Abstract
Tissue engineering has become a promising strategy for repairing damaged cartilage and bone tissue. Among the scaffolds for tissue-engineering applications, injectable hydrogels have demonstrated great potential for use as three-dimensional cell culture scaffolds in cartilage and bone tissue engineering, owing to their high water content, similarity to the natural extracellular matrix (ECM), porous framework for cell transplantation and proliferation, minimal invasive properties, and ability to match irregular defects. In this review, we describe the selection of appropriate biomaterials and fabrication methods to prepare novel injectable hydrogels for cartilage and bone tissue engineering. In addition, the biology of cartilage and the bony ECM is also summarized. Finally, future perspectives for injectable hydrogels in cartilage and bone tissue engineering are discussed.
Introduction
Cartilage and subchondral bone damage can be caused by a variety of conditions, such as trauma, arthritis, and sports-related injuries. 1,2, 3,4 It has been reported that 60% of patients examined by knee arthroscopy exhibit cartilage damage, and ~15% of people over 60 years old have some clinical symptoms of such damage.5,6 In particular, the self-healing of damaged cartilage is limited, owing to its lack of vascularization, innervation, lymphatic networks, and progenitor cells. 6,7,8,9,10,11,12 For bone tissue, despite its high vascularization, commonly used techniques for repair, such as autografting and allografting, are limited because of risks of donor-site morbidity, potential infection, and a high nonunion rate with host tissues. 13,14,15,16,17 Bone defects are one of the leading causes of morbidity and disability in elderly patients.18 Medical restoration of the damaged cartilage and bone tissue remains to be achieved. Therefore, developing a method to perfectly and permanently repair the damaged cartilage and bone tissue is of significant clinical interest for patients with cartilage lesions and bone defects.
Tissue engineering, which emerged in the early 1990s, has become one of the most commonly used approaches for cartilage and bone tissue reconstruction and regeneration.19,20,21,22 Generally, an engineered tissue is composed of a scaffold, cells, and necessary growth factors.23,24 To fully reconstruct the damaged cartilage and bone tissue, it is important to synthesize biocompatible and biodegradable scaffolds that mimic the native features of the specific tissue, successfully transport cells and growth factors to the damaged tissue, and provide support to the newly formed tissue until it matures.25 Ideally, the scaffolds of both cartilage and bone tissue engineering should be porous, highly biocompatible, nontoxic, and capable of promoting cell differentiation and new tissue formation; they should also have stable mechanical properties, degrade in response to the formation of new tissue, facilitate the diffusion of nutrients and metabolites, adhere and integrate with the surrounding native tissue, and properly fill the injured site.324,26,27,28
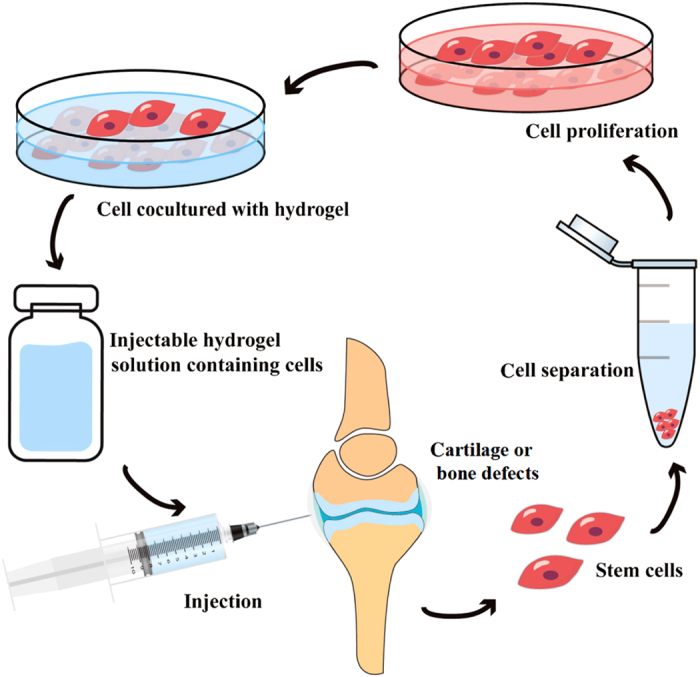
Since the 1990s, a variety of biomaterials have been investigated and tested for cartilage- and bone tissue-engineering applications. 29,30,31,32,33,34,35,36,37,38 Among all the biomaterials, hydrogels have received widespread interest, particularly for their use as scaffolds in cartilage and bone tissue engineering, owing to their structural similarity to the extracellular matrix (ECM) and their porous framework, which enables cell transplantation and proliferation.39 Hydrogels are three-dimensional (3D) cross-linked networks formed by hydrophilic homopolymers, copolymers, or macromers that swell in aqueous solution and provide an appropriate microenvironment similar to the ECM, thus facilitating the migration, adhesion, proliferation, and differentiation of chondrocytes and osteoprogenitor cells to osteoblasts, and efficiently delivering nutrients and growth factors.39–42 Recently, injectable hydrogels have attracted the attention of biomaterials scientists for cartilage- and bone tissue-engineering applications, because they can replace implantation surgery with a minimally invasive injection method and can form any desired shape, to match irregular defects.3,43–47 The schematic describing injectable hydrogels for cartilage- and bone tissue-engineering applications is illustrated in Figure 1.
Figure 1.
Schematic illustration of approaches to make injectable hydrogels for cartilage- and bone tissue-engineering applications.
Excellent biomaterials and appropriate fabrication methods play crucial roles in developing ideal injectable hydrogels that can function as scaffolds for cartilage- and bone tissue-engineering applications. A variety of biomaterials, both natural and synthetic, have been exploited to prepare injectable hydrogels; these biomaterials include chitosan,43 collagen or gelatin,48,49 alginate,50 hyaluronic acid,51 heparin,52 chondroitin sulfate,53 poly(ethylene glycol) (PEG),54 and poly(vinyl alcohol).55 Injectable hydrogels can be fabricated through both physical and chemical methods. Physically injectable hydrogels are spontaneously formed by weak secondary forces, whereas chemical hydrogels are usually formed by covalently cross-linking.56–58 On the basis of the concrete fabrication methods, injectable hydrogels can be classified as enzymatically cross-linked hydrogels,59 photo-cross-linked hydrogels,60 Schiff base cross-linked hydrogels,61 Michael addition-mediated hydrogels,62 click chemistry-mediated hydrogels,44,63 ion-sensitive hydrogels,64 pH-sensitive hydrogels,65 and temperature-sensitive hydrogels.66,67 Although injectable hydrogels prepared by different methods have been investigated for decades, there are scarcely any perfect injectable hydrogels that have been utilized in clinical regenerative medicine. Therefore, the development of an excellent injectable hydrogel for cartilage- and bone tissue-engineering applications is urgently needed. In this review, various biomaterials and fabrication methods for developing injectable hydrogels for cartilage- and bone tissue-engineering applications are discussed.
Even though many journal articles and reviews on injectable hydrogels for tissue engineering have been published, this is the first review that particularly focuses on both biomaterials and fabrication methods for developing novel injectable hydrogels, specifically for use in cartilage and bone tissue engineering. In this review, we provide a guide for selecting an appropriate biomaterial and fabrication method to prepare such injectable hydrogels. In addition, the biology of cartilage and the bony ECM is also discussed. Finally, perspectives on future injectable hydrogels for cartilage and bone tissue engineering are also discussed.
THE BIOLOGY OF CARTILAGE AND THE BONY ECM
In cartilage and bone tissue engineering, detailed understanding of the biology of cartilage and the bony ECM is crucial in realizing successful cartilage and bone tissue regeneration. Cartilage is a fiber-reinforced composite material composed of chondrocytes surrounded by specialized ECM consisting of structural and functional proteins, glycoproteins, and glycosaminoglycans assembled in unique tissue-specific 3D microenvironment architectures.68,69, 70,71 The composition and structure of cartilage tissue are always depth-dependent (Figure 2) and can be divided into four different zones on the basis of collagen fiber alignment and proteoglycan composition. 71,72,73,74 From the superficial zone to the deep zone, the proteoglycan content gradually increases. In the superficial zone, the collagen fibers are aligned parallel to the surface. Collagen fibers in the middle zone are unaligned and tangential to the cartilage surface. In the deep zone, collagen fibers are arranged radially. Finally, the collagen fibers in the calcified zone tend to arborize with little organization and mineralization.
Figure 2.
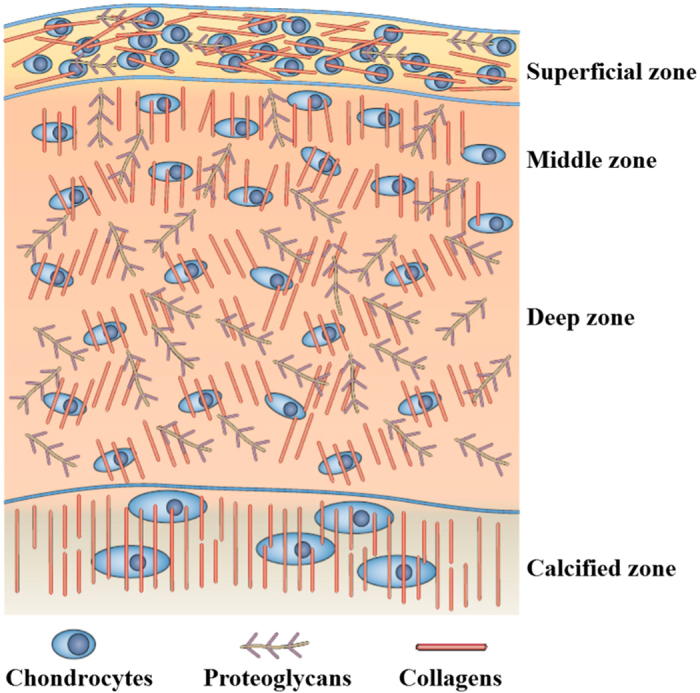
Schematic illustration of depth-dependent architecture of cartilage tissue. From the superficial zone to the deep zone, the proteoglycan content gradually increases. In the superficial zone, the collagen fibers are aligned parallel to the surface. Collagen fibers in the middle zone are unaligned and tangential to the cartilage surface. In the deep zone, collagen fibers are arranged radially. Finally, the collagen fibers in the calcified zone tend to arborize with little organization and mineralization.72
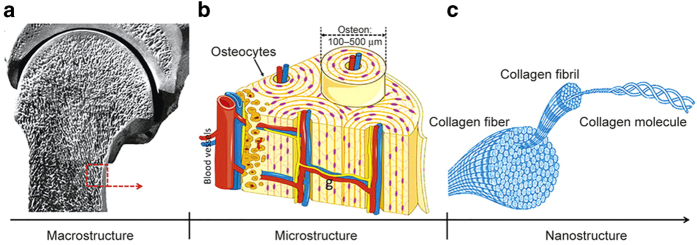
In contrast to cartilage tissue, bone is a highly vascularized biomineralized connective tissue with high mechanical strength and structural complexity.57,75 Natural bone tissue has a distinct hierarchical structural organization at the macrostructural, microstructural, and nanostructural levels (Figure 3).76,77 At the macrostructure level, bone can be distinguished into cortical bone and cancellous bone. At the microstructure level, the cortical bone is made up of repeated units of osteon, whereas the cancellous bone is composed of an interconnecting framework of trabeculae complemented with bone marrow-filled free spaces. Each osteon has 20–30 concentric layers of collagen fibers, called lamellae, which surround the central canal and contain various blood vessels and nerves. Finally, at the nanostructure level, there are large amounts of collagen fibers, calcium phosphate crystals, and non-collagenous organic proteins, which are the main components of the trabeculae and osteon units.76 The mechanical properties of bone tissue strongly depend on the specific structure and organization of the bony ECM.
Figure 3.
Schematic illustration of a distinct hierarchical structure of bone tissue. (a)At the macrostructural level, bone is composed of cortical bone and cancellous bone. (b) At the microstructural level, the cortical bone is made up of repeated units of osteon, which is characterized by 20–30 concentric layers of collagen fibers, called lamellae. The lamellae surround the central canal and contain various blood vessels and nerves. (c) At the nanostructural level, there are large numbers of collagen fibers, which are composed of periodic collagen fibrils and gaps between the collagen molecules. The calcium phosphate crystals and non-collagenous organic proteins are embedded in these gaps between collagen molecules.76
This highly organized and complicated structure of the cartilage and bone is essential to support its biological functions. The composition of both cartilage and the bony ECM is highly complex. Normally, the native cartilage ECM is composed primarily of water, type II collagen, proteoglycans, hyaluronic acid, glycosaminoglycans, and elastin.73,76, 78,79,80 Unlike cartilage ECM, the bony ECM is composed of oriented collagen I fibers and nanocrystals of carbonated hydroxyapatite, and is complemented with a number of proteoglycans, glycoproteins, and sialoproteins.81,82 All components of both cartilage and the bony ECM, which are continuously synthesized, secreted, oriented, and modified by the chondrocytes or osteoblasts that they support, are essential for chondrocyte and osteoblast growth, development, maintenance, and regulate the biological activities of the native cartilage and bone tissue.57,83,84 Under physiological conditions, the ECM exists in a state of dynamic reciprocity with chondrocytes and osteoblasts, and provides a mechanical framework for supporting the cells.70 In addition, the ECM and ECM-incorporated growth factors, together with cytokines, provide a number of functional cues that affect chondrocyte and osteoblast metabolism, and secretion. Moreover, the microenvironment provided by the ECM is dynamic and regulated by factors, such as mechanical properties, pH, oxygen concentration, and hormonal actions, that affect tissue homeostasis and possible aberrations thereof.69,85,86 Eventually, the ECM not only regulates cell adhesion, migration, growth, differentiation, and apoptosis but also takes part in cytokine activity and intracellular signaling.84,86 The complexity of the ECM is essential for specific function of the cartilage and bone tissue, and plays an important role in keeping the physiological stability of the microenvironment. Thus, design and synthesis of novel biomaterials that imitate the natural ECM are of great significance in cartilage and bone tissue engineering, and regenerative medicine.
INJECTABLE HYDROGELS PREPARED WITH DIFFERENT BIOMATERIALS
Various biomaterials have been exploited for the fabrication of injectable hydrogel scaffolds for cartilage tissue-engineering applications, including natural biomaterials and synthetic biomaterials.
Natural biomaterial-based injectable hydrogels
Natural biomaterials have been widely investigated because of their perfect biocompatibility, biodegradability, and similarity to the ECM. Natural biomaterials recently investigated for use as injectable hydrogel preparations include chitosan, collagen/gelatin, alginate, fibrin, elastin, heparin, chondroitin sulfate, and hyaluronic acid.3,46,50,52,53, 87,88,89,90,91
Chitosan-based injectable hydrogels
Chitosan is a linear polysaccharide that is derived from natural chitin, which is composed of glucosamine and N-acetylglucosamine.92,93,94, 95 Recently, chitosan has become increasingly attractive as an injectable hydrogel for cartilage repair, owing to its structural similarity to cartilage glycosaminoglycan.43,93,96 Chen et al48 have fabricated a tough chitosan–gelatin hydrogel via an in situ precipitation method. This in situ formed hydrogel exhibits improved mechanical properties, and is biodegradable and biocompatible. Naderi-Meshkinet al96 have developed a chitosan-based injectable hydrogel via the combination of chitosan, glycerol phosphate, and the cross-linking agent hydroxyethyl cellulose. Systematic investigations of the viability, proliferation, and differentiation capacity of encapsulated mesenchymal stem cells in the hydrogel have indicated that this chitosan-based injectable hydrogel has a high potential for cartilage tissue engineering. To make stimuli-responsive injectable hydrogels, chitosan is usually combined with various chemical components. By combining chitosan–glycerophosphate with different concentrations of starch, Sá-Lima et al97 have successfully prepared a novel thermoresponsive chitosan–starch hydrogel that can be used as an injectable vehicle for cell delivery. Furthermore, Moreira et al98 have reported a bioactive thermogelling chitosan-based injectable hydrogel synthesized by combining chitosan, collagen, and bioactive glass nanoparticles. Chitosan is insoluble in water, but it can be dissolved in acetic acid solution. Therefore, chitosan-based hydrogels are obtained from chitosan–acetic acid solution, which requires tedious washing steps.99 To overcome such shortcomings, water-soluble chitosan derivatives have been introduced. For example, Kamoun100 has prepared a new class of nontoxic, injectable, biodegradable materials called N-succinyl chitosan-dialdehyde starch hybrid hydrogels. These hydrogels have shorter gelation times, limited water uptake, little weight loss, and considerably tighter hydrogel structures, thus making them preferable scaffolds for cartilage tissue engineering.
Collagen/gelatin-based injectable hydrogels
Collagen is the most abundant mammalian protein in the skin, connective tissue, ligaments, bone, and cartilage of the body. 101,102,103,104 There are at least 19 types of collagen, such as type I, type II, type III, and type V. 101 Recently, naturally derived collagen has been widely used to construct collagen-based scaffolds for various biomedical applications, particularly tissue engineering, because it has the favorable property of being weakly antigenic.8,49,105 Yuan et al105 have combined type I and type II collagens to construct a favorable injectable hydrogel whose compressive modulus can be regulated by changing the type I collagen content in the hydrogel. The chondrocytes embedded in the hydrogel maintain their natural morphology and secrete cartilage-specific ECM. Funayamaet al106 have developed an injectable type II collagen hydrogel scaffold and have embedded chondrocytes in the collagen-based hydrogel and injected it into the damaged rabbit cartilage without a periosteal graft. At 8 weeks after the injection, favorable hyaline cartilage regeneration with good chondrocyte morphology was observed, and significant differences between the transplanted and control groups were observed after 24 weeks. Furthermore, collagen-based injectable hydrogels can be prepared by integrating collagen with other biomaterials. For example, Kontturiet al107 have developed an injectable, in situ forming type II collagen/hyaluronic acid hydrogel for cartilage tissue engineering. After encapsulation of chondrocytes and chondrogenic growth factor transforming growth factor-β1 into the hydrogel, the cell viability and proliferation, morphology, glycosaminoglycan production, and gene expression have been investigated. This hydrogel is able to maintain chondrocyte viability and characteristics, and it maybe a potential injectable scaffold for cartilage tissue engineering.
Gelatin is a natural protein derived from the degradation of collagen with high biocompatibility and biodegradability in physiological environments.108,109 Recently, use of gelatin to prepare injectable hydrogels has received popularity. Oh et al110 have designed and synthesized an interconnected, double thermoresponsive macroporous gelatin-based injectable hydrogel by stabilizing oil-in-water high internal phase emulsions, with gelatin-graft-poly(N-isopropyl acrylamide). In this injectable hydrogel, gelatin was chosen as the backbone of the amphiphilic graft copolymer to form high internal phase emulsions. The double thermoresponsive properties of the hydrogel promote proliferation and penetrate fibroblasts during cell seeding. Geng et al111 have prepared a gelatin-based injectable hydrogel from oxidized dextran, amino gelatin, and 4-arm PEG-acrylate through a two-step process. The attachment and spreading of preosteoblasts, as well as the encapsulated cell spreading and proliferation within the hydrogel indicate that the injectable hydrogel possesses favorable mechanical properties, biodegradability, and biocompatibility.
Hyaluronic acid-based injectable hydrogels
Hyaluronic acid, which interacts with chondrocytes through surface receptors such as CD44 and RHAMM, 112,113,114 is a linear polysaccharide in the adult cartilage ECM and is composed of disaccharide units of glucuronic acid and N-acetylglucosamine.115,116, 117 Hyaluronic acid plays very important roles in cartilage and limb bud formation, mesenchymal cell condensation, chondrocyte matrix deposition, and chondrogenic differentiation.73,118,119 Therefore, hyaluronic acid is regarded as an ideal biomaterial for cartilage tissue repair. Yu et al120 have fabricated an injectable hyaluronic acid/PEG hydrogel with excellent mechanical properties for cartilage tissue engineering. Cells encapsulated in the hydrogel in situ demonstrate high metabolic viability and proliferation. In addition, taking advantage of its biocompatibility, structural similarity to glycosaminoglycan, and ready formation of ionic complexes of chitosan, Park et al121 have successfully fabricated an injectable chitosan–hyaluronic acid hydrogel utilizing hyaluronic acid and methacrylated glycol chitosan. Chondrocytes encapsulated in the hydrogel show excellent proliferation and increased deposition of cartilaginous ECM; considering these results, this hydrogel has great potential for cartilage tissue repair.
To overcome its poor mechanical properties, fast degradation, and hydrolytic reactions, hyaluronic acid is usually modified or combined with other biomaterials for practical applications.113,122 Palumbo et al123 have designed an in situ forming hydrogel by the addition of divinyl sulfone-functionalized inulin to two types of amino-functionalized hyaluronic acid derivatives, specifically pendant ethylenediamino and amino/octadecyl hyaluronic acids. The properties of the hydrogel indicate that the presence of pendant C18 chains improves the mechanical performances of hyaluronic acid-based hydrogels and decreases their susceptibility to hyaluronidase hydrolysis. Furthermore, encapsulated bovine chondrocytes in the hydrogel result in high viability and proliferation. Domingue et al124 have used cellulose nanocrystals as nanofillers to develop a new class of reinforced hyaluronic acid-based injectable hydrogels, which comprise adipic aciddihydrazide-modified hyaluronic acid and aldehyde-modified hyaluronic acid reinforced by the aldehyde-modified cellulose nanocrystals. The biological performance of the developed hydrogel has been evaluated on the basis of the incorporation of human adipose-derived stem cells. The hydrogel has been found to possess preeminent cell-supportive properties and to spread well within the volume of gels, in addition to exhibiting pronounced proliferative activity.
Fibrin-based injectable hydrogels
Fibrin, which is regarded as a favorable cell-transplantation matrix that can enhance cell attachment, proliferation, differentiation, and migration in a 3D scaffold, is a natural fibrous protein involved in blood clotting. 125,126,127 In previous studies, fibrin, alone or in combination with other materials, has been used to synthesize scaffolds for cartilage tissue-engineering applications.128,129,130,131 Benavides et al132 have applied fibrin-based hydrogels, together with PEG and human amniotic fluid-derived stem cells, to develop a novel injectable hydrogel system that is able to induce a fibrin-driven angiogenic host response and promote in situ amniotic fluid-derived stem cell-derived neovascularization. Almeida et al133 have developed an injectable, cartilaginous ECM microparticle-functionalized fibrin-based hydrogel, which transforms growth factor transforming growth factor-β3 into a putative therapeutic for articular cartilage regeneration. The capacity of the hydrogel to promote chondrogenes is of freshly isolated stromal cells in vivo suggests that the hydrogel can induce cartilage formation and has the potential for cartilage repair, and thus may have the potential to overcome several current challenges related to cartilage tissue engineering. In addition, because alginate microbeads are stable and biocompatible, this hydrogel has been widely applied among injectable hydrogel systems for tissue regeneration.125 Hwang et al134 have developed a novel hybrid hydrogel system using alginate particles and a fibrin matrix. In this hydrogel, the introduction of alginate particles into a fibrin matrix enhances cellular mobility and proliferation, volume retention, and vascularization in vivo, thus making the injectable hybrid system a desirable approach for cartilage tissue-engineering applications.
Alginate-based injectable hydrogels
Alginate, which consists of guluronic and mannuronic acids, is a polysaccharide extracted from brown algae (Phaeophyceae).50,135,136 Alginate has become one of the most commonly used biomaterials in injectable hydrogel preparation for cartilage tissue-engineering applications, owing to its favorable scaffold forming, non-immunogenicity, and non-toxicity.135, 137,138,139 For example, Balakrishnan et al140 have produced a rapidly gelling, oxidized alginate-based injectable hydrogel by self-cross-linking periodate-oxidized alginate and gelatin in the presence of borax. The hydrogel integrates well with the cartilage tissue in addition to exhibiting negligible inflammatory and oxidative stress responses. Moreover, chondrocytes encapsulated in the hydrogel have favorable viability, and exhibit a normal phenotype in terms of proliferation and migration within the matrix, thus suggesting that the hydrogel is a promising injectable, cell-attracting adhesive scaffold for cartilage tissue engineering.
However, there is a drawback to using an injectable alginate hydrogel: it is not strong enough to maintain the structural shape of the regenerated tissue.141 Therefore, alginate is usually modified or used in combination with other biomaterials to improve its mechanical properties. Zhao et al142 have devised a fully injectable and mechanically strong calcium phosphate–alginate cement hydrogel system. The mechanical properties of the hydrogel are much better than those of previous injectable polymeric and hydrogel carriers, and the encapsulated cells are viable, exhibit osteodifferentiation, and secrete bone minerals. Furthermore, owing to its lack of cell adhesion ability, alginate is usually blended with other polymers.143,144 An injectable, biodegradable, oxidized alginate/hyaluronic acid hydrogel has been prepared by Park and Lee.143 At 6 weeks after injection of the hydrogel with primary chondrocytes into mice, effective cartilage regeneration has been observed. In another study, a class of biocompatible and biodegradable alginate-based hydrogel blend has been synthesized by using alginate and O-carboxymethyl chitosan with the addition of fibrin nanoparticles.144 Evaluation of the swelling ratio, degradation profile, compressive strength, and elastic module have indicated that alginate/O-carboxymethyl chitosan forms a preferable blend for tissue-engineering applications.
Heparin-based injectable hydrogels
Heparin, which is best known for its anticoagulant properties, is a negatively charged, highly sulfated, linear polysaccharide composed of repeating disaccharide units of 1,4-linked uronic acid and glucosamine residues. 145,146,147,148 Owing to its negatively charged functional groups, heparin can interact with proteins, including ECM proteins, growth factors, and chemokines, which plays important roles in many biological processes, such as triggering multiple downstream signaling pathways and controlling cellular proliferation, and differentiation. 149,150,151,152,153,154 As a result, heparin has widely been used for the fabrication of injectable hydrogels that control the delivery of growth factors in tissues, especially during cartilage tissue repair.153, 155,156,157, 158 For example, Jin et al159 have used horseradish peroxidase (HRP)-mediated co-cross-linking to form dextran–tyramine (Dex–TA) and heparin–tyramine injectable hydrogel conjugates whose swelling and mechanical properties can be controlled for cartilage tissue-engineering applications. Chondrocytes incorporated in the hydrogel exhibit favorable viability and proliferation, with increased production of chondroitin sulfate and abundant collagen content. In addition, heparin-based injectable hydrogels can also be combined with other scaffolds to reinforce its curative effects. Such a strategy has been attempted by Kim et al,160 who have combined the advantages of a porous gelatin-incorporated poly(L-lactide-co-ε-caprolactone) scaffold and heparin-based injectable hydrogels to produce a scaffold/hydrogel composite for delivering chondrocytes to repair partial thickness cartilage defects. Cells encapsulated in the scaffold/hydrogel composite exhibit enhanced expression of chondrogenic genes and increased the production of glycosaminoglycans. In addition, significant cartilage formation that integrates well with the surrounding natural cartilage tissue has been observed when this composite has been used to repair partial thickness defects of rabbit knees. All of these results indicate that the scaffold/hydrogel composite is a promising scaffold system for cartilage regeneration.
Elastin-based injectable hydrogels
Elastin is an insoluble, polymeric, elastic protein found in soft tissue, such as skin, blood vessels, and lungs.161,162 Currently, elastin-based biomaterials are widely used in tissue engineering, especially in fabricating injectable hydrogels for cartilage tissue engineering, because elastin not only improves local elasticity but also facilitates cellular interactions and signaling during neoplastic tissue formation.162,163 For instance, Fathi et al87 have fabricated a highly cytocompatible and injectable elastin-based hydrogel with alterable gelation characteristics, favorable mechanical properties, and good structural stability. This hydrogel is generated by the synthesis of a polymer (PNPHO) by functionalizing poly(N-isopropylacrylamide-co-polylactide-2-hydroxyethylmethacrylate-co-oligo(ethylene glycol)monomethyl ether methacrylate with succinimide ester groups, then covalently attaching elastin to PNPHO via interaction of its primary amine groups with the ester groups of PNPHO in aqueous solution. The elastin-co-PNPHO solutions are injectable and convert into hydrogels in situ at 37 °C without any cross-linking reagent. In addition, this elastin-based injectable hydrogel shows favorable structural stability and mechanical properties as well as preferable cyto-biocompatibility, thus making it a favorable candidate for cartilage tissue-engineering applications.
Chondroitin sulfate-based injectable hydrogels
Chondroitin sulfate, which is composed of sulfated disaccharide repeating units with 1–3 linkages of D-glucuronic acid and N-acetylgalactosamine, is an abundant anionic linear polysaccharide present in connective tissue and bones, and is an important component of cartilage in the body. 164,165,166,167 Chondroitin sulfate plays important roles in many biological processes such as intracellular signaling, cell recognition, the connection between ECM components and cell-surface glycoproteins, and chondrocyte phenotype regulation, as has widely been investigated in cartilage tissue engineering. 168,169,170,171 Wiltsey et al172 have developed a poly(N-isopropylacrylamide)-graft-chondroitin sulfate-based injectable hydrogel scaffold, which acts as a favorable adhesive interface with surrounding tissue. The hydrogel system has been demonstrated to have improved mechanical properties at 37 °C, enhanced adhesive tensile strength (ranging from 0.4 to 1 kPa), and no cytotoxicity to human embryonic kidney 293 cells. Chen et al173 have successfully developed a novel injectable pullulan/chondroitin sulfate composite hydrogel, synthesized under physiological conditions, for cartilage tissue engineering. The hydrogel system is very cytocompatible, enhances cell proliferation, and increases cartilaginous ECM deposition, thus showing promise for cartilage tissue repair.
Synthetic biomaterial-based injectable hydrogels
Compared with natural biomaterials, synthetic biomaterials, owing to their enhanced controllability and reproducibility, enable the systematic study of cell–matrix interactions.57 To date, several degradable synthetic polymers have been studied for the development of injectable hydrogels for cartilage tissue engineering; these polymers include PEG,114, 174,175,176,177 poly(L-glutamic acid),178,179 poly(vinyl alcohol),180 poly(propylene fumarate),181 α,β-poly(N-hydroxyethyl)-DL-aspartamide,182 PEG-poly(N-isopropyl acrylamide) (PNIPAAm),183 methoxy polyethylene glycol,184 and methoxy polyethylene glycol–poly(ε-caprolactone).185 For example, Yan et al186 have reported a novel poly(L-glutamic acid)-based injectable hydrogel. Preliminary studies of the hydrogel have demonstrated successful injectability, rapid in vivo gelling, excellent cell growth, satisfactory mechanical stability, and favorable ectopic cartilage formation. Skaalureet al187 have developed a new cartilage-specific, degradable hydrogel based on PEG and have encapsulated bovine chondrocytes from different sources in the hydrogel for cartilage tissue engineering. This new PEG-based injectable hydrogel shows promise for cartilage regeneration. Moreover, De France et al188 have designed an in situ gelling nanocomposite hydrogel based on poly(oligoethylene glycol methacrylate) and rigid rod-like cellulose nanocrystals. This injectable hydrogel possesses enhanced mechanical properties, increased stability and gelation rates, and decreased swelling ratios.
However, synthetic biomaterials are not very biocompatible, and, as compared with natural biomaterials, they lack biological activity. The most common strategy used to solve this problem is modifying or combining synthetic biomaterials with bioactive polymers. For example, Yan et al178 have fabricated a series of injectable poly(L-glutamic acid)/alginate (PLGA/ALG) hydrogels by self-cross-linking hydrazide-modified poly(L-glutamic acid) and aldehyde-modified alginate. This injectable PLGA/ALG hydrogel exhibits attractive properties for future application in cartilage tissue engineering. In addition, Yu et al120,189 have fabricated two hyaluronic acid/PEG-based injectable hydrogels. Both hydrogels possess good mechanical properties and short gelation times, and the cells encapsulated in the hydrogels exhibit high metabolic viability and proliferation, thus indicating that both hydrogels have great potential in cartilage tissue engineering.
INJECTABLE HYDROGELS FABRICATED VIA DIFFERENT APPROACHES
There are various approaches available for the fabrication of injectable hydrogels; depending on the approach used, injectable hydrogels can be divided into physical hydrogels and chemical hydrogels. Physical hydrogels are spontaneously formed by weak secondary forces, which respond to the changes in temperature, pH, or ionic concentration.63,190,191 Chemical hydrogels are produced through a variety of chemical processes, for example, enzymatic cross-linking, Schiff base cross-linking, Michael additions, click chemistry, and photo-cross-linking.44,56,62,63,192,193
Injectable hydrogels by physical methods
Temperature-sensitive injectable hydrogels
Injectable hydrogels that are sensitive to temperature changes have recently attracted substantial attention for applications in cartilage tissue engineering, because of their gelation ability at physiological temperature. These injectable hydrogels are present in aqueous form at room temperature, but they rapidly gel at physiological temperature before solidifying in the desired tissue.194,195 The threshold temperature at which hydrogels transform from a solution to a hydrogel state is defined as the lower critical solution temperature. The most useful characteristic of temperature-sensitive hydrogels is that they can undergo a phase transition without any chemical stimulus. To date, the most common explanation of the phase transition mechanism of temperature-sensitive injectable hydrogels is that when the temperature changes, there is a change in the hydration state favoring intra- and inter-molecular hydrogen bonding, thus eventually changing the hydrogel solubility.196,197 Therefore, to make injectable hydrogels that are sensitive to temperatures, temperature-sensitive polymers such as poly(lactic-co-glycolic acid)–PEG,194 poly(N,N-diethylacrylamide),195 PNIPAAm,197 and poly(ethylene glycol-b-[DL-lactic acid-co-glycolic acid]-b-ethylene glycol)198 are needed.
PNIPAAm, an inverse temperature-sensitive polymer derived from polyacrylic acid, has become one of the most commonly used temperature-sensitive polymers, owing to its rapid phase transition at its ~32 °C lower critical solution temperature. 199,200,201 However, linear PNIPAAm is not stable at physiological temperature, thus requiring the modification of other polymers to improve the stability and mechanical properties. Kloudaet al202 have studied the effects of the macromer end group, acrylate or methacrylate, and the effects of fabrication conditions on the degradative and swelling properties of PNIPAAm-based injectable hydrogels. When immersed in cell culture medium at physiological temperature, the hydrogels maintain constant swelling, and exhibit no observable degradation over 8 weeks; the methacrylated hydrogels show greater swelling than their acrylated analogs. Another temperature-sensitive PNIPAAm-based injectable hydrogel, synthesized by functionalizing PNIPAAm with methacrylate groups by degradable phosphate ester bonds, has transition temperatures between room temperature and physiological temperature.203 Making temperature-sensitive injectable hydrogels by modifying PNIPAAm with natural polymers is another strategy to optimize their stability and mechanical properties. Ren et al204 have grafted temperature-sensitive PNIPAAm onto gelatin via atom transfer radical polymerization, creating a hydrogel that successfully undergoes a sol-to-gel transition at physiological temperature. Tan et al205 have synthesized a temperature-sensitive injectable hydrogel whose lower critical solution temperature is ~35 °C, by grafting PNIPAAm-COOH with a single carboxy end group onto aminated alginate through amide bond linkages. In addition, the hydrogel is not cytotoxic and preserves the viability of the entrapped cells, thus making it suitable as a cell delivery vehicle for cartilage tissue-engineering applications.
pH-sensitive injectable hydrogels
Injectable hydrogels sensitive to pH value show significant potential in regenerative medicine. To obtain pH-sensitive injectable hydrogels, it is necessary to incorporate the hydrogel with a pH-sensitive moiety such as the polyelectrolyte N-palmitoylchitosan,65 polyacrylic acid,206 oligomeric sulfamethazine,207 and sulfamethazine oligomers (SMOs).208 For example, Shim et al209 and Kim et al191 have synthesized a pH-sensitive injectable hydrogel by adding pH-sensitive SMOs to both ends of a temperature-sensitive poly(ε-caprolactone-co-lactide)–PEG–poly(ε-caprolactone-co-lactide) (PCLA–PEG–PCLA) block copolymer. This pH-sensitive SMO–PCLA–PEG–PCLA–SMO injectable hydrogel exists in solution at high pH (pH 8.0), but rapidly changes into a stable gel under physiological conditions (pH 7.4). Kim et al191 have encapsulated human mesenchymal stem cells and recombinant human bone morphogenetic protein-2 into the hydrogels under physiological conditions and injected the mixture into the backs of mice. Histological studies observing human mesenchymal stem cell differentiation for 7 weeks have revealed mineralized tissue formation and high levels of alkaline phosphatase activity in the mineralized tissue.
Other physical injectable hydrogels
Other physical injectable hydrogels, such as ion-sensitive and stress-sensitive hydrogels, for cartilage tissue-engineering applications have also been reported. 62,63,64 For instance, Park et al64 have prepared an ionically cross-linkable hyaluronate-grafted-alginate hydrogel that easily forms gels in the presence of calcium ions and has been demonstrated to be useful in cartilage regeneration by the subcutaneous injection of primary chondrocyte-encapsulated hyaluronate-grafted-alginate into the dorsal region in a mouse model. Except for the novel methods of developing physical injectable hydrogels, determining how to improve the biocompatibility, biodegradability, mechanical properties, and the in vivo maintenance of structural integrity of correlated biomaterials are further research topics for the design of physical injectable hydrogels.
Injectable hydrogels by chemical methods
Injectable hydrogels by enzymatic cross-linking
Recently, the use of the enzymatic cross-linking method applied to the development of novel injectable hydrogels has drawn attention, owing to the fast gelation, high site specificity, ability to work at normal physiological conditions, and low cytotoxicity.210–215 Several enzyme-mediated cross-linking systems have been applied to synthesizing injectable hydrogels for cartilage tissue-engineering applications, including transglutaminase, tyrosinase, phosphopantetheinyl transferase, lysyl oxidase, plasma amine oxidase, phosphatase, thermolysin, β-lactamase, and peroxidase.215 Among them, HRP is the most commonly used enzyme in synthesizing injectable hydrogels. HRP is a single-chain β-type hemoprotein that catalyzes the conjugation of phenol and aniline derivatives in the presence of H2O2.215,216 The HRP-mediated cross-linking system covalently binds the phenol-conjugated polymers to the ECM proteins of the surrounding native tissue and thus is beneficial in maintaining the structural integrity of the wound tissue.217
Both natural and synthetic polymers that contain phenol groups or are functionalized with tyramine, tyrosine, or other aminophenol molecules can be enzymatically cross-linked by HRP (Figure 4). 218,219,220 For example, Wang et al221 have reported an HRP-mediated gelatin–hydroxyphenylpropionic acid-based injectable hydrogel for ectopic cartilage formation and early-stage osteochondral defect repair. The reported hydrogel was fabricated by oxidative coupling of hydroxyphenylpropionic acid moieties, catalyzed by HRP and H2O2. Jin et al222 have also enzymatically cross-linked Dex–TA conjugates in the presence of HRP and H2O2 to prepare an injectable hydrogel for cartilage tissue repair. Chondrocytes encapsulated in the Dex–TA hydrogels have been found to retain their viability and normal morphology after 2 weeks, and to secrete glycosaminoglycans and collagen type II after culturing for 14 and 21 days, thus indicating that the enzymatically cross-linked injectable Dex–TA hydrogels are promising for cartilage tissue-engineering applications.
Figure 4.
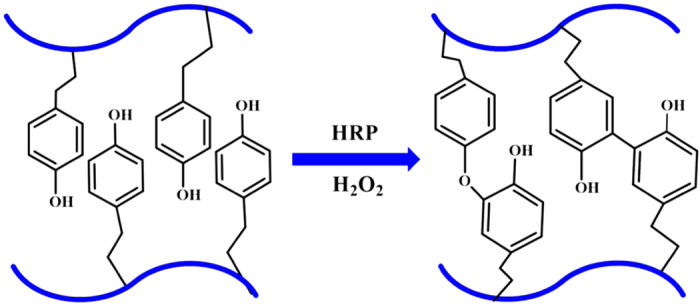
Schematic illustration of injectable hydrogels prepared by the enzymatic cross-linking method with horseradish peroxidase (HRP) and H2O2.
Injectable hydrogels by Schiff base cross-linking
Schiff base reactions have been widely used for synthesizing injectable hydrogels for cartilage regeneration applications, owing to the mild reaction conditions and high reaction rate, as well as the ability to form imine bonds between amino and aldehyde groups without any external stimuli or additional reagents under physiological conditions.92, 223,224,225,226,227,228 Chitosan is an excellent biomaterial for preparing injectable hydrogels via Schiff base cross-linking, owing to the abundant amino groups on its backbone. For example, Cheng et al229 have reported an injectable chitosan-based polysaccharide hydrogel for cell and protein delivery, which is cross-linked via an imine bond resulting from the Schiff base reaction between the amino functionalities of chitosan and the aldehyde groups of dextran aldehyde in aqueous solutions. Cao et al230 have utilized a multi-benzaldehyde-functionalized PEG analog, poly(ethylene oxide-co-glycidol)-CHO(poly(EO-co-Gly)-CHO), and glycol chitosan to successfully develop an injectable hydrogel system for cartilage tissue repair, which was chemically cross-linked through a Schiff base reaction between amino groups of glycol chitosan and aldehyde groups of poly(EO-co-Gly)-CHO under physiological conditions in situ (Figure 5). In addition, other biomaterial-based injectable hydrogels coupled by Schiff base cross-linking have been widely investigated. Most recently, Ma et al231 have developed a biodegradable and injectable polymer–liposome hydrogel by using aldehyde-modified xanthan gum and phosphatidylethanolamine liposomes, which are chemically cross-linked by a Schiff base reaction between the aldehyde groups of aldehyde-modified xanthan gum and amino groups of PE liposomes. This xanthan gum-based liposome hydrogel has many advantages, such as rapid preparation at room temperature, ready biodegradation by enzymes, excellent self-healing capability, and the ability to maintain favorable cell viability.
Figure 5.
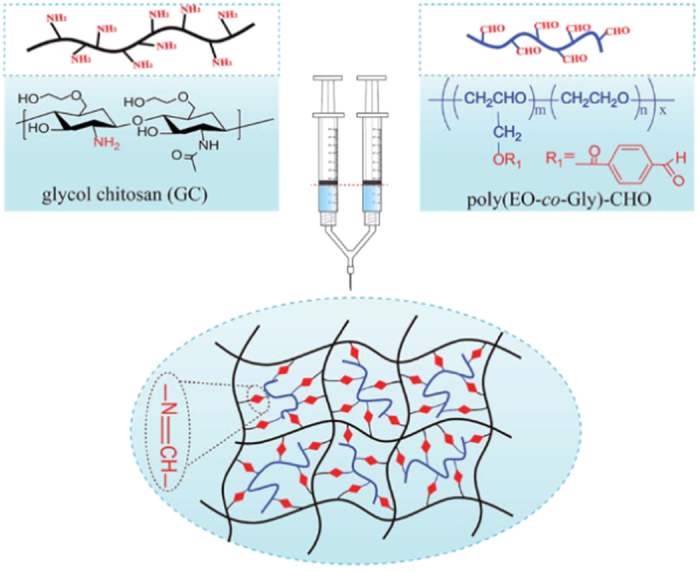
Schematic illustration of injectable hydrogels prepared by Schiff base cross-linking between aqueous solutions of GC and poly(EO-co-Gly)-CHO.230
Injectable hydrogels by Michael addition
The Michael addition reaction, which is the nucleophilic addition of a carbanion or a nucleophile to an α,β-unsaturated carbonyl compound (Figure 6), is another commonly used approach to prepare injectable hydrogels, owing to its reaction under physiological conditions and controllable reaction time.193, 232,233,234,235,236,237,238,239 Hyaluronic acid, chitosan, and PEG are frequently used biomaterials for injectable hydrogel preparation via the Michael addition reaction for cartilage tissue engineering under physiological conditions.114, 240,241,242 For example, Calogero et al243 have prepared two kinds of hyaluronic acid-based injectable hydrogels by Michael addition, using the amino derivative of hyaluronic acid (HA-EDA), α-elastin-grafted HA-EDA, and α,β-poly(N-2-hydroxyethyl)-DL-aspartamidederivatized with divinylsulfone. The swelling and degradation profile as well as its ability to incorporate viable articular chondrocytes of the injectable hydrogel indicate that this injectable hydrogel scaffold possesses desired properties for the treatment of articular cartilage damage under physiological conditions.
Figure 6.
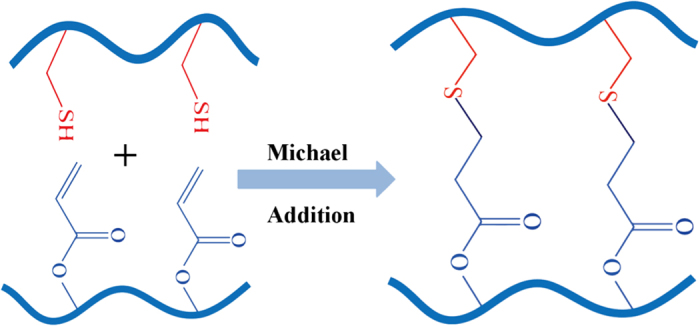
Schematic illustration of injectable hydrogels prepared by the Michael addition cross-linking method.
Injectable hydrogels by click chemistry
Click chemistry refers to a synthetic concept involving a wide range of reactions (Figure 7), including copper-catalyzed azide-alkyne cyclo-addition reactions, 244,245,246 Diels–Alder reactions,120 the thiol-ene reactions,247,248 tetrazine–norbornene chemistry,249 thiol-epoxy,250 and thiol-maleimide couplings.251 These reactions have shown great promise for the development of injectable hydrogels, owing to their rapid polymerization kinetics and low reactivity with cellular components. 252,253,254 For example, Kaga et al255 have fabricated a dendron–polymer–dendron conjugate-based injectable hydrogel through radical thiol-ene “click” reactions. In this fabrication process, the dendron–polymer conjugates were prepared through an azide-alkyne “click” reaction of alkene-containing polyester dendrons, bearing an alkyne group at their focal point, with linear PEG-bisazides. The sequential thiol-ene “click” reaction uses a tetrathiol-based cross-linker to cross-link these alkene-functionalized dendron–polymer conjugates, thus resulting in clear and transparent hydrogels.
Figure 7.
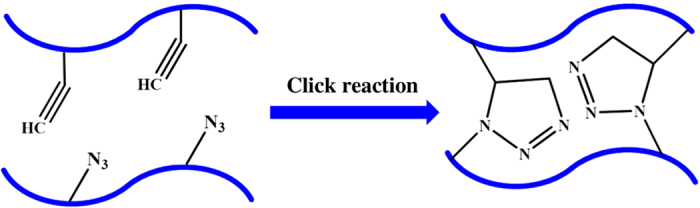
Schematic illustration of injectable hydrogels prepared by click chemistry.
Injectable hydrogels by photo-cross-linking
Photo-cross-linking is a complex process, consisting of initiation, propagation, and termination steps, triggered by electromagnetic radiation in the visible and ultraviolet regions (Figure 8).256,257 First, free radicals are created by the excitation of photoinitiators, as a result of the illumination in the initiation step. Then, long kinetic chains are cross-linked by propagating the radicals through unreacted double bonds in the propagation step, and this is followed by a termination step, which is characterized by the end of cross-linking in the 3D polymeric network.257 In recent years, photo-cross-linking methods have been widely applied to prepare injectable hydrogels for cartilage tissue engineering because of the ability to control the timing and location of cross-linking under physiological conditions. 258,259,260,261,262,263,264,265,266 For example, Papadopoulos et al267 have developed a poly(ethylene glycol)dimethacrylate copolymer-based injectable hydrogel by photo-cross-linking for cartilage tissue-engineering applications. Swine auricular chondrocytes have been encapsulated into PEGDM copolymer hydrogels composed of degradable (PEG-4,5 LA-DM) and nondegradable PEGDM macromers in a 60:40 molar ratio. The histological, biochemical, and integrative features of the neocartilage indicate that the viability, proliferation, and normal secretion of glycosaminoglycan and hydroxyproline contents of the seeded chondrocytes are maintained, and the neocartilage resembles the native swine auricular cartilage, thus indicating the promise of these hydrogels in cartilage tissue-engineering applications.
Figure 8.
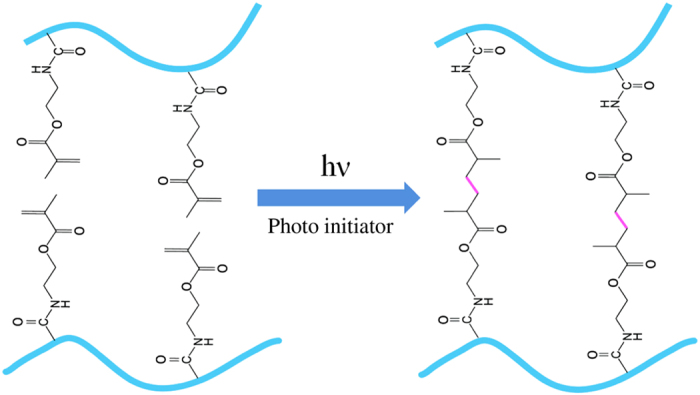
Schematic illustration of injectable hydrogels prepared by the photo-cross-linking method. Reprinted with permission from ref. 256 2009 Elsevier Publishing Group.
INJECTABLE HYDROGELS FOR BONE TISSUE ENGINEERING
Bone defects have become one of the leading causes of morbidity and disability among elderly people worldwide.268,269 Although autografting is regarded as the gold standard for bone defect repair, it is limited by the donor-site morbidity and uncertain adverse effects.270 Therefore, bone tissue engineering has attracted considerable attention from researchers as a promising strategy for repairing bone defects without the limitations and shortcomings of using either bone autografts, allografts, or xenografts.271
Recently, various injectable hydrogels with good moldability and 3D structures have been widely investigated for use in bone tissue engineering. Among the biomaterials used for preparing injectable hydrogels, alginate is one of the most investigated biomaterials used in bone tissue engineering.135 Matsuno et al272 have developed a novel injectable 3D hydrogel for bone tissue engineering that uses β-tricalcium phosphate beads and alginate as a scaffold. Mesenchymal stem cells 3D-cultured within the hydrogel have been implanted subcutaneously for in vivo experiments, and have indicated that the scaffold can favorably support osteogenic differentiation. Han et al273 have prepared an injectable calcium silicate/sodium alginate hybrid hydrogel by incorporating calcium silicate into an alginate solution. In 30 s to 10 min, this hydrogel undergoes internal in situ gelling when calcium ions are released from calcium silicate with the introduction of D-gluconic acid δ-lactone. Moreover, the hydrogel efficiently promotes the adhesion, proliferation, and differentiation of osteogenic and angiogenic cells. Chitosan is another commonly used biomaterial for synthesizing injectable hydrogels in bone tissue engineering.274 Dessi et al275 have successfully developed a thermosensitive chitosan-based hydrogel cross-linked with β-glycerophosphate and reinforced by physical interactions with β-tricalcium phosphate. The hydrogel simulates natural bone tissue and supports cellular activity and undergoes a sol–gel transition at physiological temperature with typical rheological properties. Meanwhile, owing to the properties of collagen, this hydrogel enhances cell adhesion and proliferation. Ding et al276 have incorporated collagen into the chitosan/β-glycerophosphate system to synthesize an injectable chitosan/β-glycerophosphate/collagen-based hydrogel scaffold for bone tissue engineering. Mesenchymal stem cells co-cultured in the hydrogel have been demonstrated to be capable of supporting neovascularization and osteogenic lineage differentiation. In recent years, synthetic biomaterials-based injectable hydrogels for bone tissue engineering have attracted attention. Jang et al277 have investigated an injectable in vivo forming hydrogel scaffold made of methoxy polyethylene glycol-b-polycaprolactone block copolymer for bone tissue engineering. Differentiated osteoblasts encapsulated in the hydrogel exhibit characteristic expression of osteonectin, osteopontin, and osteocalcin. Vo et al278 have designed an N-isopropylacrylamide/gelatin microparticle-composite hydrogel. The gelatin microparticles incorporated in the hydrogel enhance bony bridging and mineralization within the defect and direct bone-implant contact. After encapsulation of mesenchymal stem cells in the hydrogel, significant tissue infiltration and osteoid formation have been observed, thus suggesting that the hydrogel system facilitate bone ingrowth and integration.
To improve the mechanical properties and mineralization of the scaffold in bone tissue engineering, inorganic materials are usually introduced with hybrid hydrogels. Given that hydroxyapatite (HA) is one of the major inorganic components in bone tissue,279 Fu et al280 have prepared a novel three-component injectable thermosensitive hydrogel composite composed of triblock PEG–PCL–PEG copolymer, collagen, and nanohydroxyapatite. This hydrogel composite has a good interconnected porous structure in addition to excellent thermosensitivity. Furthermore, in vivo studies have demonstrated that the PECE/collagen/nanohydroxyapatite hydrogel has good biocompatibility and exhibits better performance in guided bone regeneration than in the self-healing process, thus indicating its great promise for bone tissue engineering. Furthermore, Jiao et al281 have synthesized an in situ cross-linkable citric acid-based biodegradable PEG maleate citrate/HA hydrogel. Huang et al282 have fabricated an injectable nanohydroxyapatite/glycol chitosan/hyaluronic acid composite hydrogel. MC-3T3-E1 cells incorporated in the hydrogel attach and spread well after 7 days of co-incubation, thus suggesting that the hydrogel’s potential application in bone tissue engineering. Lin et al283 have designed an injectable and thermosensitive hydrogel composite composed of poly(lactic acid-co-glycolic acid)-g-PEG and HA for its potential application in bone tissue engineering. The addition of HA into the hydrogel enhances the mechanical properties and bioactivity of the hydrogel. Most recently, an injectable alginate/HA hydrogel scaffold, combined with gelatin microspheres (GMs), has been reported by Yan et al.284 In this hydrogel, HA and GMs successfully improve the mechanical properties of the scaffold, thus demonstrating that the HA and GMs double-integrated alginate-based hydrogel has a suitable physical performance and bioactive properties. Thus, the hydrogel shows great potential for local treatment of pathologies involving bone defects. Moreover, taking advantage of the structural and regulatory cellular functions of zinc (Zn) and its ability to promote osteoblastogenesis and suppress osteoclastogenesis,285 Niranjan et al286 have reported a thermosensitive hydrogel, containing Zn, chitosan, and β-glycerophosphate, for bone tissue engineering. Furthermore, Dhivyaet al287 have designed an injectable thermosensitive zinc-doped chitosan/nanohydroxyapatite/β-glycerophosphate-based hydrogel. In vivo studies in a ratbone-defect model system have indicated the potential of the hydrogel for accelerating bone formation at molecular and cellular levels. Other inorganic materials such as nanosilica and Bioglass have been studied for the preparation of hybrid hydrogel systems.288,289 For example, Vishnu Priya et al290 have developed an injectable hydrogel system by using chitin and poly(butylene succinate) loaded with fibrin nanoparticles and magnesium-doped Bioglass. This hydrogel system enhances the initiation of differentiation and expression of alkaline phosphatase and osteocalcin, thus indicating its promise for regenerating irregular bone defects.
Conclusions and perspectives
Injectable hydrogels are promising scaffolds for cartilage and bone tissue engineering, owing to their minimal invasive properties and ability to match irregular defects. In this review, we summarized many novel injectable hydrogels prepared by a variety of biomaterial and fabrication techniques for cartilage- and bone tissue-engineering applications. First, injectable hydrogels fabricated from both natural biomaterials and synthetic biomaterials were reviewed. Natural biomaterials such as chitosan, collagen/gelatin, alginate, fibrin, elastin, heparin, and hyaluronic acid are among the most commonly used biomaterials for the preparation of injectable hydrogels, owing to their perfect cyto-biocompatibility, biodegradability, low cytotoxicity, and similarity to the natural cartilage and bony ECMs. However, injectable hydrogels synthesized from natural biomaterials usually lack mechanical strength, thus limiting their potential utilization. In contrast, synthetic biomaterials-based injectable hydrogels have favorable stability and mechanical properties, but have poor biocompatibility and bioactive properties. Then, various preparation methods of injectable hydrogels, including both physical and chemical methods, were highlighted. Physical hydrogels can be easily fabricated, owing to their sensitivity to external stimuli such as temperature, pH, ion concentration, and stress. Although physical injectable hydrogels can easily be produced and have low cytotoxicity, they usually have a slow response time and low stability. In contrast, injectable hydrogels prepared via chemical methods show favorable stability under physiological conditions and excellent mechanical properties, but they have adverse effects in vivo, owing to chemical reactions.
Over the past several years, there have been many studies focused on synthesizing novel injectable hydrogels for cartilage and bone repair. However, many challenges remain to be addressed in fabricating injectable hydrogels to optimally achieve cartilage and bone regeneration. The major challenge of developing injectable hydrogels for cartilage and bone tissue engineering is the design of bioactive scaffolds that have perfect biocompatibility, biodegradability, stability, and favorable mechanical properties for 3D cell culture, and are able to support nutrient transportation and growth factor delivery. To address this challenge, first, bioactive biomaterials that can be used to prepare novel injectable hydrogels should be developed. Most recently, attempts at using glycopolypeptide,291 silk,292 carrageenan,293 pectin,294 and even the ECM295 to synthesize injectable hydrogels have attracted attention. Second, advanced fabrication methods require further development, primarily to improve the mechanical properties and physiological stability, and to decrease the cytotoxicity and adverse effects of the hydrogels in vivo. Finally, the development of a methodology to integrate the merits of the various biomaterials and fabrication methods for the preparation of injectable hydrogels will play an important role in the clinical applications of hydrogels in cartilage and bone tissue engineering.
Acknowledgments
This work was supported by NSFC (nos 61471168, 61571187,61301043, and 61527806), China Postdoctoral Science Foundation (2016T90403), and the Economical Forest Cultivation and Utilization of 2011 Collaborative Innovation Center in Hunan Province [(2013) 448].
Footnotes
The authors declare no conflict of interest.
References
- Walker KJ, Madihally SV. Anisotropic temperature sensitive chitosan-based injectable hydrogels mimicking cartilage matrix. J Biomed Mater Res B Appl Biomater 2015; 103: 1149–1160. [DOI] [PubMed] [Google Scholar]
- Söntjens SHM, Nettles DL, Carnahan MA et al. Biodendrimer-based hydrogel scaffolds for cartilage tissue repair. Biomacromolecules 2006; 7: 310–316. [DOI] [PubMed] [Google Scholar]
- Ren K, He C, Xiao C et al. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials 2015; 51: 238–249. [DOI] [PubMed] [Google Scholar]
- Cancedda R, Dozin B, Giannoni P et al. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol 2003; 22: 81–91. [DOI] [PubMed] [Google Scholar]
- Hjelle K, Solheim E, Strand T et al. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 2002; 18: 730–734. [DOI] [PubMed] [Google Scholar]
- Vilela CA, Correia C, Oliveira JM et al. Cartilage repair using hydrogels: a critical review of in vivo experimental designs. ACS Biomater Sci Eng 2015; 1: 726–739. [DOI] [PubMed] [Google Scholar]
- Liao J, Shi K, Ding Q et al. Recent developments in scaffold-guided cartilage tissue regeneration. J Biomed Nanotechnol 2014; 10: 3085–3104. [DOI] [PubMed] [Google Scholar]
- Yuan T, Zhang L, Li K et al. Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J Biomed Mater Res B Appl Biomater 2014; 102: 337–344. [DOI] [PubMed] [Google Scholar]
- Buckwalter J. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther 1998; 28: 192–202. [DOI] [PubMed] [Google Scholar]
- Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science 2012; 338: 917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch J, Venkatesan J, Rey-Rico A et al. Current progress in stem cell-based gene therapy for articular cartilage repair. Curr Stem Cell Res Ther 2015; 10: 121–131. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ouyang H, Dass CR et al. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res 2016; 4: 15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson RE, Silva MJ. Skeletal blood flow in bone repair and maintenance. Bone Res 2013; 1: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flierl MA, Smith WR, Mauffrey C et al. Outcomes and complication rates of different bone grafting modalities in long bone fracture nonunions: A retrospective cohort study in 182 patients. J Orthop Surg Res 2013; 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury 2005; 36 (Suppl 3): S20–S27. [DOI] [PubMed] [Google Scholar]
- Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury 2007; 38 (Suppl 1): S75–S80. [DOI] [PubMed] [Google Scholar]
- Marenzana M, Arnett TR. The key role of the blood supply to bone. Bone Res 2013; 1: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhao L, Liu J et al. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res 2014; 2: 14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TG, Shin H, Lim DW. Biomimetic scaffolds for tissue engineering. Adv Funct Mater 2012; 22: 2446–2468. [Google Scholar]
- Khan WS, Malik A. Stem cell therapy and tissue engineering applications for cartilage regeneration. Curr Stem Cell Res Ther 2012; 7: 241–242. [DOI] [PubMed] [Google Scholar]
- Grottkau BE, Lin Y. Osteogenesis of adipose-derived stem cells. Bone Res 2013; 1: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JR, Liang H, Dickinson M et al. Xylan hemicellulose improves chitosan hydrogel for bone tissue regeneration. Polym Adv Technol 2016; 27: 1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni V, Tibrewal S, Bissell L et al. The role of tissue engineering in achilles tendon repair: a review. Curr Stem Cell Res Ther 2015; 10: 31–36. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shang S, Li C. Aligned biomimetic scaffolds as a new tendency in tissue engineering. Curr Stem Cell Res Ther 2016; 11: 3–18. [DOI] [PubMed] [Google Scholar]
- Malda J, Visser J, Melchels FP et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater 2013; 25: 5011–5028. [DOI] [PubMed] [Google Scholar]
- Balakrishnan B, Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem Rev 2011; 111: 4453–4474. [DOI] [PubMed] [Google Scholar]
- Huang CC, Ravindran S, Yin Z et al. 3-D self-assembling leucine zipper hydrogel with tunable properties for tissue engineering. Biomaterials 2014; 35: 5316–5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater 2005; 4: 518–524. [DOI] [PubMed] [Google Scholar]
- Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science 2012; 336: 1124–1128. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xia K, Lu Z et al. Efficient and facile synthesis of gold nanorods with finely tunable plasmonic peaks from visible to near-IR range. Chem Mater 2014; 26: 1794–1798. [Google Scholar]
- Deng Y, Wang M, Jiang L et al. A comparison of extracellular excitatory amino acids release inhibition of acute lamotrigine and topiramate treatment in the hippocampus of ptz-kindled epileptic rats. J Biomed Nanotechnol 2013; 9: 1123–1128. [DOI] [PubMed] [Google Scholar]
- Shin SR, Li YC, Jang HL et al. Graphene-based materials for tissue engineering. Adv Drug Deliv Rev 2016; 105: 255–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lu Z, Li X et al. Methoxy poly(ethylene glycol) conjugated denatured bovine serum albumin micelles for effective delivery of camptothecin. Polym Chem 2012; 3: 1958. [Google Scholar]
- Fan C, Wang D-A. A biodegradable PEG-based micro-cavitary hydrogel as scaffold for cartilage tissue engineering. Eur Polym J 2015; 72: 651–660. [Google Scholar]
- Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 2003; 24: 4337–4351. [DOI] [PubMed] [Google Scholar]
- Fan J, He N, He Q et al. A novel self-assembled sandwich nanomedicine for NIR-responsive release of NO. Nanoscale 2015; 7: 20055–20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Huang Y, Zhang L et al. Preparation of gold nanorods using 1,2,4-trihydroxybenzene as a reducing agent. J Nanosci Nanotechnol 2015; 15: 6230–6235. [DOI] [PubMed] [Google Scholar]
- Zhang L, Webster TJ. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009; 4: 66–80. [Google Scholar]
- Slaughter BV, Khurshid SS, Fisher OZ et al. Hydrogels in regenerative medicine. Adv Mater 2009; 21: 3307–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B, Kim S, Lin B et al. Cartilaginous extracellular matrix-modified chitosan hydrogels for cartilage tissue engineering. ACS Appl Mater Interfaces 2014; 6: 20110–20121. [DOI] [PubMed] [Google Scholar]
- Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules 2011; 12: 1387–1408. [DOI] [PubMed] [Google Scholar]
- Yazdimamaghani M, Vashaee D, Assefa S et al. Hybrid macroporous gelatin/bioactive-glass/nanosilver scaffolds with controlled degradation behavior and antimicrobial activity for bone tissue engineering. J Biomed Nanotechnol 2014; 10: 911–931. [DOI] [PubMed] [Google Scholar]
- Jin R, Moreira Teixeira LS, Dijkstra PJ et al. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 2009; 30: 2544–2551. [DOI] [PubMed] [Google Scholar]
- Sivashanmugam A, Arun Kumar R, Vishnu Priya M et al. An overview of injectable polymeric hydrogels for tissue engineering. Eur Polym J 2015; 72: 543–565. [Google Scholar]
- Tan H, Li H, Rubin JP et al. Controlled gelation and degradation rates of injectable hyaluronic acid-based hydrogels through a double crosslinking strategy. J Tissue Eng Regen Med 2011; 5: 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Wang C, Lai RC et al. An improved injectable polysaccharide hydrogel: modified gellan gum for long-term cartilage regeneration in vitro. J Mater Chem 2009; 19: 1968–1977. [Google Scholar]
- Wei Y, Hu Y, Hao W et al. A novel injectable scaffold for cartilage tissue engineering using adipose-derived adult stem cells. J Orthop Res 2008; 26: 27–33. [DOI] [PubMed] [Google Scholar]
- Shen Z-S, Cui X, Hou R-X et al. Tough biodegradable chitosan-gelatin hydrogels via in situ precipitation for potential cartilage tissue engineering. RSC Adv 2015; 5: 55640–55647. [Google Scholar]
- Hong Y, Gong Y, Gao C et al. Collagen-coated polylactide microcarriers/chitosan hydrogel composite: injectable scaffold for cartilage regeneration. J Biomed Mater Res A 2008; 85: 628–637. [DOI] [PubMed] [Google Scholar]
- Bidarra SJ, Barrias CC, Granja PL. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater 2014; 10: 1646–1662. [DOI] [PubMed] [Google Scholar]
- Dorsey SM, McGarvey JR, Wang H et al. MRI evaluation of injectable hyaluronic acid-based hydrogel therapy to limit ventricular remodeling after myocardial infarction. Biomaterials 2015; 69: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim HJ, Thambi T, Lee DS. Heparin-based temperature-sensitive injectable hydrogels for protein delivery. J Mater Chem B 2015; 3: 8892–8901. [DOI] [PubMed] [Google Scholar]
- Wang F, Li Z, Khan M et al. Injectable, rapid gelling and highly flexible hydrogel composites as growth factor and cell carriers. Acta Biomater 2010; 6: 1978–1991. [DOI] [PubMed] [Google Scholar]
- Alexander A, Ajazuddin, Khan J et al. Poly(ethylene glycol)-poly(lactic-co-glycolic acid) based thermosensitive injectable hydrogels for biomedical applications. J Control Release 2013; 172: 715–729. [DOI] [PubMed] [Google Scholar]
- Ossipov DA, Piskounova S, Hilborn J. Poly(vinyl alcohol) cross-linkers for in vivo injectable hydrogels. Macromolecules 2008; 41: 3971–3982. [Google Scholar]
- Overstreet DJ, Dutta D, Stabenfeldt SE et al. Injectable hydrogels. J Polym Sci Pol Phys 2012; 50: 881–903. [Google Scholar]
- Amini AA, Nair LS. Injectable hydrogels for bone and cartilage repair. Biomed Mater 2012; 7: 024105. [DOI] [PubMed] [Google Scholar]
- Binetti VR, Fussell GW, Lowman AM. Evaluation of two chemical crosslinking methods of poly(vinyl alcohol) hydrogels for injectable nucleus pulposus replacement. J Appl Polym Sci 2014; 131: 40843. [Google Scholar]
- Jin R, Teixeira LS, Dijkstra PJ et al. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran-hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 2010; 31: 3103–3113. [DOI] [PubMed] [Google Scholar]
- Lin C-C, Ki CS, Shih H. Thiol-norbornene photoclick hydrogels for tissue engineering applications. J Appl Polym Sci 2015; 132: 41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rodrigues J, Tomas H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem Soc Rev 2012; 41: 2193–2221. [DOI] [PubMed] [Google Scholar]
- Tan H, Marra KG. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010; 3: 1746–1767. [Google Scholar]
- Ko DY, Shinde UP, Yeon B et al. Recent progress of in situ formed gels for biomedical applications. Prog Polym Sci 2013; 38: 672–701. [Google Scholar]
- Park H, Woo EK, Lee KY. Ionically cross-linkable hyaluronate-based hydrogels for injectable cell delivery. J Control Release 2014; 196: 146–153. [DOI] [PubMed] [Google Scholar]
- Chiu YL, Chen SC, Su CJ et al. pH-triggered injectable hydrogels prepared from aqueous N-palmitoyl chitosan: in vitro characteristics and in vivo biocompatibility. Biomaterials 2009; 30: 4877–4888. [DOI] [PubMed] [Google Scholar]
- Choi BG, Park MH, Cho S-H et al. Thermal gelling polyalanine-poloxamine-polyalanine aqueous solution for chondrocytes 3D culture: Initial concentration effect. Soft Matter 2011; 7: 456–462. [Google Scholar]
- Yeon B, Park MH, Moon HJ et al. 3D culture of adipose-tissue-derived stem cells mainly leads to chondrogenesis in poly(ethylene glycol)-poly(L-alanine) diblock copolymer thermogel. Biomacromolecules 2013; 14: 3256–3266. [DOI] [PubMed] [Google Scholar]
- Badylak SF, Weiss DJ, Caplan A et al. Engineered whole organs and complex tissues. Lancet 2012; 379: 943–952. [DOI] [PubMed] [Google Scholar]
- Benders KE, van Weeren PR, Badylak SF et al. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol 2013; 31: 169–176. [DOI] [PubMed] [Google Scholar]
- Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res 2014; 163: 268–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhu J, Liu F et al. Reduced EGFR signaling enhances cartilage destruction in a mouse osteoarthritis model. Bone Res 2014; 2: 14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JA, Cobelli N, Santambrogio L. Consequences of metabolic and oxidative modifications of cartilage tissue. Nat Rev Rheumatol 2015; 11: 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials 2011; 32: 8771–8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra J, Andrades JA, Guerado E et al. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev 2010; 16: 617–627. [DOI] [PubMed] [Google Scholar]
- Tan R, Feng Q, She Z et al. In vitro and in vivo degradation of an injectable bone repair composite. Polym Degrad Stab 2010; 95: 1736–1742. [Google Scholar]
- Gong T, Xie J, Liao J et al. Nanomaterials and bone regeneration. Bone Res 2015; 3: 15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel J, Woodruff MA, Epari DR et al. Bone regeneration based on tissue engineering conceptions—A 21st century perspective. Bone Res 2013; 1: 216–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mow V, Guo X. Mechano-electrochemical properties of articular cartilage: Their inhomogeneities and anisotropies. Annu Rev Biomed Eng 2002; 4: 175–209. [DOI] [PubMed] [Google Scholar]
- Bobick BE, Chen FH, Le AM et al. Regulation of the chondrogenic phenotype in culture. Birth Defects Res C Embryo Today 2009; 87: 351–371. [DOI] [PubMed] [Google Scholar]
- Svensson A, Nicklasson E, Harrah T et al. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005; 26: 419–431. [DOI] [PubMed] [Google Scholar]
- Alford AI, Kozloff KM, Hankenson KD. Extracellular matrix networks in bone remodeling. Int J Biochem Cell Biol 2015; 65: 20–31. [DOI] [PubMed] [Google Scholar]
- Cordonnier T, Sohier J, Rosset P et al. Biomimetic materials for bone tissue engineering—state of the art and future trends. Adv Eng Mater 2011; 13: B135–B150. [Google Scholar]
- Ahadian S, Sadeghian RB, Salehi S et al. Bioconjugated hydrogels for tissue engineering and regenerative medicine. Bioconjug Chem 2015; 26: 1984–2001. [DOI] [PubMed] [Google Scholar]
- Sell S, Barnes C, Smith M et al. Extracellular matrix regenerated: tissue engineering via electrospun biomimetic nanofibers. Polym Int 2007; 56: 1349–1360. [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol 1982; 99: 31–68. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 2006; 22: 287–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi A, Mithieux SM, Wei H et al. Elastin based cell-laden injectable hydrogels with tunable gelation, mechanical and biodegradation properties. Biomaterials 2014; 35: 5425–5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant TD, Desai AP, Banerjee S et al. An in situ forming collagen-PEG hydrogel for tissue regeneration. Acta Biomater 2012; 8: 124–132. [DOI] [PubMed] [Google Scholar]
- Williams C, Budina E, Stoppel WL et al. Cardiac extracellular matrix-fibrin hybrid scaffolds with tunable properties for cardiovascular tissue engineering. Acta Biomater 2015; 14: 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tian H, Chen X. Hyaluronic acid based injectable hydrogels for localized and sustained gene delivery. J Control Release 2015; 213: E140–E141. [DOI] [PubMed] [Google Scholar]
- Ji X, Yang W, Wang T et al. Coaxially electrospun core/shell structured poly(L-lactide) acid/chitosan nanofibers for potential drug carrier in tissue engineering. J Biomed Nanotechnol 2013; 9: 1672–1678. [DOI] [PubMed] [Google Scholar]
- Tan H, Chu CR, Payne KA et al. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009; 30: 2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino AD, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005; 26: 5983–5990. [DOI] [PubMed] [Google Scholar]
- Yang W, Fu J, Wang T et al. Chitosan/Sodium tripolyphosphate nanoparticles: preparation, characterization and application as drug carrier. J Biomed Nanotechnol 2009; 5: 591–595. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhang Z, Wang G et al. Preparation of chitosan-sodium sodium tripolyphosphate nanoparticles via reverse microemulsion-ionic gelation method. J Bionanosci 2015; 9: 301–305. [Google Scholar]
- Naderi-Meshkin H, Andreas K, Matin MM et al. Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol Int 2014; 38: 72–84. [DOI] [PubMed] [Google Scholar]
- Sá-Lima H, Caridade SG, Mano JF et al. Stimuli-responsive chitosan-starch injectable hydrogels combined with encapsulated adipose-derived stromal cells for articular cartilage regeneration. Soft Matter 2010; 6: 5184–5195. [Google Scholar]
- Moreira CD, Carvalho SM, Mansur HS et al. Thermogelling chitosan-collagen-bioactive glass nanoparticle hybrids as potential injectable systems for tissue engineering. Mater Sci Eng C Mater Biol Appl 2016; 58: 1207–1216. [DOI] [PubMed] [Google Scholar]
- Yang X, Liu Q, Chen X et al. Investigation of PVA/ws-chitosan hydrogels prepared by combined γ-irradiation and freeze-thawing. Carbohydr Polym 2008; 73: 401–408. [Google Scholar]
- Kamoun EA. N-succinyl chitosan-dialdehyde starch hybrid hydrogels for biomedical applications. J Adv Res 2016; 7: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm 2001; 221: 1–22. [DOI] [PubMed] [Google Scholar]
- Parmar PA, Chow LW, St-Pierre JP et al. Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration. Biomaterials 2015; 54: 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez CM, Panitch A, Chmielewski J. A collagen peptide-based physical hydrogel for cell encapsulation. Macromol Biosci 2011; 11: 1426–1431. [DOI] [PubMed] [Google Scholar]
- Ackermann B, Steinmeyer J. Collagen biosynthesis of mechanically loaded articular cartilage explants. Osteoarthritis Cartilage 2005; 13: 906–914. [DOI] [PubMed] [Google Scholar]
- Yuan L, Li B, Yang J et al. Effects of composition and mechanical property of injectable collagen I/II composite hydrogels on chondrocyte behaviors. Tissue Eng Part A 2016; 22: 899–906. [DOI] [PubMed] [Google Scholar]
- Funayama A, Niki Y, Matsumoto H et al. Repair of full-thickness articular cartilage defects using injectable type II collagen gel embedded with cultured chondrocytes in a rabbit model. J Orthop Sci 2008; 13: 225–232. [DOI] [PubMed] [Google Scholar]
- Kontturi LS, Järvinen E, Muhonen V et al. An injectable, in situ forming type II collagen/hyaluronic acid hydrogel vehicle for chondrocyte delivery in cartilage tissue engineering. Drug Deliv Transl Res 2014; 4: 149–158. [DOI] [PubMed] [Google Scholar]
- Santoro M, Tatara AM, Mikos AG. Gelatin carriers for drug and cell delivery in tissue engineering. J Control Release 2014; 190: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Li L, Li W et al. Three-dimensional dynamic fabrication of engineered cartilage based on chitosan/gelatin hybrid hydrogel scaffold in a spinner flask with a special designed steel frame. Mater Sci Eng C Mater Biol Appl 2015; 55: 384–392. [DOI] [PubMed] [Google Scholar]
- Oh BH, Bismarck A, Chan-Park MB. Injectable, interconnected, high-porosity macroporous biocompatible gelatin scaffolds made by surfactant-free emulsion templating. Macromol Rapid Commun 2015; 36: 364–372. [DOI] [PubMed] [Google Scholar]
- Geng X, Mo X, Fan L et al. Hierarchically designed injectable hydrogel from oxidized dextran, amino gelatin and 4-arm poly(ethylene glycol)-acrylate for tissue engineering application. J Mater Chem 2012; 22: 25130–25139. [Google Scholar]
- Evanko SP, Tammi MI, Tammi RH et al. Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev 2007; 59: 1351–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-D, Kim D-H, Son Y-J. Three-dimensional porous scaffold of hyaluronic acid for cartilage tissue engineering. Stud Mechanobiol Tissue Eng Biomater 2011; 8: 329–349. [Google Scholar]
- Jin R, Moreira Teixeira LS, Krouwels A et al. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via Michael addition: an injectable biomaterial for cartilage repair. Acta Biomater 2010; 6: 1968–1977. [DOI] [PubMed] [Google Scholar]
- Balazs EA, Watson D, Duff IF et al. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis Rheum 1967; 10: 357–376. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, McDonald JA. Hyaluronan-is bigger better? Am J Respir Cell Mol Biol 2000; 23: 431–433. [DOI] [PubMed] [Google Scholar]
- Muzzarelli RA, Greco F, Busilacchi A et al. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: a review. Carbohydr Polym 2012; 89: 723–739. [DOI] [PubMed] [Google Scholar]
- Knudson CB. Hyaluronan and CD44: strategic players for cell-matrix interactions during chondrogenesis and matrix assembly. Birth Defects Res C Embryo Today 2003; 69: 174–196. [DOI] [PubMed] [Google Scholar]
- Astachov L, Vago R, Aviv M et al. Hyaluronan and mesenchymal stem cells: from germ layer to cartilage and bone. Front Biosci (Landmark Ed) 2011; 16: 261–276. [DOI] [PubMed] [Google Scholar]
- Yu F, Cao X, Li Y et al. An injectable hyaluronic acid/PEG hydrogel for cartilage tissue engineering formed by integrating enzymatic crosslinking and Diels-Alder “click chemistry”. Polym Chem 2014; 5: 1082–1090. [Google Scholar]
- Park H, Choi B, Hu J et al. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater 2013; 9: 4779–4786. [DOI] [PubMed] [Google Scholar]
- Barbucci R, Lamponi S, Borzacchiello A et al. Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials 2002; 23: 4503–4513. [DOI] [PubMed] [Google Scholar]
- Palumbo FS, Fiorica C, Di Stefano M et al. In situ forming hydrogels of hyaluronic acid and inulin derivatives for cartilage regeneration. Carbohydr Polym 2015; 122: 408–416. [DOI] [PubMed] [Google Scholar]
- Domingues RM, Silva M, Gershovich P et al. Development of injectable hyaluronic acid/cellulose nanocrystals bionanocomposite hydrogels for tissue engineering applications. Bioconjug Chem 2015; 26: 1571–1581. [DOI] [PubMed] [Google Scholar]
- Zhou H, Xu HH. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials 2011; 32: 7503–7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyrich D, Brandl F, Appel B et al. Long-term stable fibrin gels for cartilage engineering. Biomaterials 2007; 28: 55–65. [DOI] [PubMed] [Google Scholar]
- Sha'ban M, Yoon SJ, Ko YK et al. Fibrin promotes proliferation and matrix production of intervertebral disc cells cultured in three-dimensional poly(lactic-co-glycolic acid) scaffold. J Biomater Sci Polym Ed 2008; 19: 1219–1237. [DOI] [PubMed] [Google Scholar]
- Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev 2008; 14: 199–215. [DOI] [PubMed] [Google Scholar]
- Snyder TN, Madhavan K, Intrator M et al. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J Biol Eng 2014; 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dare EV, Griffith M, Poitras P et al. Genipin cross-linked fibrin hydrogels for in vitro human articular cartilage tissue-engineered regeneration. Cells Tissues Organs 2009; 190: 313–325. [DOI] [PubMed] [Google Scholar]
- Choi JW, Choi BH, Park SH et al. Mechanical stimulation by ultrasound enhances chondrogenic differentiation of mesenchymal stem cells in a fibrin-hyaluronic acid hydrogel. Artif Organs 2013; 37: 648–655. [DOI] [PubMed] [Google Scholar]
- Benavides OM, Brooks AR, Cho SK et al. In situ vascularization of injectable fibrin/poly(ethylene glycol) hydrogels by human amniotic fluid-derived stem cells. J Biomed Mater Res A 2015; 103: 2645–2653. [DOI] [PubMed] [Google Scholar]
- Almeida HV, Eswaramoorthy R, Cunniffe GM et al. Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration. Acta Biomater 2016; 36: 55–62. [DOI] [PubMed] [Google Scholar]
- Hwang CM, Ay B, Kaplan DL et al. Assessments of injectable alginate particle-embedded fibrin hydrogels for soft tissue reconstruction. Biomed Mater 2013; 8: 014105. [DOI] [PubMed] [Google Scholar]
- Venkatesan J, Bhatnagar I, Manivasagan P et al. Alginate composites for bone tissue engineering: a review. Int J Biol Macromol 2015; 72: 269–281. [DOI] [PubMed] [Google Scholar]
- Zhang F, Li X, He N et al. Antibacterial properties of ZnO/calcium alginate composite and its application in wastewater treatment. J Nanosci Nanotechnol 2015; 15: 3839–3845. [DOI] [PubMed] [Google Scholar]
- Park H, Kang SW, Kim BS et al. Shear-reversibly crosslinked alginate hydrogels for tissue engineering. Macromol Biosci 2009; 9: 895–901. [DOI] [PubMed] [Google Scholar]
- Ruvinov E, Cohen S. Alginate biomaterial for the treatment of myocardial infarction: progress, translational strategies, and clinical outlook: from ocean algae to patient bedside. Adv Drug Deliv Rev 2016; 96: 54–76. [DOI] [PubMed] [Google Scholar]
- Follin B, Juhl M, Cohen S et al. Human adipose-derived stromal cells in a clinically applicable injectable alginate hydrogel: phenotypic and immunomodulatory evaluation. Cytotherapy 2015; 17: 1104–1118. [DOI] [PubMed] [Google Scholar]
- Balakrishnan B, Joshi N, Jayakrishnan A et al. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater 2014; 10: 3650–3663. [DOI] [PubMed] [Google Scholar]
- Kretlow JD, Young S, Klouda L et al. Injectable biomaterials for regenerating complex craniofacial tissues. Adv Mater 2009; 21: 3368–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Weir MD, Xu HH. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 2010; 31: 6502–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Lee KY. Cartilage regeneration using biodegradable oxidized alginate/hyaluronate hydrogels. J Biomed Mater Res A 2014; 102: 4519–4525. [DOI] [PubMed] [Google Scholar]
- Jaikumar D, Sajesh KM, Soumya S et al. Injectable alginate-O-carboxymethyl chitosan/nano fibrin composite hydrogels for adipose tissue engineering. Int J Biol Macromol 2015; 74: 318–326. [DOI] [PubMed] [Google Scholar]
- Casu B. Structure and biological activity of heparin. Adv Carbohydr Chem Biochem 1985; 43: 51–134. [DOI] [PubMed] [Google Scholar]
- Tae G, Kim Y-J, Choi W-I et al. Formation of a novel heparin-based hydrogel in the presence of heparin-binding biomolecules. Biomacromolecules 2007; 8: 1979–1986. [DOI] [PubMed] [Google Scholar]
- Liang Y, Kiick KL. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater 2014; 10: 1588–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram M, Qi Y, Shriver Z et al. Rational design of low-molecular weight heparins with improved in vivo activity. Proc Natl Acad Sci USA 2003; 100: 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammadov R, Mammadov B, Guler MO et al. Growth factor binding on heparin mimetic peptide nanofibers. Biomacromolecules 2012; 13: 3311–3319. [DOI] [PubMed] [Google Scholar]
- Guillame-Gentil O, Semenov O, Roca AS et al. Engineering the extracellular environment: strategies for building 2D and 3D cellular structures. Adv Mater 2010; 22: 5443–5462. [DOI] [PubMed] [Google Scholar]
- Hudalla GA, Murphy WL. Biomaterials that regulate growth factor activity via bioinspired interactions. Adv Funct Mater 2011; 21: 1754–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tang H, Kowitsch A et al. Novel mineralized heparin-gelatin nanoparticles for potential application in tissue engineering of bone. J Mater Sci Mater Med 2014; 25: 669–680. [DOI] [PubMed] [Google Scholar]
- Go DH, Joung YK, Lee SY et al. Tetronic-oligolactide-heparin hydrogel as a multi-functional scaffold for tissue regeneration. Macromol Biosci 2008; 8: 1152–1160. [DOI] [PubMed] [Google Scholar]
- Wang T, Ji X, Jin L et al. Fabrication and characterization of heparin-grafted poly-l-lactic acid-chitosan core-shell nanofibers scaffold for vascular gasket. ACS Appl Mater Interfaces 2013; 5: 3757–3763. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Ishihara M, Obara K et al. Controlled release of fibroblast growth factor-2 from an injectable 6-O-desulfated heparin hydrogel and subsequent effect on in vivo vascularization. J Biomed Mater Res A 2006; 78: 364–371. [DOI] [PubMed] [Google Scholar]
- Fujita M, Ishihara M, Shimizu M et al. Therapeutic angiogenesis induced by controlled release of fibroblast growth factor-2 from injectable chitosan/non-anticoagulant heparin hydrogel in a rat hindlimb ischemia model. Wound Repair Regen 2007; 15: 58–65. [DOI] [PubMed] [Google Scholar]
- Lee J, Choi WI, Tae G et al. Enhanced regeneration of the ligament-bone interface using a poly(L-lactide-co-epsilon-caprolactone) scaffold with local delivery of cells/BMP-2 using a heparin-based hydrogel. Acta Biomater 2011; 7: 244–257. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim SE, Kang SS et al. The use of de-differentiated chondrocytes delivered by a heparin-based hydrogel to regenerate cartilage in partial-thickness defects. Biomaterials 2011; 32: 7883–7896. [DOI] [PubMed] [Google Scholar]
- Jin R, Moreira Teixeira LS, Dijkstra PJ et al. Chondrogenesis in injectable enzymatically crosslinked heparin/dextran hydrogels. J Control Release 2011; 152: 186–195. [DOI] [PubMed] [Google Scholar]
- Kim M, Hong B, Lee J et al. Composite system of PLCL scaffold and heparin-based hydrogel for regeneration of partial-thickness cartilage defects. Biomacromolecules 2012; 13: 2287–2298. [DOI] [PubMed] [Google Scholar]
- Annabi N, Mithieux SM, Weiss AS et al. Cross-linked open-pore elastic hydrogels based on tropoelastin, elastin and high pressure CO2. Biomaterials 2010; 31: 1655–1665. [DOI] [PubMed] [Google Scholar]
- Ozsvar J, Mithieux SM, Wang R et al. Elastin-based biomaterials and mesenchymal stem cells. Biomater Sci 2015; 3: 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annabi N, Fathi A, Mithieux SM et al. The effect of elastin on chondrocyte adhesion and proliferation on poly (varepsilon-caprolactone)/elastin composites. Biomaterials 2011; 32: 1517–1525. [DOI] [PubMed] [Google Scholar]
- Knutson JR, Iida J, Fields GB et al. CD44/chondroitin sulfate proteoglycan and alpha 2 beta 1 integrin mediate human melanoma cell migration on type IV collagen and invasion of basement membranes. Mol Biol Cell 1996; 7: 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DA, Varghese S, Sharma B et al. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater 2007; 6: 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi P, Bhat S, Nayak V et al. Study of different delivery modes of chondroitin sulfate using microspheres and cryogel scaffold for application in cartilage tissue engineering. Int J Polym Mater Po 2014; 63: 859–872. [Google Scholar]
- Jo S, Kim D, Woo J et al. Development and physicochemical evaluation of chondroitin sulfate-poly(ethylene oxide) hydrogel. Macromol Res 2011; 19: 147–155. [Google Scholar]
- Strehin I, Nahas Z, Arora K et al. A versatile pH sensitive chondroitin sulfate-PEG tissue adhesive and hydrogel. Biomaterials 2010; 31: 2788–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Kim S, Noh I. Synthesis of in situ chondroitin sulfate hydrogel through phosphine-mediated Michael type addition reaction. Macromol Res 2012; 20: 968–976. [Google Scholar]
- Liao J, Qu Y, Chu B et al. Biodegradable CSMA/PECA/graphene porous hybrid scaffold for cartilage tissue engineering. Sci Rep 2015; 5: 9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li K, Xiao W et al. Preparation of collagen-chondroitin sulfate-hyaluronic acid hybrid hydrogel scaffolds and cell compatibility in vitro. Carbohydr Polym 2011; 84: 118–125. [Google Scholar]
- Wiltsey C, Kubinski P, Christiani T et al. Characterization of injectable hydrogels based on poly(N-isopropylacrylamide)-g-chondroitin sulfate with adhesive properties for nucleus pulposus tissue engineering. J Mater Sci Mater Med 2013; 24: 837–847. [DOI] [PubMed] [Google Scholar]
- Chen F, Yu S, Liu B et al. An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci Rep 2016; 6: 20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, He Q, Liu Y et al. Light-responsive biodegradable nanomedicine overcomes multidrug resistance via NO-enhanced chemosensitization. ACS Appl Mater Interfaces 2016; 8: 13804–13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, He N, Fu J et al. Preparation of porous core-shell poly l-lactic acid/polyethylene glycol superfine fibres containing drug. J Nanosci Nanotechnol 2015; 15: 9911–9917. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xia K, Deng Y et al. Methoxy poly(ethylene glycol) conjugated doxorubicin micelles for effective killing of cancer cells. J Nanosci Nanotechnol 2014; 14: 6458–6460. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lu Z, Bai Y et al. PEGylated denatured bovine serum albumin modified water-soluble inorganic nanocrystals as multifunctional drug delivery platforms. J Mater Chem B 2013; 1: 1289. [DOI] [PubMed] [Google Scholar]
- Yan S, Wang T, Feng L et al. Injectable in situ self-cross-linking hydrogels based on poly(L-glutamic acid) and alginate for cartilage tissue engineering. Biomacromolecules 2014; 15: 4495–4508. [DOI] [PubMed] [Google Scholar]
- Yang W, He N, Li Z. Rapamycin release study of porous poly(L-lactic acid) scaffolds, prepared via coaxial electrospinning. J Nanosci Nanotechnol 2016; 16: 9404–9412. [Google Scholar]
- Bonakdar S, Emami SH, Shokrgozar MA et al. Preparation and characterization of polyvinyl alcohol hydrogels crosslinked by biodegradable polyurethane for tissue engineering of cartilage. Mat Sci Eng C 2010; 30: 636–643. [Google Scholar]
- Kallukalam BC, Jayabalan M, Sankar V. Studies on chemically crosslinkable carboxy terminated-poly(propylene fumarate-co-ethylene glycol)-acrylamide hydrogel as an injectable biomaterial. Biomed Mater 2009; 4: 015002. [DOI] [PubMed] [Google Scholar]
- Sun S, Cao H, Su H et al. Preparation and characterization of a novel injectable in situ cross-linked hydrogel. Polym Bull 2009; 62: 699–711. [Google Scholar]
- Alexander A, Ajazuddin, Khan J et al. Polyethylene glycol (PEG)-poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur J Pharm Biopharm 2014; 88: 575–585. [DOI] [PubMed] [Google Scholar]
- Hyun H, Park S, Kwon D et al. Thermo-responsive injectable MPEG-polyester diblock copolymers for sustained drug release. Polymers 2014; 6: 2670–2683. [Google Scholar]
- Kwon JS, Yoon SM, Kwon DY et al. Injectable in situ-forming hydrogel for cartilage tissue engineering. J Mater Chem B 2013; 1: 3314–3321. [DOI] [PubMed] [Google Scholar]
- Yan S, Zhang X, Zhang K et al. Injectable in situ forming poly(L-glutamic acid) hydrogels for cartilage tissue engineering. J Mater Chem B 2016; 4: 947–961. [DOI] [PubMed] [Google Scholar]
- Skaalure SC, Chu S, Bryant SJ. An enzyme-sensitive PEG hydrogel based on aggrecan catabolism for cartilage tissue engineering. Adv Healthc Mater 2015; 4: 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De France KJ, Chan KJ, Cranston ED et al. Enhanced mechanical properties in cellulose nanocrystal-poly(oligoethylene glycol methacrylate) injectable nanocomposite hydrogels through control of physical and chemical cross-linking. Biomacromolecules 2016; 17: 649–660. [DOI] [PubMed] [Google Scholar]
- Yu F, Cao X, Li Y et al. Diels-Alder crosslinked HA/PEG hydrogels with high elasticity and fatigue resistance for cell encapsulation and articular cartilage tissue repair. Polym Chem 2014; 5: 5116–5123. [Google Scholar]
- Liu H, Liu J, Qi C et al. Thermosensitive injectable in situ forming carboxymethyl chitin hydrogel for three-dimensional cell culture. Acta Biomater 2016; 35: 228–237. [DOI] [PubMed] [Google Scholar]
- Kim HK, Shim WS, Kim SE et al. Injectable in situ-forming pH/thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng Part A 2009; 15: 923–933. [DOI] [PubMed] [Google Scholar]
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev 2012; 64: 18–23. [DOI] [PubMed] [Google Scholar]
- Yang J-A, Yeom J, Hwang BW et al. In situ-forming injectable hydrogels for regenerative medicine. Prog Polym Sci 2014; 39: 1973–1986. [Google Scholar]
- Nagahama K, Takahashi A, Ohya Y. Biodegradable polymers exhibiting temperature-responsive sol-gel transition as injectable biomedical materials. React Funct Polym 2013; 73: 979–985. [Google Scholar]
- Sood N, Bhardwaj A, Mehta S et al. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv 2016; 23: 758–780. [DOI] [PubMed] [Google Scholar]
- Yu R, Zheng S. Poly(acrylic acid)-grafted poly(N-isopropyl acrylamide) networks: preparation, characterization and hydrogel behavior. J Biomater Sci Polym Ed 2011; 22: 2305–2324. [DOI] [PubMed] [Google Scholar]
- Ashraf S, Park H-K, Park H et al. Snapshot of phase transition in thermoresponsive hydrogel PNIPAM: role in drug delivery and tissue engineering. Macromol Res 2016; 24: 297–304. [Google Scholar]
- Lee PY, Cobain E, Huard J et al. Thermosensitive hydrogel PEG-PLGA-PEG enhances engraftment of muscle-derived stem cells and promotes healing in diabetic wound. Mol Ther 2007; 15: 1189–1194. [DOI] [PubMed] [Google Scholar]
- Vo TN, Ekenseair AK, Spicer PP et al. In vitro and in vivo evaluation of self-mineralization and biocompatibility of injectable, dual-gelling hydrogels for bone tissue engineering. J Control Release 2015; 205: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte Campos DF, Drescher W, Rath B et al. Supporting biomaterials for articular cartilage repair. Cartilage 2012; 3: 205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Cheng W, Shao Z et al. Synthesis and characterization of temperature-sensitive hydrogels. E-Polymers 2015; 15: 353–360. [Google Scholar]
- Klouda L, Perkins KR, Watson BM et al. Thermoresponsive, in situ cross-linkable hydrogels based on N-isopropylacrylamide: Fabrication, characterization and mesenchymal stem cell encapsulation. Acta Biomater 2011; 7: 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson BM, Kasper FK, Engel PS et al. Synthesis and characterization of injectable, biodegradable, phosphate-containing, chemically cross-linkable, thermoresponsive macromers for bone tissue engineering. Biomacromolecules 2014; 15: 1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Wang Y, Ma S et al. Effective bone regeneration using thermosensitive poly(N-isopropylacrylamide) grafted gelatin as injectable carrier for bone mesenchymal stem cells. ACS Appl Mater Interfaces 2015; 7: 19006–19015. [DOI] [PubMed] [Google Scholar]
- Tan R, She Z, Wang M et al. Thermo-sensitive alginate-based injectable hydrogel for tissue engineering. Carbohyd Polym 2012; 87: 1515–1521. [Google Scholar]
- Lima GGD, Campos L, Junqueira A et al. A novel pH-sensitive ceramic-hydrogel for biomedical applications. Polym Advan Technol 2015; 26: 1439–1446. [Google Scholar]
- Huynh CT, Nguyen MK, Jeong IK et al. Synthesis, characteristics and potential application of poly(beta-amino ester urethane)-based multiblock co-polymers as an injectable, biodegradable and ph/temperature-sensitive hydrogel system. J Biomater Sci Polym Ed 2012; 23: 1091–1106. [DOI] [PubMed] [Google Scholar]
- Shim WS, Yoo JS, Bae YH et al. Novel injectable pH and temperature sensitive block copolymer hydrogel. Biomacromolecules 2005; 6: 2930–2934. [DOI] [PubMed] [Google Scholar]
- Shim WS, Kim JH, Park H et al. Biodegradability and biocompatibility of a pH- and thermo-sensitive hydrogel formed from a sulfonamide-modified poly(epsilon-caprolactone-co-lactide)-poly(ethylene glycol)-poly(epsilon-caprolactone-co-lactide) block copolymer. Biomaterials 2006; 27: 5178–5185. [DOI] [PubMed] [Google Scholar]
- Lee F, Chung JE, Kurisawa M. An injectable enzymatically crosslinked hyaluronic acid-tyramine hydrogel system with independent tuning of mechanical strength and gelation rate. Soft Matter 2008; 4: 880–887. [DOI] [PubMed] [Google Scholar]
- Kurisawa M, Lee F, Wang L-S et al. Injectable enzymatically crosslinked hydrogel system with independent tuning of mechanical strength and gelation rate for drug delivery and tissue engineering. J Mater Chem 2010; 20: 5371–5375. [Google Scholar]
- Park KM, Lee Y, Son JY et al. In situ SVVYGLR peptide conjugation into injectable gelatin-poly(ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for enhancement of endothelial cell activity and neo-vascularization. Bioconjug Chem 2012; 23: 2042–2050. [DOI] [PubMed] [Google Scholar]
- Kuo KC, Lin RZ, Tien HW et al. Bioengineering vascularized tissue constructs using an injectable cell-laden enzymatically crosslinked collagen hydrogel derived from dermal extracellular matrix. Acta Biomater 2015; 27: 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Lin C, Cao A. Enzyme-mediated fast injectable hydrogels based on chitosan-glycolic acid/tyrosine: Preparation, characterization, and chondrocyte culture. Polym Chem 2014; 5: 391–398. [Google Scholar]
- Teixeira LS, Feijen J, van Blitterswijk CA et al. Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials 2012; 33: 1281–1290. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Uyama H, Kimura S. Enzymatic polymerization. Chem Rev 2001; 101: 3793–3818. [DOI] [PubMed] [Google Scholar]
- Moreira Teixeira LS, Bijl S, Pully VV et al. Self-attaching and cell-attracting in situ forming dextran-tyramine conjugates hydrogels for arthroscopic cartilage repair. Biomaterials 2012; 33: 3164–3174. [DOI] [PubMed] [Google Scholar]
- Gohil SV, Brittain SB, Kan H-M et al. Evaluation of enzymatically crosslinked injectable glycol chitosan hydrogel. J Mater Chem B 2015; 3: 5511–5522. [DOI] [PubMed] [Google Scholar]
- Furtmuller PG, Zederbauer M, Jantschko W et al. Active site structure and catalytic mechanisms of human peroxidases. Arch Biochem Biophys 2006; 445: 199–213. [DOI] [PubMed] [Google Scholar]
- Hou J, Li C, Guan Y et al. Enzymatically crosslinked alginate hydrogels with improved adhesion properties. Polym Chem 2015; 6: 2204–2213. [Google Scholar]
- Wang LS, Du C, Toh WS et al. Modulation of chondrocyte functions and stiffness-dependent cartilage repair using an injectable enzymatically crosslinked hydrogel with tunable mechanical properties. Biomaterials 2014; 35: 2207–2217. [DOI] [PubMed] [Google Scholar]
- Jin R, Moreira Teixeira LS, Dijkstra PJ et al. Enzymatically crosslinked dextran-tyramine hydrogels as injectable scaffolds for cartilage tissue engineering. Tissue Eng Part A 2010; 16: 2429–2440. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tao L, Li S et al. Synthesis of multiresponsive and dynamic chitosan-based hydrogels for controlled release of bioactive molecules. Biomacromolecules 2011; 12: 2894–2901. [DOI] [PubMed] [Google Scholar]
- Xin Y, Yuan J. Schiff's base as a stimuli-responsive linker in polymer chemistry. Polym Chem 2012; 3: 3045–3055. [Google Scholar]
- Li Z, Yuan B, Dong X et al. Injectable polysaccharide hybrid hydrogels as scaffolds for burn wound healing. RSC Adv 2015; 5: 94248–94256. [Google Scholar]
- Jia Y, Li J. Molecular assembly of Schiff Base interactions: construction and application. Chem Rev 2015; 115: 1597–1621. [DOI] [PubMed] [Google Scholar]
- Sun J, Xiao C, Tan H et al. Covalently crosslinked hyaluronic acid-chitosan hydrogel containing dexamethasone as an injectable scaffold for soft tissue engineering. J Appl Polym Sci 2013; 129: 682–688. [Google Scholar]
- Li L, Ge J, Ma PX et al. Injectable conducting interpenetrating polymer network hydrogels from gelatin-graft-polyaniline and oxidized dextran with enhanced mechanical properties. RSC Adv 2015; 5: 92490–92498. [Google Scholar]
- Cheng Y, Nada AA, Valmikinathan CM et al. In situ gelling polysaccharide-based hydrogel for cell and drug delivery in tissue engineering. J Appl Polym Sci 2014; 131: 39934. [Google Scholar]
- Cao L, Cao B, Lu C et al. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized PEG analogues for cartilage tissue engineering. J Mater Chem B 2015; 3: 1268–1280. [DOI] [PubMed] [Google Scholar]
- Ma Y-H, Yang J, Li B et al. Biodegradable and injectable polymer-liposome hydrogel: a promising cell carrier. Polym Chem 2016; 7: 2037–2044. [Google Scholar]
- Lih E, Yoon KiJ, Jin Woo B et al. An in situ gel-forming heparin-conjugated PLGA-PEG-PLGA copolymer. J Bioact Compat Pol 2008; 23: 444–457. [Google Scholar]
- Censi R, Fieten PJ, di Martino P et al. In situ forming hydrogels by tandem thermal gelling and michael addition reaction between thermosensitive triblock copolymers and thiolated hyaluronan. Macromolecules 2010; 43: 5771–5778. [DOI] [PubMed] [Google Scholar]
- Lin C, Zhao P, Li F et al. Thermosensitive in situ-forming dextran-pluronic hydrogels through Michael addition. Mat Sci Eng C-Mater 2010; 30: 1236–1244. [Google Scholar]
- Mather BD, Viswanathan K, Miller KM et al. Michael addition reactions in macromolecular design for emerging technologies. Prog Polym Sci 2006; 31: 487–531. [Google Scholar]
- Yu Y, Deng C, Meng F et al. Novel injectable biodegradable glycol chitosan-based hydrogels crosslinked by Michael-type addition reaction with oligo(acryloyl carbonate)-b-poly(ethylene glycol)-b-oligo(acryloyl carbonate) copolymers. J Biomed Mater Res A 2011; 99: 316–326. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan J, Krishnan UM, Sethuraman S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol Adv 2014; 32: 449–461. [DOI] [PubMed] [Google Scholar]
- Sepantafar M, Maheronnaghsh R, Mohammadi H et al. Stem cells and injectable hydrogels: synergistic therapeutics in myocardial repair. Biotechnol Adv 2016; 34: 362–379. [DOI] [PubMed] [Google Scholar]
- Kim M, Lee JY, Jones CN et al. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials 2010; 31: 3596–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Wang L, Deng L et al. Performance optimization of injectable chitosan hydrogel by combining physical and chemical triple crosslinking structure. J Biomed Mater Res A 2013; 101: 684–693. [DOI] [PubMed] [Google Scholar]
- Rodell CB, MacArthur JW, Dorsey SM et al. Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv Funct Mater 2015; 25: 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard CD, O'Shea TM, Siegwart DJ et al. An injectable thiol-acrylate poly(ethylene glycol) hydrogel for sustained release of methylprednisolone sodium succinate. Biomaterials 2011; 32: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorica C, Palumbo FS, Pitarresi G et al. Injectable in situ forming hydrogels based on natural and synthetic polymers for potential application in cartilage repair. RSC Adv 2015; 5: 19715–19723. [Google Scholar]
- Testa G, Di Meo C, Nardecchia S et al. Influence of dialkyne structure on the properties of new click-gels based on hyaluronic acid. Int J Pharm 2009; 378: 86–92. [DOI] [PubMed] [Google Scholar]
- Kaga S, Yapar S, Gecici EM et al. Photopatternable “clickable” hydrogels: “orthogonal” control over fabrication and functionalization. Macromolecules 2015; 48: 5106–5115. [Google Scholar]
- DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater 2009; 8: 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Long H, Malkoch M et al. Characterization of well-defined poly(ethylene glycol) hydrogels prepared by thiol-ene chemistry. J Polym Sci Pol Chem 2011; 49: 4044–4054. [Google Scholar]
- Dong Y, Saeed AO, Hassan W et al. "One-step" preparation of thiol-ene clickable PEG-based thermoresponsive hyperbranched copolymer for in situ crosslinking hybrid hydrogel. Macromol Rapid Commun 2012; 33: 120–126. [DOI] [PubMed] [Google Scholar]
- Alge DL, Azagarsamy MA, Donohue DF et al. Synthetically tractable click hydrogels for three-dimensional cell culture formed using tetrazine-norbornene chemistry. Biomacromolecules 2013; 14: 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cengiz N, Rao J, Sanyal A et al. Designing functionalizable hydrogels through thiol-epoxy coupling chemistry. Chem Commun 2013; 49: 11191–11193. [DOI] [PubMed] [Google Scholar]
- Arslan M, Gevrek TN, Sanyal A et al. Cyclodextrin mediated polymer coupling via thiol-maleimide conjugation: facile access to functionalizable hydrogels. RSC Adv 2014; 4: 57834–57841. [Google Scholar]
- Hermann CD, Wilson DS, Lawrence KA et al. Rapidly polymerizing injectable click hydrogel therapy to delay bone growth in a murine re-synostosis model. Biomaterials 2014; 35: 9698–9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Li J, Cui M et al. In situ "clickable" zwitterionic starch-based hydrogel for 3D cell encapsulation. ACS Appl Mater Interfaces 2016; 8: 4442–4455. [DOI] [PubMed] [Google Scholar]
- Hacker MC, Nawaz HA. Multi-functional macromers for hydrogel design in biomedical engineering and regenerative medicine. Int J Mol Sci 2015; 16: 27677–27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga S, Gevrek TN, Sanyal A et al. Synthesis and functionalization of dendron-polymer conjugate based hydrogels via sequential thiol-ene “click” reactions. J Polym Sci Pol Chem 2016; 54: 926–934. [Google Scholar]
- Jeon O, Bouhadir KH, Mansour JM et al. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 2009; 30: 2724–2734. [DOI] [PubMed] [Google Scholar]
- Ifkovits JL, Burdick JA. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng 2007; 13: 2369–2385. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ma G, Shi S et al. Photopolymerized water-soluble chitosan-based hydrogel as potential use in tissue engineering. Int J Biol Macromol 2011; 48: 408–413. [DOI] [PubMed] [Google Scholar]
- Hu J, Hou Y, Park H et al. Visible light crosslinkable chitosan hydrogels for tissue engineering. Acta Biomater 2012; 8: 1730–1738. [DOI] [PubMed] [Google Scholar]
- Elisseeff J, McIntosh W, Fu K et al. Controlled-release of IGF-I and TGF-β1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res 2001; 19: 1098–1104. [DOI] [PubMed] [Google Scholar]
- Cho IS, Cho MO, Li Z et al. Synthesis and characterization of a new photo-crosslinkable glycol chitosan thermogel for biomedical applications. Carbohydr Polym 2016; 144: 59–67. [DOI] [PubMed] [Google Scholar]
- Censi R, Schuurman W, Malda J et al. A printable photopolymerizable thermosensitive p(HPMAm-lactate)-peg hydrogel for tissue engineering. Adv Funct Mater 2011; 21: 1833–1842. [Google Scholar]
- Huang Z, Liu X, Chen S et al. Injectable and cross-linkable polyphosphazene hydrogels for space-filling scaffolds. Polym Chem 2015; 6: 143–149. [Google Scholar]
- Kim HD, Heo J, Hwang Y et al. Extracellular-matrix-based and Arg-Gly-Asp-modified photopolymerizing hydrogels for cartilage tissue engineering. Tissue Eng Part A 2015; 21: 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G, Wang Y, Li J et al. Synthesis and characterization of injectable photocrosslinking poly (ethylene glycol) diacrylate based hydrogels. Polym Bull 2008; 61: 91–98. [Google Scholar]
- Chou AI, Akintoye SO, Nicoll SB. Photo-crosslinked alginate hydrogels support enhanced matrix accumulation by nucleus pulposus cells in vivo. Osteoarthr Cartilage 2009; 17: 1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos A, Bichara DA, Zhao X et al. Injectable and photopolymerizable tissue-engineered auricular cartilage using poly(ethylene glycol) dimethacrylate copolymer hydrogels. Tissue Eng Part A 2011; 17: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud KE. Epidemiology of fracture risk with advancing age. J Gerontol A Biol Sci Med Sci 2013; 68: 1236–1242. [DOI] [PubMed] [Google Scholar]
- Borgstrom F, Lekander I, Ivergard M et al. The international costs and utilities related to osteoporotic fractures study (ICUROS)-quality of life during the first 4 months after fracture. Osteoporos Int 2013; 24: 811–823. [DOI] [PubMed] [Google Scholar]
- Dosier CR, Uhrig BA, Willett NJ et al. Effect of cell origin and timing of delivery for stem cell-based bone tissue engineering using biologically functionalized hydrogels. Tissue Eng Part A 2015; 21: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khojasteh A, Fahimipour F, Eslaminejad MB et al. Development of PLGA-coated beta-TCP scaffolds containing VEGF for bone tissue engineering. Mater Sci Eng C Mater Biol Appl 2016; 69: 780–788. [DOI] [PubMed] [Google Scholar]
- Matsuno T, Hashimoto Y, Adachi S et al. Preparation of injectable 3D-formed β-tricalcium phosphate bead/alginate composite for bone tissue engineering. Dent Mater J 2008; 27: 827–834. [DOI] [PubMed] [Google Scholar]
- Han Y, Zeng Q, Li H et al. The calcium silicate/alginate composite: Preparation and evaluation of its behavior as bioactive injectable hydrogels. Acta Biomater 2013; 9: 9107–9117. [DOI] [PubMed] [Google Scholar]
- Ma G, Yang D, Li Q et al. Injectable hydrogels based on chitosan derivative/polyethylene glycol dimethacrylate/N,N-dimethylacrylamide as bone tissue engineering matrix. Carbohydr Polym 2010; 79: 620–627. [Google Scholar]
- Dessi M, Borzacchiello A, Mohamed TH et al. Novel biomimetic thermosensitive beta-tricalcium phosphate/chitosan-based hydrogels for bone tissue engineering. J Biomed Mater Res A 2013; 101: 2984–2993. [DOI] [PubMed] [Google Scholar]
- Ding K, Zhang YL, Yang Z et al. A promising injectable scaffold: The biocompatibility and effect on osteogenic differentiation of mesenchymal stem cells. Biotechnol Bioproc E 2013; 18: 155–163. [Google Scholar]
- Jang JY, Park SH, Park JH et al. In vivo osteogenic differentiation of human dental pulp stem cells embedded in an injectable in vivo-forming hydrogel. Macromol Biosci 2016; 16: 1158–1169. [DOI] [PubMed] [Google Scholar]
- Vo TN, Shah SR, Lu S et al. Injectable dual-gelling cell-laden composite hydrogels for bone tissue engineering. Biomaterials 2016; 83: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Guo G, Gong C et al. Injectable biodegradable thermosensitive hydrogel composite for orthopedic tissue engineering. 1. Preparation and characterization of nanohydroxyapatite/poly(ethylene glycol)-poly(ε-caprolactone)-poly(ethylene glycol) hydrogel nanocomposites. J Phys Chem B 2009; 113: 16518–16525. [DOI] [PubMed] [Google Scholar]
- Fu S, Ni P, Wang B et al. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials 2012; 33: 4801–4809. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Gyawali D, Stark JM et al. A rheological study of biodegradable injectable PEGMC/HA composite scaffolds. Soft Matter 2012; 8: 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhang X, Wu A et al. An injectable nano-hydroxyapatite (n-HA)/glycol chitosan (G-CS)/hyaluronic acid (HyA) composite hydrogel for bone tissue engineering. RSC Adv 2016; 6: 33529–33536. [Google Scholar]
- Lin G, Cosimbescu L, Karin NJ et al. Injectable and thermosensitive PLGA-g-PEG hydrogels containing hydroxyapatite: preparation, characterization and in vitro release behavior. Biomed Mater 2012; 7: 024107. [DOI] [PubMed] [Google Scholar]
- Yan J, Miao Y, Tan H et al. Injectable alginate/hydroxyapatite gel scaffold combined with gelatin microspheres for drug delivery and bone tissue engineering. Mater Sci Eng C Mater Biol Appl 2016; 63: 274–284. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Weitzmann MN. Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-kappaB activation. Mol Cell Biochem 2011; 355: 179–186. [DOI] [PubMed] [Google Scholar]
- Niranjan R, Koushik C, Saravanan S et al. A novel injectable temperature-sensitive zinc doped chitosan/beta-glycerophosphate hydrogel for bone tissue engineering. Int J Biol Macromol 2013; 54: 24–29. [DOI] [PubMed] [Google Scholar]
- Dhivya S, Saravanan S, Sastry TP et al. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J Nanobiotechnol 2015; 13: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas TE, Piwowarczyk W, Pamula E et al. Injectable self-gelling composites for bone tissue engineering based on gellan gum hydrogel enriched with different bioglasses. Biomed Mater 2014; 9: 045014. [DOI] [PubMed] [Google Scholar]
- Lewandowska-Łańcucka J, Fiejdasz S, Rodzik Ł et al. Bioactive hydrogel-nanosilica hybrid materials: a potential injectable scaffold for bone tissue engineering. Biomed Mater 2015; 10: 015020. [DOI] [PubMed] [Google Scholar]
- Vishnu Priya M, Sivshanmugam A, Boccaccini AR et al. Injectable osteogenic and angiogenic nanocomposite hydrogels for irregular bone defects. Biomed Mater 2016; 11: 035017. [DOI] [PubMed] [Google Scholar]
- Ren K, He C, Li G et al. In situ forming glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. J Control Release 2015; 213: E64–E65. [DOI] [PubMed] [Google Scholar]
- Wang X, Partlow B, Liu J et al. Injectable silk-polyethylene glycol hydrogels. Acta Biomater 2015; 12: 51–61. [DOI] [PubMed] [Google Scholar]
- Popa EG, Caridade SG, Mano JF et al. Chondrogenic potential of injectable kappa-carrageenan hydrogel with encapsulated adipose stem cells for cartilage tissue engineering applications. J Tissue Eng Regen Med 2015; 9: 550–563. [DOI] [PubMed] [Google Scholar]
- Munarin F, Guerreiro SG, Grellier MA et al. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 2011; 12: 568–577. [DOI] [PubMed] [Google Scholar]
- Wu J, Ding Q, Dutta A et al. An injectable extracellular matrix derived hydrogel for meniscus repair and regeneration. Acta Biomater 2015; 16: 49–59. [DOI] [PubMed] [Google Scholar]