Significance
Recently, small interfering RNAs have been used to specifically target point-mutated KRAS, yet without sufficiently discriminating its wild-type counterpart. Here, we describe an innovative approach based on the development of artificial microRNAs able to efficiently target mutated KRAS, leaving their normal counterpart unaffected and preventing major side effects.
Keywords: artificial microRNA, KRAS, RNAi
Abstract
Mutated protein-coding genes drive the molecular pathogenesis of many diseases, including cancer. Specifically, mutated KRAS is a documented driver for malignant transformation, occurring early during the pathogenesis of cancers such as lung and pancreatic adenocarcinomas. Therapeutically, the indiscriminate targeting of wild-type and point-mutated transcripts represents an important limitation. Here, we leveraged on the design of miRNA-like artificial molecules (amiRNAs) to specifically target point-mutated genes, such as KRAS, without affecting their wild-type counterparts. Compared with an siRNA-like approach, the requirement of perfect complementarity of the microRNA seed region to a given target sequence in the microRNA/target model has proven to be a more efficient strategy, accomplishing the selective targeting of point-mutated KRAS in vitro and in vivo.
Single point mutations, categorized according to their ability to affect the structure and function of the translated protein, are involved in numerous diseases, including cancer (1). Specifically, Kras, a GTPase protein, is frequently mutated in codon 12 of its coding sequence (CDS) in large fractions of specific human cancer types (2, 3). For example, the G12S mutation, in which a single nucleotide modification leads to the substitution of a glycine (Gly) for a serine (Ser), confers constitutive activation of KRAS, inducing cell proliferation (4–6). Additionally, the G12S mutation induces resistance to all drugs targeting upstream of any pathway in which KRAS is involved (i.e., gefitinib) (7). Several attempts to target KRAS-mutant cancers have used small noncoding RNA (sncRNA), in particular, small-interfering RNA (siRNA) targeting KRAS pathways (8). Given the intrinsic limitations of siRNA targeting, there are not effective siRNA-based strategies to specifically target KRAS mutations without affecting KRAS WT (9), and such indiscriminate targeting is likely to be toxic (10–12). Alternatively, use of microRNAs (miRNAs), a class of endogenous 22- to 24-bp-long sncRNAs involved in multiple cellular processes, pathways, and diseases, including cancer (13), could provide a more tailored targeted therapy design. Generally, miRNAs target the 3′ untranslated region (UTR) of mRNA transcripts, despite having also been proven to bind the CDS, as well as the 5′ UTR (14) like siRNAs, causing translation inhibition and/or inducing mRNA degradation (15). Nevertheless, unlike siRNAs, miRNAs exhibit only partial complementarity to the binding sequence on their targets. The efficacy of the interaction in this partial binding is based on a 6- to 8-nucleotide (nt)-long region on the 5′ end of the miRNA sequence termed the “seed” sequence. A single mismatch in the seed region (specifically in positions 2–7) can dramatically compromise the interaction between an miRNA and its target (16, 17). Furthermore, the common presence of a central bulge in the binding interaction supports the importance of a perfect seed match to assure the stability of the duplex (18). Leveraging these important traits, we designed a set of artificial miRNAs (amiRs) (19) with seed regions that perfectly match an 8-nt sequence encompassing the single point-mutation in the CDS of the KRAS (G12S and G12D), as well as BRAF (V600D) mRNAs, while imperfectly matching KRAS WT and BRAF WT transcripts. We have developed an miRNA-based methodology for the selective targeting of single point-mutated gene transcripts. Here, we demonstrate the impact of an amiR on pathways affected by KRAS G12S in non-small-cell lung cancer (NSCLC), while also investigating the possibility of off-target biological effects.
Results
Design of amiRs for Selective mRNA Targeting.
To generate a set of artificial RNA-based molecules for selective targeting of specific point-mutated KRAS mRNAs, while sparing WT counterparts, we elected to use an amiR-based design, rather than an siRNA-based one, leveraging the innate miRNA seed-mediated target recognition (16, 17). We had reported the effectiveness of amiRs for gene silencing (19). The rationale for an amiR-based approach stems from the ability of this type of molecule to better discriminate between two targets that differ only by a single base in its seed target region (SI Appendix, Fig. S1A), as opposed to the use of siRNAs, whose function may not be greatly affected by a single mismatch (SI Appendix, Fig. S1B). We thus decided to test our method by designing a set of 22-bp-long amiRs to target mutated KRAS, one of the best-investigated oncogenes in human cancer. We equipped our amiRs with seed sequences perfectly matching the region of KRAS mRNA, including the G-to-A point mutation (14) at codon 12 (G12S) and, as a consequence, mismatching for the same region in WT KRAS. Specifically, the amiRs were designed such that the seed mismatch on KRAS WT would fall on different seed bases, between and including nucleotide positions 2 and 7. The remaining portions of the amiRs were designed to favor a binding site with the KRAS target characterized by both a central bulge preceded by the seed region as well as a single 3′ supplementary site (20) that confers a greater specificity (21) (Fig. 1A). The WT and mutated (G12S) KRAS portions of the coding region were, respectively, cloned into a PGL3 control reporter vector and tested for luciferase activity, after cotransfection with the amiR-KS set. Each of the designed amiRs was superior in suppressing KRAS G12S expression vs. KRAS WT (Fig. 1B), thus showing that seed-driven design can discriminate between mutant and WT, with the exact position of the mismatch within the seed region as almost irrelevant for targeting efficiency.
Fig. 1.
amiRs specifically targeting KRAS G12S. (A) Representation of the amiR KS set designed for KRAS G12S targeting (six sequences) and corresponding match with the KRAS binding site in the CDS including the G12S mutation (Left) and the WT version (Right), respectively. (B) Graph of luciferase activity percentage for amiR KS set sequences cotransfected with the KRAS WT (green) and KRAS G12S (orange) CDS portions, respectively, cloned downstream of the luciferase reporter gene and including the corresponding amiR KS binding site. (C) Representation of amiR- and siRNA-KS3 matching the same binding site on the KRAS WT and G12S CDS, respectively. (D) Graph of luciferase activity percentage for amiR- and siRNA-KS3 sequences cotransfected with the KRAS WT (green) and KRAS G12S (orange) CDS portions, respectively. (E–G) Chromatogram of sequenced KRAS endogenously expressed in H1299 and H292 cell lines (blue dot) and A549 cell line (orange dot), respectively, all compared with the WT KRAS reference sequence (green dot) (Left); Western blots show the expression of endogenous KRAS in all cell lines after the transfection of amiR- and siRNA-KS3, respectively (Right). *P < 0.05; **P < 0.01; ***P < 0.001. Scr, scramble; seq., sequence.
Among the amiRs from the KRAS G12S (KS) set, we selected amiR-KS3 for its strong ability to most significantly repress KRAS G12S, while not affecting KRAS WT expression (Fig. 1B and SI Appendix, Fig. S2A). To prove the hypothesis that only a molecule with miRNA characteristics (seed region followed by central bulge) can discriminate the two forms of KRAS (point-mutated and WT), we created an siRNA with a sequence identical to amiR-KS3, except for the bulge, thus displaying perfect target complementarity, in compliance with siRNA structure and function (22) (Fig. 1C). siRNA- and amiR-KS3 were, respectively, tested on KRAS WT and KRAS G12S by luciferase assay to verify their effects on the two distinct KRAS forms. The siRNA-KS3 could not discriminate between KRAS WT and KRAS G12S, causing strong repression of both. On the contrary, amiR-KS3 was confirmed to selectively target KRAS G12S (Fig. 1D).
We then tested the effect of amiR-KS3 in both KRAS WT and KRAS mutated cancer cell lines. For this purpose, we used H1299 and H292 NSCLC cell lines that express KRAS WT, as well as A549, a NSCLC cell line that homozygously expresses endogenous KRAS G12S (Fig. 1 E–G, Left). amiR- and siRNA-KS3 were, respectively, transfected in all three cell lines, and KRAS levels were assessed by Western blot. Our results show that amiR-KS3 targeted the mutant form of KRAS, but not its WT, as opposed to the siRNA-KS3, which did not distinguish between the two KRAS versions (Fig. 1 E–G, Right). The effect of the amiR-KS set on endogenous KRAS G12S and WT mRNA expression on H1299 and A549 cell lines was further validated by RT-PCR, and amiR-KS set expression was confirmed by using custom probes (SI Appendix, Fig. S2 B–E). The vast majority of the KS-set amiRs suppressed KRAS G12S in A549, while not down-regulating KRAS WT in H1299.
To further test the efficacy of our approach, we designed two additional sets of amiRs, an amiR-KD set for the KRAS G-to-A point mutation in codon 12 (G12D) and an amiR-VD set for the BRAF GU-to-UA double mutation in codon 600 (V600D), comprising 6 and 5 amiR sequences, respectively, targeting mutated versions of KRAS or BRAF mRNAs, and evaluated their efficacy by luciferase assays, as described for KRAS G12S. The sequences of these amiRs and their binding sites on the target sequences are shown in SI Appendix, Fig. S3A for KRAS G12D and in SI Appendix, Fig. S4A for BRAF V600D. We performed a luciferase assay using reporter plasmids (KRAS WT and G12D and BRAF WT and BRAF V600D coding regions, respectively) cotransfected with each amiR from each set. As shown in SI Appendix, Fig. S3B and S4B, the amiRs designed to target KRAS G12D and BRAF V600D, respectively, repressed the luciferase activity of the mutated versions of KRAS and BRAF better than they repressed the respective WT versions. We also validated the KD set of amiRs by transfecting each amiR into pancreatic carcinoma cell lines BX-PC3 (expressing KRAS WT) and PC1 (expressing only KRAS G12D-mutant) and tested for endogenous Kras expression levels by Western blot (SI Appendix, Fig. S3C). Similarly, we transfected the amiR-VD set into melanoma cell lines SK-Mel2 (expressing BRAF WT) and WM266 (expressing only BRAF V600D mutant) and verified the endogenous Braf expression levels (SI Appendix, Fig. S4C). In both cases, a number of the designed amiRs were able to effectively down-regulate the point-mutated versions of the Kras and Braf endogenous proteins, while not down-regulating their WT counterparts.
amiR-KS3 Inhibits KRAS G12S Activity.
The mutant forms of KRAS drive the induction of the cell cycle by activating the ERK and PI3K pathways (11, 23, 24). We first evaluated the effects of amiR-KS3 in the A549/KRAS G12S cell line by comparing the expression of 729 genes of the nCounter PanCancer Pathways Panel, in biological triplicates, with effects of a commercial KRAS siRNA (SI Appendix, Fig. S5 A–C). KRAS was significantly down-regulated in both cases. We performed a functional enrichment analysis of the significantly deregulated genes by using the Ingenuity Pathway Analysis (IPA) software and focused our attention on the ERK/MAPK signaling and cell-cycle G1/S checkpoint regulation pathways, strictly linked to the KRAS pathway activity in lung cancer (24, 25). Our results show how both molecules (siRNA-KRAS and amiR-KS3) were significantly involved in these two pathways (SI Appendix, Fig. S5D).
We elected to validate our enrichment predictions by transfecting amiR- and siRNA-KS3, respectively, evaluating the phosphorylation levels of Erk1/2 and Akt in the A549, H292, and H1299 NSCLC cell lines. In Fig. 2A, we show that only amiR-KS3 resulted in down-regulation of the KRAS G12S effect on its downstream effectors, whereas the siRNA-KS3 acted indiscriminately on KRAS WT (H1299 and H292) and KRAS G12S (A549). Additionally, siRNA KS3 indiscriminately reduced cell viability in KRAS WT and KRAS mutant-expressing cell lines (SI Appendix, Fig. S6A).
Fig. 2.
amiR-KS3 specifically reduces the proliferation of the A549 G12S mutated cell line. (A) H1299 and H292 expressing KRAS WT and A549 expressing KRAS G12S transfected with amiR- and siRNA-KS3, respectively; phospho-AKT and -Erk1/2 levels detected by Western blot in all three cell lines, with total AKT and total ERK1/2 measured by Western blot as expression control. (B) Bar graphs of cell-density growth assays (Lower) measured after 48 and 72 h from transfection of amiR-KS3 and scramble control in H1299 and H292 KRAS WT, as well as in A549 KRAS G12S cell lines. (B, Upper) Representative image of the cell density assays for each cell line. (C) Cell viability assay after 48 and 72 h from transfection of amiR-KS3 and scramble control in H1299 and H292 KRAS WT, as well as in A549 KRAS G12S cell lines. (D) Graph of cellular growth assay after 48, 72, and 96 h from transfection of amiR-KS3 and scramble control in H1299 and H292 KRAS WT, as well as in A549 KRAS G12S cell lines. (E) Flow cytometry analysis using propidium iodide (PI) after 48 h from transfection of amiR-KS3 and scramble control in H1299 and H292 KRAS WT, as well as in A549 KRAS G12S cell lines. *P < 0.05; **P < 0.01; ***P < 0.001. Scr, scramble.
The H1299, H292, and A549 (KRAS G12S) cell lines were also transfected with amiR-KS3 and evaluated for cell growth density (Fig. 2B), cell viability (Fig. 2C), and cell proliferation (Fig. 2D) time course assays. We showed that amiR-KS3 caused reduction of all three functions only in KRAS-mutated A549 cells. Finally, we performed a cell-cycle study demonstrating that amiR-KS3 maintained KRAS-mutant cells in the G1/G0 phase (Fig. 2E and SI Appendix, Fig. S6B). The results thus show the ability of amiR-KS3 to affect the cell cycle only in A549 cells, whereas no significant effect was recorded in the H1299 and H292 KRAS WT cell lines.
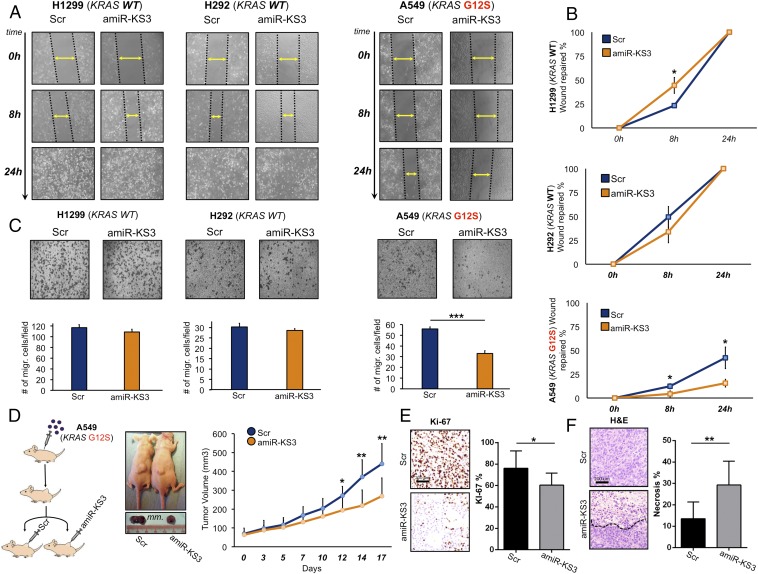
Additionally, several studies have shown that mutated KRAS induces tumor cell migration through the activation of the MAPKs and PI3K/AKT pathways (26, 27). We assessed the effects of amiR-KS3 on cell migration in both KRAS WT and mutated NSCLC cell lines. As shown in Fig. 3 A and B by wound healing assay, amiR-KS3 led to reduced migration of A549 (KRAS G12S), whereas no effect was noted in H1299 and H292 (KRAS WT). We also conducted a transwell migration assay in all three cell lines to reinforce our results (Fig. 3C).
Fig. 3.
amiR-KS3 effects on A549 KRAS G12S mutated cell line migration, as well as in vivo proliferation and necrosis. (A) “Wound healing” migration assays measured after 8 and 24 h from transfection of amiR-KS3 and scramble control in H1299, H292, and A549 cell lines. (B) Wound repair percentage graphs relative to A. (C) Bar graphs (Lower) of transwell migration assays (Upper) measured after 24 h from transfection of amiR-KS3 and scramble control in in H1299, H292, and A549 cell lines. (D, Left) Mouse xenograft A549 injection protocol. (D, Center) Tumor sizes for two representative mice, respectively picked from the scramble and amiR-KS3 groups at the end of a 17-d treatment. (D, Right) Tumor volume increase graph after 17 d of treatment for two groups of 10 mice each (5 male and 5 female), scramble and amiR-KS3 intratumoral-injected, respectively. (E and F) Percentage of Ki-67 proliferation marker-positive tumor cells (E) and graph representing the mean percentage of necrotic areas on the whole tumor area (F), for xenograft mice tumors after 17 d of scramble and amiR-KS3 treatment, respectively (Right). (E and F, Left) Representative image of Ki-67 expression (immunohistochemistry) (E) and of H&E stain (F) in scramble and amiR-KS3–treated xenograft tumors. The necrosis border is represented by a black dotted line. *P < 0.05; **P < 0.01; ***P < 0.001. Migr., migrated; Scr, scramble.
Finally, we tested amiR-KS3 effects in vivo. Through intratumoral injection of A549 (KRAS G12S) cells, we induced overexpression of amiR-KS3, which resulted in marked inhibition of tumor growth and cell proliferation, as assessed by the Ki-67 proliferative index, starting at 12 d of treatment (Fig. 3 D and E). A significant enhanced tumor necrosis was also noted in the amiR-KS3–treated group (Fig. 3F). We confirmed the levels of amiR-KS3 in the xenograft tumors by quantitative RT-PCR (RT-qPCR) at day 17 (SI Appendix, Fig. S7A). Together, our results clearly show that our amiR design strategy allows development of molecules to effectively target KRAS G12S, perturbing its biological effects in vitro and in vivo, without significantly affecting KRAS WT.
amiR-KS3 Induces Gefitinib Sensitivity.
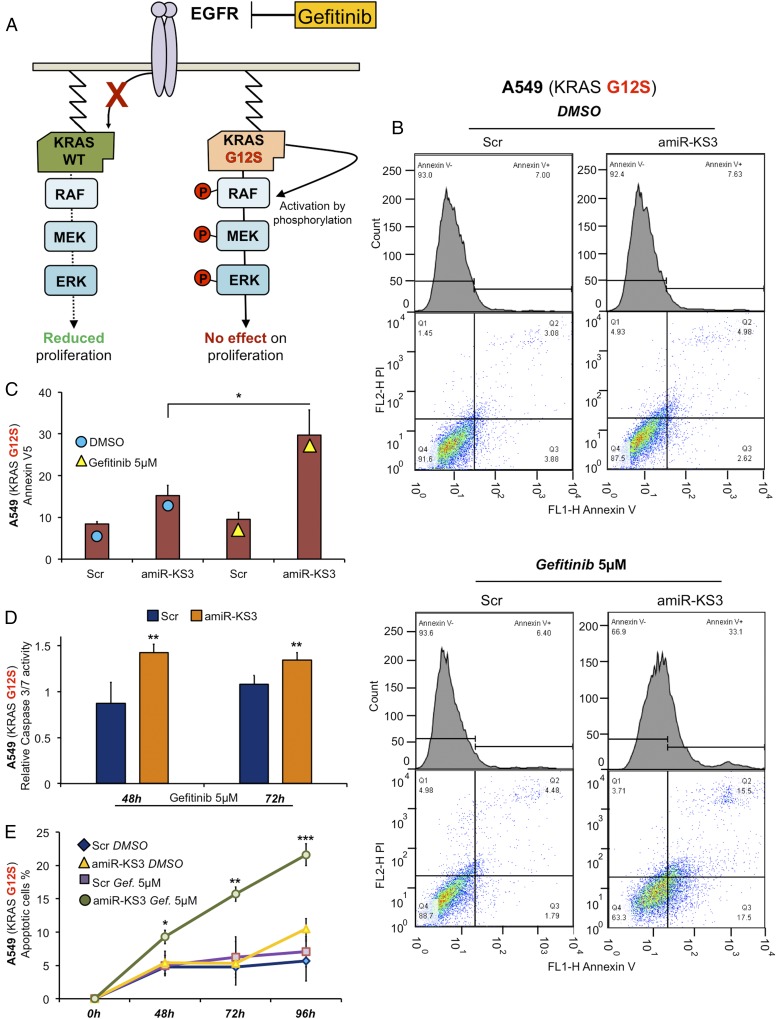
Gefitinib acts on the EGF receptor by inhibiting its pathway activation, thereby reducing proliferation and increasing apoptosis (28, 29). A549 cells are gefitinib-resistant because of the KRAS G12S mutation (30–32) (Fig. 4A). We thus tested the ability of amiR-KS3 to sensitize A549 cells to gefitinib treatment. We overexpressed amiR-KS3 in A549 cells and assessed the percentage of apoptotic cells through the Annexin V-5 assay. As shown in Fig. 4B, A549 cells treated with amiR-KS3 and gefitinib (5 μM) showed an increased apoptotic fraction vs. scrambled control, amiR-KS3/DMSO, or scrambled control/gefitinib (Fig. 4C). We also performed a time course treatment with gefitinib, evaluating apoptosis through caspase 3/7 activity (Fig. 4D) and Trypan Blue assays (Fig. 4E), confirming the Annexin V-5 results. In conclusion, the results confirm the ability of amiR-KS3 to induce gefitinib sensitivity in A549 NSCLC cell lines by targeting KRAS G12S.
Fig. 4.
amiR-KS3 induces gefitinib sensitivity in an A549 KRAS G12S mutated cell. (A) Effects of gefitinib on proliferation when KRAS is WT (green) and G12S mutated (orange). (B) Representative plots of Annexin V5 assay on A549 KRAS G12S transfected with amiR-KS3 and scramble and treated with DMSO (Upper) and gefitinib 5 μM (Lower) for 48 h. (C) Bar graph representing the results shown in B. (D) Caspase 3/7 activity assay after amiR-KS3 and scramble transfection, respectively, and treated with DMSO and gefitinib 5 μM for 48 and 72 h. Each bar is normalized to its own corresponding DMSO-treated. (E) Apoptotic cell percentage of A549 treated with DMSO and gefitinib 5 μM for 48, 72, and 96 h after Trypan Blue cell viability test. Statistical significance for amiR-KS3 with gefitinib (green) was calculated in relation to amiR-KS3 with DMSO (yellow). *P < 0.05; **P < 0.01; ***P < 0.001. Gef., gefitinib; Scr, scramble.
amiR-KS3 Does Not Present Off-Targets.
Evidence shows that endogenous miRNAs possess multiple targets (33). It has also been proven that the presence of one or more perfect matches in 3′ UTR sequences of unintended targets can lead to considerable off-target effects, as with siRNAs when acting as miRNAs (34, 35). Because our artificial RNA molecules were designed to act as endogenous miRNAs, possessing a central bulge, they also display extensive complementarity to the corresponding target sequence, like siRNAs, thus potentially reducing off-targeting. Considering the “hybrid” nature of our molecules, we thus decided to evaluate the targeting specificity of amiR-KS3, estimating its potential off-targeting effects. To accomplish this, we again transfected amiR-KS3 into H1299 and H292 (KRAS WT) cell lines, respectively, to evaluate the expression of 729 genes of the nCounter PanCancer Pathways Panel, in biological triplicates (SI Appendix, Fig. S7B). Because of the general effects of endogenous miRNAs in gene regulation, we only considered the down-regulated transcripts in both experiments, as well as in the previous transfection in A549 cells, obtaining 65 genes in A549, 25 in H1299, and 68 in H292 as potential targets of amiR-KS3. Remarkably, KRAS results down-regulated only in A549 (KRAS G12S) (Fig. 5A). All down-regulated transcripts from all three experiments were then intersected together as shown in Fig. 5B and SI Appendix, Fig. S7C. We then performed a targeting prediction consensus using the miRNA target prediction tools miRanda (36), PITA (37), and miRiam (38) for amiR-KS3 on all 5′ UTR, CDS, and 3′ UTR human transcript sequences, obtaining three distinct sets of putative direct targets (Dataset S1). Each of these three sets was then intersected with each of the three sets of significantly down-regulated genes. As shown in Fig. 5B and SI Appendix, Fig. S7C, only very few transcripts were shared among at least two of the three sets of significantly down-regulated genes. Moreover, only three of these transcripts resulted as putative direct targets (Dataset S2), indicating the absence of a diffused off-targeting phenomena.
Fig. 5.
Assessment of off-target effects for amiR-KS3. (A) Heat maps of down-regulated transcripts detected through the nCounter PanCancer Pathways Panel in H1299, H292 (KRAS WT), and A549 KRAS G12S NSCLC cell lines. (B) Venn diagrams of down-regulated genes after amiR-KS3 transfection in A549, H1299, and H292 cell lines, respectively. (C) Sylamer hypergeometric statistical enrichment landscape plots for three words corresponding to the 6-bp 3′ UTR sequences complementary to the 6-mer amiR-KS3 canonical and two offset seeds (listed according to enrichment, above; or depletion, below; from left to right in each graph), in transfection experiments in A549, H1299, and H292 cell lines, respectively. In each case, the x axis represents the sorted gene list from most down-regulated (C, Left) to most up-regulated (C, Right). The y axis shows the hypergeometric significance for each word at each leading bin. Positive values indicate enrichment [−log10(P value)], and negative values indicate depletion [log10(P value)]. The horizontal red dotted lines in each graph represent E value thresholds (Bonferroni corrected) of 0.01 for both enriched and depleted significances. Scr, scramble.
To more formally evaluate the off-targeting effects in an unbiased manner, we bioinformatically evaluated the statistical enrichment of binding sites for amiR-KS3 across the transcriptome of investigated cell types. We thus used the Sylamer software (39) on the list of transcripts of both panels (Dataset S3), as well as on the same panel previously run for amiR-KS3 transfection in the A549 KRAS G12S cell line. Specifically, Sylamer was used to determine the hypergeometric statistical enrichment of three 6-bp 5′ UTR, CDS, and 3′ UTR sequences complementary to the 6-mer amiR-KS3 canonical, as well as two offset seeds (33), across the three transcript lists, each ranked from most down-regulated to most up-regulated compared with a control scrambled transfection (Fig. 5C and SI Appendix, Fig. S8). Results show that in all three cell lines (in 5′ UTR, CDS, and 3′ UTR), there was no occurrence bias for any of the three 6-mer amiR-KS3 seeds, thus showing no off-target signals from the expression data.
Discussion
The development of molecules to specifically target oncogenes has in recent years shifted to the use of noncoding RNAs, such as siRNA and miRNA (40). Our laboratory has previously developed an approach for the design of amiRs, thus leveraging on their natural targeting for the down-regulation of multiple transcripts of choice in multiple biological pathways (24). In the present work, we provide a design methodology for a class of amiRs able to target a point-mutated transcript of choice, discriminating it from its WT version specifically in the location of the seed interaction within the binding site.
KRAS targeting represents a particularly important opportunity for impacting cancer therapy, given that KRAS mutations are involved in the development of several types of cancers (3). Attempts at targeting point-mutated KRAS have consisted of siRNAs directly targeting this oncogene and associated pathways. However, there are currently no methodologies for the efficient targeting of single point-mutated transcripts, discriminating them from their WT form. To accomplish this goal, we demonstrated that an miRNA-like design is necessary, as opposed to an siRNA-like one, to specifically discriminate the point-mutated form from the WT, allowing such methodology to be applied in the case of other important point-mutated transcripts. Despite our miRNA-like design results being diversely effective compared with an siRNA one, our approach could replace such siRNA-oriented strategies for specific point-mutated gene repression in light of the greater specificity it provides. For this reason, we have designed such molecules in a very specific manner, assuring an exact matching in the seed segment, as well as at the 3′ end of the binding region (as with siRNA), yet with a central bulge. Such an approach further proves the necessity for perfect complementarity of the seed region with the target sequence (as in endogenous miRNAs).
Having tested our amiRs on a mutation of KRAS, G12S, we have identified a best-performing candidate to verify its efficacy in inhibiting the cancerous pathways associated with the G12S mutation. We, thus, demonstrate, in vitro and in vivo, that our design creates molecules that can neutralize specific pathways characterized by point-mutated oncogenes, inhibiting proliferation and migration and inducing necrosis, while also providing proof of effectiveness in the ability to sensitize gefitinib-resistant cell lines to gefitinib.
Additionally, we have analyzed gene expression in NSCLC cell lines transfected with our amiR-KS3 and carrying KRAS WT and G12S, respectively, and, through computational analyses, we have detected no relevant off-target effects.
The main goal of our methodology thus aims for its application to several other point-mutated oncogenes in addition to KRAS, potentially also providing a major advantage to RNA-based therapy for cancer in regard to delivery. In fact, the advantages of our strategy lie in our artificial molecules requiring an efficient, yet nonspecific, delivery, having little to no biological effect on cells expressing only the WT form of the desired target. Considering recent technology developed for the delivery of small RNAs, such as nanoparticles and liposomes (41), our method would provide a strategy that could allow one to compensate for the lack of specificity of the delivery system, through its ability to exclusively affect specific targets expressed only in cancer cells. Furthermore, coupling our strategy with more specific delivery systems (i.e., associating antibodies or aptamers) could even additionally reduce any potential “leakage” of such systems.
We have clearly shown that our methodology for the design of artificial noncoding RNAs can be used to effectively and exclusively target point-mutated KRAS. The application of such a design technique can be extended to other point-mutated oncogenes and to other diseases caused by point-mutation gain of function, providing a major advantage compared with other RNA-based therapies.
Methods
Cell Culture, Transfection, and Chemicals.
All cell lines (H292, A549, H1299, BX-PC3, PC1, SK-Mel2, WM266, and Hek-293) were grown in RPMI with 10% FBS, l-glutamine, and antibiotics (Invitrogen). All of the transfections were performed by using Lipofectamine 3000 (Invitrogen) as suggested by the manufacturer. All cell lines used were transfected at 60% (72 and 96 h experiment) or 80% confluence in 60-mm or 96-well plates with a serum-free medium without antibiotics and then transfected with 100 nmol of artificial-premiR oligonucleotides (Qiagen), custom siRNA KS3 (Qiagen), Kras-siRNA (Santa Cruz), or negative control for 48, 72, or 96 h. pGL3-Control (Promega) Kras-CDS12WT, pGL3-Control Kras-CDS12S (mutated in G12S), pGL3-Control Kras-CDS12D, pGL3-Control B-RAFCDS600WT, and pGL3-Control B-RAFCDS600D (mutated in V600D) were transfected as described in Luciferase Assay.
RNA Extraction.
Total RNA was extracted with TRIzol solution (Invitrogen), according to the manufacturer’s instructions, followed by an RNA extraction kit (NORGEN).
Western Blot Analysis.
H292, A549, H1299, BX-PC3, PC1, SK-Mel2, and WM266 cells were seeded and grown in appropriate medium with 10% FBS in p60 plates for 24 h before the transfection. After transfection, cells were washed with cold PBS and collected for RNA and protein extraction. Cells for protein extraction were subjected to lysis in a lysis buffer (50 mM Tris⋅HCl, 1 mM EDTA, 20 g/L SDS, 5 mM DTT, and 10 mM phenylmethylsulfonyl fluoride), and then equal amounts of protein lysates (50 mg each) and rainbow molecular weight marker (Bio-Rad Laboratories) were separated by 4–20% SDS/PAGE and then electrotransferred to nitrocellulose membranes. The membranes were blocked with a buffer containing 5% nonfat dry milk or BSA (depending on the antibody) in Tris-buffered saline with 0.1% Tween-20 for at least 1 h and incubated overnight with antibodies at 4 °C. After a second wash with Tris-buffered saline with 0.1% Tween 20, the membranes were incubated with peroxidase-conjugated secondary antibodies (GE Healthcare, Amersham) and developed with an enhanced chemiluminescence detection kit (Pierce).
Antibody Used for Western Blot Analysis.
Vinculin (Abcam) was used as a loading control. K-RAS was from Abcam; pAkt pERK1/2, PARP antibodies were from Cell Signaling Technologies (Danvers); BRAF was from Santa Cruz.
Real-Time qRT-PCR.
Real-time PCR was performed by using a standard TaqMan PCR Kit protocol on an Applied Biosystems 7900HT Sequence Detection System (Applied Biosystems). The 10-μL PCR included 0.67 μL of RT product, 1 μL of TaqMan Universal PCR Master Mix (Applied Biosystems), 0.2 mM TaqMan probe, 1.5 mM forward primer, and 0.7 mM reverse primer. The reactions were incubated in a 96-well plate at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. All reactions were run in triplicate. The threshold cycle (Ct) is defined as the fractional cycle number at which the fluorescence passes the fixed threshold. The comparative Ct method for relative quantization of gene expression (Applied Biosystems) was used to determine miRNA expression levels. The y axis represents the 2(″DCt), or the relative expression of different miRs. amiR expression was calculated relative to U44 and Kras relative to GAPDH expression. Experiments were carried out in triplicate for each data point, and data analysis was performed by using software (Bio-Rad). All custom probes were from Applied Biosystems.
Luciferase Assay.
To generate Kras and B-RAF luciferase reporter constructs, a part of CDSs were amplified by PCR and cloned downstream of the luciferase CDS in the PGl3Ctr vector at the XbaI restriction site (Promega). Mutations were introduced into the miRNA-binding sites by using the QuikChange Mutagenesis Kit (Stratagene). HEK-293 cells were cotransfected with renilla plasmid, pGL3-Control Kras-CDS12WT, pGL3-Control Kras-CDS12S (mutated in G12S), pGL3-Control Kras-CDS12D, pGL3-Control B-RAFCDS600WT, and pGL3-Control B-RAFCDS600D (mutated in V600D) plasmids and amiRs by using Lipofectamine 3000 (Invitrogen). After 24 h, cells were lysed and assayed with Dual Luciferase Assay (Promega) according to the manufacturer’s instructions.
The primers used for the cloning were as follows:
K-RAS CDS forward (FW): 5′-TCTAGATCAGCGGCTCCCAGGTGC-3′
K-RAS CDS reverse (RV): 5′-TCTAGAGCCCTCCCCAGTCCTCATGTAC-3′
BRAF CDS FW: 5′-GGCTCGAGTGAAGTAGGAGTACTCAGG-3′
BRAF CDS RV: 5′-TTGCGGCCGCGCCAGCAGCTCAAT-3′
K-RAS G12S FW: 5′-GTGGTAGTTGGAGCTAGTGGCGTAGGCAAGA-3′
K-RAS G12S RV: 5′-TCTTGCCTACGCCACTAGCTCCAACTACCAC-3′
K-RAS G12D FW: 5′-CTCTTGCCTACGCCATCAGCTCCAACTACCA-3′
K-RAS G12D RV: 5′-TGGTAGTTGGAGCTGATGGCGTAGGCAAGAG-3′
BRAFCDSV600D FW: 5′-GACCCACTCCATCGAGATTTCACTGTAGCTAGACCAAAATCA-3′
BRAFCDSV600D RV: 5′-TGATTTTGGTCTAGCTACAGTGAAATCTCGATGGAGTGGGTC-3′
Transwell Migration Assay.
To measure cell migration, 8-nm pore size culture inserts (Transwell; Costar) were placed into the wells of 24-well culture plates, separating the upper and lower chambers. In the lower chamber, 400 μL of RPMI (10% FBS) was added. A549, H1299, and H292 cells were grown in RPMI containing 10% FBS to ∼60% confluence and transfected with 100 nmol of amiR-KS3 precursor molecule or negative control. After 48 h, the cells were harvested by trypsinization, and 1 × 105 cells were added to the upper chamber in no-FBS medium. After 16 h of incubation at 37 °C with 5% CO2, the cells that had migrated through the pores were fixed and colored by Brilliant Blue, and the number was quantified by counting 12 independent visual fields under the microscope (Zeiss) using 20× magnification.
In Vitro Migration Scratch.
Confluent A549, H1299, and H292 cells were transfected by using Lipofectamine 3000 (Invitrogen) with amiR-KS3 or control oligonucleotides. At 48 h after transfection, a cell scratch spatula was used to make a scratch in the cell monolayer, after which the cell monolayers were rinsed and further incubated. Pictures of the scratches were taken by using a digital camera system coupled to a microscope. The cells were incubated for 8 and 24 h, and pictures of the scratches were taken by using a digital camera system coupled to a microscope. To determine the migration distance (micrometers), we used a software image analysis program, ImageJ (a version of NIH Image, https://imagej.nih.gov/ij/), as the reduction of the wide of the open area.
Cell Death and Cell Proliferation Assays.
Counting.
A549, H1299, and H292 cells, at an appropriate time after transfection, were harvested by trypsinization, and the number of them was counted on a hemocytometer.
AlamarBlue assay.
Cells were plated in 96-well plates in triplicate and transfected as described. Cell viability was examined after 48 and/or 72 h with alamarBlue (Bio-Rad), according to the manufacturer’s protocol. Metabolically active cells were detected by adding 10 μL to each well. After 1 h of incubation, the plates were analyzed in a Multilabel Counter (Bio-Rad).
Growth density assay.
The cells were harvested 8 h after transfection by trypsinization, and 5 × 105 of them were seeded into 10-mm plates in triplicates. The cells were then stained and fixed, and density was assessed by using the QuantityOne program (Bio-Rad).
Cell-cycle analysis.
The cells, 48 h after transfection, were trypsinized, washed in PBS, and fixed with ice-cold 70% ethanol while vortexing. Cells were rehydrated in PBS and stained 30 min at room temperature with propidium iodide (PI; 50 mg/mL PI and 0.5 mg/mL RNase in PBS). Samples were analyzed on a FACSCalibur flow cytometer (BD Bioscience). Percentages of cells in the G0/G1, S, and G2/M phases of the cell cycle were calculated by using the MODFIT-LT software (Verity Software House).
Apoptosis.
Apoptosis was assessed by using Annexin V-FITC apoptosis detection kits followed by flow-cytometric analysis and caspase 3/7 activity. A549 cells were transfected and, after 24 h, treated with 5 μM gefinitib. After incubation, cells were washed with cold PBS and removed from the plates by trypsinization. The resuspended cells were washed with cold PBS and stained with FITC-conjugated annexin V antibody according to the manufacturer’s instructions (Roche Applied Science). Samples were allowed to stand for 15 min in the dark and immediately analyzed. Samples were analyzed on a FACSCalibur flow cytometer (BD Bioscience). The percentage of apoptotic cells was calculated by using FlowJo software.
For detection of caspase 3/7 activity, cells were cultured and transfected in 96-well plates; after 24 h, they were treated with 5 μM gefinitib and analyzed by using the Caspase-Glo 3/7 Assay kit (Promega) according to the manufacturer’s instructions. The percentage of caspase activity was corrected for background levels found in the corresponding untreated controls.
In Vivo Studies.
The animal studies were approved by The Ohio State University Institutional Animal Care and Use Committee (OSU-IACUC). Viable A549 cells were injected s.c. (total cells 7 × 106) into the left flanks of 6-wk-old nude mice. After 1 wk, we started to intratumorally transfect 11 μg (final concentration) of scrambled oligonucleotides or amiR-KS3, by using TurboFect in vivo Transfection Reagent (Invitrogen), according to the protocol of the manufacturer. We treated three times a week for 17 d. Tumor sizes were measured before each treatment. Three days after the last treatment, mice were killed, and tumors were excised; a number of the tumors collected were lysed for RNA extraction, and the rest was put in formalin along with paraffin. Tumor volumes were determined by using the equation V (in mm3) = A × B 2/2, where A is the largest diameter and B is the perpendicular diameter.
Tissue Analysis.
The tissue was processed in a Leica ASP 300S Tissue Processor for ∼5 h. Samples were then embedded in Leica Surgipath Paraplast Plus embedding medium. The tissue was cut at 5 μm and placed on white frosted, positive-charged slides. Slides were placed on a slide warmer at 66 °C for ∼15 min. The slides were then placed in the Leica Autostainer XL by using the standard H&E staining program. Slides were coverslipped by using Tissue-Tek Coverslipping Film.
For immunohistochemistry, all sections were stained by using a Bond Rx autostainer (Leica). Briefly, slides were baked at 65 °C for 15 min, and automated software performed dewaxing, rehydration, antigen retrieval, blocking, primary antibody incubation, postprimary antibody incubation, detection (DAB), and counterstaining by using Bond reagents (Leica). Samples were then removed from the machine, dehydrated through ethanols and xylenes, mounted, and coverslipped. An antibody for the following marker was diluted in Antibody diluent (Leica): rabbit antibody K-i67 (1:200; Abcam).
Differential Expression Analysis in amiR-KS3 and siRNA-KS3 Transfections.
We performed differential expression analysis of 729 genes of the nCounter PanCancer Pathways Panel, in biological triplicates. Sixty nanograms of total RNA was hybridized overnight with nCounter Reporter (20 μL) probes in hybridization buffer and in excess of nCounter Capture probes (5 μL) at 65 °C for 16−20 h. After overnight hybridization, excess probes were removed by using two-step magnetic beads-based purification on an automated fluidic handling system (nCounter Prep Station). Biotinylated capture probe-bound samples were immobilized and recovered on a streptavidin-coated cartridge. The abundance of specific target molecules was then quantified by using the nCounter digital analyzer. Individual fluorescent barcodes and target molecules present in each sample were recorded with a CCD camera by performing a high-density scan (600 fields of view). Normalization was performed by using the nSolver Analysis Software (Version 3.0; NanoString Technologies), applying the geometric mean of the negative controls for negative background subtraction; the positive controls for the technical normalization; and of the Housekeeping genes for the biological normalization in all samples, as recommended by NanoString. P values were calculated by using the LIMMA package from the Bioconductor R project. P values were used to rank all genes, retaining those under a significant threshold of 0.05 and with average expression of >50 counts, after negative background subtraction, within triplicates of at least one of the compared conditions. These data were used to generate heat maps through the employment of the R package heatmap.2.
We created the Venn diagrams that represent the intersection between the sets of down-regulated genes by using the web-based tool InteractiVenn (www.interactivenn.net/index2.html).
Consensus Targeting Prediction for amiR-KS3.
We respectively predicted binding sites on 5′ UTR, CDS, and 3′ UTR sequences (UCSC.hg19) of down-regulated genes through a consensus of three miRNA target prediction tools: miRanda36 (Version 3.3a), PITA37 (Version 6.0), and miRiam38, our in-house tool, enhanced with the scoring function described in Laganà et al. (19). Standard parameters were used for the tools miRanda and PITA. miRiam’s parameters were set to detect canonical binding sites only (6-mer, 7-mer-A1, 7-mer-m8, and 8-mer), allowing no mismatches in the seed (e.g., wobble pairs). miRiam’s scoring function is based on the combination of the tree-based multiple linear regression learning system M5P with CTree and takes into account six different features of miRNA/target interactions: type of seed match, miRNA nucleotide composition, pairing of the three regions of the miRNA, adenylate–uridylate (AU) content of the binding site and its flanking regions, structural accessibility of the binding site, and presence of AU-rich element and cytoplasmic polyadenylation element motifs upstream of the binding sites.
Pathway Enrichment Analysis After amiR-KS3 and siRNA-KS3 Transfection in A549 Cell Lines.
We performed functional enrichment analysis on the retained set of differentially expressed genes (mentioned above) by using the software IPA by Ingenuity. Settings used included experimentally observed data for human species, specifically, pathways exclusively associated to the A549 NSCLC cell line.
Off-Target Analysis for amiR-KS3.
The 729 genes of the nCounter PanCancer Pathways Panel were ranked from most down-regulated to most up-regulated to generate a ranked transcript list. Sylamer software39 was then used to respectively assess 5′ UTR, CDS, and 3′ UTR amiR-KS3 seed match enrichment P values across the ranked transcript list. Three FASTA files, respectively, containing 5′ UTR, CDS, and 3′ UTR sequences of refseq transcripts (UCSC.hg19), were provided to Sylamer, along with the ranked transcript list, after they were purged of duplicate sequences (bioinfo5.ugr.es/srnatoolbox) and sequences referring to noncoding RNAs, redundant segments were eliminated through the web tool purge-sequence of the RSAT package (rsat-tagc.univ-mrs.fr/rsat/), and, finally, low-complexity regions were masked with the tool RepeatMasker by using default settings (www.repeatmasker.org/). Sylamer settings were 6-bp motifs, fixed number of bins 40, Markov correction 4, scan restricted to sequences in the ranked gene list, and searching for words (option –words) corresponding to the three possible 6-nt motifs representing the reverse complementary sequences of the three 6-nt-long seeds of amiR-KS3, from positions 1–6, 2–7, and 3–8 from the 5′ end.
Supplementary Material
Acknowledgments
We thank Prof. F. Kay Hubner for her useful support during the drafting process and Prof. Patrick Nana-Sinkam for his precious suggestions during the experimental design and the scientific discussion. This work was supported by National Institutes of Health Grant NIH U01 CA166905-04. D.V. was supported by Italian Foundation for Cancer Research Grant 16572.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620562114/-/DCSupplemental.
References
- 1.Vogelstein B, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malumbres M, Barbacid M. RAS oncogenes: The first 30 years. Nat Rev Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 3.Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov. 2014;13:828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinlan MP, Settleman J. Isoform-specific ras functions in development and cancer. Future Oncol. 2009;5:105–116. doi: 10.2217/14796694.5.1.105. [DOI] [PubMed] [Google Scholar]
- 5.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72:2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson L, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 7.Misale S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan TL, et al. Development of siRNA payloads to target KRAS-mutant cancer. Cancer Discov. 2014;4:1182–1197. doi: 10.1158/2159-8290.CD-13-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormick F. KRAS as a therapeutic target. Clin Cancer Res. 2015;21:1797–1801. doi: 10.1158/1078-0432.CCR-14-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu T, et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin Cancer Res. 2012;18:2316–2325. doi: 10.1158/1078-0432.CCR-11-2381. [DOI] [PubMed] [Google Scholar]
- 11.Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: Promises and challenges. Nat Rev Drug Discov. 2014;13:928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 12.Gysin S, Salt M, Young A, McCormick F. Therapeutic strategies for targeting ras proteins. Genes Cancer. 2011;2:359–372. doi: 10.1177/1947601911412376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helwak A, Kudla G, Dudnakova T, Tollervey D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell. 2013;153:654–665. doi: 10.1016/j.cell.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer—a brief overview. Adv Biol Regul. 2015;57:1–9. doi: 10.1016/j.jbior.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye W, et al. The effect of central loops in miRNA:MRE duplexes on the efficiency of miRNA-mediated gene regulation. PLoS One. 2008;3:e1719. doi: 10.1371/journal.pone.0001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laganà A, et al. miR-Synth: A computational resource for the design of multi-site multi-target synthetic miRNAs. Nucleic Acids Res. 2014;42:5416–5425. doi: 10.1093/nar/gku202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimson A, et al. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 23.Tape CJ, et al. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell. 2016;165:910–920. doi: 10.1016/j.cell.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 25.Westcott PM, To MD. The genetics and biology of KRAS in lung cancer. Chin J Cancer. 2013;32:63–70. doi: 10.5732/cjc.012.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell PM, Der CJ. Oncogenic Ras and its role in tumor cell invasion and metastasis. Semin Cancer Biol. 2004;14:105–114. doi: 10.1016/j.semcancer.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Okudela K, et al. K-ras gene mutation enhances motility of immortalized airway cells and lung adenocarcinoma cells via Akt activation: Possible contribution to non-invasive expansion of lung adenocarcinoma. Am J Pathol. 2004;164:91–100. doi: 10.1016/S0002-9440(10)63100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4:1681–1689; discussion 1690. doi: 10.1371/journal.pmed.0040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 30.Janmaat ML, Rodriguez JA, Gallegos-Ruiz M, Kruyt FA, Giaccone G. Enhanced cytotoxicity induced by gefitinib and specific inhibitors of the Ras or phosphatidyl inositol-3 kinase pathways in non-small cell lung cancer cells. Int J Cancer. 2006;118:209–214. doi: 10.1002/ijc.21290. [DOI] [PubMed] [Google Scholar]
- 31.Pao W, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garofalo M, et al. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat Med. 2011;18:74–82. doi: 10.1038/nm.2577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 34.Jackson AL, et al. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA. 2006;12:1179–1187. doi: 10.1261/rna.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birmingham A, et al. 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat Methods. 2006;3:199–204. doi: 10.1038/nmeth854. [DOI] [PubMed] [Google Scholar]
- 36.John B, et al. Human microRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet. 2007;39:1278–1284. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- 38.Laganà A, et al. Prediction of human targets for viral-encoded microRNAs by thermodynamics and empirical constraints. J RNAi Gene Silencing. 2010;6:379–385. [PMC free article] [PubMed] [Google Scholar]
- 39.van Dongen S, Abreu-Goodger C, Enright AJ. Detecting microRNA binding and siRNA off-target effects from expression data. Nat Methods. 2008;5:1023–1025. doi: 10.1038/nmeth.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam JK, Chow MY, Zhang Y, Leung SW. siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids. 2015;4:e252. doi: 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miele E, et al. Nanoparticle-based delivery of small interfering RNA: Challenges for cancer therapy. Int J Nanomedicine. 2012;7:3637–3657. doi: 10.2147/IJN.S23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.