Abstract
Background
Racial/ethnic minority groups remain underrepresented in clinical trials. Many strategies to increase minority recruitment focus on minority communities, and emphasize common diseases such as hypertension. Scant literature focuses on minority recruitment to trials of less common conditions, often conducted in specialty clinics, and dependent on physician referrals. We identified trust/mistrust of specialist physician investigators and institutions conducting medical research and consequent participant reluctance to participate in clinical trials as key-shared barriers across racial/ethnic groups. We developed a trust-based continuous quality improvement (CQI) intervention to build trust between specialist physician investigators and community minority-serving physicians and ultimately potential trial participants. To avoid the inherent biases of non-randomized studies, we evaluated the intervention in the national Randomized Recruitment Intervention Trial (RECRUIT). This report presents the design of RECRUIT. Specialty clinic follow-up continues through April 2017.
Methods
We hypothesized that specialist physician investigators and coordinators trained in the trust-based CQI intervention would enroll a greater proportion of minority participants in their specialty clinics than specialist physician investigators in control specialty clinics. Specialty clinic was the unit of randomization. Using CQI, the specialist physician investigators and coordinators tailored recruitment approaches to their specialty clinic characteristics and populations. Primary analyses were adjusted for clustering by specialty clinic within parent trial and matching covariates.
Results
RECRUIT was implemented in four multi-site clinical trials (parent trials) supported by three NIH Institutes and included 50 associated specialty clinics from these parent trials. Using current data, we have 88% power or greater to detect a 0.15 or greater difference from the currently observed control proportion adjusting for clustering. We detected no differences in baseline matching criteria between intervention and control specialty clinics (all p-values >0.17).
Conclusions
RECRUIT was the first multi-site randomized control trial to examine the effectiveness of a trust-based CQI intervention to increase minority recruitment into clinical trials. RECRUIT’s innovations included its focus on building trust between specialist investigators and minority-serving physicians, the use of CQI to tailor the intervention to each specialty clinic’s specific racial/ethnic populations and barriers to minority recruitment, and the use of specialty clinics from more than one parent multi-site trial to increase generalizability. The effectiveness of the RECRUIT intervention will be determined after the completion of trial data collection and planned analyses.
Keywords: Minority recruitment, intervention mapping, cluster randomized trial, trust, continuous quality improvement
Background
Higher rates of morbidity and mortality are observed for most racial/ethnic minority groups for many diseases.1, 2 Inclusion of diverse racial and ethnic groups in clinical trials allows probing for differences in intervention response potentially related to genetic or environmental variability,3–8 pathophysiologic, or social factors contributing to disease activity or severity. Yet, racial/ethnic minority groups remain underrepresented in clinical trials.9–11
There is an extensive literature on facilitators and barriers to minority recruitment to clinical trials12 and on approaches to increasing minority enrollment in clinical trials.13–15 Most studies of recruitment approaches were anecdotal or used pre-post designs; few were randomized trials. Many strategies directly targeted minority communities, and focused on common diseases such as hypertension, kidney failure, or diabetes. Few strategies focused on facilitating minority recruitment to trials of less common conditions, often conducted in specialty clinics, and dependent on physician referrals.
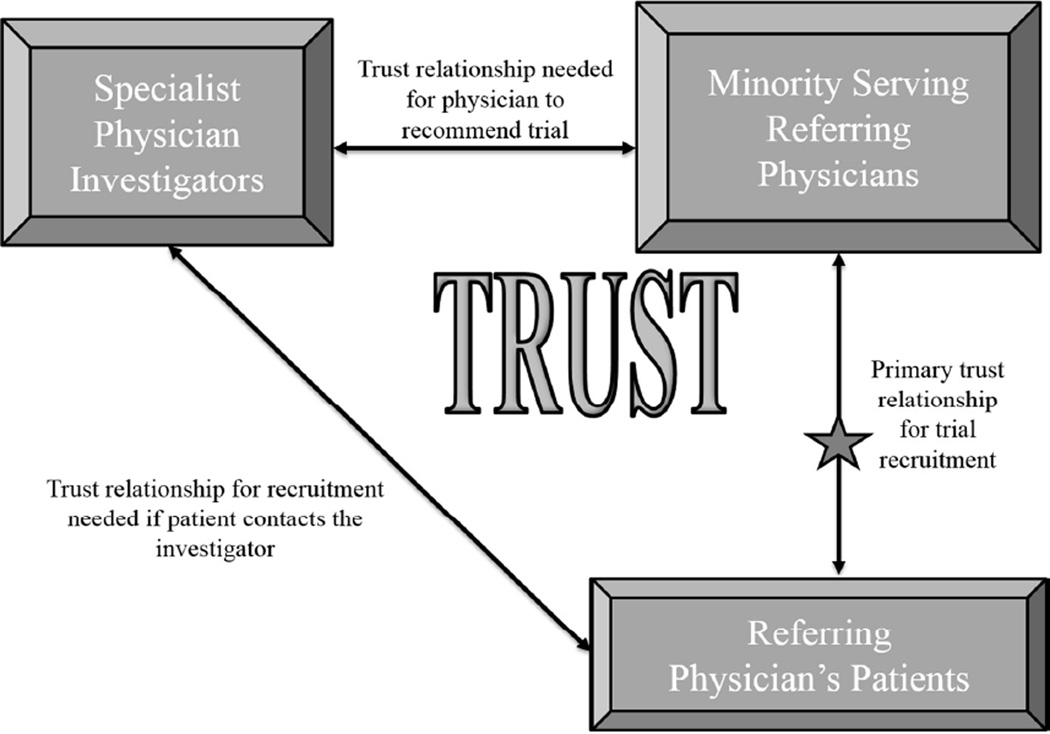
We identified patient trust/mistrust of medical researchers and institutions conducting medical research16 and consequent reluctance to participate in clinical trials as key-shared barriers across racial/ethnic minorities. The underlying reasons for mistrust differed among minority groups, but lack of trust was a common theme.17 Some minorities were more likely to participate in clinical trials if their own physician recommended consideration of the trial.18, 19 Some minority-serving community physicians shared the distrust of medical researchers observed in their patients and were reluctant to refer their patients to clinical trials in specialty clinics.20–22 To address trust-related barriers to minority recruitment in specialty clinics we developed a trust-based continuous quality improvement (CQI) intervention23 implemented in specialty clinics recruited from multi-site randomized trials (parent trials). A key intervention component was trust building between specialist physician investigators and minority-serving physicians and ultimately potential trial participants. We expected that by promoting personal trusting relationships, referrals from the minority-serving physicians would increase. We expected that a patient’s trust in his/her physician could be transferred, in part, to the specialist investigator, and that a referred patient would be likely to engage in trial participation (Figure 1).
Figure 1.
Trust Triangle
To avoid the inherent biases of non-randomized studies and provide Level 1 clinical trial evidence24, 25 we evaluated the trust-based CQI minority recruitment intervention in the National Institute on Minority Health and Health Disparities (NIMHD)-funded Randomized Recruitment Intervention Trial (RECRUIT). In this report, we describe the complex, innovative RECRUIT design and intervention, and baseline characteristics of the participating specialty clinics, specialist investigators, and specialty clinic coordinators. Parent trial identification began in January 2012 and recruitment of associated specialty clinics into RECRUIT concluded in April 2015. RECRUIT follow-up continues through April 2017, precluding early presentation of trial results in order to avoid bias and reporting of misleading results.
Methods
Primary hypothesis
We tested the hypothesis that intervention specialty clinics trained in RECRUIT’s trust-based CQI intervention that emphasized building trust between specialist investigators and minority-serving physicians would enroll a greater proportion of minority participants than control specialty clinics. We defined minority participants as individuals from racial/ethnic groups historically underrepresented in clinical trials, including Blacks/African Americans, Hispanics/Latinos, Asians, American Indians/Alaska Natives, and Native Hawaiians/Other Pacific Islanders.26 Race/ethnicity was determined using methods from the parent trials (e.g., participant-response to pre-set questions, open-ended self-identification, or both).
Trial design and eligibility
RECRUIT was a cluster randomized trial that included eligible “parent trials” and their associated specialty clinics. In soliciting “parent” trials whose specialty clinics were randomized to RECRUIT, the following parent trial characteristics were required: parent trial recruitment relied primarily on physician referrals to specialty clinics; increasing minority recruitment was a parent trial goal; a randomized design was employed by the parent trial; the parent trial included at least six eligible specialty clinics, each expected to recruit at least 10 trial participants; the parent trial sponsor was committed to minority recruitment and endorsed RECRUIT participation; and transportation assistance for parent trial participants was available or RECRUIT funds could be used.
Specialty clinics from the eligible parent trials were invited to participate in RECRUIT if they met the following criteria: at least 20% of the population in the trial age group residing within 30 miles of the specialty clinic were minorities; for an ongoing trial, no more than 15% of parent trial participants enrolled at the specialty clinic at the time of randomization to RECRUIT were minorities; the specialty clinic needed to recruit at least 10 additional participants; the specialist investigator (Principal Investigator or Co-Principal Investigator at the specialty clinic) and specialty clinic coordinator consented to participate in RECRUIT and in RECRUIT’s intervention training if randomized to the intervention group.
Recruitment of parent trials and associated specialty clinics
The RECRUIT principal investigator visited project scientists within NIH Institutes, and had multiple contacts with clinical trials’ researchers. The NIMHD Project Scientist announced the opening of RECRUIT for parent trial participation to Project Scientists across NIH. The RECRUIT team also contacted trials identified through http://www.clinicaltrials.gov. Once the leaders of an eligible parent trial agreed to participate in RECRUIT, we contacted eligible associated specialty clinics. We invited specialist investigators and coordinators to a webinar about RECRUIT requirements for participation. RECRUIT team members documented reasons for refusal by parent trials and specialty clinics.
Incentives
Parent trial coordinating centers and consenting intervention specialty clinics each received $22,000 as partial reimbursement for time and effort. Consenting control specialty clinics each received $5,000. Reimbursements were processed using “site agreements” or sub-contracts. Funds supported recruitment efforts or any institutionally allowed expense other than tuition or equipment costing $5,000 or more. All specialist investigators and coordinators who completed key informant interviews (Table 1) at the end of RECRUIT received a $250 Amazon gift card if permitted by their institution.
Table 1.
Data Collection
| Instrument | Primary Data Collected | Completed by | Time Point |
|---|---|---|---|
|
Specialty Clinic GIS Information |
Population percentages within 30 miles of specialty clinic, minority, foreign born, non-English speaking; other socio- economic and healthcare access markers |
RECRUIT Team from Census Data | Before determination of eligibility |
|
Specialist Investigator Demographics Form |
Demographics, clinical trial experience, Average number of patients on protocol for any trial at a specialty clinic |
Specialist Investigator | Baseline, consenting specialty clinics |
|
Coordinator Demographics Form |
Demographics, education, clinical trial experience |
Coordinator | Baseline, consenting specialty clinics |
|
Self-Efficacy and Outcome Expectations Questionnaires36 |
Confidence in planning effective recruitment, implementing a plan to recruit minorities, communicating with patients, communicating with recruitment sources |
Specialist Investigators and Coordinators (separate forms) |
Kick Off End of modules End of intervention |
| Enrollment Logs | Patient age category, gender, ethnicity, and date of enrollment |
Parent Trial Coordinating Center | Monthly |
| Screening Logs** | Race/Ethnicity, age category, gender referral source, reason for refusal |
Coordinator or Parent Trial Coordinating Center |
Monthly |
|
Recruitment Activity Checklists** |
Frequency of common recruitment activity types |
Coordinator | Monthly |
|
Patient Satisfaction Survey78,79 ** |
Anonymous satisfaction rating on interactions with clinic staff, convenience of visit, and comfort level |
All screened potential participants and enrolled parent trial participants (voluntary) |
Every Screening and Study Visit |
|
Key Informant Interview67 |
Semi-structured open-ended questions about minority recruitment strategies and intervention components |
Interviewer independent of RECRUIT Team interviewing Specialist investigators’ and coordinators’ from consenting specialty clinics |
End of the RECRUIT intervention |
Specialty clinics are followed for two years from enrollment in RECRUIT or until the end of recruitment for their parent trial, whichever occurs first.
Randomization and masking
Specialty clinic was the unit of randomization. The RECRUIT team randomized consenting specialty clinics from eligible parent trials to receive RECRUIT’s trust-based CQI intervention or to control using stratification by parent trial. Within the parent trial, consenting specialty clinics were pair-matched on total percent minorities within a 30-mile radius of the specialty clinic, and geographic region. Within each pair, we randomly allocated clinics to intervention or control. We randomized individual specialty clinics joining late without matching using simple randomization. Because the trial was unmasked, we asked intervention, specialty clinic staff not to discuss the intervention or training modules with the control, specialty clinic staff. Control specialty clinic staff received no additional training or advice from the RECRUIT team. All specialty clinic staff in both the intervention and control specialty clinics followed any additional recruitment procedures advocated by the parent trials.
Developing intervention components and pilot testing
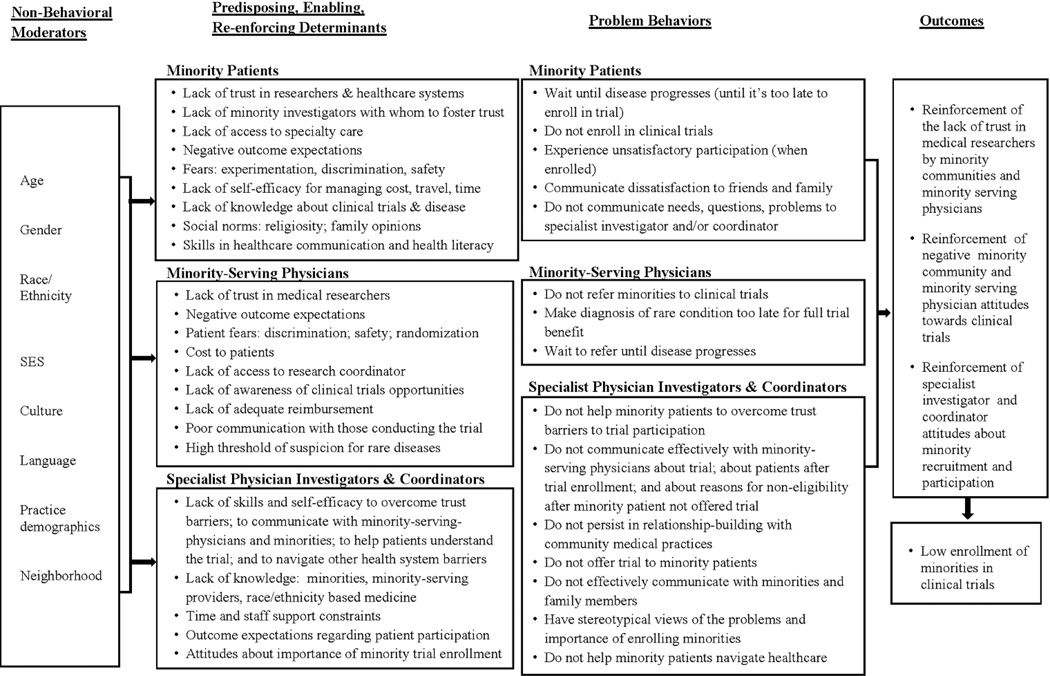
We employed Intervention Mapping, a systematic framework to develop and test the behavioral intervention.27 Following intervention mapping procedures we developed a logic model of the problem of low minority recruitment (Figure 2)27, 28 after review of empirical evidence from the literature17–19, 29 and our own past research.16, 22, 30–32 The logic model described reasons for underrepresentation of minorities in clinical trials related to behaviors of minority patients, minority-serving physicians, specialist physician investigators, and coordinators in specialty clinics.
Figure 2.
Logic Model of potential determinants of low-minority recruitment
Next we identified theory-based methods33–36 and practical applications to address specialist investigator and coordinators’ knowledge, skills, outcome expectations, and self-efficacy for building trust with minority-serving physicians and with potential minority participants into trials. Based on these methods and practical applications we created RECRUIT’s intervention content and materials. Delivery of the intervention components was organized as described in a literature review by Eisenberg.37 We used CQI methods to encourage specialty clinic staff to tailor the intervention to the specific barriers and populations under study at their clinic. We included methods from patient navigation38 in the intervention to reduce instrumental barriers (e.g. cost, childcare, transportation) for minority participants.
We pilot tested RECRUIT’s intervention in nine domestic clinics from the ASPirin in Reducing Events in the Elderly trial.39 After the pilot, the RECRUIT team increased training emphasis on the key concept of increasing trust between specialist investigators and minority-serving physicians, shortened the intervention training, and included more follow-up with intervention specialty clinic staff.
Intervention delivery
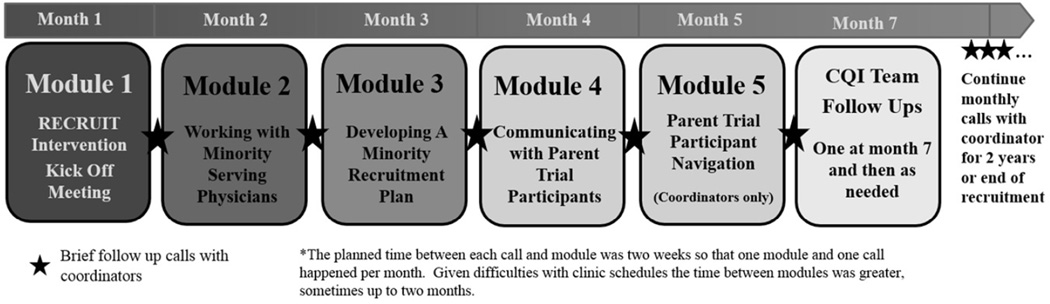
Figure 3 outlines the final form of RECRUIT’s intervention delivery timeline and components. RECRUIT’s intervention guided specialist investigators and coordinators in making process changes to enhance trust and increase minority recruitment. CQI teams, a specialist investigator and coordinator, at each intervention specialty clinic implemented changes to the recruitment process within their clinics and added other members to the CQI team if needed. Beyond strongly encouraging direct contact between specialist investigators and minority-serving physicians, the RECRUIT team did not prescribe particular recruitment strategies, but offered approaches for specialty clinics to identify recruitment barriers and develop site-specific recruitment strategies. Group meetings and webinars were usually held with CQI teams in specialty clinics from the same parent trial. The RECRUIT team provided make-up sessions if needed.
Figure 3.
Intervention Timeline
Module 1, the Kick-Off was a mandatory, in-person, six-hour session with RECRUIT team members and the CQI specialty clinic teams. The session emphasized the importance of minority recruitment, and the central role of minority serving physicians in minority recruitment efforts. The RECRUIT team presented available information on potential minority differences in incidence and prevalence of the parent trial condition, and potential differences in response to the parent trial treatment. The RECRUIT team presented the NIH/FDA perspective on minority recruitment, a discussion of current knowledge about minority recruitment strategies, and information on the importance of the Trust Triangle (Figure 1) with an emphasis on building trust between specialist investigators and minority serving physicians.
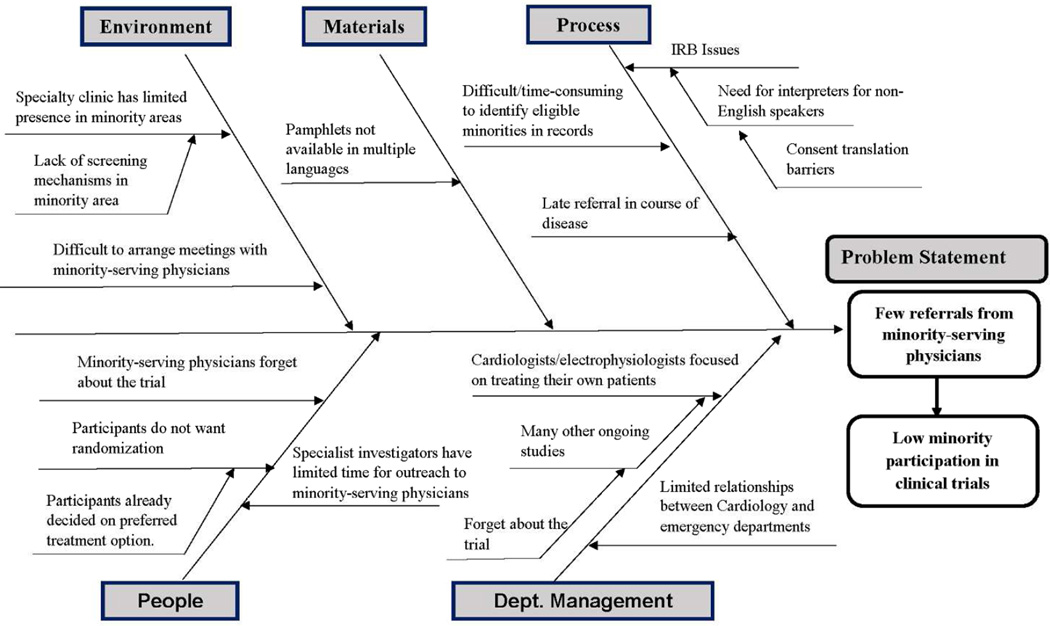
To facilitate specialty clinic-specific tailoring, each CQI team developed a flow-chart40 of its current minority recruitment process, and a fishbone diagram40 detailing potential barriers to building relationships with minority-serving physicians and other barriers to increasing minority recruitment. The fishbone diagram shown in Figure 4 aggregated barriers identified by various CQI teams in the Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA). Not all CABANA specialty clinics shared the same barriers. Each CQI team left the Kick-Off with a draft fishbone specific to their own specialty clinic and ideas for increasing referrals from minority serving physicians. Each specialty clinic received maps from the US Census describing the distribution of minority groups within 30 miles of their specialty clinic where minority-serving physicians might be located. The CQI teams developed a Plan-Do-Study-Act approach23 to improve minority-serving physician referrals and minority recruitment at their specialty clinic. CQI team members were cautioned not to attempt multiple changes at the same time. After Module 1, each module began with the CQI teams’ progress update.
Figure 4.
Fishbone Example of Barriers from Across Arrhythmia Specialty Clinics.
Modules 2 through 5 were webinars. Most webinars included videos modeling possible approaches to minority-serving physicians or minority participants. Module 2 focused on communications with minority-serving physicians and stressed the importance of personal contact by the specialist investigators. CQI team members were advised to avoid sending brochures, or letters, or sending coordinators to the minority-serving practices before making physician-to-physician contacts. A video modeled a specialist investigator’s discussion with a minority-serving physician using physician-centered communication skills, i.e., use of open-ended questions to draw-out concerns of minority-serving physicians, restating concerns so the minority-serving physicians knew they were understood, and then addressing the concerns.
In Module 3, the CQI teams presented their revised fishbone diagrams and CQI plans to other CQI teams for feedback. Module 4 focused on increasing patient-centered communication skills using the same open-ended method described in Module 2 for physician-centered communication. Videos modeled a specialist investigator asking open-ended questions, and addressing a minority patient’s concerns about participation, randomization, and about being “a guinea pig.”
Module 5, for coordinators, focused on instrumental aspects of patient navigation. A video modeled a coordinator using patient-centered communication to identify and address patient concerns about instrumental barriers to participation (e.g. time, childcare, cost, etc.). Coordinators developed a list of resources at their institutions to address instrumental barriers to trial participation including patient navigators, if available at the specialty clinic.
On a group call two months after the completion of the modules, CQI teams shared successful strategies and were encouraged to continue updating and evaluating their strategies. Until RECRUIT participation ended for a CQI team, a RECRUIT team member held individual monthly calls with specialty clinic coordinators to continue the Plan-Do-Study-Act process by providing encouragement and help with problem solving, and reminders regarding the trust-based approach being tested. Additional unstructured CQI team calls were added as needed. The RECRUIT Statistical Center provided CQI teams periodic reports on cumulative screening, and recruitment statistics for all intervention specialty clinics in their parent trial. Throughout RECRUIT participation, specialty clinic staff distributed satisfaction surveys to screened patients and trial participants and CQI teams received quarterly tabulations of their satisfaction surveys. Control specialist investigators and coordinators received patient satisfaction summaries after RECRUIT completion.
Data collection
Follow-up in specialty clinics continued for two years from RECRUIT enrollment or until the end of parent trial recruitment, whichever occurred first. Table 1 lists the trial data collected and the timing of data collection.
Specialty physicians and coordinators in the intervention group completed questionnaires on self-efficacy and outcome expectations at baseline and at the end the intervention training, and at the end of their participation in RECRUIT. At the end of formal data collection for RECRUIT, all specialist investigators and coordinators were asked to participate in a key informant interview by an interviewer, independent of the RECRUIT team.
Planned data analyses
Primary outcome
Specialty clinic was the unit of analysis. Analysis was by intent to treat. The primary outcome was the proportion of minority participants recruited across intervention and control specialty clinics respectively. In specialty clinics, proportions were based on de-identified data provided by the parent trial coordinating centers. Analysis used a generalized estimating equations analytical framework with logit link function and independence working correlation matrix to account for clustering by specialty clinic within parent trial and matching variables as covariates. The 50 clusters (specialty clinics) enrolled exceed the 40 clusters required for valid inference when applying generalized estimating equations using the sandwich estimator.41 Specialty clinic matching criteria were included as covariates in analysis rather than using a matched-pairs analysis to avoid a reduction in power for limited gain.42
Secondary outcomes
Intervention and control specialty clinics were compared on percent minorities screened, percent minorities enrolled of those minorities screened, patients’ satisfaction scores, and recruitment activities logs. Generalized estimating equations methods accounted for clustering with appropriate choice of link functions, and assumed an independence working correlation matrix. Scales measuring self efficacy and outcome expectations of intervention specialist investigators and coordinators were examined as predictors of minority recruitment. In analyses of scales there was no adjustment for clustering unless more than one investigator or coordinator participated per specialty clinic.
Key informant interviews were transcribed verbatim and analyzed thematically using a qualitative data analysis program, Atlas.ti (7.5.10 Version). Thematic content analysis was based on an inductive coding process to identify and aggregate themes by their hierarchical relationships. Qualitative data were used to enhance understanding of specialty clinics’ experiences with minority recruitment and with the RECRUIT trial.
We planned additional descriptive sub-group analyses of the primary and secondary outcomes, screening and recruitment logs, and recruitment data by race/ethnicity, parent trial, and specialty clinics’ characteristics. These sub-group analyses had limited power given the expected sample sizes.
Planned approach to missing data
If a specialty clinic was dropped by a parent trial, we used percent minorities enrolled at that time. If a specialty clinic dropped out of RECRUIT, we used data provided by the parent trial coordinating center on percent minorities recruited at the end of the RECRUIT trial period. Formal tests of hypotheses for secondary outcomes used multiple imputation for missing values. Missing data in intervention group self-efficacy and outcome expectations scales due to employee turnover were considered missing completely at random.
Sample size
RECRUIT was powered to detect a 0.10 absolute difference from a range of control proportions of minorities recruited (0.05 to 0.15), assuming a 2-sample test and an intra-cluster (specialty clinic) correlation, ICC, of 0.10. An ICC of 0.1 has been a commonly used benchmark in medical studies.43, 44 With a two-sided alpha of 0.05, 30 specialty clinics per group, and an average cluster size of 10 participants per specialty clinic, a power of 83% or greater could be achieved to detect a difference if one existed. RECRUIT was not powered to detect differences in secondary outcomes or sub-groups.
Human subjects
All participating specialist investigators and coordinators provided written informed consent according to The University of Texas Health Science Center at Houston Institutional Human Subjects Review Board procedures. Parent trial coordinating centers received local Institutional Review Board (IRB) approval to send non-protected health information recruitment and screening data to RECRUIT. All eligible specialty clinics (intervention or control) obtained local IRB approval to participate in their parent trial and obtained additional IRB approval before distributing anonymous participant satisfaction surveys to screened and enrolled participants, or sending non-protected health information screening and recruitment activity logs to RECRUIT. If a screened patient or trial participant provided a survey comment related to safety or well-being, concerns were reported immediately to the specialty clinic, regardless of intervention or control status. Participant consent to treatment in the parent trials was obtained separately by the respective parent trial coordinating centers’ and specialty clinics. Data were not collected on minority-serving physicians beyond the number of physicians approached as provided in recruitment activities logs and screening logs.
Data and safety monitoring
Parent trials used their own Data and Safety Monitoring Committees. The RECRUIT funding institute considered RECRUIT’s intervention to be minimal risk so an external Data and Safety Monitoring Committee was not required. RECRUIT’s Steering Committee, comprised of the principal investigator, two external investigators, and two NIMHD program officials, provided general study oversight.
Results
Four participating multi-site parent trials were enrolled in RECRUIT, funded by three NIH Institutes (National Cancer Institute [2 parent trials], National Heart, Lung, and Blood Institute, and National Institute of Neurological Disorders and Stroke). The parent trials studied four disease entities (secondary colorectal cancer prevention; bone marrow transplant outcomes in leukemia, high-risk multiple myeloma, and myelodysplastic syndrome; atrial fibrillation; and early Parkinson’s disease), enhancing the generalizability of the RECRUIT trial findings.
Recruitment of parent trials
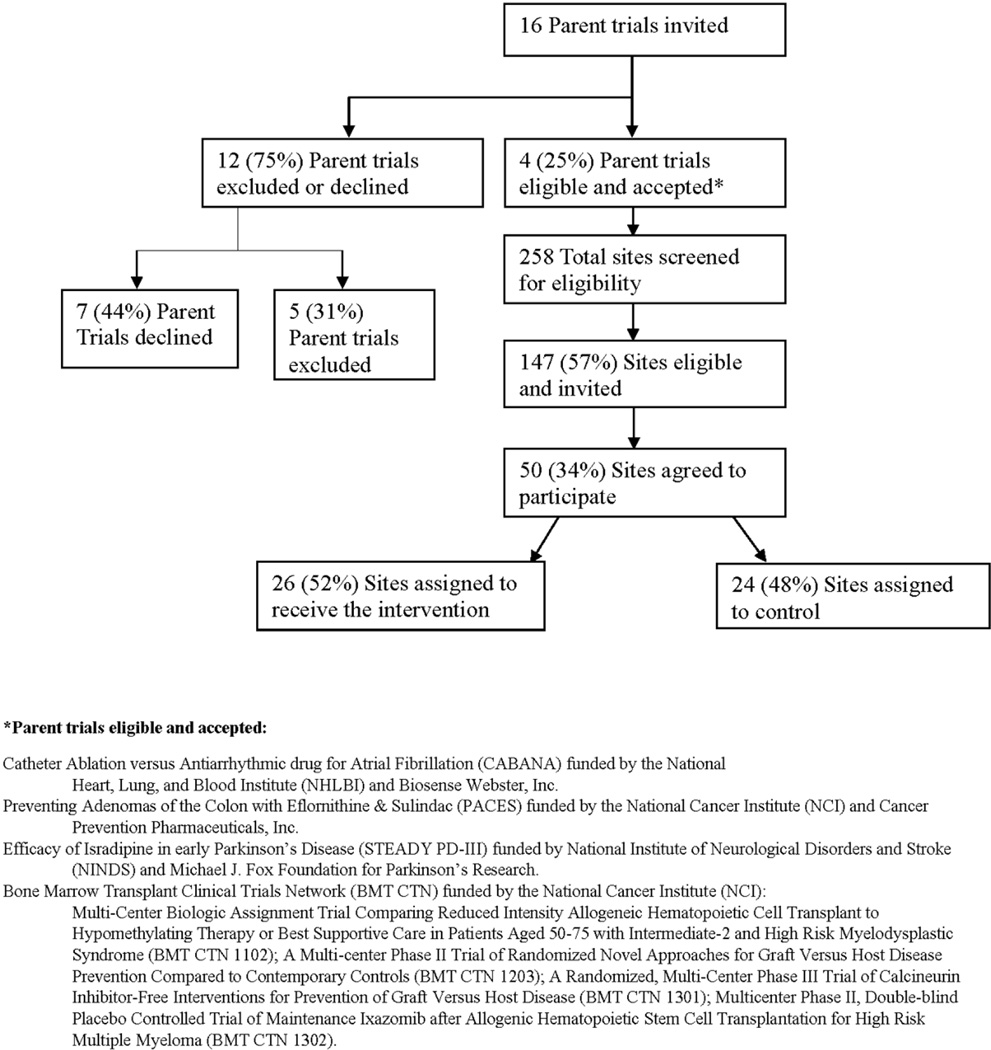
The CONSORT Diagram for RECRUIT (Figure 5) summarized acceptances, refusals/ineligibles, and final enrollment. A total of 26 intervention specialty clinics and 24 control clinics were randomized. Dropouts and losses to follow-up will be reported in the final CONSORT diagram after trial completion. Three of the four consenting parent trials were in research networks and planned to recruit a varying number of participants (336, 753, 1340 and 2,200). Of the 12 trials ineligible or refusing, 58% were part of research networks and planned to recruit a median number 1600 participants (range, 80 to 10,000). Reasons for parent trial ineligibility included the following: not enough interested specialty clinics; already hired a professional recruitment coordinator; recruitment close to completion; no anticipated problems with minority recruitment. Reasons for parent trial refusal included the following: reluctance to give any specialty clinic an unfair advantage (fixed allowed recruitment goals per specialty clinic); did not want investigators to use trial start-up time on minority recruitment strategies or have “interference” from RECRUIT; the trial sponsor was “hesitant” to interact with RECRUIT; the trial sponsor wanted all specialty clinics to receive the intervention.
Figure 5.
RECRUIT CONSORT Diagram
Recruitment of specialty clinics
Of specialty clinic Principal Investigators refusals, sixty-six did not respond after initial contact, ten refused without providing a reason, eleven stated they did not have enough time or resources, four were not interested, and two said they did not have a large enough minority population in the vicinity of their specialty clinics. In one specialty clinic, the specialist investigator coordinated parent trial recruitment and could have contaminated control specialty clinics. One specialty clinic specialist investigator had a general recruitment system planned and did not want to add RECRUIT’s intervention if randomized to the intervention group. Some specialist investigators expressed concerns about attending the in-person kick-off meeting.
Eligible specialty clinic refusals were similar in percent minorities within 30 miles to clinic acceptances (p > 0.21). Fewer specialty clinics in the Southwest and West were acceptances than those located elsewhere, 42.3% of 97 versus 26% of 50, but a statistically significant difference was not detected (p > 0.17).
Baseline characteristics of enrolled specialty clinics
We could not detect a difference in matching characteristics between intervention and control specialty clinics (all p-values > 0.17, Table 2). Specialist investigators (N=70) were on average 49.7 years of age with a standard deviation (SD) of ±0.8. Coordinators (N=86) were on average 39.7 years of age (SD ±0.8). Seventy-one percent of the specialist investigators and 13% of the coordinators were male. Sixty percent of the specialist investigators and 71% of coordinators were non-Hispanic white. Ninety percent of the coordinators had college or advanced degrees. We detected no statistically significant demographic differences between intervention and control specialist investigators or between intervention and control coordinators (all p-values > 0.20).
Table 2.
Baseline Specialty Clinic Characteristics Pooled Across Parent Trials and Specialty Clinics
| Baseline Specialty Clinic Characteristics |
Intervention Specialty Clinics (N = 26) n (%) |
Control Specialty Clinics (N = 24) n (%) |
P- value++ |
|---|---|---|---|
| Specialty clinic geographic location+ | |||
| Northeast | 6 (23.1) | 7 (29.2) | 0.17 |
| Southeast | 8 (30.8) | 7 (29.2) | |
| Midwest and Northwest | 4 (15.3) | 5 (20.8) | |
| Southwest and West | 8 (30.8) | 5 (20.8) | |
|
Percent minorities enrolled in previous trials+† |
|||
| 0% | 6 (23.1) | 7 (29.2) | 0.94 |
| 1% –< 10% | 1 (3.8) | - | |
| 10% –< 20% | 6 (23.1) | 3 (12.5) | |
| 20% or more | 9 (34.6) | 11 (45.8) | |
| Specialty clinic not in a previous trial or | 4 (15.4) | 3 (12.5) | |
| data not available | |||
|
Percent minorities within 30 miles of specialty clinic+,* |
|||
| 17% - < 20% | 1 (3.9) | 1 (4.2) | 0.31 |
| 20% –< 40% | 14 (53.8) | 13 (54.1) | |
| 40% –< 60% | 7 (26.9) | 9 (37.5) | |
| 60% or more | 4 (15.4) | 1 (4.2) | |
|
Foreign born population percentage within 30 miles of specialty clinic* |
|||
| 0% –< 10% | 7 (26.9) | 6 (25.0) | 0.85 |
| 10% –< 20% | 9 (34.6) | 11 (45.8) | |
| 20% –< 30% | 6 (23.1) | 2 (8.2) | |
| 30% or more | 4 (15.4) | 5 (20.8) | |
|
Non-English speaking population percentage within 30 miles of specialty clinic*,** |
|||
| 0% –< 10% | 7 (26.9) | 8 (33.3) | 0.22 |
| 10% –< 20% | 7 (26.9) | 7 (29.2) | |
| 20% –< 30% | 5 (19.2) | 3 (12.5) | |
| 30% or more | 7 (26.9) | 6 (25.0) | |
|
Number of specialty clinic patients seen per week for any trial†,+++ |
|||
| Mean (SD) | 78.9 (16.0) | 41.2 (6.3) | 0.07 |
| Median | 46 | 32.5 |
Baseline matching criteria used in randomization.
Information obtained from Investigator Demographics Form.
P-values were adjusted for stratification by trial except for comparison of means.
P-value was adjusted for clustering and stratification by trial.
Information from US Census.
Non-English speaking population (speak a language other than English at home).
Conclusions
RECRUIT was the first multi-center randomized controlled trial to examine the effectiveness of a trust-based CQI intervention to increase minority recruitment into clinical trials. As a strength, trial conclusions were more generalizable due to the use of multiple parent trials. Choice of a CQI approach was informed by empirical evidence suggesting that continuing medical education, a passive method for driving healthcare provider change, has not been effective.45 While there have been some applications of CQI46–48 and patient navigation49 methods to recruitment, none of the studies were randomized trials of recruitment strategies and most did not focus exclusively on minority recruitment or on building trust.
A particular challenge of trials in specialty clinics is the racial/ethnic distribution of the specialists. In RECRUIT, sixty percent of the specialist investigators and 71% of coordinators were non-Hispanic white. Data from the Parkinson’s disease minority recruitment trial31 and other studies50 cast some doubt on the need for racial and ethnic concordance between specialist investigators and potential trial participants. In the Parkinson’s disease trial31 the high minority enrolling specialist investigators and coordinators were predominantly Caucasian non-Hispanic. RECRUIT community advisory groups, consulted in planning the trial, stressed the need for respect from specialists, rather than concordance.
Parent trial and specialty clinic recruitment took longer than anticipated, due in part to reluctance of identified parent trial leaders or specialty clinic investigators to participate. Other than logistics, the main reasons for refusal among parent trials and specialty clinics were similar to reasons voiced by minorities refusing to participate in medical research (e.g. lack of trust in the RECRUIT team, concern about randomization, or concern that increasing minority recruitment would require too many resources or be too costly).
As a limitation, it is possible that only the most motivated specialty clinic investigators and coordinators agreed to participate. However, specialty clinics were randomly allocated to intervention and control.
We recruited ten fewer specialty clinics than planned. Updating our power estimate using data from August 2016,41, 51 if a difference exists we have 62% power to detect an absolute 0.10 difference from the observed control estimate, 74% power for a 0.12 difference, 88% power for a 0.15 difference, and 95% power for a 0.18 difference, sufficient to detect a clinically meaningful difference. Participant recruitment remains in progress at some specialty clinics with all clinics completing RECRUIT by the end of April 2017. The average cluster size per specialty clinic is likely to increase, potentially increasing the power to detect the hypothesized range of differences.
If successful, we cannot the separate components of the intervention that contributed to success. The key informant interviews, screening and recruitment activity logs, and analysis of intervention group mediators may provide guidance on important components of the intervention including qualitative information related to specific racial/ethnic groups, and assessment of possible contamination across intervention and control specialty clinics.
In conclusion, RECRUIT was one of a small number of randomized trials of minority recruitment strategies. RECRUIT’s innovative design included its focus on building trust between specialist investigators and minority-serving physicians, the use of CQI to tailor the intervention to each specialty clinic’s specific racial/ethnic populations and barriers to minority recruitment, and the use of more than one parent trial to increase generalizability. The efficacy of the RECRUIT intervention will be determined once trial data collection and analyses are complete.
Acknowledgments
Funding
Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number U24MD006941. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONSORT 2010 checklist of information to include when reporting a randomised trial*
Design and implementation of a multi-site cluster-randomized minority recruitment trial: RECRUIT-Tilley BC
Footnotes
Trial Registration: clinicaltrials.gov NCT01911208
References
- 1.Frist WH. Overcoming disparities in U.S. health care. Health Aff (Millwood) 2005;24:445–451. doi: 10.1377/hlthaff.24.2.445. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. CDC Health Disparities and Inequalities Report—United Sates, 2013. Report no. MMWR. 2013;62(Suppl 3):85–149. [PubMed] [Google Scholar]
- 3.Collins FS. What we do and don’t know about ‘race’, ‘ethnicity’, genetics and health at the dawn of the genome era. Nat Genet. 2004;36:S13–S15. doi: 10.1038/ng1436. [DOI] [PubMed] [Google Scholar]
- 4.Poolsup N, Li Wan Po A, Knight TL. Pharmacogenetics and psychopharmacotherapy. J Clin Pharm Ther. 2000;25:197–220. doi: 10.1046/j.1365-2710.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- 5.Taylor JS, Ellis GR. Racial differences in responses to drug treatment: implications for pharmacotherapy of heart failure. Am J Cardiovasc Drugs. 2002;2:389–399. doi: 10.2165/00129784-200202060-00004. [DOI] [PubMed] [Google Scholar]
- 6.Bjornsson TD, Wagner JA, Donahue SR, et al. A Review and Assessment of Potential Sources of Ethnic Differences in Drug Responsiveness. J Clin Pharmacol. 2003;43:943–967. doi: 10.1177/0091270003256065. [DOI] [PubMed] [Google Scholar]
- 7.Temple R, Stockbridge NL. BiDil for heart failure in black patients: The US. Food and Drug Administration perspective. Ann Intern Med. 2007;146:57–62. doi: 10.7326/0003-4819-146-1-200701020-00010. [DOI] [PubMed] [Google Scholar]
- 8.Park IU, Taylor AL. Race and ethnicity in trials of antihypertensive therapy to prevent cardiovascular outcomes: a systematic review. Ann Fam Med. 2007;5:444–452. doi: 10.1370/afm.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 10.Burke JF, Brown DL, Lisabeth LD, et al. Enrollment of women and minorities in NINDS trials. Neurology. 2011;76:354–360. doi: 10.1212/WNL.0b013e3182088260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen MS, Lara PN, Dang JHT, et al. Twenty years post-NIH Revitalization Act: Enhancing minority participation in clinical trials (EMPaCT): Laying the groundwork for improving minority clinical trial accrual. Cancer. 2014;120:1091–1096. doi: 10.1002/cncr.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 13.Curry L, Jackson J. Recruitment and retention of diverse ethnic and racial groups in health research: An evolving science. In: Curry L, Jackson J, editors. The science of inclusion: Recruiting and retaining racial and ethnic elders in health research. The Gerontological Society of America: 2004. p. 1. [Google Scholar]
- 14.Sinclair S, Hayes-Reams P, Myers HF, et al. Recruiting African Americans for health studies: lessons from the Drew-RAND center on health and aging. J Ment Health Aging. 2000;6:639–651. [Google Scholar]
- 15.UyBico SJ, Pavel S, Gross CP. Recruiting vulnerable populations into research: a systematic review of recruitment interventions. J Gen Intern Med. 2007;22:852–863. doi: 10.1007/s11606-007-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mainous AG, 3rd, Smith DW, Geesey ME, et al. Development of a measure to assess patient trust in medical researchers. Ann Fam Med. 2006;4:247–252. doi: 10.1370/afm.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104:e16–e31. doi: 10.2105/AJPH.2013.301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levkoff S, Sanchez H. Lessons learned about minority recruitment and retention from the Centers on Minority Aging and Health Promotion. Gerontologist. 2003;43:18–26. doi: 10.1093/geront/43.1.18. [DOI] [PubMed] [Google Scholar]
- 19.A Research!America poll of U.S. adults conducted in partnership with Zogby Analytics. Clinical Trials: Poll Data of Minority Populations [Google Scholar]
- 20.Lynch GF, Gorelick PB, Raman R, et al. A pilot survey of African-American physician perceptions about clinical trials. J Natl Med Assoc. 2001;93:8S–13S. [PMC free article] [PubMed] [Google Scholar]
- 21.Gorelick PB, Harris Y, Burnett B, et al. The recruitment triangle: reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African-American Antiplatelet Stroke Prevention Study (AAASPS) J Natl Med Assoc. 1998;90:141–145. [PMC free article] [PubMed] [Google Scholar]
- 22.Mainous AG, 3rd, Smith DW, Geesey ME, et al. Factors influencing physician referrals of patients to clinical trials. J Natl Med Assoc. 2008;100:1298–1303. doi: 10.1016/s0027-9684(15)31508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shortell SM, Bennett CL, Byck GR. Assessing the impact of continuous quality improvement on clinical practice: what it will take to accelerate progress. Milbank Q. 1998;76:593–624. doi: 10.1111/1468-0009.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:305–310. doi: 10.1097/PRS.0b013e318219c171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1989;95:2S–4S. [PubMed] [Google Scholar]
- 26.Office of Management and Budget (OMB) Directive No. 15, Race and Ethnic Standars for Federal Statistics and Administrative Reporting. 1977. May 12, [PubMed] [Google Scholar]
- 27.Bartholomew LK, Parcel G, Kok G, et al. Health promotion planning: An intervention mapping approach. 2nd. San Francisco, CA: John Wiley & Sons, Inc.; 2006. [Google Scholar]
- 28.Green L, Kreuter M. Health promotion planning: an educational and ecological approach. 4th. NY: McGraw-Hill Higher Education; 2005. [Google Scholar]
- 29.Albrecht TL, Eggly SS, Gleason ME, et al. Influence of clinical communication on patients’ decision making on participation in clinical trials. J Clin Oncol. 2008;26:2666–2673. doi: 10.1200/JCO.2007.14.8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornton LR, Amorrortu RP, Smith DW, et al. Exploring willingness of elder Chinese in Houston to participate in clinical research. Contemp Clin Trials Commun. 2016;4:33–38. doi: 10.1016/j.conctc.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilley BC, Mainous AG, 3rd, Elm JJ, et al. A randomized recruitment intervention trial in Parkinson’s disease to increase participant diversity: early stopping for lack of efficacy. Clin Trials. 2012;9:188–197. doi: 10.1177/1740774512436881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford ME, Siminoff LA, Pickelsimer E, et al. Unequal burden of disease, unequal participation in clinical trials: solutions from African American and Latino community members. Health Soc Work. 2013;38:29–38. doi: 10.1093/hsw/hlt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prochaska JO, Redding CA, Evers KE. The transtheoretical model and stages of change. In: Glanz K, Rimer BK, Viswanath K, editors. Health behavior and health education: Theory, research, and practice. 4. San Francisco, CA: John Wiley & Sons; 2008. p. 97. [Google Scholar]
- 34.Petty RE, Barden J, Wheeler SC. The elaboration likelihood model of persuasion: Health promotions that yield sustained behavioral change. In: DiClemente R, Crosby RA, Kegler MC, editors. Emerging theories in health promotion practice and research. San Francisco: Jossey-Bass; 2002. pp. 71–99. [Google Scholar]
- 35.Locke EA, Shaw KN, Saari LM, et al. Goal setting and task performance: 1969–1980. Psychol Bull. 1981;90:125. [Google Scholar]
- 36.Bandura A. Social cognitive theory: an agentic perspective. Annu Rev Psychol. 2001;52:1–26. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Eisenberg JM, Williams SV. Cost containment changing physicians’ practice behavior. Can the fox learn to guard the chicken coop? JAMA. 1981;246:2195–2201. [PubMed] [Google Scholar]
- 38.Vargas RB, Ryan GW, Jackson CA, et al. Characteristics of the original patient navigation programs to reduce disparities in the diagnosis and treatment of breast cancer. Cancer. 2008;113:426–433. doi: 10.1002/cncr.23547. [DOI] [PubMed] [Google Scholar]
- 39.ASPREE Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013;36:555–564. doi: 10.1016/j.cct.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLaughlin CP, Kaluzny AD. Continuous quality improvement in health care: Theory, implementations, and applications. 3rd. Jones & Bartlett Learning; 2005. [Google Scholar]
- 41.Murray DM, Varnell SP, Blitstein JL. Design and analysis of group-randomized trials: a review of recent methodological developments. Am J Public Health. 2004;94:423–432. doi: 10.2105/ajph.94.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleinbaum DG, Kuppler LL, Morenstern H. Epidemiologic research: Principles and quantitative methods. John Wiley & Sons; 1982. pp. 386–391. [Google Scholar]
- 43.Donner A. Sample size requirements for stratified cluster randomization designs. Stat Med. 1992;11:743–750. doi: 10.1002/sim.4780110605. [DOI] [PubMed] [Google Scholar]
- 44.Donner A, Klar N. Design and analysis of cluster randomization trials in health research. London, UK: Arnold Publishing Co; 2000. [Google Scholar]
- 45.Akbari A, Mayhew A, Al-Alawi MA, et al. Interventions to improve outpatient referrals from primary care to secondary care. Cochrane Database Syst Rev. 2008;(4):CD005471. doi: 10.1002/14651858.CD005471.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tilley BC, Lyden PD, Brott TG, et al. Total quality improvement method for reduction of delays between emergency department admission and treatment of acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Arch Neurol. 1997;54:1466–1474. doi: 10.1001/archneur.1997.00550240020008. [DOI] [PubMed] [Google Scholar]
- 47.Baraniuk S, Tilley BC, del Junco DJ, et al. Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial: design, rationale and implementation. Injury. 2014;45:1287–1295. doi: 10.1016/j.injury.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ENNACT. Education Network to Advance Cancer Clinical Trials (ENACCT) A quality improvement program to improve cancer clinical trial recruitment, accrual, and retention: Lessons learned from the National Clinical Trials Pilot Breakthrough Collaborative. 2012. Nov, [Google Scholar]
- 49.Ghebre RG, Jones LA, Wenzel JA, et al. State-of-the-science of patient navigation as a strategy for enhancing minority clinical trial accrual. Cancer. 2014;120(Suppl 7):1122–1130. doi: 10.1002/cncr.28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fryer CS, Passmore SR, Maietta RC, et al. The symbolic value and limitations of racial concordance in minority research engagement. Qual Health Res. 2016;26:830–841. doi: 10.1177/1049732315575708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu S, Crespi CM, Wong WK. Comparison of methods for estimating the intraclass correlation coefficient for binary responses in cancer prevention cluster randomized trials. Contemp Clin Trials. 2012;33:869–880. doi: 10.1016/j.cct.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]