Abstract
Estrogen receptors α (ESR1) and β (ESR2) play central roles in folliculogenesis and therefore in reproductive biology. In the present study, two single nucleotide polymorphisms (SNPs) were identified in the ESR1 and ESR2 genes using PCR-single strand conformation polymorphism (PCR-SSCP) and DNA sequencing. One of the identified SNPs, a T1101C transition located within exon 4 of the ESR1 gene, was significantly associated with hen-housed egg production (HHEP) at 30, 43, 57 and 66 weeks of age (P<0.05), and egg weight (EW) at 30 weeks (P<0.05). Another SNP, a G1755A transition leading to a non-synonymous substitution (valine 459-to-isoleucine) located within exon 8 of the ESR2 gene, was also markedly correlated with the HHEP at 30, 43, 57 and 66 weeks of age (P<0.05), and EW at 30 weeks (P<0.05). A greater proportion of the additive variance was explained by the SNPs for most of the associated egg production traits (>1%). Furthermore, the results of the combined genotype-based association analysis supported the finding that the two SNPs were associated with the traits under a study. Taken together, our findings suggest that the two sequence variations in the ESR1 and ESR2 genes may provide promising genetic markers for the early selection and prediction of advantageous phenotypes in chicken breeding.
Keywords: Dagu hens, egg production trait, estrogen receptors α, estrogen receptors β, polymorphisms
One of the major objectives of selection in genetic breeding programs in laying hens is to improve egg productivity [43]; however, most egg production traits in chicken, including those in hen-houses, egg weight and age of sexual maturity are inherited as polygenic traits, with low to moderate heritability, leading to difficulty in the estimation of the level of genetic improvement [5, 30, 33, 43]. Many modern chicken breeds have been generated by conventional breeding methods (such as family selection assisted by self-selection), with improved egg production traits, a key focus. However, the high costs and low effectiveness of the conventional breeding approach in chicken using pedigree breeding and selection, has been disappointing for poultry breeders and researchers. Fortunately, with recent progresses in molecular biology technologies, breeding for target traits can be fast-tracked by the utilization of marker-assisted selection (MAS), focusing on improving egg productivity in chickens. A candidate gene approach is a cost-effective means of investigating associations between gene polymorphisms and quantitative trait loci that are responsible for variations in traits of interest [26, 33, 35, 42, 47]. This approach enables more efficient breeding selection for egg production traits in chickens. Therefore, identification and application of potential candidate genes and associated genotypes can have significant economic implications and have become increasingly important in poultry breeding programs [27, 44, 48]. However, more candidate genes and single nucleotide polymorphisms (SNPs) are required for MAS in chicken breeding. Currently, accumulating evidence has confirmed that estrogen receptor 1 (ESR1) and estrogen receptor 2 (ESR2) genes are involved in ovarian follicular development, and ovulation in chicken and mice [13, 14, 18, 19], with a potential impact on egg production traits in laying hens.
Estrogen is essential for folliculogenesis, with independent functions attributed to each of the two estrogen receptors [14]. Estrogen plays a pivotal role as an intrafollicular modulator by stimulating granulosa cell proliferation and facilitating the differentiative actions of FSH and LH on these cells [13, 34]. Estrogen receptors (ERs) are ligand-activated transcription factors, which are stabilized in the cytosol and translocate to the nucleus upon ligand binding and dimerization in addition to their involvement of membrane ER-centered signaling triggered by estrogen [28, 32, 40]. The expression of ESR1 (ER alpha) and ESR2 (ER beta) mRNA was examined in the ovaries of laying hens; the expression of ESR1 was higher than that of ESR2 [22]. The ESR1 gene is located on chromosome 3 and contains eight exons encoding a 589-amino acid protein (ERα) [23]. Previous studies on mice with targeted disruption in the ERα gene have indicated the presence of other estrogen binding elements, given that ovarian follicular growth appears to be unperturbed up to the secondary and antral stages [12]. In quails, the marked expression of ERα mRNA in the granulosa layer of the largest follicle may be related to the role of estrogens in cell proliferation and protein synthesis in the oviduct [21]. The results of recent studies revealed that adult female mutant mice that lack ERα in the neurons during the neonatal period fail to exhibit estrous cycles or negative feedback [10], whereas the differential expression of the ESR1 gene in chicken is involved in asymmetric ovarian development [19]. In addition, a recent study demonstrated that variants in the ESR1 gene, revealed using SNP analysis, were associated with laying traits in quails [41].
The chicken ESR2 gene, located on chromosome 5, has been cloned and found to comprise of eight exons encoding a 472-amino acid protein (ERβ), which plays a predominant role in estrogen activity in the chicken ovary [13, 23]. ERβ, expressed predominantly by the ovarian granulosa cells, is required for antrum formation, preovulatory follicle maturation and ovulation in follicle development [16, 20]. However, ERα and ERβ play different roles in folliculogenesis [13], where the proliferative action of estrogen is transmitted preferentially via ERα, whereas the differentiative effects of estrogen are mediated principally by ERβ [6, 7]. Ovulatory defects have been linked to polymorphisms in human ERβ [39]. Thus, these data indicate that sequence mutations in ESR1 and ESR2 may have a great influence on chicken ovarian development and egg laying performance. Recent studies have shown that a polymorphism in the first intron of the ESR1 gene was associated with egg laying traits (such as the onset of egg laying, egg production at the age of 300 days and egg-laying sequence) in Guizhou local blue-shell chickens [9, 50]. Accordingly, chicken ESR1 and ESR2 were proposed as candidate genes for egg production traits.
The Chinese Dagu chicken is an important poultry resource that is widely distributed in the northeast area of China; however, characterizations of genetic polymorphisms within the ESR1 and ESR2 encoding regions, and their possible correlation with egg production traits in Chinese breeds have been poorly investigated. In the present study, we detected two sequence variations in the coding regions of the ESR1 and ESR2 genes using the PCR-SSCP method and sequencing analysis. Associations between the newly identified single nucleotide polymorphisms (SNPs) and egg production traits were explored in local Chinese Dagu hens, and additive genetic effects of the SNP genotypes on the correlated traits were also evaluated. The purpose of this study was to determine a potential genetic marker to assist in the improvement of egg productivity in chicken breeding.
MATERIALS AND METHODS
Chicken and trait measurements
Chinese Dagu chickens were produced by the College of Animal Science and Technology of Jilin Agricultural University. This chicken breed is an important indigenous chicken genetic resource in China [8], which originated and is mainly distributed in Northeast China. These birds are adapted to cold weather conditions (the minimum temperature in winter is below −30°C outdoors) in the Northern-East area, with some excellent economic traits, such as high meat and egg qualities, relatively strong disease resistance and high egg productivity [8, 33]. In this experiment, 360 Dagu hens were sampled. They were hatched at the same time and raised in layered batteries under the same rearing conditions, which included free access to water and feed in accordance with the nutrient requirements of local Chinese chicken breeds and ration standard (including the Dagu chicken) established and published by the Chinese government, NY/T 33-2004, China. Approaching 16 weeks of age, the birds were reared in individual cages under a constantly maintained condition. As previously reported [33], the birds were exposed to a 16L:8D photoperiod, with lights on at 5:00 am. With the onset of egg-laying, all eggs were collected and recorded daily, with egg weights determined one day each week. Body weight was recorded following feed and water restrictions at 30 and 43 weeks of age, with the individual laying performance calculated. Egg production traits examined in this study included hen-housed egg production (egg laying number) at 30, 43, 57 and 66 weeks of age, and egg weight and body weight were determined at 30 and 43 weeks of age. All animal experiments were performed in accordance with laws of the People’s Republic of China regarding animal protection.
DNA extraction and PCR amplification
At 300 days of age, peripheral blood was sampled from individuals of the 360 Chinese Dagu hens via the wing vein. Genomic DNA was extracted using a standard phenol-chloroform method, and DNA purity was examined using 1% agarose gel electrophoresis and ultraviolet-spectrophotometry, with final concentrations between 2 and 10 ng/µl detected. Primers for PCR were designed according to the published ESR1 (GenBank accession No. NM_205183.2) and ESR2 (Accession No. NM_204794.2) mRNA sequences in chicken. The primer pairs (Table 1) utilized to amplify fragments of the ESR1 and ESR2 genes were screened after examination, from a clutch of primers that cover almost the whole coding region of each candidate gene.
Table 1. Primers used to amplify chicken ESR1 and ESR2 fragments.
| Genes | Sequences of primers (5′–3′) | Product length (Location) | Annealing temperature (°C) |
|---|---|---|---|
| ESR1 | F: GGGAGGCTTCTTCTACAGA | 121 bp | 55 |
| R: AAGGCACTGACCATCTGT | (1,016–1,136 bp) | ||
| ESR2 | F: ATGACTTGCTGCTGGAGA | 103 bp | 57 |
| R:CAGACCTGGAAATGTGAAAC | (1,696–1,798 nt) |
PCR was performed in a total volume of 50 µl, containing 25 µl of 2X Taq Master Mix (CWBIO, Beijing, China), 1 µl each primer (100 nM), 1 µl template DNA (25 to 50 ng) and 22 µl RNase-free Water. The PCR conditions were as follows: 94°C for 2 min, followed by 35 cycles at 94°C for 30 sec for denaturing, 55°C (57°C) for 30 sec for annealing (see Table 1), 72°C for 30 sec for extension and a final extension at 72°C for 2 min.
Cloning of PCR products, sequencing and alignment
PCR products were purified with the Wizard prep PCR purification system (Promega, Madison, WI, U.S.A.). The amplified products were then cloned into the Promega pGEM-T easy vector for sequencing commercially, according to the methods published by Sambrook and Russell [36]. For each sampled bird, two independent PCRs were conducted, with sequences analyzed using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome) to confirm the amplification of the expected chicken ESR1 and ESR2 gene fragments. The obtained sequences were then aligned using DNAMAN software version 6.0 (LynnonBiosoft, San Ramon, CA, U.S.A.) to identify nucleotide variations.
Genotyping by PCR-SSCP and reconstruction of combined genotypes
To determine ESR1 and ESR2 gene polymorphisms, the confirmed PCR products were further analyzed using the single-strand conformation polymorphism (SSCP) assay, as previously described [33]. Briefly, each 10-µl PCR product was mixed with 6 µl loading dye (0.025% bromophenol blue, 0.025% xylene cyanol, 98% deionized formamide, 2% glycerin and 10 mM ethylenediaminetetraacetic acid [EDTA]). Samples were denatured at 99°C for 10 min, cooled rapidly on ice and then loaded on 10% polymerized gels (acrylamide:bisacrylamide, 39:1; 0.75 mm thickness) 16 × 18 cm in size. Electrophoresis was carried out at 110 V for 5 hr in 1X tris-borate-EDTA (TBE) buffer at 20°C under an air-conditioning unit. After silver staining, the gels were examined under upper white by gel photography system (GeneSnap from SynGene). To avoid false positive/negative results due to artificial manipulation in the experiment, each sample was confirmed by repeated examinations. Combined genotypes were reconstructed based on the genotyping data obtained from all 360 birds using the PHASE program [38].
Polymorphism evaluation
Frequencies of genotypes and alleles at each SNP site were calculated, with each polymorphism evaluated for Hardy–Weinberg equilibrium using a Pearson’s goodness-of-fit chi-square test (degree of freedom=1). Gene homozygosity (Ho), heterozygosity (He), effective number of alleles (Ne) and polymorphism information content (PIC) were statistically analyzed using the POPGENE v. 1.32 software [46].
Marker-trait association analysis
Associations of single polymorphisms and combined genotypes with laying performance traits were evaluated using the General Linear Model (GLM) procedure in SPSS 18.0. The following linear mixed effects model was used in the analysis:
Yijk = µ + Li + Gj + Fk + eijk
where, Yijk is the phenotypic value of the target trait, such as hen-housed egg production and egg weight, µ is the population mean, Li is the fixed effect of the line, Gj is the fixed effect of the SNP genotype or combined genotype, Fk is the random effect of the family, and eijk is the residual. Type III sum of squares was used in each test. Values were considered significant at P<0.05 and presented as least square means ± standard errors (SE).
Predicted SNP genotype effects
For the SNP(s) that showed significant association with the egg-laying traits, differences between the means of each genotype and allelic frequencies were used to estimate additive effects [17]. The percentage of additive genetic variance (%Vj) explained by the SNPs was determined using the following formula:
%Vj = 100pjqjαj ^ 2 / Vg
where p and q are the allele frequencies for the jth SNP estimated across the entire population; αj is the estimated additive effect of the jth SNP (allele substitution effect) on the trait under analysis; and Vg is the restricted maximum likelihood (REML) estimate of the (poly-) genetic variance for the trait.
RESULTS
Confirmation of the amplified nucleotide sequence
Two targeted fragments were amplified using screened primers specific for Chinese Dagu hens, in which a 121-bp PCR amplicon for ESR1 and a 103-bp fragment for ESR2 were obtained, respectively. The purified PCR products were then cloned into the Promega pGEM-T easy vector for sequencing. Alignments of the amplified sequences with the corresponding template sequences (GenBank No. NM_205183.2 for ESR1 and NM_204794.2 for ESR2 gene) were accomplished using the BLAST software provided by the NCBI server. Following confirmation of primer specificity, whereby the sizes of the PCR amplicons corresponded to the expected sequences of the candidate genes (Table 1), the cloned PCR products were identified by aligning with the direct genomic PCR products from the same chicken. No more than two allelic sequences were observed for all examined birds, confirming that the primer pairs specifically amplified the targeted genes.
Genotyping by PCR-SSCP and reconstruction of combined genotypes
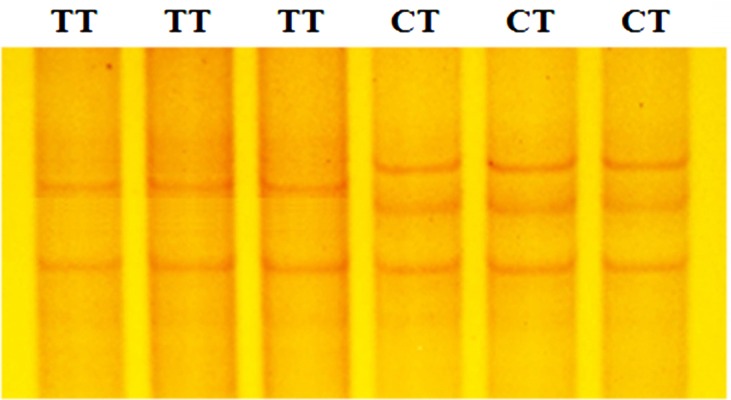
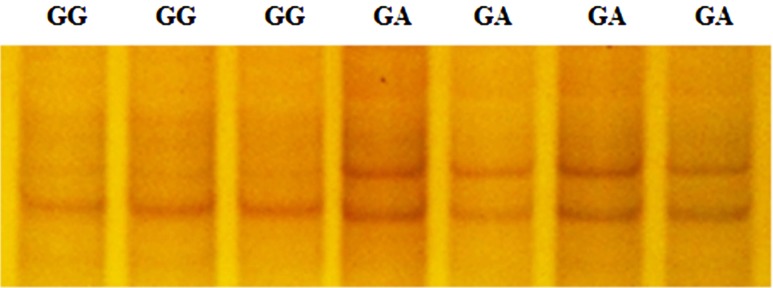
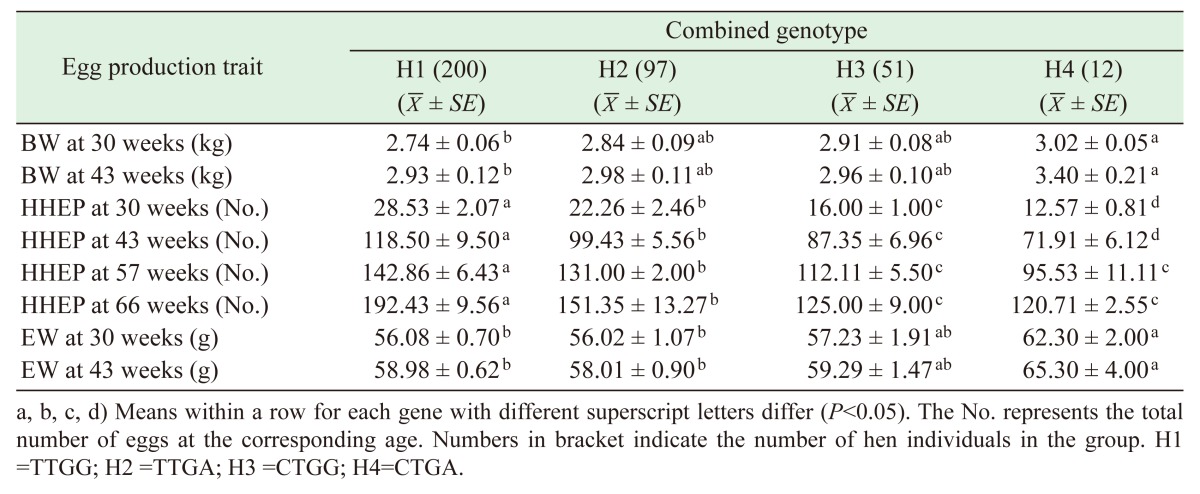
Genotyping results obtained by PCR-SSCP analysis revealed that the TT and CT genotypes were classified for the ESR1 genomic fragment (Fig. 1), and the GG and GA genotypes, for the ESR2 sequence within the Dagu hen population (Fig. 2). The combined genotype was reconstruction based on these genotype data, and four combined genotypes (H1, H2, H3 and H4) were determined among the 360 birds sampled (Table 6). The combined genotype present at the highest frequency was H1 (TTGG; 0.56), with H2 (TTGA) being the next most frequent (0.27), followed by H3 (CTGG; 0.14) and H4 (CTGA; 0.03).
Fig. 1.
Polymerase chain reaction- single strand conformation polymorphism band patterns of the ESR1 gene.
Fig. 2.
Polymerase chain reaction- single strand conformation polymorphism band patterns of the ESR2 gene.
Table 6. Associations between the combined genotypes and egg production traits in Dagu hens.
Polymorphism of the target sequences
Two single nucleotide polymorphisms (SNPs) were identified, corresponding to the PCR-SSCP banding patterns of the ESR1 and ESR2 genes following sequence alignment. In the ESR1 gene, a T/C transition at nucleotide position 1,101 of exon 4 was found and named SNP T1101C. However, this T/C transition leads to a synonymous substitution (alanine 301 to alanine). For this SNP, the hens sampled were typed as either TT or CT based upon the SSCP banding pattern. In the ESR2 gene fragment, a G/A transition at nucleotide position 1,755 was detected in exon 8 and named SNP G1755A. This transition results in a non-synonymous substitution of valine 459 to isoleucine with birds categorized as having GG and GA genotypes following PCR-SSCP analysis.
Frequencies of genotype and alleles at the SNP locus
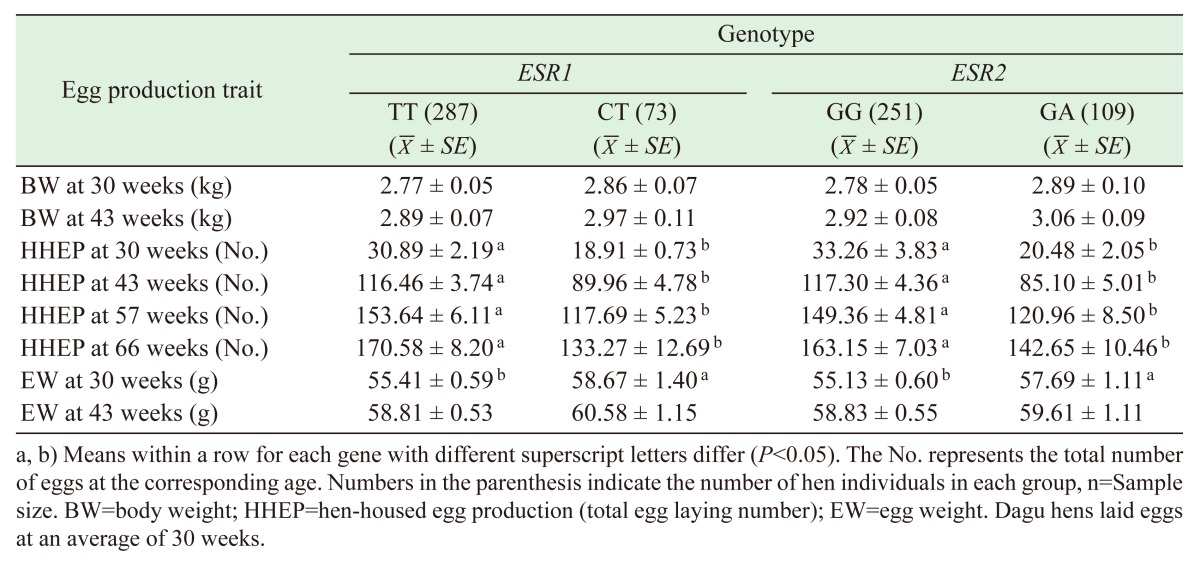
Genotype and allelic frequencies of SNPs at the ESR1 and ESR2 loci in the Dagu chicken population are shown in Table 2. At SNP T1101C of the ESR1 gene (designated as the E1 locus), the frequency of allele T was significantly higher than that of allele C, with the frequency of genotype TT being higher than that of genotype CT in this population. At SNP G1755A of the ESR2 (E2 locus), the frequency of allele G was higher than that of allele A, with the frequency of genotype GG being higher than that of genotype GA. Polymorphisms at both SNP loci were evaluated and were found to exhibit significant genetic disequilibrium between the T and C alleles of ESR1 and the G and A alleles of ESR2 (P<0.05).
Table 2. Genotypic and allelic frequency at the SNP ESR1 and ESR2 loci in the Dagu chicken population.
| SNP | Genotype | No. of chickens |
Genotype frequency |
Allele | Allele frequency | χ2 |
|---|---|---|---|---|---|---|
| T1101C (ESR1) | TT | 287 | 0.797 | T | 0.898 | 4.773 a |
| CT | 73 | 0.203 | C | 0.102 | ||
| G1755A (ESR2) | GG | 251 | 0.697 | G | 0.849 | 9.123 a |
| GA | 109 | 0.303 | A | 0.151 |
a) P<0.05 was accepted as statistically significant when the data were analyzed using a Pearson’s goodness-of-fit chi-square test (degree of freedom=1).
As shown in Table 3, gene homozygosity (Ho) was higher than gene heterozygosity (He) for both E1 locus and E2 locus, with effective allele numbers of 1.190 (E1) and 1.351 (E2). The value of PIC for He at the E2 locus was higher than that at the E1 locus; however, polymorphism was not high and only varied from moderate (0.226) to low (0.147).
Table 3. Polymorphism information analysis of the ESR1 and ESR2 genes in the Dagu chicken population.
| SNP | Gene homozygosity (Ho) | Gene heterozygosity (He) | Effective allele number (Ne) | Polymorphism information content (PIC) |
|---|---|---|---|---|
| T1101C (ESR1) | 0.841 | 0.159 | 1.19 | 0.147 |
| G1755A (ESR2) | 0.74 | 0.26 | 1.351 | 0.226 |
Association of the SNP genotypes with laying performance and the predicted SNP genotype effects
Results of the association analysis between SNPs and egg production traits are shown in Table 4. The SNP T1101C genotype TT was significantly associated with higher HHEP at 30, 43, 57 and 66 weeks of age (P<0.05), and with the lower EW at 30 weeks (P<0.05). Moreover, the SNP G1755A genotype GG was also markedly associated with the higher HHEP at 30, 43, 57 and 66 weeks of age (P<0.05), and with the smaller EW at 30 weeks (P<0.05). Furthermore, a large percentage of the additive variance was explained by the SNP for its significant association with the traits (>1%), respectively (Table 5). However, no significant difference was observed between the TT and CT or between the GG and GA genotypes regarding the BW at 30 and 43 weeks (P>0.05) or EW at 43 weeks (P>0.05).
Table 4. Association between polymorphism in chicken ESR1 and ESR2 genes and egg production traits in Dagu hens.
Table 5. Percentages of additive genetic variance explained by the SNPs identified in fragments of chicken ESR1 and ESR2 genes.
| Egg production trait | Percentage of additive genetic variance explained by the SNPs (%) |
|
|---|---|---|
| T1101C (ESR1) | G1755A (ESR2) | |
| BW at 30 weeks (kg) | - | - |
| BW at 43 weeks (kg) | - | - |
| HHEP at 30 weeks (No.) | 0.736 | 0.572 |
| HHEP at 43 weeks (No.) | 1.644 | 0.599 |
| HHEP at 57 weeks (No.) | 2.358 | 0.877 |
| HHEP at 66 weeks (No.) | 5.59 | 5.007 |
| EW at 30 weeks (g) | 0.12 | 0.463 |
| EW at 43 weeks (g) | - | - |
As shown in Table 6, the combined genotype H1 was found to be significantly correlated with the highest HHEP at 30, 43, 57 and 66 weeks of age (P<0.05), but with lower BW and EW at 30 and 43 weeks (P<0.05). In contrast, the H2 type was notably associated with the higher HHEP at 30, 43, 57 and 66 weeks of age (P<0.05), and with the smaller EW at 30 and 43 weeks (P<0.05). However, H4 was found to be significantly associated with the lowest HHEP at 30, 43, 57 and 66 weeks of age (P<0.05), and with higher BW and EW at 30 and 43 weeks (P<0.05).
DISCUSSION
Egg-laying performance is one of the most important economic traits in the poultry industry; improvement in egg production traits is a key goal of breeding programs and has been attracting increasing interest. However, most of the egg production traits in chicken, are inherited polygenically, with low to moderate heritability [5, 30, 43]; moreover, they obtained only from sexually mature females. This makes genetic improvements in males more difficult to estimate using traditional methods, which depend on a breeding value assessment. To explore methods that are more effective than the traditional breeding approach, many SNP detection techniques, including the PCP-SSCP genotyping analysis, have been established and are currently utilized. Nevertheless, because egg production traits are polygenically inherited, more associated target genes and favorable alleles are required for egg-laying improvement. Therefore, the ESR1 and ESR2 genes were screened and used to study genetic associations with egg production traits.
To explore the potential association between the ESR1 and ESR2 genes with the egg production traits, including BW, HHEP and EW, we first examined SNPs in the coding region of the ESR1 and ESR2 genes in the Chinese Dagu chicken population, in which the main breeding goal focused on HHEP trait improvement. In the present study, two SNPs in the chicken ESR1 and ESR2 fragments were identified; the frequency of allele T at the E1 locus was higher than that of allele C, and the frequency of genotype TT was higher than that of genotype CT. Moreover, genotype CC was missing at the E1 locus. For the E2 locus, the frequency of allele G was significantly higher than that of allele A and the frequency of genotype GG was higher than that of genotype GA, and the homologous AA genotype was missing. This phenomenon may be explained by several factors: (i) alleles T at E1 or G at E2 may be tightly linked with either an advantageous allele or with an artificially selected economically favorable trait, such as a higher HHEP and/or EW, as demonstrated above. As a result, the levels of homozygotes with genotype TT or GG were either promoted under natural selection pressures or were artificially selected for favorable agricultural attributes; (ii) both the SNPs were confirmed to be under genetic disequilibrium in this study. This was due to either allele T or G being predominant under artificial and/or natural genetic selection pressure, thereby becoming more common than other alleles in this population; (iii) the egg production traits of the local Dagu populations sampled in this study have been developed with the aim of enhancing early sexual maturity and egg laying number traits for the last six generations. During Dagu chicken breeding, the T or G alleles may be coincidently linked with one or more of the selected breeding traits, thereby presenting a possible explanation for the higher allelic frequencies; (iv) the number of birds examined in each population may have been insufficient to demonstrate the true event, and therefore, an extreme allele frequency was estimated as a result.
Previous studies have demonstrated that Ne and PIC are important genetic parameters that indicate the level of intra-population genetic variation [1, 29]. The results of the present study show that the two SNPs exhibited low polymorphism, with the mean PIC value being lower than 0.25; however, allele homozygosity was higher than 0.7, indicating that the predominant allele had been subjected to selection pressure. Continuous selection aiming to obtain a favorable trait may lead to an increase in allelic frequencies; simultaneously, it brought about Hardy–Weinberg disequilibrium at the linked allele locus. This result may be partially explained by the following data on the trait association analyses.
Age at first egg is a critical factor for egg-laying performance in poultry. For the Dagu hens sampled here, the average age at first egg was 30 weeks, indicating the sexual maturity of the entire chicken population. Therefore, the BW, HHEP and EW in 30-week-old Dagu hens were qualified in this study. Based upon the genotype-based association analysis, the newly identified TT genotype at the E1 locus and GG genotype at the E2 locus were found to be significantly associated with higher HHEP at 30, 43, 57 and 66 weeks of age, and with the smaller EW at 30 weeks of age under a study (P<0.05), respectively. This was coincident with the negative correlation observed between HHEP and EW. Furthermore, to analyze the genetic effects of the SNPs on the associated traits, including HHEP and EW, and considering that additive variance is an important component of genetic variance in the expression of traits related to egg production [3, 5, 17], the percentage of additive genetic variance explained by the SNPs was estimated in this study. The results of the present study indicated that a larger proportion of variance was explained by these markers (>1%), especially for the phenotypes including HHEP at 43, 57 and 66 weeks of age by the ESR1 locus and HHEP at 66 weeks of age by the ESR2 locus. Although a relatively smaller proportion of variance (<1%) was explained by these markers for the phenotypes, including the HHEP and EW at 30 weeks of age, the effects of allele substitution on the egg production traits were not neglected.
In the present study, four combined genotypes (H1–H4) were detected, and the association analysis of combined genotypes showed that ESR1 and ESR2 polymorphisms are significantly associated with egg production traits in Dagu chickens. Moreover, the results of the combined genotype-based association analysis were consistent with the significant effect detected by the genotype-based association analysis. The combined genotype H1, which carried the favorable T and G SNP alleles, was significantly associated with the highest HHEP at 30, 43, 57 and 66 weeks of age, and with the lower EW at 30 and 43 weeks of age (P<0.05). Interestingly, each of the alleles (T and G) was coincidentally found to be predominant at the locus; this may be due to the artificial selection for favorable HHEP traits in the last six generations. Collectively, these results support a strong association between the two variations in ESR1 and ESR2 with the egg production traits, via some functional genetic mechanisms.
The biological actions of estrogens are mediated via two distinct intranuclear estrogen receptor proteins, ERα and ERβ [11, 14]. The carboxyl-terminal ligand-binding domains (LBDs) of ERs are conserved, as suggested by the similar affinities of the two ERs for 17β-estradiol [25], which is encoded by exon 8 of the ER gene. The amino-terminal domain of ERβ is shorter than that of ERα, but is well conserved between rat, mouse and human ERβ, suggesting an evolutionary constraint and a functional importance [14]. A single Tyr 443-to-Asn substitution within LBD leads to changes in the dimerization and transcription activation functions of ERβ [41, 49]. In the present study, SNP G1755A of the ESR2 gene resulted in a Val 459-to-Ile mutation within the LBD and was found to be associated with variation in egg production traits, indicating that changes in the LBD of ERβ may directly affect the folliculogenesis or follicular growth and development in the ovaries of chicken, and consequently, influence phenotype (egg production traits). This SNP within the ESR2 gene might represent a promising molecular marker for the early selection of individuals or families with favorable phenotypes in chicken breeding.
In addition, variations in the ESR1 genes have been associated with the natural defense of eggs against bacterial penetration by increasing cuticle deposition in chickens [4]. These findings are useful for the further investigation of possible genetic effects of ESR1 and ESR2 gene polymorphisms, and isotype-selective ESR agonists on the physiology of folliculogenesis, follicular growth and ovarian functional transformation [20]. Additionally, in some cases, these variations indicate abnormal estrogen secretion or deficiency [37]. In recent years, accumulating evidence has indicated that the role of estrogen receptor sequence variants is associated with bone mineral density and affects estradiol levels [15, 24, 31]. This increases the risk of HBV-related acute liver failure [45] and endometrial cancer in humans [2]. Hence, the results of the present study are also likely to be of considerable veterinary, clinical and biological importance.
Taken together, our data reinforce the conclusion that the two sequence variations in the ESR1 and ESR2 genes are significantly associated with HHEP and EW in Chinese Dagu chickens, and represent promising genetic markers for the early selection and prediction of advantageous phenotypes in chicken breeding programs. Additionally, these data also suggest that polymorphisms of the ESR1 and ESR2 genes might be further explored as indicators for relevant diseases or for the occurrence of ovarian dysfunction.
Acknowledgments
This work was supported by the National Natural Science Funds (No.31672407 and No. 31272431), the Project of Science and Technology Development Plan of Jilin Province (No. 20170101019JC), the China Agriculture Research System (No. CARS-42), the National High Technology Research and Development Program (No. 2011AA100305) and the Jilin Provincial Agriculture Research System of China.
REFERENCES
- 1.Abdalhag M. A., Zhang T., Fan Q. C., Zhang X. Q., Zhang G. X., Wang J. Y., Wei Y., Wang Y. J.2015. Single nucleotide polymorphisms associated with growth traits in Jinghai yellow chickens. Genet. Mol. Res. 14: 16169–16177. doi: 10.4238/2015.December.8.6 [DOI] [PubMed] [Google Scholar]
- 2.Ashton K. A., Proietto A., Otton G., Symonds I., McEvoy M., Attia J., Gilbert M., Hamann U., Scott R. J.2009. Estrogen receptor polymorphisms and the risk of endometrial cancer. BJOG 116: 1053–1061. doi: 10.1111/j.1471-0528.2009.02185.x [DOI] [PubMed] [Google Scholar]
- 3.Almasy L., Blangero J.2010. Variance component methods for analysis of complex phenotypes. Cold Spring Harb. Protoc. 2010: top77. doi: 10.1101/pdb.top77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain M. M., McDade K., Burchmore R., Law A., Wilson P. W., Schmutz M., Preisinger R., Dunn I. C.2013. Enhancing the egg’s natural defence against bacterial penetration by increasing cuticle deposition. Anim. Genet. 44: 661–668. doi: 10.1111/age.12071 [DOI] [PubMed] [Google Scholar]
- 5.Biscarini F., Bovenhuis H., Ellen E. D., Addo S., van Arendonk J. A. M.2010. Estimation of heritability and breeding values for early egg production in laying hens from pooled data. Poult. Sci. 89: 1842–1849. doi: 10.3382/ps.2010-00730 [DOI] [PubMed] [Google Scholar]
- 6.Britt K. L., Findlay J. K.2002. Estrogen actions in the ovary revisited. J. Endocrinol. 175: 269–276. doi: 10.1677/joe.0.1750269 [DOI] [PubMed] [Google Scholar]
- 7.Britt K. L., Kerr J., O’Donnell L., Jones M. E. E., Drummond A. E., Davis S. R., Simpson E. R., Findlay J. K.2002. Estrogen regulates development of the somatic cell phenotype in the eutherian ovary. FASEB J. 16: 1389–1397. doi: 10.1096/fj.01-0992com [DOI] [PubMed] [Google Scholar]
- 8.Chen G. G.2004. Poultry Genetic Resources in China. 1st ed., pp. 15−16. Shanghai Science and Technology Press, Shanghai (in Chinese). [Google Scholar]
- 9.Chen X., Wei X., Xu H. Q., Zhang Y., Sun J., Yang Z. C.2012. Analysis on the association of intron 1 of ESR1 gene with early egg production performance in chicken. Anim. Husbandry Vet. Med. 5: 6–9 (in Chinese). [Google Scholar]
- 10.Cheong R. Y., Porteous R., Chambon P., Abrahám I., Herbison A. E.2014. Effects of neuron-specific estrogen receptor (ER) α and ERβ deletion on the acute estrogen negative feedback mechanism in adult female mice. Endocrinology 155: 1418–1427. doi: 10.1210/en.2013-1943 [DOI] [PubMed] [Google Scholar]
- 11.Cheung E., Schwabish M. A., Kraus W. L.2003. Chromatin exposes intrinsic differences in the transcriptional activities of estrogen receptors alpha and beta. EMBO J. 22: 600–611. doi: 10.1093/emboj/cdg037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond A. E., Baillie A. J., Findlay J. K.1999. Ovarian estrogen receptor alpha and beta mRNA expression: impact of development and estrogen. Mol. Cell. Endocrinol. 149: 153–161. doi: 10.1016/S0303-7207(98)00247-0 [DOI] [PubMed] [Google Scholar]
- 13.Drummond A. E., Fuller P. J.2010. The importance of ERbeta signalling in the ovary. J. Endocrinol. 205: 15–23. doi: 10.1677/JOE-09-0379 [DOI] [PubMed] [Google Scholar]
- 14.Drummond A. E., Fuller P. J.2012. Ovarian actions of estrogen receptor-β: an update. Semin. Reprod. Med. 30: 32–38. doi: 10.1055/s-0031-1299595 [DOI] [PubMed] [Google Scholar]
- 15.Durusu Tanriover M., Bora Tatar G., Uluturk T. D., Dayangac Erden D., Tanriover A., Kilicarslan A., Oz S. G., Erdem Yurter H., Sozen T., Sain Guven G.2010. Evaluation of the effects of vitamin D receptor and estrogen receptor 1 gene polymorphisms on bone mineral density in postmenopausal women. Clin. Rheumatol. 29: 1285–1293. doi: 10.1007/s10067-010-1548-6 [DOI] [PubMed] [Google Scholar]
- 16.Emmen J. M. A., Couse J. F., Elmore S. A., Yates M. M., Kissling G. E., Korach K. S.2005. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER)alpha and ERbeta null mice indicate a role for ERbeta in follicular maturation. Endocrinology 146: 2817–2826. doi: 10.1210/en.2004-1108 [DOI] [PubMed] [Google Scholar]
- 17.Falconer D. S., Trudy F. C., Mackay T. F. C.1996. Introduction to Quantitative Genetics. 4th ed., Longmans Green, Harlow. [Google Scholar]
- 18.González-Morán M. G., González-Arenas A., Germán-Castelán L., Camacho-Arroyo I.2013. Changes in the content of sex steroid hormone receptors in the growing and regressing ovaries of Gallus domesticus during development. Gene. Comp. Endocr. 189: 51–58. [DOI] [PubMed] [Google Scholar]
- 19.González-Morán M. G.2014. Changes in the cellular localization of estrogen receptor alpha in the growing and regressing ovaries of Gallus domesticus during development. Biochem. Biophys. Res. Commun. 447: 197–204. doi: 10.1016/j.bbrc.2014.03.122 [DOI] [PubMed] [Google Scholar]
- 20.Hegele-Hartung C., Siebel P., Peters O., Kosemund D., Müller G., Hillisch A., Walter A., Kraetzschmar J., Fritzemeier K. H.2004. Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc. Natl. Acad. Sci. U.S.A. 101: 5129–5134. doi: 10.1073/pnas.0306720101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hrabia A., Ha Y., Shimada K.2004. Expression of estrogen receptor alpha mRNA in theca and granulosa layers of the ovary in relation to follicular growth in quail. Folia Biol. (Krakow) 52: 191–195 (in Krakow). doi: 10.3409/1734916044527458 [DOI] [PubMed] [Google Scholar]
- 22.Hrabia A., Wilk M., Rzasa J.2008. Expression of alpha and beta estrogen receptors in the chicken ovary. Folia Biol. (Krakow) 56: 187–191 (in Krakow). doi: 10.3409/fb.56_3-4.187-191 [DOI] [PubMed] [Google Scholar]
- 23.International Chicken Genome Sequencing Consortium2004. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432: 695–716. doi: 10.1038/nature03154 [DOI] [PubMed] [Google Scholar]
- 24.Jeedigunta Y., Bhoomi Reddy P. R., Kolla V. K., Munshi A., Ananthapur V., Narasimulu G., Akka J.2010. Association of estrogen receptor alpha gene polymorphisms with BMD and their affect on estradiol levels in pre- and postmenopausal women in south Indian population from Andhra Pradesh. Clin. Chim. Acta 411: 597–600. doi: 10.1016/j.cca.2010.01.026 [DOI] [PubMed] [Google Scholar]
- 25.Kuiper G. G., Lemmen J. G., Carlsson B., Corton J. C., Safe S. H., van der Saag P. T., van der Burg B., Gustafsson J. A.1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 139: 4252–4263. [DOI] [PubMed] [Google Scholar]
- 26.Linville R. C., Pomp D., Johnson R. K., Rothschild M. F.2001. Candidate gene analysis for loci affecting litter size and ovulation rate in swine. J. Anim. Sci. 79: 60–67. doi: 10.2527/2001.79160x [DOI] [PubMed] [Google Scholar]
- 27.Liu W. J., Sun D. X., Yu Y., Li G., Tang S. Q., Zhang Y., Wang Y. C., Zhang Y.2010. Association of Janus kinase 2 polymorphisms with growth and reproduction traits in chickens. Poult. Sci. 89: 2573–2579. doi: 10.3382/ps.2010-00988 [DOI] [PubMed] [Google Scholar]
- 28.Lu Q., Pallas D. C., Surks H. K., Baur W. E., Mendelsohn M. E., Karas R. H.2004. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor α. Proc. Natl. Acad. Sci. U.S.A. 101: 17126–17131. doi: 10.1073/pnas.0407492101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luikart G., Sherwin W. B., Steele B. M., Allendorf F. W.1998. Usefulness of molecular markers for detecting population bottlenecks via monitoring genetic change. Mol. Ecol. 7: 963–974. doi: 10.1046/j.1365-294x.1998.00414.x [DOI] [PubMed] [Google Scholar]
- 30.Luo P. T., Yang R. Q., Yang N.2007. Estimation of genetic parameters for cumulative egg numbers in a broiler dam line by using a random regression model. Poult. Sci. 86: 30–36. doi: 10.1093/ps/86.1.30 [DOI] [PubMed] [Google Scholar]
- 31.Mitra S., Desai M., Khatkhatay M. I.2006. Association of estrogen receptor alpha gene polymorphisms with bone mineral density in postmenopausal Indian women. Mol. Genet. Metab. 87: 80–87. doi: 10.1016/j.ymgme.2005.06.025 [DOI] [PubMed] [Google Scholar]
- 32.Moriarty K., Kim K. H., Bender J. R.2006. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology 147: 5557–5563. doi: 10.1210/en.2006-0729 [DOI] [PubMed] [Google Scholar]
- 33.Qin N., Liu Q., Zhang Y. Y., Fan X. C., Xu X. X., Lv Z. C., Wei M. L., Jing Y., Mu F., Xu R. F.2015. Association of novel polymorphisms of forkhead box L2 and growth differentiation factor-9 genes with egg production traits in local Chinese Dagu hens. Poult. Sci. 94: 88–95. doi: 10.3382/ps/peu023 [DOI] [PubMed] [Google Scholar]
- 34.Richards J. S.1980. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol. Rev. 60: 51–89. [DOI] [PubMed] [Google Scholar]
- 35.Rothschild M. F., Soller M.1997. Candidate gene analysis to detect genes controlling traits of economic importance in domestic livestock. Probe 8: 13–20. [Google Scholar]
- 36.Sambrook J., Russell D. W.2001. Molecular Cloning: A Laboratory Manual, 3rd ed. Cold Spring Harbor Laboratory Press, New York. [Google Scholar]
- 37.Shaw N. D., Histed S. N., Srouji S. S., Yang J., Lee H., Hall J. E.2010. Estrogen negative feedback on gonadotropin secretion: evidence for a direct pituitary effect in women. J. Clin. Endocrinol. Metab. 95: 1955–1961. doi: 10.1210/jc.2009-2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephens M., Smith N. J., Donnelly P.2001. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 68: 978–989. doi: 10.1086/319501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sundarrajan C., Liao W. X., Roy A. C., Ng S. C.2001. Association between estrogen receptor-beta gene polymorphisms and ovulatory dysfunctions in patients with menstrual disorders. J. Clin. Endocrinol. Metab. 86: 135–139. [DOI] [PubMed] [Google Scholar]
- 40.Tesarik J., Mendoza C.1997. Direct non-genomic effects of follicular steroids on maturing human oocytes: oestrogen versus androgen antagonism. Hum. Reprod. Update 3: 95–100. doi: 10.1093/humupd/3.2.95 [DOI] [PubMed] [Google Scholar]
- 41.Tremblay G. B., Tremblay A., Labrie F., Giguère V.1998. Ligand-independent activation of the estrogen receptors alpha and beta by mutations of a conserved tyrosine can be abolished by antiestrogens. Cancer Res. 58: 877–881. [PubMed] [Google Scholar]
- 42.Tuiskula-Haavisto M., Honkatukia M., Vilkki J., de Koning D. J., Schulman N. F., Mäki-Tanila A.2002. Mapping of quantitative trait loci affecting quality and production traits in egg layers. Poult. Sci. 81: 919–927. doi: 10.1093/ps/81.7.919 [DOI] [PubMed] [Google Scholar]
- 43.Venturini G. C., Savegnago R. P., Nunes B. N., Ledur M. C., Schmidt G. S., El Faro L., Munari D. P.2013. Genetic parameters and principal component analysis for egg production from White Leghorn hens. Poult. Sci. 92: 2283–2289. doi: 10.3382/ps.2013-03123 [DOI] [PubMed] [Google Scholar]
- 44.Wu Y., Pan A. L., Pi J. S., Pu Y. J., Du J. P., Liang Z. H., Shen J.2015. SNP analysis reveals estrogen receptor 1 (ESR1) gene variants associated with laying traits in quails. Arch. Tierzucht 58: 441–444. [Google Scholar]
- 45.Yan Z., Tan W., Dan Y., Zhao W., Deng C., Wang Y., Deng G.2012. Estrogen receptor alpha gene polymorphisms and risk of HBV-related acute liver failure in the Chinese population. BMC Med. Genet. 13: 49. doi: 10.1186/1471-2350-13-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeh F. C., Yang R. C., Boyle T. B. J., Ye Z. H., Mao J. X.1997. POPGENE, the User-friendly Shareware for Population Genetic Analysis. Molecular Biology & Biotechnology Centre, University of Alberta, Edmonton. [Google Scholar]
- 47.Yuan J., Sun C., Dou T., Yi G., Qu L., Qu L., Wang K., Yang N.2015. Identification of promising mutants associated with egg production traits revealed by genome-wide association study. PLOS ONE 10: e0140615. doi: 10.1371/journal.pone.0140615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L., Li D. Y., Liu Y. P., Wang Y., Zhao X. L., Zhu Q.2012. Genetic effect of the prolactin receptor gene on egg production traits in chickens. Genet. Mol. Res. 11: 4307–4315. doi: 10.4238/2012.October.2.1 [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q. X., Borg A., Wolf D. M., Oesterreich S., Fuqua S. A.1997. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 57: 1244–1249. [PubMed] [Google Scholar]
- 50.Zhou J., Yu D. B., Hong K. Y., Wang F., Du W. X.2008. Single nucleotide polymorphisms in FSHβ and ESRα genes of Blue Egg-shell chicken and association with early egg production. Acta Agriculture Boreali-Sinica 5: 49–52 (in Chinese). [Google Scholar]