Significance
miRNAs are important components of gene regulatory networks and affect all aspects of cell biology by controlling the stability and translation efficiency of their target mRNAs. Here, we identified the mRNA cap-binding eIF4E-related protein 4EHP as an effector of miRNA-mediated translation repression. Through screening for protein interactions in cells via the BioID method, we identified 4EHP as a component of the CCR4–NOT/DDX6/4E-T axis. Direct interaction between 4E-T and 4EHP increases the latter’s cap-binding affinity, suggesting that this interaction potentiates its competition with the eIF4F complex for binding to the mRNA 5′ cap. Our findings suggest that 4EHP facilitates the formation of a closed-loop structure between the 3′ UTR of the mRNA and its 5′ cap, which causes repression of mRNA translation.
Keywords: microRNA, 4EHP, 4E-T, CCR4–NOT, mRNA translation
Abstract
MicroRNAs (miRNAs) play critical roles in a broad variety of biological processes by inhibiting translation initiation and by destabilizing target mRNAs. The CCR4–NOT complex effects miRNA-mediated silencing, at least in part through interactions with 4E-T (eIF4E transporter) protein, but the precise mechanism is unknown. Here we show that the cap-binding eIF4E-homologous protein 4EHP is an integral component of the miRNA-mediated silencing machinery. We demonstrate that the cap-binding activity of 4EHP contributes to the translational silencing by miRNAs through the CCR4–NOT complex. Our results show that 4EHP competes with eIF4E for binding to 4E-T, and this interaction increases the affinity of 4EHP for the cap. We propose a model wherein the 4E-T/4EHP interaction engenders a closed-loop mRNA conformation that blocks translational initiation of miRNA targets.
MicroRNAs (miRNAs) are short, ∼22-nucleotide noncoding RNAs that affect gene expression in most eukaryotes. miRNAs mediate posttranscriptional silencing by guiding the miRNA-induced silencing complex (miRISC), an assembly of Argonautes and GW182/TNRC6 proteins, to target mRNAs. Target recognition initiates a succession of events: mRNA translational repression, deadenylation, and mRNA decay (1). miRNAs impair the function of eIF4F, a three-subunit complex composed of eIF4E, the m7GTP (cap)-interacting factor; eIF4G, a scaffolding protein; and eIF4A, a DEAD-box RNA helicase (2–5). The silencing activity of miRISC is mediated by the CCR4–NOT deadenylase complex through the scaffolding subunit, CNOT1 (6–8). CNOT1 recruits the DDX6 and 4E-T (eIF4E transporter, also known as EIF4ENIF1) proteins, which are important for miRNA-mediated silencing (9–16). The 4E-T protein is a conserved eIF4E-binding protein, which directly binds to the dorsal surface of eIF4E through its canonical eIF4E-binding YX4LL (Y30TKEELL) motif and impairs the eIF4E/eIF4G interaction and translation initiation (17). The 4E-T protein also facilitates the decay of CCR4–NOT-targeted mRNAs by linking the 3′-terminal mRNA decay machinery to the cap via its interaction with eIF4E (13).
In mammals, eIF4E is the best-studied member of a family of proteins composed of eIF4E (eIF4E1), 4EHP (4E-homologous protein; eIF4E2), and eIF4E3. The 4EHP and eIF4E proteins share 28% sequence identity (18, 19). The 4EHP protein is ubiquitously expressed, and it is 5–10 times less abundant than eIF4E in a number of mammalian cell lines (18–20). Like eIF4E, 4EHP binds to 4E-T, but in sharp contrast to eIF4E, it does not associate with eIF4G (18, 21). The 4EHP protein has a 30- to 100-fold weaker affinity for the cap than eIF4E due to a two-amino acid substitution in its cap-binding pocket (22).
The 4EHP protein has primarily been documented as a translation repressor. In the Drosophila embryo, 4EHP associates with the RNA binding protein Bicoid to repress caudal mRNA translation (23). Similarly, 4EHP also represses the hunchback mRNA by binding to the nanos repressive element complex, which consists of nanos, pumilio, and brain tumor proteins (24). A similar mechanism functions in the mouse, where 4EHP binds the Prep1 RNA-binding protein and inhibits Hoxb4 mRNA translation (25). Moreover, 4EHP forms a translational repressor complex with GIGYF2 (Grb10-interacting GYF protein 2), which acts as a cofactor in translational repression and mRNA decay of tristetraprolin-targeted mRNAs (26, 27).
In this work, we demonstrate that 4EHP interacts with the mRNA-silencing machinery and engenders miRNA-mediated translational repression. Our data support a model wherein 4EHP interactions with miRISC/CCR4–NOT lead to the translational repression of miRNA targets.
Results
The 4EHP Protein Is a Component of the miRISC Effector Machinery.
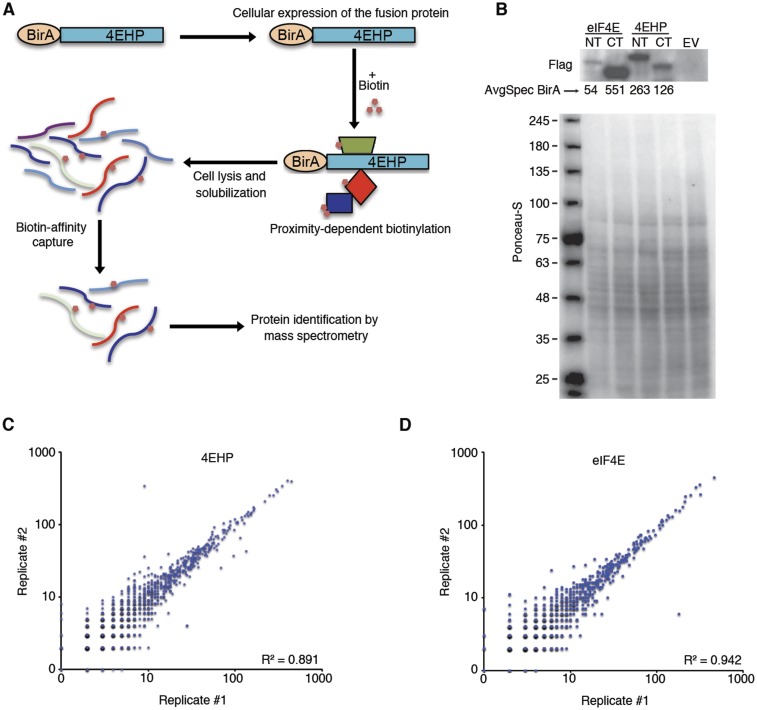
The specificity of eIF4E and 4EHP for their mRNA targets and their affinities for the cap are influenced by binding partners (2, 26, 28). Thus, we used the BioID assay (29) to identify the eIF4E and 4EHP interacting proteins. We created stable cell lines expressing 4EHP or eIF4E fused in-frame with an abortive biotin ligase BirA* (R118G) (Fig. S1 A and B). The 4EHP and eIF4E proteins were used as baits to biotinylate and capture interactors and proteins in close proximity. Biotinylated proteins were isolated by using streptavidin-affinity purification under denaturing conditions and analyzed by mass spectrometry (MS). Each bait protein was fused at its N or C terminus to BirA*. Two independent replicates for each construct were analyzed (four total for each bait protein) alongside negative controls. The MS data were processed with the SAINT (Significance Analysis of Interactome) computational tool (30), using several controls for statistical analysis to assign confidence scores to interaction pairs (Dataset S1 and SI Materials and Methods). Data were highly consistent across replicates (mean correlation coefficient R2 = 0.89 and 0.94 for 4EHP and eIF4E, respectively; Fig. S1 C and D). BioID identified 30 high-confidence targets for 4EHP and 8 for eIF4E [false discovery rate (FDR) ≤ 1%; Fig. 1A and Dataset S1]. The list of proteins identified for 4EHP is consistent with previous reports showing that 4EHP interacts with GIGYF1, GIGYF2, ZNF598, and 4E-T proteins (26). Known interactions with eIF4E, such as 4E-BP1 (EIF4EBP1), eIF4G1, and eIF4G3, were also detected (31).
Fig. S1.
Analysis of 4EHP and eIF4E-proximal proteins by BioID assay. (A) Summary of workflow used to identify BirA*–4EHP and BirA*–eIF4E proximal proteins through BioID. (B) Lysates from cells used in Fig. 1A were analyzed by Western blot with anti-Flag antibody. CT (C-terminal) and NT (N-terminal) indicate the location of BirA* fusion protein in relation to the bait proteins. Extract from cells transfected by an empty vector (EV) is also shown. The spectral counts for the BirA tag are indicated for each bait. Total protein staining (Ponceau S) was used for loading control. (C and D) Correlation between replicates in BirA*–4EHP and –eIF4E BioID assays. R2 indicates Pearson correlation.
Fig. 1.
Proteomic identification of the 4EHP and eIF4E proximal proteins. (A) High-confidence protein interactions discovered by BioID for the indicated baits in HEK293 cells. CT (C-terminal) and NT (N-terminal) indicate the location of BirA* fusion protein in relation to the Bait protein. An average of two independent experiments for each tagged variant is presented. Interacting proteins were categorized according to their known functions. Avg-Spec shows the spectral counts for each indicated prey. BFDR, Bayesian FDR. A complete list of the proximal proteins for each bait is presented in Dataset S1. (B) GO analysis of the BirA*–4EHP proximal proteins. The 10 most significantly enriched biological processes identified by using prohits-viz.lunenfeld.ca/ running g:Profiler (51) software are presented. A complete list of the enriched biological processes and molecular pathways is presented in Dataset S2. (C) HEK293T cells were transiently transfected with control or 3xFlag-4EHP plasmids. Two days later, cytoplasmic cell lysate was immunoprecipitated by using anti-Flag antibody in the presence of RNase A. LE, long exposure; SE, short exposure. (D) Cytoplasmic cell lysate from HEK293T cells was immunoprecipitated by using anti-DDX6 or IgG antibodies. (E) HEK293T cells were transiently transfected with control or HA-PATL-1 plasmid. Two days later, cytoplasmic cell lysate was immunoprecipitated by using anti-HA antibody. Western blot was performed by using the specified antibodies.
Gene ontology (GO) analysis showed that “translation” was the biological process with the most significant representation among 4EHP interactions (Fig. 1B and Dataset S2). In contrast to eIF4E, BioID with 4EHP identified several known miRNA cofactors, including DDX6, CNOT1, PATL-1, and TNRC6A/B, the scaffolding proteins of miRISC (1). To confirm that 4EHP physically interacts with these proteins, we carried out coimmunoprecipitation (co-IP) experiments. Because of the poor quality of the commercially available anti-4EHP antibodies for IP assay, lysates prepared from human embryonic kidney (HEK) 293T cells transfected with a vector expressing 3xFlag–4EHP, or an empty vector, were immunoprecipitated with anti-Flag antibody, followed by Western blot. Flag-tagged 4EHP coprecipitated 4E-T and the CNOT1 subunit of the CCR4–NOT complex (Fig. 1C). Likewise, endogenous 4EHP coprecipitated with endogenous DDX6, as well as HA-tagged PATL-1, a physical partner of CCR4–NOT, DDX6, and 4E-T (12, 32, 33) (Fig. 1 D and E). Together, these data demonstrate that 4EHP associates with several key proteins involved in miRISC/CCR4–NOT-mediated gene silencing.
The 4EHP Protein Effects miRNA-Dependent Translational Repression.
The association of 4EHP with components of the miRISC and its effector machinery (CCR4–NOT, PATL-1, 4E-T, and DDX6) raised the possibility that 4EHP plays a role in miRNA-mediated silencing. To investigate this possibility, we used a luciferase construct (Fig. 2A) fused to the wild-type (WT) 3′ UTR of the Hmga2 mRNA (an endogenous target of the let-7 miRNA) or a modified version with point mutations disrupting all seven target sites (34). Reporter constructs were transfected into mouse embryonic fibroblasts (MEFs) from WT or 4EHP-knockout (KO) mice (Fig. S2A) (26). The reporter containing the WT 3′ UTR of Hmga2 was repressed 4.4-fold in WT MEFs compared with the mutated, control reporter (Fig. 2A and Fig. S2B). This repression was significantly less (1.6-fold) in 4EHP-KO cells, demonstrating that 4EHP is required for the efficient silencing of the Hmga2 3′ UTR reporter by the let-7 miRNA. We also examined the impact of 4EHP depletion on silencing of two additional miRNA reporters, in the U251 human glioblastoma cell line and in the human cervical adenocarcinoma HeLa cell line. A luciferase construct containing a portion of the E2f 3′ UTR, which is repressed by the miR-17/20a paralogs in control U251 cells, was significantly less repressed upon knockdown of 4EHP (1.5-fold in shCTR compared with 1.1-fold repression in sh4EHP; Fig. 2B and Fig. S2C). Similarly, depletion of 4EHP (si4EHP) in HeLa cells significantly derepressed a luciferase reporter harboring three miR-19a binding sites, in comparison with cells treated with a control siRNA (siCTR) (1.6-fold in siCTR compared with 1.2-fold repression in si4EHP; Fig. S2 D and E). Derepression was not due to the differential expression of the miRNAs in 4EHP-depleted cells (Fig. S2F). Repression of both miR-17/20a– and miR-19a–targeted reporters in the control cells was less pronounced than that of the Hmga2 3′ UTR construct, likely due to fewer miRNA-binding sites in the respective UTRs (seven miRNA-binding sites for Hmga2 compared with three for miR-19a and two for miR-17/20a reporters). Together, these data show that 4EHP promotes miRNA-mediated silencing by several miRNAs and in various mammalian cell lines.
Fig. 2.
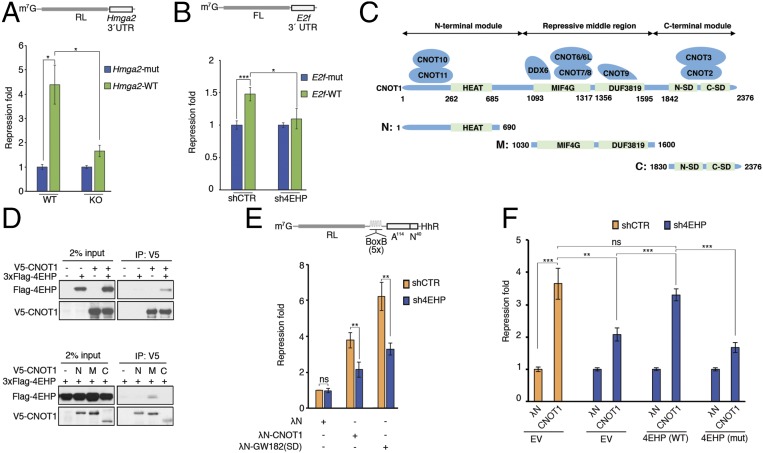
miRISC/CCR4–NOT-mediated translational silencing is impaired by 4EHP depletion. (A, Upper) Schematic representation of the RL-Hmga2 3′ UTR reporter. (A, Lower) WT and 4EHP-KO MEFs were cotransfected with RL-Hmga2 3′ UTR (WT) or mutant (Mut), along with Firefly luciferase (FL). Luciferase activity was measured 24 h after transfection. Renilla luciferase (RL) values were normalized against FL levels, and repression fold was calculated for the RL-Hmga2 3′ UTR (WT) relative to RL-Hmga2 3′ UTR (Mut) level for each population. The same data are shown as relative RL/FL levels in Fig. S2B. (B, Upper) Schematic representation of the FL-E2f 3′ UTR reporter. (B, Lower) shCTR and sh4EHP U251 cells were cotransfected with pGL3-FL-E2f 3′ UTR (WT) or a variant with mutations disrupting the two miR-17/20a–binding sites (Mut), along with RL. FL values were normalized against RL levels, and repression fold was calculated for the FL-E2f 3′ UTR (WT) relative to FL-E2f 3′ UTR (Mut) level for each population. (C) Diagram of full-length CNOT1 and the N-terminal, middle, and C-terminal fragments used in D. The binding partners in CNOT1 are also depicted and the main domains are shown in green. Amino acid positions at domain boundaries are indicated below the protein outlines. (D, Upper) Vectors expressing V5-CNOT1 and 3xFlag–4EHP (or control plasmid) were transfected into HEK293T cells. IP of V5-CNOT1 from RNase A-treated extracts was performed by using anti-V5 antibody. Purified proteins were analyzed by Western blot. (D, Lower) HEK293T cells were transfected with vectors expressing V5-CNOT1 fragments (as described in C): N, N-terminal region (amino acids 1–690); M, middle repressive module (amino acids 1,030–1,600); and C, C-terminal region (amino acids 1,830–2,376) and 3xFlag-4EHP. Extracts were subjected to anti-V5 IP, and the eluted fractions were analyzed by Western blot. (E) The 4EHP protein mediates translational repression by tethered CNOT1 and GW182(SD). (E, Upper) Schematic representation of the RL-5boxB-A114-N40-HhR reporter. Control HEK293T cells (shCTR) or cells depleted of 4EHP (sh4EHP) were cotransfected with vectors expressing either λN-CNOT1, λN-GW182(SD) or λN control, along with RL-5boxB-A114-N40-HhR or RL-A114-N40-HhR, and FL. RL luminescence was normalized against the FL level. (E, Lower) Fold repression of RL-5boxB-A114-N40-HhR relative to RL-A114-N40-HhR expression is shown. Repression of RL-5boxB-A114-N40-HhR by λN alone in shCTR cells was set as 1. (F) Rescue assay for λN-CNOT1–dependent silencing was performed, as described in E, in cells depleted of 4EHP. shCTR and sh4EHP HEK293T cells were transfected with the indicated plasmids in combination with constructs expressing shRNA-resistant versions of 3xFlag–4EHP (WT), 3xFlag–4EHPW124A cap-binding mutant (Mut), or empty vector (EV). The experiments illustrated in A, B, E, and F are represented as mean values (±SD) of three independent experiments. The P value was determined by two-tailed Student's t test. ns, nonsignificant. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. S2.
The 4EHP-dependent translational repression by the miRISC/CCR4–NOT complex. (A) Lysates from WT and 4EHP-KO MEFs were analyzed by Western blot with the indicated antibodies. (B) Expression of RL-Hmga2 3′ UTR reporter in WT and 4EHP KO MEFs. Data from Fig. 2A are represented as relative RL/FL levels. Values show relative RL activities (RL/FL), with normalized RL-Hmga2 3′ UTR (Mut) levels set equal to 100% for each cell population. (C) Western blot analysis of protein lysates from U251 cells (Fig. 2B) with the indicated antibodies. (D, Upper) Schematic representation of the FL-3xmiR-19a reporter. (D, Lower) Repression of FL-3xmiR-19a reporter in 4EHP-depleted HeLa cells. Cells were transfected by nontargeting siRNAs (siCTR) or by siRNAs targeting 4EHP (si4EHP). Two days after transfection, cells were cotransfected by pmiRGLO-FL-3xmiR-19a (WT) or a variant containing mutations in the three miR-19a–binding sites (Mut). Luciferase activity was measured 24 h after transfection. Firefly values were normalized against Renilla levels, and repression fold was calculated for FL-3xmiR-19a (WT) relative to FL-3xmiR-19a (Mut) level for each population. (E) Extracts from the HeLa cells used in D were analyzed by Western blot with the indicated antibodies. (F) Quantitative PCR analysis of the indicated miRNAs in control and 4EHP-depleted cells. Expression of let-7a, miR-19a, miR-17a, and miR-20a was measured in the indicated cell lines by using TaqMan miRNA quantitative RT-PCR. miRNA levels were normalized to 18S expression values, and the results are presented as fold changes (control cells set as 1). (G) Western blot analysis of the shCTR and sh4EHP lysates from Fig. 2E. A total of 20 µg of extracts were loaded on a 4–12% gradient SDS/PAGE gel, and Western blot was performed by using the specified antibodies. (H, Upper) Schematic depiction of λN-CNOT1 tethered to RL-5boxB-A114-N40-HhR reporter mRNA. (H, Lower) HEK293T cells were transfected by nontargeting siRNAs (siCTR) or by siRNAs targeting 4E-T (si4E-T). Two days after transfection, cells were cotransfected by a mixture of plasmids to measure to silencing activity of λN-CNOT1 as described in Fig. 2E. Repression fold of RL-5boxB-A114-N40-HhR relative to RL-A114-N40-HhR expression is shown. Repression of RL-5boxB-A114-N40-HhR by λN alone was set as 1 in each cell population. (I) Lysates from H were analyzed by Western blot. (J) Lysates from Fig. 2F were analyzed by Western blot with the indicated antibodies. The experiments illustrated in B, D, F, and H are represented as mean values (±SD) of three independent experiments. The P value was determined by two-tailed Student's t test. ns, nonsignificant. *P < 0.05; **P < 0.01; ***P < 0.001.
CNOT1, the scaffolding subunit of the CCR4–NOT complex, is an established player in miRNA-mediated silencing (6–8). We therefore sought to examine the interaction of 4EHP with CNOT1. To this end, we transfected HEK293T cells with plasmids encoding 3xFlag–4EHP and V5-tagged full-length or truncated variants of CNOT1 (Fig. 2C) and examined their interaction by co-IP. The 4EHP protein coprecipitated with either full-length CNOT1 or the middle domain (M) of CNOT1 (Fig. 2D). Importantly, the middle domain also interacts with 4E-T via DDX6 (9, 10, 14–16). To investigate whether 4EHP plays a role in translation repression by the CCR4–NOT complex, we used the λN-BoxB tethering approach (35). We used a Renilla luciferase (RL) reporter containing five BoxB hairpins in its 3′ UTR. The 3′ end of the reporter contains a self-cleaving hammerhead ribozyme (HhR) to generate an internalized poly(A) stretch of 114 nucleotides, followed by 40 nucleotides, to prevent deadenylation and subsequent degradation (36). The reporter was cotransfected into HEK293T cells along with a plasmid encoding a fusion of CNOT1 to a λN peptide, which binds to BoxB elements. Silencing through CNOT1 was examined by comparing the luciferase activity in cells wherein 4EHP was depleted via shRNA (sh4EHP) with nondepleted control cells (shCTR). CNOT1 tethering to the BoxB reporter in shCTR cells resulted in a 3.7-fold repression of RL activity, whereas depletion of 4EHP resulted in significant derepression of the reporter (1.8-fold; Fig. 2E and Fig. S2G). Similarly, siRNA knockdown of 4E-T partially relieved the repression exerted by tethered CNOT1 (1.8-fold vs. 2.9-fold repression in siCTR) (Fig. S2 H and I). We also examined the role of 4EHP in the repressive activity of the C-terminal silencing domain of human GW182/TNRC6C [GW182(SD); amino acids 1,382–1,690], which functions through CNOT1 recruitment (6–8). Consistent with the effects on the miRNA reporters and with the CNOT1 tethering experiment, 4EHP depletion relieved the repression exerted by tethered GW182(SD) (1.8-fold derepression; Fig. 2E and Fig. S2G).
We next examined the importance of the cap-binding activity of 4EHP for miRNA-mediated silencing. We performed a complementation assay using tethered λN-CNOT1 in the presence of WT 4EHP or the 4EHPW124A mutant, which is incapable of binding to the cap (19). Transient transfection of shRNA-resistant 3xFlag–4EHP in 4EHP-knockdown cells restored λN-CNOT1–mediated translation repression (Fig. 2F and Fig. S2J), whereas transfection with 4EHPW124A did not. These results demonstrate that cap binding by 4EHP is required for translation repression effected through CCR4–NOT.
The 4EHP and eIF4E Proteins Compete for Binding to 4E-T.
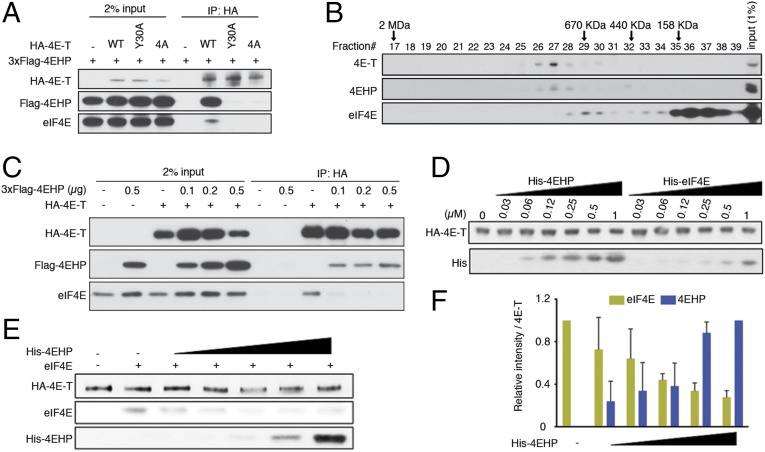
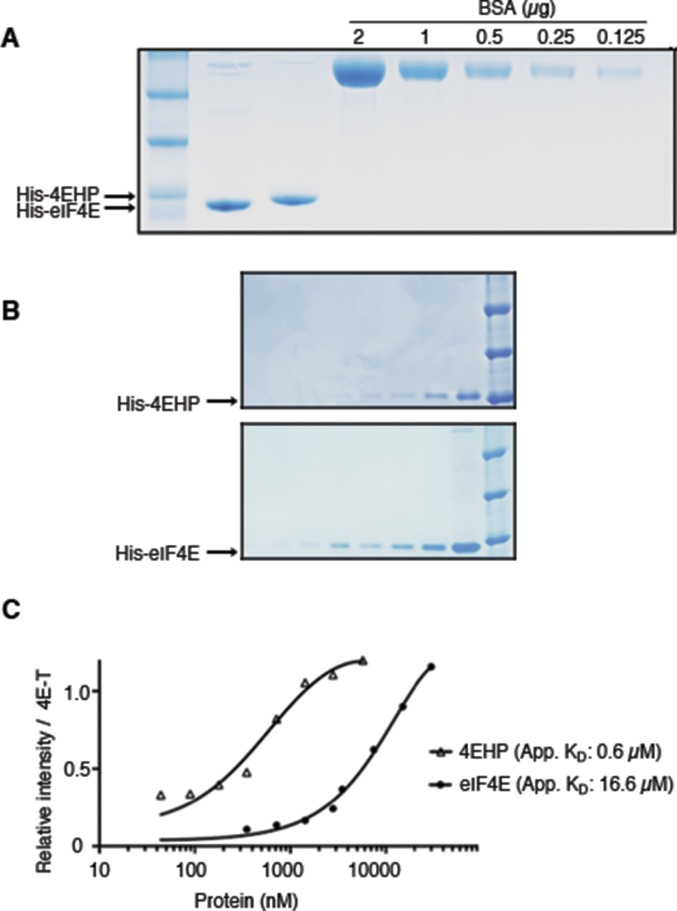
The 4E-T protein is enriched among 4EHP BioID targets (Fig. 1A), but 4E-T also interacts with eIF4E, and both eIF4E and 4EHP bind the same eIF4E-binding motif of 4E-T (Y30TKEELL) (21). We compared the effects of mutations in this motif on the interactions of 4E-T with 4EHP and eIF4E. We cotransfected HEK293T cells with constructs encoding 3xFlag–4EHP and either WT HA-tagged 4E-T or 4E-T bearing point mutations in the Y30TKEELL motif (Y30→A and 4A; Y30TKEELL→A30AKEEAA) and tested their interactions by IP. WT 4E-T coimmunoprecipitated with 4EHP and eIF4E, but these interactions were lost with either mutant of the YTKEELL motif (Fig. 3A). To analyze the distribution of the endogenous 4E-T with eIF4E and 4EHP proteins among native protein complexes, we performed size-exclusion chromatography of HEK293T lysates. The 4E-T protein associated with 4EHP at a molecular mass of ∼1 MDa, whereas eIF4E was detected in lower-molecular-mass fractions (Fig. 3B). We thus hypothesized that 4E-T may bind better to 4EHP than to eIF4E. To address this hypothesis, we performed an IP assay in transfected HEK293T cells, which coexpressed constant amounts of HA–4E-T and increasing amounts of Flag-4EHP (Fig. 3C). Although the interaction between HA–4E-T and endogenous eIF4E could be detected in the absence of exogenous 4EHP, overexpression of 4EHP dramatically reduced 4E-T binding to eIF4E (Fig. 3C). To further examine these interactions, we used recombinant 4EHP, eIF4E, and 4E-T proteins and performed in vitro binding assays. We compared the 4E-T/4EHP interaction with the 4E-T/eIF4E interaction in vitro by using constant amounts of HA–4E-T and increasing concentrations of His-4EHP or -eIF4E (Fig. S3 A and B). In such conditions, 4EHP was 20–30 times more potent than eIF4E in binding to 4E-T (Fig. 3D and Fig. S3C). To corroborate the preference of 4E-T for 4EHP over eIF4E, we performed an in vitro displacement assay using preassociated HA–4E-T/eIF4E immobilized on anti-HA agarose beads. An excess amount of eIF4E was incubated with the 4E-T–bound beads to saturate the binding sites on 4E-T. Preassembled eIF4E/4E-T complexes were next incubated with increasing amounts of recombinant 4EHP. In such conditions, addition of 1.8 µM 4EHP displaced >60% of eIF4E bound to 4E-T (Fig. 3 E and F). Together these results suggest that 4EHP has a competitive advantage over eIF4E for binding to 4E-T.
Fig. 3.
The 4EHP protein competes with eIF4E for binding to 4E-T. (A) HEK293T cells were transfected with vectors expressing HA-tagged WT 4E-T or the eIF4E/4EHP-binding mutant variants and 3xFlag–4EHP (or control vector). Lysates were immunoprecipitated by using the anti-HA antibody. Western blot was performed by using the specified antibodies. (B) Fractionation of endogenous 4E-T, 4EHP, and eIF4E by size-exclusion chromatography. A total of 5 mg of proteins from HEK293T cells was loaded onto a Superose 6 column and run at a flow rate of 0.5 mL/min. Fractions of 0.5 mL were collected, and 50 µL of each fraction was analyzed by Western blot. The elution position of the molecular size markers is shown. A total of 50 µg (1%) of input was used for Western blot. (C) HEK293T cells were transfected with vectors expressing HA–4E-T and increasing amounts of 3xFlag–4EHP. Anti-HA IP and Western blot were performed as described in A. (D) Binding assays with 4E-T/4EHP and 4E-T/eIF4E complexes. Constant amounts of the HA–4E-T–bound beads were incubated with increasing concentrations of recombinant His-4EHP or His-eIF4E (0, 0.03, 0.06, 0.12, 0.25, 0.5, and 1 µM). After washing the beads, the retained complexes were eluted and analyzed by Western blot with anti-His and -HA antibodies. (E) In vitro displacement assay. Preassembled eIF4E/4E-T complexes bound on anti-HA agarose beads were incubated with increasing amounts of 4EHP (0, 0.11, 0.22, 0.44, 0.89, and 1.78 µM). Proteins were eluted and analyzed by Western blot. (F) Bar graph, obtained by densitometry analysis of Western blot data from E, shows quantified intensities of eIF4E and 4EHP signals normalized against 4E-T. The data are expressed as mean values (±SD) of two independent experiments.
Fig. S3.
Analysis of recombinant 4EHP and eIF4E. (A) SDS/PAGE and Coomassie brilliant blue staining of purified His-4EHP and -eIF4E proteins. (B) Quantification of purified His-4EHP and -eIF4E proteins by SDS/PAGE and Coomassie staining. (C) Binding assay with 4E-T/4EHP and 4E-T/eIF4E complexes. Similar to Fig. 3D, constant amounts of the 4E-T–bound beads were incubated with increasing concentrations of recombinant His-4EHP (0, 66, 85, 160, 234, 468, 930, 3,750, and 7,500 nM) or His-eIF4E (0, 234, 468, 930, 1,875, 3,750, 7,500, 15,000, and 30,000 nM). After washing the beads, the retained complexes were eluted and analyzed as described in Fig. 3D. For each point across the titration range, the signal intensity was quantified by densitometry and analyzed by curve fitting using nonlinear regression. The graph reports the quantified intensities of eIF4E and 4EHP signals normalized against 4E-T.
Interaction with 4E-T Increases the Affinity of 4EHP to m7GTP Cap.
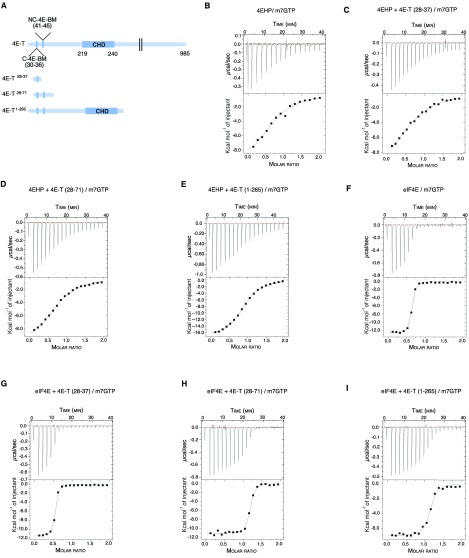
In addition to the canonical YTKEELL motif, both 4EHP and eIF4E interact with a secondary noncanonical sequence of 4E-T (21). However, the contribution of the noncanonical motif appears more important for the interaction of 4E-T with 4EHP (21). We thus hypothesized that the extended interaction surface of 4E-T and its greater affinity for 4EHP (Fig. 3) may influence the ability of 4EHP to bind the m7GTP cap. To address this hypothesis, we performed an isothermal titration calorimetry (ITC) assay to examine whether the interaction of 4EHP with 4E-T peptides impacted the 4EHP affinity for the cap. We used three different 4E-T peptides: one encoding the canonical YTKEELL motif alone (4E-T28–37; Fig. S4A), the same sequence in combination with the noncanonical motif (4E-T28–71), and another, which covers the entire N-terminal extremity (4E-T1–265; Fig. S4A). The 4E-T peptides were preincubated with recombinant 4EHP protein and titrated with m7GTP. The affinity of 4EHP for m7GTP was 8.0 ± 0.56 × 10−6 M in the absence of 4E-T peptides (Table 1 and Fig. S4B). Adding the 4E-T28–37 peptide did not significantly change this affinity. However, the affinity of 4EHP for the m7GTP cap was fourfold greater in the presence of the N terminus of 4E-T (KD of 5.9 ± 0.7 × 10−6 M and 1.8 ± 0.4 × 10−6 M for 4E-T28–71 and 4E-T1–265, respectively; Table 1 and Fig. S4 B–E). In comparison, the affinity of eIF4E for the m7GTP cap (KD of 8.8 ± 1.1 × 10−8 M) remained virtually unchanged by the 4E-T peptides (Table 1 and Fig. S4 F–I). Together, these results suggest that 4E-T interactions with 4EHP enhance the binding of the latter to the m7GTP cap structure.
Fig. S4.
Thermodynamic parameters for the interaction of 4EHP and eIF4E with m7GTP in presence of 4E-T peptides. (A) Schematic diagram of full-length 4E-T and 4E-T peptides used in ITC experiments. The canonical (C), noncanonical (NC), and Cup-homology domain (CHD) of the 4E-T protein are depicted. We used three different 4E-T peptides harboring either the canonical YTKEELL motif alone (4E-T28–37), in combination with the noncanonical motif (4E-T28–71), or covering the entire N-terminal extremity including the eIF4E-binding motifs and the CUP-homology domain (4E-T1–265). The 4E-T peptides were incubated with recombinant 4EHP or eIF4E proteins and titrated with m7GTP. (B–I) ITC profiles of 4EHP + m7GTP (B), 4EHP + 4E-T28–37 + m7GTP (C), 4EHP + 4E-T28–71 + m7GTP (D), 4EHP + 4E-T1–265 + m7GTP (E), eIF4E + m7GTP (F), eIF4E + 4E-T28–37 + m7GTP (G), eIF4E+ 4E-T28–71 + m7GTP (H), and eIF4E + 4E-T1–265 + m7GTP (I). The reaction cell contained 200 μL of 30 μM protein and was titrated with 19 injections of 2 μL of 300 μM m7GTP. B–I, Upper show the raw data (μcal⋅s−1), and B–I, Lower represents the integrated data (kcal⋅mol−1 injectant) of heat changes. The binding isotherm was fit with a binding model that uses a single set of independent sites to determine the thermodynamic binding constants and stoichiometry (see Table 1 for details).
Table 1.
Thermodynamic parameters for the interaction of eIF4E and 4EHP with m7GTP in the presence of 4E-T peptides, as determined by ITC
| Protein/peptide | KD, M | ∆H, kcal/mol | −T∆S, kcal/mol | Molar ratio |
| 4EHP/m7GTP | 8.0 ± 0.56 × 10−6 | −10.8 ± 1.5 | 1.26 | 1.10 ± 0.07 |
| 4EHP + 4E-T28–37/m7GTP | 7.8 ± 1.0 × 10−6 | −10.2 ± 0.4 | 3.32 | 0.91 ± 0.02 |
| 4EHP + 4E-T28–71/m7GTP | 5.9 ± 0.7 × 10−6 | −10.8 ± 0.3 | 3.81 | 0.95 ± 0.01 |
| 4EHP + 4E-T1–265/m7GTP | 1.8 ± 0.4 × 10−6 | −17.1 ± 0.2 | 9.42 | 1.02 ± 0.006 |
| eIF4E/m7GTP | 8.8 ± 1.1 × 10−8 | −11.12 ± 0.1 | 1.66 | 1.02 ± 0.12 |
| eIF4E + 4E-T28–37/m7GTP | 8.2 ± 0.4 × 10−8 | −9.0 ± 0.1 | 0.43 | 0.98 ± 0.007 |
| eIF4E + 4E-T28–71/m7GTP | 7.3 ± 2.5 × 10−8 | −18.4 ± 0.5 | 8.60 | 1.03 ± 0.01 |
| eIF4E + 4E-T1–265/m7GTP | 6.2 ± 3.0 × 10−8 | −22.3 ± 0.3 | 12.70 | 1.04 ± 0.08 |
See Fig. S4A for a schematic description of the peptides.
SI Materials and Methods
List of Antibodies, siRNAs, and shRNAs.
The following antibodies were used: rabbit anti-eIF4E2 (4EHP) (Genetex, catalog no. GTX103977), mouse anti-eIF4E (BD Biosciences, catalog no. 610270), rabbit anti-eIF4ENIF1 (4E-T; Abcam, catalog no. ab55881), rabbit anti-DDX6 (Bethyl Laboratories, catalog no. A300-460A), rabbit anti-CNOT1 (Proteintech, catalog no. 14276-1-AP), mouse anti–α-Tubulin (Santa Cruz, catalog no. sc-23948), mouse anti–β-actin (Sigma, catalog no. A5441), mouse anti-Flag (Sigma, catalog no. F3165), rabbit anti-HA (Sigma, catalog no. H6908), anti-V5 tag (Invitrogen, catalog no. R960-25), and anti-His tag (Abcam, catalog no. ab18184).
The following siRNA and shRNAs were used: ON-TARGETplus Nontargeting Control Pool (Dharmacon, catalog no. L-001810-10-05), eIF4ENIF1 (4E-T) siRNA SMARTpool (Dharmacon, catalog no. L-013237-01), EIF4E2 siRNA SMARTpool (Dharmacon, catalog no. L-019870-01), Non-Targeting shRNA Controls (Sigma, catalog no. SHC002), and EIF4E2 shRNA (Sigma, catalog no. TRCN0000152006).
Plasmid Cloning.
For recombinant protein expression, the 4E-T1–265 and full-length 4EHP ORF contained in BamHI–NotI cDNA fragments were cloned into the pGEX-2T and pProEX HTb vectors in frame with the GST or His tag (6xHis), respectively. To generate the pCI-RLuc-5BoxB-A114-N40-HhR vector, a fragment containing the A114-N40-HhR was obtained by PCR from the pAWH-RL-let-7-A114-N40-HhR (36) (Addgene plasmid 50555) and inserted into the pCI-RLuc-5BoxB. A similar strategy was used to generate the pRL-A114-N40-HhR vector. To create λN-V5 expression plasmids, PCR was used to construct cDNAs encoding CNOT1 (amino acids 1–2,376, 1–690, 1,030–1,600, and 1,830–2,376), which were inserted as XhoI–XbaI fragments into the pCI-λN-V5 plasmid. λN-HA-GW182(SD) (TNRC6C; amino acids 1,382–1,690) was described (7). The 3xFlag–4EHP-overexpressing plasmid was created by cloning the 4EHP ORF into the p231 pcDNA5 FRT TO 3xFlag vector using NheI and BamHI restriction enzymes. The 4EHP-NT-BirA and eIF4E-NT-BirA plasmids were created by cloning the relevant ORF into the pcDNA5 FRT/TO Flag-BirAR118G using the BsrI and NotI or AscI and NotI restriction enzymes, respectively. Similarly, the 4EHP-CT-BirA and eIF4E-CT-BirA plasmids were created by cloning the relevant ORF into the pcDNA5 FRT/TO MCS-BirAR118G-Flag using the BsrI and NotI or AscI and NotI restriction enzymes, respectively.
Generation of Stable Cell Lines for BioID Assay.
Flp-In T-REx HEK293 cells were transfected by using jetPRIME transfection reagent (Polyplus) according to the manufacturer’s specifications. Briefly, cells were seeded in six-well plates with DMEM supplemented with 5% FBS, 5% Cosmic calf serum, and 100 U/mL Pen/Strep and transfected with 100 ng of pcDNA5-BAIT_PROTEIN-BirA*-FLAG and 1 µg of POG44 (Flp recombinase). Transfected cells were passaged into 10-cm plates, and 48 h after transfection were treated with hygromycin B (200 µg/mL) for stable selection of integrated cells. Selection medium was changed every 2–3 d until clear visible colonies were present. Mixed clonal populations were pooled and scaled up into three 15-cm plates (one to freeze and two for BioID).
BioID; Affinity Purification, and Trypsin Digestion.
For BioID experiments, stable cells were grown to ∼75% confluency. Bait expression vectors and biotin were induced simultaneously (1 µg/mL tetracycline and 50 µM biotin). After 24 h of treatment, cells were rinsed once on the plate with ∼20 mL of PBS, then scraped into 1 mL of PBS. Cell pellets were collected by centrifugation (500 × g for 5 min) and stored at −80 °C for further processing. Cell pellets were thawed on ice, and their weight was measured. A 4:1 (vol/wt) ratio of ice-cold lysis buffer was added to the cells (50 mM Tris⋅HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.4% SDS, 1.5 mM MgCl2, 1 mM EGTA, benzonase, and Sigma protease inhibitors). Cells were dispersed with a P1000 pipette tip (∼10–15 aspirations) and subjected to a rapid freeze/thaw cycle (dry ice to 37 °C water bath). Lysates were rotated at 4 °C for 30 min and then centrifuged at 16,000 × g for 20 min at 4 °C. Supernatant was collected (with 20 µL of aliquot saved for Western blot) into new tubes for affinity purification (AP). Samples were incubated with 20 µL (packed beads) of streptavidin-Sepharose (GE) (equilibrated in lysis buffer) with rotation overnight at 4 °C. Beads were collected (500 × g for 2 min), the supernatant was discarded, and the beads were transferred to new tubes in 500 µL of lysis buffer. Beads were washed once with SDS wash buffer (50 mM Tris⋅HCl, pH 7.5, and 2% SDS), twice with lysis buffer, and three times with 50 mM ammonium bicarbonate (pH 8.0; ABC) (all wash volumes = 500 µL with centrifugations at 500 × g for 30 s). Beads were resuspended in 100 µL of ABC containing 1 µg of sequencing-grade trypsin and gently mixed at 37 °C for 4 h. A total of 1 µg fresh trypsin was added, and the samples were rotated overnight. Supernatant was collected (500 × g for 2 min), and the beads were washed with 100 µL of molecular biology-grade H2O and pooled with peptides. Digestion was terminated by acidification with formic acid (50 µL of 10% stock = 2% final concentration). Samples were then centrifuged (16,000 × g for 5 min), and ∼90% of the sample was transferred to a new tube and dried with vacuum centrifugation.
MS.
Dried peptides were resuspended in 10 µL of 2% formic acid, and 1.5 µL was analyzed by MS. Samples were injected by autosampler onto a spray tip formed from a fused silica capillary column (0.75 µm i.d., 350 µm o.d.) using a laser puller. The column had previously been loaded with 10–12 cm of C18 reversed-phase material (ZorbaxSB; 3.5 µm) by pressure bomb loading in MeOH and preequilibrated with buffer A. The column was placed in-line with a 5600 TripleTOF mass spectrometer (Sciex) equipped with a nanoelectrospray ion source connected in-line to a NanoLC-Ultra 2D plus HPLC system (Eksigent). Buffer A was 0.1% formic acid in water; buffer B was 0.1% formic acid in ACN. The HPLC gradient delivered an acetonitrile gradient over 120 min (2–35% buffer B over 85 min, 40–60% buffer B over 5 min, 60–90% buffer B over 5 min, hold buffer B at 90% 8 min, and return to 2% B at 105 min). The instrument was operated in the data-dependent acquisition mode with 1 MS scan (250 ms; mass range 400–1,250) followed by up to 20 MS/MS scans (50 ms each). Only candidate ions that were between two and five charge states were considered, and ions were dynamically excluded for 20 s with a 50-mDa window. The isolation width = 0.7, and minimum threshold = 200.
MS Data Analysis.
All raw (WIFF and WIFF.SCAN) files were saved in our local interaction proteomics LIMS, ProHits (46). mzXML files were generated from raw files by using the ProteoWizard converter (version 3.0.4468) and SCIEX converter (version 1.3 beta), implemented within ProHits. The searched database contained the human complement of the RefSeq protein database (version 57) complemented with SV40 large T-antigen sequence (72,226 sequences searched, including reversed sequences). mzXML files were searched by Mascot (version 2.3.02) and Comet (version 2016.01 revision 2) using the following parameters: up to two missed trypsin cleavage sites, methionine oxidation, and asparagine/glutamine deamidation as variable modifications. The fragment mass tolerance was 0.15 Da, and the mass window for the precursor was ±40 ppm with charges of 2+ to 4+ (both monoisotopic mass). Search engine results were analyzed by using the Trans-Proteomic Pipeline (TPP version 4.6 OCCUPY revision 3) (47) via iProphet (48). Peptides were initially matched to proteins, and this information was then used to map peptides to genes (protein and gene IDs were acquired from RefSeq). If peptides were shared between multiple genes, spectral counts were assigned exclusively to those genes with unique peptide assignments proportionally to the evidence for that assignment. If peptides matched only to genes without unique peptide assignments, spectral counts were divided equally between those genes (49). SAINTexpress (version 3.6.1) (30) was used to calculate the probability that identified proteins were significantly enriched above background contaminants using spectral counting (semisupervised clustering) through comparing bait runs to a series of negative control runs. Bait proteins were run as biological duplicates and analyzed alongside 12 independent negative control runs compressed to 6 (consisting of purifications from cells expressing 3xFlag only (6 runs) and BirA-Flag only (6 runs). A BFDR of 1% or less was considered a “high-confidence” proximity interaction.
Visualization in Fig. 1A was generated by using tools now available at prohits-viz.lunenfeld.ca/ (50). Essentially, each prey detected as a high confidence proximity interactor with at least one bait (FDR ≤ 1%) is displayed, alongside its quantitative values across all baits. Quantitation is encoded by the node color (mapping to averaged spectra, capped to 50), and relative quantification across the baits is encoded by node size. Statistical significance of each bait–prey interaction is encoded as edge color. GO analysis was performed within ProHits-viz running g:Profiler (51).
Lentivirus Production.
A total of 8 × 106 HEK293FT (Thermo Scientific, catalog no. R70007) cells were cultured in a 10-cm dish for 24 h in high-glucose DMEM supplemented with 10% (vol/vol) FBS. Medium was replaced by OptiMEM (Thermo Scientific, catalog no. 51985091) 30 min before transfection. Lentivirus particles were produced by transfecting the HEK293FT cells using Lipofectamine 2000 and 10 µg of shRNA plasmid, 6.5 µg of psPAX2 (Addgene, plasmid 12260), and 3.5 µg of pMD2.G (Addgene, plasmid 12259) packaging plasmids. At 5 h after transfection, the medium was replaced with fresh high-glucose DMEM supplemented with 10% (vol/vol) FBS. Supernatant was collected at 48 h after transfection, replaced with fresh medium, and harvested again after 24 h. Viral particles were cleared by filtration (0.45 µm; Fisher Scientific, catalog no. 09-720-005) and used to infect the cells directly in the presence of 6 µg/mL polybrene (Sigma, catalog no. H9268).
miRNA Isolation and Quantitation.
Total RNA were isolated by using the mirVana miRNA Isolation Kit (Thermo Scientific) according to the manufacturer’s recommendations. TaqMan miRNA assays were used to quantify the expression levels of mature let-7a (Thermo Scientific, catalog no. 000377), miR-19a (Thermo Scientific, catalog no. 000395), miR-17a (Thermo Scientific, catalog no. 002308), and miR-20a (Thermo Scientific, catalog no. 000580). Total RNA (10 ng) was reverse-transcribed by the TaqMan microRNA reverse transcription kit (Thermo Scientific) in a reaction mixture containing the respective miR-specific stem-loop reverse-transcription (RT) primer. The detection of mature miRNAs was performed by using a universal PCR master mix containing TaqMan primers without AmpErase UNG. The 18S rRNA (Thermo Scientific, 4333760T) was amplified as an internal control. The relative quantitation of each miRNA was performed by using the comparative Ct method. All RT reactions, including no-template controls, were run in triplicate.
Luciferase Assays.
For experiments with miRNA reporters, U251, HeLa, and MEFs were cotransfected in a 24-well plate with 20 ng of pGL3-E2f 3′ UTR (52), pmiRGLO-3xmiR-19 (53), and pIS1-Hmga2 3′ UTR (34), respectively. The pIS1 and pGL3 reporters were cotransfected with 5 ng of FL and RL plasmids, respectively. In tethering experiments, shCTR or sh4EHP HEK293T cells were transfected with 50 ng of RL-5BoxB-A114-N40-HhR, 10 ng of FL, and 50 ng of λN-fusion constructs per well in a 24-well plate by using Lipofectamine 2000 (Thermo Scientific, catalog no. 11668019) according to the manufacturer’s instructions. For complementation experiments, 10 ng of pcDNA5-3xFlag-4EHP (WT or Mut) were also added to the transfection mixtures. For 4EHP and 4E-T knockdown, 2 × 106 cells were plated in a 10-cm culture dish and transfected with a final concentration of 25 nM siRNA duplexes using Lipofectamine 2000 according to the manufacturer’s instructions. After 24 h, cells were plated in a 24-well plate and transfected a second time with the plasmid mixture as described. Cells were lysed 24 h after transfection. Luciferase activities were measured with the Dual-Luciferase Reporter Assay System (Promega) in a GloMax 20/20 luminometer (Promega). RL activity was normalized to the activity of coexpressed FL, and the normalized RL values are shown as repression fold relative to the indicated control.
Expression and Purification of Recombinant Proteins.
pEt15b-eIF4E (1–217) and pProEX HTb-4EHP (1–245) were transformed into Rossetta2 (DE3) competent cells (Novagen) for expression. Cells were grown to an OD of ∼0.8 at 37 °C. Expression of eIF4E and 4EHP proteins were induced by the addition of 0.5 mM isopropylthio-β-galactoside and grown for an additional 16 h at 25 °C. Cells were harvested and suspended in 20 mL of lysis buffer per liter of cell cultured (lysis buffer: 20 mM Hepes-KOH, pH 7.6, 0.1 mM EDTA, 0.5 mM TCEP, 10% glycerol, 300 mM KCl, 15 mM imidazole, and 0.5% IGEPAL CA-630) containing lysozyme, RNase, and DNase and PMSF. The cells were disrupted by sonication and centrifuged for 45 min at 75,000 × g. The supernatant was applied onto Ni-NTA agarose beads (Qiagen) equilibrated in lysis buffer. The beads were then washed with 10 column volume of lysis buffer, and proteins were eluted in buffer containing 20 mM Hepes-KOH (pH 7.6), 0.1 mM EDTA, 0.5 mM TCEP, 300 mM KCl, and 250 mM imidazole. Proteins were dialyzed overnight in buffer containing 20 mM Hepes-KOH (pH 7.6), 0.1 mM EDTA, 0.5 mM TCEP, and 75 mM KCl and applied onto a Heparin Sepharose 6 fast flow (GE Healthcare). Heparin beads were washed with 10 column volumes of binding buffer and eluted in buffer containing 500 mM KCl. The concentrated eluate was applied for size-exclusion chromatography to a Superdex75 16/10 preparative column (GE Healthcare) equilibrated with buffer containing 20 mM Hepes-KOH (pH 7.6), 0.5 mM TCEP, and 100 mM KCl.
Binding and Displacement Assays.
HA-4ET plasmid (5 µg) was transfected into HEK293H cells (5.5 × 106 cells in a 10-cm plate) and collected 24 h after transfection. Cells were suspended in 1 mL of lysis buffer containing 20 mM Hepes-KOH (pH 7.6), 200 mM KCl, 5 mM MgCl2, 0.5 mM TCEP, 0.5% IGEPAL CA-630, protease inhibitors, RNase, and DNase. Cells were lysed for 5 min on ice, followed by centrifugation at 18,000 × g for 5 min. The supernatant (5 mL) was incubated with 100 µL of preequilibrated pierce anti-HA magnetic beads (Thermo Scientific) for 2 h at 4 °C. Collected beads were washed with lysis buffer containing 0.2% IGEPAL CA-630 (×10 column volumes) and inverted 20 times. The HA–4E-T–containing beads were then equally distributed in several tubes and incubated with increasing concentrations of His-4EHP (to determine 4EHP–4ET binding) or His-eIF4E (to determine eIF4E–4ET binding) for 1 h at 4 °C. Beads were collected and washed three times with 5 column volumes of binding buffer and suspended in SDS/PAGE loading buffer for Western blot using His antibody (cell signaling 1:2,000). Band density was determined by using ImageJ, and the graphs were made in Prism7.
For the displacement assay, expression and purification of HA–4E-T were performed as described above. Clean HA-4ET bound to HA-magnetic beads were incubated with excess of eIF4E protein 1 h at 4 °C. The excess of eIF4E was carefully removed, and the magnetic beads were washed twice with binding buffer (20 mM Hepes–KOH, pH 7.6, 150 mM KCl, 0.25 mM TCEP, and 0.2% IGEPAL CA-630). HA-4ET/eIF4E magnetic beads were equally distributed in several tubes and were incubated with increasing concentrations of His-4EHP protein for 1 h at 4 °C. Beads were collected and washed three times with 2 column volumes of binding buffer and suspended in SDS/PAGE loading buffer for Western blot. These experiments were performed in the presence of cap analog. For this method, eIF4E or 4EHP was incubated with m7GTP (1:3) molar ratio for 10 min on ice before being added to HA-4ET beads for binding or displacement assays.
Size-Exclusion Chromatography.
HEK293T cells (20 × 106) were collected by centrifugation and resuspended in 600 µL of lysis buffer containing 25 mM Hepes–KOH (pH 7.4), 150 mM KCl, 75 mM KOAc, 2 mM MgCl2, and 0.5% IGEPAL CA-630, supplemented with complete EDTA-free protease inhibitor mixture and 1 mM NaF, 1 mM Na3VO4, and 1 mM β-glycerophosphate phosphatase inhibitors. The lysate was clarified by centrifugation at 20,000 × g for 10 min at 4 °C and filtered on Nanosep MF spin columns (PALL, catalog no. ODM45C34). A total of 5 mg of the extract was directly loaded onto a 24-mL Superose 6 column (Increase HR 10/300, GE Healthcare Life Sciences), preequilibrated with lysis buffer and run in the same buffer at a flow rate of 0.5 mL/min. Molecular mass calibration was carried out by using the Gel Filtration HMW Calibration Kit (Healthcare Life Sciences, catalog no. 28-4038-42). Fractions of 0.5 mL were collected and analyzed by Western blot.
ITC.
Experiments were carried out on a MicroCal microcalorimetry systems (GE Healthcare Life Science) in 20 mM Hepes–KOH (pH 7.6), 0.5 mM TCEP, and 100 mM KCl at 20 °C. The reaction cell contained 200 μL of 30 µM protein and was titrated with 19 injections of 2 μL of 300 µM m7GTP. The binding isotherm was fit with a binding model that uses a single set of independent sites to determine the thermodynamic binding constants and stoichiometry.
Discussion
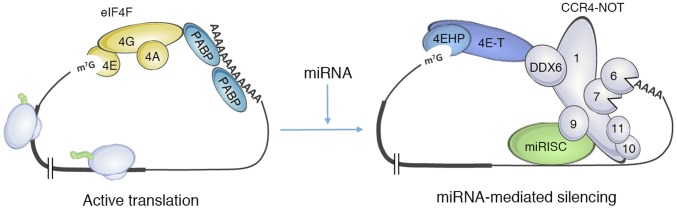
Here, we describe a role for 4EHP in miRNA-mediated translation repression. Our results demonstrate that miRISC recruits 4EHP to target mRNAs through the CCR4–NOT complex. We propose that these interactions engender a closed-loop mRNP structure (Fig. 4), which resembles the cap-to-tail closed-loop mRNA conformation (37). A similar mechanism of translational inhibition had been described in Drosophila embryos wherein an interaction between 4EHP and Bicoid bridges the 5′ and 3′ ends of caudal mRNA (23). Thus, 4EHP-mediated mRNA looping appears as a general repressive mechanism implicated in various posttranscriptional regulatory pathways. This mechanism can also be used by other RNA-binding proteins, such as pumilio family members, tristetraprolin, and nanos, which recruit CCR4–NOT to mRNAs (38).
Fig. 4.
Model of 4EHP-mediated translation repression by miRNAs. The recruitment of 4EHP to the miRNA target mRNA through the CCR4–NOT/DDX6/4E-T axis promotes its binding to the cap. The assembly of this complex is likely to initiate the formation of a closed-loop structure (Right) resembling the cap-to-tail closed-loop mRNA conformation involving eIF4G/PABP interaction (Left).
An additional layer of complexity in understanding the exact mechanism of 4EHP action stems from the interaction of 4EHP with other protein partners. For instance, the GIGYF1/2 and 4E-T proteins bind to the same motif on the 4EHP protein and thus GIGYF1/2 must compete with 4E-T for this binding site. The GIGYF1/2–4EHP complex had been characterized as a translational repressor, notably as a cofactor of tristetraprolin (26, 27). Interestingly, human GIGYF1/2 and the related yeast protein Smy2 also associate with CCR4–NOT (39, 40). Furthermore, it was reported that GIGYF2 coimmunoprecipitates with AGO2, and tethering of GIGYF2 to an mRNA leads to its silencing (41). Therefore, it is conceivable that the miRISC/CCR4–NOT axis may engage 4EHP via several parallel or exclusive interactions, such as GIGYF1/2 and 4E-T. Additional details of the recruitment of 4EHP by CCR4–NOT remain to be determined.
Our model provides a tenable mechanism of miRNA-mediated translation repression, but further investigation is necessary to fully resolve the interactions that occur at the cap of miRNA targets. An important conundrum is that the affinity of recombinant eIF4E for the cap is 30- to 100-fold greater than that of recombinant 4EHP. Although binding to 4E-T increases 4EHP’s affinity for the cap by approximately fourfold, it remains much weaker than eIF4E (Fig. S4 and Table 1). The solution to this discrepancy may be explained by additional interactions that prevail within the native miRISC effector complex and are absent in the in vitro assays. Other interactions may further improve the affinity of 4EHP for the cap. Further studies of the protein–protein interactions identified in our BioID survey may help resolve this conundrum. Alternatively, because the affinity of 4E-T for 4EHP is greater than that for eIF4E, it is possible that the interaction of 4E-T with 4EHP increases the concentration of 4EHP near the cap to compete with eIF4E for binding to the cap.
It was recently reported that 4E-T is recruited to mRNAs targeted by CCR4–NOT and that 4E-T functions as an important cofactor of the mRNA decay machinery (13). This finding raises the intriguing possibility that 4E-T may play a dual role in miRNA-mediated silencing: interaction of 4E-T with 4EHP potentiates translation repression, whereas its direct binding to eIF4E enables mRNA decay. Therefore, it would be important to determine the relative importance of eIF4E/4E-T vs. 4EHP/4E-T contributions under different circumstances.
Although significant progress had been made in understanding how miRNAs instigate mRNA deadenylation and decay, the mechanisms by which they repress translation remained unclear (1). Several recent studies demonstrated translational repression as an early step of miRNA-mediated silencing, which is followed by mRNA deadenylation and decay (4, 42, 43). Multiple studies now have shown that miRNAs interfere with translation initiation, specifically with cap recognition by eIF4E (3–5), and may induce the dissociation of eIF4E and eIF4G from target mRNAs (44). The model outlined here wherein 4E-T/4EHP interactions potentiate an interaction with the cap shines a new light on prior findings.
Materials and Methods
Cell Lines and Culture Conditions.
MEFs, HeLa, U251, HEK293FT, and H and T cells were routinely maintained in DMEM supplemented with 10% FBS and penicillin/streptomycin in a humidified atmosphere of 5% CO2 at 37 °C. Flp-In T-REx HEK293 cells (Thermo Scientific, catalog no. R78007) were grown in high-glucose DMEM (Thermo Scientific, catalog no. 11965118) supplemented with 5% FBS, 5% Cosmic calf serum, 100 U/mL penicillin, 100 µg/mL streptomycin, 2 mM l-glutamine, 100 µg/mL zeocin, and 15 µg/mL blasticidin. Cell lines expressing inducible BirA-4EHP or -eIF4E were generated as described (45) and selected and maintained in medium supplemented with 100 µg/mL hygromycin. Expression of tagged proteins was induced for 24 h by addition of tetracycline to 1 µg/mL final concentration.
Extract Preparation and Immunoprecipitation.
Cells were resuspended in a lysis buffer containing 25 mM HEPES-KOH, pH 7.4, 150 mM KCl, 75 mM KOAc, 2 mM MgCl2, 0.5% NP40, supplemented with complete EDTA-free protease inhibitor cocktail and 1 mM NaF, 1 mM Na3VO4, and 1 mM β-glycerophosphate phosphatase inhibitors, and incubated for 20 min on ice. The lysate was clarified by centrifugation at 15,000 × g for 10 min at 4 °C. One milligram of extract was used for immunoprecipitation with the indicated antibodies. Thirty microliters of preequilibrated protein G-agarose (Roche) and RNase A (Thermo Scientific) were added, and the mixtures were rotated overnight at 4 °C. Beads were washed four times with lysis buffer and directly resuspended in protein sample buffer.
Supplementary Material
Acknowledgments
We thank Devon Merkley, Pudchalaluck Panichnantakul, and Eliana Sacher for technical assistance; Maayan Shapiro, Mark A. Hancock, and Nadeem Siddiqui for discussions; and Chris Rouya, Masahiro Morita, T. Yamamoto, N. Gehring, and Y. Tomari for reagents. This work was supported by Canadian Institute of Health Research (CIHR) Grants MOP-7214 (to N.S.), MOP 123352 (to T.F.D.), and FDN 143301 (to A.-C.G.); Terry Fox Research Institute Grant TFF-122868 (to N.S.); and National Science and Engineering Research Council Grant RGPIN-2014-06434 (to A.-C.G.). T.F.D. is supported by the Fonds de la Recherche en Santé du Québec (FRQS) Chercheur-Boursier Senior Salary Award. S.M.J. is the recipient of a CIHR postdoctoral fellowship. C.C. is supported by FRQS and Fondation pour la Recherche Médicale postdoctoral fellowships. E.M.-C. is supported by Groupe de Recherche Axé sur la Structure des Protéines. G.G.H. is supported by a Parkinson Canada Basic Research Fellowship.
Footnotes
The authors declare no conflict of interest.
Data deposition: The dataset has been deposited in the MassIVE database (ID MSV000080503; available for FTP download at massive.ucsd.edu/ProteoSAFe/status.jsp?task=766a1eeee437427a95e3ca76806876b3). The dataset has also been deposited in the ProteomeXchange Consortium, proteomecentral.proteomexchange.org (identifier PXD005789).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701488114/-/DCSupplemental.
References
- 1.Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet. 2015;16:421–433. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 2.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pillai RS, et al. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 4.Mathonnet G, et al. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science. 2007;317:1764–1767. doi: 10.1126/science.1146067. [DOI] [PubMed] [Google Scholar]
- 5.Humphreys DT, Westman BJ, Martin DI, Preiss T. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc Natl Acad Sci USA. 2005;102:16961–16966. doi: 10.1073/pnas.0506482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun JE, Huntzinger E, Fauser M, Izaurralde E. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol Cell. 2011;44:120–133. doi: 10.1016/j.molcel.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Chekulaeva M, et al. miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat Struct Mol Biol. 2011;18:1218–1226. doi: 10.1038/nsmb.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabian MR, et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat Struct Mol Biol. 2011;18:1211–1217. doi: 10.1038/nsmb.2149. [DOI] [PubMed] [Google Scholar]
- 9.Ozgur S, et al. Structure of a human 4E-T/DDX6/CNOT1 complex reveals the different interplay of DDX6-binding proteins with the CCR4-NOT complex. Cell Reports. 2015;13:703–711. doi: 10.1016/j.celrep.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Waghray S, Williams C, Coon JJ, Wickens M. Xenopus CAF1 requires NOT1-mediated interaction with 4E-T to repress translation in vivo. RNA. 2015;21:1335–1345. doi: 10.1261/rna.051565.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamenska A, et al. Human 4E-T represses translation of bound mRNAs and enhances microRNA-mediated silencing. Nucleic Acids Res. 2014;42:3298–3313. doi: 10.1093/nar/gkt1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamenska A, et al. The DDX6-4E-T interaction mediates translational repression and P-body assembly. Nucleic Acids Res. 2016;44:6318–6334. doi: 10.1093/nar/gkw565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura T, et al. The eIF4E-binding protein 4E-T is a component of the mRNA decay machinery that bridges the 5′ and 3′ termini of target mRNAs. Cell Reports. 2015;11:1425–1436. doi: 10.1016/j.celrep.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, et al. A DDX6-CNOT1 complex and W-binding pockets in CNOT9 reveal direct links between miRNA target recognition and silencing. Mol Cell. 2014;54:737–750. doi: 10.1016/j.molcel.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Mathys H, et al. Structural and biochemical insights to the role of the CCR4-NOT complex and DDX6 ATPase in microRNA repression. Mol Cell. 2014;54:751–765. doi: 10.1016/j.molcel.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Rouya C, et al. Human DDX6 effects miRNA-mediated gene silencing via direct binding to CNOT1. RNA. 2014;20:1398–1409. doi: 10.1261/rna.045302.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dostie J, Ferraiuolo M, Pause A, Adam SA, Sonenberg N. A novel shuttling protein, 4E-T, mediates the nuclear import of the mRNA 5′ cap-binding protein, eIF4E. EMBO J. 2000;19:3142–3156. doi: 10.1093/emboj/19.12.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi B, Cameron A, Jagus R. Characterization of mammalian eIF4E-family members. Eur J Biochem. 2004;271:2189–2203. doi: 10.1111/j.1432-1033.2004.04149.x. [DOI] [PubMed] [Google Scholar]
- 19.Rom E, et al. Cloning and characterization of 4EHP, a novel mammalian eIF4E-related cap-binding protein. J Biol Chem. 1998;273:13104–13109. doi: 10.1074/jbc.273.21.13104. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm M, et al. Mass-spectrometry-based draft of the human proteome. Nature. 2014;509:582–587. doi: 10.1038/nature13319. [DOI] [PubMed] [Google Scholar]
- 21.Kubacka D, et al. Investigating the consequences of eIF4E2 (4EHP) interaction with 4E-transporter on its cellular distribution in HeLa cells. PLoS One. 2013;8:e72761. doi: 10.1371/journal.pone.0072761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuberek J, et al. Weak binding affinity of human 4EHP for mRNA cap analogs. RNA. 2007;13:691–697. doi: 10.1261/rna.453107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho PF, et al. A new paradigm for translational control: inhibition via 5′-3′ mRNA tethering by Bicoid and the eIF4E cognate 4EHP. Cell. 2005;121:411–423. doi: 10.1016/j.cell.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Cho PF, et al. Cap-dependent translational inhibition establishes two opposing morphogen gradients in Drosophila embryos. Curr Biol. 2006;16:2035–2041. doi: 10.1016/j.cub.2006.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villaescusa JC, et al. Cytoplasmic Prep1 interacts with 4EHP inhibiting Hoxb4 translation. PLoS One. 2009;4:e5213. doi: 10.1371/journal.pone.0005213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morita M, et al. A novel 4EHP-GIGYF2 translational repressor complex is essential for mammalian development. Mol Cell Biol. 2012;32:3585–3593. doi: 10.1128/MCB.00455-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu R, Olsen MT, Webb K, Bennett EJ, Lykke-Andersen J. Recruitment of the 4EHP-GYF2 cap-binding complex to tetraproline motifs of tristetraprolin promotes repression and degradation of mRNAs with AU-rich elements. RNA. 2016;22:373–382. doi: 10.1261/rna.054833.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uniacke J, et al. An oxygen-regulated switch in the protein synthesis machinery. Nature. 2012;486:126–129. doi: 10.1038/nature11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012;196:801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teo G, et al. SAINTexpress: Improvements and additional features in Significance Analysis of INTeractome software. J Proteomics. 2014;100:37–43. doi: 10.1016/j.jprot.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 32.Marnef A, Standart N. Pat1 proteins: A life in translation, translation repression and mRNA decay. Biochem Soc Trans. 2010;38:1602–1607. doi: 10.1042/BST0381602. [DOI] [PubMed] [Google Scholar]
- 33.Barišić-Jäger E, Kręcioch I, Hosiner S, Antic S, Dorner S. HPat a decapping activator interacting with the miRNA effector complex. PLoS One. 2013;8:e71860. doi: 10.1371/journal.pone.0071860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron-Benhamou J, Gehring NH, Kulozik AE, Hentze MW. Using the lambdaN peptide to tether proteins to RNAs. Methods Mol Biol. 2004;257:135–154. doi: 10.1385/1-59259-750-5:135. [DOI] [PubMed] [Google Scholar]
- 36.Fukaya T, Tomari Y. MicroRNAs mediate gene silencing via multiple different pathways in Drosophila. Mol Cell. 2012;48:825–836. doi: 10.1016/j.molcel.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 37.Kahvejian A, Roy G, Sonenberg N. The mRNA closed-loop model: The function of PABP and PABP-interacting proteins in mRNA translation. Cold Spring Harb Symp Quant Biol. 2001;66:293–300. doi: 10.1101/sqb.2001.66.293. [DOI] [PubMed] [Google Scholar]
- 38.Chapat C, Corbo L. Novel roles of the CCR4-NOT complex. Wiley Interdiscip Rev RNA. 2014;5:883–901. doi: 10.1002/wrna.1254. [DOI] [PubMed] [Google Scholar]
- 39.Ash MR, et al. Conserved beta-hairpin recognition by the GYF domains of Smy2 and GIGYF2 in mRNA surveillance and vesicular transport complexes. Structure. 2010;18:944–954. doi: 10.1016/j.str.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 40.Ajiro M, et al. Involvement of RQCD1 overexpression, a novel cancer-testis antigen, in the Akt pathway in breast cancer cells. Int J Oncol. 2009;35:673–681. [PubMed] [Google Scholar]
- 41.Kryszke MH, Adjeriou B, Liang F, Chen H, Dautry F. Post-transcriptional gene silencing activity of human GIGYF2. Biochem Biophys Res Commun. 2016;475:289–294. doi: 10.1016/j.bbrc.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Béthune J, Artus-Revel CG, Filipowicz W. Kinetic analysis reveals successive steps leading to miRNA-mediated silencing in mammalian cells. EMBO Rep. 2012;13:716–723. doi: 10.1038/embor.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Djuranovic S, Nahvi A, Green R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336:237–240. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zekri L, Kuzuoğlu-Öztürk D, Izaurralde E. GW182 proteins cause PABP dissociation from silenced miRNA targets in the absence of deadenylation. EMBO J. 2013;32:1052–1065. doi: 10.1038/emboj.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spitzer J, Landthaler M, Tuschl T. Rapid creation of stable mammalian cell lines for regulated expression of proteins using the Gateway® recombination cloning technology and Flp-In T-REx® lines. Methods Enzymol. 2013;529:99–124. doi: 10.1016/B978-0-12-418687-3.00008-2. [DOI] [PubMed] [Google Scholar]
- 46.Liu G, et al. ProHits: Integrated software for mass spectrometry-based interaction proteomics. Nat Biotechnol. 2010;28:1015–1017. doi: 10.1038/nbt1010-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deutsch EW, et al. A guided tour of the Trans-Proteomic Pipeline. Proteomics. 2010;10:1150–1159. doi: 10.1002/pmic.200900375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shteynberg D, et al. iProphet: Multi-level integrative analysis of shotgun proteomic data improves peptide and protein identification rates and error estimates. Mol Cell Proteomics. 2011;10:M111.007690. doi: 10.1074/mcp.M111.007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu G, et al. Data independent acquisition analysis in ProHits 4.0. J Proteomics. 2016;149:64–68. doi: 10.1016/j.jprot.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knight JD, et al. A web-tool for visualizing quantitative protein-protein interaction data. Proteomics. 2015;15:1432–1436. doi: 10.1002/pmic.201400429. [DOI] [PubMed] [Google Scholar]
- 51.Reimand J, et al. g:Profiler-a web server for functional interpretation of gene lists (2016 update) Nucleic Acids Res. 2016;44:W83–W89. doi: 10.1093/nar/gkw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 53.Mayya VK, Duchaine TF. On the availability of microRNA-induced silencing complexes, saturation of microRNA-binding sites and stoichiometry. Nucleic Acids Res. 2015;43:7556–7565. doi: 10.1093/nar/gkv720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.