Abstract
Background
In the debate on reconstruction of the radiated breast, there is little information on associated healthcare resource use. We used nationwide data to examine healthcare resource use associated with implant and autologous reconstruction. We hypothesized that failure rates would contribute the most to higher average cumulative cost with either reconstruction method.
Methods
From the 2009 – 2013 MarketScan Commercial Claims and Encounters database, we selected radiated breast cancer patients who underwent implant or autologous reconstruction. In a 24-month follow-up period, we tallied and described the cumulative costs of healthcare services used. We then used regression models stratified by reconstruction method to estimate the influence of failure on cumulative cost of reconstruction.
Results
There were 2964 patients in the study. A majority of patients (78%) underwent implant reconstruction. The unadjusted mean cost for implant and autologous reconstructions were $22,868 and $30,527 respectively. Thirty-two percent of implant reconstructions failed compared to 5% for autologous cases. Twelve percent of the implant reconstructions had ≥ 2 failures and required subsequent autologous reconstruction. The cost of implant reconstruction failure requiring a flap was $47,214 and $48,344 for autologous failures. In aggregate, failures constituted > 20% of the cumulative costs of implant reconstruction compared to < 5% for autologous reconstruction.
Conclusion
More than 1 in 10 patients who had implant reconstruction in the setting of radiation therapy to the breast eventually required a flap for failure. These findings make a case for autologous reconstruction being primarily considered in radiated patients who have this option available.
Keywords: Autologous Breast Reconstruction, Cost, Implant Breast Reconstruction, Radiation, Resource Use
INTRODUCTION
The use of radiation therapy as an adjunct in node-positive breast cancer treatment and the rates of breast reconstruction among patients treated with radiation therapy have increased recently.1,2 However, the increased rate of breast reconstruction among radiated patients is tilted disproportionately towards implant-based reconstruction.3–5 A recent examination of immediate breast reconstruction trends among radiated patients showed an approximately 25% increase in implant reconstruction among these patients in the decade between 2000 and 2010 with a concomitant and equivalent decrease in autologous reconstruction in the same period.4
The effects of radiation therapy pose significant challenges in breast reconstruction as a result of the damage done to chest wall tissue. In general, there is agreement that implant reconstruction exposes radiated patients to comparatively higher associated morbidity than with non-radiated patients.6, 7 It is also generally accepted that autologous flaps for breast reconstruction offer the advantage of replacing radiation-exposed breast and chest wall tissue with arguably superior outcomes.8–10 However, the clear recent trend in favor of implant breast reconstruction in radiated patients suggests that proponents believe that success rates, aesthetic outcomes and patient satisfaction are reasonable with this option. This view is supported in the literature.7, 11–13 Although several studies show failures rates up to 45% among radiated patients with implant reconstruction, other studies have reported considerably better outcomes.11–13 This divergence in published literature indicates that more information would be beneficial in the debate about reconstructing the radiated breast especially for patients with no contraindications to all available reconstruction options.
In the current healthcare reform dispensation that emphasizes alternative payment models to achieve cost containment, a useful metric to add to the debate about reconstruction of the radiated breast is the relative use of healthcare resources between options. Hence, the high failure and morbidity rates widely reported with implant reconstruction in radiated patients warrant examination from the cost perspective. We used nationwide longitudinal data to examine healthcare resource use associated with 2 different methods of breast reconstruction: implant and autologous reconstruction. We hypothesized that among radiated breast cancer patients, failure rates will be higher with implant reconstruction. Additionally, failure rates would contribute the most to higher average cumulative cost with either reconstruction method.
METHODS
Data Source and Study Cohort
We used data from the 2009 – 2013 Truven Health MarketScan® Research Databases that includes the Commercial Claims and Encounters Database and the Medicare Supplemental and Coordination of Benefits Database. These longitudinal databases capture patient-level utilization of medical services, and payments across healthcare settings. They represent the healthcare utilization of approximately 50 million active employees, early retirees, Medicare-eligible retirees with employer-provided Medicare Supplemental plans, and their dependents each year.
The first inclusion criterion was claims for radiation therapy associated with breast cancer diagnosis. (See Supplemental Digital Content 1, Appendix 1, which shows the Diagnoses and procedure codes for breast cancer, breast reconstruction and radiation therapy, insert link) Secondly, selected patients had medical claims for mastectomy and breast reconstruction procedures also associated with breast cancer diagnosis between January 1st, 2009 and December 31th, 2012. This period allowed a 24-month follow-up for each patient enabling us to capture healthcare encounters and the associated use of healthcare resources following the index reconstruction procedure. Other inclusion criteria include: female sex, age ≥ 18 years of age and continuous enrolment for at least 24 months following the index procedure. In order to simplify our comparisons, we included patients who had implant only or autologous only procedures and excluded those who had a combination of both autologous and implant techniques in the index reconstruction episode e.g. implants or tissue expanders with a latissimus dorsi flap. We used International Classification of Diseases, 9th version codes (ICD-9 codes) and the Current Procedural Terminology codes (CPT codes) to identify specific diagnosis and procedures, respectively (See Supplemental Digital Content 1, Appendix 1, insert link).
Components of Healthcare Resource Use
For each patient we delineated components of healthcare resource use in the longitudinal course of breast reconstruction over the 24-month follow-up period and tallied the costs i.e. reimbursements from payers associated with each component.14–16 These components included: 1) the index procedure of reconstruction e.g. initial autologous reconstruction procedure or tissue expander placement (See Supplemental Digital Content 1, Appendix 1, insert link), 2) secondary procedures including but not limited to tissue expander to implant exchange or procedures for symmetry. (See Supplemental Digital Content 2, Appendix 2, which shows the Codes for secondary procedures, insert link.) Other components include: 3) complications associated with breast reconstruction (See Supplemental Digital Content 3, Appendix 3 which shows the Diagnosis and procedural codes (ICD-9/CPT) for complications and reconstruction failure, insert link.), 4) inpatient readmissions/Emergency Department (ED) visits associated with breast reconstruction and 5) reconstruction failures.
Analyses
We described socio-economic, demographic and clinical variables in the study population according to reconstruction type (implant/autologous). We also included the 5 above listed components of healthcare resource use in the descriptions by reconstruction type. (Table 1) Secondly, examining each group of reconstruction type in aggregate, we calculated the percentage contribution of each of the 5 components of healthcare resource to the cumulative cost of reconstruction. (Figure 1)
Table 1.
Patient demographic and clinical characteristics (n=2,964)
| Patient characteristics | Implant Reconstruction (n/%) |
Autologous Reconstruction (n/%) |
|---|---|---|
| Total | 2306 (100%) | 658 (100%) |
| Age | ||
| 18–34 | 130 (6%) | 32 (5%) |
| 35–44 | 628 (27%) | 155 (24%) |
| 45–54 | 927 (40%) | 275 (42%) |
| 55–64 | 480 (21%) | 155 (24%) |
| 65 and older | 141 (6%) | 41 (6%) |
| Quartile of median house income | ||
| Quartile1 (<=$46,910) | 347 (15%) | 97 (15%) |
| Quarile2 ($46,910 to $51,920) | 381 (17%) | 111 (17%) |
| Quartile3 ($51,920 to $58,900) | 660 (29%) | 192 (29%) |
| Quartile4 (> $58,900) | 660 (29%) | 169 (26%) |
| Missing | 258 (11%) | 89 (14%) |
| Comorbidity score | ||
| Quartile1 (<=12) | 585 (25%) | 226 (34%) |
| Quartile2 (13–20) | 628 (27%) | 157 (24%) |
| Quartile3 (21–27) | 652 (28%) | 155 (24%) |
| Quartile4 (>=27) | 441 (19%) | 120 (18%) |
| Region | ||
| North east | 507 (22%) | 142 (22%) |
| North central | 532 (23%) | 113 (17%) |
| South | 755 (33%) | 250 (38%) |
| West | 469 (20%) | 142 (22%) |
| Missing | 43 (2%) | 11 (2%) |
| Timing of radiation | ||
| Before reconstruction | 1135 (49%) | 489 (74%) |
| After reconstruction | 1171 (51%) | 169 (26%) |
| Timing of reconstruction | ||
| Immediate | 1866 (81%) | 322 (49%) |
| Delayed | 440 (19%) | 336 (51%) |
| Bilateral | ||
| No | 1659 (72%) | 570 (87%) |
| Yes | 647 (28%) | 88 (13%) |
| Complications | ||
| No | 1165 (51%) | 399 (61%) |
| Yes | 1141 (49%) | 259 (39%) |
| ED visits | ||
| No | 1905 (83%) | 564 (89%) |
| Yes | 401 (17%) | 94 (14%) |
| Readmissions | ||
| No | 1644 (71%) | 531 (81%) |
| Yes | 662 (29%) | 127 (19%) |
| Failures | ||
| No | 1566 (68%) | 623 (95%) |
| 1 | 459 (20%) | 25 (4%) |
| ≥2 | 281 (12%) | 10 (2%) |
Figure 1.
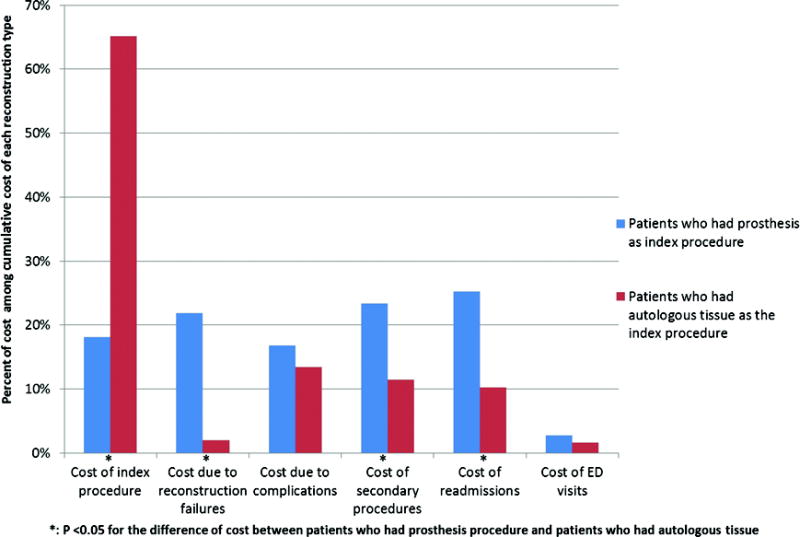
Proportional representation of costs of utilization for implant and autologous reconstruction
Then we used generalized linear models (GLM) stratified by reconstruction type to evaluate the association between reconstruction failure (key predictor variable) and the cumulative cost of reconstruction i.e. the tally of the 5 components of healthcare resource use (dependent variable). (Table 2) The GLMs eliminate the requirement for a normal distribution of the response variable, cost, for proper model fitting. From these models, we predicted the mean adjusted cumulative costs for implant and autologous patients. We also predicted the adjusted cumulative costs for patients with complications, readmissions/ED visits and reconstruction failures for each type of reconstruction. (Table 2) We ran an additional stratified linear model consisting of failed implant patients with the cumulative cost of reconstruction as the dependent variable and the method of subsequent reconstruction: autologous vs. non autologous as the main predictor. (Table 3) From this model, we predicted the adjusted cumulative cost for failed implant patients who required subsequent autologous reconstruction. (Table 3) There was no need to run such a model for autologous patients because in radiated patients who failed autologous reconstruction subsequent reconstruction is usually autologous. Therefore, the cumulative cost of subsequent reconstruction is reflected in the stratified model for autologous patients.
Table 2.
Adjusted estimation of the cumulative cost of reconstruction for implant and autologous patients
| Variables | Mean difference in cost (CI)‡ (Implants) |
Adjusted mean cost‡ (Implants) |
Mean difference in cost (CI)‡ (Autologous) |
Adjusted mean cost‡ (Autologous) |
|---|---|---|---|---|
| Failures | ||||
| No | Ref. | 14,284 | Ref. | 29,199 |
| 1 | 10,037 (6,792, 13,782) | 24,321 | 19,144 (1,932, 45,871) | 48,344 |
| ≥2 | 18,313 (12,837, 24,895) | 32,598 | –§ | –§ |
| Bilateral | ||||
| No | Ref. | 21,347 | Ref. | 30,086 |
| Yes | 6,074 (2,892, 9,673) | 27,421 | 4,131 (−4,184, 15,116) | 34,217 |
| Complications | ||||
| No | Ref. | 15,973 | Ref. | 25004 |
| Yes | 12,276 (9,126, 15,820) | 28,249 | 11,705 (4,981, 19,937) | 36,709 |
| ED visits | ||||
| No | Ref. | 20,203 | Ref. | 29,590 |
| Yes | 7,351 (3,523, 11,796) | 27,554 | 2,544 (−5,448, 13,182) | 32,134 |
| Readmissions | ||||
| No | Ref. | 12,886 | Ref. | 26,887 |
| Yes | 20,428 (16,315, 25,120) | 33,314 | 13,667 (4,301, 25.845) | 40,554 |
Select variables are presented in this table. Full tables showing all independent variables can be found in Appendix 4.
Costs in US dollars
Secondary failures were negligible and hence resulted in unreliable point estimates
Table 3.
Adjusted estimation of the cumulative cost of reconstruction for implant patients who required a subsequent autologous flap
| Variables | Mean difference in cost (CI)‡ | Adjusted mean cost‡ |
|---|---|---|
| Whether had any additional flap | ||
| No | Ref. | 23,090 |
| Yes | 24,123 (17,384, 31,984) | 47,214 |
| Bilateral | ||
| No | Ref. | 39,219 |
| Yes | 12,626 (6,433, 19,658) | 51,845 |
| Complications | ||
| No | Ref. | 32,186 |
| Yes | 20,762 (14,930, 27,317) | 52949 |
| ED visits | ||
| No | Ref. | 37,469 |
| Yes | 8,687 (2,226, 16,200) | 46,157 |
| Readmissions | ||
| No | Ref. | 17,156 |
| Yes | 25,685 (19,737, 32,591) | 42,841 |
Select variables are presented in this table. Full tables showing all independent variables can be found in Appendix 4.
Costs in US dollars
RESULTS
There were 2,964 patients who met the inclusion criteria for the study. Seventy-eight percent (2,306) had implant reconstruction and 22% (658) had autologous reconstruction. Table 1 shows the social, demographic and clinical characteristics of patients by reconstruction type (implant/autologous). Twenty percent of implant patients had 1 reconstruction failure and 12% had ≥ 2 failures; these categories for autologous patients were 4% and 1% respectively. Finally, 37.5% of implant failures (12% of all implant patients) had subsequent reconstruction by autologous method.
Figure 1 shows that in the autologous reconstruction group, the index reconstructive procedure contributed most (approximately 65%) to the cumulative cost of reconstruction and failures contributed < 5% to the cumulative cost. In the implant reconstruction group, readmissions contributed the most (approximately 25%) to the cumulative cost and failures contributed > 20% to the cumulative cost. (Figure 1)
Tables 2 and 3 show the results of the linear regression models described in the methods section and the adjusted predicted mean cumulative costs associated with key independent variables (Linear regression tables 2 and 3 with full a complement of independent variables are presented in Appendix 4, see Supplemental Digital content 4, insert link). The unadjusted mean cumulative cost regardless of failure for implant reconstruction was $22,868. For autologous patients, the unadjusted mean cumulative cost regardless of failure was $30,527. For implant reconstruction patients with no failures (68% of all implant patients), the adjusted mean cumulative cost was $14,284. For autologous reconstruction patients with no failures (95% of all autologous patients), the adjusted mean cumulative cost was $29,199. When we examined implant reconstruction patients with failures in a stratified analysis (32% of all implant patients), patients who did not have subsequent autologous reconstruction (20% of all implant patients) had an adjusted mean cumulative cost of $23,090 and for those who had subsequent autologous reconstruction (12% of all implant patients) the adjusted mean cumulative cost was $47,214. Lastly, the adjusted mean cumulative cost was $48,344 for autologous patients who had failures with subsequent reconstruction (5% of all autologous patients).
DISCUSSION
In this study we examined a national sample of breast cancer patients who received radiation as part of their treatment, and reconstruction with implant or autologous methods. We studied their breast reconstruction course longitudinally tracking use of healthcare resources reflected in reimbursements made by payers and patient out-of-pocket payments.
First, the results of the study agree with existing literature that implant reconstruction is more common in radiated patients.3–5 Seventy-eight percent of patients who met inclusion criteria underwent implant reconstruction as the index attempt at breast reconstruction. Secondly, using this nationwide longitudinal data, the results also demonstrate a failure rate of implant reconstruction in radiated patients within the ranges reported in previous studies.13, 17–18 On average, implant based breast reconstructions were cheaper than autologous breast reconstructions (unadjusted mean cumulative cost, $22,868 vs $30,527). However, the study results demonstrate that aggregately, among radiated patients who undergo implant reconstruction, reconstruction failures contribute over 4 times as much to the cumulative cost of reconstruction (> 20%) compared to the cost of failures among patients having autologous reconstruction (< 5%). (Figure 1) Finally, our results show that the majority of patients in each group: implant (68%) and autologous (95%) had reconstruction without failure. Costs for implant and autologous reconstruction in patients without failures were $14,284 and $29,199 respectively. Implant failure that requires autologous reconstruction cumulatively costs $47,214/patient and autologous failure cumulatively costs $48,344/patient. Considering that 12% of implant patients compared to 5% of autologous patients required subsequent autologous reconstruction after failure, the total costs of failures extrapolated to the population were $13 million and $2 million respectively.
Even with the substantial evidence, including results from this study, demonstrating superior success rates with autologous reconstruction in radiated patients8–10, one must acknowledge that not all radiated patients are candidates for autologous breast reconstruction. Arguably then, the debate about appropriate choices in radiated breast reconstruction should be centered on patients who are candidates for all breast reconstruction options including implant and autologous methods. Generally, studies on reconstruction of the radiated breast do not address the reasons or indications for implant reconstruction in the cohorts studied and this study is no exception to that trend. However, a few studies in existing literature and the results from this study highlight that at least roughly 1 in 10 patients who undergo implant reconstruction and had a failure had a subsequent reconstruction with an autologous component.9, 18 This finding indicates that autologous reconstruction was a possible initial option for these patients. According to the results from this study, one can argue that these patients potentially incurred excess reconstruction cost. The mean adjusted cost for 95% of the patients who underwent successful autologous reconstruction on index attempt was $29,199 compared to the $47,214 for the 12% of implant reconstruction patients who had failures and subsequent reconstruction with an autologous component. The potential excess reconstruction cost ($18,015/patient for a total of $13 million) stems from the possibility that most of these patients could have undergone successful autologous reconstruction on index attempt.
One of the most heralded components of the recent healthcare reform agenda is cost containment through payment reforms with much emphasis on alternative payment models (APM) such as bundled payments i.e. global payments for episodes of care.19 Typically with APM arrangements, providers (hospitals/physicians) share in the financial risks for costs that overrun the allotted payment for episodes of care.19 Clearly, healthcare resource use and their associated costs can only make up part of the equation in the choice regarding breast reconstruction among radiated patients who have both implant and autologous options available to them. There is informed patient preference i.e. radiated patients aware of the increased risk of morbidity and failure with implant reconstruction that choose to undergo implant reconstruction for individual reasons. Moreover, other factors such as the growing concentration of surgeons with expertise in autologous reconstruction in fewer centers may also contribute to limiting the options some radiated patients have.16 Lastly, surgeon preferences play a considerable role. Studies have objectively shown a reimbursement to effort ratio that favors implant reconstruction compared to autologous reconstruction and, plastic surgeons admitting a preference for implant reconstruction because of this favorable reimbursement/effort ratio.20, 21 However, some findings from this study, such as the aggregately higher contribution of failures (> 20%) to the mean cumulative cost of implant reconstruction in radiated patients and the potential excess cost of implant failures in radiated patients that subsequently undergo autologous reconstruction, are typical of factors with which providers will increasingly have to grapple. These factors will increasingly figure in decisions about how best to deploy healthcare resources to achieve successful outcomes for patients in a dispensation where cost containment features prominently in healthcare delivery.
There were some limitations in our methods. The components of healthcare resource use for breast reconstruction that we employed in estimating cumulative costs in this study are not standardized. Moreover there are no standard methods, to our knowledge, for estimating healthcare resource use and the associated costs for breast reconstruction. However, from our clinical knowledge of the course of breast reconstruction and with methods from previous related studies, we created an exhaustive list of potential uses of healthcare resources in the course of breast reconstruction (See Supplemental Digital Content 2, Appendix 2, insert link and Supplemental Digital Content 3, Appendix 3, insert link) to provide reasonable estimates of cumulative cost.14– 16 Additionally, as is commonly the case with secondary data from claims and administrative databases, we do not have variables that definitively describe patient or surgeon factors that influence decision-making. However, this study was less about the appropriateness of the method used for reconstruction of the radiated breast and more an attempt to highlight what the relative resource use was for each method in order to add to current knowledge on the subject. Finally, it was not possible to determine a range of failure rates and costs based on individual providers as providers are not uniquely designated in the database. We reported national average failure rates meaning that some providers will have higher and others lower failure rates that would be reflected in costs accordingly. However, the average failure rates we reported are well within the range reported in existing literature.13
CONCLUSION
Our results demonstrate that implant reconstruction is much more widely used in radiated breast cancer patients than autologous methods. Implant reconstructions are on average cheaper than autologous reconstructions. Among the radiated patients studied however, implant reconstruction had significantly higher rates of failures compared to autologous methods with more than 1 in 10 patients who had implant reconstruction requiring a flap for failure. Although failures resulted in substantially more healthcare resource use in both autologous and implant based reconstruction, the added cost incurred was six times more among the patients with implant based reconstruction compared to patients with autologous reconstruction, a reflection of the significant difference in the respective failure rates. These findings make a case for autologous reconstruction being primarily considered in radiated patients who have this option available.
Supplementary Material
Supplemental Digital Content 1, See Appendix 1 which shows the Diagnoses and procedure codes for breast cancer, breast reconstruction and radiation therapy, insert link.
Supplemental Digital Content 2, See Appendix 2 which shows the Codes for secondary procedures, insert link.
Supplemental Digital Content 3, See Appendix 3 which shows the Diagnosis and procedural codes (ICD-9/CPT) for complications and reconstruction failure, insert link.
Supplemental Digital Content 4, See Appendix 4 which shows the Linear regression tables (2 and 3) with full complement of independent variables, insert link.
Acknowledgments
Support for this study was provided in part by grants from the Michigan Institute for Clinical and Health Research (to Adeyiza O Momoh and Erika D Sears), and a Mid-career Investigator Award in Patient-Oriented Research (2K24 AR053120-06) (to Kevin C Chung) Dr. Jennifer F Waljee receives research funding from the Agency for Healthcare Research and Quality (K08 1K08HS023313-01), the American College of Surgeons, and the American Foundation for Surgery of the Hand.
Footnotes
Financial interest: No authors have financial interests to declare.
References
- 1.Yao K, Liederbach E, Lutfi W, et al. Increased utilization of postmastectomy radiotherapy in the United States from 2003 to 2011 in patients with one to three tumor positive nodes. J Surg Oncol. 2015;112(8):809–814. doi: 10.1002/jso.24071. [DOI] [PubMed] [Google Scholar]
- 2.Frasier LL, Holden S, Holden T, et al. Temporal Trends in Post-Mastectomy Radiation Therapy and Breast Reconstruction. JAMA Oncol. 2016;2(1):95–101. doi: 10.1001/jamaoncol.2015.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in the use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32(9):919–926. doi: 10.1200/JCO.2013.52.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131(1):15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal S, Kidwell KM, Farberg A, et al. Immediate reconstruction of the radiated breast: recent trends contrary to traditional standards. Ann Surg Oncol. 2015;22(8):2551–2559. doi: 10.1245/s10434-014-4326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam TC, Hsieh F, Boyages J. The effects of postmastectomy adjuvant radiotherapy on immediate two-stage prosthetic breast reconstruction: a systematic review. Plast Reconstr Surg. 2013;132(3):511–518. doi: 10.1097/PRS.0b013e31829acc41. [DOI] [PubMed] [Google Scholar]
- 7.Codeiro PG, Albornoz CR, McCormick B, et al. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg. 2014;134(4):588–595. doi: 10.1097/PRS.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 8.Jhaveri JD, Rush SC, Kostroff K, et al. Clinical outcomes of postmastectomy radiation therapy after immediate breast reconstruction. Int J Radiat Oncol Biol Phys. 2008;72(3):859–865. doi: 10.1016/j.ijrobp.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 9.Berry T, Brooks S, Sydow N, et al. Complication rates of radiation on tissue expander and autologous tissue breast reconstruction. Ann Surg Oncol. 2010;17(Suppl 3):202–210. doi: 10.1245/s10434-010-1261-3. [DOI] [PubMed] [Google Scholar]
- 10.Wong JS, HO AY, Kaelin CM, et al. Incidenc of major corrective surgery after postmastectomy breast reconstruction and radiation therapy. Breast J. 2008;14(1):49–54. doi: 10.1111/j.1524-4741.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy CM, Pusic AL, Disa JJ, et al. Unilateral postoperative chest wall radiotherapy in bilateral tissue expander/implant reconstruction patients: a prospective outcomes analysis. Plast Reconstr Surg. 2005;116(6):1642–1647. doi: 10.1097/01.prs.0000187794.79464.23. [DOI] [PubMed] [Google Scholar]
- 12.Ho A, Codeiro PG, Disa JJ, et al. Long-term outcomes in breast cancer patients undergoing immediate 2-stage expander/implant reconstruction and postmastectomy radiation. Cancer. 2011;118(9):2552–2559. doi: 10.1002/cncr.26521. [DOI] [PubMed] [Google Scholar]
- 13.Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol. 2014;21(1):118–124. doi: 10.1245/s10434-013-3284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isreali R, Funk S, Reaven NL. Comparative analysis of 18-month outcomes and costs of breast reconstruction flap procedures. Plast Reconstr Surg. 2014;133(3):471–479. doi: 10.1097/PRS.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 15.Lad SP, Babu R, Baker AA, et al. Complications, reoperation rates and health-care cost following surgical treatment of lumbar spondylolisthesis. J Bone Joint Surg Am. 2013;95(21):e162 1–10. doi: 10.2106/JBJS.L.00730. [DOI] [PubMed] [Google Scholar]
- 16.Albornoz CR, Cordeiro PG, Mehrara BJ, et al. Economic implications of recent trends in the U.S. immediate autologous breast reconstruction. Plast Reconstr Surg. 2014;133(3):463–470. doi: 10.1097/PRS.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 17.Spear SL, Baker JL. Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg. 1995;96(5):1119–1123. [PubMed] [Google Scholar]
- 18.Hirsch EM, Seth AK, Dumanian GA, et al. Outcomes of tissue expander/implant breast reconstruction in the setting of prereconstruction radiation. Plast Reconstr Surg. 2012;129(2):354–361. doi: 10.1097/PRS.0b013e31823ae8b1. [DOI] [PubMed] [Google Scholar]
- 19.Press MJ, Rajkumar R, Conway PH. Medicare’s new bundled payments: design, strategy, and evolution. JAMA. 2016;315(2):131–132. doi: 10.1001/jama.2015.18161. [DOI] [PubMed] [Google Scholar]
- 20.Sando IC, Chung KC, Kidwell KM, et al. Comprehensive breast reconstruction in an academic surgical practice: an evaluation of financial impact. Plast Reconstr Surg. 2014;134(6):1131–1139. doi: 10.1097/PRS.0000000000000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulkarni AR, Sears ED, Atisha DM, et al. Use of autologous and microsurgical breast reconstruction by U.S. plastic surgeons. Plast Reconstr Surg. 2013;132(3):534–541. doi: 10.1097/PRS.0b013e31829ae03e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1, See Appendix 1 which shows the Diagnoses and procedure codes for breast cancer, breast reconstruction and radiation therapy, insert link.
Supplemental Digital Content 2, See Appendix 2 which shows the Codes for secondary procedures, insert link.
Supplemental Digital Content 3, See Appendix 3 which shows the Diagnosis and procedural codes (ICD-9/CPT) for complications and reconstruction failure, insert link.
Supplemental Digital Content 4, See Appendix 4 which shows the Linear regression tables (2 and 3) with full complement of independent variables, insert link.


