Significance
The prokaryotic clustered regularly interspaced short palindromic repeats (CRISPR)-associated (Cas) systems have been widely used as tools for various genome-engineering applications. The most popular CRISPR-Cas system makes use of the Cas9 endonuclease from Streptococcus pyogenes (SpCas9) and a single-guide RNA (sgRNA). The SpCas9/sgRNA complex cleaves double-stranded DNA sequences specified by sgRNA and protospacer adjacent motif (PAM). This study explores the mechanism by which SpCas9/sgRNA efficiently locates the correct target DNA sequence. The results show that high efficiency of DNA interrogation can be explained, in part, by destabilization of double-stranded DNA conformation in the vicinity of PAM. The data also suggest that the local DNA destabilization is mediated by SpCas9/sgRNA affinity for a single-stranded DNA segment adjacent to PAM and by hindering of duplex DNA entry into the SpCas9/sgRNA interior.
Keywords: Cas9, CRISPR, DNA interrogation, protein–DNA interactions, fluorescence spectroscopy
Abstract
The prokaryotic clustered regularly interspaced short palindromic repeats (CRISPR)-associated 9 (Cas9) endonuclease cleaves double-stranded DNA sequences specified by guide RNA molecules and flanked by a protospacer adjacent motif (PAM) and is widely used for genome editing in various organisms. The RNA-programmed Cas9 locates the target site by scanning genomic DNA. We sought to elucidate the mechanism of initial DNA interrogation steps that precede the pairing of target DNA with guide RNA. Using fluorometric and biochemical assays, we studied Cas9/guide RNA complexes with model DNA substrates that mimicked early intermediates on the pathway to the final Cas9/guide RNA–DNA complex. The results show that Cas9/guide RNA binding to PAM favors separation of a few PAM-proximal protospacer base pairs allowing initial target interrogation by guide RNA. The duplex destabilization is mediated, in part, by Cas9/guide RNA affinity for unpaired segments of nontarget strand DNA close to PAM. Furthermore, our data indicate that the entry of double-stranded DNA beyond a short threshold distance from PAM into the Cas9/single-guide RNA (sgRNA) interior is hindered. We suggest that the interactions unfavorable for duplex DNA binding promote DNA bending in the PAM-proximal region during early steps of Cas9/guide RNA–DNA complex formation, thus additionally destabilizing the protospacer duplex. The mechanism that emerges from our analysis explains how the Cas9/sgRNA complex is able to locate the correct target sequence efficiently while interrogating numerous nontarget sequences associated with correct PAMs.
Prokaryotic clustered regularly interspaced short palindromic repeats (CRISPR)-associated (Cas) gene systems acquire fragments of foreign DNA (protospacers) and insert them as spacers into the CRISPR array in the prokaryotic host genome. Upon subsequent encounters, the complex of Cas proteins with CRISPR RNA (crRNA) bearing the spacer sequence binds and cleaves foreign DNA containing the matching sequence (1–3), thus providing the host organism with adaptive hereditable immunity. The DNA endonuclease Cas9 of type II CRISPR-Cas systems is targeted to specific DNA sequences by a 20-base crRNA spacer that binds to the complementary strand of protospacer DNA, displacing the noncomplementary strand to form an R-loop (4, 5). Both binding and cleavage of target DNA by the Cas9/crRNA complex require a short protospacer adjacent motif (PAM) located immediately downstream of the targeted sequence (6). For the most commonly used Streptococcus pyogenes Cas9 (SpCas9), the PAM sequence is 5′-NGG (7). The perfect match between guide RNA and seven to 12 bases of target DNA at the immediate 5′ side of PAM (“seed” region) is the most critical for DNA binding and cleavage, although limited mispairing in the distal bases is tolerated (8). Two RNA molecules (crRNA and a transactivating crRNA (tracrRNA)) required to guide SpCas9 to targets can be replaced with a single-guide RNA (sgRNA) composed of fused crRNA and tracrRNA molecules (7). The Cas9/sgRNA system is widely used for genome editing in various organisms (9, 10). A Cas9 derivative (dCas9) that lacks endonuclease activity but can still bind to targets (7) is used for transcriptome modulation (11) and visualization of genomic loci in live cells (12).
Given the large size of the eukaryotic genome, the numerous successes of genome editing with Cas9 imply that it must search DNA for target sequences with remarkably high speed. Studies on dynamics of CRISPR-Cas9 genome interrogation in vitro and in living cells show that 3D diffusion dominates Cas9 genome searching and that off-target binding events are, on average, short-lived (6, 13–15). Biochemical and single-molecule experiments indicate that DNA strand separation and RNA/DNA heteroduplex formation initiate at the protospacer end proximal to PAM and proceed toward the distal end (6). Analyses of Cas9/sgRNA complexes with DNA targets containing mismatches with guide RNA suggest that the Cas9/PAM interaction can facilitate initial protospacer DNA strand separation near PAM and enable initial base pairing between the target DNA strand and the RNA guide sequence (6). Cas9 contains a loop (“phosphate lock loop”) that interacts with the target strand DNA phosphate close to PAM and may stabilize target DNA immediately upstream of PAM in an unwound conformation (16). It was proposed that the interaction between the phosphate and the loop promotes local unpairing in early interrogation complexes (16). Substitutions of phosphate lock loop amino acids considerably affect cleavage of partially mismatched DNA targets but only modestly reduce the rate of cleavage of DNA perfectly complementary to guide RNA (16). This observation seems to suggest that some other mechanisms apart from the phosphate lock loop contacts with DNA may mediate the PAM-induced DNA destabilization. We sought to elucidate mechanistic details of initial DNA interrogation steps that precede the pairing of target DNA with the guide RNA spacer. Using fluorometric and biochemical assays, we studied Cas9/sgRNA interactions with a set of model DNA substrates that likely mimic interactions in early intermediate Cas9/sgRNA–DNA interrogation complexes. Our data indicate that Cas9/sgRNA interaction with PAM confers high affinity for unpaired DNA segments adjacent to PAM even in the absence of pairing between the crRNA guide and the protospacer. This result suggests that Cas9/sgRNA binding to PAM favors separation of nearest base pairs of the protospacer. The destabilization of the DNA duplex is achieved primarily due to interactions of Cas9/sgRNA with nontarget DNA strand nucleotides and steric hindering of duplex DNA entry into the Cas9/sgRNA interior. The data also suggest that interaction of the target DNA strand with the phosphate lock loop is subsequent to the initial DNA destabilization step and is coupled with the DNA/RNA pairing.
Results
Experimental Setup.
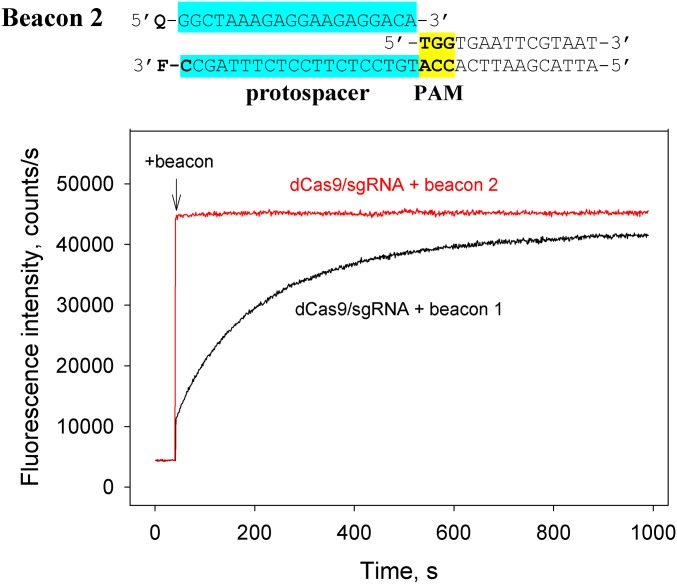
To investigate the roles of distinct Cas9/sgRNA–DNA interactions during interrogation of DNA, we measured binding affinities of S. pyogenes dCas9/sgRNA to a set of fully double-stranded, partially single-stranded, or bubbled model substrates (Fig. S1) that mimic likely early Cas9/sgRNA–DNA intermediates on the pathway to the final R-loop complex. Similar methodology has been applied to study mechanisms of duplex DNA destabilization by RNA polymerases and a variety of enzymes that use base flipping to gain access to bases in the double-stranded DNA (17, 18). To determine equilibrium dissociation constant (Kd) values of dCas9/sgRNA complexes with various probes quantitatively, we measured the ability of these probes to affect the rate of dCas9/sgRNA binding to “Cas9 beacons,” fluorescently labeled DNA fragments containing the protospacer and PAM, competitively (19). Cas9 binding to a beacon results in a readily detectable increase in fluorescence intensity. The rate of fluorescence increase depends on the beacon sequence and structure, which can be varied easily for particular applications (19, 20). Schematic representations of the beacon assay and structures of Cas9 beacons 1 and 2 used in this work are shown in Fig. 1 and Fig. S2. Beacon 1 consists of two fully complementary oligonucleotides, and beacon 2 consists of three oligonucleotides: Its nontarget strand contains a discontinuity between PAM and the protospacer. Beacon 1 contains a T1C substitution at the position immediately adjacent to PAM, whereas the sequence of the protospacer segment of beacon 2 fully matches the sgRNA spacer sequence. The kinetics of beacon 2 binding to dCas9 are much faster than the kinetics of beacon 1 binding to dCas9 (Fig. S2). Because measurement of slower beacon-binding rates is more convenient, we used beacon 1 in experiments with dCas9 (binding takes several minutes), whereas beacon 2 was used in experiments with the intrinsically slower mutant Cas9KG/sgRNA complex (16).
Fig. S1.
Structures of sgRNA and DNA probes used. The probe numbers used in this study are shown to the left of the sequences. The PAM sequences are highlighted in yellow.
Fig. 1.
Quantitative measurement of DNA binding to Cas9 using Cas9 beacons. (A) Principle of the Cas9 beacon assay. The circles labeled F and Q indicate the fluorophore and quencher. (B) Effect of competitor DNA probes 1–3 that bore no sgRNA guide sequence complementarity but contained different numbers of PAMs on the kinetics of beacon 1 binding to dCas9/sgRNA. The concentrations of dCas9/sgRNA and beacon 1 were 5 nM and 1 nM, respectively. The concentrations of DNA probes 1–3 were 500 nM. The structure of beacon 1 is shown above the panel. The PAM and protospacer sequences are highlighted in red and blue, and the mismatched protospacer bases are highlighted in yellow. Errors are SDs (n = 3).
Fig. S2.
Time dependencies of the fluorescence signal upon addition of 1 nM beacon 1 or 1 nM beacon 2 to 5 nM dCas9/sgRNA. The structure of beacon 2 is shown above the panel. The PAM and protospacer sequences are highlighted in yellow and blue, respectively.
The structures of sgRNA, target DNA, and other DNA probes used in this work are presented in Fig. S1. The parental “target probe” is a 47-bp DNA duplex containing a protospacer fully complementary to the sgRNA spacer sequence. Probe 1 is a nontarget derivative of the 47-bp duplex in which the protospacer segment has no complementarity to the sgRNA spacer. Both probes contain a single trinucleotide sequence, TGG, that serves as the PAM in the target probe. Other probes are fragments or derivatives of probe 1. There are four NGG trinucleotides in probe 2. Probe 3 does not have any NGG sequences. Representative competition experiments with the dCas9/sgRNA, target DNA, and probes 1–3 are shown in Fig. 1B. The target DNA fragment very effectively competed with beacon 1 for binding to the dCas9/sgRNA, and no increased fluorescence from the beacon was observed upon preincubation of the dCas9/sgRNA with this probe (10 nM). Significant inhibition of beacon 1 binding by probes 1–3 was reached at much higher probe concentrations. Probe 1 (500 nM) decreased the rate of beacon binding by 1.7-fold. Probe 2 was a more efficient competitor, inhibiting the beacon-binding rate by 3.3-fold. The inhibition effect caused by probe 2 was proportional to probe concentration in the range of 200–1,000 nM (Fig. S3). The calculated Kd values for the binding of probes 1 and 2 to dCas9/sgRNA were 780 nM and 210 nM, respectively. Because probe 3 lacks both the PAM and the protospacer, it was not expected to be specifically recognized by the dCas9/sgRNA. Indeed, this probe at a concentration of 500 nM decreased the binding rate of the beacon by just 1.2-fold. Overall, the results indicate, in agreement with earlier data (6), that the Cas9/sgRNA is capable of recognizing PAM sequences in the absence of protospacers matching the sgRNA spacer but that the binding affinity is very low.
Fig. S3.
Effect of different concentrations of DNA probe 2 that contained four PAM sequences but bore no sgRNA guide sequence complementarity on the kinetics of beacon 1 binding to dCas9/sgRNA. The concentrations of dCas9/sgRNA and beacon 1 were 5 nM and 1 nM, respectively. Concentrations of probe 2 are indicated.
Interaction of dCas9/sgRNA with Partially Mismatched and Gapped Substrates Without Complementarity to sgRNA Spacer.
Unpairing of a short PAM-proximal segment of protospacer (1 or 2 bp) is a critical step in DNA interrogation that may determine the overall rate of target location (6, 21). We surmised that DNA substrates noncomplementary to sgRNA spacer but carrying short mismatched “bubble” segments near PAM may mimic the DNA structure in early interrogation complexes and could be used as tool to study the target location/DNA interrogation mechanism. Assaying of dCas9/sgRNA interaction with probes 4–9 (derivatives of probe 1 containing mismatched segments adjacent to PAM) showed that 1-bp and 2-bp bubbles in probes 4 and 5, respectively, increase affinity about 10-fold and 100-fold compared with the parent probe (Fig. 2 A and B and Fig. S4). Further extension of mismatched segments in probes 6 and 7 only slightly improved the affinity (about twofold). In contrast, mismatches in positions distant from PAM (probes 8 and 9) had no significant effect on the binding (Fig. 2 A and B). These data indicate that dCas9/sgRNA specifically binds with high affinity and in a spacer sequence-independent manner to double-stranded DNA bearing short unpaired segments adjacent to PAM. The increased affinity should stimulate unpairing of a few base pairs nearest to PAM during the early stages of target interrogation even in the absence of pairing between the crRNA guide and the protospacer.
Fig. 2.
Competition assays with partially mismatched and gapped DNA probes. All probes bore no sgRNA guide sequence complementarity, and the concentrations of all probes were 200 nM. (A) Representative data on effect of competitor DNA probes 4–9 that contained PAM mismatched “bubble” segments either adjacent to or distant from PAM on the kinetics of beacon 1 binding to dCas9/sgRNA. (B) Quantification of competition data shown in A. (C) Effect of gapped DNA probes 10 and 11 on the kinetics of beacon 1 binding to dCas9/sgRNA. Bars are means, and errors are SDs (n = 3 or n = 4).
Fig. S4.
EMSA assay of dCas9/sgRNA binding to probes 5 and 2 (A) and quantification of binding data for probe 5 fitted to a standard binding isotherm (B). DNA probes were prepared using target strand oligonucleotides 5′ end-labeled with [32P]. Reactions containing 20 nM DNA probes and indicated concentrations of dCas9/sgRNA in the binding buffer [20 mM Tris⋅HCl (pH 7.9), 100 mM NaCl, 5% glycerol, 0.1 mM DTT, and 1 mM MgCl2] were incubated for 15 min at 25 °C before being resolved by 6% native PAGE at 4 °C (0.5 × TBE buffer). Radioactive DNA was visualized by phosphorimaging, quantified with ImageQuant, and analyzed with SigmaPlot software.
Next, we measured the Kd for dCas9/sgRNA binding to gapped derivatives of probe 5 lacking either target or nontarget strand parts of the 2-bp-long bubble (Fig. 2C). The affinity of probe 10 lacking the nontarget strand segment was 16-fold lower than the affinity of probe 5. This result indicates that dCas9/sgRNA has a significant affinity for the nontarget strand protospacer segment adjacent to PAM in the context of bubble probe 5. In contrast, removing nucleotides from the target strand made probe 11 affinity 1.7-fold higher than the affinity of parental probe 5, implying that the unpaired segment in the target strand is slightly inhibitory for the binding. To exclude the possibility that these results are specific for probe 5, we measured Kd values for dCas9/sgRNA binding to probe 12 and its gapped derivatives (probes 13 and 14). These probes are similar to, correspondingly, probes 5, 10, and 11 but have different sequences in the mismatched/gapped region (Fig. S1). As seen in Fig. 2C and Fig. S5, the effects caused by deletion of target or nontarget strand segments of the bubble are similar in both cases, although the Kd for probe 12 is higher than for probe 5 (17 nM and 7.1 nM, respectively). Overall, these data suggest that initial local DNA melting is driven by interactions between Cas9/sgRNA and nontarget strand nucleotides adjacent to PAM, whereas target strand nucleotides likely do not play a significant role in this process.
Fig. S5.
Effect of DNA probes 12, 13, and 14 on the kinetics of beacon 1 binding to dCas9/sgRNA. All probes bore no sgRNA guide sequence complementarity. Errors are SDs (n = 3). The concentrations of all competitor DNA probes were 200 nM.
We also measured dCas9/sgRNA affinities for bubble probe 15, whose mismatched target strand bases are complementary to sgRNA bases at the −1 and −2 positions and to its gapped derivative (probe 16) lacking the nontarget strand part in its 2-bp-long bubble (Fig. S6). The affinity of dCas9/sgRNA to probe 15 is considerably (13-fold and 30-fold) higher than for similarly constructed probes 5 and 12, respectively (Fig. 2C and Figs. S5 and S6), which are fully noncomplementary to sgRNA. Removal of the nontarget strand bubble part in the context of probes 15 and 16 decreased the affinity by 2.7-fold (Fig. S6), which is a smaller effect than the effects observed with probes 5 and 12 and their gapped derivatives, probes 10 and 13 (Fig. 2C and Fig. S5). This result suggests that the initial RNA–DNA hybrid formation near PAM leads to some weakening of Cas9/sgRNA interaction with nontarget strand nucleotides neighboring PAM.
Fig. S6.
Effect of DNA probes 15 and 16 on kinetics of beacon 1 binding to dCas9/sgRNA. In contrast to other probes, mismatched target strand bases of probes 15 and 16 are complementary to sgRNA bases at the −1 and −2 positions. Errors are SDs (n = 3). The concentrations of competitor DNA probes were 20 nM.
Because probe 12 bears unpaired T’s in both strands of the mismatched segment, the interaction of dCas9/sgRNA with this probe was also examined using KMnO4 probing that specifically reveals unpaired thymines. KMnO4 sensitivity of unpaired target strand T did not noticeably change upon the addition of the dCas9/sgRNA to probe 12 (Fig. 3). In contrast, the sensitivity of a T located in the nontarget strand segment of the bubble significantly decreased in the presence of dCas9/sgRNA (Fig. 3), suggesting that this base becomes buried inside the protein upon the binding of dCas9/sgRNA.
Fig. 3.
Examination of dCas9/sgRNA interactions with duplex and mismatched bubble probes using a permanganate reactivity assay. (Left) KMnO4 probing of dCas9/sgRNA complexes with duplex probe 2 reveals no KMnO4-sensitive thymines. (Right) Effect of dCas9/sgRNA on KMnO4 reactivity of thymines on the target (t) and nontarget (nt) strand unpaired segments of bubble probe 12. The red arrow indicates a thymine whose reactivity to KMnO4 significantly decreased in the presence of dCas9/sgRNA.
Structural analysis suggested that Cas9/sgRNA binding to PAM may lead to the formation of a stably unpaired DNA region near PAM even in the absence of complementary base pairing between target DNA and sgRNA (16). We tested this possibility by probing the dCas9/sgRNA complex with probe 2 (contains four PAM sequences and bears a T within 2 bp upstream of each of the PAMs) with KMnO4. A high dCas9/sgRNA concentration (0.5 μM) was used in this experiment to ensure effective binding. However, no KMnO4-sensitive thymines were detected (Fig. 3), suggesting that initial noncomplementary interactions between dCas9/sgRNA and target DNA do not lead to stable local DNA strand separation, at least in the context of the probe 2 sequence. We surmise that initial noncomplementary interactions may transiently stabilize the short-lived opened DNA conformation resulting from DNA breathing (22–24), giving it a sufficient lifetime to allow dynamic motions of the complex needed for RNA–DNA base pairing near PAM to occur.
Double-Stranded DNA Upstream of Position −2 Is Inhibitory to Cas9/sgRNA Binding.
DNA bending and other forms of DNA distortion have been proposed to facilitate DNA opening in various biological systems (25). In crystal structures of Cas9/sgRNA bound to target DNA, the DNA duplex containing the PAM sequence is sharply bent toward the sgRNA spacer/DNA protospacer heteroduplex that passes through a central channel in the protein (16, 26). Thus, the trajectory of duplex DNA in the vicinity of PAM may become altered in early intermediate complexes, which could favor local strand separation during R-loop complex formation (Discussion). To test this conjecture, we conducted competition binding experiments with duplex probes 17–21 that had the same downstream boundary but different upstream edges (Fig. S1). Strikingly, a fragment of probe 1 truncated immediately upstream of PAM (probe 17) had ∼70-fold higher affinity (Kd = 11 nM) than probe 1 (Fig. 4). The substitution of a G for a T at the +2 position of probe 17 virtually eliminated the competition effect, proving that probe 17 binding was PAM-dependent. Probe 18 bearing a 2-bp segment upstream of PAM showed nearly the same affinity as probe 17. Further extensions of the upstream edge to −3 and −4 (probes 19 and 20) led to 2.5-fold and 4.5-fold drops in the affinity, whereas probe 21 bearing a 7-bp segment upstream of PAM was a weak competitor, similar to probe 1 (Fig. 4).
Fig. 4.
Competition assays using double-stranded DNA probes with upstream edges at different positions. (A) Effect of competitor DNA probes 17–21 on the kinetics of beacon 1 binding to dCas9/sgRNA. Positions of upstream edges are shown in parentheses. (B) Quantification of competition data shown in A. Bars are means, and errors are SDs (n = 3). All probes bore no sgRNA guide sequence complementarity. The concentrations of all competitor DNA probes were 200 nM.
The data thus show that intrinsic interaction of Cas9/sgRNA with PAM is, in fact, quite strong. However, the presence of the upstream DNA duplex beyond a certain point significantly weakens this interaction, suggesting that an element of the effector complex hinders the entry of duplex DNA beyond a certain threshold distance from PAM into the Cas9/sgRNA interior.
The Cas9 Phosphate Lock Loop Does Not Play a Significant Role in the Initial Destabilization of the Target but Promotes Target DNA/sgRNA Pairing Immediately Downstream of PAM.
Structural studies suggest that local unpairing of the target DNA duplex can be facilitated by interaction between the phosphate lock loop of Cas9 and the +1 phosphate on the target strand of the protospacer (16). However, the data presented above did not reveal dCas9/sgRNA affinity for unpaired target strand positions −1/−2 in the context of substrates noncomplementary to sgRNA. This finding suggests that phosphate lock loop is not involved in initial destabilization of target DNA duplex proximal to PAM. To test this idea, we compared the affinities of DNA probes 5, 10, 11, 15, 17, and 18 for dCas9 and a Cas9 mutant Cas9KG (which carries a substitution of the Lys1107-Ser1109 loop with a Lys-Gly dipeptide) in which the phosphate lock loop interaction with the +1 phosphate is destroyed (16). The affinities of probes 5, 10, 11, 17, and 18 for Cas9KG were seven- to 20-fold lower than for dCas9 (Table 1). This drop in affinity cannot be explained solely by the difference in the strength of dCas9 and Cas9KG binding to the +1 phosphate because probe 17 lacks a protospacer segment and has no terminal phosphate groups. Because Lys1107 interacts with PAM (16, 26), it seems possible that weakening of this interaction in the Cas9KG complexes may be the main cause of its lower affinity for the probes. The deletions of nontarget or target strand single-stranded bubble segments in the context of probes 5, 10, and 11 produce similar decreases and increases in affinities to both dCas9 and Cas9KG (Table 1 and Fig. S7). This result indicates that the phosphate lock loop does not influence the mode dCas9/sgRNA interactions with probe 5, and therefore is unlikely to promote duplex unpairing significantly in the PAM-proximal region of the target.
Table 1.
Comparison of Kd values for dCas9/sgRNA and Cas9KG/sgRNA binding to DNA probes
| Probe | KddCas9, nM | KdCas9KG, nM | KdCas9KG/KddCas9 |
| 5 | 7.1 ± 0.9 | 140 ± 18 | 20 |
| 10 | 120 ± 14 | 850 ± 160 | 7.2 |
| 11 | 4.3 ± 0.47 | 63 ± 5.5 | 15 |
| 15 | 0.53 ± 0.08 | 90 ± 13 | 170 |
| 17 | 11 ± 1.8 | 78 ± 10 | 7.1 |
| 18 | 12 ± 1.8 | 82 ± 14 | 7.0 |
The probes bear no sgRNA guide sequence complementarity except for probe 15, which contains complementary to sgRNA bases at the −1 and −2 positions of the target strand. Errors are SDs (n = 3 or n = 4).
Fig. S7.
Representative data on Cas9KG binding to DNA probes. Effect of DNA probes 5, 10, and 11 on the kinetics of beacon 2 binding to Cas9KG/sgRNA. The concentrations of Cas9KG/sgRNA and beacon 2 were 5 nM and 1 nM, respectively. The concentrations of DNA probes 9–11 were 200 nM. Errors are SDs (n = 3).
In contrast to other probes, mismatched target strand bases of probe 15 are complementary to sgRNA bases at the −1 and −2 positions. Notably, probe 15 had nearly 200-fold lower affinity for Cas9KG/sgRNA than for dCas9/sgRNA (Table 1). This difference in affinity is much larger than the differences observed with other probes. Furthermore, the affinity of Cas9KG for probe 15 is only ∼1.5-fold higher than for a similarly constructed probe 5 that is fully noncomplementary to sgRNA, whereas the affinity of dCas9/sgRNA is 13-fold higher (Table 1). These observations indicate that the complementary interactions between template strand bases in the −1 and −2 positions and sgRNA are impaired in the Cas9KG/sgRNA complex with probe 15. This finding suggests that the interaction of the +1 phosphate with the phosphate lock loop promotes the initial pairing of sgRNA with the target strand. In contrast, destabilization of the target duplex that precedes the DNA–RNA pairing occurs without the involvement of the phosphate lock loop/DNA interactions.
Discussion
The results reported here, together with earlier data, clarify the process of target interrogation by the Cas9/sgRNA complex. Consistent with earlier data (6), we find that dCas9/sgRNA binds duplex DNA probes with a single PAM sequence and with no complementarity to the sgRNA spacer sequence weakly (with about micromolar affinity). We show that affinity to similar DNA probes bearing short unpaired segments immediately close to PAM is much higher despite the absence of complementarity between unpaired regions and corresponding positions of sgRNA. In contrast, unpaired segments in positions distant from PAM have no significant effect on dCas9/sgRNA affinity for DNA. Thus, Cas9/sgRNA interaction with PAM favors separation of neighboring base pairs, which should cause a shift in the equilibrium between the duplex and unpaired conformations of the target. This result can be explained, in part, by Cas9/sgRNA affinity for unpaired segments of nontarget strand DNA close to PAM. In contrast, the Cas9 phosphate lock loop interaction with the target strand +1 phosphate is linked to target DNA/sgRNA pairing immediately upstream of PAM but does not play a significant role in the initial duplex destabilization. Thus, the Cas9/sgRNA interactions with PAM-proximal sequences of DNA probes not complementary to sgRNA studied here differ from those interactions revealed in crystal complexes, where the target DNA strand was hybridized with the complementary sgRNA (16, 26–28). Overlays of Cas9/sgRNA-DNA and Cas9/sgRNA structures reveal that in the Cas9/sgRNA structure, domains 2 and 3 of the helical recognition lobe sterically occlude the central channel in which the RNA/DNA hybrid is located in the Cas9/sgRNA–DNA complexes (16, 21, 26). This finding indicates that a structural rearrangement within the helical recognition lobe must occur upon target DNA binding and R-loop formation to avoid a steric clash between target DNA strand and the helical recognition lobe (21, 29). We hypothesize that during the initial DNA interrogation steps preceding the DNA/sgRNA pairing, the helical recognition lobe maintains a conformation similar to the conformation seen in the Cas9/sgRNA complex (21), which should impede double-stranded DNA access to the central channel. This proposal is consistent with our data showing that the presence of duplex DNA beyond 2 bp upstream of PAM strongly decreases Cas9/sgRNA affinity for DNA (Fig. 4). We further envision that this steric obstacle promotes DNA bending and destabilization of the PAM-proximal duplex in the initial interrogation complex, as schematically shown in Fig. 5. It would be of interest to determine whether similar inhibitory interactions exist in other CRISPR effector complexes with DNA.
Fig. 5.
Proposed mechanism for early DNA interrogation by Cas9/sgRNA. Cas9/sgRNA binding to PAM (Cas9/DNA interactions are shown as triangles) leads to bending of a protospacer segment adjacent to PAM due to the impedance of DNA access into the central Cas9 channel by a part of the helical recognition lobe (dark cylinder). The DNA bending and Cas9 interactions with nontarget strand nucleotides facilitate transient separation of a few protospacer base pairs nearest to PAM, which consequently promotes pairing between target strand bases in the melted protospacer region and complementary sgRNA bases. The initial sgRNA/DNA pairing is linked to the interaction of the +1 phosphate on the target DNA strand with the phosphate lock loop of Cas9 (violet triangle).
Materials and Methods
Proteins.
S. pyogenes Cas9 protein derivatives dCas9 (catalytically inactive Cas9 harboring point mutations D10A/H840A) and Cas9KG (a Cas9 derivative with a substitution of the Lys1107-Ser1109 loop with a Lys-Gly dipeptide) were expressed in Escherichia coli BL21(DE3) using the expression plasmid pMJ841 (Addgene; https://www.addgene.org/) and a plasmid encoding Cas9KG (a gift from M. Jinek, University of Zurich, Zurich; described in ref. 16). The proteins were purified essentially as previously described (7).
sgRNA and DNA Probes.
sgRNA was prepared as previously described (19). DNA probes were prepared from unmodified and chromophore-labeled DNA oligonucleotides synthesized by Integrated DNA Technologies. Double-stranded DNA probes and Cas9 beacon DNA constructs were formed by mixing equimolar amounts of synthetic complementary strands in a buffer containing 40 mM Tris (pH 7.9) and 100 mM NaCl heated for 2 min at 90 °C, with the reactions slowly cooled to 20 °C. The PAM-distal ends of the beacon target and nontarget strands were labeled with fluorescein and Iowa Black R-FQ, respectively.
Fluorometric Measurements.
Fluorescence measurements were performed using a QuantaMaster QM4 spectrofluorometer (PTI) in binding buffer [20 mM Tris⋅HCl (pH 7.9), 100 mM NaCl, 5% glycerol, 0.1 mM DTT, and 1 mM MgCl2] containing 0.02% Tween 20 at 25 °C. Final assay mixtures (800 μL) contained 5 nM Cas9 protein, 8 nM sgRNA, 1 nM labeled Cas beacon construct, and competitor DNA substrates at various concentrations. The fluorescein fluorescence intensities were recorded with an excitation wavelength of 498 nm and an emission wavelength of 520 nm. Time-dependent fluorescence changes were monitored after addition of a negligible volume of Cas beacon to a cuvette, followed by manual mixing; the mixing dead time was 15 s. Competition experiments were analyzed to determine the Kd of competitor DNA (SI Materials and Methods). The Kd values presented are averages obtained from at least three individual experiments.
KMnO4 Probing.
For in vitro KMnO4 probing experiments, the double-stranded DNA target fragments were prepared using radioactively 5′ end-labeled with [32P]-γATP oligonucleotides corresponding to either a nontarget or target strand. The labeled DNA fragments were purified on Micro Bio-Spin 6 columns (BioRad). The dCas9/sgRNA complexes were formed in a final volume of 10 μL and contained 600 nM dCas9, 600 nM sgRNA (where indicated), and 100 nM DNA template in a reaction buffer [30 mM Tris⋅HCl (pH 8.0), 40 mM KCl, and 10 mM MgCl2]. The dCas9 and sgRNA were mixed and incubated for 10 min at room temperature, followed by the addition of DNA target. The reactions were incubated for the next 10 min at 37 °C and treated with 1 mM KMnO4 at room temperature for 1 min. The reactions were terminated by the addition of 300 mM β-mercaptoethanol, followed by ethanol precipitation and 20 min of treatment with 10% piperidine at 95 °C. Reaction products were next treated with chloroform to remove piperidine, precipitated in ethanol, dissolved in 8 mL of urea-formamide loading buffer, and resolved in 7% polyacrylamide denaturing gel.
SI Materials and Methods
Analysis of Competition Binding Assays.
All beacon-binding reactions in the presence of DNA competitors (probes) 1–21 (Fig. S1) were at least 90% complete in 5 h, indicating that affinity to the beacon was much higher than to competitors and that beacon binding to Cas9/sgRNA was practically irreversible. In all experiments, concentrations of competitors were significantly higher than concentrations of either Cas9/sgRNA or beacon (5 nM and 1 nM, respectively). Competition experiments were analyzed with Felix software (PTI) essentially as described by Kuznedelov et al. (20). The analysis was performed assuming that the rate of beacon binding in the absence of competitor (V0) is proportional to the Cas9/sgRNA concentration, whereas the binding rate in the presence of competitor (V1) is proportional to concentration of Cas9/sgRNA molecules that remain unbound to competitor; that is and , where Cas9/sgRNA DNA is the Cas9/sgRNA complex with competitor and X is a proportionality coefficient. Consequently, the concentration of Cas9/sgRNA DNA was calculated from Eq. S1:
| [S1] |
The Kd for Cas9/sgRNA binding to DNA probes was calculated from the chemical equilibrium (Eq. S2):
| [S2] |
The V0 and V1 rates were determined from the initial stage of the beacon-binding reactions. The rate values were calculated as slopes of the kinetic curve segments from the mixing point to the time points at which beacon binding was about 15% complete.
The Kd calculation procedure assumes that the equilibrium binding between Cas9/sgRNA and the competitor is reached during the reaction incubation time. This assumption is validated by the observation that preincubation of Cas9/sgRNA with competitors 1–21 for either 2 min or 30 min yielded identical beacon-binding curves. We note that the equilibrium balance between free and competitor-bound Cas9/sgRNA fractions may become disturbed during a late stage of beacon binding if the Cas9/sgRNA complex with competitor dissociates slowly. Calculation of V0 and V1 from the initial stage of the beacon-binding reaction allows one to avoid this potential complication.
Comparison of Beacon Assay and Electrophoretic Mobility Shift Assay Data.
Electrophoretic mobility shift assay (EMSA) binding assays with probes 2 and 5 were performed to verify the results from the beacon assay (Fig. S4A). The EMSA measurements agree well with the beacon assay data in that the Cas9/sgRNA affinity for probe 5 is considerably (about 30-fold) higher than for probe 2 (Figs. 1B and 2 A–C). The absolute Kd value for probe 5 calculated from the EMSA data (Fig. S4B) is about twofold higher than the absolute Kd value obtained using the beacon assay (Fig. 2C).
Acknowledgments
We thank M. Jinek for the generous gift of a plasmid encoding Cas9KG. This study was supported by NIH Grant GM10407; Russian Science Foundation Grant 14-14-00988; Ministry of Education and Science of Russian Federation Project 14.B25.31.0004; and Skoltech institutional support (all to K.S.). Funding for an open access charge was provided by Skoltech.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619926114/-/DCSupplemental.
References
- 1.Barrangou R, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 2.Brouns SJ, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–964. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garneau JE, et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468:67–71. doi: 10.1038/nature09523. [DOI] [PubMed] [Google Scholar]
- 4.Jore MM, et al. Structural basis for CRISPR RNA-guided DNA recognition by Cascade. Nat Struct Mol Biol. 2011;18:529–536. doi: 10.1038/nsmb.2019. [DOI] [PubMed] [Google Scholar]
- 5.Szczelkun MD, et al. Direct observation of R-loop formation by single RNA-guided Cas9 and Cascade effector complexes. Proc Natl Acad Sci USA. 2014;111:9798–9803. doi: 10.1073/pnas.1402597111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507:62–67. doi: 10.1038/nature13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu Y, et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Methods. 2013;10:957–963. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert LA, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen B, et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155:1479–1491. doi: 10.1016/j.cell.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight SC, et al. Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science. 2015;350:823–826. doi: 10.1126/science.aac6572. [DOI] [PubMed] [Google Scholar]
- 14.Ma H, et al. CRISPR-Cas9 nuclear dynamics and target recognition in living cells. J Cell Biol. 2016;214:529–537. doi: 10.1083/jcb.201604115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh D, Sternberg SH, Fei J, Doudna JA, Ha T. Real-time observation of DNA recognition and rejection by the RNA-guided endonuclease Cas9. Nat Commun. 2016;7:12778. doi: 10.1038/ncomms12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anders C, Niewoehner O, Duerst A, Jinek M. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature. 2014;513:569–573. doi: 10.1038/nature13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cámara B, et al. T7 phage protein Gp2 inhibits the Escherichia coli RNA polymerase by antagonizing stable DNA strand separation near the transcription start site. Proc Natl Acad Sci USA. 2010;107:2247–2252. doi: 10.1073/pnas.0907908107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao C, Jiang YL, Stivers JT, Song F. Dynamic opening of DNA during the enzymatic search for a damaged base. Nat Struct Mol Biol. 2004;11:1230–1236. doi: 10.1038/nsmb864. [DOI] [PubMed] [Google Scholar]
- 19.Mekler V, Minakhin L, Semenova E, Kuznedelov K, Severinov K. Kinetics of the CRISPR-Cas9 effector complex assembly and the role of 3′-terminal segment of guide RNA. Nucleic Acids Res. 2016;44:2837–2845. doi: 10.1093/nar/gkw138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuznedelov K, et al. Altered stoichiometry Escherichia coli Cascade complexes with shortened CRISPR RNA spacers are capable of interference and primed adaptation. Nucleic Acids Res. 2016;44:10849–10861. doi: 10.1093/nar/gkw914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang F, Zhou K, Ma L, Gressel S, Doudna JA. Structural biology. A Cas9-guide RNA complex preorganized for target DNA recognition. Science. 2015;348:1477–1481. doi: 10.1126/science.aab1452. [DOI] [PubMed] [Google Scholar]
- 22.Guéron M, Kochoyan M, Leroy JL. A single mode of DNA base-pair opening drives imino proton exchange. Nature. 1987;328:89–92. doi: 10.1038/328089a0. [DOI] [PubMed] [Google Scholar]
- 23.Stivers JT. Extrahelical damaged base recognition by DNA glycosylase enzymes. Chemistry. 2008;14:786–793. doi: 10.1002/chem.200701501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altan-Bonnet G, Libchaber A, Krichevsky O. Bubble dynamics in double-stranded DNA. Phys Rev Lett. 2003;90:138101. doi: 10.1103/PhysRevLett.90.138101. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Martín J, Rojo F, de Lorenzo V. Promoters responsive to DNA bending: A common theme in prokaryotic gene expression. Microbiol Rev. 1994;58:268–290. doi: 10.1128/mr.58.2.268-290.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang F, et al. Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. Science. 2016;351:867–871. doi: 10.1126/science.aad8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimasu H, et al. Crystal structure of Staphylococcus aureus Cas9. Cell. 2015;162:1113–1126. doi: 10.1016/j.cell.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirano H, et al. Structure and engineering of Francisella novicida Cas9. Cell. 2016;164:950–961. doi: 10.1016/j.cell.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oakes BL, et al. Profiling of engineering hotspots identifies an allosteric CRISPR-Cas9 switch. Nat Biotechnol. 2016;34:646–651. doi: 10.1038/nbt.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]