Abstract
Transcranial photobiomodulation (PBM) also known as low level laser therapy (tLLLT) relies on the use of red/NIR light to stimulate, preserve and regenerate cells and tissues. The mechanism of action involves photon absorption in the mitochondria (cytochrome c oxidase), and ion channels in cells leading to activation of signaling pathways, up-regulation of transcription factors, and increased expression of protective genes. We have studied PBM for treating traumatic brain injury (TBI) in mice using a NIR laser spot delivered to the head. Mice had improved memory and learning, increased neuroprogenitor cells in the dentate gyrus and subventricular zone, increased BDNF and more synaptogenesis in the cortex. These highly beneficial effects on the brain suggest that the applications of tLLLT are much broader than at first conceived. Other groups have studied stroke (animal models and clinical trials), Alzheimer’s disease, Parkinson’s disease, depression, and cognitive enhancement in healthy subjects.
Keywords: Transcranial photobiomodulation, low level laser therapy, brain disorders, traumatic brain injury, stroke, Alzheimer’s disease, psychiatric diseases
1 Introduction
Light in the visible and near-infrared spectrum makes up the major part of solar radiation and is also provided by artificial light sources. Since light is widely present throughout our world, approaches that harness it for the purposes of medical treatment is hardly a new phenomenon. Photobiomodulation (PBM) involves the utilization of light from the visible and near infrared portions of the spectrum at a relatively low power density that is insufficient to heat or burn tissue. Although PBM often uses lasers as the light source, its method of action is not thermal or ablative in any way. Rather, the photons themselves stimulate chemical changes within the cells, provoking biological reactions that benefit the body in a variety of ways - including the triggering of neuroprotective responses, improvements in metabolism, blood flow, and neurogenesis, and decreases in inflammation and oxidative stress [1].
The medical benefits of low level laser therapy (LLLT) were first discovered in 1967 by Dr. Endre Mester at the Semmelweis Medical University in Hungary. Mester was trying to repeat an experiment originally described by McGuff in Boston USA, who had successfully used a ruby laser to cure malignant tumors in rats [2]. However, Mester’s laser possessed only a very small fraction of the power possessed by McGuff’s laser. Thus, he was unsuccessful in curing any tumors. However, he did observe a heightened rate of hair growth and better wound healing in the rats in which he had surgically implanted tumors, the first indication that low level laser light (rather than high power thermal lasers) could have its own distinct applications in medicine [3, 4]. Over the almost 50 years since then, LLLT or PBM has come into use for the treatment of pain [5] and wounds [6] and for cosmetic purposes such as decreasing the severity of wrinkles [7] and regrowing hair [8]. Based on the knowledge of its protective, restorative and healing abilities, research was eventually conducted into whether PBM could be used in the treatment of acute stroke [9], and from there onwards for a wide variety of other neurological conditions [10].
Generally, transcranial PBM (the name applied to PBM specifically when the light is applied to the head to treat neurological conditions) procedures involve the placement of a light source (or multiple light sources) on one or several areas of the head, with the goal of stimulating a certain part of the brain, which varies depending on the illness or disorder being treated. The light can originate from a laser or a light emitting diode (LED), and can be pulsed or continuous. Several parameters are typically employed to fully describe PBM protocols. One is the wavelength of light being used. Wavelength is important, as only certain ranges have any effect. Light in the 700–750 nm range, for example, tends to have little impact, whereas the impact of light in the 600–690 nm or 760–900 nm is far greater. Another important parameter is the irradiance or power density (mW/cm2), which equals the power of the light source in Watts divided by the area over which the light hits the body. A similar concept is energy density (sometimes called the fluence), which is provided by Joules/area (J/cm2). It should be noted that if the spot is relatively small (<< 1 cm2), the power density and energy density are likely to be misleading. For instance if the spot is only 1 mm2, a calculation might lead to claims that 1W/cm2 was delivered from a 10 mW laser. Additionally it is important to note that the treatment duration, and the frequency of repetition can have influence on the success (or lack thereof). Finally there is some evidence that pulsing the light can have a better effect than continuous wave (CW) light [11, 12]. Parameters are of vital importance in PBM due to its characteristic biphasic dose response effect [13, 14]. That is, PBM can have vastly different effects depending on such factors as wavelength, irradiance, energy density, and treatment duration. This biphasic effect adheres to the “Arndt-Schultz Law”, which states that if weak stimuli provoke a significant rise in activity, then strong stimuli will not continue to provoke ever-increasing rises. Instead, they only provoke an increase until a certain peak level is reached, after which point the activity decreases until it returns to baseline, and may even get to the point where a negative (or inhibitory) response is achieved [13, 14].
As of now, transcranial PBM research still remains in a state of relative infancy. While its positive effects have been demonstrated countless times in animal models, they have yet to be proven in broad-scope clinical testing. However, the research that does exist is very promising, strongly indicating that PBM could be a viable treatment option for a broad range of neurological diseases including stroke, traumatic brain injury, Parkinson’s disease, Alzheimer’s disease, and depression, in addition to providing cognitive enhancement for healthy subjects [10].
2 Molecular Mechanisms of Photobiomodulation
2.1 Mitochondria and Cytochrome-C Oxidase
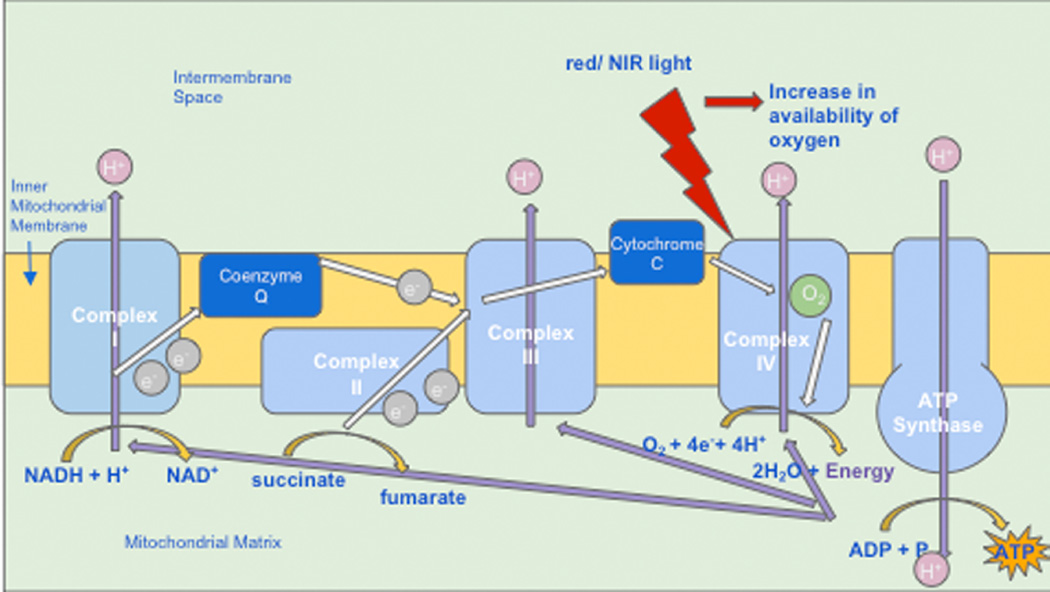
Cytochrome C oxidase (Cox) is the terminal enzyme in the electron transport chain. The electron transport chain, through a series of redox reactions, facilitates the transfer of electrons across the inner membrane of the mitochondria. As the terminal enzyme, otherwise known as “complex IV”, Cox mediates the transfer of electrons from cytochrome C to molecular oxygen (see Figure 1). In many publications, Cox has been shown to be an acceptor and transducer of signals activated by light in the red and near-infrared regions of the spectrum [15]. Specifically, exposure to this type of light, via PBM, seems to provoke an increase in the availability of electrons for the reduction of molecular oxygen in the catalytic center of Cox, increasing mitochondrial membrane potential (MMP), and increasing levels of adenosine triphosphate (ATP), cyclic adenosine monophosphate (cAMP), and reactive oxygen species (ROS), all of which indicate heightened mitochondrial functioning and cellular metabolism [1].
Figure 1.
Photobiomodulation is absorbed by cytochrome c oxidase (unit IV) in the mitochondrial électron transport chain leading to an increase in oxygen consumption and ATP synthesis. ATP, adenosine triphosphate; NADH, nicotinamide adénine dinucleotide reduced form; NIR, near-infrared red
Although other portions of the electron transfer chain, such as complexes I–IV and succinate dehydrogenase do experience increased activity as a result of PBM, Cox is still believed to be the primary photoacceptor. This notion is supported by the fact that low-level irradiation such as PBM causes increased oxygen consumption, and that the majority of oxygen consumption occurs at complex IV. It is additionally supported by the fact that the presence of sodium azide, a Cox inhibitor, renders the effects of PBM null [16].
It has been proposed that the reason PBM increases Cox activity is through the photodissociation of NO from Cox. Evidenced by the increased levels of NO following PBM, it is possible that PBM reverses the mitochondrial inhibition of cellular respiration that exists as a result of excessive NO binding . According to this theory, NO would be photodissociated from its binding sites at the copper and heme iron centers in Cox. Thus, NO would no longer prevent binding to oxygen, and enzymatic activity would be restored to normal levels, allowing for an influx of oxygen, the resumption of respiration and a burst of generation of ROS. NO can also be photo-released from other intracellular sites, including nitrosylated myoglobin and hemoglobin, producing similarly positive effects [17].
On a slightly broader scale, PBM is also believed to trigger retrograde mitochondrial signaling [18]. This term refers to the communication between the mitochondria and the nucleus of a cell (normally signals pass the other way from the nucleus to other organelles). The aforementioned mitochondrial changes result in an altered mitochondrial ultrastructure, which drives the communication. As a result, membrane permeability and ion flux at the cell membrane are altered, in turn leading to production of reactive oxygen species (ROS), and altered activity of redox-sensitive transcription factors such as activator protein-1 (AP1) and NF-κB [19].
2.2 Light/Heat Gated Ion Channels
Although evidence proving that light-gated or heat-gated ion channels could be mechanisms of action in PBM is sparse at present, it is gradually increasing [20]. PBM is thought likely to affect transient receptor potential (TRP) channels. First discovered in a Drosophila mutant as the mechanism responsible for the vision of insects, some TRP channels are now known to be sensitive to light or heat [21], in addition to a wide variety of other stimuli [22]. TRP channels are calcium channels, and are modulated by phosphoinositides [23].
The majority of research relating PBM to light/heat-gated ion channels has concentrated on the TRPV “vanilloid” subfamily of TRP channels. Evidence from studies [24–27] have led to the general consensus that TRP channels are most likely to be activated by green light or infrared light. However, because green light lacks the same penetrating ability of infrared or near-infrared light, it lacks practical clinical application. However, Ryu et al. found that exposure to infrared (2,780 nm) wavelength light attenuated TRPV1 activation, causing a decrease in generation of pain stimuli. A similar, but far less dramatic antinociceptive effect was also observed when TRPV4 was exposed to light of the same wavelength. TRPV4 was also shown to be responsive to 1,875 nm pulsed light [26]. Since water is the primary absorber of infrared in this region and is also by far the most prevalent single molecule in living systems, it is possible that minute alterations in temperature (low enough to produce no detectable tissue heating) could alter protein conformation and open the channel.
3. Tissue optics, light delivery, light sources
3.1 Does light penetrate into the brain?
It could be reasonably claimed that the brain is one of the most interesting organs, for those working in the field of tissue optics. Tisse optics describes how visible and infrared light of different wavelengths interacts with different tissues, and involves measuring tissue optical properties, namely, absorption coefficient, scattering coefficient, and anisotropy factor. One reason the brain is so interesting is the intense interest in near-infrared spectroscopy (NIRS) and diffuse optical imaging. Functional NIRS (fNIRS) using 700–900 nm light has become established as a brain imaging technique that can be compared to functional magnetic resonance imaging (fMRI) that has made such a revolutionary impact upon neurosceince in récent years [28]. Different laboratories have investigated the penetration of light of different wavelengths though the scalp and the skull, and to what depths into the brain this light can penetrate. Haeussinger et al estimated that the mean penetration depth (5% remaining intensity) of NIR light through the scalp and skull was 23:6+0:7 mm [29]. Jagdeo et al [30] used human cadaver heads (skull with intact soft tissue) to measure penetration of 830 nm light, and found penetration depended on the anatomical region of the skull (0.9% at the temporal region, 2.1% at the frontal region, and 11.7% at the occipital region). Red light (633 nm) hardly penetrated at all. Tedord et al [31] also used human cadaver heads to compare penetration of 660 nm, 808 nm, and 940nm light. They found 808 nm light was best and could reach a depth in the brain of 40–50mm.
3.2 Systemic effects?
Another manner in which PBM may affect the brain is through the provocation of broader systemic effects. That is, when PBM is used to target a specific area other than the brain, such as lower back pain, the brain can benefit remotely from the changes being caused. For example, PBM stimulates macrophages and mast cells, which can benefit the brain. Also, regardless of the treatment site, PBM can lead to the down-regulation of pro-inflammatory cytokines and the up-regulation of anti-inflammatory cytokines on a body-wide basis, meaning that the brain will still benefit from the positive effects despite not being the specific target for treatment. Another possibility is that PBM can trigger the production of a yet-unidentified extracellular signaling molecule from the mitochondria (for instance), which is then transported to enable the remote action of PBM on brain cells and other non-local cells. It should be remembered that blood platelets have large stores of ATP, and that low platelet ATP content has been linked with dépression [32].
It is in fact very likely that the beneficial effects of PBM on the brain cannot be entirely explained by penetration of photons through the scalp and skull into the brain itself. There have been some studies that have explicitly addressed this exact issue. In a study of PBM for Parkinson’s disease in a mouse model [33]. Mitrofanis and colleagues compared delivering light to the mouse head, and also covered up the head with aluminum foil so that they delivered light to the remainder of the mouse body. They found that there was a highly beneficial effect on neurocognitive behavior with irradiation to the head, but nevertheless there was also a statistically significant (although less pronounced benefit, referred to by these authors as an ‘abscopal effect”) when the head was shielded from light [34]. Moreover Oron and co-workers [35] have shown that delivering NIR light to the mouse tibia (using either surface illumination or a fiber optic) resulted in improvement in a transgenic mouse model of Alzheimer’s disease (AD). Light was delivered weekly for 2 months, starting at 4 months of age (progressive stage of AD). They showed improved cognitive capacity and spatial learning, as compared to sham-treated AD mice. They proposed that the mechanism of this effect was to stimulate c-kit-positive mesenchymal stem cells (MSCs) in autologous bone marrow (BM) to enhance the capacity of MSCs to infiltrate the brain, and clear β-amyloid plaques [36]. It should be noted that the calvarial bone marrow of the skull contains substantial numbers of stem cells [37].
3.3 Where should the light be delivered?
The obvious answer is that if one wants to affect the brain one should deliver light to the head, and more specifically, this often imeans the forehead as there is no hair present. Hair can act as a significant attenuator of light in the visible and NIR regions. Moreover light that hits the forehead penetrates to the frontal lobes of the cortex, a part of the brain that is responsible for motor function, problem solving, spontaneity, memory, language, initiation, judgement, and impulse control. However other investigators have come to the conclusion that other specific parts of the head (and therefore the underlying brain) should be specifically targeted by spatially directed spots of light. Margaret Naeser has been a leading proponent of this point of view and believes that the default mode network is critically important in the brain’s response to PBM [38]. A paper from Russia [39] treated patients with Alzheimer’s disease by threading a fiber optic all the way from the femoral artery to the middle cerebral artery to deliver red laser light (20mW) into the brain. As mentioned above the Mitrofanis laboratory in Australia has studied Parkinson’s disease in animal models, and after implanting a fiber optic into the mouse brain [40], they repeated the procedure in Macaque monkeys with Parkinson’s disease caused by MPTP administration [41].
Chinese investigators [42] and the Vielight Company in Canada [43] have long studied intranasal PBM, in which a low power LED or laser diode (<20mW) is clipped to the inside of one nostril, and have even claimed that therapeutic doses of light can penetrate to the brain. However it can be readily appreciated that systemic blood absorption, or even activation of olefactory ensheathing cells (a type of stem cell resident in the nasal mucosa [44]) could also play a role in the beneficial effects of intranasal PBM on the brain.
Laser acupuncture is often used as an alternative or as an addition to traditional Chinese acupuncture using needles [45]. Many of the applications of laser acupuncture have been for conditions that affect the brain [46] such as Alzheimer’s disease [47] and autism [48] that have all been investigated in animal models. Moreover laser acupuncture has been tested clinically [49].
3.4 Light sources
A wide array of different light sources (lasers and LEDs) have been employed for tPBM. One of the most controversial questions which remains to be conclusively settled, is whether a coherent monochromatic laser is superior to non-coherent LEDs that typically have a 30 nm band-pass (full width half maximum). Although wavelengths in the NIR region (800–1100 nm) have been the most often used, red wavelengths have sometimes been used either alone, or in combination with NIR. Power levels also vary markedly from Class IV lasers with total power outputs in the region of 10W [50], to lasers with more modest power levels (circa 1W). LEDs can also have widely varying total power levels depending on the size of the array and the number and power of the individual diodes (now diodes are available with a power of 3W each). Power densities can also vary quite substantially from the Photothera laser [51] and other class IV lasers [52], which required active cooling or constant movement (~700 mW/cm2), to LEDs in the region of 10–30 mW/cm2.
4 Mechanisms of PBM Applied to the Brain
4.1 Metabolism
Improved metabolic functioning is one of the most easily recognizable effects of PBM, and increased intracellular ATP production is one of its most strongly supported mechanisms of action. Using phosphorus magnetic resonance spectroscopy (P-MRS), significant increases in nucleotide triphosphate (NTP), a marker of cellular energy availability, have been observed following PBM [53] strongly indicating the increase in cellular ATP production to be a result of PBM. This is significant, as lowered ATP production is a hallmark of many neurological conditions such as major depressive disorder, traumatic brain injury, Parkinson’s disease, and Alzheimer’s disease, the details of which will be discussed at a later point.
4.2 Blood Flow
The production of NO as result of PBM is similarly well recorded [54]. NO is a major neuronal signaling molecule which, among other functions, possesses the ability to trigger vasodilation. To do so, it first stimulates soluble guanylate cyclase to form cyclic-GMP (cGMP). The cGMP then activates protein kinase G, leading to the reuptake of Ca2+ and the opening of calcium-activated potassium channels. Due to the subsequent fall in concentration of Ca2+, myosin light-chain kinase is prevented from phosphorylating the myosin molecule, causing the smooth muscle cells in the lining of blood vessels and lymphatic vessels to become relaxed. This vasodilation then promotes improved circulation, which in turn leads to improved cerebral oxygenation in a similar manner to that observed with pulsed electromagnetic fields [55]. As is the case with any other part of the body, improvement in blood flow significantly aids the functioning of the area in which it occurs.
4.3 Neuroprotection
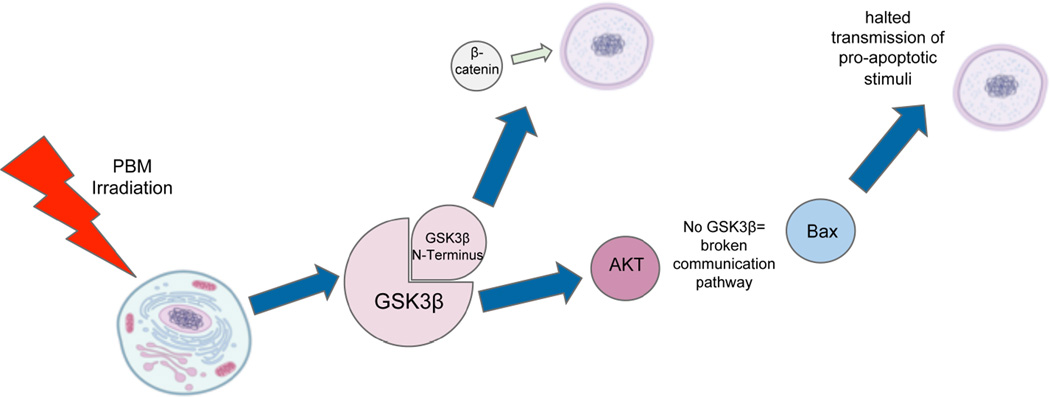
A wide variety of evidence suggests that PBM can be utilized for neuroprotection-- essentially, as a preemptive measure to protect cells from future damage and reduce ongoing damage and promote their survival and longevity. One way it achieves this result is by inhibiting the activity of glycogen synthase kinase 3β (GSK3β). To do so, it activates protein kinase B (AKT) which increases the phosphorylation level of its Ser9 amino acid residue, which then allows the N-terminus of GSK3β to bind with its own binding site. One result of this is the accumulation and translocation to the nucleus of β-catenin, which ceases to be underphosphorylated and therefore becomes more viable when GSK3β activity is inhibited. Once allowed to accumulate in the nucleus, β-catenin relies on the increased activity of TCF/LEF transcription factors to promote cellular survival [56]. This inhibition of GSK3β also helps to prevent apoptosis, the normal cell death that occurs as an organism grows. GSK3β is believed to act as a mediator between AKT and Bax, a protein which is translocated to the nucleus in the presence of pro-apoptotic stimuli to trigger the beginning of the process. However, when GSK3β is inhibited, the communication pathway between AKT and Bax is cut off. As a result, the signals for Bax translocation are inhibited [57]. This pathway is shown in Figure 2.
Figure 2.
Signaling pathway leading from light absorption to anti-apoptosis and neuroprotection. AKT, alternative term for protein kinase B; Bax, BCL2-associated X protein; GSK3β, glycogen synthase kinase 3β, PBM, photobiomodulation
PBM also demonstrates neuroprotective qualities in the form of protection against senescence. It has been observed to activate the extracellular signal-related kinase (ERK)/forkhead box protein M1 (FOXM1) pathway. The FOXM1 protein regulates the progression from the G1 to the S phase of the cell cycle, and via the activation of the ERK/FOXM1 pathway, PBM leads to the greater translocation of ERK to the nucleus and the greater accumulation of FOXM1 in the nucleus. This, in turn, causes reduced expression of the p21 protein and mitotic arrest in the G1 phase therefore slowing the overall progression of cellular senescence [58].
PBM has also been shown to be effective in protecting cells from the harmful effects of toxins. In a study done by Eells [59], irradiation with 670 nm light was successful in causing the recovery of retinal function and the prevention of histological damage in rodent models exposed to methanol. This is likely due to the fact that methanol generates the toxic metabolite formic acid, an inhibitor of Cox, and PBM is a known stimulator of Cox. A study by Wong-Riley et al [60] on the effects of PBM given post-exposure to toxins such as potassium cyanide and tetrodotoxin produced similarly successful results, especially when rats were irradiated with 670 and 830 nm light, the peaks of the Cox absorption spectrum. This further indicates that the antitoxin effect of PBM can be traced to its stimulation of Cox.
PBM is also effective in prevention of the harmful effects associated with potassium cyanide. When pretreated with 670 nm light, Liang et al. in 2006 found that neuronal expression of Bax induced by cyanide was decreased, preventing the subsequent apoptosis [56].
In addition, PBM has demonstrated the rather unique quality of affecting cells in different states of health in different ways, essentially modifying the cell in whatever way might be necessary to promote its survival. For instance, in normal cells the absorption of light by Cox leads to an increase in MMP and a brief surge in ROS production. However, in cells where MMP is low due to existing oxidative stress, excitotoxicity, or inhibition of electron transport, ROS are produced from dysfunctional mitochondria. In this case light absorption leads to an increase of MMP towards normal levels and thereby a decrease in ROS production [61]. Similarly, the typical response to PBM in healthy cells is an uptick in intracellular Ca2+ [61]. However, in cells that already contain excessive levels of Ca2+ as seen in excitotoxicity, PBM provokes the opposite reaction, lowering intracellular calcium and promoting cell survival [62].
4.4 Oxidative Stress
Oxidative stress occurs when there exists an imbalance between the production of reactive oxygen species (ROS) and the ability of the body to counteract their effects using antioxidants, leading to ROS becoming harmful when they are present in excess and for a long time. Many sources have linked oxidative stress to various neurological conditions, such as major depressive disorder [63], traumatic brain injury and Alzheimer’s disease [64].
In studies of the effect of PBM on traumatized muscle, PBM has been shown to be effective in regulating the amount of cytokine-inducible nitric oxide synthase (iNOS) produced by the cell. This is important because excessive amounts of iNOS can lead to the excessive production of NO, which would then lead to production of the reactive nitrogen species, peroxynitrite, leading to an increase in oxidative stress. Specifically, iNOS could overwhelm the positive effects of other forms of NO synthase such as endothelial nitric oxygen synthase (eNOS), which is the species primarily responsible for the vasodilating effects of PBM [65–67]
PBM has also been shown to cause increases in angiogenesis, leading to further improvements in blood flow. As demonstrated by Cury, PBM at 780 nm and 40 J/cm2 triggered an increase in the expression of the protein HIF1α and of vascular endothelial growth factor and a decrease in matrix metalloproteinase 2 activity, all of which were found to induce angiogenesis [68]. Additionally, in an in vitro study of the effects of red/NIR light on red blood cells done by Mungrue et al., NIR light was found to be quite effective in protecting red blood cells from oxidation, which is a common occurrence in brains compromised by conditions such as MDD [65].
4.5 Anti-Inflammatory effects
Inflammation is a of one of the innate immune system’s defenses against foreign bodies such as bacteria and viruses. On a cellular level, it occurs when the transcription factor NF-κB is activated. While acute inflammation can often have positive effects, chronic inflammation can have very negative effects. Many diseases, including neurodegenerative diseases and mood disorders, can be traced at least in part to chronic inflammation.
One way PBM helps to quell inflammation is through the inhibition of the cyclooxygenase 2 (COX-2) enzyme. Lim et al. found that 635 nm light irradiation at low power was able to cause such inhibition by decreasing intracellular ROS. Inhibition of COX-2 via pharmaceutical means is widely supported at current, with COX-2 inhibitors making up a significant portion of the market for non steroidal anti-inflammatory drugs (NSAIDS) [69]. Through PBM, essentially the same result is being accomplished, just with a different stimulus.
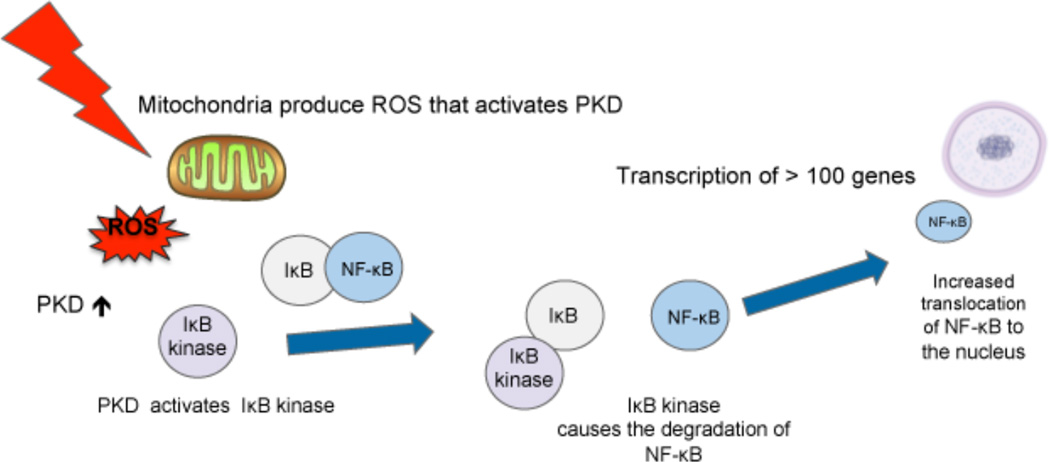
PBM can also modulate cellular levels of free NF-κB. When light is absorbed in the mitochondria there is a brief burst of ROS (as mentioned above). These ROS can activate protein kinase D that can go on to signal in the NF-κB system [70]. NF-κB is found in the cytosol bound to IκB, an inhibitor protein. Pro-inflammatory stimuli have the ability to activate IκB kinase, an upstream signaling regulator that causes the degradation of IκB. Once the IκB has been degraded, the NF-κB is free to translocate to the nucleus, where it triggers the expression of pro-inflammatory genes [19]. These signaling pathways are shown in Figure 3. However, Lim et al. found evidence suggesting that treatment with 635 nm light inhibited the NF-κB pathway, leading to the down regulation of pro-inflammatory gene expression [69].
Figure 3.
Light is absorbed by mitochondria producing a brief burst of ROS that activates PKD leading to activation of Ik-B kinase and translocation of NF-kB to the nucleus. Ik-B, inhibitor of NF-kB; NF-kB, nuclear factor kB ; PKD, protein kinase D ; ROS, reactive oxygen species.
Additionally, PBM possesses the ability to modulate levels of cytokines, proteins that act as important signaling molecules for the immune system. PBM has been shown to modulate levels of both pro and anti- inflammatory cytokines, though to explian the prevention of inflammation, its ability to modulate levels of tumor necrosis factor (TNFα) and other pro-inflammatory cytokines is especially useful.
4.6 Neurogenesis and Synaptogenesis
One of the most notable and potentially significant effects of PBM in the brain is its ability to promote both synaptogenesis and neurogenesis. This is vitally important, as many brain conditions, including TBI, neurodegenerative diseases, and mood disorders can be traced, either partially or in full, to atrophy, cell death, and poor neuronal connections in certain regions of the brain. If PBM possesses the ability to counter these effects by facilitating neural regeneration and rewiring, it could prove to be extremely promising as a novel method of treating such conditions.
One manner in which PBM promotes neuronal connectivity is BDNF (brain derived neurotrophic factor) regulation. BDNF is a protein found in the nervous system, which helps to maintain existing neurons and to encourage the growth of new neurons and synapses. Specifically, it is believed to modulate dendritic structure to facilitate improved synaptic transmission. PBM has been shown to slow attenuation of BDNF via the ERK/CREB pathway, thus positively affecting dendritic morphogenesis and improved neuronal connectivity [71]. BDNF is also a mediator of the downstream protein synapsin 1, which improves synaptogenesis by accelerating the development of neuronal fibers and maintaining synaptic contact [72]. In a study done by Meng et al [71] denser branches and increased interconnectivity between fibers were observed in neural tissue of embryonic rats following irradiation with 780 nm light, indicating increased action of these proteins. BDNF has also been linked to improvements in neuroplasticity and adaptation, which is especially important in cases of traumatic brain injury and stroke [73].
PBM has also proven to be effective as a tool for manipulating stem cells, so that they may be optimized for repairing damaged brain tissue and for improving neurogenesis overall. Specifically, it has been shown to increase cell migration, differentiation, proliferation, and viability all of which are important for the success of any sort of stem cell therapy. In the brain, PBM has the potential to activate neural stem cells, which typically lay dormant in certain areas of the brains of complex organisms once the organism has reached maturity. Once activated, the neural stem cells could facilitate the regeneration of damaged tissue. PBM additionally possesses the ability to provoke increased production of neuroprogenitor cells, which are similar in their functionality to neural stem cells and would have positive effects on neurogenesis [74].
5 PBM for Stroke
Strokes result when hemorrhages or blockages reduce blood flow to part of the brain, causing inflammation, hypoxia, and subsequent cell death. Because PBM had been shown to have positive effects on inflammation, oxidative stress, and neurogenesis, among other things, investigators hypothesized that it could be utilized to reduce damage in acute stroke patients.
5.1 Animal Studies
Initial preclinical testing began on animal models, typically investigating the ability of PBM to prevent long-term neurological damage following an induced acute stroke. Typical parameters tested were 810 nm NIR light at a calculated power on the brain surface (7.5 mW/cm2). One group, Lapchak et al., found in two separate studies that motor functioning [75] and clinical behavior ratings [76] were improved in a rabbit small clot embolic stroke model that had been irradiated 6–24 hours post embolization. Additionally, Oron et al. found in 2006 [77] that PBM applied to rat models post stroke led to significantly reduced long-term neurological deficits. They also found that numbers of newly-formed neuronal cells and migrating cells were increased post-irradiation. Successful studies such as these led to the general consensus that PBM irradiation applied in the short term post-stroke reduces subsequent neurological damage, and that further development through clinical trials would be beneficial.
5.2 Clinical Trials
The first major studies concerning PBM for stroke were the NeuroThera Effectiveness and Safety Trials (NEST), a series of 3 clinical trials which included a total of 1410 subjects receiving irradiation at 808 nm and 1 J/cm2 (on the brain) between 16 and 18 hours following an ischemic stroke. Although evidence proving the efficacy of treatment was inconclusive in NEST-3, there was a significant trend for improvement post-treatment in both NEST-1 and NEST-2. In NEST-1, 70% of patients receiving genuine treatment had successful outcomes (defined as a 9 point decrease on the National Institutes of Health Stroke Scale (NIHSS) 90 days post treatment), compared to 51% of subjects who received an identical sham treatment. In NEST-2, a higher percentage of patients receiving genuine treatment (36.3%) achieved a favorable outcome (defined as a score less than 16 the NIHSS), as compared to 30.9% of patients who received sham treatment. While this was not considered statistically significant, it still represented a positive trend. The third and last clinical trial, NEST-3, was planned to enrol 1000 patients, but the study was prematurely terminated by the DSMB for futility (an expected lack of statistical significance) [78]. The failure of NEST3 may have been due to the insufficient light pénétration to the brain, the decision to use only one tPBM treatment instead of a series of treatments [79]. Moreover, the optimum brain areas to be treated in acute stroke remain to be determined. Additionally, in all three studies PBM was demonstrated to be safe and more or less free of adverse side effects.
Research has also been done into whether laser PBM, rather than needles, can be utilized to stimulate acupuncture points for the treatment of paralysis in chronic stroke patients. In one study [80] seven patients were tested, with five seeing some increase (11–28%) in isolated motion of certain joints. Generally, the treatment was found to be most effective in those with mild to moderate paralysis, as opposed to severe. Norman Doidge, in Toronto, Canada has described the use of PBM as a component of a neuroplasticity approach to rehabilitate chronic stroke patients [81].
6 Traumatic Brain Injury (TBI)
6.1 Acute TBI
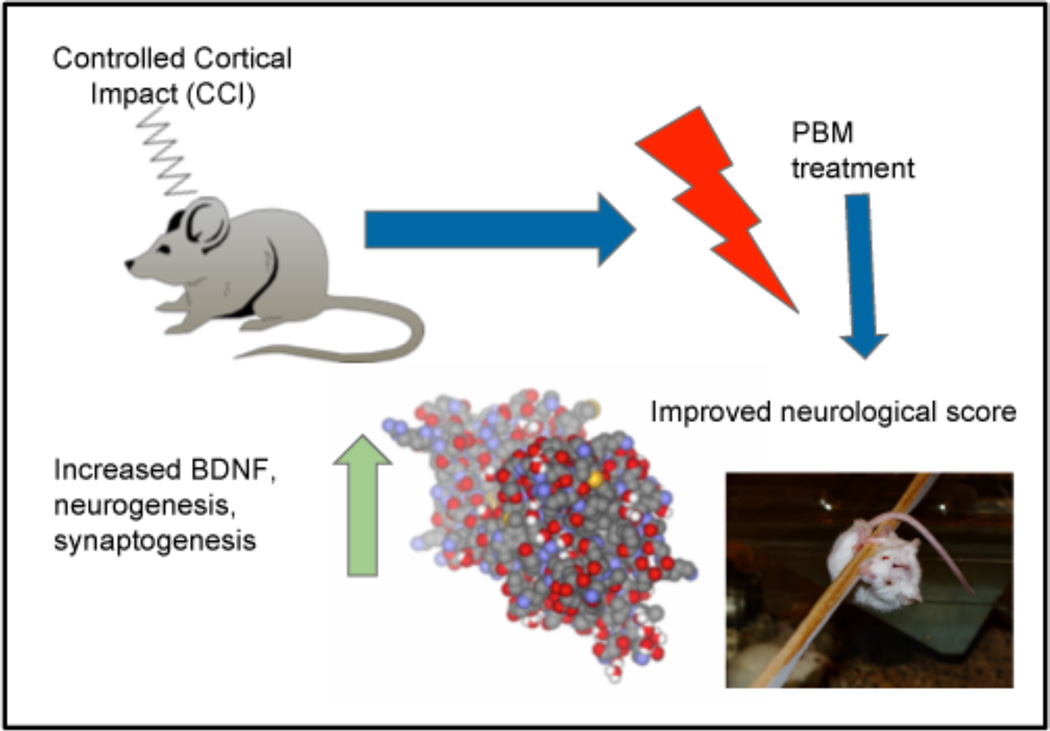
The majority of studies regarding the effects of PBM on acute TBI have been conducted on animal models. For example, in a study by Xuan et al [74]., mouse models who had been subjected to controlled cortical impact received either one or three 810 nm laser treatments following induction of TBI. The results were very promising, indicating that PBM had triggered increased rates of neurogenesis and synaptogenesis in the mouse models (see Figure 4). This finding was supported by immunofluorescence staining of sections of brain tissue from mice sacrificed at different time intervals post-treatment, which revealed markers of significant increases in the formation of neuroprogenitor cells, of cellular migration, and of BDNF levels between 7 and 28 days following PBM treatment. Additionally, mice that had received PBM saw greater improvement on the Morris Water Maze Test, indicating improved spatial memory, learning and cognitive perception. Another interesting observation from this study was the effect of lesion size in the brains of mouse models, or rather its non-effect. Despite lesion size growing over the four-week testing period, cognitive functioning improved (it should be noted that this was true of all animals, regardless of treatment received). However, the rate of improvement was accelerated in mice that received genuine treatment as opposed to sham). This observation indicates that PBM likely aided in the process of neuroplastic adaptation, or the process by which the uninjured portion of the brain takes over the functions of the injured portion. Evidence from this and other similar studies seem to point to the conclusion that PBM used as a treatment for acute TBI is effective because it accelerates the process of regeneration and repair within the brain, thus reducing long term damage and improving cognitive functioning overall.
Figure 4.
In a mouse model of traumatic brain injury caused by controlled cortical impact exposure of the head to 810 nm laser leads to improved neurological performance. At the same time there is upregulation of BDNF, neurogenesis and synaptogenesis in the brain. BDNF, brain derived neurotrophic factor; PBM, photobiomodulation.
6.2 Chronic TBI
Several common neurological characteristics have been observed in sufferers of chronic TBI. One is a long-term increase in microglial activity, leading to chronic inflammation [82]. Another is a decrease in brain interconnectivity due to abnormalities in white matter axons [83]. Specifically, chronic TBI patients seem to present with deficits in the functioning of three systems-- the default mode network (DMN), the central executive network (CEN), and the salience network (SN) (see Figure 5D). Typically, patients have impaired ability to deactivate the DMN, meaning that rapid switching between networks cannot occur, hindering overall cognitive performance. TBI is also linked with chronic sleep disturbances, which result in deficiencies in cognitive functioning, and with lower levels of ATP production in the damaged cortical tissue. However, PBM has been demonstrated to be effective in treating the majority of these symptoms in a variety of models. As previously discussed, PBM reduces inflammation, improves neural interconnectivity (via synaptogenesis), and heightens ATP production.
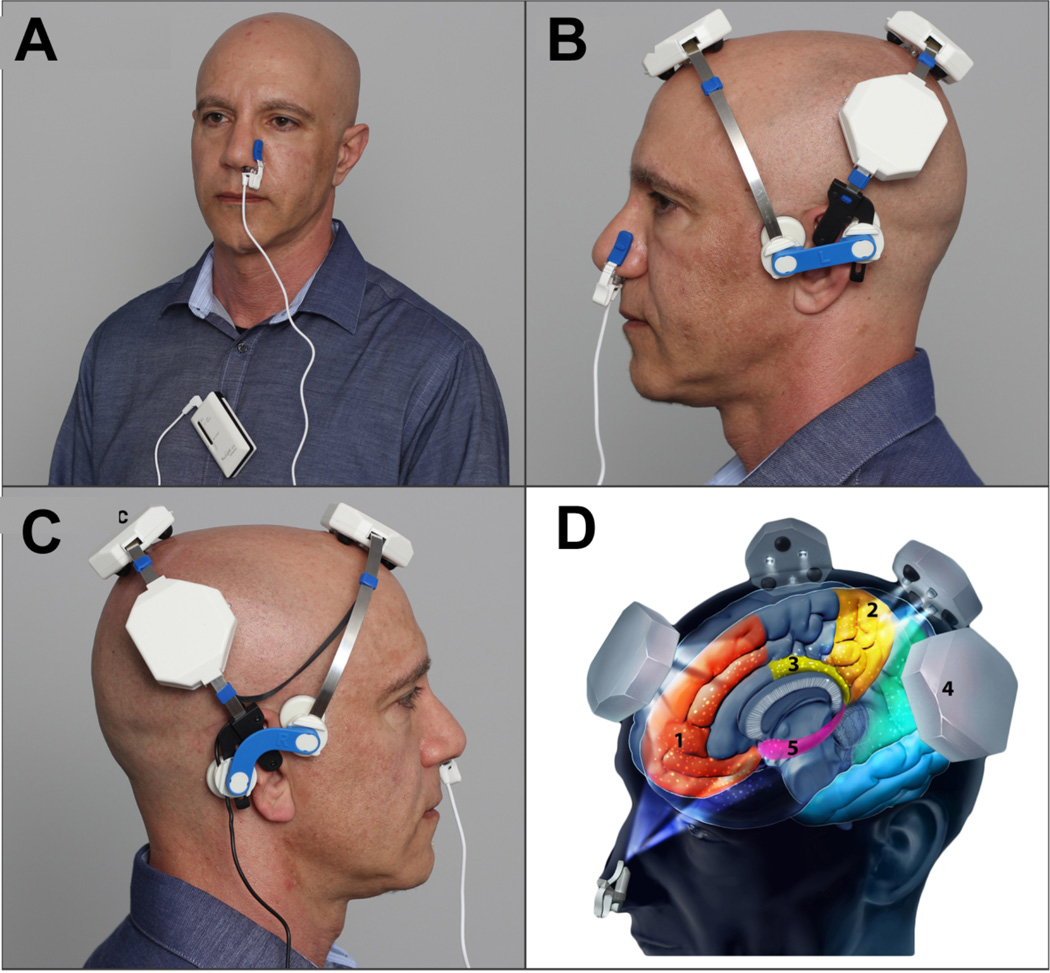
Figure 5.
(A) Vielight 810 Intranasal; (B) Vielight Neuro left view ; (C) Vielight Neuro right view; (D) 1. Mesial préfrontal cortex ; 2. Precuneus ; 3. Posterior congulate cortex; 4. Inferior pariétal lobe; 5. Hippocampus.
Initial clinical studies of PBM for chronic TBI in humans have been promising. For example, research conducted by Naeser et al. revealed that after receiving a total of 18 LED treatments at 22.2 mW/cm2, subjects saw improvements in executive functioning and verbal memory, as noted by improved scores on the Stroop test for executive functioning, Trial 4 inhibition switching, which measures ability to regulate between networks and yielded a p value of .003; and the California Verbal Learning Test II, Long Delay and Free Recall, which yielded a p value of .006 [38]. Additionally, the Naeser lab has carried out pilot research demonstrating that fMRI scans of the brains of chronic aphasia patients both before and after a series of 18 LED treatments indicate increased connectivity between nodes in all three networks affected by TBI. Although at present the majority of studies of PBM for TBI tend to be limited by size, results so far have been quite positive, and indicative of the fact that the anti-inflammatory and regenerative properties of PBM could prove to be very effective in treating TBI.
7 Parkinson’s disease
Parkinson’s disease is caused by the gradual degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc), leading to decreased control of movement over time. In relation to Parkinson’s, PBM has primarily been studied for its neuroprotective abilities. Several groups have already conducted research into the effects of PBM on mouse models of Parkinson’s disease. Mitrofanis’ group found that dopaminergic cells in the SNc were protected from toxicity caused by MPTP (a drug used to induce Parkinson’s symptoms in animal models) if PBM was administered following injection [84]. The same group found that, in tau transgenic mice, PBM decreased expression of hyperphosphorylated tau, reducing tau-caused oxidative stress subsequent cellular degeneration [85]. They went on to test a surgically implanted intracranial fiber designed to deliver either 670 nm LED (0.16 mW) or 670 nm laser (67 mW) into the lateral ventricle of the brain in MPTP-treated mice [40]. Both low power LED and high power laser were effective in preserving SNc cells, but the laser was considered to be unsuitable for long term use (6 days) due to excessive heat production. As mentioned above, these authors also reported a protective effect of abscopal light exposure (head shielded) in this mouse model [33]. Recently this group has tested their implanted fiber approach in a model of Parkinson’s disease in adult Macaque monkeys treated with MPTP [86]. Clinical evaluation of Parkinson’s symptoms (posture, general activity, bradykinesia, and facial expression) in the monkeys were improved at low doses of light (24 J or 35 J) compared to high doses (125 J) [87]. Consistent across the majority of these studies, though, has been the finding that PBM led to increased levels of tyrosine hydroxylase (TH+), which is itself indicative of improvements in the functioning of dopaminergic cells. The pathology of Parkinson’s disease has also been linked to mitochondrial dysfunction, which has been proven to be counteracted by PBM. Essentially, it seems to be a logical conclusion that PBM could be quite effective in the treatment of Parkinson’s disease in human patients.
8 Alzheimer’s disease
Alzheimer’s disease is a neurodegenerative disorder characterized by progressive memory loss and cognitive dysfunction [88]. While the exact cause remains unknown, there are several definitive characteristics. For example, hypometabolism and atrophy of such regions as the cerebral cortex, the temporal lobe, and the parietal lobe is extremely common. The brains of Alzheimer’s patients are also easily distinguishable for their numerous amyloid plaques caused by the buildup of beta-amyloid peptide (β42), around the cells, and neurofibrillary tangles, caused by accumulation of hyperphosphorylated tau protein within the cells. Vascular abnormalities have also been implicated in Alzheimer’s etiology [89], together with toxicity caused by metals such as copper, zinc and aluminum [90]
8.1 Animal Models
Previous findings regarding the neuroprotective and regenerative effects of PBM have led to numerous studies in animal models of its applicability for the treatment of Alzheimer’s disease. For example, De Taboada et al. tested the effects of PBM in a transgenic mouse model of Alzheimer’s disease (amyloid-β protein precursor, AβPP). Beginning at three months of age, PBM was administered three times a week. It was found that, in mice who had received treatment, A-beta plaque numbers were decreased and amyloid levels within the brain were reduced. Importantly tPBM mitigated the behavioral effects seen with advanced amyloid deposition and reduced the expression of inflammatory markers in the AβPP transgenic mice [91]. Purushothuman et al studied two different mouse models of AD-related pathologies: the K369I tau transgenic model (K3), engineered to develop neurofibrillary tangles, and the APPswe/PSEN1dE9 transgenic model (APP/PS1), engineered to develop amyloid plaques [92]. Mice were treated with tPBM 20 times over a four-week period and histochemistry was used to quantify AD-related pathological hallmarks and other markers of cell damage in the neocortex and hippocampus. In the K3 mice, pBM gave a reduction in hyperphosphorylated tau, neurofibrillary tangles and oxidative stress markers (4-hydroxynonenal and 8-hydroxy-2’-deoxyguanosine) to near wildtype levels in the neocortex and hippocampus, and with a restoration of expression of the mitochondrial marker cytochrome c oxidase in surviving neurons. In the APP/PS1 mice, PBM led to a reduction in the size and number of amyloid-β plaques in the neocortex and hippocampus. In a follow-up study these workers reported very similar findings in the cerebellum [93]
There have additionally been studies wherein it was found that animal models saw improvements in metabolic activity in areas of the brain typically impacted by Alzheimer’s after receiving PBM. For instance, Rojas et al. [94] found that PBM enhanced cortical oxygenation and metabolic capacity in the brains of rats, in addition to improving memory. Indeed, it seems that PBM has the ability to target Alzheimer’s disease from several different angles, making it out to be very viable as a potential treatment.
8.2 Clinical Trials
As of yet, clinical trials of PBM for Alzheimer’s remain in the very preliminary phases. However, what little research that currently exists is quite promising. The biggest clinical study came from Russia where investigators used an intravenous fiberoptic approach that was threaded upwards from the femoral artery until it reached The majority of human studies of PBM have investigated intranasal PBM, a form of PBM where the light originates from a source placed inside the nasal cavity, because it is believed to allow for better access to the regions of the brain most directly affected by Alzheimer’s. No broad studies of this sort have yet been conducted, however, there have been several extensive case studies. Lim followed four Alzheimer’s patients over the course of a year, with PBM treatment occurring in daily 25 minute sessions using the Vielight Neuro and Intranasal device (see Figure 5). Three patients received treatment with pulsed light emitting diodes at 810 nm and 13 mW/cm2. A fourth received continuous LED treatment at 633 nm and 7.5 mW/cm2. The caregivers of all four patients reported significant improvements in cognitive ability over the course of a year. While two provided only qualitative observations, the other two were rated on the Mini Mental State Examination before and after the treatment period. Both saw notable improvements in score, with one even being upgraded to the status of practically “no cognitive impairment” from his baseline “significant cognitive impairment”. While the effect of treatment on levels of amyloid beta plaques and neurofibrillary tangles cannot be determined within the scope of the research, the noted improvements in memory and cognition seem to suggest that, at the very least, PBM was slowing the neurodegenerative process. Given the frequency of treatment, and the fact that patients who did not maintain a firm treatment regimen did not see as much success, it would be a reasonable conclusion to draw that the positive effects of PBM seen here can certainly be traced, at least in part, to its pro-metabolic properties [43].
An interesting paper from Russia [39] described the use of intravascular PBM to treat 89 patients with AD who received PBM (46 patients) or standard treatment with memantine and rivastigmine (43 patients). The PBM consisted of threading a fiber-optic through a cathéter in the fémoral artery and advancing it to the to the distal site of the anterior and middle cerebral arteries and delivering 20 mW of red laser for 20–40 minutes. The PBM group had improvement in cerebral microcirculation leading to permanent (from 1–7 years) reduction in dementia and cognitive recovery.
9 Depression and Other Psychiatric Disorders
Major depressive disorder (MDD) is a psychological condition characterized by persistent low mood and anhedonia. It is believed to be caused by inadequate levels of monoamines--the neurotransmitters serotonin, norepinephrine, and dopamine. This leads to the arising of a variety of neurological symptoms, all of which contribute to the severity of the disease. For example, hypometabolism of the limbic system and the frontal lobe has been found to be very common in depressed patients [95]. Suppression of neurogenesis in the dentate gyrus [96] and hippocampal atrophy are also very common [97] and can often be traced in part to increased levels of proinflammatory cytokines [98]. Additionally, MDD patients are believed to be more susceptible to oxidative stress [99]. As previously discussed, PBM has been proven to combat these symptoms, heightening brain metabolism by stimulating ATP production, lowering levels of proinflammatory cytokines, promoting neurogenesis, and reducing oxidative stress.
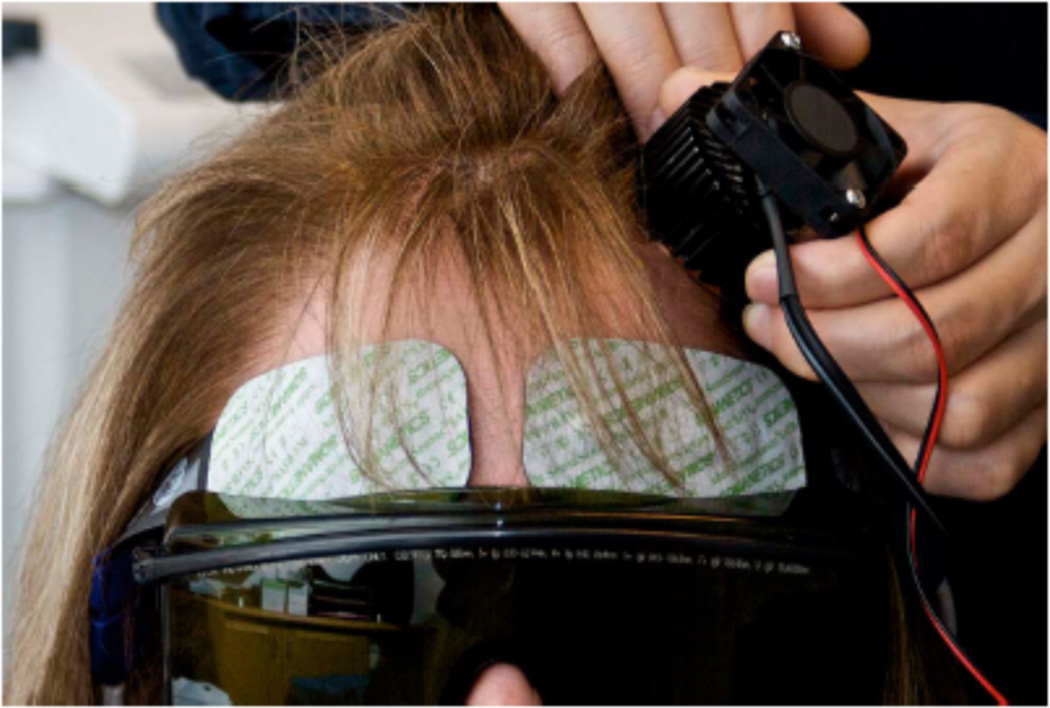
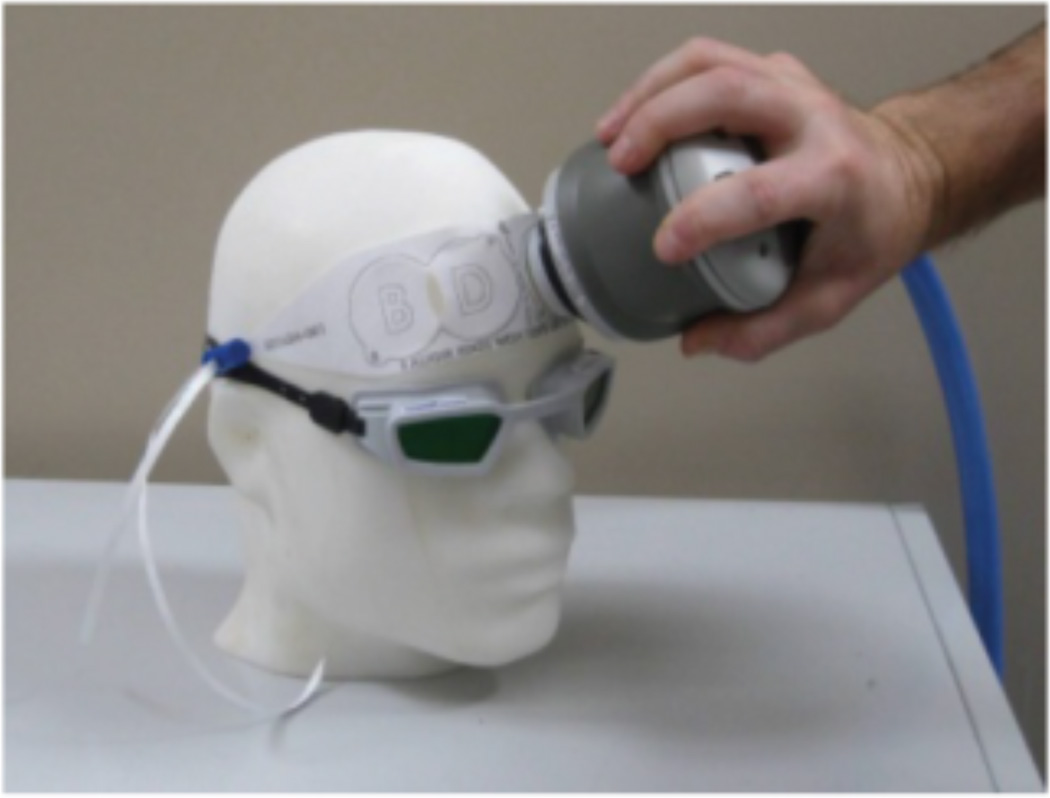
While the frequency of studies regarding PBM for depression remains low thus far, and while those that have taken place have been limited by a small sample size, there have been some very promising initial results. For example, Schiffer et al. exposed 10 treatment-resistant depressed patients to a single PBM treatment, an 810 nm, 250 mW/cm2 LED source placed at two locations on the forehead for four minutes each (see Figure 6). It was found that, after two weeks, the mean Hamilton Depression Rating Scale (HDRS) score for the group had decreased by about 10 points (23.9 +/−8.8 SD to 13.2) and 60% had achieved remission, although by the four week mark symptoms had begun to reappear [100]. Additionally, Cassano et al. [101] studied the effects of multiple PBM treatments administered over three weeks, and compared these results to those of three weeks of sham treatment, with a double-blind status being maintained for the duration of the seven week period (a washout week was designated between the two three week treatment periods). The light parameters utilized were 808nm wavelength, 700 mW/cm2 irradiance, and 84 J/cm2 fluence (see figure 7). At completion of the study, two out of four patients had achieved remission, and the mean HDRS score had decreased from the baseline of 19.8 +/− 4.35 to 13 +/− 5.35. There have also been several instances [38, 52, 102] where TBI patients receiving PBM saw decreases in their comorbid depression.
Figure 6.
810 nm LED application to the forehead for treatment of dépression and anxiety.
Figure 7.
810 nm laser application to the forehead for treatment of dépression.
10 Brain Enhancement in Normal Subjects
While many studies have noted the positive effects of PBM on cognition and memory retention, very few have studied it for the sole purpose of improving the cognitive functioning of healthy subjects. The majority of data comes from a double-blind, placebo controlled study conducted by Barrett and Gonzalez-Lima [103, 104], who tested the effect of PBM on the memory and attention of a class of 40 undergraduate students. Subjects received treatment with 1064 nm light at an irradiance of 250 mW/cm2 and a fluence of 60 J/cm2 at two different sites, or an identical sham treatment for four minutes at each site. The area of the brain targeted was the right frontal pole of the cerebral cortex. After two weeks, it was found that subjects who received genuine treatment saw noticeable cognitive improvements. For instance, they saw faster reaction times (as measured by a psychomotor vigilance test), and performed better on a memory test that involved viewing images of red and yellow squares and identifying their matches out of several options following a short waiting period. The genuine treatment group had a greater number of correct responses and took less time to select an image. Barrett and Gonzalez-Lima also noted that the genuine treatment group seemed less affected by day-to-day anxieties and stressors. Overall, it is quite logical that PBM would positively affect healthy patients. While PBM produces some effects that would only benefit an ill patient, many of its effects, including reducing oxidative stress and increasing blood flow, are quite applicable to the broader population.
11 Future Outlook
The future outlook for photobiomodulation and the brain is, taken overall, highly promising. Due to the extremely positive results obtained in studies in animal models, and the small clinical trials that have been conducted thus far, broader clinical testing of PBM and its applications for neurological conditions is certainly warranted, as much as it is necessary if PBM is to ever become a widely accessible treatment. It is worth mentioning here that trials of pharmaceutical drugs to treat brain damage due to stroke [105] or TBI [106] have largely been unsuccessful. Moreover, despite huge amounts of funding and research by both academic labs and industry, progress in discovering drugs to halt Alzheimer’s and Parkinson’s diseases has not been dramatic [107]. Perhaps it is time to give tPBM serious trials for these indications considering its established safety and notable lack of adverse effects, and relative cost-effective nature? Although psychiatric drugs such as anti-depressants are well established and among some of the world’s biggest selling pharmaceuticals, their rate of effectiveness is disappointing and they can have high rates of distressing side-effects [108, 109]. Although the testing of tPBM for depression has a lot further to go, the availability of inexpensive home-use LED devices suggests that tPBM could also be used for this indication.
Acknowledgments
MRH was supported by US NIH grants R01AI050875 and R21AI121700, Air Force Office of Scientific Research grant FA9550-13-1-0068, by US Army Medical Research Acquisition Activity grant W81XWH-09-1-0514, and by US Army Medical Research and Materiel Command grant W81XWH-13-2-0067.
Bibliography
- 1.De Freitas LF, Hamblin MR. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE Journal of Selected Topics in Quantum Electronics. 2016;22:7000417. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGuff PE, Deterling RA, Jr, Gottlieb LS. Tumoricidal effect of laser energy on experimental and human malignant tumors. The New England journal of medicine. 1965;273:490–492. doi: 10.1056/NEJM196508262730906. [DOI] [PubMed] [Google Scholar]
- 3.Mester E, Lud√°ny G, Sellyei M, Szende B, Tota J. The simulating effect of low power laser rays on biological systems. Laser Rev. 1968;1:3. [Google Scholar]
- 4.Mester E, Szende B, Gartner P. The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl) 1968;9:621–626. [PubMed] [Google Scholar]
- 5.Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374:1897–1908. doi: 10.1016/S0140-6736(09)61522-1. [DOI] [PubMed] [Google Scholar]
- 6.Houreld NN. Shedding light on a new treatment for diabetic wound healing: a review on phototherapy. ScientificWorldJournal. 2014;2014:398412. doi: 10.1155/2014/398412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41–52. [PMC free article] [PubMed] [Google Scholar]
- 8.Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2013 doi: 10.1002/lsm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang YY, Gupta A, Vecchio D, de Arce VJ, Huang SF, Xuan W, Hamblin MR. Transcranial low level laser (light) therapy for traumatic brain injury. J Biophotonics. 2012;5:827–837. doi: 10.1002/jbio.201200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naeser MA, Hamblin MR. Potential for Transcranial Laser or LED Therapy to Treat Stroke, Traumatic Brain Injury, and Neurodegenerative Disease. Photomed Laser Surg. 2011;29:443–446. doi: 10.1089/pho.2011.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ando T, Xuan W, Xu T, Dai T, Sharma SK, Kharkwal GB, Huang YY, Wu Q, Whalen MJ, Sato S, Obara M, Hamblin MR. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS ONE. 2011;6:e26212–e26220. doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashmi JT, Huang YY, Sharma SK, Kurup DB, De Taboada L, Carroll JD, Hamblin MR. Effect of pulsing in low-level light therapy. Lasers Surg Med. 2010;42:450–466. doi: 10.1002/lsm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YY, Sharma SK, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy - an update. Dose Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karu TI. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life. 2010;62:607–610. doi: 10.1002/iub.359. [DOI] [PubMed] [Google Scholar]
- 16.Poyton RO, Ball KA. Therapeutic photobiomodulation: nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov Med. 2011;11:154–159. [PubMed] [Google Scholar]
- 17.Gladwin MT, Shiva S. The ligand binding battle at cytochrome c oxidase: how NO regulates oxygen gradients in tissue. Circ Res. 2009;104:1136–1138. doi: 10.1161/CIRCRESAHA.109.198911. [DOI] [PubMed] [Google Scholar]
- 18.Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 2008;84:1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS, Hamblin MR. Low-Level Laser Therapy Activates NF-kB via Generation of Reactive Oxygen Species in Mouse Embryonic Fibroblasts. PLoS ONE. 2011;6:e22453. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang D, Spielmann A, Wang L, Ding G, Huang F, Gu Q, Schwarz W. Mast-cell degranulation induced by physical stimuli involves the activation of transient-receptor-potential channel TRPV2. Physiol Res. 2012;61:113–124. doi: 10.33549/physiolres.932053. [DOI] [PubMed] [Google Scholar]
- 21.Feng Q. Temperature sensing by thermal TRP channels: thermodynamic basis and molecular insights. Curr Top Membr. 2014;74:19–50. doi: 10.1016/B978-0-12-800181-3.00002-6. [DOI] [PubMed] [Google Scholar]
- 22.Nishida M, Hara Y, Yoshida T, Inoue R, Mori Y. TRP channels: molecular diversity and physiological function. Microcirculation. 2006;13:535–550. doi: 10.1080/10739680600885111. [DOI] [PubMed] [Google Scholar]
- 23.Rohacs T. Phosphoinositide regulation of TRP channels. Handb Exp Pharmacol. 2014;223:1143–1176. doi: 10.1007/978-3-319-05161-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang WZ, Chen JY, Yu JT, Zhou LW. Effects of low power laser irradiation on intracellular calcium and histamine release in RBL-2H3 mast cells. Photochem Photobiol. 2007;83:979–984. doi: 10.1111/j.1751-1097.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- 25.Ryu JJ, Yoo S, Kim KY, Park JS, Bang S, Lee SH, Yang TJ, Cho H, Hwang SW. Laser modulation of heat and capsaicin receptor TRPV1 leads to thermal antinociception. J Dent Res. 2010;89:1455–1460. doi: 10.1177/0022034510381394. [DOI] [PubMed] [Google Scholar]
- 26.Albert ES, Bec JM, Desmadryl G, Chekroud K, Travo C, Gaboyard S, Bardin F, Marc I, Dumas M, Lenaers G, Hamel C, Muller A, Chabbert C. TRPV4 channels mediate the infrared laser-evoked response in sensory neurons. J Neurophysiol. 2012;107:3227–3234. doi: 10.1152/jn.00424.2011. [DOI] [PubMed] [Google Scholar]
- 27.Gu Q, Wang L, Huang F, Schwarz W. Stimulation of TRPV1 by Green Laser Light. Evid Based Complement Alternat Med. 2012;2012:857123. doi: 10.1155/2012/857123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 2011;54:2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haeussinger FB, Heinzel S, Hahn T, Schecklmann M, Ehlis AC, Fallgatter AJ. Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: implications for optical neuroimaging. PLoS One. 2011;6:e26377. doi: 10.1371/journal.pone.0026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jagdeo JR, Adams LE, Brody NI, Siegel DM. Transcranial red and near infrared light transmission in a cadaveric model. PLoS One. 2012;7:e47460. doi: 10.1371/journal.pone.0047460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tedford CE, DeLapp S, Jacques S, Anders J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg Med. 2015;47:312–322. doi: 10.1002/lsm.22343. [DOI] [PubMed] [Google Scholar]
- 32.Sommer AP, Trelles MA. Light pumping energy into blood mitochondria: a new trend against depression? Photomed Laser Surg. 2014;32:59–60. doi: 10.1089/pho.2014.9866. [DOI] [PubMed] [Google Scholar]
- 33.Johnstone DM, el Massri N, Moro C, Spana S, Wang XS, Torres N, Chabrol C, De Jaeger X, Reinhart F, Purushothuman S, Benabid AL, Stone J, Mitrofanis J. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism - an abscopal neuroprotective effect. Neuroscience. 2014;274:93–101. doi: 10.1016/j.neuroscience.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 34.Johnstone DM, Mitrofanis J, Stone J. Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light. Neural Regen Res. 2015;10:349–351. doi: 10.4103/1673-5374.153673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farfara D, Tuby H, Trudler D, Doron-Mandel E, Maltz L, Vassar RJ, Frenkel D, Oron U. Low-level laser therapy ameliorates disease progression in a mouse model of Alzheimer’s disease. J Mol Neurosci. 2015;55:430–436. doi: 10.1007/s12031-014-0354-z. [DOI] [PubMed] [Google Scholar]
- 36.Oron A, Oron U. Low-Level Laser Therapy to the Bone Marrow Ameliorates Neurodegenerative Disease Progression in a Mouse Model of Alzheimer’s Disease: A Minireview. Photomed Laser Surg. 2016 doi: 10.1089/pho.2015.4072. [DOI] [PubMed] [Google Scholar]
- 37.Iwashita T, Tada T, Zhan H, Tanaka Y, Hongo K. Harvesting blood stem cells from cranial bone at craniotomy--a preliminary study. J Neurooncol. 2003;64:265–270. doi: 10.1023/a:1025684903137. [DOI] [PubMed] [Google Scholar]
- 38.Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J, Hamblin MR, Knight JA, Meehan WP, 3rd, Baker EH. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma. 2014;31:1008–1017. doi: 10.1089/neu.2013.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maksimovich IV. Dementia and Cognitive Impairment Reduction after Laser Transcatheter Treatment of Alzheimer’s Disease. World J Neurosci. 2015;5 [Google Scholar]
- 40.Moro C, Massri NE, Torres N, Ratel D, De Jaeger X, Chabrol C, Perraut F, Bourgerette A, Berger M, Purushothuman S, Johnstone D, Stone J, Mitrofanis J, Benabid AL. Photobiomodulation inside the brain: a novel method of applying near-infrared light intracranially and its impact on dopaminergic cell survival in MPTP-treated mice. J Neurosurg. 2014;120:670–683. doi: 10.3171/2013.9.JNS13423. [DOI] [PubMed] [Google Scholar]
- 41.Moro C, El Massri N, Darlot F, Torres N, Chabrol C, Agay D, Auboiroux V, Johnstone DM, Stone J, Mitrofanis J, Benabid AL. Effects of a higher dose of near-infrared light on clinical signs and neuroprotection in a monkey model of Parkinson’s disease. Brain Res. 2016;1648:19–26. doi: 10.1016/j.brainres.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Liu TCY, Cheng L, Su WJ, Zhang YW, Shi Y, Liu AH, Zhang LL, Qian ZY. Randomized, Double-Blind, and Placebo-Controlled Clinic Report of Intranasal Low-Intensity Laser Therapy on Vascular Diseases. Int J Photoenergy. 2012;2012:489713–489718. [Google Scholar]
- 43.Lim L. The Potential of Treating Alzheimer’s disease with Intranasal Light Therapy. Toronto: MedicLights Research Inc; 2013. [Google Scholar]
- 44.Duan D, Lu M. Olfactory mucosa: a rich source of cell therapy for central nervous system repair. Rev Neurosci. 2015;26:281–293. doi: 10.1515/revneuro-2014-0065. [DOI] [PubMed] [Google Scholar]
- 45.Quah-Smith I, Williams MA, Lundeberg T, Suo C, Sachdev P. Differential brain effects of laser and needle acupuncture at LR8 using functional MRI. Acupunct Med. 2013;31:282–289. doi: 10.1136/acupmed-2012-010297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quah-Smith I, Sachdev PS, Wen W, Chen X, Williams MA. The brain effects of laser acupuncture in healthy individuals: an FMRI investigation. PLoS One. 2010;5:e12619. doi: 10.1371/journal.pone.0012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutalangka C, Wattanathorn J, Muchimapura S, Thukham-Mee W, Wannanon P, Tong-un T. Laser acupuncture improves memory impairment in an animal model of Alzheimer’s disease. J Acupunct Meridian Stud. 2013;6:247–251. doi: 10.1016/j.jams.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Khongrum J, Wattanathorn J. Laser Acupuncture Improves Behavioral Disorders and Brain Oxidative Stress Status in the Valproic Acid Rat Model of Autism. J Acupunct Meridian Stud. 2015;8:183–191. doi: 10.1016/j.jams.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Quah-Smith I, Suo C, Williams MA, Sachdev PS. The Antidepressant Effect of Laser Acupuncture: A Comparison of the Resting Brain’s Default Mode Network in Healthy and Depressed Subjects During Functional Magnetic Resonance Imaging. Med Acupunct. 2013;25:124–133. doi: 10.1089/acu.2012.0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henderson TA. Multi-watt near-infrared light therapy as a neuroregenerative treatment for traumatic brain injury. Neural Regen Res. 2016;11:563–565. doi: 10.4103/1673-5374.180737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hacke W, Schellinger PD, Albers GW, Bornstein NM, Dahlof BL, Fulton R, Kasner SE, Shuaib A, Richieri SP, Dilly SG, Zivin J, Lees KR, Committees N. Investigators, Transcranial laser therapy in acute stroke treatment: results of neurothera effectiveness and safety trial 3, a phase III clinical end point device trial. Stroke. 2014;45:3187–3193. doi: 10.1161/STROKEAHA.114.005795. [DOI] [PubMed] [Google Scholar]
- 52.Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat. 2015;11:2191–2208. doi: 10.2147/NDT.S78182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cassano P, Petrie SR, Hamblin MR, Henderson TA, Iosifescu DV. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics. 2016;3:031404. doi: 10.1117/1.NPh.3.3.031404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma SK, Kharkwal GB, Sajo M, Huang YY, De Taboada L, McCarthy T, Hamblin MR. Dose response effects of 810 nm laser light on mouse primary cortical neurons. Lasers Surg Med. 2011;43:851–859. doi: 10.1002/lsm.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bragin DE, Statom GL, Hagberg S, Nemoto EM. Increases in microvascular perfusion and tissue oxygenation via pulsed electromagnetic fields in the healthy rat brain. J Neurosurg. 2015;122:1239–1247. doi: 10.3171/2014.8.JNS132083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang J, Liu L, Xing D. Photobiomodulation by low-power laser irradiation attenuates Abeta-induced cell apoptosis through the Akt/GSK3beta/beta-catenin pathway. Free Radic Biol Med. 2012;53:1459–1467. doi: 10.1016/j.freeradbiomed.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, Zhang Y, Xing D. LPLI inhibits apoptosis upstream of Bax translocation via a GSK-3beta-inactivation mechanism. J Cell Physiol. 2010;224:218–228. doi: 10.1002/jcp.22123. [DOI] [PubMed] [Google Scholar]
- 58.Ling Q, Meng C, Chen Q, Xing D. Activated ERK/FOXM1 pathway by low-power laser irradiation inhibits UVB-induced senescence through down-regulating p21 expression. J Cell Physiol. 2014;229:108–116. doi: 10.1002/jcp.24425. [DOI] [PubMed] [Google Scholar]
- 59.Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, Whelan NT, Whelan HT. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A. 2003;100:3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 61.Huang YY, Nagata K, Tedford CE, McCarthy T, Hamblin MR. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J Biophotonics. 2013;6:829–838. doi: 10.1002/jbio.201200157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang YY, Nagata K, Tedford CE, Hamblin MR. Low-level laser therapy (810 nm) protects primary cortical neurons against excitotoxicity in vitro. J Biophotonics. 2014;7:656–664. doi: 10.1002/jbio.201300125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maurya PK, Noto C, Rizzo LB, Rios AC, Nunes SO, Barbosa DS, Sethi S, Zeni M, Mansur RB, Maes M, Brietzke E. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:134–144. doi: 10.1016/j.pnpbp.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Tramutola A, Lanzillotta C, Perluigi M, Butterfield DA. Oxidative stress, protein modification and Alzheimer disease. Brain Res Bull. 2016 doi: 10.1016/j.brainresbull.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Mungrue IN, Husain M, Stewart DJ. The role of NOS in heart failure: lessons from murine genetic models. Heart Fail Rev. 2002;7:407–422. doi: 10.1023/a:1020762401408. [DOI] [PubMed] [Google Scholar]
- 66.Ahmed I, Bose SK, Pavese N, Ramlackhansingh A, Turkheimer F, Hotton G, Hammers A, Brooks DJ. Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain. 2011;134:979–986. doi: 10.1093/brain/awr028. [DOI] [PubMed] [Google Scholar]
- 67.Assis L, Moretti AI, Abrahao TB, de Souza HP, Hamblin MR, Parizotto NA. Low-level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med Sci. 2013;28:947–955. doi: 10.1007/s10103-012-1183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cury V, Moretti AI, Assis L, Bossini P, Crusca Jde S, Neto CB, Fangel R, de Souza HP, Hamblin MR, Parizotto NA. Low level laser therapy increases angiogenesis in a model of ischemic skin flap in rats mediated by VEGF, HIF-1alpha and MMP-2. J Photochem Photobiol B. 2013;125:164–170. doi: 10.1016/j.jphotobiol.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim W, Kim J, Kim S, Karna S, Won J, Jeon SM, Kim SY, Choi Y, Choi H, Kim O. Modulation of lipopolysaccharide-induced NF-kappaB signaling pathway by 635 nm irradiation via heat shock protein 27 in human gingival fibroblast cells. Photochem Photobiol. 2013;89:199–207. doi: 10.1111/j.1751-1097.2012.01225.x. [DOI] [PubMed] [Google Scholar]
- 70.Storz P, Mitochondrial ROS--radical detoxification. mediated by protein kinase D. Trends Cell Biol. 2007;17:13–18. doi: 10.1016/j.tcb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Meng C, He Z, Xing D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: implications for Alzheimer’s disease. J Neurosci. 2013;33:13505–13517. doi: 10.1523/JNEUROSCI.0918-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marte A, Messa M, Benfenati F, Onofri F. Synapsins Are Downstream Players of the BDNF-Mediated Axonal Growth. Mol Neurobiol. 2016 doi: 10.1007/s12035-015-9659-3. [DOI] [PubMed] [Google Scholar]
- 73.YYW Huang Q, Xuan W, Ando T, Xu T, Sharma SK, Kharkwal GB, Hamblin MR. Low Level Light Therapy for Traumatic Brain Injury [Google Scholar]
- 74.Xuan W, Vatansever F, Huang L, Hamblin MR. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J Biomed Opt. 2014;19:108003. doi: 10.1117/1.JBO.19.10.108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lapchak PA, Salgado KF, Chao CH, Zivin JA. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148:907–914. doi: 10.1016/j.neuroscience.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 76.Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke. 2004;35:1985–1988. doi: 10.1161/01.STR.0000131808.69640.b7. [DOI] [PubMed] [Google Scholar]
- 77.Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M, Lampl Y, Streeter J, DeTaboada L, Chopp M. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37:2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- 78.Zivin JA, Sehra R, Shoshoo A, Albers GW, Bornstein NM, Dahlof B, Kasner SE, Howard G, Shuaib A, Streeter J, Richieri SP, Hacke W. N.-. investigators, NeuroThera(R) Efficacy and Safety Trial-3 (NEST-3): a double-blind, randomized, sham-controlled, parallel group, multicenter, pivotal study to assess the safety and efficacy of transcranial laser therapy with the NeuroThera(R) Laser System for the treatment of acute ischemic stroke within 24 h of stroke onset. Int J Stroke. 2014;9:950–955. doi: 10.1111/j.1747-4949.2012.00896.x. [DOI] [PubMed] [Google Scholar]
- 79.Lapchak PA, Boitano PD. Transcranial Near-Infrared Laser Therapy for Stroke: How to Recover from Futility in the NEST-3 Clinical Trial. Acta Neurochir Suppl. 2016;121:7–12. doi: 10.1007/978-3-319-18497-5_2. [DOI] [PubMed] [Google Scholar]
- 80.Naeser MA, Stiassny-Eder D, Galler V, Hobbs J, Bachman D, Lannin L. Laser acupuncture in the treatment of paralysis in stroke patients: a CT scan lesion site study. Am J Acupuncture. 1995;23:13–28. [Google Scholar]
- 81.Doidge N. The Brain’s Way of Healing: Remarkable Discoveries and Recoveries from the Frontiers of Neuroplasticity. New York, NY: Viking Press; 2015. [Google Scholar]
- 82.Karve IP, Taylor JM, Crack PJ. The contribution of astrocytes and microglia to traumatic brain injury. Br J Pharmacol. 2016;173:692–702. doi: 10.1111/bph.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, Patel MC, Counsell SJ, Sharp DJ. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moro C, Torres N, El Massri N, Ratel D, Johnstone DM, Stone J, Mitrofanis J, Benabid AL. Photobiomodulation preserves behaviour and midbrain dopaminergic cells from MPTP toxicity: evidence from two mouse strains. BMC Neurosci. 2013;14:40. doi: 10.1186/1471-2202-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Purushothuman S, Nandasena C, Johnstone DM, Stone J, Mitrofanis J. The impact of near-infrared light on dopaminergic cell survival in a transgenic mouse model of parkinsonism. Brain Res. 2013;1535:61–70. doi: 10.1016/j.brainres.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 86.El Massri N, Moro C, Torres N, Darlot F, Agay D, Chabrol C, Johnstone DM, Stone J, Benabid AL, Mitrofanis J. Near-infrared light treatment reduces astrogliosis in MPTP-treated monkeys. Exp Brain Res. 2016 doi: 10.1007/s00221-016-4720-7. [DOI] [PubMed] [Google Scholar]
- 87.Moro C, Massri NE, Darlot F, Torres N, Chabrol C, Agay D, Auboiroux V, Johnstone DM, Stone J, Mitrofanis J, Benabid AL. Effects of a higher dose of near-infrared light on clinical signs and neuroprotection in a monkey model of Parkinson’s disease. Brain Res. 2016 doi: 10.1016/j.brainres.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 88.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer’s disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 89.Wijesinghe P, Shankar SK, Yasha TC, Gorrie C, Amaratunga D, Hulathduwa S, Kumara KS, Samarasinghe K, Suh YH, Steinbusch HW, De Silva KR. Vascular Contributions in Alzheimer’s Disease-Related Neuropathological Changes: First Autopsy Evidence from a South Asian Aging Population. J Alzheimers Dis. 2016 doi: 10.3233/JAD-160425. [DOI] [PubMed] [Google Scholar]
- 90.Shcherbatykh I, Carpenter DO. The role of metals in the etiology of Alzheimer’s disease. J Alzheimers Dis. 2007;11:191–205. doi: 10.3233/jad-2007-11207. [DOI] [PubMed] [Google Scholar]
- 91.De Taboada L, Yu J, El-Amouri S, Gattoni-Celli S, Richieri S, McCarthy T, Streeter J, Kindy MS. Transcranial laser therapy attenuates amyloid-beta peptide neuropathology in amyloid-beta protein precursor transgenic mice. J Alzheimers Dis. 2011;23:521–535. doi: 10.3233/JAD-2010-100894. [DOI] [PubMed] [Google Scholar]
- 92.Purushothuman S, Johnstone DM, Nandasena C, Mitrofanis J, Stone J. Photobiomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex - evidence from two transgenic mouse models. Alzheimers Res Ther. 2014;6:2. doi: 10.1186/alzrt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Purushothuman S, Johnstone DM, Nandasena C, Eersel J, Ittner LM, Mitrofanis J, Stone J. Near infrared light mitigates cerebellar pathology in transgenic mouse models of dementia. Neurosci Lett. 2015;591:155–159. doi: 10.1016/j.neulet.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 94.Rojas JC, Bruchey AK, Gonzalez-Lima F. Low-level light therapy improves cortical metabolic capacity and memory retention. J Alzheimers Dis. 2012;32:741–752. doi: 10.3233/JAD-2012-120817. [DOI] [PubMed] [Google Scholar]
- 95.Pandya M, Altinay M, Malone DA, Jr, Anand A. Where in the brain is depression? Curr Psychiatry Rep. 2012;14:634–642. doi: 10.1007/s11920-012-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sahay A, Drew MR, Hen R. Dentate gyrus neurogenesis and depression. Prog Brain Res. 2007;163:697–722. doi: 10.1016/S0079-6123(07)63038-6. [DOI] [PubMed] [Google Scholar]
- 97.Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 100.Schiffer F, Johnston AL, Ravichandran C, Polcari A, Teicher MH, Webb RH, Hamblin MR. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct. 2009;5:46. doi: 10.1186/1744-9081-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cassano P, Cusin C, Mischoulon D, Hamblin MR, De Taboada L, Pisoni A, Chang T, Yeung A, Ionescu DF, Petrie SR, Nierenberg AA, Fava M, Iosifescu DV. Near-Infrared Transcranial Radiation for Major Depressive Disorder: Proof of Concept Study. Psychiatry J. 2015;2015:352979. doi: 10.1155/2015/352979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morries LD, Cassano P, Henderson TA. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr Dis Treat. 2015;11:2159–2175. doi: 10.2147/NDT.S65809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. 2013;230:13–23. doi: 10.1016/j.neuroscience.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 104.Gonzalez-Lima F, Barrett DW. Augmentation of cognitive brain functions with transcranial lasers. Front Syst Neurosci. 2014;8:36. doi: 10.3389/fnsys.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kaur H, Prakash A, Medhi B. Drug therapy in stroke: from preclinical to clinical studies. Pharmacology. 2013;92:324–334. doi: 10.1159/000356320. [DOI] [PubMed] [Google Scholar]
- 106.Maas AI, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics. 2010;7:115–126. doi: 10.1016/j.nurt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kulshreshtha A, Piplani P. Current pharmacotherapy and putative disease-modifying therapy for Alzheimer’s disease. Neurol Sci. 2016;37:1403–1435. doi: 10.1007/s10072-016-2625-7. [DOI] [PubMed] [Google Scholar]
- 108.Potocnjak I, Degoricija V, Vukicevic Baudoin D, Culig J, Jakovljevic M. Cardiovascular side effects of psychopharmacologic therapy. Int J Cardiol. 2016;219:367–372. doi: 10.1016/j.ijcard.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 109.Givens CJ. Adverse Drug Reactions Associated with Antipsychotics, Antidepressants, Mood Stabilizers, and Stimulants. Nurs Clin North Am. 2016;51:309–321. doi: 10.1016/j.cnur.2016.01.013. [DOI] [PubMed] [Google Scholar]