Abstract
In June 2015, a highly fatal and acute disease broke out in a duckling farm in Hyogo Prefecture, Japan. The birds exhibited poor growth, reduced movement, lying in a dorsal recumbent position, depression, lethargy, ataxia and opisthotonus, with a high mortality rate of approximately 76%. By performing a reverse transcription-polymerase chain reaction (RT-PCR) using primers specific for duck hepatitis A virus type 1 (DHAV-1), we obtained the PCR products of a predicted size. The nucleotide sequences of the PCR products showed a >96% identity with that of the DHAV-1, HB02 strain, which was isolated in China. To our knowledge, this is the first time that the DHAV-1 virus has been isolated since its outbreak in Japan in 1963.
Keywords: DHAV-1, hepatitis, RT-PCR
Duck hepatitis is a highly lethal, acute and contagious disease with a high mortality rate in young 1–21-day-old ducklings. Pathologically, the disease is characterized by enlargement of the liver and spleen and swelling of the kidneys with some congestion of renal blood vessels [3, 7, 11]. This disease is on the list of diseases notified to the World Organisation for Animal Health.
The most common causative pathogen is a member of the newly proposed Picornavirus genus (Avihepatovirus) duck hepatitis A virus (DHAV). DHAV has been further divided into three serotypes or genotypes, including DHAV-1 (classical serotype 1), DHAV-2 (a serotype isolated in Taiwan) and DHAV-3 (a serotype isolated in South Korea and China) or DHAV genotypes A, B and C [2, 5, 12, 13]. Of these three DHAV types, the most virulent and widespread is DHAV-1, which can cause mortality of up to 95% in young ducklings within 1 week of age.
Till date, no outbreak of this disease has been reported in Japan since the last outbreak in 1963 [8]. However, an acute disease with a high mortality rate that presented as ecchymosis of the liver occurred among approximately 7-day-old ducklings in Hyogo Prefecture, Japan, in June 2015. Here, we describe the genetic identification of the causative agent of this disease and provide a genetic characterization of the isolated virus.
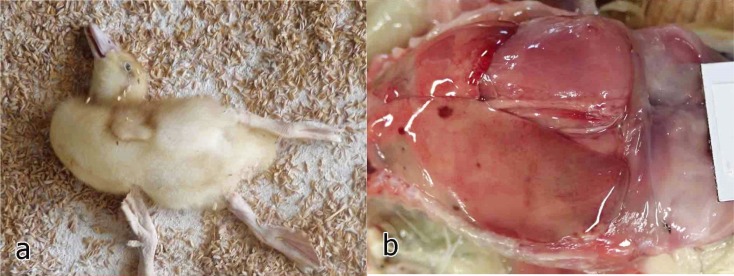
The outbreak occurred in June 2015 in a duckling farm in Hyogo Prefecture, Japan. Two or three days after 330 4-day-old ducklings were introduced to the farm, more than half of them started exhibiting poor growth, reduced movement, lying in a dorsal recumbent position, depression, lethargy, ataxia, opisthotonus and mortality (Fig. 1a). The mortality rate of the introduced flock 6 days after introduction was 76% (251/330 birds). At necropsy, the liver was irregularly or diffusely pale with a few hemorrhagic spots and rare greenish small areas (Fig. 1b). Major organs were sampled for a histological examination, and the liver was sampled for a microbiological examination.
Fig. 1.
a) Domestic duck exhibiting opisthotonus. b) A liver with irregular pale areas and a few hemorrhagic spots.
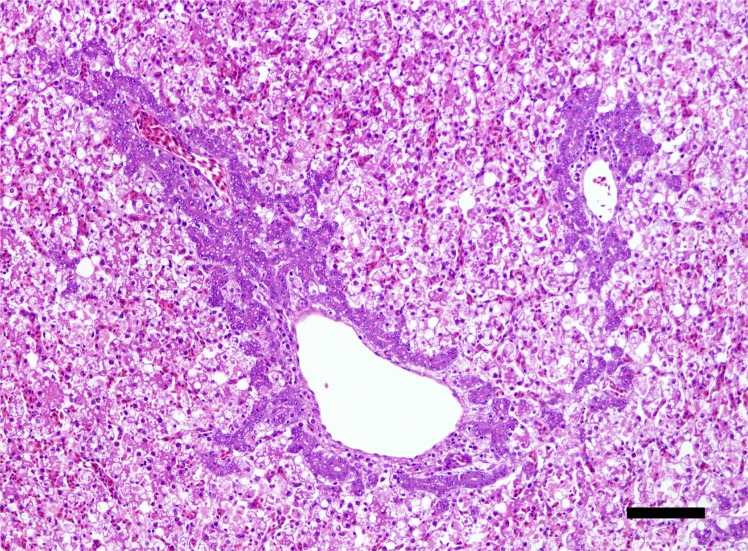
The bacteriological examination resulted in the isolation of Salmonella enteritidis from the livers of affected birds. Histologically, a diffuse degeneration and necrosis of hepatocytes was observed throughout the liver (Fig. 2). Most of hepatocytes were pale and granular cytoplasm with many fatty droplets.
Fig. 2.
Histological section of the liver showing diffuse degeneration and necrosis of hepatocytes with proliferation of basophilic bile ductular cells around the portal areas. Hematoxylin and eosin. Scale bar=100 µm.
Therefore, we attempted to isolate the duck hepatitis virus using chicken embryonated eggs. Livers were collected from affected ducklings and homogenized as a 10% (w/v) suspension in phosphate buffered saline (PBS) containing antibiotics. The homogenates were centrifuged and filtered with Millex-HV 0.45 µm (Millipore Ltd., Billerica, MA, U.S.A.). The obtained supernatant was inoculated into the allantoic cavity of 5- or 6-day-old embryonated chicken eggs and incubated at 37°C for 7 days unless the embryo had died. The inoculated eggs were then chilled to 4°C, and the allantoic fluids were harvested, and the state of the embryos was observed.
The inoculated embryos became dwarfed and exhibited bleeding and dropsy. Next, RNA was extracted from the collected allantoic fluid using a commercial kit (RNeasy Mini Kit; QIAGEN Inc., Valencia, CA, U.S.A.) according to the manufacturer’s instructions. Reverse transcription-polymerase chain reaction (RT-PCR) was performed using the One Step RT-PCR kit (QIAGEN Inc.). The following primers were used in this study [4]: DHV-1 ComF 5′-AAG-AAG-GAG-AAA-ATY-AAG-GAA-GG-3′ (sense) and DHV-1 ComR: 5′-TTG-ATG-TCA-TAG-CCC-AAS-ACA-GC-3′ (antisense). The primers were flanked a 467-bp DNA sequence in the 3D gene of DHV-1. RT was performed at 45°C for 30 min after which the enzyme was inactivated at 94°C for 5 min. PCR amplification was conducted under the following conditions: an initial denaturation for 20 sec at 94°C; followed by 40 cycles of annealing for 30 sec at 52°C, extension for 30 sec at 72°C, denaturation for 20 sec at 94°C and a final extension for 5 min at 72°C. The predicted size of the PCR products was approximately 467 bp.
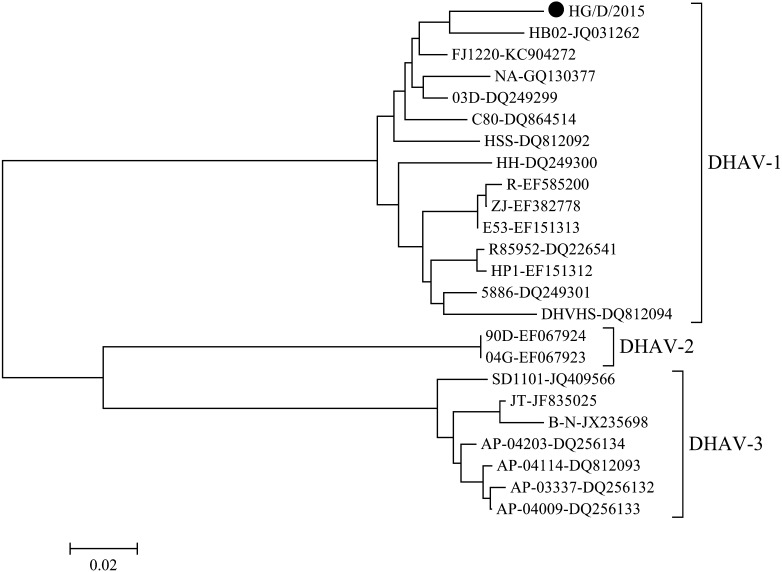
We successfully obtained the PCR products of the predicted size from all tested samples. For further confirmation, we directly determined the nucleotide sequence of the generated PCR products. The obtained PCR products of DHAV-1 were purified using Montage (Millipore), according to the manufacturer’s instructions. The purified PCR products were used as a template for sequencing in an Applied Biosystems 3100 automated DNA sequencer using dye terminator cycle sequencing chemistry (Big Dye, Applied Biosystems, Foster City, CA, U.S.A.). The purified PCR products were sequenced from both directions. The nucleotide sequences of the PCR products exhibited a 96% identity with that of the HB02 strain (GenBank Accession Number: JQ031262) of DHAV-1, which was isolated in China. The isolate was designated as the “HG/D/2015” strain. A phylogenetic tree was constructed by the neighbor-joining method [6] in MEGA6 [10]. This analysis confirmed that the HG/D/2015 strain was genetically close mainly to the viruses isolated in China (Fig. 3). Furthermore, to check dual infections with DHAV-1 and 3, we performed the PCR for detection of DHAV-3 [9]. From examined samples in this study, DHAV-3 was not detected.
Fig. 3.
Phylogenetic tree based on the 3D gene of DHAV1-3. Nucleotides 6497-6910 of the complete genome of DHAV (GenBank Accession No. JQ031262) were subjected to a phylogenetic analysis. Horizontal distances are proportional to the minimum number of nucleotide differences required to join nodes and sequences. The virus employed in this study is shown by a black circle.
This is the first duck hepatitis type 1 virus to be isolated in Japan since its outbreak in Japan in 1963. The introduction route of the virus into this affected farm is still unknown. However, subsequent outbreaks have not occurred on this affected farm, and no other outbreaks were identified in other farms until now. DHAV-1 generally infects only young ducklings in naturally occurring outbreaks [14], and once recovered, ducks may excrete the virus in their feces for up to 8 weeks after infection [14]. Furthermore, Asplin [1] suggested that wild birds act as carriers of this virus and that such bird might be responsible for new outbreaks. The phylogenetic analysis revealed that the virus isolated in this outbreak is close to the Chinese isolates, although the epidemiological relationship is unknown. This indicated the possibility that this virus was introduced from these countries into Japan by wild birds. However, an epidemiological surveillance of this virus in ducks has not been performed in Japan. To understand the epidemiology of this virus in Japan, an epidemiological surveillance of the virus would also be necessary and should include wild birds.
REFERENCES
- 1.Asplin F. D.1961. Notes on epidemiology and vaccination for virus hepatitis of ducks. Off. Int. Epizoot. Bull. 56: 793–800. [Google Scholar]
- 2.Fu Y., Pan M., Wang X., Xu Y., Yang H., Zhang D.2008. Molecular detection and typing of duck hepatitis A virus directly from clinical specimens. Vet. Microbiol. 131: 247–257. doi: 10.1016/j.vetmic.2008.03.011 [DOI] [PubMed] [Google Scholar]
- 3.Gough R. E., Borland E. D., Keymer I. F., Stuart J. C.1985. An outbreak of duck hepatitis type II in commercial ducks. Avian Pathol. 14: 227–236. doi: 10.1080/03079458508436224 [DOI] [PubMed] [Google Scholar]
- 4.Kim M. C., Kwon Y. K., Joh S. J., Kwon J. H., Kim J. H., Kim S. J.2007. Development of one-step reverse transcriptase-polymerase chain reaction to detect duck hepatitis virus type 1. Avian Dis. 51: 540–545. doi: 10.1637/0005-2086(2007)51[540:DOORTC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 5.Kim M. C., Kwon Y. K., Joh S. J., Kim S. J., Tolf C., Kim J. H., Sung H. W., Lindberg A. M., Kwon J. H.2007. Recent Korean isolates of duck hepatitis virus reveal the presence of a new geno- and serotype when compared to duck hepatitis virus type 1 type strains. Arch. Virol. 152: 2059–2072. doi: 10.1007/s00705-007-1023-0 [DOI] [PubMed] [Google Scholar]
- 6.Saitou N., Nei M.1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 7.Sandhu T. S., Calnek B. W., Zeman L.1992. Pathologic and serologic characterization of a variant of duck hepatitis type I virus. Avian Dis. 36: 932–936. doi: 10.2307/1591552 [DOI] [PubMed] [Google Scholar]
- 8.Sazawa H., Sugimori T., Shimizu T., Watanabe M.1963. Isolation and behavior of duck hepatitis virus in Japan. National Ins. Anim. Health Quaterly 3: 71–76. [Google Scholar]
- 9.Soliman M., Alfajaro M. M., Lee M. H., Jeong Y. J., Kim D. S., Son K. Y., Kwon J., Choi J. S., Lim J. S., Choi J. S., Lee T. U., Cho K. O., Kang M. I.2015. The prevalence of duck hepatitis A virus types 1 and 3 on Korean duck farms. Arch. Virol. 160: 493–498. doi: 10.1007/s00705-014-2264-3 [DOI] [PubMed] [Google Scholar]
- 10.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S.2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 30: 2725–2729. doi: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toth T. E.1969. Studies of an agent causing mortality among ducklings immune to duck virus hepatitis. Avian Dis. 13: 834–846. doi: 10.2307/1588590 [DOI] [PubMed] [Google Scholar]
- 12.Tseng C. H., Tsai H. J.2007. Molecular characterization of a new serotype of duck hepatitis virus. Virus Res. 126: 19–31. doi: 10.1016/j.virusres.2007.01.012 [DOI] [PubMed] [Google Scholar]
- 13.Wang L., Pan M., Fu Y., Zhang D.2008. Classification of duck hepatitis virus into three genotypes based on molecular evolutionary analysis. Virus Genes 37: 52–59. doi: 10.1007/s11262-008-0233-1 [DOI] [PubMed] [Google Scholar]
- 14.Woolcock P. R., Tsai H. J.2013. Duck hepatitis. pp. 422–431. In: Diseases of Poultry, 13th ed. (Swayne, D. E., Glisson, J. R., McDougald, L. R., Nolan, L.K., Suarez, D.L. and Nair, V.eds.), Wiley-Blackwell, Ames. [Google Scholar]