Abstract
In this study, the virucidal effect of a novel electrically charged disinfectant CAC-717 was investigated. CAC-717 is produced by applying an electric field to mineral water containing calcium hydrogen carbonate to generate mesoscopic crystals. Virus titration analysis showed a >3 log reduction of influenza A viruses after treatment with CAC-717 for 1 min in room temperature, while infectivity was undetectable after 15 min treatment. Adding bovine serum albumin to CAC-717 solution did not affect the disinfectant effect. Although CAC-717 is an alkaline solution (pH=12.39), upon contact with human tissue, its pH becomes almost physiological (pH 8.84) after accelerated electric discharge, which enables its use against influenza viruses. Therefore, CAC-717 may be used as a preventative measure against influenza A viruses and for biosecurity in the environment.
Keywords: antisepsis, biosecurity, calcium hydrogen carbonate, influenza virus, public health
Influenza viruses are members of the family Orthomyxoviridae. Human influenza viruses A, B and C have been recognized and are classified based on their type-specific nucleoprotein and matrix protein antigens. Type A influenza viruses are further classified into subtypes according to the antigenic properties of the hemagglutinin and neuramidase glycoproteins expressed on the surface of the virus. Because influenza viruses have segmented genomes, reassortment is a powerful method for generating genetic diversity that could facilitate interspecies transmission or the evasion of the host immune responses through an antigenic shift. Reassortment occurs when two influenza viruses infect the same cell, and pont mutation occurs during the replication within cells; both mechanisms are important for the appearance of pandemics in the human population. The virus strains responsible for the influenza pandemics of 1957 and 1968 both arose from the reassortment of genes between avian influenza viruses (AIV) and a prevailing human influenza strain [15, 16].
Disinfectants, such as hypochlorite, alkalis, oxidizing agents, alcohols and aldehyde, are all effective against AIV for a relatively short contact period [7]. However, the presence of organic materials in the liquids or the application area attenuates their disinfection [13, 14]. Control of avian influenza is extremely difficult owing to its high contagiousness. The best way to combat these infections is to enhance biosecurity. Wild migratory birds and various AIV subtypes without antigenic stability are problematic for poultry immunization [2, 5, 17]. Enhancing biosecurity at farms is important to control infectious diseases.
Slaked lime, the main component of which is calcium hydroxide, is widely used at farms in Japan. However, slaked lime can cause injury or blindness, if it comes into contact with human or animal eyes [9]. In this report, electrically charged calcium hydrogen carbonate powder was used to sterilize influenza A viruses in media. Ponrouch et al. demonstrated the feasibility of calcium plating at moderate temperature using conventional electrolytes, such as those used for lithium ion technology to produce rechargeable batteries [12]. The calcium hydrogen carbonate mesoscopic structure can serve as a battery in rechargeable electrical systems [12].
We continuously applied an electric field to mineral water containing calcium hydrogen carbonate and obtained the new electrically charged material, CAC-717. A Teflon insulation-coated electrostatic field electrode (N-800N, Mineral Activation Technical Research Center, Omuta, Japan; Japan Patent No. 5864010) was used to create the electric field, and a voltage of 2 × 104 V was used for 48 hr. CAC-717 solution in distilled water (Japan Patent No. 5778328) shows a pH of 12.39 ± 0.03 and contains calcium hydrogen carbonate particles (1,120 mg/l) and carbon complex microparticles (50–500 nm) with a mesoscopic structure.
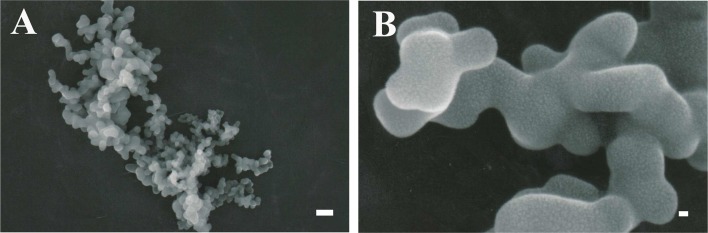
CAC-717 solution was sprayed onto glass on a hot plate and was dried for 1 min at 100°C. The dried glass was removed from the hot plate. For the scanning electron microscope observations, CAC-717 powder was coated with a thin layer of platinum. The samples were examined with a scanning electron microscope (JSM-7500, JEOL, Tokyo, Japan) [18]. Scanning electron microscope examination of samples revealed a spheroid mesoscopic structure (Fig. 1A and 1B). However, these tiny structures were not observed in non-electrically charged solutions of calcium hydrogen carbonate.
Fig. 1.
Mesoscopic structure of calcium hydrogen carbonate. (A) Scanning electron microscope image of the mesoscopic structure of calcium hydrogen carbonate. Spheroid structures are created after high-voltage treatment of aqueous solutions. Bar=100 nm. (B) Higher-magnification scanning electron microscope image of the mesoscopic structure of calcium hydrogen carbonate. Bar=10 nm.
Low-pathogenic influenza A viruses, isolated from humans (A/Aichi/2/68, H3N2) [OFL_ISL_236] or swine (A/swine/Wadayama/5/69, H3N2) [OFL_ISL_8147], were propagated in Madin-Darby canine kidney (MDCK) cells, harvested, centrifuged at 740 ×g for 15 min, aliquoted and then stored at −80°C until they were used or titrated. The virus titers before and after treatment with disinfectants were titrated in each cell culture. The virus was titrated in MDCK cells in 24-well tissue culture plates (four wells per dilution, 600 µl final volume in each well) with cell culture medium containing 2.5 µg/ml trypsin (final concentration, trypsin from bovine pancreas 180 TAME units/mg protein, MP Biomedicals, Santa Ana, CA, U.S.A.), and 50% tissue culture infective dose (TCID50) was determined by the method reported by Behrens and Kaber [8]. These experiments were repeated three times. Each virus sample was mixed with 9 times the volume of disinfectant. The mixtures were incubated at room temperature for 1 or 15 min. Following incubation, the specimens were immediately diluted in tissue culture medium, and the virus titers were determined. The virus samples mixed with 9 times the volume of distilled water were used as positive controls. A previously described numerical method was used to express the ability of the agent to inactivate viruses [5]. The virucidal index (VI) of virus inactivation is calculated by NI=tpc–ta, where tpc is the titer converted to an index of log10 of the positive control, and ta is the converted titer of the recovered virus from the disinfectant-treated sample. We defined the inactivation of viruses as effective when VI was higher than 3 [5].
To examine the reduction of virus infectivity, the TCID50 of a mixture of virus suspension and CAC-717 solution was measured. Influenza virus infectivity decreased in mixtures containing CAC-717 (Table 1). CAC-717 solution abolished influenza A viruses infectivity completely within 15 min. Furthermore, adding 10% bovine serum albumin (BSA) to CAC-717 solution did not affect the disinfectant effect on influenza A viruses (Table 1).
Table 1. Virus titer of samples after CAC-717 solution treatment.
| Sample Influenza virus | CAC-717 treatment | 10% BSA in CAC-717 solution | Treatment period |
|
|---|---|---|---|---|
| 1 min | 15 mim | |||
| A/Aichi/2/68 | - | - | 6.42a) | 6.58 |
| + | - | ≤2.25 | ≤1.50 | |
| - | + | 6.17 | 6.08 | |
| + | + | ≤2.25 | ≤1.50 | |
| A/Swine/Wadayama/5/69 | - | - | 5.5 | 5.83 |
| + | - | ≤1.67 | ≤1.50 | |
| - | + | 5.58 | 5.67 | |
| + | + | ≤1.83 | ≤1.50 | |
a) log10 TCID50 titer. The limit of the detection system was <1.50.
Human skin pH was measured with a skin pH meter (pH905, Courage Khazaka GmbH, Cologne, Germany) [1]. The pH measurement for CAC-717 solution was tested in Petri dishes. The pH measurement on skin was performed by placing the solution (0.5 ml) on a hand [1]. Initially, CAC-717 solution in dishes had a pH of 12.39 ± 0.03 (n=10) tested by skin pH meter. CAC-171 solution (0.5 ml) was placed on the skin of a human hand. After 1 min, the pH on the skin surface was measured with the skin pH meter. The pH reading on the skin changed to 8.84 ± 1.17 (n=10) (Table 2). The reading on the skin surface was decreased significantly in 1 min (P<0.005). There was no harmful effect on human skin after the experiment.
Table 2. pH of CAC-717 solution in the original Petri dishes or on human skin.
| Sample | Incubation period |
|
|---|---|---|
| Positive control | 1 min | |
| CAC-717 | 12.39 ± 0.03a) | 8.84 ± 1.17 |
a) Standard deviation of the mean. Ten samples each were tested on different areas of skin on a human hand.
In a recent report, egg CaO powder inactivated AIV even in the presence of organic materials (33% fetal bovine serum) within 3 min [10]. AIV becomes inactivated relatively easy, because its lipid envelope increases its sensitivity to hydration, detergents and surfactants [5]. It was reported that AIV (H7N2) lost 100% of its infectivity when exposed to pH 2 for 5 min, but exposure to pH 12 for 15 min had no effect on infectivity [6]. Another report showed that exposure to pH 12.3 did not inactivate AIV, whereas exposure to pH 13.0 did [19]. As shown in Table 1, CAC-717 solution inactivated the virus within 15 min.
Our results in rabbit eye toxicity test using OECD Guideline for Testing Chemicals No. 405: Acute Eye Irritation/Corrosion under the animal welfare requirements (ISO 10993-2, July 2, 2006) did not indicate any harmful effects. Three female Japanese White domestic rabbits were used to evaluate irritation caused by CAC-717 solution applied to the eyes without washing. After applying the stock solution (0.1 ml) to the conjunctival sac under the right lower eyelid, both eyelids were closed for approximately 1 sec to disperse the solution, and the animals were released for observation. Acute ocular irritation was scored 1, 3, 6, 24, 48, 72 and 96 hr after the sample application in accordance with the Draize criteria. The results revealed no eye irritation at any time, with the maximum acute ocular irritation index being 0. During the study period, the general physical conditions of the rabbits were unremarkable, with steady weight gain. Based on these findings, CAC-717 solution was determined to be a non-irritant (class 0). A rabbit skin toxicity test using the Ministry of Health, Labor and Welfare Guideline, Biological Evaluation of Medical Devices- Part 10: Test for irritation and skin sensitization (ISO 10993-10, August 1, 2010) under the same animal welfare requirement (ISO 10993-2, July 15, 2006) did not show any harmful effects. Healthy and injured skin areas were established on the backs of three female Japanese White domestic rabbits to investigate primary skin irritation caused by CAC-717 solution. A 2.5 × 2.5 cm lint dressing cloth containing 0.5 ml of the CAC-717 solution was applied to the skin as an occlusive patch and secured using non-permeable surgical tape, foam surgical tape and an elastic adhesive bandage. The skin patch was applied for 24 hr, and primary skin irritation was evaluated 3, 24 and 48 hr after removal of the patch. Distilled water was used as a control. The evaluation revealed no skin reactions at any time point, with a primary skin irritation index of 0. During the study period, the general physical condition of the rabbits was unremarkable, and their weight increased steadily. Based on these findings, the test substance was determined not to be a skin irritant. These results suggested that CAC-717 solution has good efficacy for inactivating influenza A viruses, requiring only brief contact with pathogens without damaging human or animal tissue.
The World Health Organization recommends that wiping surfaces with a 1:100 chlorine solution (known commonly as Chlorox and Eau-de-Javel) kills influenza A viruses [21]. Then, all adsorbent materials must be incinerated in heavy-duty garbage bags. The surface must be rinsed with clean water after disinfection. The main way of disinfecting hands is by washing with soap and water. Items, such as instruments used for autopsies, should be disinfected with 1:100 chlorine solution or 70% ethanol [21]. The use of strong chlorine solutions (such as 1:100 chlorine solution) is dangerous and should be avoided. The use of disinfectants registered by the U.S. Environmental Protection Agency (EPA) is also recommended. Lists of all registered disinfectants can be found at the EPA website [20]. There are approximately 400 registered disinfectants with human influenza A and/or B listed on the product label, and all will inactivate influenza viruses when used according to manufacturer’s instructions.
Our present report shows that freshly electrolyzed water may contain electrons and hydrogen oxide, together with calcium hydrogen carbonate particles. The reversible redox process observed in electrolytes containing calcium could be effective for electrolysis [12].
Another study shows that acidic electrolytic water inactivates prions completely and alkaline electrolytic water partially inactivates prions (human prion strain: Fukuoka-1) [3]. The research suggested that alkaline and acidic electrolytic water could prevent prions from propagation and amyloid aggregation. However, the mechanisms enabling this prion-inactivation effect are not well understood, although it has been suggested that free hydrogen ion or alkaline ion systems are responsible for this inactivation [11].
Even in CAC-717 solution, it is difficult to explain the disinfection as being primarily due to the alkaline effect of calcium hydrogen carbonate ions. The effects are probably caused by the free alkaline ions and by other factors present in the electrolyzed solutions, such as far-infrared rays (FIR) from mesoscopic structures, functioning as small ‘nano-batteries’. Enhanced levels of FIR were detected on mesoscopic particles under FIR imaging cameras (Furusaki et al., unpublished observations). FIR destroy nucleic acids in various microorganisms [4]. Ongoing experiments show that CAC-717 solution destroys influenza A viral RNA, detected by real-time PCR (Kirisawa et al., unpublished observations).
Cleaning and disinfection plans are a critical part of minimizing the spread of influenza A viruses during an outbreak. An effective plan should outline what should be cleaned, the frequency of cleaning, the materials and techniques, and the training of janitorial staff. The use of proper materials and techniques and clear cleaning plans will support a facility’s pandemic response. The effect of CAC-717 solution on other viruses, bacteria and microorganisms needs to be investigated to apply the technology to a wide range of areas.
DISCLOSURE
All authors declare that they have no conflicts of interest.
Acknowledgments
We thank Mr. Hikaru Natsunuma and Dr. Makoto Haritani for their help in part of this study.
REFERENCES
- 1.Ehlers C., Ivens U. I., Møller M. L., Senderovitz T., Serup J.2001. Comparison of two pH meters used for skin surface pH measurement: the pH meter ‘pH900’ from Courage & Khazaka versus the pH meter ‘1140’ from Mettler Toledo. Skin Res. Technol. 7: 84–89. doi: 10.1034/j.1600-0846.2001.70205.x [DOI] [PubMed] [Google Scholar]
- 2.Gilbert M., Xiao X., Domenech J., Lubroth J., Martin V., Slingenbergh J.2006. Anatidae migration in the western Palearctic and spread of highly pathogenic avian influenza H5NI virus. Emerg. Infect. Dis. 12: 1650–1656. doi: 10.3201/eid1211.060223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamada M.2014. Washing and sterilization method, WO2014129579A1, https://www.google.com/patents/WO2014129579A1 (in Japanese) [accessed January 20, 2016].
- 4.Kawagoe N., Oyokota S., Kikuchi Y., Takesato K.1998. Sterilizer, US5714119A, https://www.google.com/patents/US5714119 (in Japanese) [accessed January 20, 2016].
- 5.Lombardi M. E., Ladman B. S., Alphin R. L., Benson E. R.2008. Inactivation of avian influenza virus using common detergents and chemicals. Avian Dis. 52: 118–123. doi: 10.1637/8055-070907-Reg [DOI] [PubMed] [Google Scholar]
- 6.Lu H., Castro A. E., Pennick K., Liu J., Yang Q., Dunn P., Weinstock D., Henzler D.2003. Survival of avian influenza virus H7N2 in SPF chickens and their environments. Avian Dis. 47Suppl: 1015–1021. doi: 10.1637/0005-2086-47.s3.1015 [DOI] [PubMed] [Google Scholar]
- 7.Maillard J. Y., Russell A. D.1997. Viricidal activity and mechanisms of action of biocides. Sci. Prog. 80: 287–315. [PubMed] [Google Scholar]
- 8.Matumoto M.1949. A note on some points of calculation method of LD50 by Reed and Muench. Jpn. J. Exp. Med. 20: 175–179. [PubMed] [Google Scholar]
- 9.Ministry of Education Culture, Sports, Science and Technology. “Handling of the lime to use for the lines of the athletic ground”, http://www.gankaikai.or.jp/info/pdf/20080101_monbu.pdf (in Japanese) [accessed January 20, 2016].
- 10.Ota M., Toyofuku C., Thammakarn C., Sangsriratanakul N., Yamada M., Nakajima K., Kitazawa M., Hakim H., Alam M. S., Shoham D., Takehara K.2016. Calcinated egg shell as a candidate of biosecurity enhancement material. J. Vet. Med. Sci. 78: 831–836. doi: 10.1292/jvms.16-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orikasa Y., Masese T., Koyama Y., Mori T., Hattori M., Yamamoto K., Okado T., Huang Z. D., Minato T., Tassel C., Kim J., Kobayashi Y., Abe T., Kageyama H., Uchimoto Y.2014. High energy density rechargeable magnesium battery using earth-abundant and non-toxic elements. Sci. Rep. 4: 5622. doi: 10.1038/srep05622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponrouch A., Frontera C., Bardé F., Palacín M. R.2016. Towards a calcium-based rechargeable battery. Nat. Mater. 15: 169–172. doi: 10.1038/nmat4462 [DOI] [PubMed] [Google Scholar]
- 13.Quinn P. J., Markey B. K.1992. Disinfection and disease prevention in veterinary medicine. pp. 1069–1104. In: Disinfection, Sterilization, and Preservation. 5th ed. (Block, S.S. ed.) Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- 14.Sattar A. S., Springthorpe S.1999. Factors influencing the efficacy of antimicrobial agents. pp. 109–138. In: Principles and Practice of Disinfection, Preservation, and Sterilization, 3rd ed. (Russell, A.D., Hugo, W.B. and Ayliffe, G.A.J. eds.), Blackwell Science, Oxford. [Google Scholar]
- 15.Scholtissek C., Rohde W., Von Hoyningen V., Rott R.1978. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 87: 13–20. doi: 10.1016/0042-6822(78)90153-8 [DOI] [PubMed] [Google Scholar]
- 16.Stallknecht D. E., Shane S. M.1988. Host range of avian influenza virus in free-living birds. Vet. Res. Commun. 12: 125–141. doi: 10.1007/BF00362792 [DOI] [PubMed] [Google Scholar]
- 17.Suarez D. L.2012. DIVA vaccination strategies for avian influenza virus. Avian Dis. 56Suppl: 836–844. doi: 10.1637/10207-041512-Review.1 [DOI] [PubMed] [Google Scholar]
- 18.Sueyoshi M., Nakazawa M.1994. Experimental infection of young chicks with attaching and effacing Escherichia coli. Infect. Immun. 62: 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thammakarn C., Satoh K., Suguro A., Hakim H., Ruenphet S., Takehara K.2014. Inactivation of avian influenza virus, newcastle disease virus and goose parvovirus using solution of nano-sized scallop shell powder. J. Vet. Med. Sci. 76: 1277–1280. doi: 10.1292/jvms.14-0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US CDC. Interim Guidance on Environmental Management of Pandemic Influenza Virus: https://www.pandemicflu.gov/professional/hospital/influenzaguidance.html (accessed January 8, 2013).
- 21.WHO. 2006. http://www.who.int/csr/resourcse/publication/surveillance/CDS_EPR_ARO_2006_1.pdf?ud=1 (accessed April 20, 2017).