Abstract
Hyperoxaluria and oxidative stress are risk factors in calcium oxalate (CaOx) stone formation. Supplement with antioxidant could be effective in prevention of recurrent stone formation. The present study aims to evaluate the protective effects of vitamin E and vitamin C in hyperoxaluric rat. The experiment was performed in rats for 21 days. Rats were divided into 5 groups as follows: control (group 1, n=8), hyperoxaluric rats (group 2, n=8), hyperoxaluric rats with vitamin E supplement (group 3, n=7), hyperoxaluric rats with vitamin C supplement (group 4, n=7) and hyperoxaluric rats with vitamin E and C supplement (group 5, n=7). Hyperoxaluria was induced by feeding hydroxyl L-proline (HLP) 2% w/v dissolved in drinking water. Intraperitoneal 200 mg/kg of vitamin E was given in groups 3 and 5 on days 1, 6, 11 and 16, while 500 mg of vitamin C was injected intravenously in groups 4 and 5 on days 1 and 11. Renal functions and oxidative status were measured. The urinary oxalate excretion was increased in HLP supplement rats, while glomerular filtration rate, proximal water and sodium reabsorption were significantly lower in group 2 compared with a control (P<0.05). Giving antioxidants significantly lower urinary calcium oxalate crystals (P<0.05). Hyperoxaluric rats had higher plasma malondialdehyde (PMDA) and lower urinary total antioxidant status (UTAS), which were alleviated by vitamin E and/or vitamin C supplement. In conclusion, giving combination of vitamin E and vitamin C exerts a protective role against HLP-induced oxalate nephropathy.
Keywords: glomerular filtration rate, hyperoxaluria, oxidative stress, vitamin C, vitamin E
Kidney stone disease has been recognized as one of important diseases in both human and animals. Kidney stone could damage renal tubular epithelium, leading to impaired renal function. The most of the kidney stones in human are composed of calcium oxalate, which relates to calcium and oxalate metabolism in tissue and urinary tract system [11, 28]. The CaOx stone also had high incidence and recurrence rate in animals and induced oxidative stress [16, 21]. Many previous studies showed that enhanced oxidative stress was related to CaOx crystal and stone formation [7, 29]. Thus, prevention of oxidative damage may be a key role to prevent recurrent stone formation and subsequent renal dysfunction. Supplement of vitamin E (Vit E) reduces oxidative stress, oxalate excretion and CaOx crystal formation in hyperoxaluric rats receiving ethylene glycol (EG) [9, 24]. In hypertensive and hyperoxaluric patients, oral supplementation of Vit E at 400 mg/day for 9 months could normalize biochemical and kinetic properties of Tamm-Horsfall protein which inhibit CaOx crystal aggregation [23].
Vitamin C (Vit C) is another potent antioxidant which has been used to treat the oxidative stress-related diseases, including renal ischemic reperfusion injury [15] or gentamicin induced nephrotoxicity [17]. However, its metabolite is a source of endogenous oxalate production, and increased oxalate excretion and oxalate nephropathy were reported in chronic Vit C usage [6, 20]. A recent study suggested that total and supplemental Vit C ingestion was correlated with higher incident of kidney stone formation in male, but not female [5]. Inversely, there was an evidence supported that a combination of Vit E and Vit C could reduce oxidative injury in LLC-PK1 proximal tubular cells induced by oxalate [26]. Therefore, the objective of this study was to investigate the beneficial effects of using Vit E, Vit C or its combination against kidney injury and oxidative stress in hyperoxaluric rats induced by hydroxy-L-proline (HLP).
MATERIALS AND METHODS
Approvals
This study was approved and conformed to the Chulalongkorn University Animal Care and Use Committee, Faculty of Veterinary Science, Chulalongkorn University in protocol review number 1431056.
Animals and experimental protocol
All male Sprague Dawley rats weighing between 250–350 g, were obtained from National Laboratory Animal Center, Mahidol University, Thailand. Rats were housed in the 12:12 hr dark and light cycle and allowed for free access of water and food (Perfect Companion Co., Ltd., Bangkok, Thailand). All rats were allowed to acclimatize to the environment for at least 7 days before an experimental study. Rats were divided into 5 groups as follows: group 1; Control rats (n=8), group 2; Hyperoxaluric rats induced by HLP (n=8), group 3; Hyperoxaluric rats treated by Vit E (n=7), group 4; Hyperoxaluric rats treated by Vit C (n=7) and group 5; Hyperoxaluric rats treated by combination of Vit E and Vit C (n=7). Rats in group 1 received the regular drinking water, while the rest groups received 2% HLP (w/v) (ACROS ORGANICS, Morris Plains, NJ, U.S.A.) in drinking water throughout the experimental periods (21 days). Vit E (DURVET, Inc., Blue Springs, MO, U.S.A.) (200 mg/kg body weight) was given intraperitoneally on days 1, 6, 11 and 16 in groups 3 and 5 as previously described [9], while Vit C (Atlantic Laboratories Corporation Ltd., Bangkok, Thailand) (500 mg/kg body weight) was given intravenously on days 1 and 11 in groups 4 and 5. An olive oil (VIDHYASOM Co., Ltd., Bangkok, Thailand) (0.2 ml/100 g body weight, i.p.) was given to groups 1, 2 and 4 as a placebo of Vit E vehicle, and isotonic saline (0.2 ml/100 g body weight, i.v.) was given in groups 1, 2 and 3 as a placebo of Vit C vehicle. Body weight, food intake and water intake were recorded daily in all rats throughout the experiment. Each rat was housed in a metabolic cage for 24 hr and urine was collected on day 0 (U0), day 10 (U10) and day 20 (U20) for measurements of urine volume and urinalysis. The rest portion of urine was stored at −20°C for further analysis of the excretion of protein, creatinine, oxalate, citrate, electrolytes (Na, K, Cl, Ca and Mg), malondialdehyde (MDA) and total antioxidant status (TAS). Indirect blood pressure measurement was performed from tail (PowerLAb/16 SP ADInstrument and ADINSTRUMENTS NIBP Controller, ADInstruments, Bella Vista, Australia) on the next day after animals leaving metabolic cage (day 1 and day 11). After indirect measurement of blood pressure, all rats were anesthetized with intraperitoneal injection of pentobarbital sodium (60 mg/kg) for blood collection from trimly tail vein for measurements of concentrations of plasma creatinine (PCr) and blood urea nitrogen (BUN). After that, the Vit C or NSS was given on day 1 and day 11. Vit E or olive oil was administered thereafter on day 1 and day 11 without anesthesia. The additional doses of Vit E or olive oil were also given on day 6 and day 16. On day 21, all rats were subjected to a renal clearance study. Then, 5 ml of blood was collected by cardiac puncture for measurements of BUN and PCr. The rest portion of plasma was used for measurements of electrolytes (Na, K, Cl, Ca and Mg), osmolarity and plasma MDA (PMDA). Rats were euthanized using high dose of pentobarbital sodium given intravenously. Both kidneys were removed and preserved in 10% neutral buffered formalin for further histopathological investigation.
On day 21, glomerular filtration rate (GFR), effective renal plasma flow (ERPF) and tubular handling of Na and water were assessed by the clearances of inulin (CIn), para-aminohippurate (PAH; CPAH) and lithium (CLi). The procedures for assessment and calculations of renal function were described previously [1, 22].
Biochemical analyses
The hematocrit was measured using microhematocrit centrifugation. The concentrations of creatinine and BUN were determined by colorimetric method using automate analyzer (The IL ILab 650 Chemistry Analyzer, Diamond diagnostic, Holliston, MA, U.S.A.). The concentrations of inulin and PAH were measured using anthrone method [31] and Brun method [2], respectively. The osmolarity was determined using an osmometer (Advanced Instrument Inc., Norwood, MA, U.S.A.). The concentrations of sodium and potassium were determined using a flame photometer (Frame photometer 410C, Ciba Corning Inc., Corning, NY, U.S.A.) while chloride was measured using a chloridometer (Chloride analyzer 925, Ciba Corning Inc.). The Li, Ca and Mg were determined using inductively coupled plasma optical emission spectrometry (Perkin Elmer™ Optima 5400, Waltham, MA, U.S.A.). The oxalate and citrate were determined using capillary electrophoresis (P/ACE™ MDQ CE Beckman Coulter, Fullerton, CA, U.S.A.). The MDA and TAS were determined using method of Ohkawa [19] and Chrzczanowicz [4], respectively.
Urinalysis and sediment
Urine specific gravity was measured by a refractometer (Master Refractometer, ATAGO®, Tokyo, Japan), while urine pH was performed by the Urine strip test (Combur9 Test®, Roche Diagnostics GmbH, Mannheim, Germany). The sediment was examined by using 0.5 ml of urine subjected to 1,500 rpm centrifugations for 10 min. The sediment was stained using Sternheimer-Malbin stain and subjected to microscopic visualization for determination of crystal formation. The crystals in the urine were quantified by examining at X40, with five fields per sample and averaged. The crystal count was recorded as following: 0=no crystal deposits, 1=1–2 crystal deposits per field, 2=3–5 crystal deposits per field, 3=6–10 crystal deposits per field and 4=>10 crystal deposits per field.
Histopathological study
The kidney tissue segments were subjected for histologic procedure, after embedded in paraffin and cut at 5 µm thickness. The sections were stained with hematoxylin and eosin (H&E). Histopathological lesions both cortex and medulla were examined under a light microscope by a pathologist. Microscopic lesions of glomerular and tubular compartments including tubular dilatation, tubular cell flattening, tubular cell vacuolization and deposition of tubular cast were noted.
Statistical analyses
The data were expressed as mean ± standard error of mean (SEM). The data obtained in between groups were tested by one way ANOVA followed by Dunn’s post-hoc analysis. The data obtained from the same group at different time points were compared using one way repeated measured ANOVA followed by Dunnett’s Test.The relationships between parameters were performed using Pearson’s correlation. The P value less than 0.05 was considered as significant difference.
RESULTS
Body weight, food and water intake
The averages of body weight on day 21 were not different among all groups (groups 1 to 5; 378.2 ± 7.4, 397.9 ± 15.9, 365.1 ± 15.4, 407.9 ± 10.7 and 370.6 ± 14.6 g, respectively). There were also no significant differences in daily water intakes (groups 1 to 5; 28.80 ± 3.37, 39.54 ± 2.53, 24.53 ± 4.72, 35.17 ± 6.06 and 34.84 ± 4.72 ml, respectively). Only food intake in group 3 was significantly lower than that in group 1 (18.34 ± 2.15 vs 23.31 ± 0.80 g, P<0.05).
Hemodynamics and glomerular function
Indirect systolic blood pressure, mean arterial blood pressure and urine flow rate were not changed throughout the experimental study in all groups. PCr was not changed from baseline in any groups on day 10. However, on day 21, hyperoxaluric rats had significantly increased PCr (P<0.05) (Table 1). BUN was transiently elevated on day 10 in groups 2 and 3 compared with day 0 (P<0.05), but became normalized on day 21. Evidently, hyperoxaluric rats had the highest concentration of both PCr and BUN.
Table 1. The PCr and BUN (mg/dl) in groups 1–5 at days 0, 10 and 21.
| Period | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|---|
| PCr | Day 0 | 0.25 ± 0.02 | 0.30 ± 0.02 | 0.33 ± 0.02 | 0.30 ± 0.02 | 0.30 ± 0.02 |
| Day 10 | 0.33 ± 0.04 | 0.45 ± 0.03 | 0.43 ± 0.08 | 0.43 ± 0.10 | 0.31 ± 0.04 | |
| Day 21 | 0.53 ± 0.15 | 0.59 ± 0.11a) | 0.31 ± 0.03 | 0.40 ± 0.04 | 0.37 ± 0.04 | |
| BUN | Day 0 | 21.2 ± 1.4 | 16.9 ± 1.3 | 16.7 ± 0.6 | 16.6 ± 1.1 | 14.7 ± 0.8 |
| Day 10 | 20.4 ± 0.7 | 29.0 ± 4.2 a) | 23.7 ± 2.1a) | 22.2 ± 2.8 | 17.0 ± 1.7 | |
| Day 21 | 21.1 ± 5.5 | 25.7 ± 3.7 | 17.6 ± 0.9 | 20.8 ± 3.5 | 16.0 ± 1.9 |
The data were shown as mean ± SEM. a) P<0.05 in the same group when compared to day 0 using one way repeated measure ANOVA.
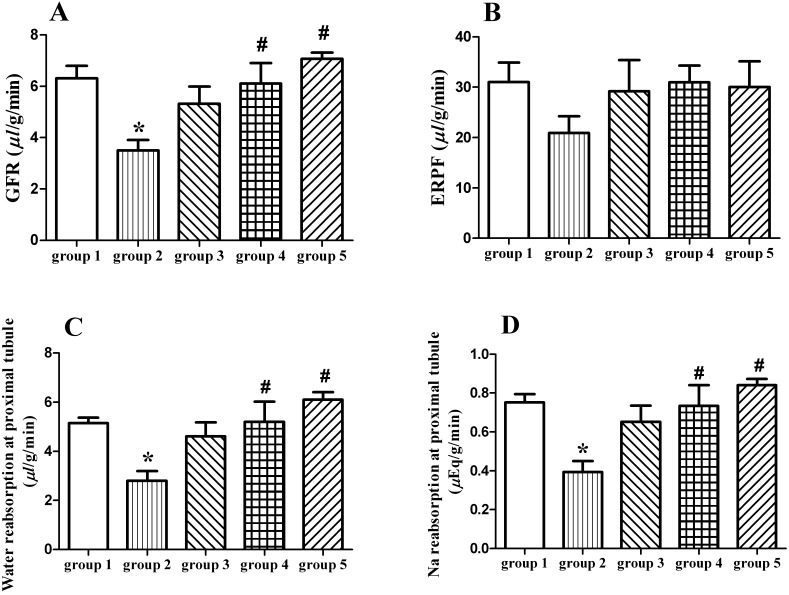
Hyperoxaluric rats had the lowest GFR and ERPF (Fig. 1A and 1B), with the highest renal vascular resistance (RVR) than any group, but filtration fraction (FF) was not different. No significant changes were found on osmolar clearance and free water clearance.
Fig. 1.
GFR (µl/g/min) (A) and ERPF (µl/g/min) (B), reabsorption of water (µl/g/min) (C) and sodium (µEq/g/min) (D) in proximal tubule of rats in groups 1−5. The data were shown as mean ± SEM. *P<0.05 compared with group 1, #P<0.05 compared with group 2.
Tubular function in water and electrolyte handling
Plasma osmolarity and levels of Na, K, Cl, Ca and Mg were not different among each group (Table 2). On day 21, hyperoxaluric rats had significant reduction in proximal water reabsorption (TC H2O) by 46% compared with the control (group 1) (Fig. 1C). There was no difference in TC H2O between group 3, 4 or 5 versus control rats. The similar results were observed in proximal tubular Na reabsorption (Fig. 1D). Comparing with hyperoxaluric rats, both proximal tubular reabsorption of water and sodium were ameliorated by Vit C treatment (groups 4 and 5). There was no difference in renal handling of both water and Na at the distal tubules, as well as the urinary excretions of Na, K, Cl, Ca and Mg among each group throughout the experiment (Table 3).
Table 2. The concentration (mmol/l) of plasma electrolytes, oxalate and citrate in groups 1–5 on day 21.
| Parameters | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|
| Na | 154 ± 6 | 146 ± 4 | 143 ± 3 | 149 ± 4 | 141 ± 0.4 |
| K | 5.5 ± 0.5 | 4.7 ± 0.2 | 5.5 ± 0.5 | 4.6 ± 0.4 | 4.7 ± 0.2 |
| Cl | 101 ± 2 | 103 ± 3 | 102 ± 1 | 102 ± 2 | 105 ± 2 |
| Ca | 3.1 ± 0.3 | 2.3 ± 0.2 | 2.6 ± 0.3 | 2.6 ± 0.3 | 2.6 ± 0.2 |
| Mg | 1.2 ± 0.1 | 0.9 ± 0.1 | 1.3 ± 0.3 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Oxalate | 0.048 ± 0.020 | 0.152 ± 0.022 a) | 0.168 ± 0.034 a) | 0.212 ± 0.034 a) | 0.121 ± 0.018 |
| Citrate | 0.356 ± 0.148 | 0.227 ± 0.137 | 0.113 ± 0.024 | 0.128 ± 0.019 | 0.121 ± 0.027 |
The data were shown as mean ± SEM. a) P<0.05 compared with group 1 using one way ANOVA.
Table 3. The urinary excretion of organic (µmol/day) and inorganic substances (µmol/day) in 5 groups of rat.
| Period | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|---|
| Na | day 0 | 2,806 ± 672 | 1,898 ± 246 | 1,590 ± 415 | 1,755 ± 289 | 1,607 ± 332 |
| day 10 | 2,242 ± 581 | 2,444 ± 198 | 1,318 ± 287 | 2,336 ± 745 | 2,233 ± 213 | |
| day 20 | 2,077 ± 287 | 2,210 ± 289 | 1,608 ± 488 | 2,468 ± 314 | 2,638 ± 454 | |
| K | day 0 | 1,315 ± 277 | 926 ± 121 | 735 ± 181 | 1,251 ± 179 | 1,098 ± 288 |
| day 10 | 1,124 ± 196 | 1,278 ± 165 | 675 ± 138 | 1,180 ± 338 | 1,154 ± 106 | |
| day 20 | 1,013 ± 148 | 1,020 ± 173 | 820 ± 247 | 1,280 ± 157 | 1,463 ± 263 | |
| Cl | day 0 | 415 ± 109 | 538 ± 131 | 199 ± 50 | 271 ± 66 | 193 ± 46 |
| day 10 | 493 ± 118 | 1,491 ± 415 | 175 ± 80 b) | 206 ± 75 | 227 ± 32 | |
| day 20 | 256 ± 61 | 844 ± 334 | 202 ± 55 | 186 ± 18 | 341 ± 75 | |
| Ca | day 0 | 7.6 ± 1.6 | 9.4 ± 2.4 | 5.1 ± 2.6 | 3.9 ± 1.1 | 4.1 ± 1.5 |
| day 10 | 15.6 ± 3.9 | 3.3 ± 0.8 | 4.0 ± 1.8 | 8.7 ± 6.6 | 5.8 ± 2.1 | |
| day 20 | 15.3 ± 5.2 | 7.4 ± 3.9 | 3.5 ± 1.7 | 4.6 ± 2.1 | 4.1 ± 2.5 | |
| Mg | day 0 | 42.3 ± 12.4 | 64.5 ± 32.9 | 27.8 ± 12.3 | 43.7 ± 21.7 | 51.9 ± 26.1 |
| day 10 | 70.0 ± 12.4 | 44.9 ± 11.8 | 24.8 ± 7.3 | 49.4 ± 22.6 | 24.6 ± 11.1 | |
| day 20 | 48.9 ± 13.3 | 43.6 ± 16.5 | 14.9 ± 6.4 | 28.1 ± 6.1 | 7.8 ± 6.5 | |
| Oxalate | day 0 | 13.6 ± 5.5 | 10.7 ± 2.5 | 15.8 ± 3.4 | 13.4 ± 1.4 | 11.8 ± 3.5 |
| day 10 | 7.8 ± 1.6 | 57.9 ± 9.1 a,c) | 23.2 ± 5.8 b) | 68.2 ± 26.9 a) | 24.6 ± 3.9 | |
| day 20 | 16.5 ± 6.7 | 56.6 ± 9.6 a,c) | 17.6 ± 7.2 b) | 77.0 ± 16.3 a,c) | 37.8 ± 8.9 c) | |
| Citrate | day 0 | 45.2 ± 12.8 | 32.5 ± 6.5 | 37.7 ± 8.9 | 32.8 ± 5.4 | 36.5 ± 12.6 |
| day 10 | 32.1 ± 10.6 | 11.9 ± 3.8 c) | 19.9 ± 9.3 | 16.9 ± 7.3 | 46.7 ± 11.8 b) | |
| day 20 | 41.7 ± 7.9 | 20.3 ± 5.0 | 15.8 ± 8.4 | 23.3 ± 4.0 | 31.8 ± 6.7 |
The data were shown as mean ± SEM. a) P<0.05 compared to group 1; b) P<0.05 compared to group 2 using one way ANOVA; c) P<0.05 in the same group when compared to day 0 using one way repeated measure ANOVA.
Plasma and urinary excretion of citrate and oxalate
Plasma level of citrate was slightly lower in Vit E or C supplemented rats (groups 3, 4 and 5) compared with the control and hyperoxaluric rats (Table 2). In the aspect of plasma oxalate level, groups 2, 3 and 4 rats had significant higher than control. Amongst these groups, hyperoxaluric rats treated by Vit C (group 4) exerted the highest plasma oxalate level. However, plasma oxalate level in HLP-induced hyperoxaluric rats appeared to be lower by combined Vit C and E supplement (group 5) compared with giving Vit C or Vit E alone. At day 20, the oxalate excretion was significantly elevated in all hyperoxaluric groups, except for group 3, while urinary citrate excretion in hyperoxaluric group was lower than control group (Table 3).
Crystal formation
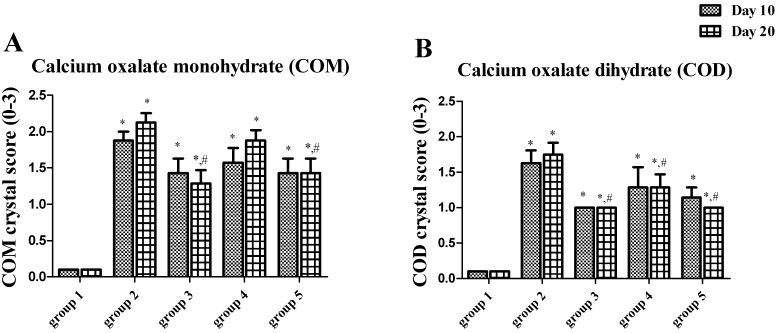
All hyperoxaluric groups had significant higher crystal deposition than the control on day 10 and day 20. The highest crystal score was observed in group 2, while Vit E supplement appeared to alleviate crystal deposition (groups 3 and 5) (Fig. 2).
Fig. 2.
COM (A) and COD (B) crystal scores from a urinalysis study of rats in groups 1−5. *P<0.05 when compared with group 1, #P<0.05 when compared with group 2.
Oxidative status
At the end of the experiment, group 2 showed significantly higher PMDA concentration than the control (Table 4), but no difference in urine MDA excretion observed between each group. Inversely, UTAS was lowest in group 2 rats, while either Vit C or Vit E treatment appeared to improve UTAS in hyperoxaluric rats (groups 3, 4 and 5).
Table 4. The oxidative status in plasma and urine in 5 groups of rat.
| Period | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|---|
| PMDA (nmol/ml) | day 21 | 1.115 ± 0.271 | 3.262 ± 0.510 a) | 2.243 ± 0.475 | 1.663 ± 0.407 | 1.958 ± 0.335 |
| UMDAV (nmol/day) | day 0 | 0.291 ± 0.0684 | 0.329 ± 0.0999 | 0.224 ± 0.0576 | 0.280 ± 0.069 | 0.412 ± 0.105 |
| day 10 | 0.155 ± 0.0304 | 0.314 ± 0.0634 | 0.231 ± 0.0387 | 0.348 ± 0.135 | 0.302 ± 0.042 | |
| day 20 | 0.198 ± 0.033 | 0.280 ± 0.0627 | 0.265 ± 0.060 | 0.262 ± 0.044 | 0.466 ± 0.124 | |
| UTAS (%) | day 0 | 81.94 ± 1.552 | 80.78 ± 1.217 | 78.44 ± 2.741 | 79.06 ± 1.126 | 80.05 ± 1.008 |
| day 10 | 82.09 ± 1.379 | 23.67 ± 4.808 a,c) | 81.09 ± 2.730 b) | 76.66 ± 3.949 b) | 80.22 ± 1.234 b) | |
| day 20 | 83.00 ± 1.783 | 29.74 ± 4.710 a,c) | 80.13 ± 1.565 b) | 77.73 ± 2.138 b) | 78.70 ± 1.015 b) |
The data were shown as mean ± SEM. a) P<0.05 compared to group 1; b) P<0.05 compared to group 2 using one way ANOVA; c) P<0.05 in the same group when compared to day 0 using one way repeated measure ANOVA.
Histopathology results
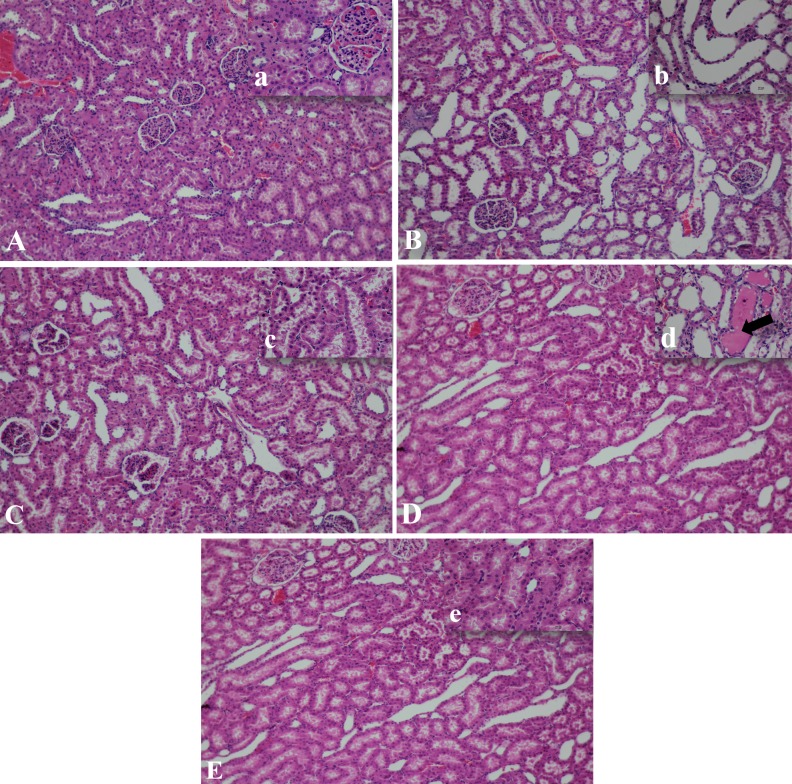
There was no remarkable lesion of glomerulus in all groups (Fig. 3). However, group 2 rats developed moderate to severe flattened tubular epithelial cells in proximal, distal and collecting duct regions (Fig. 3B, b). Antioxidant treatment appeared to alleviated tubular injury, as milder tubular epithelial lesions were observed (Fig. 3C, c−3E, e).
Fig. 3.
Histopathology pictures of kidneys in 5 groups of rats were shown (H&E staining, mag 10 × (capital letters), inset mag 40 × (small letters). Group 1, no remarkable lesions of both glomerulus and tubules were found (A, a). Group 2, the moderate to severe tubular degeneration; marked flattened tubular epithelium and dilated lumen of proximal and distal convoluted tubules (B, b). Group 3, mild tubular degeneration and dilatation were found compared with group 2 (C, c). Group 4, mild lesions of tubular cells and protein cast deposit in lumen were found (arrow) (D, d). Group 5, no remarkable lesion of tubular cell was noted (E, e).
The correlation between renal functions and oxidative stress
The GFR and the proximal tubular reabsorption of water and sodium have negative and significant correlations with PMDA (r= –0.41, r= –0.369 and r= –0.363, P<0.05, respectively), while the positive and significant correlations were observed with UTAS (r=0.22, r=0.533 and r=0.549, P<0.05, respectively).
DISCUSSION
To induce hyperoxaluria in rats, we avoided to use ethylene glycol since its metabolites are nephrotoxic. Oral HLP ingestion was substituted [13]. Hyperoxaluric rats (group 2) were confirmed by elevation of both plasma and urine excretion of oxalate. We observed that hyperoxaluric rats developed deterioration of kidney function since the increased plasma BUN and creatinine, as well as decreased GFR and ERBF compared with the control. This impaired kidney function should be subclinical, since there were no changes in body weight, food and water intake, blood pressure or urine flow rate. Since a previous study, using ethylene glycol or high dose HLP, showed the alteration of these well-being indices, we believed that the severity of illness is dose and time dependent [9, 13, 30]. Reduction in GFR may in part be due to increased intra-capsular hydrostatic pressure, since using 5% HLP induced hyperoxaluria in rats revealed crystals deposition in cortex, medulla and papillary tip at 28 days [13]. Reduction in GFR, RBF and cortical microvascular blood flow were also detected in an EG model [10].
Regarding tubular function, hyperoxaluric rats developed impaired water and sodium reabsorption at the proximal tubular region. This might be assumed that high urinary oxalate concentration disturbs proximal tubular epithelial cell. Although we could not have identified the distal tubular cell dysfunction, the severe flattened tubular epithelial cells throughout the nephron obviously indicated pan-tubular epithelial cell injury. We also found decrease in citrate excretion and calcium oxalate crystal deposition in hyperoxaluric rats.
Oxidative stress was clearly occurred in hyperoxaluric rats, since our data revealed higher plasma MDA and lower urinary TAS than control rats. Previous studies reviewed that oxidative stress is associated with crystal formation and kidney stone formation. Multifactorial conditions including oxalate-induced free radical production cause damaged tubular epithelial cells [11] which subsequently induce crystal deposition and stone formation [9]. Several studies also reported that crystal and stone can aggravate oxidative stress as well [8, 9, 12, 25, 27]. According to this, oxidative stress appears to be a vicious cycle for urolithiasis.
Comparing with distal tubule, the proximal tubule is more susceptible for injury caused by ischemia, oxidative stress or toxin due to low anaerobic glycolysis [3]. Moreover, this site had low level of expression of antioxidant enzyme gene [14]. In the present study, we observed the increased oxidative stress in hyperoxaluric rats, with the proximal tubular structural and functional derangement. Our results supported that the proximal tubular epithelial cells were markedly injured by hyperoxaluria.
Both Vit E and Vit C are accounted as high potent natural antioxidants. It was regarded that Vit E is lipid-soluble while Vit C is water-soluble, and both of them have cooperative and synergistic effect in free radical scavenging [18]. Our study showed that isolated Vit E supplement in hyperoxaluric rats induced poor food intake at the end of our study, but ameliorated glomerular dysfunction since the normalization of elevated plasma creatinine, impaired GFR and ERPF. Vit E also rescued proximal tubular dysfunction, crystal deposition, oxidative stress and alleviated histocytopathic change in tubular epithelium observed in hyperoxaluric rats. Supplementation of Vit E did not affect plasma oxalate level, but appeared to reduce both urinary citrate and oxalate excretion.
On the other hand, isolated Vit C supplement in hyperoxaluric rats did not distress the general well-being of hyperoxaluric rats. Vit C instigated glomerular and tubular impairment, oxidative status and histocytopathic change of tubule. However, Vit C induced higher plasma oxalate and oxalata excretion, while it had no effect on crystal deposition.
Combination of Vit E and C supplement appeared to exert the most beneficial effects. Our results showed that all glomerular and tubular dysfunction, oxidative status, crystal deposition and histocytopathic alteration were recovered. The poor feeding and highly elevated plasma oxalate were also improved.
In summary, we demonstrated in the present study that HLP drinking in rats caused hyperoxaluria, which resulted in nephropathy by affecting both glomerular and proximal tubular damages, and being evidenced not only reduction in GFR and tubular reabsorptions of Na and water but also elevation of crystal formation and renal histopathologic lesion. This study was demonstrated in an in vivo study for the first time that hyperoxaluria-induced renal impairment could be significantly prevented by administration of Vit C and Vit E in combination.
Acknowledgments
This study was supported by the 100th Anniversary Chulalongkorn University Fund for Doctoral Scholarship.
REFERENCES
- 1.Buranakarl C., Kitjtawonrat A., Pondeenana S., Sunyasujaree B., Kanchanapangka S., Chaiyabutr N., Bovee K. C.2003. Comparison of dipyridamole and fosinopril on renal progression in nephrectomized rats. Nephrology (Carlton) 8: 80–91. doi: 10.1046/j.1440-1797.2003.00141.x [DOI] [PubMed] [Google Scholar]
- 2.Brun C.1951. A rapid method for the determination of para-aminohippuric acid in kidney function tests. J. Lab. Clin. Med. 37: 955–958. [PubMed] [Google Scholar]
- 3.Chevalier R. L.2016. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am. J. Physiol. Renal Physiol. 311: F145–F161. doi: 10.1152/ajprenal.00164.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrzczanowicz J., Gawron A., Zwolinska A., de Graft-Johnson J., Krajewski W., Krol M., Markowski J., Kostka T., Nowak D.2008. Simple method for determining human serum 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity - possible application in clinical studies on dietary antioxidants. Clin. Chem. Lab. Med. 46: 342–349. doi: 10.1515/CCLM.2008.062 [DOI] [PubMed] [Google Scholar]
- 5.Ferraro P. M., Curhan G. C., Gambaro G., Taylor E. N.2016. Total, dietary, and supplemental vitamin C intake and risk of incident kidney stones. Am. J. Kidney Dis. 67: 400–407. doi: 10.1053/j.ajkd.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurm H., Sheta M. A., Nivera N., Tunkel A.2012. Vitamin C-induced oxalate nephropathy: a case report. J. Community Hosp. Intern. Med. Perspect. 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habibzadegah-Tari P., Byer K. G., Khan S. R.2006. Reactive oxygen species mediated calcium oxalate crystal-induced expression of MCP-1 in HK-2 cells. Urol. Res. 34: 26–36. doi: 10.1007/s00240-005-0007-3 [DOI] [PubMed] [Google Scholar]
- 8.Huang H. S., Ma M. C., Chen J.2009. Low-vitamin E diet exacerbates calcium oxalate crystal formation via enhanced oxidative stress in rat hyperoxaluric kidney. Am. J. Physiol. Renal Physiol. 296: F34–F45. doi: 10.1152/ajprenal.90309.2008 [DOI] [PubMed] [Google Scholar]
- 9.Huang H. S., Chen J., Chen C. F., Ma M. C.2006. Vitamin E attenuates crystal formation in rat kidneys: roles of renal tubular cell death and crystallization inhibitors. Kidney Int. 70: 699–710. doi: 10.1038/sj.ki.5001651 [DOI] [PubMed] [Google Scholar]
- 10.Huang H. S., Ma M. C., Chen J., Chen C. F.2003. Changes in renal hemodynamics and urodynamics in rats with chronic hyperoxaluria and after acute oxalate infusion: role of free radicals. Neurourol. Urodyn. 22: 176–182. doi: 10.1002/nau.10055 [DOI] [PubMed] [Google Scholar]
- 11.Khan S. R.1995. Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol. Res. 23: 71–79. doi: 10.1007/BF00307936 [DOI] [PubMed] [Google Scholar]
- 12.Khan S. R.2013. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J. Urol. 189: 803–811. doi: 10.1016/j.juro.2012.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan S. R., Glenton P. A., Byer K. J.2006. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int. 70: 914–923. doi: 10.1038/sj.ki.5001699 [DOI] [PubMed] [Google Scholar]
- 14.Kiyama S., Yoshioka T., Burr I. M., Kon V., Fogo A., Ichikawa I.1995. Strategic locus for the activation of the superoxide dismutase gene in the nephron. Kidney Int. 47: 536–546. doi: 10.1038/ki.1995.67 [DOI] [PubMed] [Google Scholar]
- 15.Korkmaz A., Kolankaya D.2009. The protective effects of ascorbic acid against renal ischemia-reperfusion injury in male rats. Ren. Fail. 31: 36–43. doi: 10.1080/08860220802546271 [DOI] [PubMed] [Google Scholar]
- 16.Lulich J. P., Osborne C. A., Albasan H., Koehler L. A., Ulrich L. M., Lekcharoensuk C.2013. Recent shifts in the global proportions of canine uroliths. Vet. Rec. 172: 363–368. doi: 10.1136/vr.101056 [DOI] [PubMed] [Google Scholar]
- 17.Moreira M. A., Nascimento M. A., Bozzo T. A., Cintra A., da Silva S. M., Dalboni M. A., Mouro M. G., Higa E. M.2014. Ascorbic acid reduces gentamicin-induced nephrotoxicity in rats through the control of reactive oxygen species. Clin. Nutr. 33: 296–301. doi: 10.1016/j.clnu.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 18.Niki E., Kawakami A., Yamamoto Y., Kamiya Y.1985. Oxidation of lipids. VIII. Synergistic inhibition of oxidation of phosphatidylcholine liposome in aqueous dispersion by vitamin E and vitamin C. Bull. Chem. Soc. Jpn. 58: 1971–1975. doi: 10.1246/bcsj.58.1971 [DOI] [Google Scholar]
- 19.Ohkawa H., Ohishi N., Yagi K.1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95: 351–358. doi: 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- 20.Pena de la Vega L., Lieske J. C., Milliner D., Gonyea J., Kelly D. G.2004. Urinary oxalate excretion increases in home parenteral nutrition patients on a higher intravenous ascorbic acid dose. J. Parenter. Enteral. Nutr. 28: 435–438. [DOI] [PubMed] [Google Scholar]
- 21.Sithanukul S., Shayarattanasin P., Hiranpradith V., Chansaisakorn W., Trisiriroj M., Komolvanich S., Satayatham S., Buranakarl C.2010. Blood pressure, urinary protein creatinine ratio and oxidative stress in dogs with urolithiasis. Thai. J. Vet. Med. 40: 323–340. [Google Scholar]
- 22.Suanarunsawat T., Chaiyabutr N.1996. The effect of intravenous infusion of stevioside on the urinary sodium excretion. J. Anim. Physiol. Anim. Nutr. (Berl.) 76: 141–150. doi: 10.1111/j.1439-0396.1996.tb00684.x [DOI] [Google Scholar]
- 23.Sumitra K., Pragasam V., Sakthivel R., Kalaiselvi P., Varalakshmi P.2005. Beneficial effect of vitamin E supplementation on the biochemical and kinetic properties of Tamm-Horsfall glycoprotein in hypertensive and hyperoxaluric patients. Nephrol. Dial. Transplant. 20: 1407–1415. doi: 10.1093/ndt/gfh794 [DOI] [PubMed] [Google Scholar]
- 24.Thamilselvan S., Menon M.2005. Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int. 96: 117–126. doi: 10.1111/j.1464-410X.2005.05579.x [DOI] [PubMed] [Google Scholar]
- 25.Thamilselvan S., Khan S. R., Menon M.2003. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol. Res. 31: 3–9. [DOI] [PubMed] [Google Scholar]
- 26.Thamilselvan V., Menon M., Thamilselvan S.2014. Oxalate at physiological urine concentrations induces oxidative injury in renal epithelial cells: effect of α-tocopherol and ascorbic acid. BJU Int. 114: 140–150. doi: 10.1111/bju.12642 [DOI] [PubMed] [Google Scholar]
- 27.Tosukhowong P., Yachantha C., Sasivongsbhakdi T., Ratchanon S., Chaisawasdi S., Boonla C., Tungsanga K.2008. Citraturic, alkalinizing and antioxidative effects of limeade-based regimen in nephrolithiasis patients. Urol. Res. 36: 149–155. doi: 10.1007/s00240-008-0141-9 [DOI] [PubMed] [Google Scholar]
- 28.Tosukhowong P., Boonla C., Ratchanon S., Tanthanuch M., Poonpirome K., Supataravanich P., Dissayabutra T., Tungsanga K.2007. Crystalline composition and etiologic factors of kidney stone in Thailand: update 2007. Asian Biomed. 1: 87–95. [Google Scholar]
- 29.Tungsanga K., Sriboonlue P., Futrakul P., Yachantha C., Tosukhowong P.2005. Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol. Res. 33: 65–69. doi: 10.1007/s00240-004-0444-4 [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi S., Wiessner J. H., Hasegawa A. T., Hung L. Y., Mandel G. S., Mandel N. S.2005. Study of a rat model for calcium oxalate crystal formation without severe renal damage in selected conditions. Int. J. Urol. 12: 290–298. doi: 10.1111/j.1442-2042.2005.01038.x [DOI] [PubMed] [Google Scholar]
- 31.Young M. K., Jr., Raisz L. G.1952. An anthrone procedure for determination of inulin in biological fluids. Proc. Soc. Exp. Biol. Med. 80: 771–774. doi: 10.3181/00379727-80-19758 [DOI] [PubMed] [Google Scholar]