Abstract
We have determined the primary structure of the [2Fe-2S]ferredoxin of the anaerobic protist Trichomonas vaginalis. This protein, situated in the hydrogenosome, is composed of 93 amino acids. A comparison of T. vaginalis ferredoxin with greater than 80 other ferredoxins shows the closest similarity to [2Fe-2S]putidaredoxin of the aerobic bacterium Pseudomonas putida and a lesser one to mitochondrial [2Fe-2S]ferredoxins of vertebrates. This similarity is reflected in the overall primary structure and in the spacing of cysteine residues coordinating the iron-sulfur center. The primary structure, but not the environment of the iron-sulfur center, also shows similarity with [2Fe-2S]ferredoxins of photosynthetic organisms and halobacteria. We have cloned and analyzed the T. vaginalis ferredoxin gene. The gene is present in a single copy and devoid of introns. It gives rise to a transcript with unusually short 5' and 3' untranslated regions of 16 and 18 nucleotides, respectively. DNA sequence analysis of the gene predicts an additional 8 amino acids at the amino terminus which are absent from the purified protein. This amino-terminal region of the protein is characterized by properties typical of mitochondrial presequences.
Full text
PDF
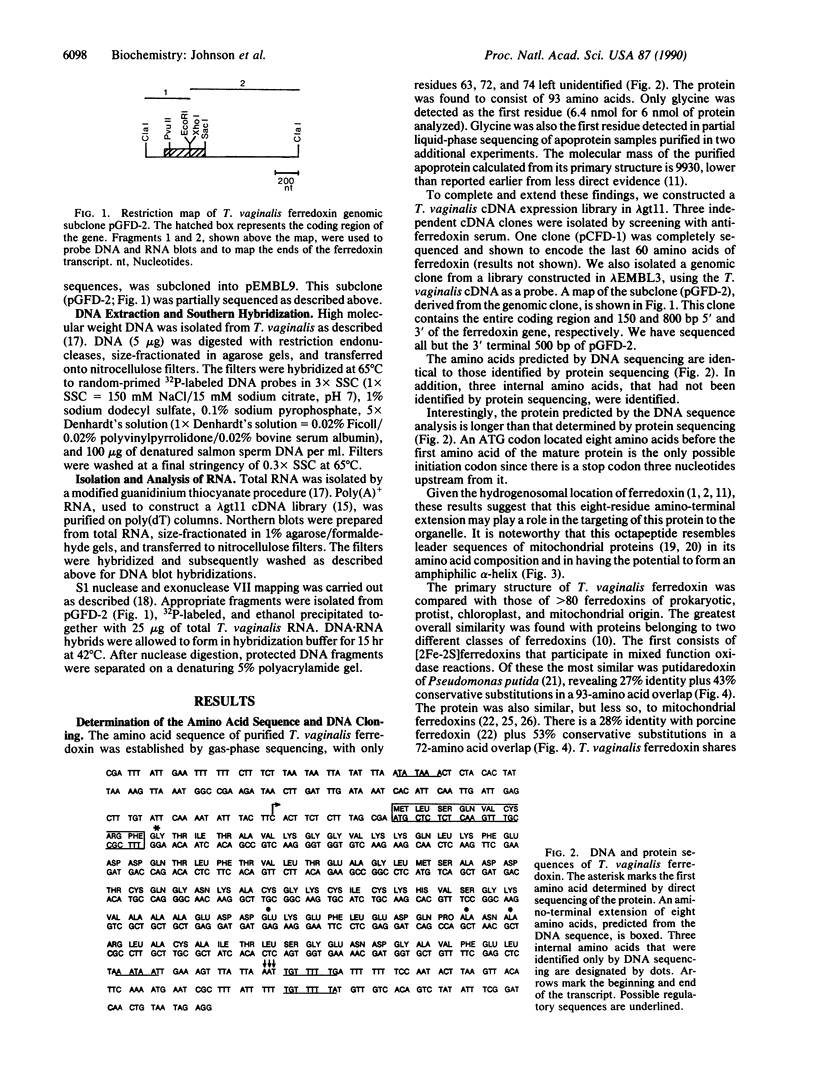
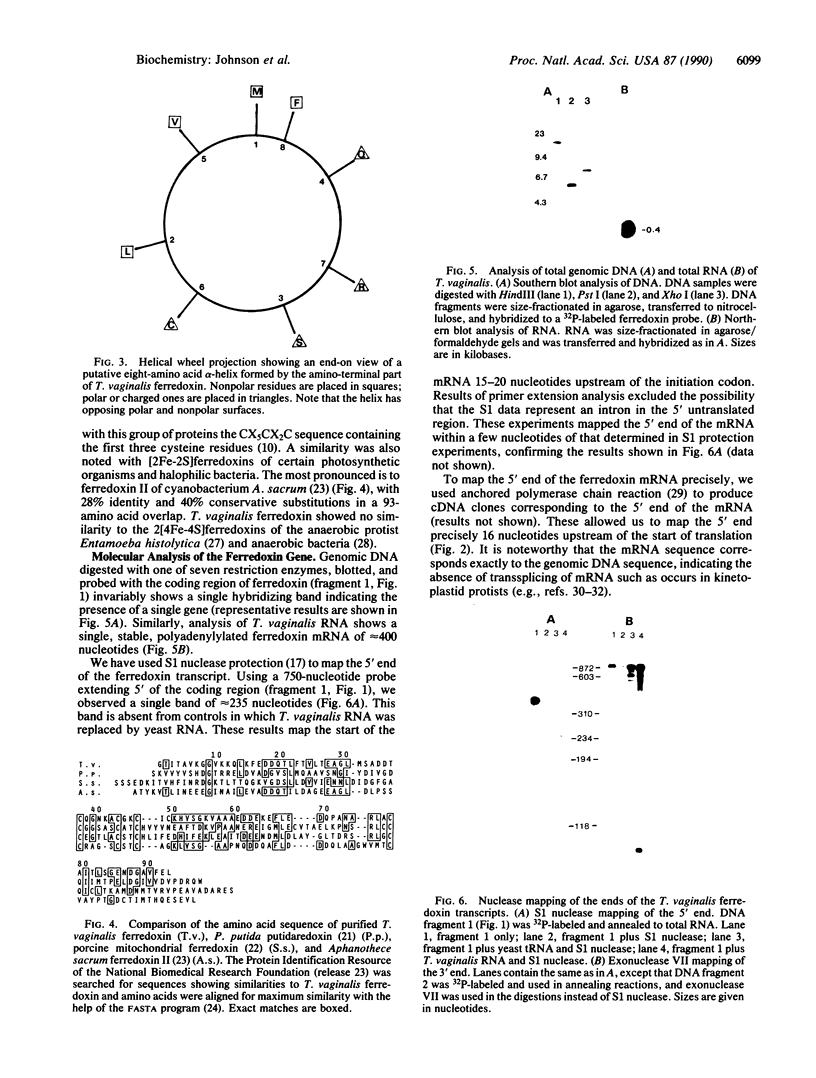


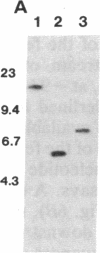
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya A. S., Manjula B. N. Dihydroxypropylation of amino groups of proteins: use of glyceraldehyde as a reversible agent for reductive alkylation. Biochemistry. 1987 Jun 16;26(12):3524–3530. doi: 10.1021/bi00386a041. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The simultaneous symbiotic origin of mitochondria, chloroplasts, and microbodies. Ann N Y Acad Sci. 1987;503:55–71. doi: 10.1111/j.1749-6632.1987.tb40597.x. [DOI] [PubMed] [Google Scholar]
- Chapman A., Cammack R., Linstead D. J., Lloyd D. Respiration of Trichomonas vaginalis. Components detected by electron paramagnetic resonance spectroscopy. Eur J Biochem. 1986 Apr 1;156(1):193–198. doi: 10.1111/j.1432-1033.1986.tb09567.x. [DOI] [PubMed] [Google Scholar]
- Cupp J. R., Vickery L. E. Identification of free and [Fe2S2]-bound cysteine residues of adrenodoxin. J Biol Chem. 1988 Nov 25;263(33):17418–17421. [PubMed] [Google Scholar]
- Edman U., Meza I., Agabian N. Genomic and cDNA actin sequences from a virulent strain of Entamoeba histolytica. Proc Natl Acad Sci U S A. 1987 May;84(9):3024–3028. doi: 10.1073/pnas.84.9.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D. G., Hunt L. T., Yeh L. S., Barker W. C. New perspectives on bacterial ferredoxin evolution. J Mol Evol. 1985;22(1):20–31. doi: 10.1007/BF02105801. [DOI] [PubMed] [Google Scholar]
- Graves M. C., Mullenbach G. T., Rabinowitz J. C. Cloning and nucleotide sequence determination of the Clostridium pasteurianum ferredoxin gene. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1653–1657. doi: 10.1073/pnas.82.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase T., Wakabayashi S., Wada K., Matsubara H. Amino acid sequence of Aphanothece sacrum Ferredoxin II (minor component). Structural characteristics and evolutionary implications. J Biochem. 1978 Mar;83(3):761–770. doi: 10.1093/oxfordjournals.jbchem.a131970. [DOI] [PubMed] [Google Scholar]
- Huber M., Garfinkel L., Gitler C., Mirelman D., Revel M., Rozenblatt S. Nucleotide sequence analysis of an Entamoeba histolytica ferredoxin gene. Mol Biochem Parasitol. 1988 Oct;31(1):27–33. doi: 10.1016/0166-6851(88)90142-9. [DOI] [PubMed] [Google Scholar]
- Kagimoto K., McCarthy J. L., Waterman M. R., Kagimoto M. Deduced amino acid sequence of mature chicken testis ferredoxin. Biochem Biophys Res Commun. 1988 Aug 30;155(1):379–383. doi: 10.1016/s0006-291x(88)81096-9. [DOI] [PubMed] [Google Scholar]
- Kirk-Mason K. E., Turner M. J., Chakraborty P. R. Evidence for unusually short tubulin mRNA leaders and characterization of tubulin genes in Giardia lamblia. Mol Biochem Parasitol. 1989 Aug;36(1):87–99. doi: 10.1016/0166-6851(89)90204-1. [DOI] [PubMed] [Google Scholar]
- Koga H., Yamaguchi E., Matsunaga K., Aramaki H., Horiuchi T. Cloning and nucleotide sequences of NADH-putidaredoxin reductase gene (camA) and putidaredoxin gene (camB) involved in cytochrome P-450cam hydroxylase of Pseudomonas putida. J Biochem. 1989 Nov;106(5):831–836. doi: 10.1093/oxfordjournals.jbchem.a122939. [DOI] [PubMed] [Google Scholar]
- Laird P. W., Zomerdijk J. C., de Korte D., Borst P. In vivo labelling of intermediates in the discontinuous synthesis of mRNAs in Trypanosoma brucei. EMBO J. 1987 Apr;6(4):1055–1062. doi: 10.1002/j.1460-2075.1987.tb04858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczak R., Gorrell T. E., Müller M. Hydrogenosomal ferredoxin of the anaerobic protozoon, Tritrichomonas foetus. J Biol Chem. 1983 Oct 25;258(20):12427–12433. [PubMed] [Google Scholar]
- Meyer J., Bruschi M. H., Bonicel J. J., Bovier-Lapierre G. E. Amino acid sequence of [2Fe-2S] ferredoxin from Clostridium pasteurianum. Biochemistry. 1986 Oct 7;25(20):6054–6061. doi: 10.1021/bi00368a033. [DOI] [PubMed] [Google Scholar]
- Mittal S., Zhu Y. Z., Vickery L. E. Molecular cloning and sequence analysis of human placental ferredoxin. Arch Biochem Biophys. 1988 Aug 1;264(2):383–391. doi: 10.1016/0003-9861(88)90303-7. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Müller M. Energy metabolism of protozoa without mitochondria. Annu Rev Microbiol. 1988;42:465–488. doi: 10.1146/annurev.mi.42.100188.002341. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picado-Leonard J., Voutilainen R., Kao L. C., Chung B. C., Strauss J. F., 3rd, Miller W. L. Human adrenodoxin: cloning of three cDNAs and cycloheximide enhancement in JEG-3 cells. J Biol Chem. 1988 Mar 5;263(7):3240–3244. [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Reeves R. E., Guthrie J. D., Lobelle-Rich P. Entamoeba histolytica: isolation of ferredoxin. Exp Parasitol. 1980 Feb;49(1):83–88. doi: 10.1016/0014-4894(80)90059-4. [DOI] [PubMed] [Google Scholar]
- Roise D., Schatz G. Mitochondrial presequences. J Biol Chem. 1988 Apr 5;263(10):4509–4511. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., van Binsbergen J., Weisbeek P. The plant ferredoxin precursor: nucleotide sequence of a full length cDNA clone. Nucleic Acids Res. 1985 May 10;13(9):3179–3194. doi: 10.1093/nar/13.9.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbüchel A., Müller M. Anaerobic pyruvate metabolism of Tritrichomonas foetus and Trichomonas vaginalis hydrogenosomes. Mol Biochem Parasitol. 1986 Jul;20(1):57–65. doi: 10.1016/0166-6851(86)90142-8. [DOI] [PubMed] [Google Scholar]
- Struhl K. Molecular mechanisms of transcriptional regulation in yeast. Annu Rev Biochem. 1989;58:1051–1077. doi: 10.1146/annurev.bi.58.070189.005155. [DOI] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. A linear double-stranded RNA in Trichomonas vaginalis. J Biol Chem. 1985 Mar 25;260(6):3697–3702. [PubMed] [Google Scholar]
- Whatley J. M., John P., Whatley F. R. From extracellular to intracellular: the establishment of mitochondria and chloroplasts. Proc R Soc Lond B Biol Sci. 1979 Apr 11;204(1155):165–187. doi: 10.1098/rspb.1979.0020. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]