Significance
The prevention of weight gain in adulthood is a public health challenge, particularly given the difficulty of losing weight. Data on freshmen were collected at the beginning and end of the academic year, and baseline blood samples were studied to find markers of incident weight gain. A metabolite, erythritol, was elevated at the beginning of the year in freshmen who went on to gain weight, fat, and abdominal fat compared with freshmen with stable weight. Erythritol is a sugar substitute low-calorie sweetener, and prior studies claimed no endogenous synthesis. We report a previously unrecognized metabolism of glucose to erythritol, and given the association between erythritol and weight gain, research is needed to understand whether and how this pathway contributes to weight gain risk.
Keywords: erythritol, metabolomics, pentose-phosphate pathway, adiposity, weight gain
Abstract
Metabolomic markers associated with incident central adiposity gain were investigated in young adults. In a 9-mo prospective study of university freshmen (n = 264). Blood samples and anthropometry measurements were collected in the first 3 d on campus and at the end of the year. Plasma from individuals was pooled by phenotype [incident central adiposity, stable adiposity, baseline hemoglobin A1c (HbA1c) > 5.05%, HbA1c < 4.92%] and assayed using GC-MS, chromatograms were analyzed using MetaboliteDetector software, and normalized metabolite levels were compared using Welch’s t test. Assays were repeated using freshly prepared pools, and statistically significant metabolites were quantified in a targeted GC-MS approach. Isotope tracer studies were performed to determine if the potential marker was an endogenous human metabolite in men and in whole blood. Participants with incident central adiposity gain had statistically significantly higher blood erythritol [P < 0.001, false discovery rate (FDR) = 0.0435], and the targeted assay revealed 15-fold [95% confidence interval (CI): 13.27, 16.25] higher blood erythritol compared with participants with stable adiposity. Participants with baseline HbA1c > 5.05% had 21-fold (95% CI: 19.84, 21.41) higher blood erythritol compared with participants with lower HbA1c (P < 0.001, FDR = 0.00016). Erythritol was shown to be synthesized endogenously from glucose via the pentose-phosphate pathway (PPP) in stable isotope-assisted ex vivo blood incubation experiments and through in vivo conversion of erythritol to erythronate in stable isotope-assisted dried blood spot experiments. Therefore, endogenous production of erythritol from glucose may contribute to the association between erythritol and obesity observed in young adults.
In the fall of 2015, an estimated 3.3 million high-school graduates enrolled in postsecondary education as first-time college freshmen (1), and the transition to a residential college environment is associated with weight gain. About 75% of the population experiences weight gain during this transition (2, 3), but there have been few efforts to identify biomarkers of risk that could guide prevention efforts. A study (4) in monozygotic twins discordant for body mass index (BMI) reported divergence at about the age of 18 y, corresponding to a time in life when the environment shifts, and further underscoring the importance of young adulthood in the lifetime trajectory of adiposity and as a window of opportunity for prevention (5).
Observational studies of young adults focus on behavioral/environmental risk factors for adiposity gain, with few studies reporting biological markers in relation to either cross-sectional and/or longitudinal changes in adiposity or body weight. A recent overview of intervention studies to prevent weight gain in young adults (6) identified 37 studies; the majority assessed diet, physical activity, and behaviors, with only 10 studies directly measuring changes in weight, BMI [weight (kilograms)/height (square meters)] and/or body composition, and none of the studies measured biological markers. Existing prediction or risk scores integrate across the various domains contributing to weight gain risk, including demographic, anthropometric, behavioral, psychological, diet-related, and physical activity (7), but do not incorporate biological markers.
Metabolomics approaches, which measure the final downstream products of the complex interactions between genetic, environmental, and pharmacological influences, have been used to study obesity as a phenotype, but few studies have investigated metabolite profiles that are predictive of risk of future weight gain in young adults who are not currently overweight or obese. A recent study (8) investigated the metabolic signature associated with adiposity and adiposity change in participants aged 16–39 y. This study focused on a prespecified set of 67 metabolites, including lipoproteins, inflammatory markers, fatty acids, and glycolysis precursors. Branched chain amino acids (BCAAs), lipoprotein-related metabolites, and glycolysis-related metabolites were all positively associated with change in BMI. Studies in older adults also identified the BCAA-related signature, and reported higher leucine/isoleucine and valine in obese compared with nonobese individuals (4, 9–11). Furthermore, experimental research using metabolomic profiling identified causal effects of weight gain on multiple blood metabolites, including elevation of BCAAs, very low-density lipoprotein (8, 10, 12), triacylglycerol (10), C-reactive protein (8, 10), and insulin-like growth factor (10). However, the role of antecedent metabolomic markers in weight gain and the human adiposity phenotype is not established.
We investigated the relation of the metabolome to incident adiposity gain in young adults over the first year of college. A nontargeted gas chromatography coupled to mass spectrophotometry (GC-MS) approach was used to identify novel metabolites associated with incident adiposity gain, and the endogenous synthesis of a biomarker associated with adiposity gain was investigated by stable isotope-assisted metabolomics approaches. Finally, in vivo and ex vivo stable isotope-assisted metabolomics approaches were used to investigate whether the risk biomarker originated from the diet and/or through endogenous synthesis.
Methods
Study Sample.
The study used data from a recently completed longitudinal cohort study of college freshmen residing on campus during their freshman year (2011–2012) at a university in the northeastern United States. Study participants were selected through stratified random sampling of the incoming freshman class to recruit equal numbers of males and females and to represent the characteristics of the full incoming class. Participants were aged 18–19 y, about 50% were female, and about 10.5% were overweight (BMI > 25) at the study baseline. The study was approved by the Cornell University Institutional Review Board, and all participants provided informed consent.
Collection of Participant Data.
Data were collected during participant study visits and included anthropometric measurements (height, weight, and waist and hip circumferences) using standardized methods and plasma for metabolomics (nonfasting), which was collected within the participants’ first 3 d on campus and at the end of the academic year (mean follow-up time = 35 wk, SD = 1.5). Adiposity, measured via dual-energy X-ray absorptiometry (DXA; Hologic Inc.), was assessed within the first 2 wk and at the end of the academic year.
Blood was collected into EDTA vacutainers (BD), stored at 4 °C for 30–90 min, centrifuged at a relative centrifugal force of 1,300 × g for 10 min, divided into three to four cryovials, and transferred to –80 °C freezers for storage. Hemoglobin A1c (HbA1c), a marker of usual glycemia and long-term glycemic control, was measured in whole blood using the Dimension Xpand Plus Integrated Chemistry System (Siemens).
Phenotypes related to cardiometabolic risk groups were defined as follows: (i) incident central adiposity gain defined by changes in all three indicators: weight increase > 0.5 kg, DXA truncal adiposity increase > 200 g, and waist circumference increase > 0.5 cm (n = 66); (ii) stable adiposity defined by minimal changes in the same three markers of adiposity, namely, body weight, DXA truncal adiposity, and waist circumference (n = 16); (iii) HbA1c in the top 25% of baseline distribution (HbA1c > 5.05%, or ∼32 nmol/mol; n = 21); and (iv) HbA1c in the bottom 10% of baseline distribution (HbA1c < 4.92%, or ∼30 nmol/mol; n = 7). Within each phenotype group, individual participant blood samples were combined to form a pooled sample for metabolomics analysis, and triplicate aliquots of each pool were assayed. To investigate dose–response associations, subpools were created and assayed in triplicate. For the HbA1c groups, subpools were defined by the median of the distribution of individual values (two subpools for each HbA1c group). The incident central adiposity gain group was divided into three subgroups based on the degree of central adiposity gain (the three subpools are described in SI Appendix, Supplemental Methods). Plasma aliquots were express-shipped on dry ice for metabolomics assays conducted at the Luxembourg Centre for Systems Biomedicine, University of Luxembourg.
Data Collection: Metabolomics.
Metabolite extraction and chemical derivatization were followed by GC-MS to yield high-dimensional data that were analyzed by MetaboliteDetector software (13) and subsequently statistically evaluated by R (release 3.1.2). All samples were assayed in triplicate to provide estimates of technical variation. Before statistical analysis, metabolite levels were normalized by reference pools (every metabolite level divided by the mean signal of the same metabolite level in a pooled sample of two measured pools chronologically closest to the measured sample). First, a nontargeted metabolomics assay of 305 metabolites was conducted. Second, an optimized assay of 107 metabolites was designed based on the first set of findings, and starting over with freshly prepared pools, the four original groups and the subpools were assayed. Signals that persisted were considered as validated. Third, a targeted and quantitative GC-MS analysis was conducted on the subset of metabolites of interest, including fructose, leucine, isoleucine, valine, lactic acid, and erythritol. For this purpose, external standard solution series were produced containing the metabolites of interest.
Statistical Analysis.
In the nontargeted GC-MS analysis, multiple testing was accounted for by calculating false discovery rate (FDR) Q-values (14); the threshold of FDR = 0.2 was set a priori for statistical significance. The metabolome at study baseline in participants with incident central adiposity gain was compared with the metabolome of participants with stable adiposity. The metabolome of participants with higher HbA1c (HbA1c > 5.05%) at the study baseline was compared with the metabolome of participants with lower HbA1c (HbA1c < 4.9%). Welch’s t test was used to test for differences in the mean metabolites between groups, and the nominal P value was adjusted for multiple comparisons to yield the FDR. The FDR Q-values were computed separately for the comparison of the weight groups and for the comparison of the glycemia groups. Discovery and quantitative assay results were analyzed using the same statistical methods.
Human and Cell Studies of Erythritol Metabolism.
Blood incubation experiments.
To investigate erythritol metabolism in humans, an ex vivo stable isotope-assisted blood incubation experiment was performed on five healthy male volunteers. Before blood collection, basal glucose levels were determined with a commercially available glucometer (Diamond Mini; ForaCare). From each volunteer, 1 mL of whole blood was collected via finger prick into K2EDTA-coated vacutainers (BD) and aliquoted in 300-μL portions. Each aliquot was supplemented with a 1 M [U13C]glucose, [6-13C1]glucose, [1,2-13C2]glucose, or [3,4-13C2]glucose (CLM-1396-1, CLM-2717-PK, CLM-504, or CLM-6750-MPT-PK; Cambridge Isotopes) solution to a final total glucose (labeled + unlabeled) concentration of 15 mM. The volume of the 1 M tracer solution added to the blood depended on the blood glucose concentration measured before and was adjusted to reach a final concentration of 15 mM glucose in all samples. After spiking the tracer, the blood samples were incubated at 37 °C under continuous shaking on an orbital shaker at a mixing frequency of 600 rpm (ThermoMixer comfort with thermoblock to cover 1.5-mL reaction vessels, Eppendorf).
Whole-blood samples were collected 5 min before addition of the tracers and 1, 15, 30, 60, and 120 min after addition of the tracers. For metabolite extraction, each sample was extracted in triplicate by mixing 10 μL of whole blood with 90 μL of ice-cold extraction fluid [8:1 methanol/water mixture (vol/vol)] containing 5 μg/mL pentanedioic-d6 acid (D-5227; C/D/N Isotopes, Inc.) as an internal standard. After mixing at 1,400 rpm on an orbital shaker (Eppendorf Thermomixer comfort) for 5 min at 4 °C and subsequent centrifugation at 21.000 × g at 4 °C for 10 min, 70 μL of supernatant was transferred into a GC glass vial with a microinsert (5–250 μL) and dried in a refrigerated rotary vacuum evaporator (Labconco) at −4 °C for 3 h before GC-MS measurement.
Dried blood spot analysis.
To evaluate erythritol synthesis from glucose in vivo, 2 g of [U13C]glucose (CLM-1396-MPT; Cambridge Isotopes) was administered orally to three healthy male donors and dried blood samples were collected via finger prick at T0 and at 5, 15, 30, 45, 60, 90, 120, and 180 min after ingestion on dried blood spot cards (Whatman protein saver cards, Z699519-100EA; Sigma–Aldrich). To analyze the impact of erythritol intake on human glucose metabolism in vivo, 50 g of commercially available erythritol (Erythrit, Charge: 435FET8B77; Natur Total) was ingested 2 min before the 45-min time point. The dried blood spot cards were allowed to dry at room temperature for 3 h, transferred to zip-lock foil bags containing a desiccant, and stored at 4 °C until metabolite extraction.
The exact blood sampling times and blood glucose concentrations after ingestion of [U13C]glucose were monitored by an in-house Android application and glucometers (Diamond Mini; ForaCare). For each card (representing one time point), dried blood metabolites were extracted in triplicate. For each replicate, two punch-outs with a diameter of 3 mm were added to 80 μL of ice-cold extraction fluid [8:1 methanol/water mixture (vol/vol)] containing 1 μg/mL [U13C]ribitol (ALD-062; Omicron Biochemicals, Inc.) as an internal standard, vortexed at 4 °C for 10 min at 1,400 rpm on an orbital shaker (ThermoMixer comfort, Eppendorf), and centrifuged at 4 °C for 10 min at 16,000 × g; 60 μL of supernatant was transferred into GC glass vials with a microinsert (5–250 μL) and dried in a refrigerated vacuum concentrator (Labconco) overnight at −4 °C.
In addition, to analyze whether erythritol is oxidized to erythronate in vivo, erythritol and erythronate levels were quantified by standard addition. Twenty-five microliters of defined standard solutions was pipetted onto each spot of the dried blood spot cards and extracted in accordance with the extraction protocol used for dried blood spots.
GC-MS measurements.
Automated sample derivatization was performed using a GERSTEL multipurpose sampler. Dried samples were dissolved in 15 μL of pyridine, containing 20 mg/mL methoxyamine hydrochloride, at 40 °C for 90 min under shaking. After adding 15 μL of N-methyl-N-trimethylsilyl-trifluoroacetamide (Macherey-Nagel), samples were incubated at 40 °C for 30 min under continuous shaking.
GC-MS analysis was performed using an Agilent 7890A gas chromatograph coupled to an Agilent 5975C inert XL Mass Selective Detector (both from Agilent Technologies). A sample volume of 1 μL was injected into a split/splitless inlet in split mode (10:1) at 270 °C. The gas chromatograph was equipped with a 30-m DB-35MS (i.d. = 250 μm, film thickness = 0.25 μm) capillary column plus 5-m DuraGuard capillary in front of the analytical column (Agilent J&W GC Column). Helium was used as carrier gas with a constant flow rate of 1.2 mL⋅min−1. The GC oven temperature was held at 90 °C for 1 min and increased to 320 °C at 15 °C⋅min−1, and then held at that temperature for 8 min. The total run time for each sample was 24.333 min. The transfer line temperature was set constantly to 280 °C. The mass selective detector was operating under electron ionization at 70 eV. The MS source was held at 230 °C, and the quadrupole was held at 150 °C. Full scan mass spectra were acquired from m/z 70 to m/z 800. For precise absolute quantification, GC-MS measurements of the derivatives of interest were additionally performed in selected ion monitoring mode (SI Appendix, Supplemental Methods).
Deconvolution of mass spectra, peak picking, integration, and retention index calibration were performed using MetaboliteDetector software. Compounds were identified using an in-house mass spectra library. The following deconvolution settings were applied: peak threshold = 5, minimum peak height = 5, bins per scan = 10, deconvolution width = five scans, no baseline adjustment, minimum of 15 peaks per spectrum, and no minimum required base peak intensity. Retention index calibration was based on a C10-C40 even n-alkane mixture (68281; Sigma-Aldrich).
Results
Sample Description.
In the longitudinal study of 264 freshmen members of the class of 2015, 65% (n = 172) of participants had data available at both the beginning and the end of the academic year. In participants with two data points, 75% gained weight (>0.5 kg) over the year; on average, the weight gain was a 3.6% increase in body weight.
There were no participants with a medical history of diabetes, other cardiometabolic disease, or insulin use. Sixty-six participants had incident central adiposity gain; these participants increased 4.0 kg (SD = 2.0) in weight, 3.9 cm (SD = 2.0) in waist circumference, and 2.6% (SD = 1.5) in DXA-derived truncal adiposity. In contrast, 16 participants were stable on the adiposity indicators, with an average weight change of 0.6 kg (SD = 1.1), waist circumference change of 0.4 cm (SD = 1.7), and DXA-derived truncal adiposity change of −0.1% (SD = 0.5).
Given that the sample was young and healthy, the HbA1c levels were within normal limits (4.0–6.0% for ages ≥ 18 y). Thus, a distributional approach was used to define two phenotype groups for comparisons: the top 25% of the baseline HbA1c distribution [n = 21, HbA1c > 5.05%, mean = 5.66% (SD = 0.18)] and the bottom 10% [n = 7, HbA1c < 4.92%, mean = 4.80% (SD = 0.084)].
At study baseline, the phenotype groups were similar in DXA-derived adiposity indicators and anthropometry measurements (Table 1). The higher HbA1c group and the group with incident central adiposity gain weighed slightly more than the other groups at the baseline. At study baseline, each of the phenotype groups was near the population median on weight-for-age (range: 45th–52nd percentile); slightly above the population median on height-for-age (52nd–60th percentile); and thus, on average, below the population median on BMI-for-age (38th–48th percentile).
Table 1.
Baseline characteristics by phenotype group
| Characteristic | Increased central adiposity* | Stable weight and adiposity† | Higher usual glycemia‡ | Lower usual glycemia§ |
| (n = 66) | (n = 16) | (n = 21) | (n = 7) | |
| Sex, % female | 47.0 | 56.3 | 57.1 | 71.4 |
| Nationality, % US | 90.9 | 93.8 | 90.5 | 85.7 |
| Age, y | 18.1 (0.3) | 18.1 (0.3) | 18.0 (0.2) | 18.0 (0) |
| Weight, kg | 64.2 (12.7) | 60.9 (12.3) | 63.3 (12.4) | 59.4 (12.3) |
| Height, cm | 171 (11) | 170 (9) | 169 (9) | 169 (11) |
| BMI, kg/m2 | 21.8 (3.2) | 21.0 (2.4) | 22.0 (3.1) | 20.6 (1.6) |
| Waist circumference, cm | 72.4 (7.6) | 71.8 (8.2) | 72.0 (7.8) | 70.1 (5.7) |
| Hip circumference, cm | 95.6 (7.8) | 94.1 (6.3) | 95.3 (6.7) | 94.4 (7) |
| Weight-for-age, percentile | 50.3 (27.4) | 45.2 (27.5) | 51.8 (24.5) | 45.8 (27.5) |
| Height-for-age, percentile | 55.3 (30.1) | 54.6 (31.6) | 52.1 (32.4) | 59.5 (30.6) |
| BMI-for-age, percentile | 44.8 (27.1) | 39.0 (23.3) | 48.5 (23.4) | 38.5 (18.4) |
| Total body fat, % | 19.1 (7.3) | 20.4 (6.6) | 20.6 (8.1) | 20.4 (6.6) |
| Truncal body fat, % | 15.9 (7.3) | 16.5 (6.3) | 17.3 (8.3) | 15.8 (6.7) |
| Fat mass index,¶ kg/m2 | 4.4 (2.2) | 4.4 (1.6) | 4.7 (2.2) | 4.3 (1.3) |
| Glycosylated hemoglobin, % | 5.3 (0.3) | 5.4 (0.4) | 5.7 (0.2) | 4.8 (0.1) |
Values are mean (SD) unless otherwise noted.
Defined as change in three indicators (weight increase > 0.5 kg, DXA truncal adiposity increase > 200 g, and waist circumference increase > 0.5 cm; n = 66).
Defined as minimal change in three indicators (weight, DXA truncal adiposity, and waist circumference; n = 16).
Defined as top 25% of baseline HbA1c [n = 21; HbA1c > 5.05%, mean = 5.66% (SD = 0.18)].
Defined as bottom 10% of baseline HbA1c [n = 7; HbA1c < 4.92%, mean = 4.80% (SD = 0.084)].
Fat mass index numerator is the mass of total body adipose tissue.
Metabolites Predictive of Incident Central Adiposity Gain Phenotype.
Five metabolites differed between the stable adiposity and incident central adiposity gain groups at a nominal P < 0.05 (Table 2). The difference in one metabolite, meso-erythritol (nominal P value = 0.0004, FDR = 0.0435), reached the FDR threshold for statistical significance, and the concentration of meso-erythritol was 13.4-fold greater in the baseline pooled blood aliquots from participants with incident central adiposity gain compared with pooled blood aliquots from participants who maintained a stable adiposity phenotype. The differences between the phenotype pools for fructose and an unidentified metabolite were near the significance threshold after adjusting for multiple comparisons (both nominal P = 0.006, FDR = 0.23).
Table 2.
Comparison of semiquantitative metabolite level in the central adiposity gain group versus the stable weight and adiposity group: Metabolites with a nominal P < 0.05
| Metabolite | Incident central adiposity* | Stable adiposity† | Nominal P value | FDR Q-value | Higher usual glycemia‡ | Lower usual glycemia§ | Nominal P value | FDR Q-value |
| Erythritol | 0.1395 | 0.0104 | 0.0004 | 0.0421 | 0.277 | 0.012 | 1.51E-06 | 0.00015 |
| Fructose | 0.0587 | 0.0271 | 0.0064 | 0.2208 | 0.050 | 0.116 | 0.0006 | 0.02770 |
| 1742¶ | 0.0184 | 0.0079 | 0.0060 | 0.2171 | ||||
| 1297¶ | 0.0097 | 0.0135 | 0.0120 | 0.3115 | ||||
| 2470¶ | 0.0089 | 0.0060 | 0.0308 | 0.5323 | ||||
| Octadecanoic acid | 0.203 | 0.284 | 0.0227 | 0.41392 | ||||
| Lactic acid | 1.987 | 2.721 | 0.0233 | 0.41789 | ||||
| Glycerol | 0.140 | 0.181 | 0.0260 | 0.43348 | ||||
| Valine | 0.436 | 0.350 | 0.0430 | 0.49758 | ||||
| 1175¶ | 0.094 | 0.154 | 0.0468 | 0.50685 | ||||
| 2278¶ | 0.096 | 0.117 | 0.0471 | 0.50754 | ||||
| Leucine | 0.216 | 0.159 | 0.0475 | 0.50845 |
Metabolite semiquantitative findings are reported for the optimized assay, which validated results based on the nontargeted assay.
Defined as change in three indicators (weight increase > 0.5 kg, DXA truncal adiposity increase > 200 g, and waist circumference increase > 0.5 cm; n = 66).
Defined as minimal change in three indicators (weight, DXA truncal adiposity, and waist circumference; n = 16).
Defined as top 25% of baseline HbA1c [n = 21; HbA1c > 5.05%, mean = 5.66% (SD = 0.18)].
Defined as bottom 10% of baseline HbA1c [n = 7; HbA1c < 4.92%, mean = 4.80% (SD = 0.084)].
Unidentified metabolites represented with their corresponding retention indices.
These metabolites, and several other candidate metabolites, were analyzed further in a targeted GC-MS approach with absolute quantification of erythritol, fructose, lactic acid, and three BCAAs (SI Appendix, Table S1). The signal for erythritol was confirmed in a targeted GC-MS approach with absolute quantification of erythritol; the concentration of erythritol was 14.7-fold greater [95% confidence interval (CI): 13.27, 16.25] in incident central adiposity gain pooled samples compared with pooled samples of participants with stable adiposity [60.8 (SE = 3.1) vs. 4.1 (SE = 0.0) μmol/L; P < 0.0001]. The concentration of fructose was 2.2-fold greater in the baseline pooled blood aliquots from participants with incident central adiposity gain compared with the stable adiposity group [46.2 (SE = 0.9) vs. 27.8 (SE = 1.6); P = 0.022]. The concentrations of leucine, isoleucine, and valine were higher in the incident central adiposity gain phenotype group, but these findings did not reach statistical significance (P = 0.76, P = 0.64, and P = 0.55, respectively).
Finally, the phenotype of incident central adiposity gain was refined to investigate dose–response patterns for the metabolites of interest. The concentration of meso-erythritol varied by tertiles of the central adiposity score (SI Appendix, Fig. S2); the highest meso-erythritol concentration was in the lowest central adiposity change subgroup (n = 26), and the pattern indicated lower meso-erythritol with greater central adiposity change. There was no difference in the concentration of meso-erythritol between the stable adiposity phenotype subgroups.
Metabolite Profile Associated with Higher Glycemia Phenotype.
The metabolomic profile between phenotype groups defined by higher versus lower glycemia was compared using HbA1c concentrations at the study baseline (Table 2), and nine metabolites had nominal P values <0.05. The group with higher glycemia had a 22-fold greater concentration of meso-erythritol (nominal P < 0.0001, FDR = 0.0002) and about half the concentration of fructose (nominal P = 0.0006, FDR = 0.0302) compared with the lower glycemia phenotype. Valine and leucine concentration differences reached the nominal P value threshold, but not the FDR threshold, and isoleucine concentration differences were consistent in direction, although not statistically significant (P = 0.0620, FDR = 0. 5346; SI Appendix, Table S4).
Based on these findings, six metabolites were further analyzed in a targeted GC-MS approach with absolute quantification of erythritol, fructose, lactic acid, valine, leucine, and isoleucine. The meso-erythritol concentration was 20.6-fold greater (95% CI: 19.84, 21.41) in the higher glycemia group [105.6 (SE = 1.4) vs. 5.1 (SE = 0.1) μmol/L; P = 0.0024]. Fructose and lactic acid had significantly lower concentrations in the higher glycemia group (nominal P = 0.0444 and nominal P = 0.0088, respectively; SI Appendix, Table S2). The concentrations of the BCAAs were higher in the higher glycemia group, but findings did not reach the statistical significance threshold.
In a further analysis of the dose–response, both glycemia phenotype groups were split at the median and the quantitative assay was repeated in the four resulting pools (SI Appendix, Fig. S2). Meso-erythritol concentrations were about the same in the two halves of the lower glycemia group, but in the higher glycemia subgroup, meso-erythritol was higher in the top 12.5% of the baseline HbA1c distribution (HbA1c ≥ 5.64%).
Erythritol Is Synthesized from Glucose in Vivo and in Vitro.
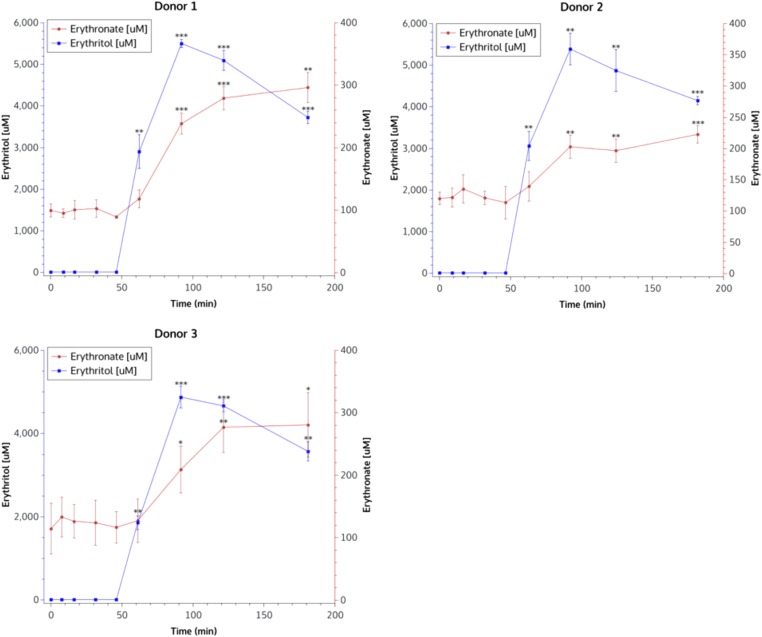
Given that erythritol was associated with subsequent gain in central adiposity gain and with higher HbA1c (measured concurrent with the metabolite), further experiments were conducted to assess whether erythritol interacted with metabolic pathways, whether it affected glucose metabolism, and whether there was evidence for endogenous production of erythritol. In vivo stable isotope-assisted dried blood spot studies in three male volunteers showed evidence that erythritol is synthesized from glucose in red blood cells after ingestion of [U13C]glucose (SI Appendix, Fig. S3). However, due to low erythritol signals and, consequently, low labeled erythritol enrichment patterns, this pathway was validated in an ex vivo blood incubation setup. Erythronate was shown to be produced from erythritol, and there was no evidence that glucose metabolism was perturbed after ingestion of 50 g of erythritol. An immediate increase in erythritol blood concentrations was observed, followed by an increase of erythronate blood concentrations (Fig. 1). Erythritol was oxidized to erythrose, which was fleeting, and then further oxidized to erythronate. Overall, although a large portion of consumed erythritol was assumed to be excreted in urine, about 5–10% was oxidized through the pathway to erythronate.
Fig. 1.
Quantitative GC-MS measurement of erythritol and erythronate in dried blood spots from three healthy donors. After ingestion of 50 g of erythritol (blue lines), an immediate increase of erythronate concentrations was observed (red lines).
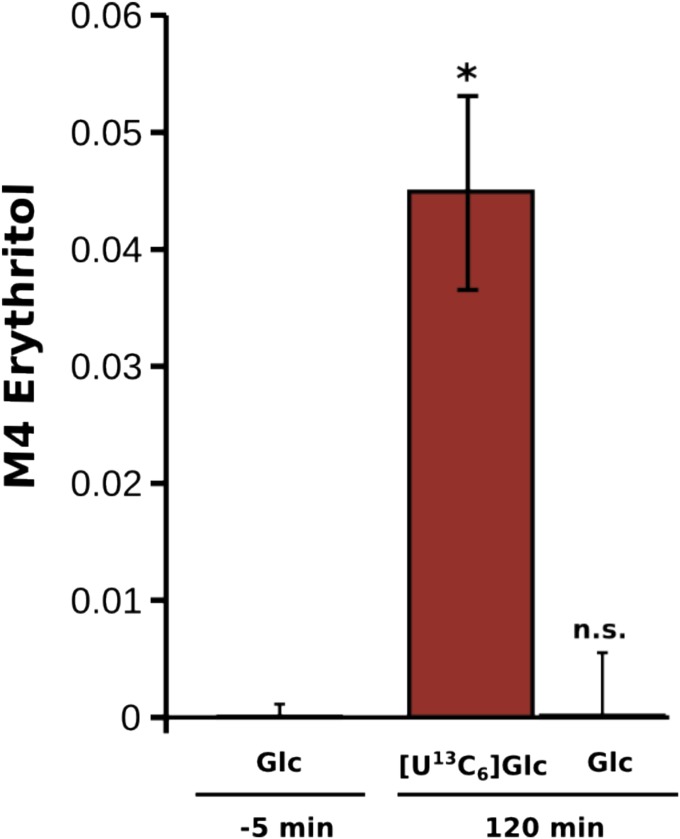
To analyze if erythritol can be synthesized from glucose by human metabolism, a stable isotope-based labeling strategy was used in human blood cells. In case of an endogenous synthesis capacity of erythritol in human (blood) cells, the production of isotopically enriched erythritol after supplementation of a [U13C]glucose tracer was expected. To test this hypothesis, whole blood was incubated with [U13C]glucose, polar metabolites were sampled after 120 min of incubation, and mass isotopomer distributions (MIDs) of erythritol were determined.
A significant increase of fully labeled erythritol was observed after 120 min compared with the time point before adding the [U13C]glucose tracer (−5 min) (Fig. 2). In contrast to these changes, no significant changes could be observed after incubation with unlabeled [U12C]glucose. These results highlight that human metabolism provides all of the biochemical capabilities required to synthesize endogenous erythritol from glucose.
Fig. 2.
M4 erythritol enrichment after supplementing [U13C6]glucose in whole blood. Significant amounts of fully labeled (M4) erythritol were synthesized by endogenous activity in the blood cells 120 min after tracer addition (*P = 0.01 by Welch’s t test). n.s., not significant (P > 0.05).
Erythritol Is Synthesized from Glucose via the Pentose Phosphate Pathway.
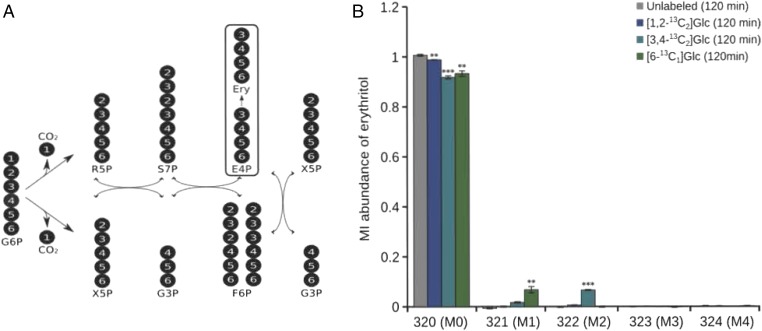
The biochemical pathway for the conversion of glucose into erythritol was investigated. Erythritol is the reduced form of the monosaccharide erythrose, which, in its phosphorylated form, is an intermediate of the reductive pentose-phosphate pathway (PPP). For this reason, it was proposed that glucose is phosphorylated by hexokinase and then oxidized to gluconate-6-phosphate in the oxidative branch of the PPP. After oxidative decarboxylation by gluconate-6-phosphate dehydrogenase, ribulose-5-phosphate is produced. During this step, the first carbon atom of glucose is lost as CO2. Further conversions by transketolase and transaldolase in the reductive part of the PPP eventually produce erythrose-4-phosphate (E4P) (Fig. 3A). It is well known that these reactions take place in almost all human cell types, including erythrocytes (15). The next step is either the reduction of E4P to erythritol-4-phosphate, followed by the action of a phosphatase to release erythritol, or the removal of the phosphate first to release erythrose, which is then reduced to erythritol. To validate this assumption, the labeling experiments were repeated in whole blood, but with three [13C]glucose tracers labeled at specific positions (Fig. 3A). In conclusion, these experiments validated that human blood cells convert glucose into erythritol and, based on the positional labeling experiments, most likely via E4P, and thus the PPP (Fig. 3B).
Fig. 3.
Atom transitions within the PPP and erythritol labeling from glucose. (A) Erythritol (Ery) is synthesized from glucose via the PPP from glucose-6-phosphate (G6P). First, a [1,2-13C2]glucose tracer, which contains stable isotopes at the first two positions, was used. Based on the atom transitions of the oxidative and reductive PPP, no significant isotopic enrichment in erythritol was expected. Second, a [3,4-13C2]glucose tracer was applied; in this case, the synthesis of M2 erythritol isotopologs was predicted. Third, a [6-13C1]glucose tracer was applied. (B) First, incubation of human blood cells with [1,2-13C2]glucose did not produce significant amounts of enriched erythritol. Second, as expected, twofold-labeled (M2) erythritol isotopologs emerged after addition of the [1,2-13C2]glucose tracer to the blood cells. Third, the expected appearance of onefold-labeled (M1) erythritol isotopologs was observed after the [6-13C1]glucose tracer was applied. The amounts of [1,2-13C2]glucose (1,2-13C2 Glc) yielding nonlabeled (M0) erythritol, [3,4-13C2]glucose (3,4-13C2 Glc) yielding M2 erythritol, and [6-13C1]glucose (6-13C1 Glc) yielding M1 erythritol are shown after incubation with the respective tracer for 120 min (**P < 0.01 and ***P < 0.001 by Student’s t test). F6P, fructose 6-phosphate; G3P, glyceraldehyde 3-phosphate; R5P, ribose 5-phosphate; S7P, sedoheptulose 7-phosphate; X5P, xylulose 5-phosphate.
Discussion
We conducted a metabolomics study to identify a metabolic profile predictive of adverse changes in body habitus, specifically incident gain in central adiposity, which is a phenotype with known cardiometabolic risk (16, 17). We found consistent differences for erythritol and fructose over several analytic steps that indicated overall positive associations between those metabolites and incident central adiposity gain in young adults. We also found the concentration of erythritol was higher in the group with higher glycemia compared with the group with lower glycemia at study baseline. Conversely, fructose and lactic acid had lower blood concentrations in the higher glycemia group. The concentrations of BCAAs (isoleucine, leucine, and valine) did not differ between the central adiposity phenotype groups, but these metabolites had higher concentrations in the higher glycemia phenotype group. BCAAs reportedly perturb insulin signaling (18), and experimental feeding of BCAAs, with and without a concurrent high-fat diet, contributes to insulin resistance in animal models (10).
Overall, the human and cell studies of erythritol support the heretofore unrecognized metabolism of glucose to erythritol. These findings are in contrast to previously published studies that reported erythritol is not metabolized in humans (19). Hiele et al. (19) showed that ingestion of 25 g of 13C-labeled glucose and lactitol led to altered human metabolism; in contrast, they reported that after ingestion of 25 g of 13C-labeled erythritol, no alterations of the human metabolism could be observed and erythritol was not further metabolized. We showed that there is metabolism of erythritol to erythronate.
In the examination of subgroups of the incident central adiposity gain group, we compared the concentration of erythritol across three levels of central adiposity gain, defined by the degree of change. We observed the greatest concentration of erythritol in the subgroup with the least degree of central adiposity change. These data provide some evidence of a stepwise relationship between erythritol and central adiposity gain; the inverse association may indicate that erythritol is associated with an overall risk of increase in central adiposity, but not with the magnitude of central adiposity gain. Replication of this study is needed to verify the direction and magnitude of this association. Also, future research characterizing specific foods or food groups that contribute to exogenous erythritol exposure, or variation in human endogenous erythritol synthesis, could improve the understanding of erythritol exposure, and lead to a better understanding of factors contributing to endogenous erythritol concentration, increased adiposity, or both.
Erythritol is a sugar alcohol that occurs naturally in a variety of foods (e.g., pear, watermelon), is 60–80% as sweet as sucrose, and is an approved low-calorie sweetener food additive (20, 21). US survey data estimate that the typical intake of erythritol is about 1 g⋅d−1 (22). Erythritol is a four-carbon polyol, and up to 90% of the ingested quantity is rapidly absorbed through the gut lumen (19), with the unabsorbed fraction possibly (23) subject to fermentation by gut microbes (20, 22). Clinical feeding studies of 0.8–1 g of erythritol per kilogram of body weight show that ∼90% of the dose is excreted (urine) in 24–48 h (20). In animal models, plasma erythritol levels peak about 30–60 min postingestion, with 99% disappearance from the plasma within 24 h (24). Erythritol exists endogenously in human tissues, with plasma levels of ∼9.8 μmol/L (25), and although a prior study claimed that endogenous production of erythritol was null (19), we demonstrated that erythritol is endogenously synthesized by human metabolism in vivo. After ingestion of a [U13C]glucose tracer, isotopically enriched erythritol was detected in the blood of the donor. We hypothesized that at least some of the erythritol production takes place in blood cells because, after transport by the enterocytes, these cells are the first that are in contact with the tracer, and the finding that these primary human blood cells can synthesize detectable amounts of isotopic labeled erythritol after incubation with stable isotope tracers supported this hypothesis. Due to the determined MIDs, erythritol is most probably synthesized from E4P, an intermediate of the PPP.
Although no previous studies are directly comparable to this study, a few studies of weight gain in young adults include biomarkers or look for predictive metabolomic profiles. Würtz et al. (8) investigated 82 prespecified metabolites to understand the causal effect of BMI and of adiposity change on the metabolome in a large consortium study of 16- to 39-y-olds. They identified causal associations of BMI change with changes in lipid-related, inflammation-related, and BCAA metabolites. Prior research also found an association of artificial sweeteners with both metabolic dysfunction (26, 27) and weight gain (28–30), but these studies were based on consumption and the conclusions are likely to be affected by both confounding and reverse causality (persons already gaining weight choose to consume artificially sweetened beverages to prevent further weight gain). The biological mechanisms for the association between artificial sweetener and weight gain are proposed to relate to gut microbiota metabolism (31). Several recent studies of erythritol loading reported no effect on glucose (32, 33), consistent with our findings. A study in patients with diabetes reported that erythritol and enrichment of another PPP biomarker (ribose) were increased in patients with diabetic retinopathy, and suggested that polyol pathway flux may yield biomarkers of clinical risk in diabetes (34).
We also found that a higher baseline concentration of plasma fructose was associated with the incident central adiposity gain phenotype. Fructose is a normal component of the human diet and is naturally concentrated in sweet fruits, although fructose is also extracted from beets, cane, and sucrose, and is concentrated as high-fructose corn syrup for use as a sweetener. The possible causal link between fructose intake and human weight gain is controversial (35), and we could not identify any published studies that used metabolomics to study the association of fructose with subsequent weight gain. We also found higher lactic acid concentrations associated with the incident central adiposity gain phenotype, and this finding may be driven by the fructose finding, given evidence from isotopic tracer studies of fructose, which show that approximately one-fourth of ingested fructose is converted to lactic acid within hours (36).
Previous studies used metabolomic approaches to construct metabolic “fingerprints” associated with obesity, and repeatedly show higher concentrations of BCAAs associated with obesity (8, 37, 38) across all age ranges and with risk for insulin resistance in adolescents (37). In our study, concentrations of BCAAs were higher in the higher glycemia phenotype group, supporting previous studies that implicate BCAAs in long-term glucose economy. No differences in concentration were found in the incident central adiposity gain versus stable adiposity phenotype groups, suggesting that increased BCAAs in circulation are not associated with subsequent adiposity gain.
A limitation of this work is the use of pools of plasma for all comparisons because it is possible that a subset of the individuals within a pool could drive the overall levels of metabolites measured in the pool. A pooling approach was adopted because it offered a cost-effective strategy to investigate the relation between the metabolome and complex phenotypes in an understudied population. In addition, pooling is advantageous to reduce overall variation when the biological variation between individual samples is greater than the technical variation between replicate assays from the same pool (39). In this study, we had information on technical variation (pool replicates), but we had no information on biological variability among individual samples. The FDR-corrected statistical tests presented herein are not as robust as tests that account for both biological and technical variation (39). However, the consistency of the erythritol results in both the central adiposity and usual glycemia comparisons lends strength to the findings, and the cluster of metabolites has biological plausibility.
This pool-based study delivers two unique results about metabolite abundance related to a one-time measurement of glycemia and longitudinal increase in central adiposity. Although the two pools within a comparison are mutually exclusive, there is minor overlap across two of the comparisons (12 of the 66 participants with central adiposity gain are also in the higher baseline glycemia group). Strengths of this work include a large sample representative of university freshmen, longitudinal data to define the phenotype using both anthropometry and gold-standard DXA methods, and the use of rigorous protocols for all data collection.
In conclusion, we found a positive association between circulating levels of erythritol at the study baseline and the incidence of central adiposity gain in nonobese adults aged 18 y and studied over 9 mo. Moreover, we demonstrated that erythritol is constitutively synthesized in humans from glucose, most probably via E4P, which is an intermediate of the PPP. Further research is needed to understand the meaning of these findings, including the interplay between variation in endogenous erythritol synthesis and exogenous exposure to erythritol-containing foods. Further research to confirm these findings in a new cohort and to identify metabolic profiles that predict changes in adiposity in early adulthood present a unique opportunity to identify novel targets for prevention, which are very important, given the well-known difficulty of losing weight once it is gained.
Supplementary Material
Acknowledgments
This research was supported by Cornell University and by NIH Institutional Research Training Grant T32-DK-7158-38. Stable isotope-assisted metabolomics assays were funded by the Fonds National de la Recherche Luxembourg (ATTRACT A10/03). Neither Cornell University nor the NIH had any role in the design, analysis, or writing of this article.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620079114/-/DCSupplemental.
References
- 1.National Center for Education Statistics & Integrated Postsecondary Education Data System 2012 Projections of Education Statistics to 2021. Table 30. Available at https://nces.ed.gov/programs/projections/projections2021/tables/table_30.asp). Accessed April 18, 2017.
- 2.Vella-Zarb RA, Elgar FJ. The ‘freshman 5’: A meta-analysis of weight gain in the freshman year of college. J Am Coll Health. 2009;58:161–166. doi: 10.1080/07448480903221392. [DOI] [PubMed] [Google Scholar]
- 3.Crombie AP, Ilich JZ, Dutton GR, Panton LB, Abood DA. The freshman weight gain phenomenon revisited. Nutr Rev. 2009;67:83–94. doi: 10.1111/j.1753-4887.2008.00143.x. [DOI] [PubMed] [Google Scholar]
- 4.Naukkarinen J, Rissanen A, Kaprio J, Pietiläinen KH. Causes and consequences of obesity: The contribution of recent twin studies. Int J Obes. 2012;36:1017–1024. doi: 10.1038/ijo.2011.192. [DOI] [PubMed] [Google Scholar]
- 5.Nelson MC, Story M, Larson NI, Neumark-Sztainer D, Lytle LA. Emerging adulthood and college-aged youth: An overlooked age for weight-related behavior change. Obesity (Silver Spring) 2008;16:2205–2211. doi: 10.1038/oby.2008.365. [DOI] [PubMed] [Google Scholar]
- 6.Laska MN, Pelletier JE, Larson NI, Story M. Interventions for weight gain prevention during the transition to young adulthood: A review of the literature. J Adolesc Health. 2012;50:324–333. doi: 10.1016/j.jadohealth.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steffen A, et al. Development and validation of a risk score predicting substantial weight gain over 5 years in middle-aged European men and women. PLoS One. 2013;8:e67429. doi: 10.1371/journal.pone.0067429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Würtz P, et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 2014;11:e1001765. doi: 10.1371/journal.pmed.1001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochhar S, et al. Probing gender-specific metabolism differences in humans by nuclear magnetic resonance-based metabonomics. Anal Biochem. 2006;352:274–281. doi: 10.1016/j.ab.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 10.Newgard CB, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szymanska E, et al. Gender-dependent associations of metabolite profiles and body fat distribution in a healthy population with central obesity: Towards metabolomics diagnostics. Omics. 2012;16:652–667. doi: 10.1089/omi.2012.0062. [DOI] [PubMed] [Google Scholar]
- 12.Wahl S, et al. Multi-omic signature of body weight change: Results from a population-based cohort study. BMC Med. 2015;13:48. doi: 10.1186/s12916-015-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiller K, Metallo C, Stephanopoulos G. Elucidation of cellular metabolism via metabolomics and stable-isotope assisted metabolomics. Curr Pharm Biotechnol. 2011;12:1075–1086. doi: 10.2174/138920111795909096. [DOI] [PubMed] [Google Scholar]
- 14.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser A Stat Soc. 1995;57:289–300. [Google Scholar]
- 15.Wood T. Physiological functions of the pentose phosphate pathway. Cell Biochem Funct. 1986;4:241–247. doi: 10.1002/cbf.290040403. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I. Influence of age on the relation between waist circumference and cardiometabolic risk markers. Nutr Metab Cardiovasc Dis. 2009;19:163–169. doi: 10.1016/j.numecd.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Jago R, Mendoza JA, Chen T, Baranowski T. Longitudinal associations between BMI, waist circumference, and cardiometabolic risk in US youth: Monitoring implications. Obesity (Silver Spring) 2013;21:E271–E279. doi: 10.1002/oby.20080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tremblay F, Lavigne C, Jacques H, Marette A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr. 2007;27:293–310. doi: 10.1146/annurev.nutr.25.050304.092545. [DOI] [PubMed] [Google Scholar]
- 19.Hiele M, Ghoos Y, Rutgeerts P, Vantrappen G. Metabolism of erythritol in humans: Comparison with glucose and lactitol. Br J Nutr. 1993;69:169–176. doi: 10.1079/bjn19930019. [DOI] [PubMed] [Google Scholar]
- 20.Munro IC, et al. Erythritol: An interpretive summary of biochemical, metabolic, toxicological and clinical data. Food Chem Toxicol. 1998;36:1139–1174. doi: 10.1016/s0278-6915(98)00091-x. [DOI] [PubMed] [Google Scholar]
- 21.Goossens JRH. Erythritol: A new bulk sweetener. International Journal of Food Ingredients. 1994;1/2:27–33. [Google Scholar]
- 22.Bernt WO, Borzelleca JF, Flamm G, Munro IC. Erythritol: A review of biological and toxicological studies. Regul Toxicol Pharmacol. 1996;24:S191–S197. doi: 10.1006/rtph.1996.0098. [DOI] [PubMed] [Google Scholar]
- 23.Arrigoni E, Brouns F, Amadò R. Human gut microbiota does not ferment erythritol. Br J Nutr. 2005;94:643–646. doi: 10.1079/bjn20051546. [DOI] [PubMed] [Google Scholar]
- 24.Noda K, Nakayama K, Modderman J. Fate of erythritol after single oral administration to rats and dogs. Regul Toxicol Pharmacol. 1996;24:S206–S213. doi: 10.1006/rtph.1996.0100. [DOI] [PubMed] [Google Scholar]
- 25.Niwa T, Tohyama K, Kato Y. Analysis of polyols in uremic serum by liquid chromatography combined with atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A. 1993;613:9–14. doi: 10.1016/0378-4347(93)80191-6. [DOI] [PubMed] [Google Scholar]
- 26.Pereira MA. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk. Adv Nutr. 2014;5:797–808. doi: 10.3945/an.114.007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suez J, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 28.Fowler SP, et al. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16:1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- 29.Hampton T. Sugar substitutes linked to weight gain. JAMA. 2008;299:2137–2138. doi: 10.1001/jama.299.18.2137. [DOI] [PubMed] [Google Scholar]
- 30.Vanselow MS, Pereira MA, Neumark-Sztainer D, Raatz SK. Adolescent beverage habits and changes in weight over time: Findings from Project EAT. Am J Clin Nutr. 2009;90:1489–1495. doi: 10.3945/ajcn.2009.27573. [DOI] [PubMed] [Google Scholar]
- 31.Payne AN, Chassard C, Lacroix C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: Implications for host-microbe interactions contributing to obesity. Obes Rev. 2012;13:799–809. doi: 10.1111/j.1467-789X.2012.01009.x. [DOI] [PubMed] [Google Scholar]
- 32.Shin DH, et al. Glycemic effects of rebaudioside A and erythritol in people with glucose intolerance. Diabetes Metab J. 2016;40:283–289. doi: 10.4093/dmj.2016.40.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wölnerhanssen BK, et al. Gut hormone secretion, gastric emptying, and glycemic responses to erythritol and xylitol in lean and obese subjects. Am J Physiol Endocrinol Metab. 2016;310:E1053–E1061. doi: 10.1152/ajpendo.00037.2016. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, et al. Plasma metabonomic profiling of diabetic retinopathy. Diabetes. 2016;65:1099–1108. doi: 10.2337/db15-0661. [DOI] [PubMed] [Google Scholar]
- 35.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 36.Sun SZ, Empie MW. Fructose metabolism in humans - what isotopic tracer studies tell us. Nutr Metab (Lond) 2012;9:89. doi: 10.1186/1743-7075-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCormack SE, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013;8:52–61. doi: 10.1111/j.2047-6310.2012.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore SC, et al. Human metabolic correlates of body mass index. Metabolomics. 2014;10:259–269. doi: 10.1007/s11306-013-0574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kendziorski C, Irizarry RA, Chen K-S, Haag JD, Gould MN. On the utility of pooling biological samples in microarray experiments. Proc Natl Acad Sci USA. 2005;102:4252–4257. doi: 10.1073/pnas.0500607102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.