized. Leaf gas exchange measurements were made at a photosynthetically active radiation of 1200 μmol m−2 s−1, CO2 of 400 µl L−1, and under ambient temperature and humidity. Values are the means of four replicates per clone measured over 20 days. The averages of each parameter across all measurements are shown. Commercial cultivars in aAustralia and bChina refer to a complex derivative of Saccharum officinarum×Saccharum spontaneum. #Transplanted from rhizomes instead of stem cuttings.
Fig. 1.
Weather conditions during the experimental period. (A) Daily average temperature and (B) humidity, and (C) maximum (on an hourly basis) solar radiation during the experimental period. Plants were transplanted on 21 December 2013 and measured between 28 March 2014 and 12 September 2014. Days of measurement are indicated with tick marks on the top border of panel C.
Experimental design
The experimental design was a randomized complete block design with four blocks. Three blocks comprised plants grown in pots, and one block consisted of plants grown in the ground. Each clone was represented by one pot or plant within each of the four blocks. All plants were grown outdoors under ambient conditions (Fig. 1). Single-bud stem cuttings of each clone were first transplanted into bowls on 21 December 2013 for germination until the three- to four-leaf stage, and then transferred on 15 January 2014 into 22.1 L pots (35 cm height and 50 cm diameter), filled with the local nursery soil, a typical subtropical red earth. For clones which had lateral buds reluctant to sprout, rhizomes were used instead of stem cuttings (Table 1). Two plants per clone were transplanted into each pot. Plants grown in the ground were established similarly to potted ones.
Potted plants were well watered daily, while in-ground plants were flood-irrigated once every month. Calcium superphosphate and urea (LvBao, Kunming, China) were applied as base fertilizer when the seedlings and rhizomes were planted into the pots or in the field. Subsequently, slow-release fertilizer (LvBao) was applied near the stem base every 4–5 weeks. Weeds were removed by hand, and herbicide (Paraquat; Syngenta, Guangzhou, China) was sprayed onto the weeds, avoiding the clones. Granular pesticide (Bisultap, Kunming, China) for insect stem borer control was applied in the same way as the base fertilizer. Liquid pesticide (chlorantraniliprole-thiamethoxam; Syngenta) was sprayed onto the plants 3 days after they were transplanted. Subsequent pesticide applications were employed as required.
Measurements
Leaf gas exchange measurements were conducted over the course of the experiment using a portable open gas exchange system (LI-6400; Li-Cor, Lincoln, NE, USA) to determine A, gs, Ci, the ratio of intercellular to ambient CO2 (Ci/Ca), and instantaneous leaf-level TEi (A/gs). Measurements were conducted between 10:00 and 14:00 on attached, last fully-expanded leaves. Measurements were made at a photosynthetic photon flux density of 1200 μmol m−2 s−1, a CO2 concentration of 400 μl L−1, and ambient temperature and humidity. Across all measurements, leaf temperature varied between 24 and 42°C, and leaf-to-air vapour pressure deficit ranged between 1 and 6 kPa. Before each measurement, the leaf was allowed to stabilize until it reached a steady state of CO2 uptake. Leaf gas exchange was measured for all plants on 20 different days spread between 28 March 2014 and 12 September 2014. On several dates, not all pots were measured, with a total of 1325 measurements being made.
Partitioning of TEi variations
Genotypic effects for TEi were partitioned into two independent sources of variation: one source attributed to variation in stomatal conductance (TEgs) and the other attributed to variation in photosynthetic capacity (TEpc). This partitioning followed the concepts described by Gilbert et al. (2011), but with some modifications. Given the general curvilinear relationship between gs and A resulting in a negative relationship between TEi and gs, on average, genotypes with low gs measures are expected to have higher TEi than genotypes with high gs. Hence, the impact of gs on TEi may be due to stomatal closure contributing to lower Ci and higher TEi, as explained by Condon et al. (2002), among others. Accordingly, for each measurement of leaf gas exchange, TEgs was defined as the TEi expected if the photosynthetic capacity was determined from a reference (A versus gs) function for the measured value of gs, and expressed as a deviation from the mean TEi based on all observations. Unlike Gilbert et al. (2011), the reference (A versus gs) function was derived from observations from all genotypes rather than just those from an individual comparator genotype. TEpc for each measurement point was defined as the deviation of the actual observed TEi from the TEgs (i.e. TEi – TEgs; the deviation of the observed TEi from the expected population average at the measured conductance level).
Data analysis
Data were analysed using SAS version 9.4, PROC MIXED, and PROC GLM procedures. All measurements for each trait (A, gs, Ci, TEi, TEpc, TEgs) were pooled for analysis and total variance was partitioned into dates and genotypes main effects, clone × date interaction effects, and residual ‘error’ variation. In the mixed model analyses, dates and genotypes were considered as random effects and variance components estimated accordingly using the restricted maximum likelihood method. P values for significance testing of genotype effects were obtained from the PROC GLM procedure using the same partitioning of effects.
Broad sense heritability (Hb) for traits was determined from Falconer and Mackay (1996):
| (6) |
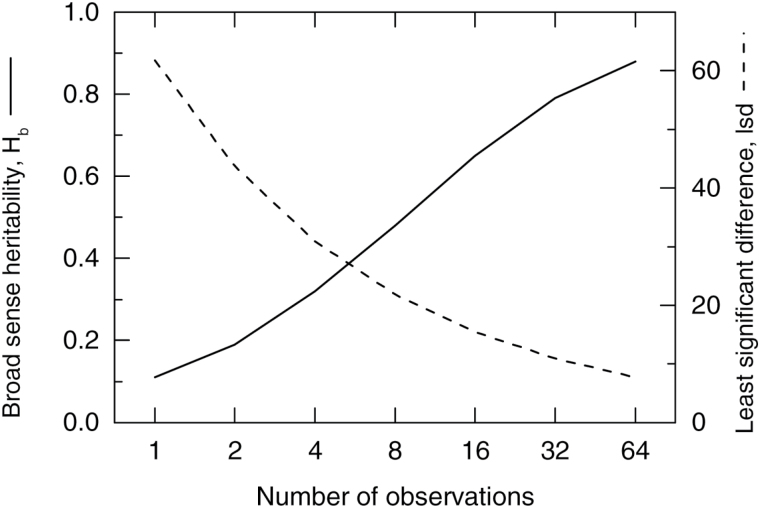
where σg2 and σp2 are the genotypic and phenotypic variance, σp2 = σg2 + σg.date2/d + σe2/n, σg.date2 = clone × date interaction variance, d = number of different dates of measurement, σe2 = error variance, and n = total number of observations per genotype. For the purpose of estimating Hb, it was assumed that n = 63 (this value in fact ranged from 63 to 70). Hb and least significant differences were also determined for TEi for a range of different n values (Fig. 2), to simulate expected values if different numbers of observations were made in future experiments involving screening of more germplasm.
Fig. 2.
Predicted effect of number of observations on Hb and least significant difference.
The genotypic coefficient of variation (GCV, %), an indicator of relative genotypic variation, was determined for each trait from:
| (7) |
where σg is the standard deviation of the genotypic effect, is the population mean, and σg2 is the genotypic variance. For the measures of TEgs and TEpc where averages are near zero (because these values are expressed as deviations from the average of all observations), the value used for was that for TEi.
Expected genotypic gain from different combinations of measurement number and number of clones was predicted using the following standard formula for gain from selection (Falconer and Mackay, 1996):
| (8) |
where i = intensity of selection; obtained from Appendix Table A in the text by Falconer and Mackay (1996), h = square root of broad sense heritability, and σg = genotypic standard deviation.
Pearson’s correlation coefficients among the various parameters were calculated using SPSS 19.0. The correlations among gas exchange parameters including A, gs, Ci, and TEi were evaluated for all the data collected in the germplasm experiment.
Results
Variation in measurements
Significant genotypic variation was observed for leaf-level A, gs, Ci, TEi, TEpc, and TEgs among the clones (Tables 1 and 2). A 2.5-fold variation in A was observed, with values ranging from an average of 10 μmol m−2 s−1 in Yunnan95-35 (Miscanthus sinensis) to 24.9 μmol m−2 s−1 in India2 (Saccharum spontaneum). Similarly, a 2.6-fold proportional variation was observed for gs, with this parameter ranging from an average of 0.07 mol m−2 s−1 in Guangxi79-8 (M. floridulus) to 0.185 mol m−2 s−1 in India2 (S. spontaneum). Smaller, but significant genotypic variations in Ci and TEi (1.6-fold and 1.7-fold, respectively) were also observed. Yunnan97-4 (Erianthus fulvus) exhibited the highest average Ci (159 μL L−1) and lowest average TEi (129 μmol CO2 mol−1 H2O), while Hainan92-84 (E. arundinaceus) exhibited the most extreme values at the opposite end of the observed variation (Ci = 102 μmol mol−1 and TEi = 164 μmol CO2 mol−1 H2O).
Table 2.
Summary statistics from analysis of variance, and estimates of Hb on the basis of all data or a single measurement
| Statistic |
A
(μmol m−2 s−1) |
gs (mol m−2 s−1) |
Ci (μL L−1) |
TEi (A/gs) (μmol mol−1) |
TEgs (μmol mol−1) |
TEpc (μmol mol−1) |
|---|---|---|---|---|---|---|
| GCV (%) | 25.3 | 27.4 | 8.6 | 5.3 | 4.0 | 2.9 |
| σclones2 | 19.1*** | 0.00122*** | 132*** | 55.8*** | 32.1*** | 16.9*** |
| σclone × dates2 | 4.81** | 0.00029** | 5.1 ns | 7.9 ns | 4.92** | 0.52 ns |
| σerror2 | 36.3 | 0.00278 | 1198 | 470 | 63.0 | 183.1 |
| Hb (all data basis) | 0.96 | 0.96 | 0.87 | 0.88 | 0.94 | 0.91 |
| Hb (single measure basis) | 0.32 | 0.29 | 0.10 | 0.10 | 0.21 | 0.14 |
ns = not significant; **P < 0.05; ***P < 0.001. Analysis was carried out as described in the “Materials and methods”.
Measurements of A and gs had much higher GCVs compared with Ci and TE (Table 2). Variation due to clone × date interaction was relatively small for all measurements compared with genotypic variance (Table 2), indicating that clones responded reasonably similarly to each other across the range of conditions experienced during the course of the experiment. Error variance was large relative to genotypic variance (about 2-fold larger for A and gs and 8-fold larger for TE) but Hb based on all data was high (>0.8) for all measurements (Table 2), due to the large number (≥63) of observations made on each clone (Fig. 2). This demonstrated accurate discrimination among the clones for this number of measurements (i.e. >85% of variation in observed genotype means attributed to genotypic effects).
Expected values of Hb on the basis of different numbers of measurements can be used to help develop optimal strategies for future germplasm screening experiments, and these are indicated in Fig. 2. In any particular screening effort with a finite set of resources available for measurements (i.e. assuming there is a particular maximum number of measurements that may be made), there exists a compromise between the desire to obtain a high accuracy of characterizing each individual genotype through more measurements and increasing numbers of genotypes which may be screened. From a breeding programme perspective, the aim is usually to maximize the gain from selection (Equation 8), and this can be calculated for different experimental configurations (e.g. genotype number × number of measurements per genotype) (Falconer and Mackay, 1996). Predicted gain from selection is given in Table 3 for different options for screening clones and assuming that a fixed number of 3000 measurements can be made.
Table 3.
Predicted gain in TEi from selection of the top 10 clones
| Number of measurements per clone | Number of clones able to be screened | Gain in TEi from selecting the top ranked 10 clones for TEi (μmol mol−1) |
|---|---|---|
| 1 | 3000 | 7.4 |
| 2 | 1500 | 9.2 |
| 4 | 750 | 11.1 |
| 8 | 375 | 12.1 |
| 16 | 188 | 12.3 |
| 32 | 94 | 11.7 |
| 64 | 47 | 9.2 |
Predictions are for different combinations of measurements per genotypes × number of genotypes, and assuming a fixed number of 3000 measurements are made.
Relationships between gas exchange parameters
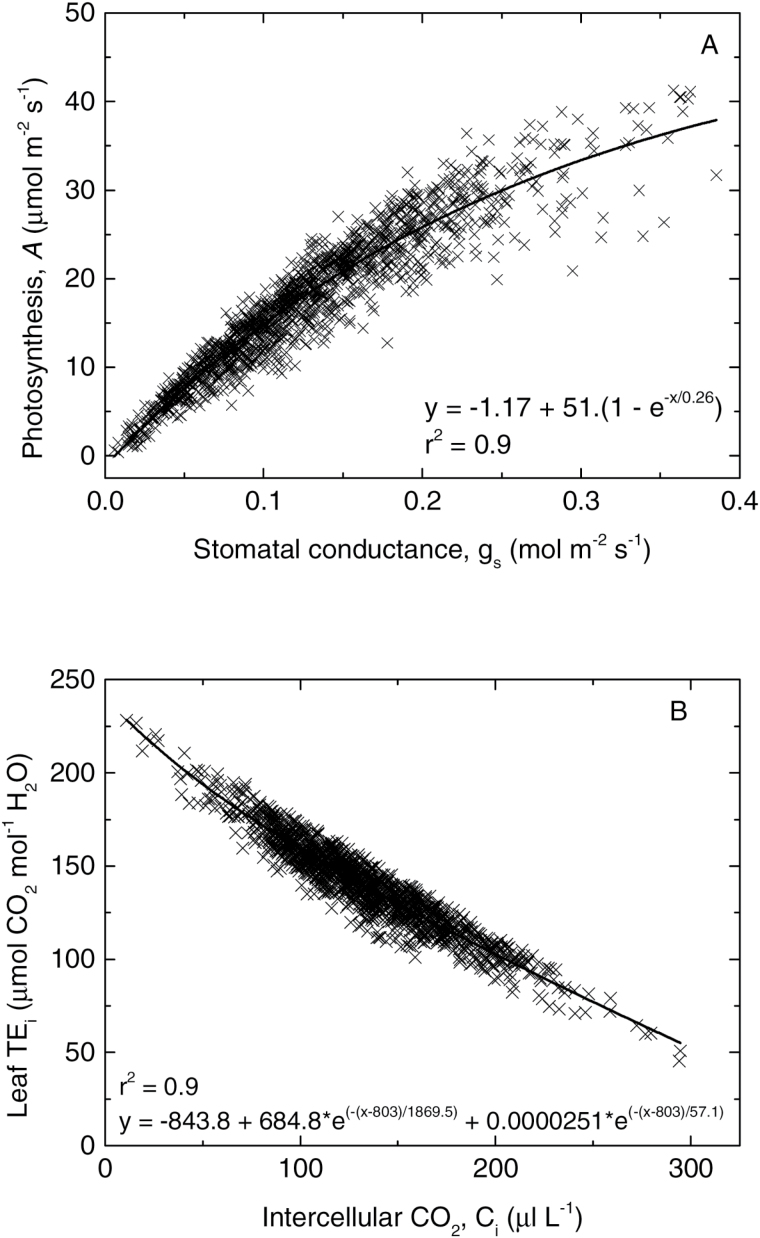
There was a strong positive correlation between A and gs (Table 4). As expected, this relationship was curvilinear (Fig. 3A) and consequently resulted in low values of gs associated with higher TEi (A/gs) (as reported elsewhere), and a negative relationship between gs and TEi (Table 4). As expected, there was also a strong negative relationship between Ci and TEi (Fig. 3B, Table 4).
Table 4.
Correlations among leaf gas exchange parameters
| A | gs | TEi | Ci | TEgs | TEpc | |
|---|---|---|---|---|---|---|
| A | 1.00 | |||||
| gs | 0.94 | 1.00 | ||||
| TEi | −0.13 | −0.41 | 1.00 | |||
| Ci | −0.18 | 0.12 | −0.95 | 1.00 | ||
| TEgs | −0.63 | −0.73 | 0.40 | −0.19 | 1.00 | |
| TEpc | 0.23 | −0.02 | 0.85 | −0.92 | −0.14 | 1.00 |
Pearson’s correlation coefficients for each measurement are shown based on all data collected. All correlations are statistically significant (P < 0.001, n = 1325).
Fig. 3.
Relationship between leaf gas exchange parameters. (A) The relationship between A and gs and (B) the relationship between TEi and Ci, for all data collected. The solid line is a best fit exponential equation for all data points.
Variation in components of leaf TEi
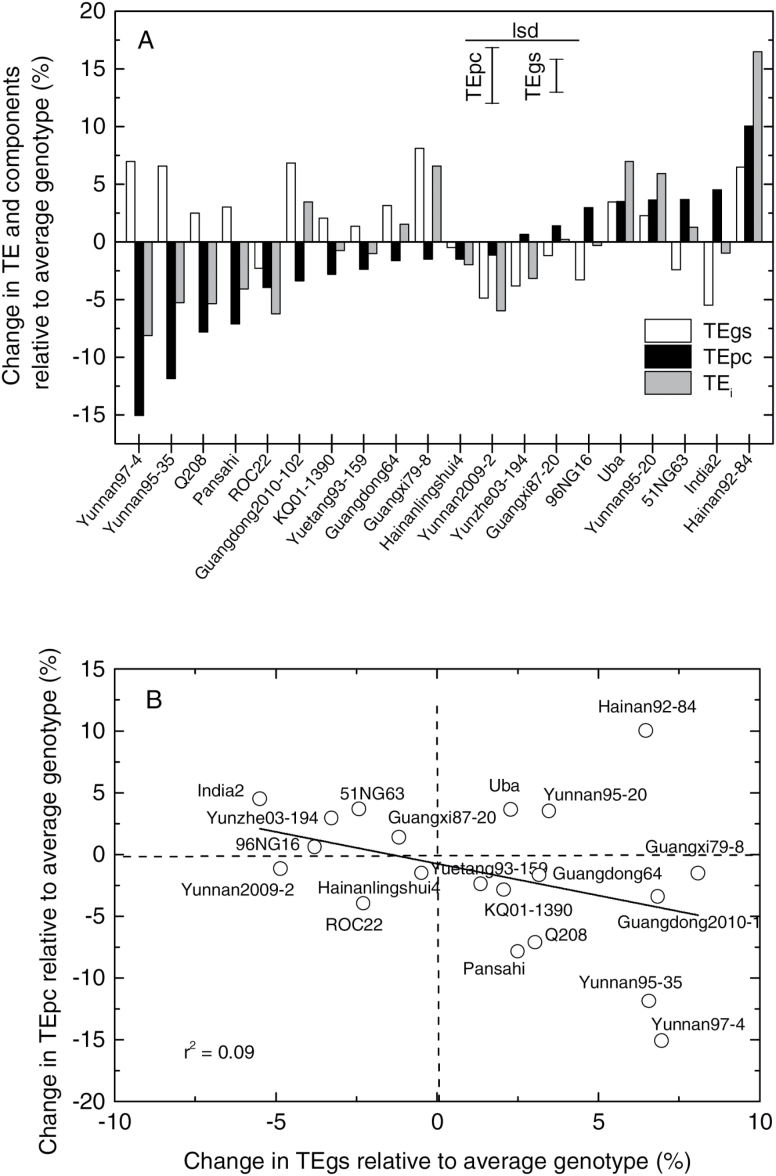
Variation in both TEgs and TEpc contributed to overall variation in TEi among the genotypes (Table 2, Fig. 4). Genotypic variation in TEgs was slightly larger than in TEpc (Table 2), but overall TEi was more strongly correlated with TEpc than TEgs (Table 4). TEpc and TEgs were only weakly related (Table 4, Fig. 4B). The TEi and TEpc levels of the two commercial cultivars (Q208 and ROC22) included in this study were clearly exceeded by some of the other wild clones (Fig. 4). The genotype with the highest observed TEi (Hainan92-84, E. arundinaceus) had a higher than average TEgs and very high TEpc (Fig. 4). Two other clones, Yunnan95-20 (E. rockii) and Uba (S. sinense), also showed high TEpc associated with high TEi (Fig. 4).
Fig. 4.
Relationship between components of TEi. (A) Changes in TEpc and TEgs for each genotype expressed relative to the average of all genotypes. (B) Relationship between genotype performance for TEpc and TEgs.
Discussion
The results from this study indicate that differences in photosynthetic capacity contribute to a significant portion of the variation in TEi, rather than only differences attributed to stomatal conductance. This is important because it means that, with concerted breeding and selection, high levels of TEi should be attainable without necessarily low levels of conductance, and therefore without necessarily sacrificing high rates of photosynthesis and productivity (Blum, 2009).
The set of clones included in this experiment was a small sample of those available in the main Chinese germplasm collections, but the results obtained in this study are encouraging in supporting further surveys of germplasm for high TE. Several wild species, most notably the E. arundinaceus clone Hainan92-84, exhibited high TEpc compared with popular Australian (Q208) and Chinese (ROC22) commercial cultivars included in this experiment. E. arundinaceus has been of interest among sugarcane breeders for its reputed ability to produce high biomass in dry environments (Jackson and Henry, 2011), although there appears to be little or no substantive data supporting this reputation. It would seem likely that other, possibly more extreme, levels of TEpc may be observed in larger-scale screening of germplasm. Genotypic variances found for A, gs, and Ci in this study were similar in magnitude to those found in a study of sugarcane-related germplasm by Jackson et al. (2016), but partitioning of TEi into components due to variation in conductance or photosynthetic capacity was not reported in that study.
The data obtained here may be used to design optimal methods for further larger-scale screening of germplasm collections and progeny derived from any breeding efforts. Results obtained from this study suggested about 16 measurements per clone would represent a near-optimal approach in further screening efforts, balancing the competing benefits of screening a large number of clones versus a large number of measurements to decrease experimental error variance. The small magnitude of clone × date interaction for TEi and TEpc is a positive result in that it suggests that relative ranking of clones for these traits was reasonably consistent under different weather conditions (at least within the range of conditions experienced in this study).
The experiment was conducted under conditions of high water availability with minimal or no water stress. Responses measured under these conditions are relevant to water-limited production environments because in many field crops a high proportion of total water use across a whole cropping growth cycle usually occurs on days when water uptake is largely meeting crop demand (Ritchie, 1973), and this is also expected to be the case for most commercial sugarcane production environments. In related work, using a modified version of the APSIM sugarcane model (Inman-Bamber et al., 2016), we estimated that >70% of water is used in typical rain-fed sugarcane production environments in Australia on days when the crop is non-stressed (data not shown). In other words, in commercial production environments a small proportion of transpiration is expected to take place on days when there is moderate-to-severe water stress, because of stomatal closure. Therefore, in relation to impacting on final yield, increasing TEi on days when there is no or mild stress will be proportionally more important than the same increase in TEi during times of moderate or severe stress. For any given growth rate, genotypes which have a higher TEi under non-stress conditions will use water at a slower rate and therefore have a delayed onset of water stress and greater total biomass production at harvest in environments in which periods of water stress occur.
Reduced gs (either generally or in response to any environmental factors) will act to reduce Ci levels and hence increase intrinsic TE (via equation 5). This may contribute to the well-known curvilinear relationship between A and gs (Fig. 3A), leading to a negative relationship between TEi and gs, which was also clearly observed in this study (Table 4). The possibility of negative genotypic correlations between conductance and TEi has been highlighted as a major complication and limitation in using TE as a selection criterion (e.g. Blum, 2005, 2009; Condon et al., 2004). Genotypes with high TEi arising through reduced conductance may have reduced total transpiration during the entire crop growth cycle, leading to reduced biomass and yield (through equation 1). However, the partitioning of variation of TEi into that expected due to the general curvilinear relationship observed between A and gs and deviations from this relationship attributed to differences in photosynthetic capacity should allow for different weightings to be placed on these two components in breeding and selection. Variation in TEi predominately arising from high photosynthetic capacity would be expected to be a more prized target than that due to low conductance, which is likely to be associated with low productivity except perhaps under the most extreme water-limited environments. This includes clones Hainan92-84 (E. arundinaceus) and Uba (S. sinense), which both exhibited high TEi associated with high TEpc in this study (Fig. 4).
There were two minor differences in the methodology used in this study to partition genotypic TEi effects than in that previously used by Gilbert et al. (2011). First, in our analysis, the mean response of all genotypes was used as the reference response rather than that of an individual reference genotype. Second, TEpc for each genotype was estimated based on its typical operating gs value rather than the mean gs of the reference genotype. Our methodology gave estimates for both TEpc and TEgs, which correlated strongly with those of Gilbert et al. (2011) (r = 0.81 for TEpc and r = 0.8 for TEgs) across genotypes. However, one possible advantage of our approach for future screening of germplasm is that it does not require estimation of the curvilinear A versus gs function, which avoids the requirement of a large number of data points for each genotype to accurately establish this relationship. The approach used in this report requires only that estimates of the typical operating levels of A and gs be made for each genotype, which, assuming similar conditions to those experienced in this study are encountered and based on suggestions above, may require about 16 measurements to maximize selection gains. This should facilitate faster screening of larger genotypic populations than if the A versus gs relationship needs to be accurately characterized for each genotype.
Overall, the results suggest that heritable genotypic variation in TEi measured on individual leaves exists within sugarcane-related germplasm and could be targeted in breeding programmes. However, one important reservation in pursuing this strategy at this stage is the uncertainty about the extent to which genotypic variation in TEi measured in plants in pots will translate to whole-plant or crop-level TE (TEP) in the field. According to (Farquhar et al., 1989), TEP can be expressed as:
| (9) |
where ϕc is the proportion of carbon lost from the shoot at night or from non-photosynthetic tissues such as roots, during the day and night; and ϕw is the proportion of unproductive water loss such as cuticular water losses or nocturnal stomatal water losses. This expression highlights the importance of whole-plant biomass partitioning, a trait that can vary genetically and exert significant mediator effect between leaf-level and crop-level TE. At the leaf level, equations 4 and 9 also highlight environmental interactions with TEi. In particular, two mechanisms may act to reduce the correlation between measurements made in experiments, such as those reported here, and genotypic effects in field production environments. First, genotypic variation in conductance sensitivity to increasing vapour pressure deficits, which has been examined in a range of crops (e.g. Fletcher et al., 2007; Sinclair et al., 2008; Sadok and Sinclair, 2009; Devi et al., 2010; Gholipoor et al., 2010; Gilbert et al., 2011; Belko et al., 2012; Belko et al., 2013; Yang et al., 2012; Gholipoor et al., 2013), may provide an additional source of genotypic variation in TE in addition to TEi. Second, any de-coupling between variation in stomatal conductance and transpiration at a crop canopy scale (Jarvis and McNaughton, 1986) will act to moderate differences occurring at the leaf level. Jackson et al. (2016) found that leaf-level TE was correlated with whole-plant TE but the plants were not grown to produce a full canopy. In a dense canopy with unstirred and humid air, stomata can exert little influence over transpiration above very low levels of conductance (e.g. Bange, 1953; Jarvis and McNaughton, 1986). By contrast, in leaves in sparse canopies and subjected to high air movement, stomata exert a high level of control over water loss. In young sugarcane crops in spring prior to wet seasons and when water availability often greatly reduces growth, it may be expected that there would be strong coupling between stomata and whole-canopy transpiration. However, further studies need to be done to examine the extent to which genotypic variation in TE measured at the leaf level in pots translates to whole-crop differences in the field.
Conclusions
This study was undertaken as part of a larger project aiming at exploring the usefulness of screening for physiological traits in large-scale breeding programmes. We found significant genotypic variation in leaf TEi that was due to the joint contribution of variation in gs and photosynthetic capacity at normal conductance operating levels. We identified a number of genotypes possessing high TEi as a result of high photosynthetic capacity, which could have significant breeding value because this measure should not be negatively associated with the reduced growth rates caused by lower gs. We also established that a small number of simple leaf gas exchange measurements is sufficient to efficiently screen TEi and apportion the variation related to the photosynthetic component. For practical applications in breeding, investigations should now turn to establishing narrow sense heritability of these traits as well as the leaf-to-field translational potential of the TEi trait reported in this study.
Acknowledgements
This research was supported by funding from the Yunnan Provincial Science and Technology Department for High-End Project: A Study on Sugarcane Drought-resistant Breeding Technology and Genetic Improvement (2012HA001).
OG was partially supported by the Hawkesbury Institute for the Environment, Western Sydney University.
Glossary
Abbreviations:
- A
CO2 assimilation rate
- Ca
ambient CO2 concentration
- Ci
intercellular CO2 concentration
- E
H2O vapour transpiration
- ea
ambient H2O vapour pressures
- ei
H2O vapour pressures inside the leaf
- GCV
genotypic coefficient of variation
- gs
stomatal conductance
- gs_CO2
stomatal conductance for CO2
- gs_H2O
stomatal conductance for H2O vapour
- Hb
broad sense heritability
- TE
transpiration efficiency
- TEgs
transpiration efficiency estimated from variation in stomatal conductance
- TEi
intrinsic transpiration efficiency
- TEL
leaf-level transpiration efficiency
- TEP
transpiration efficiency of whole plant
- TEpc
transpiration efficiency estimated from variation in photosynthetic capacity
- ϕc
proportion of consumptive carbon lost
- ϕw
proportion of unproductive water loss.
References
- Bange GGJ. 1953. On the quantitative explanation of stomatal transpiration. Acta Botanica Neerlandica 2, 255–297. [Google Scholar]
- Belko N, Zaman-Allah M, Cisse N, Diop NN, Zombre G, Ehlers JD, Vadez V. 2012. Lower soil moisture threshold for transpiration decline under water deficit correlates with lower canopy conductance and higher transpiration efficiency in drought-tolerant cowpea. Functional Plant Biology 39, 306–322. [DOI] [PubMed] [Google Scholar]
- Belko N, Zaman-Allah M, Diop NN, Cisse N, Zombre G, Ehlers JD, Vadez V. 2013. Restriction of transpiration rate under high vapour pressure deficit and non-limiting water conditions is important for terminal drought tolerance in cowpea. Plant Biology 15, 304–316. [DOI] [PubMed] [Google Scholar]
- Berding N, Roach BT. 1987. Germplasm collection, maintenance, and use. In: Heinz D, ed, Sugarcane improvement through breeding, Vol. 143 Amsterdam: Elsevier, p 210. [Google Scholar]
- Blum A. 2005. Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Australian Journal of Agricultural Research 56, 1159. [Google Scholar]
- Blum A. 2009. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Research 112, 119–123. [Google Scholar]
- Cai Q, Aitken KS, Fan YH, Piperidis G, Jackson P, McIntyre CL. 2005. A preliminary assessment of the genetic relationship between Erianthus rockii and the “Saccharum complex” using microsatellite (SSR) and AFLP markers. Plant Science 169, 976–984. [Google Scholar]
- Cardona CA, Quintero JA, Paz IC. 2010. Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresource Technology 101, 4754–4766. [DOI] [PubMed] [Google Scholar]
- Condon A, Richards R. 1993. Exploiting genetic variation in transpiration efficiency in wheat: an agronomic view. In: Ehleringer JR AE , Hall GD, Farquhar, eds, Stable Isotopes and Plant Carbon–Water Relations. San Diego, CA: Academic Press, 435–450. [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. 2002. Improving intrinsic water-use efficiency and crop yield. Crop Science 42, 122–131. [DOI] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. 2004. Breeding for high water-use efficiency. Journal of Experimental Botany 55, 2447–2460. [DOI] [PubMed] [Google Scholar]
- Devi MJ, Sinclair TR, Vadez V. 2010. Genotypic variation in peanut for transpiration response to vapor pressure deficit. Crop Science 50, 191–196. [Google Scholar]
- Falconer DS, Mackay TFC. 1996. Introduction to Quantitative Genetics. Harlow, UK: Longmans Green. [Google Scholar]
- FAO 2015. FAOSTAT http://faostat3.fao.org/download/Q/QC/E (accessed December 2015).
- Farquhar GD, Ehleringer JR, Hubick KT. 1989. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40, 503–537. [Google Scholar]
- Fletcher AL, Sinclair TR, Allen LH. 2007. Transpiration responses to vapor pressure deficit in well watered ‘slow-wilting’ and commercial soybean. Environmental and Experimental Botany 61, 145–151. [Google Scholar]
- Ghannoum O. 2016. How can we breed for more water use-efficient sugarcane? Journal of Experimental Botany 67, 557–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipoor M, Choudhary S, Sinclair T, Messina C, Cooper M. 2013. Transpiration response of maize hybrids to atmospheric vapour pressure deficit. Journal of Agronomy and Crop Science 199, 155–160. [Google Scholar]
- Gholipoor M, Prasad PV, Mutava RN, Sinclair TR. 2010. Genetic variability of transpiration response to vapor pressure deficit among sorghum genotypes. Field Crops Research 119, 85–90. [Google Scholar]
- Gilbert ME, Zwieniecki MA, Holbrook NM. 2011. Independent variation in photosynthetic capacity and stomatal conductance leads to differences in intrinsic water use efficiency in 11 soybean genotypes before and during mild drought. Journal of Experimental Botany 62, 2875–2887. [DOI] [PubMed] [Google Scholar]
- Inman-Bamber NG, Jackson PA, Stokes CJ, Verrall S, Lakshmanan P, Basnayake J. 2016. Sugarcane for water-limited environments: enhanced capability of the APSIM sugarcane model for assessing traits for transpiration efficiency and root water supply. Field Crops Research 196, 112–123. [Google Scholar]
- Jackson P, Basnayake J, Inman-Bamber G, Lakshmanan P, Natarajan S, Stokes C. 2016. Genetic variation in transpiration efficiency and relationships between whole plant and leaf gas exchange measurements in Saccharum spp. and related germplasm. Journal of Experimental Botany 67, 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P, Henry R. 2011. Erianthus. In: Kole C, ed. Wild Crop Relatives: Genomic and Breeding Resources. Berlin: Springer-Verlag, pp 97–107. [Google Scholar]
- Jarvis PG, McNaughton K. 1986. Stomatal control of transpiration: scaling up from leaf to region. Advances in Ecological Research 15, 49. [Google Scholar]
- Mukherjee SK. 1957. Origin and distribution of Saccharum. Botanical Gazette 119, 55–61. [Google Scholar]
- Passioura J. 1977. Grain yield, harvest index, and water use of wheat. Journal of the Australian Institute of Agricultural Science 43, 117–120. [Google Scholar]
- Passioura J, Angus J. 2010. Improving productivity of crops in water-limited environments. Advances in Agronomy 106, 37–75. [Google Scholar]
- Richards RA, Rebetzke GJ, Condon AG, van Herwaarden AF. 2002. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Science 42, 111–121. [DOI] [PubMed] [Google Scholar]
- Ritchie J. 1973. Influence of soil water status and meteorological conditions on evaporation from a corn canopy. Agronomy Journal 65, 893–897. [Google Scholar]
- Roach B. 1989. Origin and improvement of the genetic base of sugarcane. Proc Aust Soc Sugar Cane Technology 11, 34–47. [Google Scholar]
- Robertson MJ, Wood AW, Muchow RC. 1996. Growth of sugarcane under high input conditions in tropical Australia. I. Radiation use, biomass accumulation and partitioning. Field Crops Research 48, 11–25. [Google Scholar]
- Sadok W, Sinclair TR. 2009. Genetic variability of transpiration response to vapor pressure deficit among soybean cultivars. Crop Science 49, 955–960. [Google Scholar]
- Sinclair TR. 2012. Is transpiration efficiency a viable plant trait in breeding for crop improvement? Functional Plant Biology 39, 359. [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Zwieniecki MA, Holbrook NM. 2008. Low leaf hydraulic conductance associated with drought tolerance in soybean. Physiologia Plantarum 132, 446–451. [DOI] [PubMed] [Google Scholar]
- Vadez V, Kholova J, Medina S, Kakkera A, Anderberg H. 2014. Transpiration efficiency: new insights into an old story. Journal of Experimental Botany 65, 6141–6153. [DOI] [PubMed] [Google Scholar]
- Yang Z, Sinclair TR, Zhu M, Messina CD, Cooper M, Hammer GL. 2012. Temperature effect on transpiration response of maize plants to vapour pressure deficit. Environmental and Experimental Botany 78, 157–162. [Google Scholar]