Abstract
The effect of different cooking and preservation methods on the nutritional and phytochemical composition of the mushroom, Amanita zambiana, was investigated. Fresh mushrooms were boiled in water, fried, or microwaved. In addition, fresh mushrooms were either air‐dried for 7 days or frozen at −20°C for 14 days. The protein, lipid, carbohydrate, and phenolic content of the treated mushrooms were measured and compared to the fresh mushroom contents. Frying increased the protein (2.01% ± 0.2% [fresh mushroom] to 2.23% ± 0.09%), lipid (14.68% ± 0.9% to 15.56% ± 0.34%), and carbohydrate (0.89% ± 0.01% to 2.69% ± 0.03%) content, while microwaving increased the protein (2.01% ± 0.2% to 3.64% ± 0.08%) and carbohydrate content (0.89% ± 0.01% to 2.26% ± 0.09%). Boiling only increased the carbohydrate content (0.89% ± 0.01% to 1.71% ± 0.05%) of the mushroom and significantly decreased (p < .05) the phenolic content (8.77 ± 0.1 to 1.46 ± 0.2 mg gallic acid equivalent (GAE)/g mushroom). Drying resulted in significant increase (p < .05) in protein (2.01 ± 0.2% to 24.36 ± 0.09%), carbohydrate (0.89% ± 0.01% to 58.67% ± 3.29%), and phenolic contents (8.77 ± 0.1 to 119.8 ± 0.7 mg GAE/g mushroom), while freezing only increased the carbohydrate content (0.89% ± 0.01% to 1.77% ± 0.03%). From the three cooking methods studied, frying is recommended as the most effective cooking procedure in retaining or enhancing the mushroom nutrients, while drying is a better preservation method than freezing.
Keywords: Amanita zambiana, cooking, mushroom, nutrition, preservation
1. Introduction
Wild edible mushrooms have been a part of human diet in many regions of the world for centuries due to their pleasant taste and aroma as well as their nutritional values. Mushrooms contain large amounts of dietary fiber, proteins, vitamins, and minerals. A number of mushrooms are even consumed for medicinal purpose as they contain valuable bioactive compounds that include phenolic compounds. Phenolic compounds are secondary metabolites and are a potent source of antioxidants. Mushrooms are becoming an increasingly important source of food and medicinal purposes due to their phenolic and antioxidant contents (Wang & Xu, 2014; Caglarirmak, 2011; Kuka, Cakste, Galoburda, & Sabovics, 2014; Moon & Lo, 2013; Oktay et al., 2015).
Studies have shown that mushrooms contain proteins especially amino acids that are essential to human health. Mushrooms are rich in leucine and lysine amino acids, which are commonly lacking in many staple cereal foods. Proteins are essential for general growth, body tissue repair, and even maintenance of healthy cells. Some mushroom proteins have antibacterial and anticancer properties (Cheung, 2008; Wani, Bodha, & Wani, 2010; Xu et al., 2014). Crude protein content has been reported to vary from 10% to 40% in some of the common edible mushrooms (Barros, Cruz, Baptista, Estevinho, & Ferreira, 2008; Diez & Alvarez, 2001; Longvah & Deosthale, 1998). Carbohydrates are also an important component of mushrooms constituting between 50% and 60% of the dry matter. Mushroom carbohydrates have been found in many cases to have anticancer properties (Kalac, 2012). While fats increase palatability of foods by retaining and absorbing flavors, they are essential in diet (Shadung, Mphosi, & Mashela, 2012), even though low values of fats ranging from 2% to 6% of dry matter are required. Furthermore, fats are important in the biological and structural functioning of cells and help in transportation of fat‐soluble vitamins, which are nutritionally essential. Mushrooms have fat in their cell wall, which is important for storage of vitamin D. Mushrooms contain mainly the healthy unsaturated fats like oleic and linoleic acids, that are found at low levels but have beneficial value when consumed (Cheung, 2008).
Amanita zambiana is an edible mushroom which falls in the Amanita genus. The mushroom consists of a cap that is brown over the disk, paler toward margin, and white at the margin. A. zambiana is known as the Zambian Slender Caesar in English and like all Amanita species is mycorrhizal, associating with trees in the genus Brachystegia. The geographic distribution of A. zambiana is confined to South Central Africa, including southern Zaire (De Roman, 2010; Ryvarden, Piearce, & Masuka, 1994). Prior to its naming in 1980, the mushroom may have been referred to as the closely related, south Asian species A. hemibapha. A survey of Amanita species in sub‐Saharan Africa indicates that the species described as Amanita loosii from Zaire represent an earlier name for the species (Harkonen, 2002; Sharp, 2011). The mushroom is an early season species locally dubbed the Christmas mushroom, although it may also appear in a brief secondary flush toward the end of the rains (Pegler & Piearce, 1980; Ryvarden et al., 1994). A. zambiana is one of the best known edible fungi throughout the region with masses of the mushrooms dominating roadside market stalls around December. The species, significantly contributes to the Zimbabwean household food security, when in season (Garwe, Munzara‐Chawira, & Kusena, 2009; Ryvarden et al., 1994).
In Zimbabwe, mushrooms are commonly cooked before being consumed or they are preserved either by drying or freezing before cooking. Different preservation and cooking methods have been found to have a significant effect on the final nutritional composition of different mushrooms (Aishah & Rosli, 2013; Kumar, Singh, & Singh, 2013; Pogon, Jaworsaka, Duda‐Chodak, & Maciejaszek, 2013). Preservation is essential for extension of shelf life and for value addition. Studies have shown that preservation may improve nutritional and sensory quality substantially, hence add value to mushrooms (Chelela, Chacha, & Matemu, 2014). There is lack of data available on how the different preservation and cooking methods affect the composition of locally edible mushrooms, including A. zambiana. This study therefore aimed at evaluating the effects of different cooking and preservation methods on the total proteins, lipids, carbohydrates, and phytochemicals of A. zambiana. The information contributes toward determining the best cooking and preservation method that retains most of the nutrients and phytochemicals in A. zambiana.
2. Materials and Methods
2.1. Sample collection and identification of the mushrooms
Fresh mushroom (Amanita zambiana) fruit bodies were collected from Marondera in Zimbabwe. Identification of the mushroom was done by comparing the photographical, morphological, and anatomical characteristics with the description given in the manuals on identification of mushrooms in Southern Africa (Ryvarden et al., 1994; Sharp, 2011).
2.2. Sample preparation
Fresh raw mushrooms were cleaned by running tap water, followed by distilled water, and then exposed to different cooking and preservation treatments.
2.2.1. Boiling
Thirty grams of fresh mushroom pieces were placed in a pot with 300 ml of boiling water (ordinary cooking). Boiling was done until the mushroom was tender and the boiled mushroom was ground using a mortar and pestle.
2.2.2. Microwaving
Thirty grams of mushroom pieces were placed in a glass dish and cooked in a microwave oven for 2 min at 900 watts. The microwaved mushroom sample was ground with a mortar and pestle.
2.2.3. Frying
Thirty grams of mushroom pieces were placed in a frying pan with 100 ml of hot cooking oil while stirring until they became crisp tender. Cooking oil was removed by filtration and the sample was ground using a mortar and pestle.
2.2.4. Air drying
Three hundred grams of fresh mushrooms were cut into small pieces, placed on a clean bond paper, and left to air dry for 7 days. The air‐dried mushroom sample was ground until the sample passed through a 0.1‐mm sieve.
2.2.5. Freezing
Fifty grams of fresh mushroom fruit bodies were cut into small pieces, placed in sealable plastic bags, and frozen in a −20°C freezer for 14 days. The frozen samples were removed from the freezer, allowed to thaw, and ground using a mortar and pestle.
2.3. Nutritional analysis
2.3.1. Determination of crude protein (Kjeldahl method)
One and a half grams of the air‐dried or 3.0 g of treated mushroom samples were digested in a Kjeldahl digestion flask by boiling with 25 ml of concentrated sulfuric acid and 10 g of Kjeldahl catalyst tablet until the mixture was clear. After cooling the flasks, 400 ml of cold water and 100 ml of 40% sodium hydroxide were added. The contents were distilled until 200 ml of solution was collected, and mixed with 50 ml of boric acid with indicator. The solution was titrated with 0.097 mol/L HCl which had been standardized with sodium carbonate. The protein content was calculated as follows:
where: A is the volume of 0.097 mol/L hydrochloric acid titrated minus volume of blank.
2.3.2. Determination of crude lipid
Lipids were extracted from 5 g of mushroom samples for 4 hr with hexane using the Soxhlet apparatus, after which hexane was evaporated to dryness. The weight of crude lipid was obtained from the difference between the initial and the final weight.
2.3.3. Determination of total carbohydrate
The total carbohydrate content was determined by the calorimetric method as described by Albalasmeh, Berhe, and Ghezzehei (2013) with modifications. Briefly, 2 g of ground mushroom was dissolved in 50 ml of water and centrifuged at 1000 rpm for 2 min. The mushroom extract was rapidly mixed in a test tube with 3 ml of concentrated sulfuric acid and vortexed for 30 s. After cooling, absorbance of the solution was read at 315 nm with a UV spectrophotometer. Glucose (ranging from 0 to 1 mg/ml) was used to construct a calibration curve (R 2 = 0.9982).
2.4. Phytochemical analysis
2.4.1. Preparation of methanol extracts
Ground mushroom of 10 g was mixed with 40 ml of 98% methanol and the sample was placed in an incubator shaker at 180 rpm and 37°C for 48 hr. This extraction procedure was repeated for the fresh, frozen, and differently cooked mushroom samples. The frozen sample was allowed to thaw before being ground with a mortar and pestle. For the dried mushroom sample, 1 g was dissolved in 10 ml of 98% methanol and placed in an incubator shaker at 180 rpm and 37°C for 24 hr. All the extraction mixtures were filtered through Whatman No. 2 filter paper and the filtrate was used directly for analysis.
2.4.2. Determination of total phenol
The total phenolic content was determined by the Folin–Ciocalteu calorimetric method as described by Barros, Baptista, Correia, Morais, and Ferreira (2007). A quantity of 1 ml of the filtrate was mixed with 1 ml of 10% v/v of Folin–Ciocalteu reagent, followed by 1 ml of 7.5% sodium carbonate. The mixture was adjusted to 10 ml with distilled water. The mixture was allowed to stand for 90 min in the dark and the absorbance was read at 725 nm. Gallic acid (ranging from 0 to 0.3 mg/ml) was used to construct the standard curve (R 2 = 0.9982) and the results were expressed as mg of gallic acid equivalents.
2.5. Statistical analysis
All the analyses were done in triplicate. The data were expressed graphically and as means ± standard deviation. Graph Pad Prism 5.03 software was used for statistical analysis of data and ANOVA (one‐way analysis of variance) was done. Bonferroni's multiple comparison tests were used for comparisons between means and the significant difference between means was accepted at p ≤ .05.
3. Results and Discussion
3.1. Effects of different preservation and cooking methods on crude protein content
Mushrooms are seasonal, found mostly in the rainy season, and are highly perishable. Preservation is essential for extension of shelf life and for value addition. The dried, fresh, and frozen mushroom samples gave different protein content of 24.36% ± 0.09%, 2 .01% ± 0.19%, and 1.62% ± 0.16%, respectively. There was a significant increase in protein content (p < .05) with drying and no significant difference (p < .05) with freezing (Fig. 1a). An increase in proteins with drying might be due to the fact that drying removes water, hence concentrating the nutrients that are left. The increase in protein content obtained after drying is similar to the results obtained in other studies (Breene, 1990; Alam et al., 2007). Protein content of dried mushrooms ranges from 19% to 39% (Breene, 1990), similar to the value obtained in this study.
Figure 1.
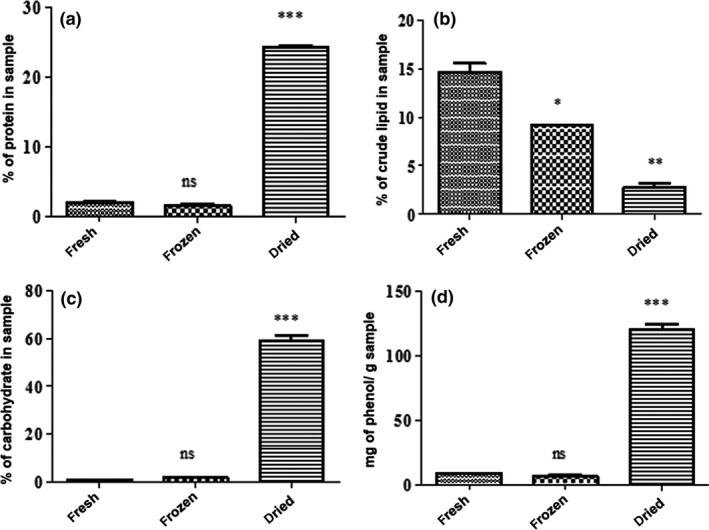
Effect of different preservation methods on: (a) crude protein content, (b) crude lipid content, (c) total carbohydrate content, and (d) total phenolic content of A. zambiana mushroom. ***p < .001, **p < .01 and *p <. 0001 compared to the fresh sample (one‐way ANOVA followed by Bonferroni's multiple comparison test). Results are expressed as ± standard deviation of three measurements
Consumption of wild edible mushrooms is increasing due to a good content of carbohydrates, fats, vitamins, minerals, and proteins, which contain all essential amino acids. Mushrooms have a good nutritional value, particularly as a source of protein that can enrich human diets, especially in developing countries where animal protein may not be available or costly (Colak, Faiz, & Sesli, 2009; Ezeibekwe, Ogbonnaya, Unamba, & Osuala, 2009). The boiled, fresh, microwaved, and fried mushroom samples gave different protein content of 1.62% ± 0.08%, 2.01% ± 0.2%, 3.64% ± 0.08%, and 2.23% ± 0.09%, respectively. There was a significantly high protein content (p < .05) in the microwaved sample compared to the fresh mushroom but there was a decrease in the boiled sample (Fig. 2a). The decrease in the boiled mushroom sample might be due to solubilization and leaching out of the nitrogenous substances during the boiling treatment. The increase in crude protein with microwaving and frying might be due to increase in protein availability as a result of enzyme hydrolysis of insoluble protein (Echendu, Obizoba, & Anyika, 2009). A similar trend was observed in studies done by Bliss (1975) who reported that the increase in protein could be a result of enzymatic hydrolysis which may cause release of free amino acids.
Figure 2.
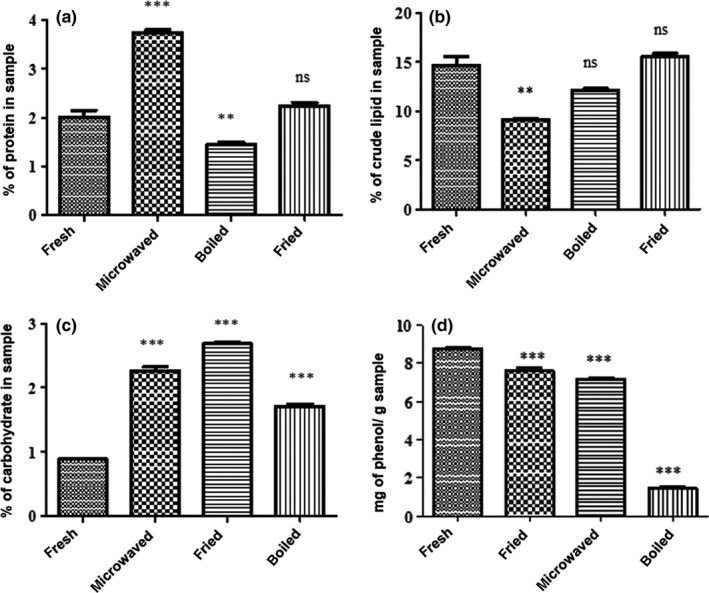
Effect of different cooking methods on: (a) crude protein content, (b) crude lipid content, (c) total carbohydrate content, and (d) total phenolic content of A. zambiana mushroom. ***p < .001 and **p < .01 compared to the fresh sample (one‐way ANOVA followed by Bonferroni's multiple comparison test). Results are expressed as ± standard deviation of three measurements
3.2. Effect of different preservation and cooking methods on crude lipid content
The fresh, frozen, and dried mushroom samples gave lipid content of 14.68% ± 0.9%, 9.16% ± 0.06%, and 2.8% ± 0.4%, respectively. There was a significant decrease (p < .05) in crude lipid content with all the preservation methods (Fig. 1b). According to Barros et al. (2007), dried samples of different mushroom species have lower total lipid composition than their corresponding fresh and frozen samples, similar to the trend found in this study. The decrease in the lipid content might be due to an increase in lipase activity and denaturation of the lipid fraction during the preservation treatment.
The results shown in Fig. 2b indicate the effect of different cooking methods on the lipid content of A. zambiana. The fresh, microwaved, fried, and boiled mushroom samples gave percentage lipid content of 14.68% ± 0.9%, 9.15% ± 0.13%, 15.56% ± 0.34%, and 12.12% ± 0.2%, respectively. There was a significant decrease (p < .05) in the lipid content in the microwaved mushroom sample. Kylen and McCready (1975) reported a similar trend of lipid decrease with cooked mushrooms. The decrease in lipids might be due to the denaturation and breakdown of the lipids into glycerol and fatty acids (Igbedioh, Olugbemi, & Akpapunan, 1994). There was no significant change (p < .05) in the boiled and fried samples compared to the fresh sample. The boiling method retained more of the lipids than microwaving which might be due to the extremely high temperatures in the microwaving treatment than the boiling temperature. Hence, more lipids were denatured during the microwaving treatment as compared to boiling.
3.3. Effect of different preservation and cooking methods on total carbohydrate content
The fresh, dried, and frozen mushroom samples gave carbohydrate content of 0.89% ± 0.01%, 58.67% ± 3.29%, and 1.77% ± 0.03%, respectively. There was a significant increase (p < .05) in total carbohydrate content with drying (Fig. 1c). The increase in carbohydrates with drying might be due to a decrease in moisture content, hence concentrating the nutrients. There was no significant increase (p < .05) with freezing. A similar trend was observed by Longvah and Deosthale (1998). The composition of edible mushroom carbohydrates ranges from 35% to 70% dry weight and varies with species (Diez & Alvarez, 2001) and the carbohydrate content of dried mushroom sample found in this study falls within the range.
The fresh, boiled, fried, and microwaved mushroom samples gave carbohydrate content of 0.89% ± 0.01%, 1.71% ± 0.05%, 2.69% ± 0.03%, and 2.26% ± 0.09%, respectively (Fig. 1c). There was a significant increase (p < .05) in carbohydrate content with all the cooking methods. The fried sample had a higher yield in carbohydrates followed by microwaving and boiling. The increase in carbohydrate content with cooking might be due to the destruction of mushroom cell walls that causes an increase in solubility of carbohydrates in water. A smaller increase of carbohydrates in boiled sample might be due to the fact that when the cell walls are destroyed, the carbohydrates might have leached into the boiling water before the extraction for analysis. The fried sample showed the highest increase, maybe because carbohydrates are insoluble in oil; hence, no leaching of carbohydrates during the cooking process.
3.4. Effect of different preservation and cooking methods on total phenolic content
The fresh, dried and frozen mushroom samples gave total phenolic content of 8.77 ± 0.1, 119.8 ± 0.7, and 6.58 ± 0.6 mg GAE/g mushroom, respectively (Fig. 1d). There was a significant increase (p < .05) in total phenolics with drying. This might be due to the changes in extractability of polyphenols as a result of plant cell wall destruction after drying treatment, thus bound polyphenols may be released more easily in the dried sample than the corresponding fresh sample. Drying may supply mushrooms with good enough energy to improve extraction without destroying the phenolic structures. There was no significant change (p < .05) in total phenolic content with freezing.
Phenolic compounds are bioactive compounds with lignin and flavonoid contents and are crucial due to the free radical scavenging activity. Bioactive compounds protect the body from reactive oxygen species that can cause cell damage (Kosanic, Rankovic, & Dasic, 2013; Oksana, Marian, Mahendra, & Bo, 2012). There exists consensus of various researchers that phenolics are the main antioxidants in mushrooms (Kalac, 2012). The total phenolics obtained for the fresh, microwaved, fried, and boiled mushroom samples were 8.77 ± 0.1, 7.17 ± 0.06, 7.62 ± 0.2, and 1.46 ± 0.2 mg GAE/g, respectively (Fig. 2d). There was a general decrease in total phenolic content with cooking. The reduction in phenolic content after cooking treatments might be due to the fact that some polyphenols are heat labile and are degraded upon heating (Rakic et al., 2007). The decrease was significantly higher (p < .05) in the boiled sample than all other cooking treatments done in this study. Greatest losses observed in the boiling treatment could be due to leaching out and denaturation of polyphenols. The decrease in total phenolics with cooking is similar to reports from other studies (Barros et al., 2007; Sun, Bai, & Zhuang,2012).
4. Conclusion
Proper preservation of food is important, making it available all season, but the preservation methods need to be appropriate in keeping the nutritional value of the food. Drying was shown to increase phenolics, proteins, carbohydrates, and decrease lipids, while freezing decrease lipids and causes little change in carbohydrates, phenolics, and proteins. Preservation was observed to have a positive impact on the nutrient and phytochemical composition of the mushrooms. Of the two preservation methods in this study, drying was found to significantly increase the amount of phenolics, carbohydrates, and proteins compared to freezing, hence is a better preservation method for mushrooms than freezing. The choice of food processing methods is also important in maintaining the nutritional values. In this study, microwaving was observed to increase the protein and carbohydrate content, while decreasing phenolics and lipids. Frying increased carbohydrates, proteins, and lipids, and decreased phenolics, while boiling decreased proteins, lipids, and phenolics, and increased carbohydrates. The boiling treatment retained lipids and lost proteins and phenolics more than any other cooking treatment and showed a relatively lower increase in carbohydrates. This means boiling results in the loss of most nutritional and phytochemical components, but retains lipids which are not needed by the body in large amounts, hence boiling is less recommended for mushroom A. zambiana. Microwaving increased proteins more than other cooking treatments, retained phenolics, and lost more lipids than the boiling treatment. However, frying increased more carbohydrates and lipids and retained more phenolics than other cooking treatments, while retaining more proteins than the boiling method. This makes frying a better way of cooking mushrooms as it retained most of the nutrients. This study therefore shows that, among the tested cooking methods, the best method of cooking mushrooms that retains or enhances nutritional benefits is frying followed by microwaving.
Conflict of Interest
None declared.
Acknowledgment
The study was funded by the Fogarty International Center Training Grant (MTRCBSA – 2D43TW001587‐10A2) and the University of Zimbabwe Research Funds.
Reid T, Munyanyi M, Mduluza T. Effect of cooking and preservation on nutritional and phytochemical composition of the mushroom Amanita zambiana . Food Sci Nutr. 2017;5:538–544. https://doi.org/10.1002/fsn3.428
References
- Aishah, M. S. , & Rosli, W. W. (2013). Effect of different drying techniques on the nutritional values of oyster mushroom (Pleurotus Sajor‐caju). Sains Malaysiana, 42, 937–941. [Google Scholar]
- Alam, N. , Khan, A. , Hossain, M. S. , Amin, S. M. R. , & Khan, L. A. (2007). Nutritional analysis of dietary mushroom Pleurotus florida Eger and Pleurotus sajor‐caju (Fr.) Singer. Bangladesh Journal of Mushroom, 1, 1–7. [Google Scholar]
- Albalasmeh, A. A. , Berhe, A. A. , & Ghezzehei, T. A . (2013). A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry.Carbohydrate Polymers, 97, 253–261. [DOI] [PubMed] [Google Scholar]
- Barros, L. , Baptista, P. , Correia, D. M. , Morais, J. S. , & Ferreira, I. C. F. R. (2007). Effects of conservation treatment and cooking on the chemical composition and anti‐oxidant of Portuguese wild edible mushrooms. Journal of Agricultural and Food Chemistry, 55, 4781–4788. [DOI] [PubMed] [Google Scholar]
- Barros, L. , Cruz, T. , Baptista, P. , Estevinho, L. M. , & Ferreira, I. C. F. R. (2008). Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chemical Toxicology, 46, 2742–2747. [DOI] [PubMed] [Google Scholar]
- Bliss, F. A. (1975). Cowpeas in Nigeria: Nutritional Improvement of Food Legumes (Breeding, Milner, M ed. New York: John Wiley and Sons. [Google Scholar]
- Breene, W. M. (1990). Nutritional and medicinal value of specialty mushrooms. Journal of Food Protection, 53, 883–894. [DOI] [PubMed] [Google Scholar]
- Caglarirmak, N . (2011). Edible mushrooms: An alternative food item. Proc. Of the 7th International Conf. on Mushroom Biology and Mushroom Products (ICMBMP 7), 4–7 October 2011, Arcachon, France, 1, 548–554. [Google Scholar]
- Chelela, B. L. , Chacha, M. , & Matemu, A. (2014). Wild edible mushroom value chain for improved livelihoods in southern highlands of Tanzania. American Journal of Research Communication, 2, 1–14. [Google Scholar]
- Cheung, P. C. K . (2008). Nutritional value and health benefits of mushrooms. In Mushrooms as Functional Foods. In Cheung P. C. K. (Eds.). New Jersey: John Wiley & Sons, Inc. [Google Scholar]
- Colak, A. , Faiz, O. , & Sesli, E. (2009). Nutritional composition of some wild edible mushrooms. Turkish Journal of Biochemistry, 34, 25–31. [Google Scholar]
- De Roman, M. (2010). The contribution of wild fungi to diet, income and health: A world review In Rai M., & Kovics G. (Eds.), Progress in Mycology. Netherlands: Springer. [Google Scholar]
- Diez, V. A. , & Alvarez, A. (2001). Compositional and nutritional studies on two wild edible mushrooms from northwest Spain. Food Chemistry, 75, 417–422. [Google Scholar]
- Echendu, C. A. , Obizoba, I. C. , & Anyika, J. U. (2009). Effects of germination on chemical composition of ground bean (Kerstingiella geocarpa harm) seeds. Pakistan Journal of Nutrition, 8, 1849–1854. [Google Scholar]
- Ezeibekwe, I. O. , Ogbonnaya, C. I. , Unamba, C. I. N. , & Osuala, O. M. (2009). Proximate analysis and mineral composition of edible mushrooms in parts of south eastern Nigeria. Report and Opinion, 1, 32–36. [Google Scholar]
- Garwe, D. , Munzara‐Chawira, A. , & Kusena, K . (2009). State of plant genetic resources for food and agriculture in Zimbabwe (1996–2008) (Report). Department of Agricultural Research for Development, Ministry of Agriculture, Mechanization and Irrigation Development.
- Harkonen, M. (2002). Mushroom collecting in Tanzania and Hunan (Southern China): Inherited wisdom and folklore of two different cultures In Watling R., Frankland J. C., Ainsworth A. M., Isaac S., & Robinson C. H. (Eds.), Tropical Mycology: Macromycetes. New York: CABI Publishing. [Google Scholar]
- Igbedioh, S. O. , Olugbemi, K. T. , & Akpapunan, M. A. (1994). Effects of processing methods on phytic acid level and some constituents in bambara groundnut (Vigna subterranea) and pigeon pea (Cajanus cajan). Food Chemistry, 50, 147–151. [Google Scholar]
- Kalac, P. (2012). Chemical composition and nutritional value of European species of wild growing mushrooms In Andres S., & Baumann N. (Eds.), Mushrooms: Types, Properties and Nutrition. New York: Nova Science Publishers. [Google Scholar]
- Kosanic, M. , Rankovic, B. , & Dasic, M. (2013). Antioxidant and antimicrobial properties of mushrooms. Bulgarian Journal of Agricultural Science, 19, 1040–1046. [Google Scholar]
- Kuka, M. , Cakste, I. , Galoburda, R. , & Sabovics, M . (2014).Chemical composition of Latvian wild edible mushroom Cantharellus cibarius. Foodbalt. 2014, 9th Baltic Conference on Food Science and Technology, 8–9 May 2014, Jelgava, Latvia. [Google Scholar]
- Kumar, A. , Singh, M. , & Singh, G. (2013). Effect of different pretreatments on the quality of mushrooms during solar drying. Journal of Food Science and Technology, 50, 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kylen, A. M. , & McCready, R. M. (1975). Nutrients in seeds and sprouts of alfalfa, lentil, mungbeans and soybeans. Journal of Food Science, 40, 1008–1009. [Google Scholar]
- Longvah, T. , Deosthale, Y. G. (1998). Composition and nutritional studies on edible wild mushroom from northeast India. Food Chemistry, 63, 331–334. [Google Scholar]
- Moon, B. , & Lo, Y. M. (2013). Conventional and novel applications of edible mushrooms in today's food industry. Journal of Food Processing and Preservation, 38, 2146–2153. [Google Scholar]
- Oksana, S. , Marian, B. , Mahendra, R. , & Bo, S. H. (2012). Review: Plant phenolic compounds for food, pharmaceutical and cosmetics production. Journal of Medicinal Plants Research, 6, 2526–2539. [Google Scholar]
- Oktay, Y. , Zehra, C. , Abdu, Q. L. , Huseyin, S. , & Meltem, M. (2015). Wild edible mushrooms as a natural source of phenolics and antioxidants. Journal of Food Biochemistry, 39, 148–154. [Google Scholar]
- Pegler, D. N. , & Piearce, G. D. (1980). The edible mushrooms of Zambia. Kew Bulletin, 35, 475–491. [Google Scholar]
- Pogon, K. , Jaworsaka, G. , Duda‐Chodak, A. , & Maciejaszek, I. (2013). Influence of the culinary treatment on the quality of Lactarius deliciosus . Foods, 2, 238–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic, S. , Petrovic, S. , Kukuc, J. , Jadranin, M. , Tesevic, V. , Povrenovic, D. , & Siler‐Marinkovic, S. (2007). Influence of thermal treatment on phenolic compounds and antioxidant properties of oak acorns from Serbia. Food Chemistry, 104, 830–834. [Google Scholar]
- Ryvarden, L. , Piearce, G. D. , & Masuka, A. J . (1994). An introduction to the larger fungi of South Central Africa. Baobab Books Publishers (a division of Academic Books (Pvt.) Ltd., Harare, Zimbabwe). ISBN 0‐908311‐52‐4. [Google Scholar]
- Shadung, K. G. , Mphosi, M. S. , & Mashela, P. W. (2012). Influence of drying method and location on proximate chemical composition of African metallic wood boring beetle, Sternocera Orissa (Coleoptera: Buprestidae) in Republic of South Africa. African Journal of Food Science, 6, 155–158. [Google Scholar]
- Sharp, C. (2011). A pocket guide to mushroom in Zimbabwe. Bulawayo: Directory Publishers. ISBN 978‐0‐7974‐4727‐1. [Google Scholar]
- Sun, L. , Bai, X. , & Zhuang, Y . (2012). Effect of cooking methods on total phenolic contents and anti‐oxidant activities of four Boletus mushrooms. Kunming, China: Research Center of Food Engineering, College of Chemistry and Engineering, Kunming University of Science and Technology. [Google Scholar]
- Wang, Y. , & Xu, B. (2014). Distribution of antioxidant activities and total phenolic contents in acetone, ethanol, water and hot water extracts from 20 edible mushrooms via sequential extraction. Austin Journal of Nutrition and Food Sciences, 2, 1009–1013. [Google Scholar]
- Wani, B. A. , Bodha, R. H. , & Wani, A. H. (2010). Review: Nutritional and medicinal importance of mushrooms. Journal of Medicinal Plants Research, 4, 2598–2604. [Google Scholar]
- Xu, C.‐J. , Wang, Y.‐Z. , Niu, B.‐N. , Liu, B. , Li, Y.‐B. , Wang, X.‐M. , & Lu, S.‐L. (2014). Isolation and characterization of a novel lectin with mitogenic activity from Pleurotus ferulae . Pakistan Journal of Pharmaceutical Sciences, 27, 983–989. [PubMed] [Google Scholar]


