Abstract
Autoimmune blistering diseases are diverse group of conditions characterized by blisters in the skin with or without mucosal lesions. There may be great degree of clinical and histopathological overlap; hence, advanced immunological tests may be necessary for more precise diagnosis of these conditions. Direct immunofluorescence microscopy is the gold standard tests to demonstrate the tissue-bound antibodies and should be done in all cases. Magnitude of antibody level in patient’ serum can be assessed by indirect immunofluorescence and enzyme linked immunosorbent assay. In this article we have reviewed the various techniques that are available in the diagnosis of autoimmune blistering diseases.
Keywords: Autoimmune bullous diseases, direct immunofluorescence, ELISA
What was known?
Direct immunofluorescence is the gold standard test in the diagnosis of autoimmune blistering diseases
Indirect immunofluorescence in serial dilution is a useful technique to monitor the disease activity in certain conditions
Biopsy specimen can be transported to the laboratory in Michel's medium.
Introduction
Autoimmune bullous diseases (AIBDs) are a heterogeneous group of conditions characterized clinically by blisters and erosions in the skin with or without mucosal involvement.[1] Autoantibodies in AIBDs target components of the epidermis or dermoepidermal junction (DEJ) that are responsible for the maintenance of cell–to-cell adhesion.[2,3] Inflammatory cascade that follows antigen-antibody reaction ultimately leads to blister formation in the skin. Depending on the level of blister formation, AIBDs are classified into intraepidermal and subepidermal types.
AIBDs are potentially life-threatening conditions; accurate diagnosis of these conditions is essential not only from the treatment perspective but also to prognosticate these patients. In clinical practice, physicians often face difficulty in categorizing specific subclasses of AIBDs because of their rarity and heterogeneous symptoms.[4] For example, IgA pemphigus may resemble subcorneal pustular dermatosis (SPD) clinically. Paraneoplastic pemphigus (PNP) may mimic pemphigus vulgaris (PV) and Stevens–Johnson syndrome.[5] Bullous pemphigoid (BP) may be indistinguishable from dermatitis herpetiformis (DH) or linear IgA disease (LAD). Occasionally, atypical presentation of an AIBDs may pose a diagnostic delay, as in cases of BP manifesting as prurigo nodularis-like phenotype (“pemphigoid nodularis”).
Cytologic smear (“Tzanck test”) and histopathological examination of blister are used routinely in many centers in India to support the clinical diagnosis. Histopathological examination from an early vesicle reveals the site of cleavage formation and presence, intensity and composition of inflammatory infiltrate.[6] However, these tests often fail to pinpoint the precise diagnosis in every case. Tests directed at the detection of tissue bound or circulating antibodies should be done for the final confirmation of the diagnosis. These include immunofluorescence (IF) microscopy, enzyme-linked immunosorbent assay (ELISA), and immunoblotting (IB). In this article, we make an attempt to give an overview of practical utility of these advanced tests in the diagnosis of AIBDs.
Immunofluorescence Tests
IF has become an indispensable tool in the diagnosis of AIBDs and has been widely used to supplement the clinical and histological features of various vesiculobullous disorders.[7] IF technique utilizes fluorescent-labeled antibodies to detect specific target antigens. Coons et al. first introduced this technique in the 1940s with the use of blue fluorescing compound, anthracene.[8] In 1964, Beutner and Jordon used the indirect IF (IIF) technique to demonstrate the circulating antibodies in the sera of pemphigus patients.[9] Since then, the use of IF has grown to be an integral part of a dermatopathology laboratory and has made a great impact in diagnosis and management of AIBDs. It not only confirms the diagnosis but also provides a platform on which other advanced tests such as ELISA and IB can be done, if the facilities are available. For example, if IF shows intercellular staining pattern (ICS), as in pemphigus, one would ask for desmoglein 1 and 3 (Dsg 1 and Dsg 3) ELISA or do IB using epidermal extracts. On the other hand, if IF results show basement membrane zone (BMZ) staining pattern (as in BP), one would consider BP ELISA or IB using dermal extracts [Figure 1]. It is always prudent to follow this algorithmic approach while making a diagnosis of AIBDs.
Figure 1.
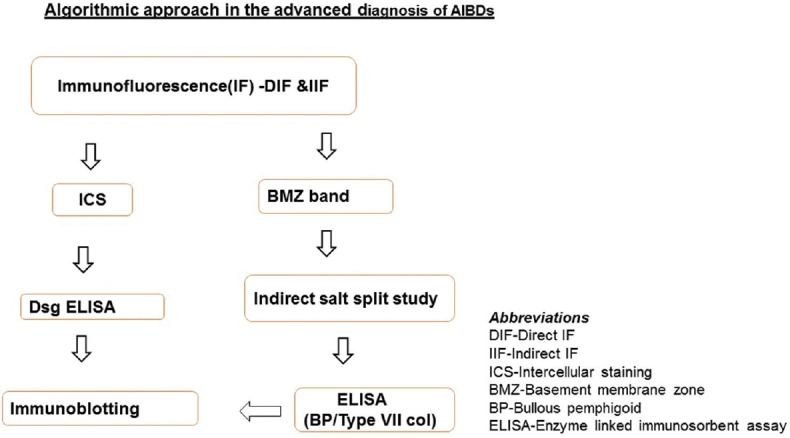
Algorithmic steps in the advanced diagnosis of autoimmune blistering diseases
Techniques of immunofluorescence
There are three basic techniques in IF: direct, indirect, and complement fixation method.
Direct immunofluorescence
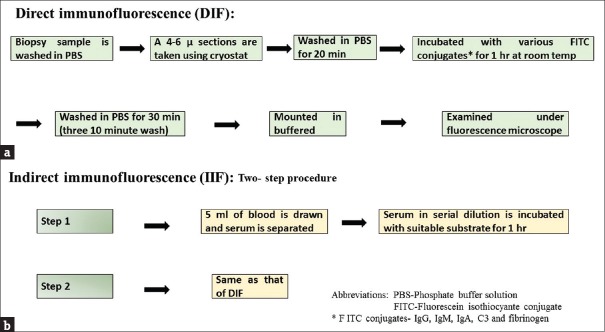
It is the gold standard test which will clinch the diagnosis of AIBDs by identifying the antibodies that are bound in vivo to the tissue antigens in biopsy specimen.[10] Details of the technique are depicted in Figure 2. Interpretation of direct IF (DIF) results is based on five features: the primary sites of immune deposition (e.g., ICS or BMZ), class of immunoglobulin, pattern of immune deposit (linear or granular), number of immune deposits, and deposition in other sites besides the main site. With the use of these parameters, a pattern approach can lead to an accurate diagnosis in majority of specimens.[4]
Figure 2.
(a) Schematic diagram showing important steps in the direct immunofluorescence test. (b) Steps involved in indirect immunofluorescence
Indirect immunofluorescence
IIF is a two-step serological technique [Figure 2] for the detection of circulating antibodies. Patient's serum is diluted in serial dilutions (starting at 1:10) with phosphate-buffered saline and incubated with a suitable substrate. The sensitivity of IIF is generally low when compared to DIF and depends on the substrate used. However, the sensitivity can be increased using combination of substrates, for example, normal human skin (NHS) and monkey esophagus (MO) in pemphigus.[11,12] It has been shown that a calcium-containing buffer solution enhances the sensitivity of IIF in pemphigus group of patients by two or more doubling dilutions.[13]
Complement fixation
This technique is obsolete now, especially with the availability of ELISA. This complex technique was used to identify circulating complement-fixing antibodies in pemphigoid gestationis (PG). Circulating complement-fixing antibodies are an immunopathological hallmark of PG and are seen along the DEJ by IIF microscopy on human skin after preincubation with a complement source.[14]
Modifications in direct immunofluorescence
Outer root sheath of anagen hair is structurally analogous to the epidermis and hence may contain pemphigus antigens. DIF of anagen hair has shown ICS staining in 85% of patients with pemphigus.[15] DIF of Tzanck smear has also been studied and found to be useful in pemphigus.[16]
Salt-split technique
This technique involves incubation of skin in 1M sodium chloride for 48–72 h and subsequently splitting the skin between the epidermis and dermis at the level of lamina lucida.[17] In our laboratory, we prefer to incubate the skin substrate in a vial containing 1 M sodium chloride in a rotator at 4 0C in a refrigerator. After overnight incubation, the skin substrate is gently teased to separate epidermis from dermis. This simple technique is extremely useful to subcategorize subepidermal immunobullous diseases (SIBDs); few antigens will remain on the epidermal side (‘roof’) of the split while others will relocate to the dermal side (‘floor’)[17] Based on the location of BMZ band with respect to the split, SIBDs may be classified into “roof-” or “floor-” binding pattern. For example, BP and inflammatory form of epidermolysis bullosa acquisita (EBA) shows clinicopathological overlap and DIF is indistinguishable in these two conditions. However, salt-split technique (SST) helps distinguish these two conditions as antibodies in BP are “epidermal” binding while EBA shows “dermal-” binding antibodies [Figure 3]. It can be performed on the patient's skin (direct SST) or using NHS which is then incubated with patients’ serum (indirect SST). Indirect SST is preferred as it is easier and is similar in accuracy to direct SST. Direct SST is done in cases where IIF tests are negative.[18]
Figure 3.

(a) Salt-split study showing linear basement membrane zone band on the epidermal side of the split (“roof pattern”) with IgG (red circle representing the level of split. (b) Linear basement membrane zone band on the dermal side (“floor pattern”) of the split with IgG (red circle denotes the level of split) (FITC, ×200)
Site of biopsy
A 3–5 mm punch biopsy is ideal for DIF study. Biopsy should preferably be taken from clinically normal-looking, perilesional skin. Biopsy from the inflamed skin and the area too close to the blister should be avoided as the immune deposits are partially or completely degraded in these areas giving rise to false-negative results.[19] Caution must be exercised to avoid formalin contamination; if two biopsies are considered (for routine histopathology and DIF), first biopsy should be taken for IF, as a touch of formalin may render the biopsy specimen unsuitable for DIF. If the disease is widespread, biopsy is preferably taken from the nonsun-exposed skin such as inner aspect of the upper arm or buttock. The latter is the preferred site of biopsy in DH.[20]
Transportation
Using Michel's transport medium (pH 7.0–7.2), biopsy specimens can be transported to the distant test destination site. Samples in Michel's media can be stored for up to 28 days; electron microscopy studies of normal skin maintained in Michel's medium have shown remarkable preservation of all components of the BMZ, including the ultrastructure of the basal keratinocytes, DEJ, and papillary dermis.[21] Alternatively, normal saline can be used if biopsy sample can be shipped to the IF laboratory within 24 h.[22] Recently, it has been shown that skin biopsy samples meant for immunofluorescence can be stored in honey; immunoreactants are preserved for up to 20 days making it an alternate transport medium for direct immunofluorescence studies.[23]
Antigen identification using epidermolysis bullosa skin
In SIBDs, autoantibodies are produced against the constituents of BMZ; the same molecule may be deficient in hereditary epidermolysis bullosa (EB). Using a panel of EB skin (with a known deficient BMZ protein) as a substrate and serum of patients with SIBDs, one can detect the target antigens in certain SIBDs using a modified IIF.[24] This technique may especially be useful to differentiate “floor-”binding SIBDs such as EBA, p-200 pemphigoid, and laminin 332 pemphigoid. Serum of patients with EBA will have antibodies against collagen Type VII; this protein is deficient in recessive dystrophic EB (RDEB) skin. Hence, EBA patient's serum does not show BMZ band in RDEB skin while it shows band in other types of EB skin, thus confirming the diagnosis of EBA [Figure 4]. The advantage of the antigen identification technique is that it is relatively simple to perform compared to other tests directed at antigen detection, such as IB. However, a major limitation of the technique is the availability of suitable skin substrates from patients with EB.
Figure 4.
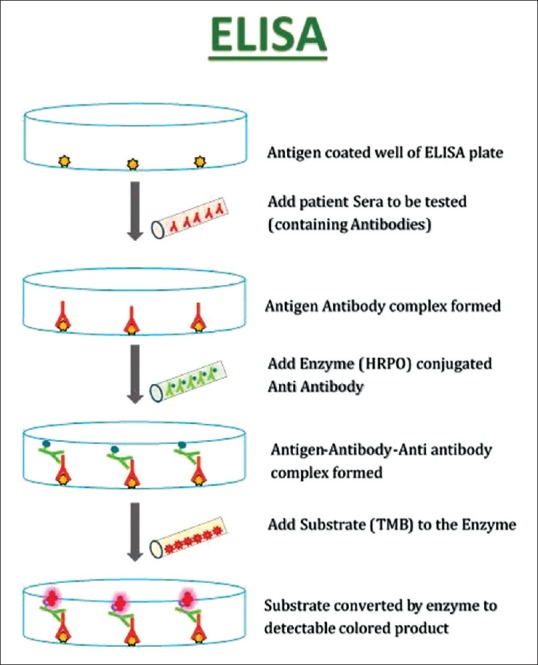
Schematic diagram of an enzyme-linked immunosorbent assay system. Intensity of final yellow color is related to the amount of circulating antibodies
Serrated pattern analysis
Serration pattern analysis by routine DIF has been shown to be useful in distinguishing BP from EBA.[25] The “u-serration” pattern confirms the diagnosis of EBA. In all other SIBDs, the antigens are located in the lamina lucida or above, so the immune deposits follow the rootlets of the basal keratinocytes showing the “n-serration” pattern.[25,26] The advantage of this technique is that this enables one to distinguish BP from EBA even if indirect SST and ELISA are negative. However, this technique requires great precision, for example, thinner frozen sections and higher magnification (≤600-fold) and difficult to carry out in the routine laboratory. Serration pattern analysis is of particular importance in EBA, since only 40%–60% of serum samples react by IIF microscopy.
Use of BIOCHIP mosaic slides
BIOCHIP mosaic slides have been found to be useful in screening autoantibodies in AIBDs patients. These ready to use slides are available commercially and contain six different substrates (monkey esophagus, primate salt-split skin, recombinant BP180 NC16A, membrane-bound Dsg 1 ectodomain, Dsg 3 ectodomain, and the C-terminal globular domain of BP230) in a miniature field. Technically, this is a modified IIF wherein serum from patients with suspected AIBD is added to these slides and examined under fluorescence microscopy. The advantage of this technique is that it is a useful tool to screen autoantibodies in AIBDs as well as to identify the target antigen. This technique avoids the need to take frozen sections of a suitable substrate. Thus, BIOCHIP mosaic is a simple, standardized, and readily available novel tool that will further facilitate the diagnosis of AIBDs. Validation of the BIOCHIP showed high specificity and high sensitivity for PV, pemphigus foliaceus (PF), and BP.[27]
Enzyme-linked immunosorbent assay
In recent years, recombinant and cell-derived forms of the target antigens have been applied in the development of sensitive and specific ELISA for the detection of circulating autoantibodies. The principle of ELISA technique is depicted in Figure 4. The commercially available ELISA plates are precoated with recombinant antigen. So far, ELISA systems are available for the diagnosis of pemphigus and pemphigoid diseases.[28]
ELISA system has several advantages. It is a quantitative method for measuring specific circulating antibody levels, similar to IIF. Hence, ELISA is useful both in the diagnosis as well as to monitor the disease activity in AIBD such as pemphigus. However, unlike IIF which is observer dependent, ELISA plates are read automatically and results do not depend on the observer.[10] The procedure is quite simple; several samples can be tested simultaneously and the results can be obtained rapidly. It is useful to distinguish PF (anti-Dsg 1 antibodies only) from PV (anti-Dsg 3 with or without anti-Dsg 1 antibodies, depending on the clinical phenotype of PV). Disadvantage of ELISA is that it may not detect all cell surface antibodies involved in the pathogenesis of AIBD, whereas the IIF method may detect antibodies directed against a variety of other nonpathogenic antigens present in normal epithelium. For example, negative result for Dsg 1 and 3 does not rule out the possibility of other rarer subtypes of pemphigus with antibodies to other antigens as in case of PNP and IgA pemphigus.[29] Hence, ELISA should be used as a complementary test to IIF and not as a substitute.
Immunoblotting and immunoprecipitation
These techniques provide information on the molecular weight of the target antigen in AIBDs. The antigen source for IB is epidermal or dermal extracts, whereas cultured keratinocytes are the antigenic source for immunoprecipitation (IP). The proteins (antigens) are separated according to their molecular weight by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and the gel is then transferred to nitrocellulose paper. Serum to be tested is then added to nitrocellulose paper and development of a band at a particular molecular weight indicates the target antigen which can be easily inferred by matching it with a control. For example, if the serum from a patient with a SIBD labels a dermal protein of 290 kDa, the target antigen is likely to be Type VII collagen and the diagnosis is EBA. However, these techniques are time-consuming, labor-intensive that require skilled personnel and can realistically be performed only in specialized centers. Most IP techniques involve the use of radioisotopes, limiting their practical use. In addition, SDS destroys the conformational epitopes, and hence, IB is not useful in the diagnosis of pemphigus since most epitopes on Dsg 1 and 3 are conformational.[10,28]
Immunoelectron microscopy
Immunoelectron microscopy (IEM) demonstrates the precise ultrastructural location of antigens in AIBDs. It is akin to DIF and IIF except that visualization of antibodies involves the use of electron-dense gold-labeled antibody probes. When the gold labeling colocalizes with a known protein, the antigen can be indirectly inferred. IEM can be carried out in the same biopsy sample that has been sent for DIF in Michel's medium.[29]
Laboratory Diagnosis of Intraepidermal Autoimmune Bullous Diseases
Pemphigus is the prototype of intraepidermal AIBDs. Autoantibodies in pemphigus target desmosomal protein that connects neighboring keratinocytes; loss of cell-to-cell adhesion leads to “acantholysis.”[30,31]
Pemphigus vulgaris
PV is the most common type of pemphigus and accounts for 70% of all pemphigus cases in India, Malaysia, China, and the Middle East.[31,32] The disease typically begins in the mouth with patients complaining of painful oral erosions (mucosal type of PV). Subsequently, majority of patients will develop flaccid blisters and erosions predominantly on the scalp, face, and trunk (mucocutaneous type). DIF of perilesional skin shows ICS of epidermis with IgG+/− C3 in a characteristic “fish net or chicken wire” pattern [Figure 5a].[18] DIF is positive in 90%–100% of patients with active disease if an appropriate biopsy specimen has been obtained.[4,19] The intensity of DIF coincides fairly well with clinical activity, but a few patients may continue to have positive DIF despite clinical inactivity of pemphigus.[19] IIF using NHS as a substrate shows a pattern similar to DIF in 70% of patients with PV. The sensitivity of IIF using human skin has shown a sensitivity level of 83% as compared to 90% when MO has been used as substrate. However, the sensitivity of IIF can be increased to 100% using a combination of substrates rich in Dsg 1 (NHS) and rich in Dsg 3 (monkey esophagus).[11] ELISA detects the circulating autoantibodies directed against Dsg 1 and 3. Mucosal dominant PV will have only anti-Dsg 3 antibodies, while in mucocutaneous subtype, patients will have circulating antibodies against both Dsg 1 and 3.[33] ELISA reactivity correlates with the disease activity; hence, Dsg ELISA is useful both for the diagnosis and to monitor the disease activity in pemphigus patients.[27]
Figure 5.
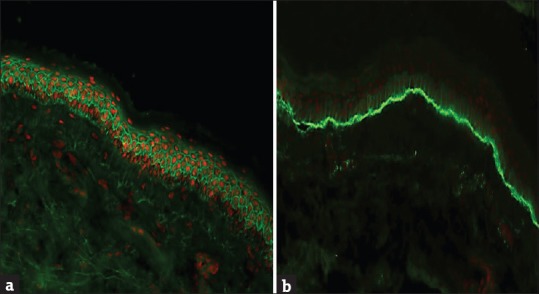
(a) Intercellular staining of epidermis with IgG in pemphigus and (b) linear staining of basement membrane zone with C3 in bullous pemphigoid (FITC ×200)
Pemphigus foliaceus
PF is characterized by superficial erosions, crusting, and scaling predominantly of seborrheic areas of the body. Sometimes, the lesions may be generalized leading to exfoliative dermatitis.[34] In PF, antibodies are directed against Dsg 1 which is expressed strongly within the superficial layers of the epidermis. Hence, the blister is located subcorneally in PF.[35] Dsg 1 may not be as critical for cell adhesion in the oral mucosa, which accounts for the low incidence of oral lesions in PF. DIF pattern in PF is similar to PV; occasionally, the staining may be more intense in superficial epidermal layers. NHS (rich in Dsg 1) is an ideal substrate for IIF in PF; ELISA detects antibodies only against Dsg 1 in PF.[36]
Pemphigus erythematosus
Pemphigus erythematosus (PE, also known as Senear–Usher syndrome) is a variant of PF which exhibits immunological coexistence of PF and lupus erythematosus (LE).[37] DIF in PE shows “dual” pattern of staining - ICS (“pemphigus like”) in the epidermis along with granular BMZ (“LE like”) staining with IgG. IgG is detected in nearly 100% of patients whereas C3 is present in 50%–100% of cases.
IgA pemphigus
ICS of epidermis with IgA is diagnostic of IgA pemphigus. Occasionally, the staining is more intense in the upper epidermis. Two variants of IgA pemphigus have been described: intraepidermal neutrophilic dermatosis (IEN) type and SPD type.[36] In IEN type, the blisters occur in the lower epidermis, whereas in SPD type, acantholysis occurs in the upper epidermis. Circulating IgA antibodies may be present in approximately 50% of patients.[4] The target antigen of the SPD type is desmocollin 1, whereas the antigen of the IEN type is still unknown.[38] Rare cases showed antibodies to either Dsg 1 or Dsg 3.[39]
Paraneoplastic pemphigus
PNP is characterized by recalcitrant mucosal erosions and a polymorphous skin rash (lichenoid, erythema multiforme like, or pemphigus like) which occur in the setting of an underlying malignancy (usually lymphoproliferative) and is associated with significant mortality and morbidity.[40] Autoantibodies in PNP typically target Dsg 3 and proteins of the plakin family, including envoplakin, periplakin, plectin, desmoplakin 1 and 2, and BP230 [Figure 6].[41] The pattern of fluorescence at the BMZ is similar to BP. ICS deposition in PNP is sometimes weak or diffuse and nonspecific.[4] Antibodies in PNP bind to the desmosomes of both simple as well as transitional epithelia. Hence, IIF using rat bladder as a substrate confirms the diagnosis of PNP and it has a sensitivity and specificity of 86% and 98%, respectively [Figure 7].[40] Recently, various ELISA systems using recombinant forms of envoplakin and periplakin have been developed; an ELISA based on the recombinant N-terminus of envoplakin is commercially available and has a sensitivity and specificity of 82% and 100%, respectively.[28]
Figure 6.
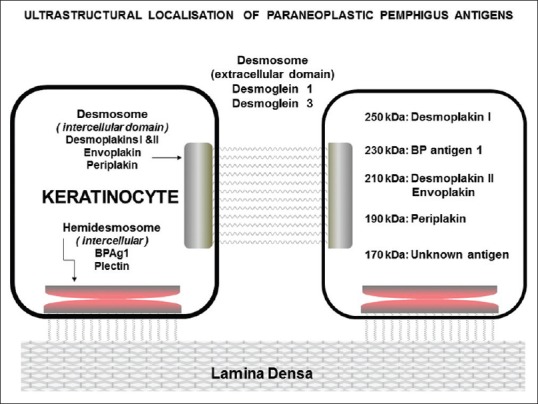
Schematic diagram showing target antigens of paraneoplastic pemphigus with their molecular weights
Figure 7.
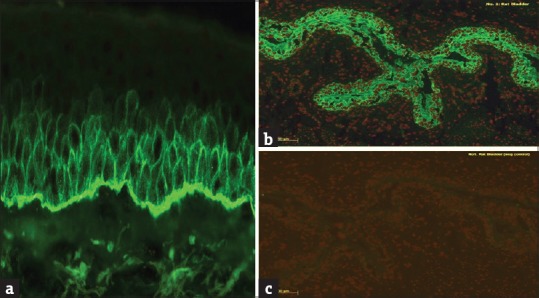
(a) Direct immunofluorescence showing dual pattern (“intercellular staining and basement membrane zone”) of staining with IgG in paraneoplastic pemphigus (FITC, ×40). (b) Indirect immunofluorescence using rat bladder showing staining of transitional epithelium. (c) Negative control in rat bladder (FITC, ×200)
Drug-induced pemphigus
Approximately two-thirds of patients with drug-induced pemphigus will have PF-like picture while the rest will have PV-like manifestations.[4] Some patients do not have any evidence of autoantibody production, and in these patients, thiol groups are thought to bind directly to intercellular bridges and destroy them. Depending on the clinical phenotype, DIF and IIF may resemble PF or PV.[42]
Laboratory Diagnosis of Subepidermal Autoimmune Bullous Diseases
Autoantibodies in SIBDs target constituents of BMZ [Figure 8]; DIF characteristically shows linear staining of BMZ in all these conditions except DH which shows granular IgA deposits over the tips of dermal papillae. DIF is extremely sensitive technique in the diagnosis of SIBDs with a positive and negative predictive value of approximately 100%.[4]
Figure 8.
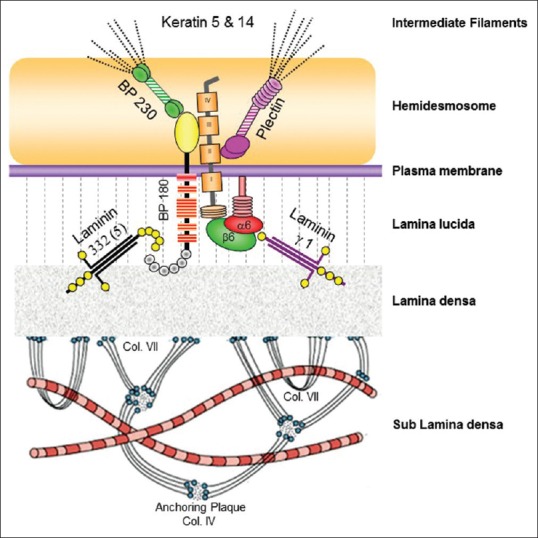
Schematic diagram showing various antigens at dermoepidermal junction. Common antigenic targets in subepidermal immunobullous diseases are BP180 (bullous pemphigoid, mucous membrane pemphigoid, pemphigoid gestationis, and LAD), BP230 (bullous pemphigoid), integrins α6 β4 (mucous membrane pemphigoid), laminin 332 (mucous membrane pemphigoid), laminin γ1 (p-200 pemphigoid) and collagen VII (epidermolysis bullosa acquisita)
Bullous Pemphigoid
BP is the prototype of SIBD in which autoantibodies are produced against hemidesmosomal proteins, namely, BP180 (Type XVII collagen or BPAG2) and less commonly against BP230 (BPAG1). BP230 is a hemidesmosomal protein, located in the basal keratinocytes, while BP180 is a transmembranous molecule. Autoantibodies in the majority of patients with BP recognize an epitope located in the aminoterminal of noncollagenous domain of BP180 (“NC16A”).[43]
The diagnosis of BP should be considered in all elderly patients who present with a generalized pruritic eruption with widespread blister formation.[44] Blisters in patients with BP are tense in nature, 1–3 cm in diameter and are seen on the background of normal or erythematous background. Lesions are distributed predominantly over the trunk, abdomen, and proximal extremities. Mucosal involvement is seen in about 30% of the patients.[45]
DIF of perilesional skin typically demonstrates linear staining of BMZ with C3+/− IgG in nearly 100% of patients [Figure 5b]. Deposition of C3 with significantly higher intensity than IgG strongly favors the diagnosis of pemphigoid group of diseases (BP, mucous membrane pemphigoid [MMP], and PG).[4] The main immunoglobulin implicated in BP is IgG4.[46] Antibodies against IgA and IgE isotypes have also been described. In fact, IgA is the second common immunoglobulin class (after IgG) that is detected in patients with BP.[28] However, the relevance of IgA and IgE autoantibodies in the diagnosis of BP needs to be elucidated.[14]
Circulating IgG anti-BMZ autoantibodies can be detected by IIF in about 50%–75% of the patients with BP, but the sensitivity increases to 80% when salt-split skin is used as a substrate. SST in BP reveals staining of IgG on the epidermal side (“roof pattern”) of the split. A “roof-”binding pattern may also characteristically be seen in other SIBDs in which autoantibodies are produced against BP180 (LAD, PG, and MMP).[47] Occasionally, a combined pattern (both “roof” and “floor”) may be seen in BP; this may be due to recognition of additional epitopes toward the carboxy-terminal (C-terminal) of BP180 molecule. Interestingly, IgG reactivity with C-terminal epitopes appears to be associated with the presence of both skin and mucosal lesions.[48]
ELISA system using the recombinant NC16A of BP180 has been found to be highly sensitive and specific and its values correlate with the disease activity.[49,50] Autoantibodies against BP230 can also be detected by ELISA in about 60%–70% of patients with BP.[51] Globular C-terminal domain that mediates interaction with keratin filaments is the immunodominant region of BP230 and autoantibodies against this epitope are found in 80% of BP230-positive sera. A combined C- and N-terminal-coated ELISA kit is also commercially available.[28]
Mucous Membrane Pemphigoid
MMP, known previously as cicatricial pemphigoid (CP), is a unique SIBD characterized by predominant involvement of the mucous membrane and tendency for scarring. Clinically, MMP is a chronic and progressive disease that most frequently affects the oral cavity (85% of patients), followed by conjunctivae (65%), skin (25%–30%), nasal cavity (20%–40%), anogenital area (20%), pharynx (20%), larynx (5%–10%), and esophagus (5%–15%). Clinical severity is highly variable and can range from subtle oral lesions and conjunctival injection to pervasive, very painful mucosal involvement and devastating esophageal and conjunctival disease.[52] DIF shows linear staining of BMZ with IgG, C3 and in some patients IgA. IIF on salt-split skin may detect circulating autoantibodies in only 50% of the patients and is usually present in low titer. BMZ staining may be seen on the roof, floor, or both on indirect salt-split study, reflecting the antigen heterogeneity of MMP [Figure 9a]. IgA autoantibodies can be detected in about 60% of serum samples and a combined IgA and IgG reactivity is associated with more severe disease than with IgG autoantibodies alone.[53]
Figure 9.
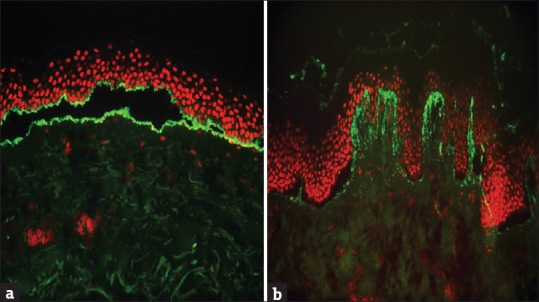
(a) Linear staining of basement membrane zone with IgG (on either side of the split) in mucous membrane pemphigoid. (b) Granular staining of dermal papillae with IgA in dermatitis herpetiformis (FITC, ×200)
BP180 is the most common target antigen of MMP. However, in contrast to BP, C-terminal epitope of BP180 is predominantly recognized by MMP serum. Only about 50% of BP180-reactive serum target NC-16A domain (i.e., the main target epitope in BP). Laminin 332 is the other target antigen of MMP (previously known as antiepiligrin CP); it is a heterotrimer consisting of three chains – α3, β3, and γ2. IIF on salt-split skin shows dermal-binding pattern in patients who have autoantibodies against laminin 332; occasionally, IIF may be negative in this subset of patients. It is very important to identify anti-laminin 332 antibodies in MMP patients since a significant proportion of them may have underlying malignancy. One of the simple techniques to identify anti-laminin 332 antibodies is by modified IIF technique using Herlitz junctional EB skin (JEB-H) as a substrate. Since laminin 332 is completely deficient in JEB-H skin, anti-laminin 332 serum fails to stain BMZ, thus confirming the diagnosis. Occasionally, antibodies may be directed against α6β4 integrin. Autoantibodies to α6 have been described in patients with oral involvement while ocular involvement is associated with antibodies to β2 unit.
IP with radiolabeled human keratinocytes is the most sensitive method for the detection of anti-laminin 332 antibodies, whereas IB with extracellular matrix of cultured human keratinocytes seems to be the most practical alternative.[54]
Pemphigoid Gestationis
PG, formerly known as herpes gestationis (HG), is a rare SIBD of pregnancy and the peripartum period. The patient initially presents with pruritic urticarial papules and annular plaques, followed by vesicles and bullae. The most common site of eruption is abdomen (especially periumbilical area in 90% of patients) and thighs.[55] The major target antigen in PG is BP180 similar to BP. Hence, DIF findings are same as that of BP and shows linear BMZ band with C3 in 100% of cases; IgG may be seen in 30% cases. Routine IIF studies are negative. However, circulating HG factor may be detected using complement fixation technique in approximately 50% of the patients.[4] ELISA system detects autoantibodies against BP180 NC16A in 90% of patients; only 10% of sera have reactivity against BP230.[14]
Linear IgA Disease
Linear IgA disease (LAD) is an acquired SIBD, characterized immunopathologically by linear deposition of IgA along the BMZ. The disease affects both children (previously known as chronic bullous disease of childhood) and adults. Autoantibodies in LAD are directed against the cleaved ectodomain of BP180 molecule, i.e., LAD-1 (120 KDa molecule) and LABD-97.[56] The classical primary lesions of LAD are clear or hemorrhagic blisters developing on normal or erythematous skin. Blisters in childhood LAD are often arranged in an annular fashion around the central crust giving a “strings of pearl” appearance.[56,57] DIF of perilesional skin shows linear staining of BMZ with IgA. Occasionally, other classes of immunoglobulin, especially IgG, may be present along the BMZ and may lead to diagnostic confusion. This type of “dual” staining of IgA and IgG has been reported in children and is known as “mixed immunobullous disease of childhood.”[58] Circulating IgA autoantibodies can be detected by IIF in both children (75% of cases) and adults (30% of cases).[56] IIF on salt-split skin shows “roof pattern” in majority of patients. However, combined pattern (“roof and floor”) or exclusive “floor” has been reported in few patients. The heterogeneous IIF pattern is due to varied antigenic targets in these patients.[57]
Dermatitis Herpetiformis
DH is a chronic, recurrent, pruritic, papulovesicular disease. All patients with DH have gluten-sensitive enteropathy and observe worsening of their condition after taking gluten-rich diet. DH is a polymorphous skin disease characterized by 1–3 mm papules, papulovesicles, crusted erosions, and excoriations typically distributed over the extensor aspect of elbows and knees, buttocks, and shoulders.[59] DIF characteristically shows granular deposition of IgA on the top of the dermal papillae and occasionally along the DEJ [Figure 9b]. Fibrillar deposits of C3 are seen in approximately half of the cases.[60] Circulating IgA antiendomysium antibodies can be demonstrated in the affected patients. The antiendomysium antibodies are directed against the tissue transglutaminase (TG2) which is homogenous to epidermal transglutaminase (TG3).[18]
Epidermolysis Bullosa Acquisita
EBA is characterized by the presence of autoantibodies of IgG class and less often by IgA class against Type VII collagen, a major component of anchoring fibrils located in the lamina densa and sublamina densa region.[61,62] Two major phenotypic presentation of EBA are classical, mechanobullous type and inflammatory type. In the classical type, blisters develop in the trauma-prone surfaces and have a tendency to heal with scarring and milia formation. Inflammatory form of EBA is indistinguishable from BP clinically; widespread tense blisters develop on an erythematous skin and are seen over the trunk, body folds, and extremities. In both types, mucosal lesions are seen in about 50% of the affected cases.[14] DIF demonstrates linear deposition of IgG and less commonly C3, along the BMZ. Deposits of multiple conjugates at the BMZ are also more frequently seen in the setting of EBA compared to BP. Autoantibodies of IgA class are seen in two-thirds of patients and IgM in half of the cases. In a subgroup (<10% of all EBA) of patients, autoantibodies are restricted to the IgA isotype – these patients tend to display the inflammatory phenotype (IgA-EBA).[14,63]
IIF is either negative or positive in low titer in classic form of the disease. This is in contrast to the inflammatory form which may show higher titer of antibodies in the serum. Using salt-split skin, antibodies binding to the dermal side (“floor pattern”) of the split can be demonstrated in 40%–60% of cases.[25,63,64] Antigen identification using RDEB skin or serration pattern analysis can be done for further confirmation of EBA. Recently, several ELISA systems based on the immunodominant NC1 domain of Type VII collagen (alone or in combination with NC2) have been established with high sensitivity (66%–100%) and specificity (98.2%–100%).[64] These ELISA systems are useful both for the diagnosis of EBA and evaluation of disease severity.[27]
Laminin γ1 Pemphigoid (p-200 Pemphigoid)
This is newly described type of SIBDs in which autoantibodies are directed against p-200 (laminin γ1), present at the interface between lamina lucida and lamina densa. Clinically, it is indistinguishable from BP. DIF features are similar to BP; however, IIF on salt-split skin shows a dermal-binding (“floor”) pattern resembling EBA.[65] Recently, ELISA has been developed to detect the circulating antibodies against C-terminus of laminin γ1 chain with a sensitivity and specificity of 70% and 98.7%, respectively.[27,65]
Monitoring of Patients with Autoimmune Bullous Diseases
The goal of therapy in AIBDs is to achieve clinical and immunological remission. Clinical remission could be achieved in most patients with immunosuppressive agents. The most difficult management decision is how to maintain remission with the least number of medications. DIF performed during the periods of clinical remission is valuable in the management of the disease as negative DIF may be viewed as a state of immunological remission. Patients in remission may refuse to undergo repeated skin biopsies. DIF of hair may be an ideal choice of substrate in such patients as it is a simple and noninvasive test with high degree of sensitivity and specificity.[66] IIF has been widely used to monitor the serological activity of pemphigus patients on treatment. The titer of antibodies in the sera of patients is in many instances proportionate of the severity of the disease. In recent years, ELISA systems have been used to monitor the immunological activity of the disease and ELISA values have been found to correlate the disease activity in PV, PF, and BP.[27] Treatment needs to be continued in patients who show immunological activity (as detected by IF/ELISA) even when they have reached the phase of clinical remission to avoid the possible risk of relapse; treatment in these patients may be discontinued when the serum autoantibodies are undetectable by these techniques.
Conclusion
An algorithmic approach is necessary for the accurate diagnosis of AIBDs. Histopathological findings on biopsy specimen should always be confirmed by performing DIF microscopy. Sera of patients positive on DIF microscopy should be obtained for IIF study. Various IF findings in AIBDs are depicted in Figure 10. Antigen identification using hereditary EB skin is a useful technique to further differentiate “floor-”binding AIBDs; however, availability of a suitable EB substrate may be an issue. Many ELISA systems are now available and are being used widely in many laboratories; these tests are useful both in confirming the diagnosis and to monitor the disease prognosis. IB tests to identify the target antigen are available only in few research centers in the world now and they are expensive, time-consuming, and require a lot of technical expertise.
Figure 10.
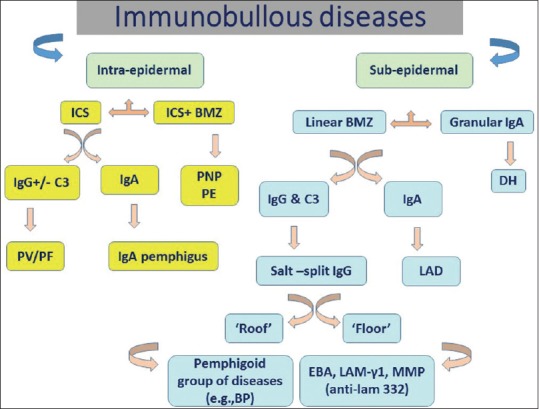
Algorithmic chart showing immunofluorescence findings in various autoimmune bullous diseases
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
Honey may be used as an alternate transport medium of skin biopsy specimen for immunofluorescence studies
Whenever possible diagnosis of autoimmune blistering disease should be confirmed by advanced immunological tests.
References
- 1.Schmidt E, Zillikens D. The diagnosis and treatment of autoimmune blistering skin diseases. Dtsch Arztebl Int. 2011;108:399–405. doi: 10.3238/arztebl.2011.0405. I-III. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kárpáti S, Amagai M, Prussick R, Cehrs K, Stanley JR. Pemphigus vulgaris antigen, a desmoglein type of cadherin, is localized within keratinocyte desmosomes. J Cell Biol. 1993;122:409–15. doi: 10.1083/jcb.122.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanley JR. Cell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous diseases due to defective epidermal cell adhesion. Adv Immunol. 1993;53:291–325. doi: 10.1016/s0065-2776(08)60503-9. [DOI] [PubMed] [Google Scholar]
- 4.Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803–22. doi: 10.1067/mjd.2001.117518. [DOI] [PubMed] [Google Scholar]
- 5.Anhalt GJ, Kim SC, Stanley JR, Korman NJ, Jabs DA, Kory M, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990;323:1729–35. doi: 10.1056/NEJM199012203232503. [DOI] [PubMed] [Google Scholar]
- 6.Kneisel A, Hertl M. Autoimmune bullous skin diseases. Part 2: Diagnosis and therapy. J Dtsch Dermatol Ges. 2011;9:927–47. doi: 10.1111/j.1610-0387.2011.07809.x. [DOI] [PubMed] [Google Scholar]
- 7.Buch AC, Kumar H, Panicker N, Misal S, Sharma Y, Gore CR. A cross-sectional study of direct immunofluorescence in the diagnosis of immunobullous dermatoses. Indian J Dermatol. 2014;59:364–8. doi: 10.4103/0019-5154.135488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47:200. [Google Scholar]
- 9.Mohan KH, Pai S, Rao R, Sripathi H, Prabhu S. Techniques of immunofluorescence and their significance. Indian J Dermatol Venereol Leprol. 2008;74:415–9. doi: 10.4103/0378-6323.42898. [DOI] [PubMed] [Google Scholar]
- 10.Harman KE. New laboratory techniques for the assessment of acquired immunobullous disorders. Clin Exp Dermatol. 2002;27:40–6. doi: 10.1046/j.0307-6938.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- 11.Harman KE, Gratian MJ, Bhogal BS, Challacombe SJ, Black MM. The use of two substrates to improve the sensitivity of indirect immunofluorescence in the diagnosis of pemphigus. Br J Dermatol. 2000;142:1135–9. doi: 10.1046/j.1365-2133.2000.03538.x. [DOI] [PubMed] [Google Scholar]
- 12.Ng PP, Thng ST, Mohamed K, Tan SH. Comparison of desmoglein ELISA and indirect immunofluorescence using two substrates (monkey oesophagus and normal human skin) in the diagnosis of pemphigus. Australas J Dermatol. 2005;46:239–41. doi: 10.1111/j.1440-0960.2005.00191.x. [DOI] [PubMed] [Google Scholar]
- 13.Matis WL, Anhalt GJ, Diaz LA, Rivitti EA, Martins CR, Berger RS. Calcium enhances the sensitivity of immunofluorescence for pemphigus antibodies. J Invest Dermatol. 1987;89:302–4. doi: 10.1111/1523-1747.ep12471587. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320–32. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- 15.Rao R, Dasari K, Shenoi S, Balachandran C. Demonstration of pemphigus-specific immunofluorescence pattern by direct immunofluorescence of plucked hair. Int J Dermatol. 2009;48:1187–9. doi: 10.1111/j.1365-4632.2009.04153.x. [DOI] [PubMed] [Google Scholar]
- 16.Aithal V, Kini U, Jayaseelan E. Role of direct immunofluorescence on Tzanck smears in pemphigus vulgaris. Diagn Cytopathol. 2007;35:403–7. doi: 10.1002/dc.20657. [DOI] [PubMed] [Google Scholar]
- 17.Gammon WR, Kowalewski C, Chorzelski TP, Kumar V, Briggaman RA, Beutner EH. Direct immunofluorescence studies of sodium chloride-separated skin in the differential diagnosis of bullous pemphigoid and epidermolysis bullosa acquisita. J Am Acad Dermatol. 1990;22:664–70. doi: 10.1016/0190-9622(90)70094-x. [DOI] [PubMed] [Google Scholar]
- 18.Pohla-Gubo G, Hintner H. Direct and indirect immunofluorescence for the diagnosis of bullous autoimmune diseases. Dermatol Clin. 2011;29:365–72. doi: 10.1016/j.det.2011.03.001. vii. [DOI] [PubMed] [Google Scholar]
- 19.Chhabra S, Minz RW, Saikia B. Immunofluorescence in dermatology. Indian J Dermatol Venereol Leprol. 2012;78:677–91. doi: 10.4103/0378-6323.102355. [DOI] [PubMed] [Google Scholar]
- 20.Anstey A, Venning V, Wojnarowska F, Bhogal B, Black MM. Determination of the optimum site for diagnostic biopsy for direct immunofluorescence in bullous pemphigoid. Clin Exp Dermatol. 1990;15:438–41. doi: 10.1111/j.1365-2230.1990.tb02139.x. [DOI] [PubMed] [Google Scholar]
- 21.Vaughn Jones SA, Palmer I, Bhogal BS, Eady RA, Black MM. The use of Michel's transport medium for immunofluorescence and immunoelectron microscopy in autoimmune bullous diseases. J Cutan Pathol. 1995;22:365–70. doi: 10.1111/j.1600-0560.1995.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 22.Vodegel RM, de Jong MC, Meijer HJ, Weytingh MB, Pas HH, Jonkman MF. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10. doi: 10.1186/1471-5945-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao R, Mellerio J, Bhogal BS, Groves R. Immunofluorescence antigen mapping for hereditary epidermolysis bullosa. Indian J Dermatol Venereol Leprol. 2012;78:692–7. doi: 10.4103/0378-6323.102358. [DOI] [PubMed] [Google Scholar]
- 24.Rao R, Mellerio J, Bhogal BS, Groves R. Immunofluorescence antigen mapping for hereditary epidermolysis bullosa. Indian J Dermatol Venereol Leprol. 2012;78:692–7. doi: 10.4103/0378-6323.102358. [DOI] [PubMed] [Google Scholar]
- 25.Vodegel RM, Jonkman MF, Pas HH, de Jong MC. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112–8. doi: 10.1111/j.1365-2133.2004.06006.x. [DOI] [PubMed] [Google Scholar]
- 26.Buijsrogge JJ, Diercks GF, Pas HH, Jonkman MF. The many faces of epidermolysis bullosa acquisita after serration pattern analysis by direct immunofluorescence microscopy. Br J Dermatol. 2011;165:92–8. doi: 10.1111/j.1365-2133.2011.10346.x. [DOI] [PubMed] [Google Scholar]
- 27.van Beek N, Rentzsch K, Probst C, Komorowski L, Kasperkiewicz M, Fechner K, et al. Serological diagnosis of autoimmune bullous skin diseases: Prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49. doi: 10.1186/1750-1172-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84–9. doi: 10.1016/j.autrev.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Harman KE, Gratian MJ, Seed PT, Bhogal BS, Challacombe SJ, Black MM. Diagnosis of pemphigus by ELISA: A critical evaluation of two ELISAs for the detection of antibodies to the major pemphigus antigens, desmoglein 1 and 3. Clin Exp Dermatol. 2000;25:236–40. doi: 10.1046/j.1365-2230.2000.00624.x. [DOI] [PubMed] [Google Scholar]
- 30.Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–77. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- 31.Adam BA. Bullous diseases in Malaysia: Epidemiology and natural history. Int J Dermatol. 1992;31:42–5. doi: 10.1111/j.1365-4362.1992.tb03519.x. [DOI] [PubMed] [Google Scholar]
- 32.Chams-Davatchi C, Valikhani M, Daneshpazhooh M, Esmaili N, Balighi K, Hallaji Z, et al. Pemphigus: Analysis of 1209 cases. Int J Dermatol. 2005;44:470–6. doi: 10.1111/j.1365-4632.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- 33.Venugopal SS, Murrell DF. Diagnosis and clinical features of pemphigus vulgaris. Dermatol Clin. 2011;29:373–80. doi: 10.1016/j.det.2011.03.004. vii. [DOI] [PubMed] [Google Scholar]
- 34.Groves RW. Pemphigus: A brief review. Clin Med (Lond) 2009;9:371–5. doi: 10.7861/clinmedicine.9-4-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James KA, Culton DA, Diaz LA. Diagnosis and clinical features of pemphigus foliaceus. Dermatol Clin. 2011;29:405–12. doi: 10.1016/j.det.2011.03.012. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez J, Bystryn JC. Pemphigus foliaceus associated with absence of intercellular antigens in lower layers of epidermis. Arch Dermatol. 1977;113:1696–9. [PubMed] [Google Scholar]
- 37.Porro AM, Caetano Lde V, Maehara Lde S, Enokihara MM. Non-classical forms of pemphigus: Pemphigus herpetiformis, IgA pemphigus, paraneoplastic pemphigus and IgG/IgA pemphigus. An Bras Dermatol. 2014;89:96–106. doi: 10.1590/abd1806-4841.20142459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashimoto T, Kiyokawa C, Mori O, Miyasato M, Chidgey MA, Garrod DR, et al. Human desmocollin 1 (Dsc1) is an autoantigen for the subcorneal pustular dermatosis type of IgA pemphigus. J Invest Dermatol. 1997;109:127–31. doi: 10.1111/1523-1747.ep12319025. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto T, Komai A, Futei Y, Nishikawa T, Amagai M. Detection of IgA autoantibodies to desmogleins by an enzyme-linked immunosorbent assay: The presence of new minor subtypes of IgA pemphigus. Arch Dermatol. 2001;137:735–8. [PubMed] [Google Scholar]
- 40.Frew JW, Murrell DF. Paraneoplastic pemphigus (paraneoplastic autoimmune multiorgan syndrome): Clinical presentations and pathogenesis. Dermatol Clin. 2011;29:419–25. doi: 10.1016/j.det.2011.03.018. viii. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann J, Bahmer F, Rose C, Zillikens D, Schmidt E. Clinical and immunopathological spectrum of paraneoplastic pemphigus. J Dtsch Dermatol Ges. 2010;8:598–606. doi: 10.1111/j.1610-0387.2010.07380.x. [DOI] [PubMed] [Google Scholar]
- 42.Brenner S, Goldberg I. Drug-induced pemphigus. Clin Dermatol. 2011;29:455–7. doi: 10.1016/j.clindermatol.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Magro CM, Roberts-Barnes J, Crowson AN. Direct immunofluorescence testing in the diagnosis of immunobullous disease, collagen vascular disease, and vascular injury syndromes. Dermatol Clin. 2012;30:763–98. doi: 10.1016/j.det.2012.06.008. viii. [DOI] [PubMed] [Google Scholar]
- 44.Liu HN, Su WP, Rogers RS., 3rd Clinical variants of pemphigoid. Int J Dermatol. 1986;25:17–27. doi: 10.1111/j.1365-4362.1986.tb03397.x. [DOI] [PubMed] [Google Scholar]
- 45.Di Zenzo G, Della Torre R, Zambruno G, Borradori L. Bullous pemphigoid: From the clinic to the bench. Clin Dermatol. 2012;30:3–16. doi: 10.1016/j.clindermatol.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Patton T, Plunkett RW, Beutner EH, Deng JS, Jukic DM. IgG4 as the predominant IgG subclass in pemphigoides gestationis. J Cutan Pathol. 2006;33:299–302. doi: 10.1111/j.0303-6987.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt E, della Torre R, Borradori L. Clinical features and practical diagnosis of bullous pemphigoid. Dermatol Clin. 2011;29:427–38. doi: 10.1016/j.det.2011.03.010. viii-ix. [DOI] [PubMed] [Google Scholar]
- 48.Hofmann S, Thoma-Uszynski S, Hunziker T, Bernard P, Koebnick C, Stauber A, et al. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. J Invest Dermatol. 2002;119:1065–73. doi: 10.1046/j.1523-1747.2002.19529.x. [DOI] [PubMed] [Google Scholar]
- 49.Sitaru C, Dähnrich C, Probst C, Komorowski L, Blöcker I, Schmidt E, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770–7. doi: 10.1111/j.1600-0625.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt E, Obe K, Bröcker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174–8. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida M, Hamada T, Amagai M, Hashimoto K, Uehara R, Yamaguchi K, et al. Enzyme-linked immunosorbent assay using bacterial recombinant proteins of human BP230 as a diagnostic tool for bullous pemphigoid. J Dermatol Sci. 2006;41:21–30. doi: 10.1016/j.jdermsci.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Chan LS, Ahmed AR, Anhalt GJ, Bernauer W, Cooper KD, Elder MJ, et al. The first international consensus on mucous membrane pemphigoid: Definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138:370–9. doi: 10.1001/archderm.138.3.370. [DOI] [PubMed] [Google Scholar]
- 53.Setterfield J, Shirlaw PJ, Kerr-Muir M, Neill S, Bhogal BS, Morgan P, et al. Mucous membrane pemphigoid: A dual circulating antibody response with IgG and IgA signifies a more severe and persistent disease. Br J Dermatol. 1998;138:602–10. doi: 10.1046/j.1365-2133.1998.02168.x. [DOI] [PubMed] [Google Scholar]
- 54.Lazarova Z, Sitaru C, Zillikens D, Yancey KB. Comparative analysis of methods for detection of anti-laminin 5 autoantibodies in patients with anti-epiligrin cicatricial pemphigoid. J Am Acad Dermatol. 2004;51:886–92. doi: 10.1016/j.jaad.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Intong LR, Murrell DF. Pemphigoid gestationis: Pathogenesis and clinical features. Dermatol Clin. 2011;29:447–52, ix. doi: 10.1016/j.det.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Venning VA. Linear IgA disease: Clinical presentation, diagnosis, and pathogenesis. Immunol Allergy Clin North Am. 2012;32:245–53. doi: 10.1016/j.iac.2012.04.004. vi. [DOI] [PubMed] [Google Scholar]
- 57.Mintz EM, Morel KD. Clinical features, diagnosis, and pathogenesis of chronic bullous disease of childhood. Dermatol Clin. 2011;29:459–62. doi: 10.1016/j.det.2011.03.022. ix. [DOI] [PubMed] [Google Scholar]
- 58.Powell J, Kirtschig G, Allen J, Dean D, Wojnarowska F. Mixed immunobullous disease of childhood: A good response to antimicrobials. Br J Dermatol. 2001;144:769–74. doi: 10.1046/j.1365-2133.2001.04131.x. [DOI] [PubMed] [Google Scholar]
- 59.Kárpáti S. An exception within the group of autoimmune blistering diseases: Dermatitis herpetiformis, the gluten-sensitive dermopathy. Dermatol Clin. 2011;29:463–8. doi: 10.1016/j.det.2011.03.019. x. [DOI] [PubMed] [Google Scholar]
- 60.Clarindo MV, Possebon AT, Soligo EM, Uyeda H, Ruaro RT, Empinotti JC. Dermatitis herpetiformis: Pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865–75. doi: 10.1590/abd1806-4841.20142966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodley DT, Briggaman RA, O’Keefe EJ, Inman AO, Queen LL, Gammon WR. Identification of the skin basement-membrane autoantigen in epidermolysis bullosa acquisita. N Engl J Med. 1984;310:1007–13. doi: 10.1056/NEJM198404193101602. [DOI] [PubMed] [Google Scholar]
- 62.Woodley DT, Burgeson RE, Lunstrum G, Bruckner-Tuderman L, Reese MJ, Briggaman RA. Epidermolysis bullosa acquisita antigen is the globular carboxyl terminus of type VII procollagen. J Clin Invest. 1988;81:683–7. doi: 10.1172/JCI113373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen M, Kim GH, Prakash L, Woodley DT. Epidermolysis bullosa acquisita: Autoimmunity to anchoring fibril collagen. Autoimmunity. 2012;45:91–101. doi: 10.3109/08916934.2011.606450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caux F. Diagnosis and clinical features of epidermolysis bullosa acquisita. Dermatol Clin. 2011;29:485–91. doi: 10.1016/j.det.2011.03.017. x. [DOI] [PubMed] [Google Scholar]
- 65.Groth S, Recke A, Vafia K, Ludwig RJ, Hashimoto T, Zillikens D, et al. Development of a simple enzyme-linked immunosorbent assay for the detection of autoantibodies in anti-p200 pemphigoid. Br J Dermatol. 2011;164:76–82. doi: 10.1111/j.1365-2133.2010.10056.x. [DOI] [PubMed] [Google Scholar]
- 66.Rao R, Dasari K, Shenoi SD, Balachandran C, Dinesh P. Monitoring the disease activity in pemphigus by direct immunofluorescence of plucked hair: A pilot study. Indian J Dermatol. 2013;58:164. doi: 10.4103/0019-5154.108111. [DOI] [PMC free article] [PubMed] [Google Scholar]