Abstract
The objective of this study was to describe outcomes of tuberculosis (TB) contact investigations, factors correlated with those outcomes, and current successes and ways to improve TB contact investigations. We abstracted clinic records of a representative U.S. urban sample of 1,080 pulmonary, sputum-smear(+) TB patients reported to CDC July 1996 through June 1997 and the cohort of their 6,225 close contacts. We found a median of four close contacts per patient. Fewer contacts were identified for homeless patients. A visit to the patient’s residence resulted in two additional (especially child) contacts identified. Eighty-eight percent of eligible contacts received tuberculin skin tests (TSTs). Recording the last exposure date to the infectious patient facilitated follow-up TST provision. Thirty-six percent of contacts were TST(+). Household contacts and contacts to highly smear(+) or cavitary TB patients were most likely to be TST(+). Seventy-four percent of TST(+) contacts started treatment for latent TB infection (LTBI), of whom 56% completed. Sites using public health nurses (PHNs) started more high-risk TST(−) contacts on presumptive treatment for LTBI. Using directly observed treatment (DOT) increased the likelihood of treatment completion. We documented outcomes of contact investigation efforts by urban TB programs. We identified several successful practices, as well as suggestions for improvements, that will help TB programs target policies and procedures to enhance contact investigation effectiveness.
Whereas the first priority of tuberculosis (TB) prevention and control programs is identification and treatment of all persons with active TB, the second priority is contact investigation to find persons who were exposed to TB patients and to evaluate and treat them for latent TB infection (LTBI) and active TB disease (1).
Past studies of contact investigation focused on TB transmission and identification of active TB disease among contacts, finding greater transmission of TB infection from patients having sputum smear positive(+) for acid-fast bacilli (AFB) (2–5) and prevalence of active TB in 1.3 to 1.5% of adult or household contacts (3). A contact investigation study in Australia examined outcomes other than transmission, finding an average of 6.5 contacts screened per patient, a median interval between case report and contact screening of 1 mo, 36% of contacts as TB infected, and 61% of those started on treatment for LTBI as having completed (6).
Although recommendations and suggested guidelines for contact investigation in the United States have been published (7–10), they are based on expert opinion and not on data from a comprehensive study of outcomes from contact investigations. This is the first study of a representative urban sample of adults with pulmonary, AFB sputum smear(+) TB disease and the cohort of their close contacts to examine variations in outcomes of contact investigation by TB patient, contact, and program characteristics. Study objectives were to describe outcomes of contact investigations, factors correlated with those outcomes, and current successes and possible ways to improve contact investigations.
METHODS
We used data reported to the Centers for Disease Control and Prevention (CDC) by TB program managers to estimate LTBI treatment completion for metropolitan statistical areas (MSAs) having greater than 500,000 residents. We then ranked the MSAs from the highest to the lowest estimated number of contacts completing LTBI treatment and selected those sites having a minimum estimate of 100 persons completing treatment and sufficient record keeping and management to allow data collection. The following 11 TB program sites agreed to participate: Chicago, IL; Fulton County, GA; Houston, TX; King County, WA; Los Angeles County, CA; New York City, NY; Newark, NJ; San Diego County, CA; San Francisco, CA; Santa Clara County, CA; and Shelby County, TN.
We used probability proportional to size sampling (11) to choose the numbers of persons from each site having pulmonary, AFB sputum smear(+) TB disease (for their increased likelihood of being infectious) who were older than 15 yr of age and were reported to CDC from July 1996 through June 1997. We visited each site between June 1998 and January 1999 to abstract data from public health clinic records on the selected TB patients and their contacts using standardized data collection instruments to input data directly into laptop computers. In addition, we used a semistructured instrument to interview TB program administrators and contact investigation supervisors about the organizational structure related to contact investigation at each site.
We obtained approval from CDC’s institutional review board (IRB) and from local IRBs when necessary for the study protocol. A waiver of informed consent was granted for collection of data from existing records of sampled patients and their contacts. However, informed consent was obtained from administrators who provided information about the TB program structure.
The data gathered on all contacts to the sampled TB patients comprised the cohort for analysis. Most sites categorized contacts as close, casual, or other, according to their own definitions. Some sites only listed close contacts. Data on close contacts were selected for analysis.
We used standard definitions from the Report of a Verified Case of TB for socioeconomic (i.e., race/ethnicity, homelessness, substance abuse, occupation) and medical factors [i.e., AFB sputum smear(+), cavitation on chest radiograph] believed to be correlated with outcomes (12). We created a composite variable for substance abuse that included injection or noninjection drug use or alcohol abuse. TB patients having AFB sputum smear levels of 3- or 4-plus by international classification were considered to be “highly smear(+).”
For outcomes, we defined a positive tuberculin skin test (TST) as greater than or equal to 5 mm reaction to 0.1 ml of 5 tuberculin units of purified protein derivative, or used local determinations of a positive result if no millimeter size was given. Contacts who were positive on an initial or follow-up TST during the contact investigation and who were without either disease or reported history of TST positivity were simply called TST(+). Among TST(+) contacts, those with an initial TST(−) reaction and a follow-up TST(+) were defined as “converters.” Treatment for LTBI refers to a standard regimen of isoniazid (INH) for 6 to 12 mo for contacts of persons with drug-susceptible disease, or rifampin (RIF) or other drugs for contacts of persons with drug-resistant disease. Treatment interruptions were defined as missing more than 2 wk of directly observed treatment (DOT) or more than 1 mo of self-administered treatment, and then resuming treatment. We recorded treatment completion as noted in the charts.
We analyzed the following outcomes of TB contact investigations: time between patient diagnosis and interview to identify contacts; number of identified contacts per patient; initial TST result; time between patient interview and TST; follow-up TST result; time between initial and follow-up TST; TST positivity on initial or follow up; receipt of chest radiograph; active TB among contacts; start of treatment for LTBI; and completion of treatment for LTBI among those starting. Outcomes were correlated with patient, contact, and TB program characteristics.
To analyze the factors associated with numbers of identified contacts, we used multiple linear regression. To examine associations with dichotomous outcome variables, we calculated relative risks (RRs) using Mantel-Haenszel statistics for bivariate analyses and Cox proportional hazards regression for multiple regression analyses. For the multiple regression analyses, we included all explanatory variables hypothesized to be related to the outcome variable in the initial model and used backwards selection to determine the final model. All multiple regression analyses (with the exception of TST positivity) initially included at least the following variables: age group, sex, race/ethnicity, substance abuse, homelessness, and foreign birth. For comparison with those known to have completed treatment, we combined unknown LTBI treatment completion with known treatment noncompletion because we assumed that unknown completion knowledge by TB providers most likely meant noncompletion. Only results significant at 95% confidence, along with their confidence intervals (CI), are reported.
The sites collected more data on TST(+) than on TST(−) contacts; they also collected more data for contacts identified with active TB. Consequently, we only included those variables for which we had data for both TST(+) and TST(−) contacts (sex, age, race/ethnicity, type of contact, patient characteristics, and site) to avoid differential misclassification bias in the analyses of TST positivity. To examine TST positivity of contacts to human immunodeficiency virus–positive [HIV(+)] TB patients, we only analyzed data from the seven sites that report HIV status of TB patients to CDC. For analysis of factors associated with active TB, only a descriptive analysis is provided.
RESULTS
Organizational Structure
Contact investigation procedures differed among the sites, including who conducted the contact investigation, who supervised the workers, and what screening contacts received. Outreach workers performed contact investigation at six sites, primarily public health nurses (PHNs) at three sites, and a combination of PHNs and outreach workers at two sites. TB program personnel directly supervised contact investigation workers at eight sites, whereas health department staff (usually nurses) who were not formally part of the TB program supervised them at the remaining sites.
Time to Contact Identification
We found a median interval of 6 d (average 22) between patient diagnosis and interview to identify contacts.
Description of Contacts
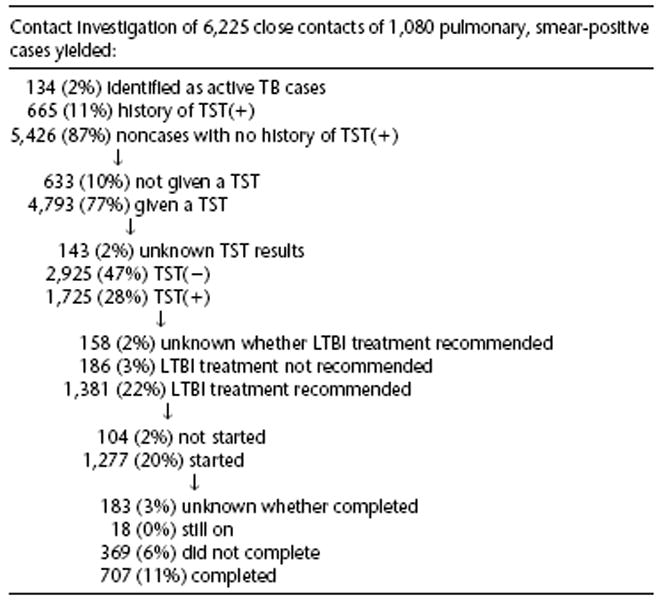
For the sample of 1,080 TB patients, the sites classified 45% (6,225) of all identified contacts as close. All sites defined household contacts as close. Table 1 describes the characteristics of the TB patients and their cohort of contacts.
TABLE 1.
CHARACTERISTICS OF PATIENTS AND THEIR CONTACTS
| Patients
|
Close Contacts
|
|||
|---|---|---|---|---|
| Characteristic | n | (%) | n | (%) |
| Total | 1,080 | (100) | 6,225 | (100) |
| Age 0–5 | 0 | (0) | 705 | (11) |
| Age 6–14 | 0 | (0) | 798 | (13) |
| Age 15–24 | 114 | (11) | 879 | (14) |
| Age 25–44 | 489 | (45) | 1,945 | (31) |
| Age 45–64 | 307 | (28) | 1,029 | (16) |
| Age 65 plus | 170 | (16) | 267 | (4) |
| White/Not Hispanic | 132 | (12) | 539 | (9) |
| Black/Not Hispanic | 439 | (41) | 1,687 | (27) |
| Hispanic | 301 | (28) | 1,506 | (24) |
| American Indian/Alaska native | 3 | (0) | 9 | (0) |
| Asian/Pacific Islander | 202 | (19) | 739 | (12) |
| Male | 742 | (69) | 2,816 | (45) |
| Female | 338 | (31) | 2,908 | (47) |
| Foreign-born | 498 | (47) | 1,302 | (21) |
| Health care worker | 24 | (2) | ND | |
| Homeless | 127 | (12) | 143 | (2) |
| Substance abuse | 324 | (30) | 117 | (2) |
| HIV(+) | 181 | (17) | 109 | (2) |
| Drug-resistant to INH or RIF | 123 | (11) | ||
| Highly smear(+) | 512 | (47) | ||
| Cavitary disease | 405 | (38) | ||
| Residence visited | 468 | (46) | ||
| Household contact | 2,664 | (43) | ||
| High-risk medical conditions* | 48 | (1) | ||
| Received DOT | 145 | (2) | ||
| Had interruptions in LTBI treatment | 489 | (8) | ||
Includes diabetes, cancer, end-stage renal disease, immunosuppression, and low body weight.
Sixty-eight percent of TB patients identified household contacts, 24% identified nonhousehold relatives, 21% identified leisure contacts, 5% identified coworkers, and 17% identified other types of close contacts. Fifty-eight percent of TB patients had been unemployed within the past 2 yr, less than 1% of whom identified work contacts compared with 11% of those who were employed. One-third of TB patients only identified household contacts.
Of the 6,225 close contacts, 43% were household contacts, 18% were relatives not living in the household, 12% were co-workers, 9% were leisure contacts, and 18% were other types of contacts.
Available Data on Contacts
The amount and type of data collected on contacts varied among the sites. The most common information collected about contacts included age, sex, close or casual status, TST dates and results, chest X-ray date and results, and LTBI treatment start and completion dates. Risk factors for TB disease (especially HIV status) and for nonadherence to LTBI treatment (e.g., substance abuse, homelessness) were often not recorded. HIV status was recorded for 13% of close contacts.
Number of Contacts Identified
We found a median of 4 (average 6) close contacts per patient, ranging from 2 to 6 among the sites. TB patients who were health care workers, had drug-resistant disease, showed cavitation on chest X-ray, and were age 15 to 44 had more identified close contacts than other TB patients (Table 2). A visit by the contact investigation worker to the patient’s residence during the investigation resulted in identification of two additional close contacts, which were likely to be children younger than 6 yr of age. Although they did not identify more close contacts overall, foreign-born patients had more household contacts than U.S.-born patients (median 3 versus 1). Fewer close contacts were identified by homeless persons, males, and Asian/Pacific Islanders with TB.
TABLE 2.
NUMBER OF CLOSE CONTACTS IDENTIFIED BY PATIENT CHARACTERISTIC
| Characteristic of Patient | Unadjusted Average | Adjusted Average* | p Value |
|---|---|---|---|
| Health care worker | 8.8 | 8.1 | 0.027 |
| Non–health care worker | 5.5 | ||
| Drug-resistant to INH or RIF | 7.5 | 7.0 | 0.001 |
| Drug-susceptible | 5.3 | ||
| Residence visited for contact investigation | 6.7 | 6.8 | < 0.001 |
| Residence not visited | 4.7 | ||
| Age 15–24 | 7.1 | 6.6 | 0.023 |
| Not age 15–24 | 5.4 | ||
| Cavitary | 6.8 | 6.6 | < 0.001 |
| Noncavitary | 4.8 | ||
| Age 25–44 | 6.0 | 6.3 | 0.002 |
| Not age 25–44 | 5.2 | ||
| Asian/Pacific Islander | 5.0 | 3.8 | 0.025 |
| Non–Asian/Pacific Islander | 5.7 | ||
| Male | 4.8 | 2.9 | < 0.001 |
| Female | 7.3 | ||
| Homeless | 2.7 | 2.2 | < 0.001 |
| Nonhomeless | 5.9 |
The multivariate linear regression model R2 = 0.10. Ten percent of the variation in the number of contacts identified is explained by the linear relationship of the variables.
No contacts were identified for 8% (88) of the sampled TB patients. Seventeen patients were dead when TB was diagnosed, 29% of whom had no identified contacts. Multiple regression analysis found that homelessness was significantly correlated with having no identified contacts (adjusted RR = 1.3, CI 1.0 to 1.5).
TST Placement
Of 5,426 contacts without active TB and with no history of TST positivity, 88% (4,793) had a TST placed, which ranged from 80% to 100% among the sites. Those who received initial TSTs were more likely to be household (RR = 1.05, CI 1.03 to 1.08) or work contacts (RR = 1.1, CI 1.07 to 1.13) and less likely to be nonhousehold relative (RR = 0.96, CI 0.94 to 0.99) or other types (RR = 0.90, CI 0.87 to 0.92) of contacts.
A median of 12 d lapsed between the patient interview and placement of TSTs on close contacts. Contact investigation workers provided TSTs earlier to children younger than 6 yr of age (median 9 d).
Fifty-four percent of 3,132 contacts initially testing negative had a follow-up TST recorded, which was given 94 (median) days after the initial TST. At the two sites that recorded the date of last exposure to the infectious patient for determination of appropriate follow-up TST date, 63% of initially TST(−) contacts had a recorded follow-up TST, compared with 45% of contacts at the remaining sites (RR = 1.4, CI = 1.3 to 1.5). Also, pediatric contacts were more likely to receive follow-up TSTs (RR = 1.3, CI = 1.2 to 1.5).
TST Positivity
Of contacts who received a TST, we found 1,512 (32%) TST(+) on initial TST and 213 (4%) TST(+) on follow-up TST, for a total positivity rate of 36%. All but 7% of TST(+) contacts had recorded TST millimeter induration sizes. Outcomes of TST by high-risk group are presented in Table 3.
TABLE 3.
OUTCOMES OF TST BY HIGH RISK GROUP
| High-risk Contacts
|
|||||
|---|---|---|---|---|---|
| Outcome | Substance Abusers | HIV(+) | Children < 6 | Foreign-born | All Close Contacts |
| TST(+), on initial | 55 | 9 | 111 | 635 | 1,512 |
| 63% | 11% | 18% | 62% | 32% | |
| TST(+), at follow-up (conversion) | 3 | 2 | 21 | 86 | 213 |
| 3% | 2% | 3% | 8% | 4% | |
| TST(−), on initial, no follow-up | 6 | 30 | 157 | 114 | 1,450 |
| 7% | 38% | 25% | 11% | 30% | |
| TST(−), on initial, and follow-up | 8 | 32 | 322 | 170 | 1,475 |
| 9% | 40% | 52% | 17% | 31% | |
| Unknown TST results | 15 | 7 | 7 | 12 | 143 |
| 17% | 9% | 1% | 1% | 3% | |
| Total | 87 | 80 | 618 | 1,017 | 4,793 |
| 100% | 100% | 100% | 100% | 100% | |
Forty-four percent of household contacts were TST(+), compared with 40% of leisure contacts, 34% of relatives not living in the same household, 29% of work contacts, and 20% of other contacts. Only 21% of children younger than 6 yr of age and 14% of known HIV(+) contacts were TST(+). TST positivity was highest for known foreign-born (71%) and substance-abusing (67%) close contacts. Among the TST(+), twice as many foreign-born compared with U.S.-born contacts had documented conversions from TST(−) to TST(+) during the contact investigation, which was true for nonhousehold as well as for household contacts. We examined whether foreign-born contacts were more likely to be contacts to cavitary or highly smear(+) patients, and found that they were not.
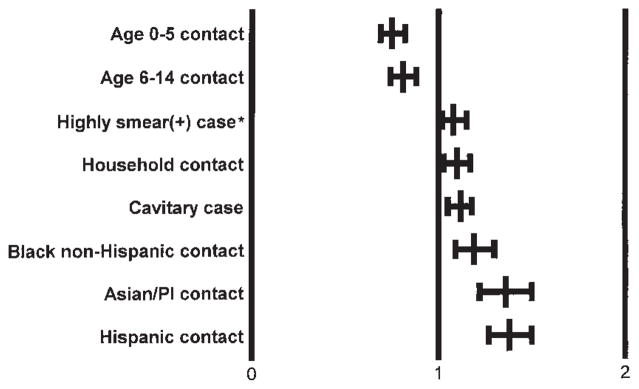
In the multiple regression analysis, we found the following types of contacts more likely to be TST(+) after controlling for site and race/ethnicity: contacts to a cavitary patient, household contacts, and contacts to a highly smear(+) patient (Figure 1). Less likely to be TST(+) were contacts less than 15 yr of age. Contacts to HIV(+) patients were less likely to be TST(+) (21% versus 33%), controlling for all the previously mentioned variables in a separate model.
Figure 1.
Risk (adjusted relative risk and 95% confidence interval) of contacts being TST(+). The referent group for each risk category is all other contacts not in the model. *The referent group is contacts to smear(+) cases who are not highly smear(+).
Receipt of Chest Radiograph
Of contacts at high risk for active TB, the following were screened with a chest X-ray: 90% of TST(+) persons, 88% of symptomatic persons, and 77% of those with a history of TST positivity. Only 72% of HIV(+) persons and 62% of children younger than 6 yr of age received a chest radiograph regardless of TST results.
Contacts with Active TB
Two percent (134) of all close contacts had active TB. Examining factors for which we had complete data, we found that contacts identified with active TB were more likely to be household contacts and children younger than 6 yr of age, and less likely to be nonhousehold relatives or work contacts. We found the following percentages of groups with known characteristics having active TB: 21% of 34 noninjection drug users; 19% of 26 correctional facility residents; 16% of 167 symptomatic contacts; 14% of 141 contacts with a history of unemployment; 13% of 109 HIV(+) persons; 13% of 95 alcohol abusers; 12% of 16 injection drug users; 5% of 705 children age 5 and younger; 4% of 1,687 blacks/non-Hispanic; 4% of 674 contacts with a history of TST positivity; 3% of 2,664 household contacts; and 3% of 3,171 contacts to highly smear(+) patients and to 2,782 cavitary patients.
Starting LTBI Treatment
There were 1,725 TST(+) close contacts who had no history of TST positivity and did not have active TB. Seventy-four percent of these contacts started LTBI treatment, including all (11) known HIV(+), TST(+) contacts. A median of 12 d passed between the last TST reading (initial or follow-up) and treatment start. The most common reason for contacts not starting LTBI treatment was uncooperativeness or refusal.
Of 117 contacts to 83 INH-resistant patients, 75% were placed on RIF, 2% on INH/RIF, 1% on RIF/pyrazinamide (PZA); the remaining 22% only had INH recorded. Fifty-two percent of 25 contacts to 31 multidrug-resistant (MDR) TB patients were most commonly placed on two drugs (i.e., ethambutol, pyrazinamide, and/or ofloxacin); the remainder only listed INH as the LTBI treatment regimen.
In multiple regression analysis of TST(+) contacts, we found that children age 6 to 14 (RR = 1.3, CI 1.1 to 1.5) and foreign-born persons (RR = 1.2, CI 1.1 to 1.3) were more likely to start LTBI treatment and contacts age 45 to 64 (RR = 0.9, CI 0.8 to 1.0) less likely.
Of high-risk close contacts without TB disease who were TST-negative, had unknown TST results, or did not receive a TST, presumptive treatment for latent TB infection was started for 45% of 539 children younger than 6 and 50% of 18 contacts with diabetes, cancer, end-stage renal disease, immunosuppression, or low body weight. Seventy-two percent of these high-risk contacts were placed on LTBI treatment presumptively at sites using PHNs, compared with 33% at sites using outreach workers (RR = 2.2, CI 1.8 to 2.6). Twenty-three percent of 84 known HIV(+) persons without TB disease who were TST(−/unknown) or who had a history of TST positivity were started on treatment presumptively and 42% completed. Sites using PHNs placed 92% of these HIV(+) contacts on LTBI treatment presumptively versus 11% at sites using outreach workers (RR = 8.2, CI 4.2 to 16.2).
Of 213 close contacts without TB disease who converted from TST(−) to TST(+) during the contact investigation, 23% were started on presumptive treatment for LTBI before the follow-up TST. In this group, children younger than 6 yr of age were more likely to have been treated presumptively (RR = 3.1, CI 1.7 to 5.7), and one of two HIV(+) converters was started on treatment presumptively. Once again, sites using PHNs rather than outreach workers were more likely to start these contacts on presumptive LTBI treatment.
Completion of LTBI Treatment
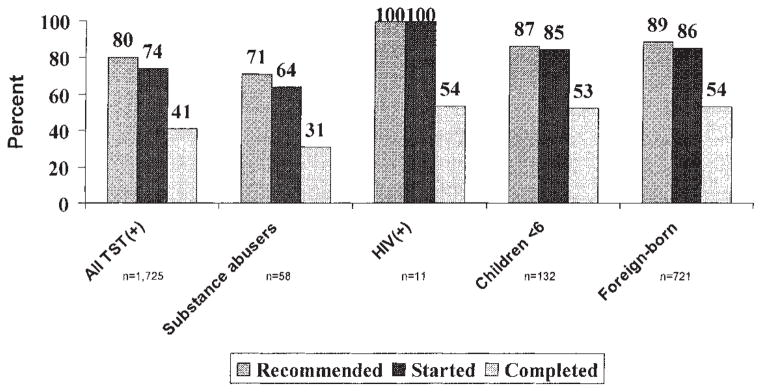
Figure 2 shows LTBI treatment recommendation, start, and completion for all TST(+) contacts and those at high risk for developing active TB. Known substance abusers had the lowest rates of LTBI treatment recommendation, start, and completion. Excluding the 18 who were still on treatment at the end of the study period, 1,259 TST(+) contacts started LTBI treatment, of whom 707 (56%) completed, 369 (29%) did not complete, and it was unknown whether 183 (14%) completed treatment. Among those not completing were 12 persons with hepatotoxicity and three persons who died; these contacts were censored from further analyses because they did not have the opportunity to complete LTBI treatment.
Figure 2.
LTBI treatment recommended, started, completed for TST(+) contacts.
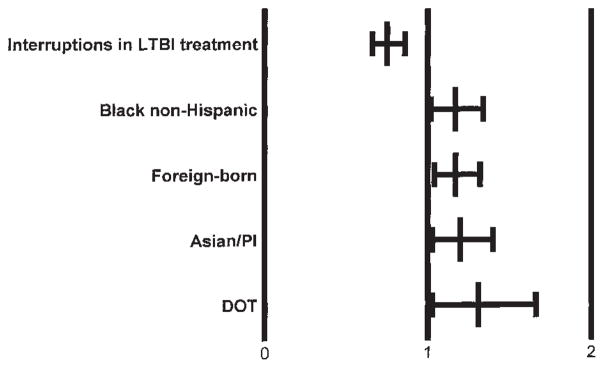
In multiple regression analysis controlling for race/ethnicity and foreign-born status, TST(+) contacts who were on DOT were more likely to complete LTBI treatment, whereas contacts who had interruptions during the course of treatment were less likely. (Figure 3). DOT was more often used with children younger than 6 yr of age. HIV(+) persons were not more likely to complete LTBI treatment; 54% of HIV(+) persons completed versus 56% of HIV(−/unknown) persons. Table 4 summarizes data outcomes for close contacts.
Figure 3.
Likelihood of TST(+) contacts completing LTBI treatment is shown as the adjusted relative risk of treatment starters and 95% confidence interval. The referent group for each risk category is all other contacts not in the model.
TABLE 4.
DATA OUTCOMES FOR CLOSE CONTACTS
|
DISCUSSION
Our study found a median of four (average of six) close contacts per patient. Eighty-eight percent of contacts without TB disease or a history of TST positivity received TSTs. Thirty-six percent of contacts who received TSTs were TST(+). Household contacts and contacts to persons having cavitary or highly smear (+) disease had the highest infection rates, controlling for race/ethnicity and site. Seventy-four percent of TST(+) contacts started treatment for LTBI, of whom 56% completed.
We examined whether the high rates of initial TST positivity and conversions among foreign-born contacts indicated recent infection or boosting from a prior TB infection or bacillus Calmette-Guérin (BCG) vaccination. Boosting occurs when a person with preexisting mycobacterial sensitivity has a negative initial TST but a positive reaction to a second TST after an interval as short as 1 wk or as long as a year or more. Although we had inadequate data on TST(−) contacts and consequently could not include foreign-born status in the TST positivity analysis, the majority of TST(+) Asian/Pacific Islander and Hispanic contacts were born in countries where TB is highly prevalent. Although crowded living conditions (indicated by greater numbers of household contacts to foreign-born TB patients) could have contributed to high TST(+) rates, the high rates persisted in nonhousehold foreign-born contacts. Also, foreign-born contacts were not more likely to be contacts to highly smear (+) or cavitary patients, which would have increased the likelihood of recent TB infection. We conclude that the high TST positivity rates among foreign-born contacts probably indicate prior infection or boosting rather than recent infection. This implies the need to balance the risk of INH hepatotoxicity (which was 1% in our study population) in prescribing LTBI treatment to TST(+) foreign-born contacts with the risk of developing active TB and for caution in making decisions to expand contact investigations to casual contacts based on high TST(+) rates in foreign-born contacts.
We identified several TB program practices or characteristics correlated with successful outcomes of contact investigation. One, the greater number of close contacts identified for drug-resistant and cavitary TB patients suggests that contact investigation workers expend greater efforts to identify close contacts of these patients. By doing this, TB programs reduce the risk of there being undiagnosed drug-resistant patients and prevent future disease among contacts, as well as identify the many infected contacts to potentially highly infectious patients. Two, a visit by the contact investigation worker to the patient’s residence results in the identification of two additional (especially child) close contacts. Three, recording the date of last exposure to the infectious patient facilitates provision of follow-up TSTs to contacts initially TST(−), which is necessary to identify all contacts likely to convert to TST(+). Four, sites that use PHNs are more likely than those using outreach workers to start TST(−/unknown) high-risk contacts, who are possibly infected but anergic, or TST converters before their follow-up TSTs on presumptive treatment for LTBI. Five, the use of DOT increases the likelihood of LTBI treatment completion.
We also identified many ways to improve contact investigations, starting with the need to consistently define a close contact, identify close contacts to homeless TB patients, collect common data regardless of the contact’s TST result or active TB, prioritize high-risk contacts, completely evaluate contacts with initial and follow-up TSTs and chest X-rays when recommended, start contacts on an appropriate drug regimen, ensure LTBI treatment completion for all eligible persons, and determine indicators to facilitate the evaluation of contact investigation quality.
Contact investigation workers should ensure that TB patients list nonhousehold as well as household close contacts and make special efforts to identify contacts to homeless persons. Possible ways to increase the number of identified contacts among the homeless include improving contact investigation worker interviewing skills and establishing trust to overcome unwillingness to provide information by homeless TB patients.
Once close contacts are identified, common data should be collected on risk factors for TB disease (especially HIV status) and for nonadherence to LTBI treatment (e.g., substance abuse, homelessness) so contacts can be prioritized. Provider knowledge of HIV status is essential for the optimal provision of presumptive treatment for latent TB infection and for the determination of the proper drug treatment regimen and coordination of care for persons dually infected with HIV and TB. Ideally, TB program staff should either be trained and prepared to offer HIV counseling and testing to close contacts at risk for HIV or should collaborate with HIV programs to offer these services.
Additional reasons for the nonprovision of initial TSTs to 12% of close contacts without TB disease or a history of TST positivity should be examined. For contacts who received initial TSTs, contact investigation workers need to completely evaluate them by recording the date of last exposure to the infectious patient and by providing follow-up TSTs, which were documented for only 54% of initially TST(−) close contacts.
Chest radiographs are recommended to diagnose active TB in HIV(+) and pediatric close contacts, regardless of TST results (13, 14). However, only two-thirds of young children and three-fourths of HIV(+) close contacts were screened by chest radiograph. Once disease is ruled out by chest X-ray, presumptive treatment for LTBI should be offered to these high-risk groups, even if they are TST(−) (13, 14). HIV(+) close contacts should complete LTBI treatment, regardless of follow-up TST results (15). For TST(+) contacts, the processing time to treatment start could be shortened by providing immediate radiographic evaluation when contacts come to clinic for their final TST reading.
Contacts to INH-resistant or MDR TB patients should be prescribed the appropriate treatment medications. Whereas it is standard practice to start contacts on INH until drug susceptibilities of the patient’s disease are known, 20% of contacts to INH-resistant patients and 44% of contacts to MDR patients remained on INH. Continuing INH for these contacts, whose only known source of TB infection is an MDR TB patient, does not reduce their risk of active TB and unnecessarily places them at risk for INH hepatotoxicity. Changes in treatment regimens should be recorded.
All efforts should be made to ensure and document LTBI treatment completion among those started, especially for contacts at high risk for disease. DOT, associated with greater treatment completion and more often used for children, should be considered for other high-risk contacts such as HIV(+) persons, diabetics, substance abusers, and homeless persons. Interruptions during the course of LTBI treatment presaged failure, which suggests the need for intensive efforts to avoid the first interruption or to increase adherence, such as by using DOT, incentives, or the new shorter LTBI treatment regimens (15). Ensuring LTBI treatment completion needs to be viewed as an integral part of the contact investigation process.
The preceding suggested improvements in contact investigation should be studied prospectively to measure increases in effectiveness, along with implementation costs.
Limitations
The lack of consistent contact definition across sites and the limited data collected on all contacts, regardless of TST result or active TB, are study limitations typical of retrospective studies of existing records. However, they reflect current procedures used in contact investigation by the major urban TB programs.
TST positivity may have been underestimated because of infected contacts who did not receive follow-up TSTs during the contact investigation. On the other hand, recent TB infection may have been overestimated because of boosting.
We did not know when contacts developed active TB nor did we have DNA fingerprint data to link secondary to primary cases. Consequently, we could not distinguish between contacts who developed active TB resulting from exposure to the index case of the contact investigation and those who were identified with active TB resulting from exposure to another patient.
Summary
This comprehensive study of a representative urban sample of adults with pulmonary, AFB smear(+) TB disease documented previously unknown outcomes of contact investigation efforts by TB programs. Working toward the goal of TB elimination in the United States, TB programs are placing renewed emphasis on contact investigation to find new cases and to ensure LTBI treatment completion in those persons recently infected (16). Knowledge of these outcomes and associated factors will help TB programs target policies and procedures to enhance contact investigation effectiveness.
Improved training of TB program staff, using the new CDC contact investigation training curriculum (17) and courses offered at the three TB model centers, will help make contact investigations more effective. In addition, provision of targeted TB screening and access to care, including LTBI treatment, to those contacts identified at high risk, such as substance abusers, correctional facility residents, unemployed persons, HIV(+) persons, children younger than 6 yr of age, and black/non-Hispanic persons may prevent cases, improve early case detection, and reduce TB transmission.
Acknowledgments
The authors acknowledge the following TB program staff for making this study possible: William Paul and Jim McAuly in Chicago, IL; Beverly DeVoe, Brian Palmer, and Ruby Lewis Hardy in Fulton County, GA; Marcos Longoria and Kathy Penrose in Houston, TX; Charles Nolan and Kim Field in King County, WA; Paul Davidson, Sue Gerber, Kathryn Koski, and Laura Knowles in Los Angeles County, CA; Paula Fujiwara, Chris Larkin, and Marie Dorsinville in New York City, NY; Eileen Napolitano and Akilah Ain in Newark, NJ; Kathy Moser in San Diego County, CA; Masae Kawamura and Tony Paz in San Francisco, CA; Karen Smith in Santa Clara County, CA, and William Moore and Linda Hamer in Shelby County, TN. They also thank the following CDC staff: Mohamed Qayad for helping develop the sampling plan; Chuck Gaines, Gail Burns Grant, Andy Heetderks, Olga Joglar, Scott Jones, and Dan Ruggiero for facilitating project implementation; Ken Dansbury for developing the data entry screens; and Ann Lanner for editorial assistance.
Footnotes
This study was a collaborative project between CDC investigators and personnel at participating sites. No grant funding was involved.
References
- 1.Centers for Disease Control and Prevention. Essential components of a tuberculosis prevention and control program: recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR. 1995;44(RR–11):2. [PubMed] [Google Scholar]
- 2.Cayla JA. Factors associated with secondary TB cases. Symposium. Investigation of Contacts to Tuberculosis Cases New York City Department of Public Health, Bureau of TB Control; New York. June 7–8, 1996. [Google Scholar]
- 3.Kritski A. Management of TB contacts in Brazil: is it a priority?. Symposium. Investigation of Contacts to Tuberculosis Cases. New York City Department of Public Health, Bureau of TB Control; New York City. June 7–8, 1996. [Google Scholar]
- 4.Shaw JB, Wynn-Williams N. Infectivity of pulmonary TB in relation to sputum status. Am Rev TB. 1954;69:724–732. doi: 10.1164/art.1954.69.5.724. [DOI] [PubMed] [Google Scholar]
- 5.Snider DE, Kelly GD, Cauthen GM, Thompson NJ, Kilburn JO. Infection and disease among contacts of tuberculosis cases with drug-resistant and drug-susceptible bacilli. Am Rev Respir Dis. 1985;132:125–132. doi: 10.1164/arrd.1985.132.1.125. [DOI] [PubMed] [Google Scholar]
- 6.MacIntyre CR, Plant AJ. Impact of policy and practice on the effectiveness of contact screening for tuberculosis. Preventive Med. 1998;27:830–837. doi: 10.1006/pmed.1998.0366. [DOI] [PubMed] [Google Scholar]
- 7.American Thoracic Society. Guidelines for the investigation and management of tuberculosis contacts. Am Rev Respir Dis. 1976;114:459–463. doi: 10.1164/arrd.1976.114.2.459. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. A strategic plan for the elimination of tuberculosis in the United States. MMWR. 1989;38(S-3):1–21. [PubMed] [Google Scholar]
- 9.Etkind SC. Contact tracing in tuberculosis. In: Reichman L, Hershfield E, editors. TB: a comprehensive international approach. Lung biology in health and disease. New York: Marcel-Dekker; 1993. pp. 275–289. [Google Scholar]
- 10.Iseman MD. Containment of tuberculosis: preventive therapy with isoniazid and contact investigation. Chest. 1979;76S:801–804. [PubMed] [Google Scholar]
- 11.Levy PS, Lemeshow S. Sampling of populations methods and applications. New York: John Wiley; 1991. pp. 244–272. [Google Scholar]
- 12.Centers for Disease Control and Prevention. Report of Verified Case of Tuberculosis Form Completion Instructions: SURVS-TB Version 2.0. Atlanta, GA: CDC; 1994. [Google Scholar]
- 13.Centers for Disease Control and Prevention. Core Curriculum on Tuberculosis: What the Clinician Should Know. Vol. 34. Atlanta: CDC; 1994. [Google Scholar]
- 14.Centers for Disease Control and Prevention. Prevention and treatment of tuberculosis among patients infected with human immunodeficiency virus: principles of therapy and revised recommendations. MMWR Recommendations and Reports. 1998;47(RR-20):37–38. [PubMed] [Google Scholar]
- 15.American Thoracic Society and Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Tuberculosis elimination revisited: obstacles, opportunities, and a renewed commitment. MMWR Recommendations and Reports. 1999;48(RR–9):7. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Self-Study Modules on Tuberculosis: Contact Investigations for Tuberculosis. Atlanta, GA: CDC; 1999. [Google Scholar]