Abstract
Dichlorodiphenyldichloroethane (DDE) and polychlorinated biphenyls (PCBs) are widespread environmental contaminants that have been postulated to increase the risk of diseases such as non-Hodgkin's lymphoma, breast cancer, as well as lead to early menopause. Studies assessing the effect of organochlorine exposure often can only measure organochlorine levels once, such as at study enrollment which may not be an etiologically relevant time period. We assessed the temporal changes in DDE and PCBs and the predictors of those changes using interview data and DDE/PCB measures collected from 123 women who were enrolled in a baseline study from 1978-1982 and followed up in 2003-2004. Baseline and follow-up organochlorine levels were compared using Spearman correlations (rs) and predictors of the rate of change in log concentration were evaluated using linear regression models. While serum concentrations dramatically declined (median follow-up to baseline concentration ratio was 16% for DDE and 45% for PCBs), baseline and follow-up measures were strongly correlated for DDE (rs=0.72) and moderately correlated for PCBs (rs=0.43). Prediction of follow-up PCB levels was substantially improved (rs=0.75) with data on initial concentration, length of lactation, baseline body mass index and percent change in body fat, while DDE prediction improved slightly (rs=0.83) with data on lactation and baseline body mass index. These findings suggest that a single organochlorine measure provides considerable information on relative ranking at distant times and that the predictive power can be improved, particularly for PCBs, with information on a few predictors.
Keywords: DDE, PCBs, organochlorine, predictors, correlation, cancer
Introduction
Extensive use of the pesticide, dichlorodiphenyltrichloroethane (DDT), beginning in the 1940s and the industrial chemical, polychlorinated biphenyls (PCBs) beginning in 1929, have led to their widespread distribution in the environment. Despite their ban since the 1970s, PCBs and the DDT metabolite, dichlorodiphenyldichloroethane (DDE), remain ubiquitous, raising concerns over their environmental and public health impact. A number of studies have evaluated associations of these compounds with human health effects, including non-Hodgkin's lymphoma (1), breast cancer (2, 3) and earlier menopause (4, 5), although clear associations have not been established.
Studies assessing the effect of these organochlorines on risk of late onset diseases or diseases with long latency periods often can only measure levels once; for example, they may be measured at enrollment into a prospective study of disease incidence. While a single measure reflects exposure accumulation and excretion of a compound up to the time of sampling, it may not provide an accurate picture of the exposure level at a time of etiologic relevance, which may be years or decades apart from the time of sampling (6, 7).
The objective of this paper is to examine the ability to predict changes in organochlorine levels from baseline forward in time over a period of decades. Few studies to date have assessed long-term intra-individual changes in DDE or PCB concentrations and the predictors of those changes. These longitudinal studies (8-13) span approximately 2 to 10 years in duration, and most have limited covariate data. Our study uses information collected from women who had enrolled in a baseline study of organochlorines (from 1978-1982) and were followed for approximately 25 years.
4.3 Methods
Study population
From 1978-1982, pregnant women (n=877) from North Carolina were recruited into the baseline study of organochlorines and child development (14). Around the time of delivery, a questionnaire including information on demographics, pre-pregnancy weight, and reproductive and medical histories was administered; maternal and cord bloods, placenta and breast milk were also collected for organochlorine determination. Additional samples were collected at subsequent study visits, including blood at the 6-week visit and milk periodically for as long as the child was breast-fed.
In 2003-2004, women were traced and invited into a follow-up study on menopause. Of 726 traceable women, 513 (70.7%) participated. Additional data including reproductive and lactation history (covering all pregnancies), height and current weight were obtained via a telephone-administered questionnaire.
A sample of 126 individuals, constituting 82% of a 30% random sample of follow-up participants who were selected for a blood draw, had DDE/PCBs assessed in the serum samples. Of these, 123 had a serum organochlorine measure at delivery from the baseline study and will be the subjects for the current study. One individual had missing pregnancy data on the follow-up questionnaire and was excluded from our predictive modeling. Informed consent and Institutional Review Board approval were obtained for both baseline and follow-up studies.
Laboratory Assays
At baseline, p,p’-DDE and PCBs were assayed using packed column-gas chromatography with electron capture detection. PCBs were quantified as the sum of two peaks on a chromatogram (15). The coefficients of variation were <12% (15). Due to the highly variable fat content in breast milk, milk concentrations were expressed as grams of organochlorine per gram of lipid, while concentrations in placenta were reported per gram of tissue and concentrations in blood were reported as μ/L.
At follow-up, organochlorines were assayed using gas chromatography coupled with mass spectrometry (GC-MS); coefficients of variation were 5.1% for DDE and 3.5%-11.4% for the various PCB congeners. Fourteen congeners were quantitated; for this study, we focused on the total of 8 congeners (PCB# 99, 118, 138, 153, 156, 170, 180, and 187) having the lowest proportion of non-detects (<18%, compared to 52-99% for the remaining 6 congeners: PCB-28, 52, 101, 105, 128, and 183) and the highest relative abundance (together comprising ~94% of all PCBs measured). Total lipids were also estimated from total cholesterol, phospholipids and triglycerides using the Phillips et al. approach (16), thereby allowing concentrations to be expressed per gram of lipid. A validation study shows that this approach gives values very similar to those obtained using the standard gravimetric lipid analysis (17).
Accounting for change in PCB quantitation methods
Though the assays have changed since the baseline study, DDE quantitation (which involves measurement of a single peak) was the same. However, PCB quantitation methods used at baseline differed from those used at follow-up and do not produce directly comparable results. To convert baseline PCB concentrations into their follow-up equivalents, we used data from 10 baseline milk samples reanalyzed by GC-MS (18). Baseline levels were multiplied by the median ratio (0.236127) of concentration using the follow-up method to concentration using the baseline method.
Handling non-detects
One individual at baseline had non-detectable serum DDE and 18 (14.6%) had non-detectable serum PCBs. Serum organochlorine concentrations below the limit of detection (LOD) at baseline were assigned estimated values using measurements from all available specimens at baseline. The concentrations from each specimen were previously scaled to reflect the concentration in milk lipids at birth of the index child and then averaged to create a composite measure for each woman (14). For this analysis, these composite measures were converted into their approximate serum equivalents by multiplying by the median ratio of serum to composite concentrations (5.0071367 for DDE and 5.4120890 for PCBs) obtained from members of the baseline population with detectable serum values. To evaluate the validity of this imputation, we compared estimated and actual levels among those with detectable concentrations in serum at delivery: mean % difference and standard deviation were 6.1 ± 36.9 for DDE and 3.8 ± 24.3 for PCBs. Pearson and Spearman correlations were high between estimated and observed values for both DDE (0.90 and 0.91, respectively) and PCBs (0.91 and 0.84). At follow-up, one individual had non-detectable serum DDE, whereas 28 (22.8%) had at least one non-detectable PCB congener. Non-detects at follow-up were assigned LOD divided by the square root of 2 (19). Since the non-detectable congeners were usually the minor ones, there were only six individuals in which the imputation method (using extremes of LOD or 0) made a >10% difference in their estimated total PCBs.
Accounting for pregnancy-related increases at baseline
As serum lipids were not available at baseline, our primary goal was to explore changes in wet weight serum organochlorine measures over time. Measures at baseline were acquired at or around the time of delivery; however, short-term changes due to pregnancy are not the changes of interest. Given the characteristic rise in lipid content across pregnancy (20, 21) and the resulting elevation in organochlorines, wet weight serum measures were adjusted to reflect estimated non-pregnant levels. Based on mean serum total lipids of 5.9 g/L among more than 2000 non-pregnant Caucasian women, aged 20-34, participating in the 1976-80 National Health and Nutrition Examination Survey (22, 23) and 8.5 g/L in a 1977-79 study of 553 pregnant Caucasian women, ages 20-41, at 36 weeks of gestation (21), we estimated a 30% increase in total lipids across pregnancy. To account for this rise, wet weight serum concentrations at baseline were reduced by an equivalent amount.
Secondary analysis: Lipid-adjusted serum measures
Though our primary analysis was based on wet weight organochlorines, we conducted a secondary analysis on estimated lipid-adjusted measures. Lipid-adjusted serum concentrations at baseline were estimated by dividing the aforementioned “composite” milk values by a correction factor (1.5) that accounts for the difference in lipid concentrations between milk and serum. This factor is the approximate ratio of concentrations in milk lipids to those in serum lipids and was used for both DDE (24, 25) and PCBs (24, 26).
Predictive modeling
Although changes in serum DDE and PCB levels over time are driven by complex processes, we posit that the time course of such changes during the period under study can be reasonably approximated by an exponential decay. Thus, our basic predictive model was:
which can be re-written as
where [Follow-up] and [Baseline] are the concentrations, respectively, at follow-up and baseline, and “follow-up time” represents years from baseline to follow-up. Slope represents change in log concentrations per unit of follow-up time. The slope can be affected by various determinants, so we modeled it using linear regression; the full model is then of the form:
where α is the intercept and βk is the regression coefficient for the k-th predictor, Xk.
Lactational transfer from mother to infant is an important route of organochlorine excretion (14, 27). In this study, lactation duration, from the follow-up questionnaire, is defined as the number of weeks she breast-fed twice or more per day. Given the diminishing role of exclusive breast-feeding as lactation proceeds, the effect of breast-feeding on the rate of organochlorine excretion should lessen later in lactation. Thus, lactation duration was treated as a continuous variable and modeled in a piecewise linear fashion with the points where the slope changes prespecified at 26 and 39 weeks of lactation. This was accomplished by defining 3 variables representing the number of weeks of lactation during the 3 intervals: 0-26th, 27th-39th, and >39th week of lactation. The number of weeks of lactation for each pregnancy from baseline to follow-up was partitioned among these three variables. For women with multiple lactations between baseline and follow-up, the contribution from each pregnancy was summed. To illustrate, for a woman with one child whose lactation lasted 60 weeks, a value of 26, 13 and 21 weeks was assigned to the first, second and third lactation variables, respectively. If she also had a second child who was breastfed for 30 weeks, then an additional 26 weeks was assigned to the first lactation variable and 4 weeks to the second, giving final values of 52, 17, and 21 for the three lactation variables. To operationalize, the 3 pieces contribute to the overall slope of organochlorine decline based on their regression coefficients: slope contribution= β1(piece 1) + β2(piece2) + β3(piece3).
Previous studies on PCBs and dioxins show that high initial concentration was associated with faster decline over time (28, 29). We included the log of initial (baseline) concentration of serum DDE or PCBs as a linear variable in the model.
Body mass index (BMI) may be associated with an individual's ability to metabolize organochlorines and other xenobiotics, with heavier individuals having slower metabolism (30). Baseline BMI was included in the model as a categorical predictor, with cutpoints at 20 and 23 kg/m2, due to its narrow distribution.
Since fat is where organochlorines are sequestered, an increase in body fat without an increase in organochlorines might dilute organochlorine concentrations. Thus, we included a measure of percent change in body fat (kg) based on a validated formula for percent body fat, which we then converted to body fat by multiplying by body weight (31):
Usual weight prior to baseline study pregnancy and current weight were obtained, respectively, from the baseline and follow-up questionnaires. Percentage change in body fat, defined as the difference in body fat from baseline to follow-up divided by baseline body fat, was included as a linear variable.
Mother's date of birth reflects the secular trend in environmental DDE/PCB levels, and hence, the exposure potential. We included calendar date of birth (measured in days with a SAS reference date of January 1, 1960) as a linear variable in the model. Another possible predictor is baseline maternal age; organochlorine metabolism may change with age. However, since age at baseline is highly correlated with date of birth (r=0.97), it was not included.
Statistical analysis
Linear regression models were fit using SAS (version 9.1). Each predictor was examined to obtain the fewest categories needed to adequately characterize the relationship between that variable and the slope. The predictors were added to the model in successive order of their assumed strength of effect. The statistical significance of each potential predictor was assessed using the F test. Spearman correlations comparing actual versus model-predicted concentrations at follow-up were used to assess the predictiveness of each model. To illustrate the model fits, model-predicted changes in organochlorine levels were plotted along with actual changes for the 122 individuals with non-missing questionnaire data.
4.5 Results
The mean age of our study sample at baseline was 29 years. Other descriptive information is shown in Table 1. Among the 122 women with pregnancy data, there were 207 births during the study period (i.e. from baseline to follow-up, including baseline pregnancy) and 39% of these are the only birth during the study period. Ninety percent of the babies were breast-fed, and 52% were breast-fed for more than 26 weeks. Approximately 90% of women had a pre-pregnancy body mass index of less than 25 kg/m2 at baseline, and all but two had increased (65% with at least a 50% increase) their body fat mass by the time of follow-up.
Table 1.
Characteristics of study population, n=123a
| Variable | N | (%) |
|---|---|---|
| Age at baseline (years) | ||
| 19 – 24 | 24 | (19) |
| 25 – 29 | 54 | (44) |
| 30 – 34 | 39 | (32) |
| 35 – 37 | 6 | (5) |
| No. of births (baseline to follow-up) | ||
| 1 | 48 | (39) |
| 2 | 56 | (46) |
| 3 | 17 | (14) |
| 6 | 1 | (1) |
| No. of babies breast-fed (baseline to follow-up)b | ||
| 0 | 12 | (10) |
| 1 | 45 | (37) |
| 2 | 48 | (39) |
| 3 | 16 | (13) |
| 6 | 1 | (1) |
| No. weeks of lactation for each birth (baseline to follow-up)b | ||
| 0 – 13 | 49 | (24) |
| 14 – 26 | 50 | (24) |
| 27 – 39 | 31 | (15) |
| 40 – 52 | 54 | (26) |
| 53 – 165 | 23 | (11) |
| Pre-pregnancy BMI (kg/m2) | ||
| 16.6 – 19.9 | 47 | (38) |
| 20 – 24.9 | 65 | (53) |
| 25 – 29.9 | 9 | (7) |
| 30 – 32.9 | 2 | (2) |
| % change in body fat | ||
| -6 – 0 | 2 | (2) |
| 1 – 24 | 13 | (11) |
| 25 – 49 | 27 | (22) |
| 50 – 99 | 49 | (40) |
| 100 – 149 | 18 | (14) |
| 150 – 294 | 14 | (11) |
One individual had missing pregnancy and lactation data
Among the 207 births delivered to 122 mothers from baseline to follow-up
At baseline, the median wet weight concentrations were 8.5 ug/L for DDE and 1.5 ug/L for PCBs (Table 2). At follow-up, medians fell to 1.2 ug/L and 0.7 ug/L, respectively; expressing each follow-up concentration as a percent of her baseline, medians were 16% for DDE and 45% for PCBs. There was a dramatic decline in DDE levels, with 90% of women having follow-up concentrations ≤30% of baseline, whereas the variability in individual changes was greater for PCBs. Changes over time in lipid-adjusted measures were of similar magnitude.
Table 2.
DDE and PCBa concentrations in serum, n=123
| Organochlorine | Percentile |
||||||
|---|---|---|---|---|---|---|---|
| Min | 10 | 25 | 50 | 75 | 90 | Max | |
| Wet weight measures | |||||||
| DDE (ug/L serum) | |||||||
| Baseline | 1.3 | 3.8 | 5.8 | 8.5 | 12.5 | 19.8 | 41.0 |
| Follow-up | 0.01 | 0.5 | 0.7 | 1.2 | 2.3 | 3.9 | 11.0 |
| Relative changeb | 0.002 | 0.07 | 0.10 | 0.16 | 0.23 | 0.29 | 0.62 |
| PCBs (ug/L serum) | |||||||
| Baseline | 0.6 | 0.9 | 1.2 | 1.5 | 2.1 | 2.7 | 5.2 |
| Follow-up | 0.2 | 0.4 | 0.5 | 0.7 | 1.0 | 1.2 | 2.2 |
| Relative changeb | 0.09 | 0.25 | 0.33 | 0.45 | 0.65 | 0.80 | 1.43 |
| Lipid-adjusted measures | |||||||
| DDE (ug/g lipid) | |||||||
| Baseline | 0.3 | 0.7 | 1.8 | 1.7 | 2.5 | 3.7 | 7.4 |
| Follow-up | 0.002 | 0.1 | 0.1 | 0.2 | 0.4 | 0.5 | 1.9 |
| Relative changeb | 0.002 | 0.06 | 0.08 | 0.12 | 0.18 | 0.22 | 0.56 |
| PCBs (ug/g lipid) | |||||||
| Baseline | 0.1 | 0.2 | 0.2 | 0.3 | 0.4 | 0.5 | 0.7 |
| Follow-up | 0.03 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.3 |
| Relative changeb | 0.08 | 0.21 | 0.29 | 0.37 | 0.51 | 0.59 | 0.96 |
PCB concentration comprises PCB-99, 118, 138, 153, 156, 170, 180, and 187
Relative change = (follow-up concentration / baseline concentration) among all 123 participants
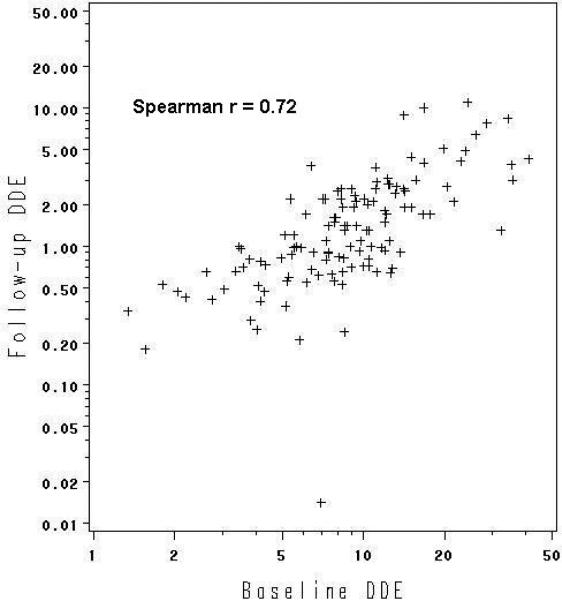
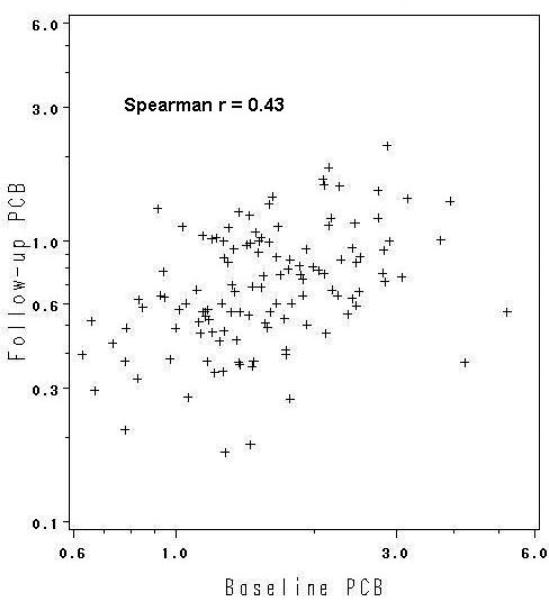
The correlations between the two wet weight measures taken more than 20 years apart were relatively strong (Figures 1a and 1b). DDE at baseline was highly correlated with DDE at follow-up (Spearman rs = 0.72), whereas the correlation for PCBs was lower, but still sizeable (rs= 0.43). Correlations were similar for lipid-adjusted measures (0.73 and 0.50, respectively).
Figure 1a.
Correlation of baseline with follow-up DDE (ug/L serum), n=123
Figure 1b.
Correlation of baseline with follow-up PCBs (ug/L serum), n=123
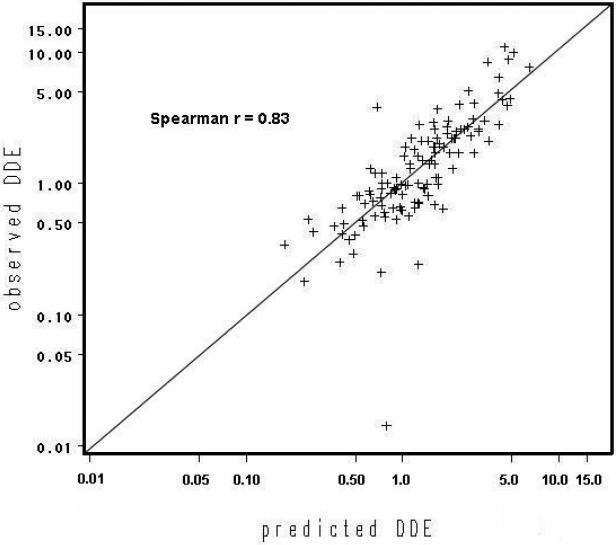
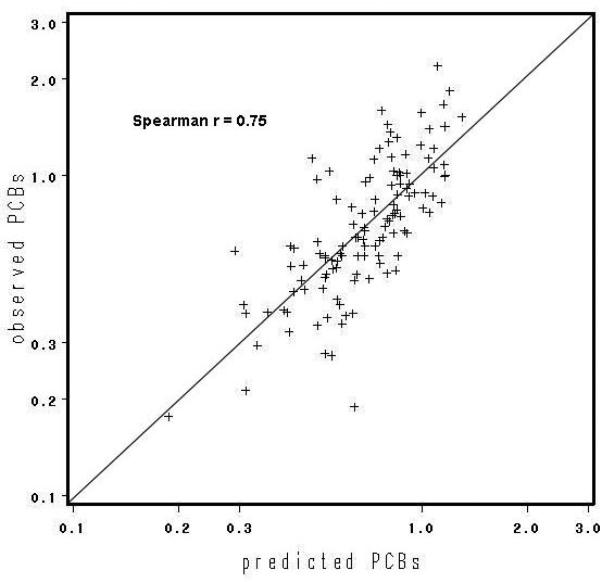
Higher initial concentration, lactation before 39 weeks, and an increase fat mass were associated with an increase in the rate of elimination of wet weight DDE and PCBs, whereas higher baseline BMI tended to decrease the rate of decay (Table 3). Similar results were found for lipid-adjusted values (not shown). Mother's date of birth is not presented, as its effect was not significant for either DDE or PCBs. Table 4 shows the statistical significance of successive additional terms in the model for the slope and the correlation of the resulting predicted follow-up concentration with observed concentration. Lactation duration and baseline BMI were the only statistically significant predictors of DDE slope, whereas for PCBs, initial concentration and percent change in body fat were also significant. Predicted follow-up concentrations from the full model were highly correlated with actual follow-up concentrations for both DDE (rs=0.83) and PCBs (rs=0.75) (Table 4, Figures 2a and 2b).
Table 3.
Full model coefficients and standard error for slope of DDE and PCB decline*, n=122
| Predictors | Regression coefficient (S.E.) |
|||
|---|---|---|---|---|
| DDE | PCB | |||
| Intercept | -0.061983 | (0.0100) | -0.012182 | (0.0036) |
| Log of initial concentration (ug/L) | -0.004270 | (0.0036) | -0.016655 | (0.0031) |
| Total weeks of breastfeeding in each lactation period | ||||
| 0-26th | -0.000202 | (0.0001) | -0.000201 | (0.0001) |
| 27th-39th | -0.000295 | (0.0003) | -0.000427 | (0.0001) |
| >39th | 0.000052 | (0.0001) | -0.000086 | (0.0001) |
| Baseline BMI (kg/m2) | ||||
| 16.6-19.9 | referent | referent | referent | referent |
| 20-22.9 | 0.005467 | (0.0053) | 0.002533 | (0.0029) |
| ≥23 | 0.031238 | (0.0063) | 0.011727 | (0.0036) |
| Percent change in body fat | -0.008504 | (0.0046) | -0.006323 | (0.0025) |
Table 4.
Description of successive models for slope, n=122
| Statistical significance (p-value) of successive terms in model for slope |
Spearman correlation for observed and predicted follow-up concentration |
||||
|---|---|---|---|---|---|
| Modela | Predictors | DDE | PCB | DDE | PCB |
| I | Intercept | n/a | n/a | 0.72 | 0.43 |
| II | Log of initial concentration (ug/L) | 0.75 | <0.01 | 0.73 | 0.46 |
| III | Weeks of lactation | 0.05 | <0.01 | 0.75 | 0.67 |
| IV | Baseline BMI | <0.01 | 0.01 | 0.82 | 0.72 |
| V | Percent change in body fat | 0.07 | 0.01 | 0.83 | 0.75 |
Each model includes all variables from the preceding model
Figure 2a.
Plot of predicted versus observed follow-up DDE (ug/L), n=122
Figure 2b.
Plot of predicted versus observed follow-up PCB (ug/L), n=122
Supplemental Figures 1a and 1b illustrate the predicted changes in DDE and PCB levels, and the effect of lactation and baseline BMI. Overall, there is a rapid decline in DDE and shallower reduction in PCB. The effect of the heaviest versus lightest category of baseline BMI was considerable for both DDE and PCBs. The drop in concentrations as a result of breast-feeding one child for one year versus no breast-feeding was evident for both compounds, although weaker for DDE. Although not shown, the effect of initial concentration (75th versus 25th percentile) on PCB decline was also notable, but the effect of an increase in fat mass was comparatively small.
4.6 Discussion
We found a substantial decline in intra-individual DDE levels over an approximate 25 year time span (1978-1982 to 2003-2004), with a smaller drop in PCB concentrations. These declines correspond with the reduction in environmental levels since the banning of DDT in 1972 and PCBs in 1977 in the United States (32-35). The larger decline in DDE compared to PCBs is similar to those of previous studies conducted among Swedish men from 1991-2001 (12) and Great Lakes fisheaters from 1982-1989 (9). In shorter studies, with medians 25.4 months (11) and 5 years of follow-up (8), the differences in the decline between DDE and PCBs were less obvious. Peak production and the restrictions on usage occurred later for PCBs than for DDT and may partly explain the smaller decline in PCBs. Differences between regions and sampling time since organochlorine restrictions may also contribute to the differences between studies.
Our baseline levels of DDE were highly correlated with those at follow-up (Spearman r=0.7) whereas correlations were lower for PCBs. Higher correlations for DDE than for PCBs were also found in the Wolff et al. study with a median of 25.4 months of follow-up (0.95 versus 0.83, respectively) (11) and the Hoyer et al. study with a maximum of 7 years follow-up (0.79 versus 0.64, respectively) (8). These differences became less evident when the specific congener PCB-153 was assessed; DDE and PCB-153 correlations were respectively 0.92 and 0.90 in a 1991-2001 study by Hagmar et al. (12) and 0.79 and 0.68 in the Hoyer et al. (8) study. The latter study also showed that correlations varied by PCB congener. Individual congeners could not be evaluated in our study since they were not measured at baseline, and the fact that total PCBs is a mix of congeners with different rates of decomposition may partly explain the lower correlations in PCBs.
A number of previous studies have used occupational cohorts or individuals exposed to very high, acute doses to estimate half-lives of organochlorines, with estimates being approximately 6-10 years for DDE (36, 37) and a few weeks to over 10 years for individual PCB congeners (38). We examined a group of women with protracted, albeit declining, environmental exposures to organochlorines, with attention to the influence of individual factors such as lactation and weight fluctuations on DDE and PCB levels. Based on the predicted curves (Supplemental Figures 1a and 1b), the apparent half-lives we predict during this time period are approximately 10 years for DDE and 25 years for PCBs, although they vary for women with different levels of the predictors. Since exposure is ongoing, although declining, in our study population, half-life estimation is expected to be longer compared to the aforementioned studies of occupational cohorts.
Breast-feeding was important in the elimination of organochlorines in previous studies of approximately 2-years (14) and 4-years of follow-up (39), and our study indicates that the effect of lactation still exists approximately 25 years later. In our study, lactation beyond 9 months had less of an impact on DDE and PCB levels than the earlier months. This change in effect across intervals of lactation is expected, given that breast-feeding is likely to diminish in the later months as solid foods become an important component of diet. The impact of one year of lactation was more dramatic for PCBs than for DDE (Supplemental Figures 1a and 1b).
We found an association between higher initial concentration and faster elimination of PCBs, consistent with studies among those occupationally exposed (29, 40). A study of 701 women environmentally exposed to PCBs (median 31 months follow-up) also reported similar findings; those whose PCBs had decreased were over 3 times more likely to have higher initial concentrations than those who had maintained their levels (10). A possible explanation for this phenomenon that is most likely in our population is higher induction of enzymes at higher doses (28). Although faster elimination of DDT from adipose tissue was associated with higher initial concentration in an experimental study of 3 individuals taking varying high level doses of DDT (41), similar data for DDE is not available.
Baseline BMI had a strong effect on the rate of change of both DDE and PCBs. This is consistent with BMI-dependent variation in DDE levels in previous studies (11, 13), showing a positive association or correlation between a single BMI measure and DDE half life. Sweeney et al. also found a small positive association between baseline BMI and an increase in PCB levels (10), although another study did not (11). It is possible that the effect of higher body mass index on the rate of decline may be associated with reduced metabolism by xenobiotic-metabolizing enzymes, such as cytochrome P450 (CYP). CYPs of the 1A, 2B and 3A family are most likely involved in the metabolism of various PCB congeners (33, 42, 43), and obesity has been associated with decreased activity in CYP 3A4 in several studies (30).
Studies assessing the influence of weight change on organochlorine change have been few, particularly for long term trends (8, 12, 44-46). Increased fat as a result of weight gain may lead to the dilution of organochlorine concentrations, thereby resulting in a negative association between body fat and serum organochlorine levels (47). Our finding of a significant effect of fat mass change for PCBs agrees with other studies (with 5 or more years of follow-up) that use relative change in body mass index (12) or absolute weight change (8) as the predictors of interest.
Mother's date of birth, and consequently age, was not a significant predictor of change for either DDE or PCBs, which mirrors the lack of association found in other studies between age at study entry and change in DDE (12) or PCB levels (10). As postulated by several theoretical models, the exposure patterns over time are a result of complex interactions between cohort-related exposures and breast-feeding patterns as well as age-dependent growth and weight/fat content, dietary intake and composition, and metabolism (47, 48). However, given the small variation in birth year and age in our study population and the fact that everyone was born prior to the ban, we would need a much larger sample size to more fully assess the effect of birth cohort and age.
Although the factors considered in our study help to explain a large portion of the variation in DDE and PCB levels over time, other potentially important covariates such as changes in diet, particularly fish consumption, were not measured. A longitudinal study (35) conducted from 1980-1995 and a serial cross-sectional study from 1973-1993 (49) of Great Lakes fisheaters and “non-eaters” showed fish consumption in the previous year to be predictive of PCB body burden. Likewise, whale blubber consumption in the Faroes Islands have been associated with increased levels of highly chlorinated PCBs (50).
A limitation of this study is that baseline and follow-up samples were analyzed by different labs at different times using different assays. The most important difference, however, is the change in PCB quantitation methods. To obtain comparable PCB values from the old and new techniques, we used conversion factors derived from a set of 10 breast milk samples spanning the range of organochlorine values. Though derived from a small sample, our conversion factor seemed reasonable, given the similarity in values and high Spearman and Pearson correlations (0.83 and 0.96, respectively) between the original and converted values.
We analyzed both estimated wet-weight and lipid-adjusted measures. These measures involved various assumptions, including those regarding methodology, lipid concentration and non-detects. Similar results for both analyses, nevertheless, provide further assurance of our outcome and conclusions.
“Weeks of lactation” were modeled as three periods of lactation duration, and additional cutpoints (at 13 weeks and 52 weeks) did not significantly alter the results. However, “weeks of lactation” alone does not fully capture the extent to which organochlorines are being transferred, as there are no data on the amount of milk expressed per breast-feeding session or the duration of weaning for all relevant pregnancies. Additionally, for women who fed multiple children, we assumed the effect of lactation would not vary across pregnancies; however, it is not possible to evaluate this assumption given the small sample size and the lack of organochlorine measurement at each pregnancy.
While our findings agree with those of previous studies, there are limits to the generalizability. Our study population consists of women who were pregnant at baseline, highly educated and mostly Caucasian (95%). These participants may differ from those not studied in terms of their exposure potential, dietary pattern, BMI, and metabolism. More importantly, our results may not be generalizable to other regions of the world with vastly different exposures, and the findings can be extrapolated beyond the time frame of our study (1978-1982 to 2003-2004) only with caution. Although our assumption of an exponential decay is plausible, the shape of the concentration curve between the two measurements cannot be determined given only two measures. Multiple measures would be required to more fully evaluate the shape. Nevertheless, our data may be useful to those conducting studies on the role of these organochlorines in the development of chronic diseases and have only one DDE/PCB measure, at perhaps, a time period that is not optimal in terms of etiologic relevance.
We have focused on predicting future levels based on past measurements. Others have attempted to predict past exposure from current concentrations (51, 52). While our model coefficients cannot be directly used for backward estimations, our study is useful in identifying a handful of important predictors to consider in such estimation models. A single measure of DDE is highly predictive of a woman's relative exposure over a time span of approximately 25 years, and can be further improved with data on lactation and baseline BMI. A single measure of PCBs is also predictive of future levels. Though initial concentration and percent body fat change contribute to the prediction of PCB, but not DDE values, lactation duration and baseline BMI are the predominant contributors to both DDE and PCBs and information on these predictors can be obtained retrospectively.
This study addressing intra-individual variations and influences on body burden of DDE and PCBs over time in a general population with relatively low level environmental exposure includes measures spanning approximately 25 years apart. Longer term trends and predictions are of interest for public health assessment as well as studies of cancer and other diseases with long latency periods, where the levels of etiologic relevance may be decades apart from the time of measurement. Future studies to replicate or confirm our findings (particular in other periods and populations) are warranted and would benefit from multiple organochlorine measures and additional information on other potential predictors.
Supplementary Material
Acknowledgement / financial support
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Conflict of interest: none declared
References
- 1.Engel LS, Lan Q, Rothman N. Polychlorinated biphenyls and non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16(3):373–6. doi: 10.1158/1055-9965.EPI-07-0055. [DOI] [PubMed] [Google Scholar]
- 2.Wolff MS, Toniolo PG. Environmental organochlorine exposure as a potential etiologic factor in breast cancer. Environ Health Perspect. 1995;103(Suppl 7):141–5. doi: 10.1289/ehp.95103s7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negri E, Bosetti C, Fattore E, La Vecchia C. Environmental exposure to polychlorinated biphenyls (PCBs) and breast cancer: a systematic review of the epidemiological evidence. Eur J Cancer Prev. 2003;12(6):509–16. doi: 10.1097/00008469-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Cooper GS, Savitz DA, Millikan R, Chiu Kit T. Organochlorine exposure and age at natural menopause. Epidemiology. 2002;13(6):729–33. doi: 10.1097/00001648-200211000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Akkina J, Reif J, Keefe T, Bachand A. Age at natural menopause and exposure to organochlorine pesticides in Hispanic women. J Toxicol Environ Health A. 2004;67(18):1407–22. doi: 10.1080/15287390490483845. [DOI] [PubMed] [Google Scholar]
- 6.Engel LS, Laden F, Andersen A, et al. Polychlorinated biphenyl levels in peripheral blood and non-Hodgkin's lymphoma: a report from three cohorts. Cancer Res. 2007;67(11):5545–52. doi: 10.1158/0008-5472.CAN-06-3906. [DOI] [PubMed] [Google Scholar]
- 7.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007;115(10):1406–14. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoyer AP, Jorgensen T, Grandjean P, Hartvig HB. Repeated measurements of organochlorine exposure and breast cancer risk (Denmark). Cancer Causes Control. 2000;11(2):177–84. doi: 10.1023/a:1008926219539. [DOI] [PubMed] [Google Scholar]
- 9.Hovinga ME, Sowers M, Humphrey HE. Historical changes in serum PCB and DDT levels in an environmentally-exposed cohort. Arch Environ Contam Toxicol. 1992;22(4):362–6. doi: 10.1007/BF00212554. [DOI] [PubMed] [Google Scholar]
- 10.Sweeney AM, Symanski E, Burau KD, et al. Changes in serum PBB and PCB levels over time among women of varying ages at exposure. Environ Res. 2001;86(2):128–39. doi: 10.1006/enrs.2001.4261. [DOI] [PubMed] [Google Scholar]
- 11.Wolff MS, Zeleniuch-Jacquotte A, Dubin N, Toniolo P. Risk of breast cancer and organochlorine exposure. Cancer Epidemiol Biomarkers Prev. 2000a;9(3):271–7. [PubMed] [Google Scholar]
- 12.Hagmar L, Wallin E, Vessby B, et al. Intra-individual variations and time trends 1991-2001 in human serum levels of PCB, DDE and hexachlorobenzene. Chemosphere. 2006;64(9):1507–13. doi: 10.1016/j.chemosphere.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 13.Wolff MS, Anderson HA. Polybrominated biphenyls: sources and disposition of exposure among Michigan farm residents, 1976-80. Eur J Oncol. 1999b;4(6):645–651. [Google Scholar]
- 14.Rogan WJ, Gladen BC, McKinney JD, et al. Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: effects of maternal factors and previous lactation. Am J Public Health. 1986;76(2):172–7. doi: 10.2105/ajph.76.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKinney JD, Moore L, Prokopetz A, Walters DB. Validated extraction and cleanup procedures for polychlorinated biphenyls and DDE in human body fluids and infant formula. J Assoc Off Anal Chem. 1984;67(1):122–9. [PubMed] [Google Scholar]
- 16.Phillips DL, Pirkle JL, Burse VW, et al. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18(4):495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 17.Bernert JT, Turner WE, Patterson DG, Jr., Needham LL. Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere. 2007;68(5):824–31. doi: 10.1016/j.chemosphere.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 18.Longnecker MP, Gladen BC, Patterson DG, Jr., Rogan WJ. Polychlorinated biphenyl (PCB) exposure in relation to thyroid hormone levels in neonates. Epidemiology. 2000;11(3):249–54. doi: 10.1097/00001648-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 20.Lockitch G. Clinical Biochemistry of Pregnancy. Critical Reviews in Clinical Laboratory Sciences. 1997;34(1):67–139. doi: 10.3109/10408369709038216. [DOI] [PubMed] [Google Scholar]
- 21.Knopp RH, Bergelin RO, Wahl PW, et al. Population-based lipoprotein lipid reference values for pregnant women compared to nonpregnant women classified by sex hormone usage. Am J Obstet Gynecol. 1982;143(6):626–37. doi: 10.1016/0002-9378(82)90107-7. [DOI] [PubMed] [Google Scholar]
- 22.NCHS . Total Serum Cholesterol Levels of Adults 20-74 Years of Age: United States, 1976-80. In: Fulwood R, Kalsbeek W, Rifkind B, Russell-Briefel R, Muesing R, editors. Vital Health and Statistics. 1986. pp. 1–59. [PubMed] [Google Scholar]
- 23.NCHS . Serum lipids of adults 20-74 years: United States, 1976-80. In: Carroll M, Sempos C, Briefel R, Gray S, Johnson C, editors. Vital Health and Statistics. 1993. pp. 1–107. [PubMed] [Google Scholar]
- 24.Steuerwald U, Weihe P, Jorgensen PJ, et al. Maternal seafood diet, methylmercury exposure, and neonatal neurologic function. J Pediatr. 2000;136(5):599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- 25.Jaraczewska K, Lulek J, Covaci A, et al. Distribution of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers in human umbilical cord serum, maternal serum and milk from Wielkopolska region. Poland. Sci Total Environ. 2006;372(1):20–31. doi: 10.1016/j.scitotenv.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 26.Inoue K, Harada K, Takenaka K, et al. Levels and concentration ratios of polychlorinated biphenyls and polybrominated diphenyl ethers in serum and breast milk in Japanese mothers. Environ Health Perspect. 2006;114(8):1179–85. doi: 10.1289/ehp.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rylander L, Dyremark E, Stromberg U, Ostman C, Hagmar L. The impact of age, lactation and dietary habits on PCB in plasma in Swedish women. Science of the Total Environment. 1997;207(1):55–61. doi: 10.1016/s0048-9697(97)00245-3. [DOI] [PubMed] [Google Scholar]
- 28.Phillips DL, Smith AB, Burse VW, et al. Half-life of polychlorinated biphenyls in occupationally exposed workers. Arch Environ Health. 1989b;44(6):351–4. doi: 10.1080/00039896.1989.9935905. [DOI] [PubMed] [Google Scholar]
- 29.Wolff MS, Fischbein A, Selikoff IJ. Changes in PCB serum concentrations among capacitor manufacturing workers. Environ Res. 1992;59(1):202–16. doi: 10.1016/s0013-9351(05)80240-3. [DOI] [PubMed] [Google Scholar]
- 30.Kotlyar M, Carson SW. Effects of obesity on the cytochrome P450 enzyme system. Int J Clin Pharmacol Ther. 1999;37(1):8–19. [PubMed] [Google Scholar]
- 31.Gallagher D, Visser M, Sepulveda D, et al. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143(3):228–39. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 32.ATSDR . Toxicological profile for DDT, DDE, and DDD. Department of Human and Health Services; Atlanta: 2002. pp. 15–19. [Google Scholar]
- 33.ATSDR . Toxicological profile for polychlorinated biphenyls. Department of Human and Health Services; Atlanta, GA: 2000. pp. 15–16. [Google Scholar]
- 34.Smith D. Worldwide trends in DDT levels in human breast milk. Int J Epidemiol. 1999;28(2):179–88. doi: 10.1093/ije/28.2.179. [DOI] [PubMed] [Google Scholar]
- 35.Tee PG, Sweeney AM, Symanski E, et al. A longitudinal examination of factors related to changes in serum polychlorinated biphenyl levels. Environ Health Perspect. 2003;111(5):702–7. doi: 10.1289/ehp.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noren K, Meironyte D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20-30 years. Chemosphere. 2000;40(9-11):1111–23. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- 37.Longnecker MP, Rogan WJ, Lucier G. The human health effects of DDT (dichlorodiphenyltrichloroethane) and PCBS (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu Rev Public Health. 1997;18:211–44. doi: 10.1146/annurev.publhealth.18.1.211. [DOI] [PubMed] [Google Scholar]
- 38.Shirai JH, Kissel JC. Uncertainty in estimated half-lives of PCBS in humans: impact on exposure assessment. Sci Total Environ. 1996;187(3):199–210. doi: 10.1016/0048-9697(96)05142-x. [DOI] [PubMed] [Google Scholar]
- 39.Sasamoto T, Horii S, Ibe A, Takada N, Shirota K. Concentration changes of PCDDs, PCDFs, and dioxin-like PCBs in human breast milk samples as shown by a follow-up survey. Chemosphere. 2006;64(4):642–9. doi: 10.1016/j.chemosphere.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 40.Taylor PR, Lawrence CE. Polychlorinated biphenyls: estimated serum half lives. Br J Ind Med. 1992;49(7):527–8. doi: 10.1136/oem.49.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan DP, Roan CC. Loss of DDT from storage in human body fat. Nature. 1972;238(5361):221–3. doi: 10.1038/238221a0. [DOI] [PubMed] [Google Scholar]
- 42.Hansen LG. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect. 1998;106(Suppl 1):171–89. doi: 10.1289/ehp.98106s1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu K, Bunce NJ. Metabolism of polychlorinated dibenzo-p-dioxins and related dioxin-like compounds. J Toxicol Environ Health B Crit Rev. 1999;2(2):183–210. doi: 10.1080/109374099281214. [DOI] [PubMed] [Google Scholar]
- 44.Roncevic N, Pavkov S, Galetin-Smith R, et al. Serum concentrations of organochlorine compounds during pregnancy and the newborn. Bull Environ Contam Toxicol. 1987;38(1):117–24. doi: 10.1007/BF01606568. [DOI] [PubMed] [Google Scholar]
- 45.Imbeault P, Chevrier J, Dewailly E, et al. Increase in plasma pollutant levels in response to weight loss in humans is related to in vitro subcutaneous adipocyte basal lipolysis. Int J Obes Relat Metab Disord. 2001;25(11):1585–91. doi: 10.1038/sj.ijo.0801817. [DOI] [PubMed] [Google Scholar]
- 46.Baris D, Kwak LW, Rothman N, et al. Blood levels of organochlorines before and after chemotherapy among non-Hodgkin's lymphoma patients. Cancer Epidemiol Biomarkers Prev. 2000;9(2):193–7. [PubMed] [Google Scholar]
- 47.Wolff MS, Britton JA, Teitelbaum SL, et al. Improving organochlorine biomarker models for cancer research. Cancer Epidemiol Biomarkers Prev. 2005b;14(9):2224–36. doi: 10.1158/1055-9965.EPI-05-0173. [DOI] [PubMed] [Google Scholar]
- 48.Alcock RE, Sweetman AJ, Juan CY, Jones KC. A generic model of human lifetime exposure to persistent organic contaminants: development and application to PCB-101. Environ Pollut. 2000;110(2):253–65. doi: 10.1016/s0269-7491(99)00298-5. [DOI] [PubMed] [Google Scholar]
- 49.He JP, Stein AD, Humphrey HE, Paneth N, Courval JM. Time trends in sport-caught Great Lakes fish consumption and serum polychlorinated biphenyl levels among Michigan Anglers, 1973-1993. Environ Sci Technol. 2001;35(3):435–40. [PubMed] [Google Scholar]
- 50.Barr DB, Weihe P, Davis MD, Needham LL, Grandjean P. Serum polychlorinated biphenyl and organochlorine insecticide concentrations in a Faroese birth cohort. Chemosphere. 2006;62(7):1167–82. doi: 10.1016/j.chemosphere.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 51.Rylander L, Stromberg U, Dyremark E, et al. Polychlorinated biphenyls in blood plasma among Swedish female fish consumers in relation to low birth weight. Am J Epidemiol. 1998;147(5):493–502. doi: 10.1093/oxfordjournals.aje.a009476. [DOI] [PubMed] [Google Scholar]
- 52.Axmon A, Rignell-Hydbom A. Estimations of past male and female serum concentrations of biomarkers of persistent organochlorine pollutants and their impact on fecundability estimates. Environ Res. 2006;101(3):387–94. doi: 10.1016/j.envres.2005.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.