Significance
Methods for studying functional genomics of ion-channel target sites of antinematodal drugs (anthelmintics) have been limited to the model nematode Caenorhabditis elegans because many techniques for studying animal parasitic nematodes have been unsuccessful. Here, we develop preparations of the human parasite Brugia malayi, which causes elephantiasis, to allow us to combine RNAi, PCR amplification of RNA from single muscle cells, computer analysis of movement, and patch-clamp electrophysiology to study the action of cholinergic anthelmintics. We find that they are not a homogenous class of drugs and that they act selectively on four distinguishable, M-, L-, P-, and N-acetylcholine ion-channel receptors. These four receptors are distinct druggable targets with different physiological functions.
Keywords: filaria, Brugia, dsRNA, nAChR, patch clamp
Abstract
Many techniques for studying functional genomics of important target sites of anthelmintics have been restricted to Caenorhabditis elegans because they have failed when applied to animal parasites. To overcome these limitations, we have focused our research on the human nematode parasite Brugia malayi, which causes elephantiasis. Here, we combine single-cell PCR, whole muscle cell patch clamp, motility phenotyping (Worminator), and dsRNA for RNAi for functional genomic studies that have revealed, in vivo, four different muscle nAChRs (M-, L-, P-, and N-). The cholinergic anthelmintics had different selectivities for these receptors. We show that motility and patch-clamp responses to levamisole and pyrantel, but not morantel or nicotine, require the unc-38 and/or unc-29 genes. Derquantel behaved as a competitive antagonist and distinguished M-nAChRs activated by morantel (Kb 13.9 nM), P-nAChRs activated by pyrantel (Kb 126 nM), and L-nAChRs activated by levamisole (Kb 0.96 µM) and bephenium. Derquantel was a noncompetitive antagonist of nicotine, revealing N-type nAChRs. The presence of four diverse nAChRs on muscle is perhaps surprising and not predicted from the C. elegans model. The diverse nAChRs represent distinguishable drug targets with different functions: Knockdown of unc-38+unc-29 (L- and/or P-receptors) inhibited motility but knockdown of acr-16+acr-26 (M- and/or N-receptors) did not.
The neglected tropical diseases caused by nematode parasites affect some 2 billion people globally, and effective vaccines are not available. Although these parasites by themselves do not produce high mortality, they produce significant morbidity that affects worker productivity and increase susceptibility to AIDS and malaria, and they are strongly associated with poverty in endemic countries (1–4). There are only a limited number of classes of anthelmintic drugs that are used to treat these diseases, and there are concerns about the development of resistance (5). There is a pressing need to advance the study of anthelmintic drugs.
Many useful studies on modes of action of anthelmintic drugs have been conducted on the model nematode, Caenorhabditis elegans, but this is not a parasite: It is separated evolutionarily by millions of years from parasitic nematodes and it does not possess “parasitism genes” (6). This separation increases the impetus for anthelmintic studies using real parasites, but until now many techniques developed in C. elegans have not have been tractable when applied to animal parasites (7).
The neuromuscular systems of nematodes, which control motility and support feeding, growth, and reproduction, are governed by significant parts of their gene pools (8, 9). Their neuromuscular systems are unlike those of vertebrates because nematode muscles send processes to the motor neurons to form synapses, rather than the other way around. Membrane ion-channels, which regulate nematode neuromuscular systems, are target sites of major classes of anthelmintic drugs, including the cholinergic anthelmintics levamisole, pyrantel, and derquantel (10–17). Here, we have combined a number of techniques for the study of muscle pentameric acetylcholine receptor channels (nAChRs) of the filarial nematode parasite, Brugia malayi. Filariasis is one of a group of neglected tropical diseases caused by clade III parasitic nematodes that are transmitted by biting insects. B. malayi adults are found in the lymphatic vessels of humans, producing lymphatic filariasis, sometimes associated with swelling of the limbs (elephantiasis). B. malayi is also a suitable model parasite because it can be maintained in laboratory rodents, unlike a more common cause of lymphatic filariasis Wuchereria bancrofti. Lymphatic filariasis occurs in some 120 million people in developing countries, and regrettably, effective treatments for adult filaria infections are limited. Although a triple combination of ivermectin, diethylcarbamazine, and albendazole seems to be effective (18), development of new treatments to kill adults is still required (19, 20). Cholinergic anthelmintics (levamisole, haloxon) are not regularly used for the treatment of B. malayi, but there are reports of their efficacy (21, 22) that have encouraged us to study their nAChRs as anthelmintic targets.
To study the functional genomics of nAChRs of B. malayi, we used the following: (i) video capture for quantitative phenotyping (23, 24); (ii) single-cell PCR to identify nAChR subunit genes expressed in muscle (25); (iii) dsRNA for RNAi knockdown of unc-38+unc-29 of nematode levamisole and pyrantel receptors (26, 27), and (iv) whole-cell patch clamp to record functional properties of muscle nAChRs (10, 28). Knockdown of acr-16+acr-26 had little effect on motility, but knockdown of unc-38+unc-29 abolished motility, suggesting in B. malayi that different physiological functions may be ascribed to different receptors. Our observations revealed that there are four types of nAChR in B. malayi muscle. Two types, which require UNC-38 and/or UNC-29 subunits, support motility: They are the L-type, which is preferentially activated by levamisole, and the P-type, which is preferentially activated by pyrantel. The two other types, which persisted in the absence of UNC-38 and UNC-29 and which did not maintain motility, were the M-type, which was preferentially activated by morantel and preferentially antagonized by derquantel, and the N-type, which was preferentially activated by nicotine. Separation of the four muscle receptors is significant and contrasts with the model nematode, C. elegans, where only N- and L-receptors are recognized (29). The four receptors could be targeted by future combinations of single receptor selective cholinergic anthelmintics or one that is equally potent on all of the receptors. Either of these approaches are anticipated to overcome or slow the development of resistance due to mutation at a single receptor.
Results
Cholinergic Anthelmintics Inhibit Whole B. malayi Motility.
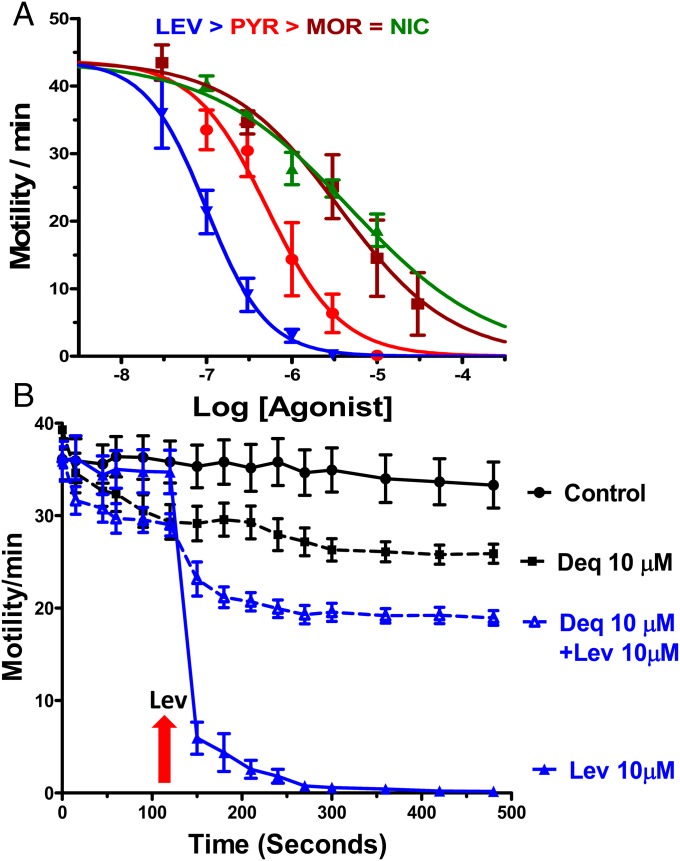
Fig. 1A shows the concentration-dependent inhibitory effects of cholinergic agonist anthelmintics on motility of B. malayi adult females. The effects of each of these agonists were rapid and occurred within 5 min of application. We quantitated the motility of the adult B. malayi by using Worminator motility measurements at 5 min to compare the potencies of the anthelmintics. Levamisole was the most potent with an IC50 of 99 ± 2 nM; pyrantel was less potent, IC50 = 516 ± 30 nM; as was morantel IC50 = 3.7 ± 0.7 µM; and nicotine, IC50 = 4.8 ± 0.8 µM. The potency series was: levamisole > pyrantel > morantel = nicotine. We also tested the effects of a cholinergic antagonist, derquantel, and found that it was the least potent of the anthelmintics on motility inhibition: Even high concentrations of derquantel (10 µM) only had a small (∼30%) inhibitory effect on motility (Fig. 1B). Interestingly, despite a poor effect on motility, derquantel antagonized and inhibited the effects of the cholinergic agonists. Fig. 1B shows the inhibitory effect of derquantel on the actions of levamisole on whole-worm motility, indicating that its low potency inhibiting motility is not due to the failure to permeate the cuticle of B. malayi because it inhibits the effects of levamisole. To examine differences between the actions of these cholinergic anthelmintics, we conducted single-muscle cell RT-PCR to identify nAChR subunits and whole-muscle cell patch clamp to characterize receptors.
Fig. 1.
(A) Concentration response plots of the effects of levamisole, pyrantel, morantel, and nicotine on motility of whole adult female worms. Each point is the mean ± SE of four worms, each from a different batches of worms. Curves were fitted with variable slope nonlinear regression model analysis in GraphPad Prism 6. Concentration response curves for different agonists: LEV, levamisole 99.2 ± 2.3 nM; PYR, pyrantel 516 ± 3 nM; MOR, morantel 3.7 ± 0.7 µM; and NIC, nicotine 4.8 ± 0.8 µM (n = 4 for each concentration of agonist, four biological replicates). (B) Effects over time on motility of whole B. malayi of: 10 µM derquantel (Deq 10 µM); derquantel pretreatment followed by 10 µM levamisole (Deq 10 µM + Lev 10 µM, levamisole at the arrow); 10 µM levamisole (Lev 10 µM, levamisole at the arrow). Note that derquantel only has a modest effect on motility by itself, but clearly, it reduces the inhibitory effect of levamisole. n = 12, four biological replicates, two-way ANOVA and Bonferroni post hoc tests: ***P < 0.001; **P < 0.01.
Single-Cell PCR Reveals Expression of Multiple nAChR Subunits.
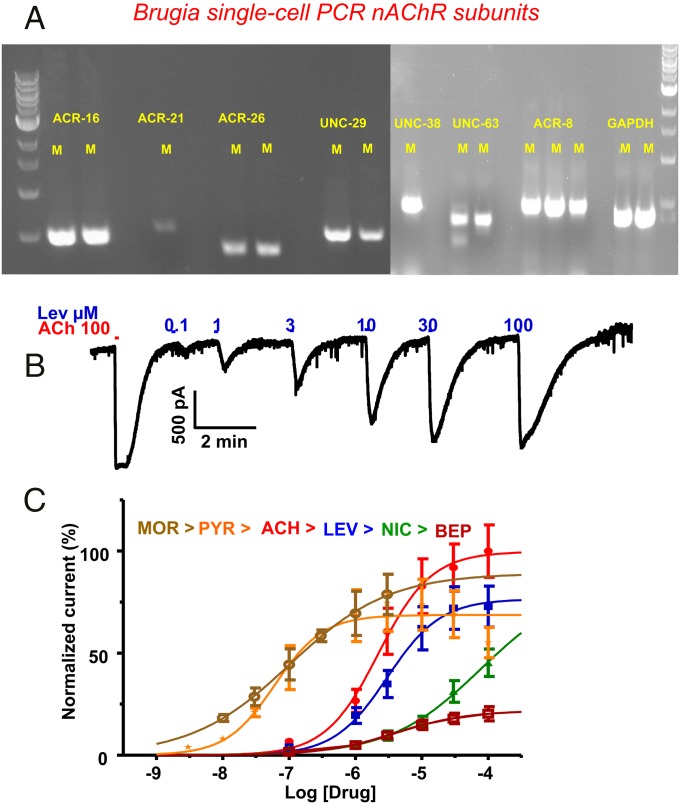
We are able to collect cytoplasm from individual somatic muscle cells of B. malayi for PCR analysis. Suction on the whole muscle-cell patch pipette following recording allowed us to collect cytoplasm from single muscle cells. After collection of cytoplasm, the 2-mm tip of the patch pipette was broken off and placed in a single-step RT-PCR mix. We designed specific primer pairs (Table S1) to look for and were able to detect amplicons in each of six muscle cells per subunit: Bma-acr-8, Bma-acr-16, Bma-acr-21, Bma-acr-8, Bma-unc-63, Bma-unc-38, Bma-unc-29, and Bma-acr-26 (Fig. 2A), along with the housekeeping gene, gapdh (30). Although we confirmed the expression of Bma-acr-27 in whole B. malayi worms, expression in single muscle cells was more difficult to detect, and amplicons were visible only in 2 of 8 single muscle cells tested (Fig. S1). Recovery of mRNA for these different subunits encouraged the view that multiple nAChRs are expressed in single B. malayi somatic muscles and that the receptor types differ from those seen in C. elegans where only levamisole-sensitive, L-type, and the nicotine-sensitive, N-type, of nAChR are observed (31). Clearly there are additional subunits present indicating the presence of additional nAChR subtypes.
Table S1.
Primer sequences used for RT-PCR and qPCR experiments
| Subunits | Primers | BP |
| Bma-unc-29 | F: 5′-GAATTCTTCCCGTTCGATGA-3′ | 183 |
| R: 5′-GATGCGTGAACGCTTATTGA-3′ | ||
| Bma-unc-38 | F: 5′-CGGGGGTGTAAGTGTGCTAT-3′ | 198 |
| R: 5′-TCGAAGGGAAACCATCTGAC-3′ | ||
| Bma-acr-16 | F: 5′-AATCGACCAGGAGTTCATCT-3′ | 200 |
| R: 5′-GACATTCAACGTCCACTGAA-3′ | ||
| Bma-acr-26 | F: 5′-GTTCTTCTTGCATTCTCGGT-3′ | 170 |
| R: 5′-TCAAATGGACCACGATGATG-3′ | ||
| Bma-acr-27 | F: 5′-TCAGTAGAACTACCAAATATTCCGGA-3′ | 201 |
| R: 5′- TATTGAAAATATGGGTGAGTGTAAG-3′ | ||
| Bma-unc-63 | F: 5′-TCGCAACTCCTTGACGTTGT-3′ | 191 |
| R: 5′-GGCACGCCAAGTATATCCCA-3′ | ||
| Bma-acr-21 | F: 5′-TGGACAGCACGGTAATCCAG-3′ | 241 |
| R: 5′-TCCCTCCGATCGTTTTCAGC-3′ | ||
| Bma-acr-8 | F: 5′-GCATCAATCCTCTTATCCGT-3′ | 218 |
| R: 5′-CCTGATGATCTATTGCTGCT-3′ | ||
| Bma-gapdh | F: 5′-GACGGAGCGGAGTATGTTGT-3′ | 207 |
| R: 5′-CAAACAATTGGTGGTGCAAG-3′ | ||
| Bma-unc-49 | F: 5′-AACTTTTCCCGATGGATTCA-3′ | 205 |
| R: 5′-CTAACCTGAAGAAGTAGTTGCCC-3′ |
BP, base pairs; F, forward; R, reverse.
Fig. 2.
(A) Single muscle cell PCR demonstrating the presence of different nAChR subunits expressed in single muscle cells. Representative gel pictures show the presence of mRNA for acr-16, acr-21, unc-29, unc-38, unc-63, acr-8, and acr-26. GAPDH was the internal control for each cell (example of n = 6, all positive for each gene). (B) Concentration-dependent effect of nicotinic agonists on B. malayi muscle cell whole cell patch-clamp recordings. Representative trace illustrating effects of increasing concentrations of levamisole on inward current, response is normalized to ACh 100 µM (first application) for EC50 calculation. (C) Concentration-response curves for different agonists. ACH, acetylcholine; BEP, bephenium; LEV, levamisole; MOR, morantel; NIC, nicotine; PYR, pyrantel; (n = 7 for each agonist).
Fig. S1.
Single muscle cell PCR demonstrating the presence of ACR-27 nAChR amplicon expressed in single muscle cell (detected in two of eight single muscle cell PCR experiments). Representative gel picture shows acr-27. GAPDH was the internal control for each cell. M, single-cell muscle cell.
Cholinergic Anthelmintic Agonist Effects on B. malayi Muscle nAChR Currents.
Fig. 2B shows a representative recording of the inward current responses to 100 µM acetylcholine followed by the effects of increasing concentrations of levamisole made on a muscle cell clamped at −40 mV: These current responses illustrate the presence of functioning nAChRs on B. malayi muscle cells. We tested other cholinergic anthelmintics in the same manner, and Fig. 2C shows log-concentration plots, normalized to the first application of 100 µM acetylcholine, for levamisole, pyrantel, morantel, nicotine, and bephenium. Acetylcholine had an EC50 of 2.4 ± 0.6 µM; pyrantel had an EC50 of 63.5 ± 0.2 nM; morantel had an EC50 of 101.8 ± 0.4 nM; levamisole had an EC50 of 3.4 ± 0.6 μM; bephenium had an EC50 of 4.2 ± 0.1 μM with a reduced maximum response and; nicotine had an EC50 of 30.1 ± 0.4 μM (n = 7 for all agonists). Thus, the EC50 potency series was pyrantel > morantel >> acetylcholine = levamisole = bephenium >> nicotine. This potency series was different from the B. malayi whole worm motility assay with pyrantel and morantel being more potent than levamisole. One reason for the difference in the potency series is the cuticle barrier of B. malayi, which may be more permeable to levamisole, which is much less polar than pyrantel and morantel at pH 7.2.
Selective Antagonist Effects of Derquantel Distinguish Four Muscle nAChRs.
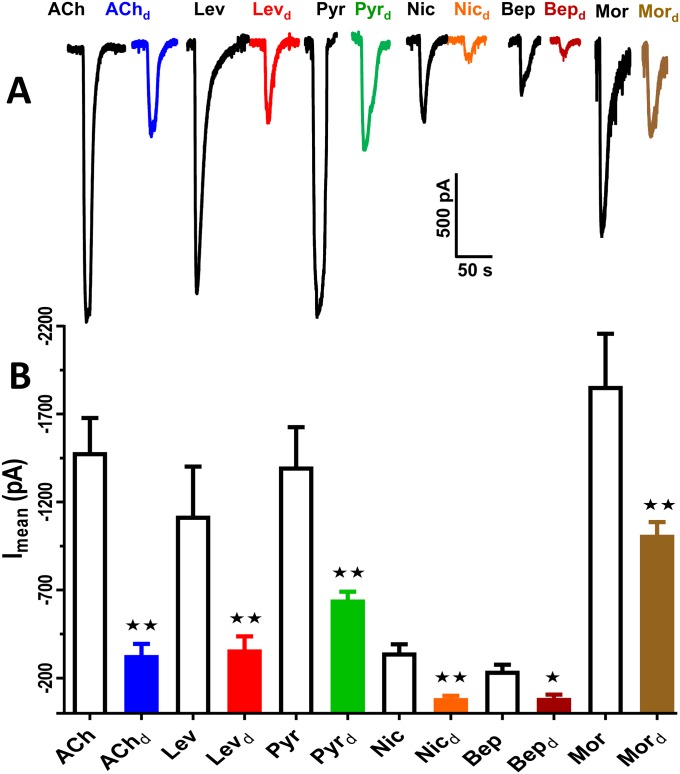
We also tested morantel, pyrantel, levamisole, bephenium, and nicotine as agonists in the presence of the selective anthelmintic antagonist derquantel (n = 5). Fig. S2A shows representative current traces demonstrating that derquantel inhibited all of the agonists. Fig. S2B shows bar charts of the mean ± SE currents produced by the different agonists without, and then with, 1 μM derquantel for morantel, or 10 μM derquantel for the other agonists. The inhibition was significant (P ≤ 0.001, two-way ANOVA and Bonferroni post hoc tests) for all of the agonists and reversible on washing.
Fig. S2.
Derquantel is a potent antagonist. (A) Representative whole cell patch clamp recordings showing inward currents induced by different nAChR agonists in the absence and the presence of derquantel. Agonist concentrations were 30 µM for all except pyrantel, which was 10 µM. The agonists were acetylcholine (ACh), levamisole (Lev), pyrantel (Pyr), nicotine (Nic), bephenium (Bep), and morantel (Mor) in the absence and then presence (d) of derquantel (10 µM was used against all agonists except for morantel, where 1 µM was used). (B) Bar chart quantifying the average reduction of inward currents induced by different agonists in the presence of derquantel. All agonists were inhibited significantly (two-way ANOVA and Bonferroni post hoc tests, P < 0.001, n = 5 for each agonist). Ach-induced currents reduced from −1,483 ± 195.8 to −330.5 ± 64.5 pA; levamisole respponse reduced from −1,338 ± 252.9 pA to −437.5 ± 72.3 pA; pyrantel response reduced from −1,401 ± 224.6 to −645.4 ± 46.4 pA; nicotine-induced currents reduced from −419 ± 63.01 to –91.04 ± 11.5 pA and bephenium-induced inward currents were suppressed from −291.7 ± 42.8 to −109.7 ± 19.8 pA, respectively. *P < 0.05; **P < 0.01.
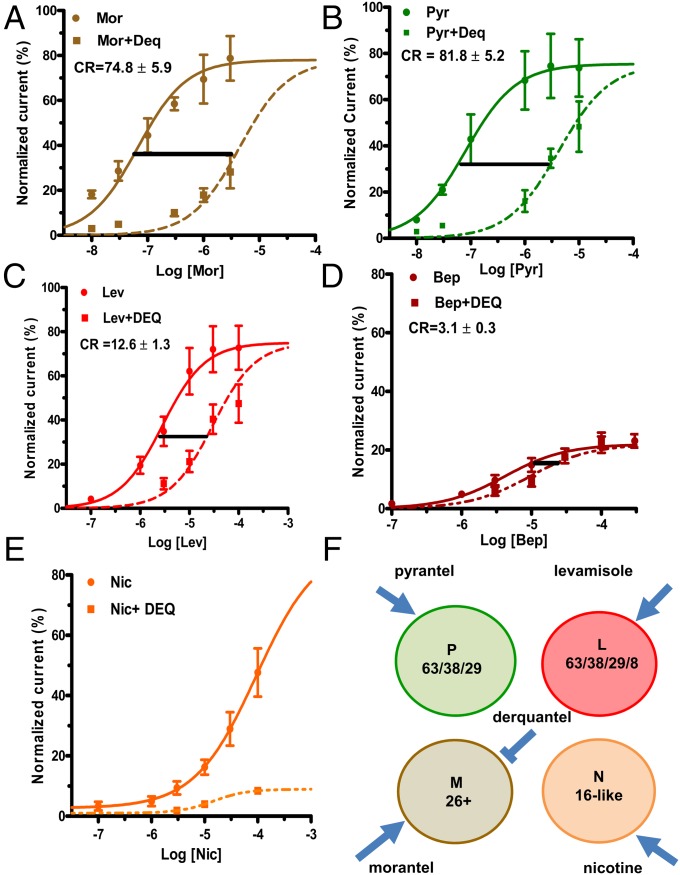
The effect of derquantel on concentration–response relationships for these agonists is shown in Fig. 3. We fitted parallel concentration–response plots to quantify and describe the inhibitory action of derquantel by using the competitive antagonist model and determined the concentration ratios (CR) from the ratios. Derquantel had a potent effect on morantel, so we used 1 µM derquantel as the antagonist concentration and found the concentration ratio to be 74.8 ± 5.9 (n = 6). We calculated the dissociation constant, Kb, for derquantel from the classic Gaddum–Schild equation:
where Xb is antagonist concentration, Kb is the antagonist dissociation constant, and CR is the concentration ratio. The Kb for derquantel using morantel as the agonist was 14 nM. We used 10 µM derquantel with the other agonists because we found that it was less potent. The pyrantel concentration-ratio was 81.8 ± 5.2 (n = 6) and the Kb was 126 nM; the levamisole CR was 12.6 ± 1.3 (n = 6) and the Kb was 956 nM; the bephenium CR was 3.1 ± 0.3 (n = 6) and the Kb was 5.4 µM. The antagonism of nicotine was noncompetitive (Fig. 3E), demonstrating that nicotine activates a different population of nAChRs to the other agonists. Two-way ANOVA showed that the differences between the agonist CRs were significant (P < 0.001), and the Bonferroni multiple comparison tests showed that the CRs for 10 µM derquantel were significantly different for the agonists except for levamisole and bephenium. These observations show that morantel, pyrantel, levamisole, and nicotine activate different populations of receptors, but that levamisole and bephenium activate populations of nAChRs that were not distinguished by derquantel.
Fig. 3.
Selective antagonist effects of derquantel on nAChR agonist concentration response relationships. Control concentration response relationship in the absence (solid line) and then in the presence (broken line) of derquantel. (A) Morantel antagonism by 1 µM derquantel. (B) Pyrantel was antagonized in the presence of 10 µM derquantel. (C) Levamisole concentration–response relationship was antagonized by 10 µM derquantel. (D) Bephenium concentration response curve was antagonized in the presence of 10 µM derquantel. (E) Derquantel suppressed the nicotine concentration response relationship in noncompetitive manner, n = 5 worms for each set of experiments (CR, concentration ratio). (F) Diagrammatic representation of the four types of nAChR: P, L, M, and N. Also shown is their selective agonists with derquantel being the most potent inhibitor on the M-receptor. The L-receptor is shown as composed of the subunits ACR-63 (63), UNC-38 (38), UNC-29 (29), and ACR-8 (8); the P-receptor is shown as composed of the subunits ACR-63 (63), UNC-38 (38), and UNC-29 (29); the M-receptor is shown as composed of the subunits ACR-26 (26) and another (+), possibly ACR-27 subunit; the N-receptor is proposed to be composed of ACR-16-like (16-like) subunits and may include ACR-21. Bep, bephenium; CR, concentration ratio; Deq, derquantel; Mor, morantel; Nic, nicotine.
The derquantel inhibitory potency sequence is morantel >> pyrantel >> levamisole. We interpret our observations to indicate that there are four separable types of nAChR present on B. malayi muscle. The receptors preferentially activated by morantel and inhibited most by derquantel we refer to as the M-receptors, the receptors preferentially activated by pyrantel as the P-receptors, and the receptors preferentially activated by levamisole as the L-receptors. Because derquantel had a noncompetitive effect on nicotine in contrast to the effects on the other agonists, it demonstrates that nicotine acts on a different population of nAChRs. We refer to the preferred population of receptors activated by nicotine as the N-receptors. Fig. 3F is a schematic representation of the main pharmacological properties of these receptors and their putative subunit compositions.
Knockdown of unc-29 and unc-38mRNA Is High and Selective.
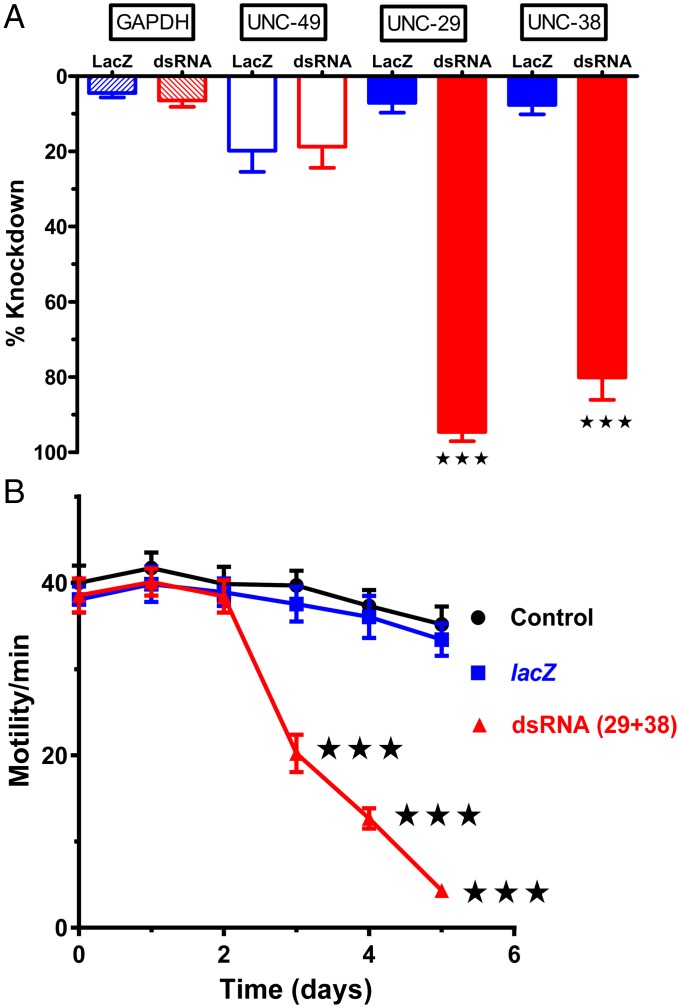
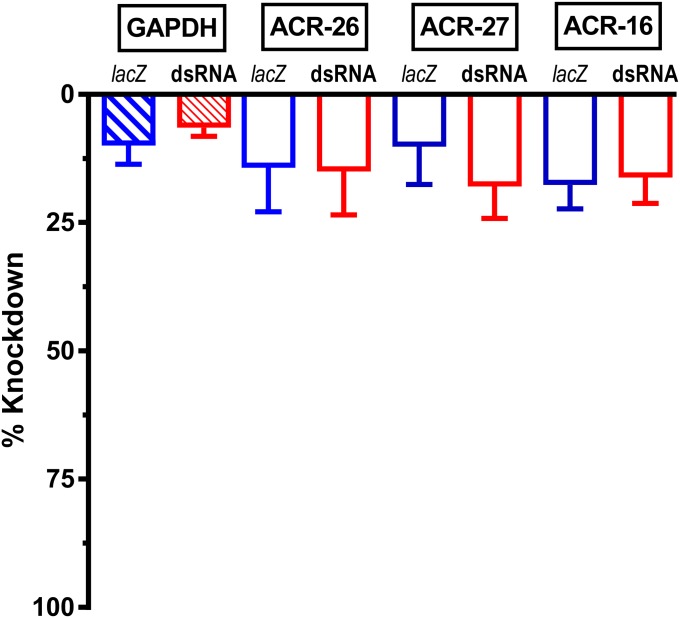
RNAi has not been tractable when applied to animal parasitic nematodes, unlike the model nematode, C. elegans (32, 33). Success using heterogeneous short interfering RNA (hsiRNA) mixtures has been reported with B. malayi (34). We found that we did not need to use hsiRNA and that a high level of mRNA knockdown was achieved when we used 30 µg/mL of double stranded RNA (dsRNA) for 4 d. We designed and screened our dsRNA platform for adult B. malayi to knock down two specific nAChR subunit transcripts selected out of a group of some 30 nAChR subunits that might be present in the whole worms (30). We targeted both UNC-29 and UNC-38 nAChR subunits, which contribute to putative levamisole-sensitive, L-type, and pyrantel-sensitive, P-type nAChRs in parasitic nematodes (14, 35). We used two negative controls: an untreated (no added dsRNA) and lacZ from E. coli as a nonspecific dsRNA control that is not expressed in part or whole in nematodes. A dual-target dsRNA mixture delivery approach worked well and produced robust results in every experiment. Fig. 4A shows the high percentage of mRNA knockdown of unc-29+unc-38 (specific) dsRNA-treated worms compared with the lacZ dsRNA-treated worms: The mRNA levels of the other controls, GABA (unc-49) and GAPDH (gpd-1), remain little affected for both treatments. The mRNA levels were measured on single worms that were the same worms used for the day 5 electrophysiology recordings described later. We achieved significant (two-way ANOVA and Bonferroni post hoc, P ≤ 0.001) knockdown of transcript levels for unc-29 (95 ± 2.8%) and unc-38 (80 ± 6.9%) in the specific dsRNA-treated worms compared with modest change in the transcript level for unc-29 (7.3 ± 3.1%) and unc-38 (7.8 ± 2.9%) in the lacZ dsRNA-treated worms. We also tested whole worms for transcript knockdown for the nAChR subunits, ACR-26, ACR-27, and ACR-16. There was no significant knockdown in either of the nonspecific or specific dsRNA-treated worms (Fig. S3).
Fig. 4.
Effects of dsRNA unc-38+unc-29 on quantitative PCR and motility analysis demonstrate a selective reduction in unc-38 and unc-29 transcript levels and motility in dsRNA mixture soaked B. malayi. (A) Bar chart demonstrating quantitative PCR results showing a significant reduction in transcript levels of unc-29 (by 95 ± 2.8%, 29 red bar) and unc-38 (by 80 ± 6.9%, 38 red bar) from the control transcript levels in lacZ dsRNA-treated worms, unc-29 (by 7.3 ± 3.1%, 29 blue bar) and unc-38 (by 7.8 ± 2.9%, 38 filled blue bar). Quantitative PCR results are also shown for GAPDH (hatched bars) and for the GABA receptor subunit transcript unc-49 (open bars). n = 8, one-way ANOVA and Bonferroni post hoc tests; ***P < 0.001. (B) Time series of motility for dsRNA-soaked worms. Black color data points (circles) representing control, blue color data points (squares) lacZ (nonspecific dsRNA), and red (triangles) color representing the worms treated with mixture of unc-29 and unc-38 dsRNA. n = 38 worms for all treatments, two-way ANOVA and Bonferroni post hoc tests; ***P < 0.001.
Fig. S3.
Bar chart demonstrating % reduction in transcript level for whole worm quantitative PCR results produced by the 29+38 dsRNA mixture (red bars) and lacZ dsRNA (blue bars)-soaked B. malayi for: acr-26 (14.3 ± 9.6% and 15.0 ± 8.5%); acr-27 (10.2 ± 7.4% and 17.9 ± 6.3%); acr-16 (17.7 ± 4.6% and 16.2 ± 5.1%), and GAPDH (9.9 ± 3.6% and 6.4 ± 1.7%). GAPDH (n = 8 worms each), acr-26 (n = 5 worms each), acr-27 (n = 5 worms each), and acr-16 (n = 5 worms each). There was no significant difference between the dsRNA lacZ and dsRNA 29+38 values, P = 0.77, two-way ANOVA.
dsRNA Knockdown of unc-29+unc-38 Produces a Motility-Inhibition Phenotype.
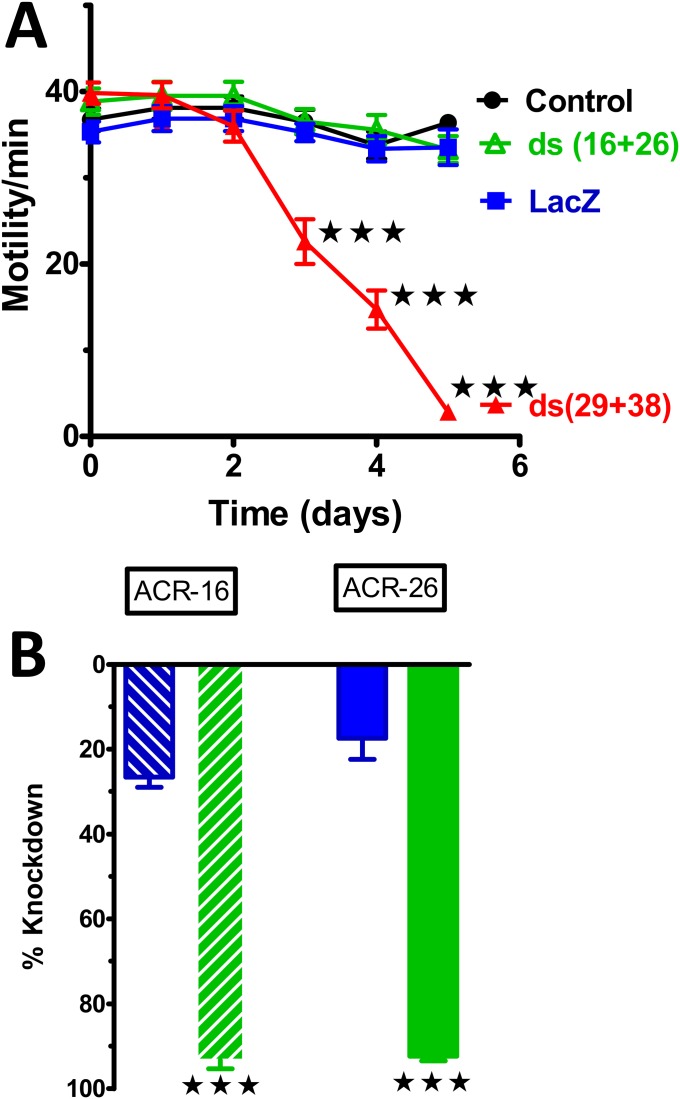
We used the Worminator video tracking system to quantify, using pixel changes, the motility of the adult female worms exposed to specific (unc-29+unc-38) and lacZ (nonspecific) dsRNA over 5 d. Fig. 4B shows the recorded motility at daily intervals for control (untreated and lacZ dsRNA-treated) worms and test (unc-38+unc-29 dsRNA-treated) worms. There was a large and significant (ANOVA, P < 0.001, n = 114) decrease in motility in the worms exposed to unc-29+unc-38 dsRNA by day 3 but only a modest decrease in motility in the control worms or lacZ-treated worms. These observations suggest that UNC-29 and/or UNC-38 are essential for maintaining the motility phenotype and that other nAChR subtypes, which do not contain UNC-29 and/or UNC-38 subunits, do not support motility. To test this hypothesis further, we conducted similar experiments and knocked down acr-16+unc-26 and found that in contrast to knockdown of unc-38+unc-29, there was little effect on motility (Fig. S4).
Fig. S4.
Effects of dsRNA acr-16+acr-26 on motility and quantitative PCR demonstrates no effect on motility following soaking for 5 d but a reduction in acr-16 and acr-26 mRNA. The effect of dsRNA acr-16+acr-26 contrasts with the effect on motility of dsRNA unc-38+unc-29. (A) Time series of motility for dsRNA soaked worms. Black (circles) representing control, blue (squares) lacZ (nonspecific dsRNA), red (triangles) (dsRNA unc-29 + unc-38), and green (triangles) (dsRNA acr-16+acr-26) color data points representing the different treatments of worms (n = 10, four biological replicates for each treatment, two-way ANOVA and Bonferroni post hoc tests; ***P < 0.001). (B) Bar chart demonstrating quantitative PCR results showing a significant reduction in transcript levels of acr-16 (hatched green, by 93 ± 2.0%) and acr-26 (filled green by 92 ± 1.1%) compared with the control transcript levels in lacZ dsRNA-treated worms, acr-16 (hatched blue by 27 ± 2.4%) and acr-27 (filled blue by 17 ± 4.9%). n = 6 worms. (unpaired t tests: ***P < 0.001; **P < 0.01; *P < 0.05). ds, double stranded.
Only Levamisole and Pyrantel Currents Are Sensitive to unc-29+unc-38 Knockdown.
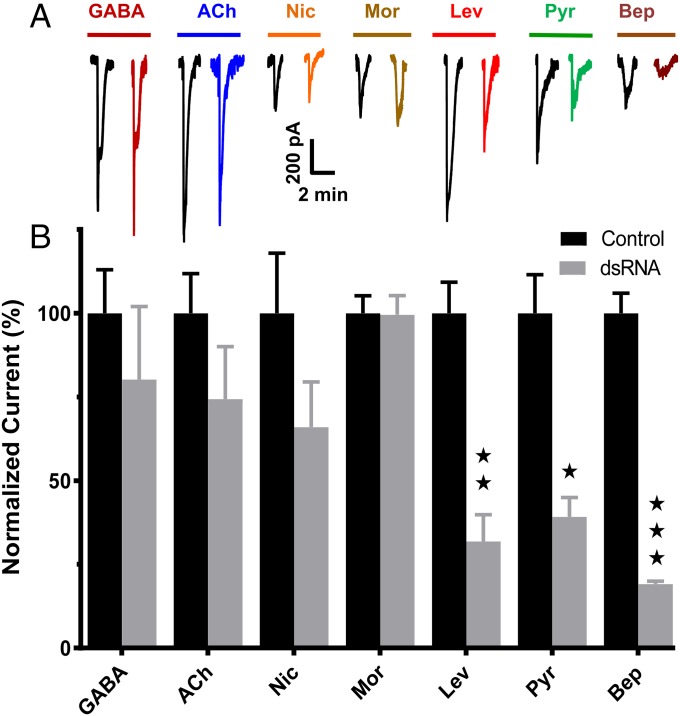
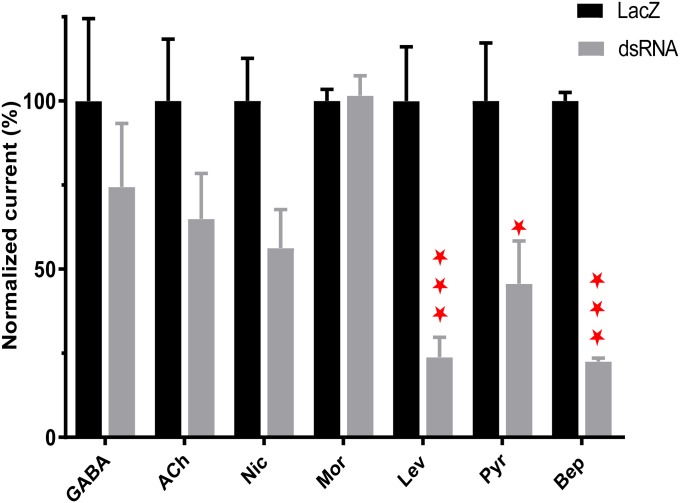
dsRNA directed-target knockdown reduced transcript and motility levels (Fig. 4) and also changed the responses to agonists (Fig. 5). We tested the whole muscle-cell current responses of the two controls (untreated and lacZ, n = 16 for both) and dsRNA-treated (unc-29+unc-38, n = 16) worms to fixed concentrations of acetylcholine, levamisole, nicotine, morantel, pyrantel, and bephenium on day 5. We included GABA, which is an inhibitory transmitter in nematodes, and which does not activate nAChRs as an additional control. Fig. 5A shows representative examples of the inward current responses to GABA, acetylcholine, nicotine, morantel, levamisole, pyrantel, and bephenium recorded at −40 mV in untreated worms and in unc-29+unc-38 dsRNA-treated worms. We compared effects of the dsRNA on each of the agonist responses. Fig. 5B shows that the current responses in unc-29+unc-38 dsRNA-treated worms were significantly reduced for levamisole, pyrantel, and bephenium compared with untreated worm responses. Similar reductions were seen when we compared lacZ controls with the dsRNA-treated worms (Fig. S5). The effects on GABA, acetylcholine, morantel, and nicotine current responses were not statistically significant, suggesting that the UNC-29 and/or UNC-38 subunits are an essential part of receptors that respond to levamisole, bephenium, and pyrantel, but these subunits have little or no part to play in the responses to the other agonists. These observations suggest that the L-type and P-type nAChRs contain UNC-38 and/or UNC-29 subunits but that the N-type and the M-type do not contain UNC-38 and/or UNC-29 subunits.
Fig. 5.
Levamisole, pyrantel and bephenium currents are sensitive to unc-29 and unc-38 knockdown. (A) Representative traces demonstrating the inward current responses to acetylcholine, levamisole, nicotine, pyrantel, bephenium and GABA at -40 mV in control untreated and dsRNA-treated worms under whole-cell patch clamp (n = 8). (B) Bar chart illustrating the agonist current responses of 29+38 dsRNA-treated worm normalized against mean current response of each agonist in control untreated worms. n = 8 worms for each agonist. Only levamisole, pyrantel, and bephenium responses were significantly inhibited when 29+38 dsRNA worm’s responses were compared with untreated control dsRNA-treated worm currents: one-way ANOVA and Bonferroni post hoc tests: ***P < 0.001; **P < 0.01; *P < 0.05. Ach, acetylcholine; Bep, bephenium; Lev, levamisole; Mor, morantel; Nic, nicotine; Pyr, pyrantel.
Fig. S5.
Bar chart showing the mean ± SE agonist current responses of dsRNA unc-29+unc-38-treated worms normalized against their mean control (lacZ-treated) currents. n = 8 worms for each agonist. Only levamisole, pyrantel, and bephenium responses were significantly inhibited when dsRNA unc-29+unc-38 worm’s responses were compared with untreated control or lacZ dsRNA-treated worms. Two-way ANOVA and Bonferroni post hoc tests: ***P < 0.001; *P < 0.05. ACh, acetylcholine; Bep, bephenium; Lev, levamisole; Mor, morantel; Nic, nicotine; Pyr, pyrantel.
No Monepantel-Sensitive Receptors on B. malayi Muscle.
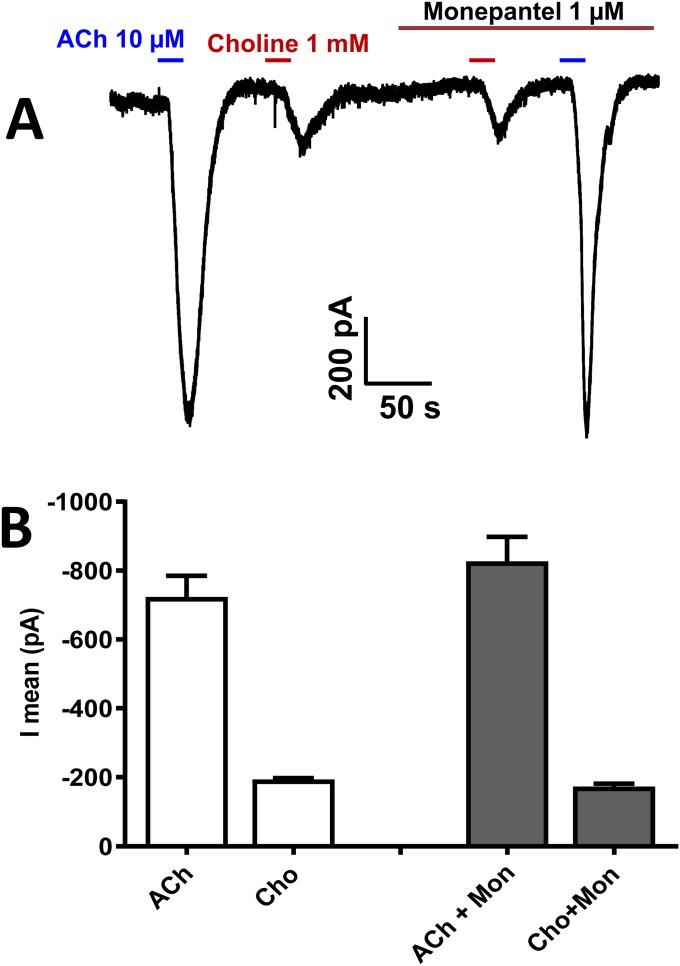
Monepantel is another cholinergic anthelmintic that acts as a positive allosteric modulator of the DEG-3 family of nAChRs that may be in nematode muscle (36, 37). We tested the effects of 1 mM choline and 10 µM monepantel on B. malayi whole-muscle currents. We found that monepantel was without effect when tested in four preparations (Fig. S6). These observations suggest the absence of monepantel receptors [DEG-3 nAChRs (MPLR-1, ACR-23, and ACR-20)] from B. malayi muscle.
Fig. S6.
Effect of monepantel on ACh and choline responses. (A) Representative voltage clamp recording illustrating inward currents induced by ACh (10 µM) and choline (1 mM) in absence and presence of monepantel (1 µM). There is little or no effect. (B) Bar chart demonstrating that monepantel had no significant effect on the acetylcholine (ACh) and choline-induced inward currents (n = 5). Cho, choline; Mon, monepantel.
Discussion
Despite there being a published genome of B. malayi (38), functional and genomic studies of the modes of action of anthelmintics in filaria have been difficult, and there have been only a few successful reports of RNAi experiments in B. malayi (34, 39–47). Here, we are able to describe a reliable RNAi method, using soaking in dsRNA that we have coupled with movement phenotyping and patch clamp to study the effects of cholinergic anthelmintics on adult B. malayi in vitro. Important components were the optimization of the dsRNA concentration and culture duration required for effective and selective RNAi knockdown. We can now conduct functional genomics related to the modes of action of anthelmintics in a significant nematode parasite; there are advantages over the use of the C. elegans model, which is evolutionarily distant and does not contain “parasite genes.” We are optimistic we can now explore functional properties of their other ion channels, effects of anthelmintics, and examine the role of individual or groups of selected genes in this major parasite. We did not find significant off-target effects with our lacZ controls and no significant knockdown of GAPDH, GABA, or other nicotinic channel subunit genes (Fig. 4 and Fig. S3).
Functional Interpretation of the Observations.
We have identified four distinct (M-, L-, P-, and N-) types of cholinergic receptors on muscle of B. malayi (Fig. 3). The dsRNA knockdown dissected out L- and P-receptors from M- and N-receptors, and derquantel dissected out M-, P-, L-, and N-receptor populations. The names for these receptor types is based on the agonists we used with derquantel as the antagonist. We point out that the agonists are not exclusive to one receptor and will have some overlapping actions, depending on the agonist concentration and receptor type. nAChRs are composed of five subunits with the ion channel in the center as the pore. These ion channels, if they are composed of five identical subunits, are described as homomeric or heteromeric if these subunits are different. In C. elegans (31, 35), only two types of muscle nAChR are recognized: the L-type, which appears to be composed of UNC-63, UNC-38, UNC-29, LEV-1, and LEV-8 subunits, and the N-type, which appears to be composed of ACR-16 subunits. There are also a range of ACR-16–like subunits, including ACR-21 present in other tissues of C. elegans (48, 49), which are anticipated to form homomeric receptors in vivo. In animal parasitic nematodes, there is a greater diversity in the nAChRs that are present on somatic muscle. In Ascaris suum, there are three separable muscle subtypes [N-, L-, and B- (27)] and four types in Oesophagostomum dentatum (11). Xenopus expression experiments suggest in Haemonchus contortus and Oesophagostomum dentatum that: the L-type (L-AChR-1) is composed of UNC-63, UNC-38, UNC-29, and ACR-8 subunits; the P-type (L-AChR-2) is composed of UNC-63, UNC-38, and UNC-29 subunits (10, 25); and the N-type in Ascaris suum is composed of ACR-16 subunits (14, 35, 50). A fourth type of muscle nAChR selectively activated by morantel and composed of ACR-26 and ACR-27 subunits has been described in Haemonchus contortus (51). We anticipate that our M-type could include nAChRs involving ACR-26 subunits and ACR-27 or other subunits.
One of the limitations of these earlier studies has been the difficulty in performing RNAi and in vivo electrophysiology on the same parasite for functional genomic studies. Here, we have been able to overcome those difficulties and were able to knockdown unc-38 and unc-29 mRNA to show that the L-types, which are sensitive to levamisole, and/or the P-types, which are sensitive pyrantel, are required to drive spontaneous motility of B. malayi. In contrast, knockdown of acr-16+acr-26 does not inhibit the motility phenotype, suggesting that the M- and N-type receptors do not drive motility. We also point out that derquantel was most potent as an inhibitor of the M-type of receptors and derquantel only had a weak effect on motility, supporting the view that the M-receptors do not drive motility. This lack of effect on motility is interesting because it raises the question of the function of the M- and N-receptors in the muscle. nAChR subunit genes are not only expressed in body muscle, but they are widely distributed and found on embryos, spermatogonia, and lateral cord of B. malayi (52); the distribution suggests that the ion-channels function in development and metabolism and not just in muscle control. nAChRs have functions outside the neuromuscular system in mammals where they are also involved in immune responses, metabolism, and early development (53). Thus, M- and N-receptors may have paracrine, immune response, and/or developmental functions in parasitic nematodes. Anthelmintics like derquantel targeting M- or other anthelmintics targeting N-receptors could have effects on development, responses to host attack, and adaptations to different environments in the host rather than just effects on motility. Future phenotypic anthelmintic screens for drug development should include growth and developmental assays as well as release of excretory substances and exosomes assay (54–56) in addition to motility screens.
Materials and Methods
Details of our methods are provided in SI Materials and Methods and are outlined here. Female B. malayi were used for studying functional genomics by using: (i) Worminator for motility phenotyping; (ii) single-cell RT-PCR to identify nAChR subunit genes expression; (iii) dsRNA for knockdown of UNC-38+UNC-29 subunits of nematode levamisole and pyrantel receptors and ACR-16+ACR-26 subunits of nicotine and morantel receptors; and (iv) whole-cell patch clamp to study functional properties of muscle nAChRs.
SI Materials and Methods
Parasites.
Only female B. malayi adults were used in this study because of their larger size and were sourced from the NIH/NIAID Filariasis Research Reagent Resource Center (FR3) College of Veterinary Medicine, University of Georgia, Athens, GA. The worms were maintained in nonphenol red Roswell Park Memorial Institute (RPMI: Invitrogen) 1640 medium supplemented with 10% heat-inactivated FBS (Fisher Scientific) and 1% penicillin-streptomycin (Invitrogen) at a temperature of 37 °C in a 5% CO2 incubator. The worms were sorted individually in 24-well microtiter plates containing 2 mL of media for the studies of the effects of dsRNA and drugs on motility.
Motility Studies.
We used the Worminator system (24), which is capable of measuring motility phenotypes and was developed by Marcellino et al. (23) to simultaneously quantify the movement of the worms in each well of a 24-well plate. The WormAssay version 1.4 software with high-definition video capture capability was used to obtain videos of the movements of each worm for mean motility units per minute readings for the worms in the microtiter plates. The effects of log concentration of agonist cholinergic anthelmintic on motility were determined after exposing individual whole worms to single concentrations of the anthelmintic for 5 min (to obtain maximum effect) and then measuring motility over 30 s. The effects of dsRNA treatment, the effects of the cholinergic anthelmintic antagonist, derquantel, and the effects of levamisole on mean motility units per minute were followed over time.
Muscle Flap and Electrophysiology.
All dissections and whole muscle cell patch-clamp recordings were made on day 5 worms at room temperature. Short cylindrical body tubes, ∼0.5 cm long, were cut from the anterior region of the worm and placed in the recording chamber filled with bath solution. The base of the chamber was a 24 × 50 mm coverslip coated with a thin layer of Sylgard. The muscle tube was first immobilized in the chamber along one side with Glushield cyanoacrylate glue (Glustitch). The body tube was then cut open longitudinally by using a tungsten needle. The resulting “muscle flap” was then glued along the cut edge, and the uterus and gut were removed by using fine forceps. The preparations were viewed under DIC optics (Nikon TE2000-U inverted light microscope 400×) making individual muscle cells visible (10, 27). The bath solution was 23 mM NaCl, 110 mM Na acetate, 5 mM KCl, 6 mM CaCl2, 4 mM MgCl2, 5 mM Hepes, 10 mM d-glucose, and 11 mM sucrose, pH adjusted to 7.2 with NaOH and ∼320 mOsmol.
Muscle flaps were then incubated in 1 mg/mL collagenase (type 1A, Sigma-Aldrich) in bath solution for 15–120 s and washed 10 times with bath solution. The patch-clamp technique was used to record whole muscle cell currents. Fire-polished patch pipettes were pulled from capillary glass (G85150T; Warner Instruments). We filled the patch pipettes with a solution containing 120 mM KCl, 20 mM KOH, 4 mM MgCl2, 5 mM Tris, 0.25 mM CaCl2, 4 mM NaATP, 5 mM EGTA, and 36 mM sucrose (pH 7.2 with KOH), ∼315–330 mOsmol. Pipettes with resistances of 3–5 MΩ were used. A 1-cm region near the tip of the electrode was covered with Sylgard to reduce background noise and improve frequency responses. Giga-ohm seals were formed before breaking the membrane with suction to make whole-cell recordings. The preparation was continuously perfused with bath solution at 2 mL/min. The muscle transmembrane currents were amplified by an Axopatch 200B amplifier (Axon Instruments), filtered at 2 kHz (three-pole Bessel filter), sampled at 25 kHz, digitized with a Digidata 1320A (Axon Instruments), and stored on a computer hard disk for later analysis.
Chemicals.
Stock concentrations of the drugs were dissolved in the bath solution, except for bephenium and pyrantel, which were made up in DMSO. The final concentration of DMSO in the bephenium and pyrantel containing perfusate was 0.1%. All of the compounds tested on the preparation were obtained from Sigma-Aldrich. Fresh 1 μM acetylcholine solution was prepared for every experiment. All other chemicals were obtained from Fisher Scientific. Monepantel was obtained as Zolvix (Elanco, 25 mg/ml) and diluted in DMSO to make a stock. Derquantel was a gift of Debra Woods, Zoetis, Kalamazoo, MI.
Data Analysis.
Whole-cell patch clamp data were analyzed with Clampfit 9.2 (Molecular Devices) and GraphPad Prism 5.0 software. The peak current responses from whole-cell recordings were used for analysis from dsRNA-treated and dsRNA-untreated worms. The response to 100 µM ACh was used to normalize the concentration–response relationships of all of the other agonists. For whole worm adult female concentration–response relationships, motility/minute was plotted against log concentration. Agonist concentrations were log10 transformed before analysis. The log agonist vs. response equation (variable slope) was used to generate concentration response curves to calculate EC50 and nH values for each agonist. The responses were plotted as the mean ± SE. To calculate the concentration ratios for the derquantel experiments, the graphs were fitted with the Hill equations with the Hill slope, nH, fixed to the same values for control and antagonist curve, the bottom of the curves were set to zero and the tops set to be identical (to produce parallel curves). Statistical analyses were performed on groups of values by using ANOVA to determine whether the group means were dissimilar; Bonferroni post hoc tests were used for multiple comparison tests to determine whether there were significant differences between groups.
dsRNA for RNAi.
dsRNA Bma-unc-38, dsRNA Bma-unc-29, dsRNA Bma-acr-16, and dsRNA Bma-acr-26 was synthesized as described in McCoy et al. (42). Target and nontarget T7 promoter-labeled PCR products were amplified by using the primers described in Table S2. These primers had a T7 promoter sequence appended to their 5′ end (5′-TAATACGACTCACTATAGGG-3′). Amplification was carried out by using Techne PRIMEG (Bibby Scientific Limited, UK) with cycling conditions: 95 °C × 5 min, 35 × (95 °C × 30 s, 55 °C × 30 s, 72 °C × 1 min), 72 °C × 10 min from sequence verified cDNA templates. dsRNA was synthesized by using T7 RiboMAX Express RNAi kit (Promega) according to the manufacturer’s instructions. The concentration and purity of dsRNA were assessed by using a spectrophotometer, and the absorbance ratio (260/280) was 1.8–2.0. Adult female B. malayi were soaked in dsRNA (30µg/mL of each subunit) for 5 d for RNAi.
Table S2.
Primer sequences used for dsRNA synthesis
| Subunits | Primers | BP |
| Bma-unc-29 | F: 5′-TGGGTACCACCAGCCATCTA-3′ | 478 |
| R: 5′-TGAGCACGAAAGTGAGCAGT-3′ | ||
| Bma-unc-38 | F: 5′-TGGGATCGATCTGTCGGACT-3′ | 401 |
| R: 5′-TGCGATGTGTCGTAGGCTTT-3′ | ||
| E. coli lacZ | F: 5′-CGTAATCATGGTCATAGCTGTTTCC-3′ | 400 |
| R: 5′-CTTTTGCTGGCCTTTTGCTC-3′ | ||
| Bma-gapdh | F: 5′-TCGTATTTGTGCGTTGTGTTTGT-3′ | 397 |
| R: 5′-TCAGCGGGATCTTTGCTGTT-3′ | ||
| Bma-acr-16 | F: 5′-CGACCAGGAGTTCATCTCTC-3′ | 200 |
| R: 5′-GAAATTGGGCTCTTTCCATT-3′ | ||
| Bma-acr-26 | F: 5′-CTCAATTAAATTCGGCTCGT-3′ | 207 |
| R: 5′-AGCGTCTTCCGTCTGATATG-3′ |
BP, base pairs; F, forward; R, reverse.
RNA Extraction and Analysis of RNA Levels by Quantitative Real-Time PCR.
A sample of the dsRNA-treated worms from each biological replicate (separate batch of worms from FR3) was used to estimate knockdown by quantitative real-time PCR (qPCR). Snap frozen worms were crushed into fine powder inside a 1.5-mL microcentrifuge tube by using Kimble Kontes Pellet Pestle (Fisher Scientific). Total RNA was extracted by using TRIZOL Reagent, and cDNA was synthesized by using SuperScript VILO Master Mix (Life Technologies) according to the manufacturer’s instructions. Target and reference (Bma-Gapdh) genes were amplified from each cDNA sample in triplicate by qPCR using the CFX96 Touch Real-Time PCR Detection System and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad). The primers used were as mentioned in Tables S1 and S2. Cycling conditions used were as follows: 95 °C × 10 min, 40 × (95 °C × 10 s, 55 °C × 30 s). PCR efficiencies were calculated by the in-built CFX96 Software Suite (Bio-Rad). Relative quantification of target gene knock-down were estimated by ∆∆Ct method (57). All of the worms used to estimate RNA knockdown after dsRNA unc-38+unc-29 treatment were then used for whole-cell recording.
Single Muscle Cell PCR.
We used whole-cell patch pipettes to collect cytoplasm from individual somatic muscle cells for PCR analysis. Single-cell RT-PCR (QIAGEN OneStep RT-PCR Kit) was used to detect expression of nicotinic receptor subunits. We used the following cycling conditions for single-cell PCR: 1 cycle (48 °C × 20 min, 94 °C × 20 min), 35 × (94 °C × 30 s, 58 °C × 30 s, 72 °C × 45 s), and 1 cycle (72 °C × 5 min). We identified Bm1_35890-Bma-unc-29, Bm1_20330-Bma-unc-38, Bm1_13586-Bma-acr-16, Bm1_48815-Bma-acr-26, Bm1_10632-Bma-acr-27, Bm1_38195-Bma-unc-63, Bm1_43040-Bma-acr-21, Bm1_40890-Bma-acr-8, and Bm1_57265A-Bma-gapdhmRNA from single body muscle cell of B. malayi. The list of primers used is provided in Table S1.
Effects of dsRNA on Motility and Electrophysiology.
dsRNA was prepared in DNA/RNase-free water and concentrated to ensure that no more than 30 µL of dsRNA unc-38+unc-29 or dsRNA lac-z or dsRNA acr-16+acr-26 was added to each well of a 24-well culture plate to produce a final concentration of 30 µg/mL of each dsRNA (60 µg/mL total dsRNA for unc-38+unc-29 and 60 µg/mL total dsRNA for acr-16+acr-26). One female worm was added to each of the wells that contained 2 mL of control RPMI 1640 medium, no phenol red, (+) L-glutamine (Gibco) media or dsRNA RPMI media. We used the Worminator system, which is capable of defining and quantifying motility phenotypes and was developed by Marcellino et al. (23) and modified by Storey et al. (24) to simultaneously quantify the movement of the B. malayi in each well of the plate. The video capture capability was used to record movements of the control, dsRNA unc-38+unc-29, dsRNA lac-z, and dsRNA acr-16+acr-26 treated worms. The treated plates were maintained for 120 h. Worminator motility readings were taken at 0.17, 4, 24, 48, 72, 96, and 120 h to estimate changes in motility. For the dsRNA unc-38+unc-29 treatment groups, we used 114 female worms in seven batches (biological replicates) for motility analysis. For the dsRNA acr-16+acr-26 treatment group, we used 10 female worms in four batches (biological replicates) for motility analysis.
Worm sections from each batch of dsRNA unc-38+unc-29, dsRNA lac-z and control treated worms were used for electrophysiology experiments on muscle flap preparation made from day 5 worms; the rest of the worm was snap frozen in liquid nitrogen, stored at −80 °C, and later used for RNA extraction and analysis of mRNA levels by qPCR.
Acknowledgments
We acknowledge the gift of derquantel from Dr. Debra Woods of Zoetis, and helpful advice on the use of the Worminator system by Dr. Bryan Bellaire. The B. malayi were sourced from the NIH/National Institute of Allergy and Infectious Diseases (NIAID) Filariasis Research Reagent Resource Center (FR3; College of Veterinary Medicine, University of Georgia). The research funding was by NIAID NIH Grant R01 AI047194-15 (to R.J.M.) and R21 AI121831 (to A.P.R.). The funding agencies had no role in the design, execution, or publication of this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAID.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619820114/-/DCSupplemental.
References
- 1.Bethony J, et al. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Hotez P. Hookworm and poverty. Ann N Y Acad Sci. 2008;1136:38–44. doi: 10.1196/annals.1425.000. [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ. Neglected diseases and poverty in “The Other America”: The greatest health disparity in the United States? PLoS Negl Trop Dis. 2007;1:e149. doi: 10.1371/journal.pntd.0000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotez PJ. Neglected infections of poverty in the United States of America. PLoS Negl Trop Dis. 2008;2:e256. doi: 10.1371/journal.pntd.0000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vercruysse J, et al. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int J Parasitol Drugs Drug Resist. 2011;1:14–27. doi: 10.1016/j.ijpddr.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coghlan A. Nematode genome evolution. WormBook. 2005:1–15. doi: 10.1895/wormbook.1.15.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilleard JS. The use of Caenorhabditis elegans in parasitic nematode research. Parasitology. 2004;128:S49–S70. doi: 10.1017/S003118200400647X. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg RM. Ion channels and drug transporters as targets for anthelmintics. Curr Clin Microbiol Rep. 2014;1:51–60. doi: 10.1007/s40588-014-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mani T, et al. Polymorphism in ion channel genes of Dirofilaria immitis: Relevant knowledge for future anthelmintic drug design. Int J Parasitol Drugs Drug Resist. 2016;6:343–355. doi: 10.1016/j.ijpddr.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson AP, Buxton SK, Martin RJ. Whole-cell patch-clamp recording of nicotinic acetylcholine receptors in adult Brugia malayi muscle. Parasitol Int. 2013;62:616–618. doi: 10.1016/j.parint.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson AP, Bjørn HE, Martin RJ. Pyrantel resistance alters nematode nicotinic acetylcholine receptor single-channel properties. Eur J Pharmacol. 2000;394:1–8. doi: 10.1016/s0014-2999(00)00135-7. [DOI] [PubMed] [Google Scholar]
- 12.Martin RJ, et al. Drug resistance and neurotransmitter receptors of nematodes: Recent studies on the mode of action of levamisole. Parasitology. 2005;131:S71–S84. doi: 10.1017/S0031182005008668. [DOI] [PubMed] [Google Scholar]
- 13.Martin RJ. Modes of action of anthelmintic drugs. Vet J. 1997;154:11–34. doi: 10.1016/s1090-0233(05)80005-x. [DOI] [PubMed] [Google Scholar]
- 14.Buxton SK, et al. Investigation of acetylcholine receptor diversity in a nematode parasite leads to characterization of tribendimidine- and derquantel-sensitive nAChRs. PLoS Pathog. 2014;10:e1003870. doi: 10.1371/journal.ppat.1003870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson SJ, Martin RJ. Levamisole-activated single-channel currents from muscle of the nematode parasite Ascaris suum. Br J Pharmacol. 1993;108:170–178. doi: 10.1111/j.1476-5381.1993.tb13458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson AP, Martin RJ. Ion-channels on parasite muscle: Pharmacology and physiology. Invert Neurosci. 2007;7:209–217. doi: 10.1007/s10158-007-0059-x. [DOI] [PubMed] [Google Scholar]
- 17.Puttachary S, et al. Derquantel and abamectin: Effects and interactions on isolated tissues of Ascaris suum. Mol Biochem Parasitol. 2013;188:79–86. doi: 10.1016/j.molbiopara.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomsen EK, et al. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of bancroftian filariasis. Clin Infect Dis. 2016;62:334–341. doi: 10.1093/cid/civ882. [DOI] [PubMed] [Google Scholar]
- 19.Kwarteng A, Ahuno ST, Akoto FO. Killing filarial nematode parasites: Role of treatment options and host immune response. Infect Dis Poverty. 2016;5:86. doi: 10.1186/s40249-016-0183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horton J, et al. An analysis of the safety of the single dose, two drug regimens used in programmes to eliminate lymphatic filariasis. Parasitology. 2000;121:S147–S160. doi: 10.1017/s0031182000007423. [DOI] [PubMed] [Google Scholar]
- 21.Zahner H, Schares G. Experimental chemotherapy of filariasis: Comparative evaluation of the efficacy of filaricidal compounds in Mastomys coucha infected with Litomosoides carinii, Acanthocheilonema viteae, Brugia malayi and B. pahangi. Acta Trop. 1993;52:221–266. doi: 10.1016/0001-706x(93)90010-9. [DOI] [PubMed] [Google Scholar]
- 22.Mak JW, Chan WC. Treatment of Brugia malayi infection with mebendazole and levamisole. Southeast Asian J Trop Med Public Health. 1983;14:510–514. [PubMed] [Google Scholar]
- 23.Marcellino C, et al. WormAssay: A novel computer application for whole-plate motion-based screening of macroscopic parasites. PLoS Negl Trop Dis. 2012;6:e1494. doi: 10.1371/journal.pntd.0001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storey B, et al. Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: “The Worminator”. Int J Parasitol Drugs Drug Resist. 2014;4:233–243. doi: 10.1016/j.ijpddr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abongwa M, et al. Pharmacological profile of Asu-ACR-16, a new homomeric nAChR widely distributed in Ascaris tissues. Br J Pharmacol. 2016;173:2463–2477. doi: 10.1111/bph.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian H, Robertson AP, Powell-Coffman JA, Martin RJ. Levamisole resistance resolved at the single-channel level in Caenorhabditis elegans. FASEB J. 2008;22:3247–3254. doi: 10.1096/fj.08-110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian H, Martin RJ, Robertson AP. Pharmacology of N-, L-, and B-subtypes of nematode nAChR resolved at the single-channel level in Ascaris suum. FASEB J. 2006;20:2606–2608. doi: 10.1096/fj.06-6264fje. [DOI] [PubMed] [Google Scholar]
- 28.Robertson AP, Puttachary S, Martin RJ. Single-channel recording from adult Brugia malayi. Invert Neurosci. 2011;11:53–57. doi: 10.1007/s10158-011-0118-1. [DOI] [PubMed] [Google Scholar]
- 29.Touroutine D, et al. acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J Biol Chem. 2005;280:27013–27021. doi: 10.1074/jbc.M502818200. [DOI] [PubMed] [Google Scholar]
- 30.Jones AK, Sattelle DB. Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. BioEssays. 2004;26:39–49. doi: 10.1002/bies.10377. [DOI] [PubMed] [Google Scholar]
- 31.Richmond JE, Jorgensen EM. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sijen T, et al. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- 33.Caldwell KN, Anderson GL, Williams PL, Beuchat LR. Attraction of a free-living nematode, Caenorhabditis elegans, to foodborne pathogenic bacteria and its potential as a vector of Salmonella poona for preharvest contamination of cantaloupe. J Food Prot. 2003;66:1964–1971. doi: 10.4315/0362-028x-66.11.1964. [DOI] [PubMed] [Google Scholar]
- 34.Landmann F, Foster JM, Slatko BE, Sullivan W. Efficient in vitro RNA interference and immunofluorescence-based phenotype analysis in a human parasitic nematode, Brugia malayi. Parasit Vectors. 2012;5:16. doi: 10.1186/1756-3305-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulin T, et al. Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc Natl Acad Sci USA. 2008;105:18590–18595. doi: 10.1073/pnas.0806933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rufener L, Mäser P, Roditi I, Kaminsky R. Haemonchus contortus acetylcholine receptors of the DEG-3 subfamily and their role in sensitivity to monepantel. PLoS Pathog. 2009;5:e1000380. doi: 10.1371/journal.ppat.1000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yassin L, et al. Characterization of the deg-3/des-2 receptor: A nicotinic acetylcholine receptor that mutates to cause neuronal degeneration. Mol Cell Neurosci. 2001;17:589–599. doi: 10.1006/mcne.2000.0944. [DOI] [PubMed] [Google Scholar]
- 38.Ghedin E, et al. Draft genome of the filarial nematode parasite Brugia malayi. Science. 2007;317:1756–1760. doi: 10.1126/science.1145406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aboobaker AA, Blaxter ML. Use of RNA interference to investigate gene function in the human filarial nematode parasite Brugia malayi. Mol Biochem Parasitol. 2003;129:41–51. doi: 10.1016/s0166-6851(03)00092-6. [DOI] [PubMed] [Google Scholar]
- 40.Ford L, et al. Characterization of a novel filarial serine protease inhibitor, Ov-SPI-1, from Onchocerca volvulus, with potential multifunctional roles during development of the parasite. J Biol Chem. 2005;280:40845–40856. doi: 10.1074/jbc.M504434200. [DOI] [PubMed] [Google Scholar]
- 41.Ford L, et al. Functional analysis of the cathepsin-like cysteine protease genes in adult Brugia malayi using RNA interference. PLoS Negl Trop Dis. 2009;3:e377. doi: 10.1371/journal.pntd.0000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCoy CJ, et al. RNA interference in adult Ascaris suum–an opportunity for the development of a functional genomics platform that supports organism-, tissue- and cell-based biology in a nematode parasite. Int J Parasitol. 2015;45:673–678. doi: 10.1016/j.ijpara.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfarr K, Heider U, Hoerauf A. RNAi mediated silencing of actin expression in adult Litomosoides sigmodontis is specific, persistent and results in a phenotype. Int J Parasitol. 2006;36:661–669. doi: 10.1016/j.ijpara.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Song C, Gallup JM, Day TA, Bartholomay LC, Kimber MJ. Development of an in vivo RNAi protocol to investigate gene function in the filarial nematode, Brugia malayi. PLoS Pathog. 2010;6:e1001239. doi: 10.1371/journal.ppat.1001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lustigman S, Zhang J, Liu J, Oksov Y, Hashmi S. RNA interference targeting cathepsin L and Z-like cysteine proteases of Onchocerca volvulus confirmed their essential function during L3 molting. Mol Biochem Parasitol. 2004;138:165–170. doi: 10.1016/j.molbiopara.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Geldhof P, et al. RNA interference in parasitic helminths: Current situation, potential pitfalls and future prospects. Parasitology. 2007;134:609–619. doi: 10.1017/S0031182006002071. [DOI] [PubMed] [Google Scholar]
- 47.Dalzell JJ, et al. RNAi effector diversity in nematodes. PLoS Negl Trop Dis. 2011;5:e1176. doi: 10.1371/journal.pntd.0001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mongan NP, et al. An extensive and diverse gene family of nicotinic acetylcholine receptor alpha subunits in Caenorhabditis elegans. Receptors Channels. 1998;6:213–228. [PubMed] [Google Scholar]
- 49.Ballivet M, Alliod C, Bertrand S, Bertrand D. Nicotinic acetylcholine receptors in the nematode Caenorhabditis elegans. J Mol Biol. 1996;258:261–269. doi: 10.1006/jmbi.1996.0248. [DOI] [PubMed] [Google Scholar]
- 50.Abongwa M, Buxton SK, Robertson AP, Martin RJ. Curiouser and curiouser: The macrocyclic lactone, abamectin, is also a potent inhibitor of pyrantel/tribendimidine nicotinic acetylcholine receptors of gastro-intestinal worms. PLoS One. 2016;11:e0146854. doi: 10.1371/journal.pone.0146854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Courtot E, et al. Functional characterization of a novel class of morantel-sensitive acetylcholine receptors in nematodes. PLoS Pathog. 2015;11:e1005267. doi: 10.1371/journal.ppat.1005267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li BW, Rush AC, Weil GJ. Expression of five acetylcholine receptor subunit genes in Brugia malayi adult worms. Int J Parasitol Drugs Drug Resist. 2015;5:100–109. doi: 10.1016/j.ijpddr.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conroy WG, Berg DK. Nicotinic receptor subtypes in the developing chick brain: Appearance of a species containing the alpha4, beta2, and alpha5 gene products. Mol Pharmacol. 1998;53:392–401. doi: 10.1124/mol.53.3.392. [DOI] [PubMed] [Google Scholar]
- 54.Buck AH, et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun. 2014;5:5488. doi: 10.1038/ncomms6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zamanian M, et al. Release of small RNA-containing exosome-like vesicles from the human filarial parasite Brugia malayi. PLoS Negl Trop Dis. 2015;9:e0004069. doi: 10.1371/journal.pntd.0004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pilotte N, Zaky WI, Abrams BP, Chadee DD, Williams SA. A novel xenomonitoring technique using mosquito excreta/feces for the detection of filarial parasites and malaria. PLoS Negl Trop Dis. 2016;10:e0004641. doi: 10.1371/journal.pntd.0004641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]