Abstract
Controlled fermentation of Sweet potato (Ipomoea batatas) var. Beauregard by yeast, Saccharomyces boulardii (MAY 796) to enhance the nutritional value of sweet potato was investigated. An average 8.00 × 1010 Colony Forming Units (CFU)/g of viable cells were obtained over 5‐day high‐solid fermentation. Yeast cell viability did not change significantly over time at 4°C whereas the number of viable yeast cells reduced significantly at room temperature (25°C), which was approximately 40% in 12 months. Overall, the controlled fermentation of sweet potato by MAY 796 enhanced protein, crude fiber, neutral detergent fiber, acid detergent fiber, amino acid, and fatty acid levels. Development of value‐added sweet potato has a great potential in animal feed and human nutrition. S. boulardii‐ fermented sweet potato has great potential as probiotic‐enriched animal feed and/or functional food for human nutrition.
Keywords: amino acid, CFU, fatty acid, fermented, Ipomoea batatas, neutral detergent fiber, viable cell
1. Introduction
Sweet Potato (Ipomoea batatas (L.) Lam) is an important crop of southern United States due to the ideal long frost‐free growing season. Globally, sweet potato is ranked at the seventh position next to rice, wheat, corn, and cassava consumption. This is primarily due to the versatile adaptation of the crop to diverse environmental and climatic conditions. Sweet potato is considered as a vegetable crop and has various food and feed applications. According to the USDA‐NASS (2015), the U.S. production topped 29 million hundredweight (cwt). Southern states dominated in production with North Carolina and California ranking top states followed by Texas and Mississippi. Sweet potatoes are rich in wide range of nutrients which include dietary fiber, vitamins, phenolic compounds, and carotenes (Ray & Tomlins, 2010). Sweet potato has been used as animal feed in poultry, pigs, and in livestock (Chittaranjan, 2007; Lebot, 2009; Woolfe, 1992). On dry basis, sweet potato is known to contain about 80% starch and a potential feedstock for bioethanol production (Santa‐Maria, Yencho, Haigler, & Sosinski, 2011).
Fermentation of vegetable products with beneficial microbes such as Lactobacillus (LA), Sacchromyces, Bacillus, etc. have been carried out for preservation and nutritional enhancement (Karovičova, Grief, Kohajdova, & Hybenova, 2001). Salt‐incorporated fermentation of cabbage, cucumber, and olives are commercially available which not only provide preservation but also enhance the nutritional quality (Maifreni, Marino, & Conte, 2004). Some of the Asian LA‐fermented vegetables such as radish, mustard, and cauliflowers are known to enhance flavor and preserve some biochemicals such as carotenes and phenolics (Shivashankara, Isobe, Al‐Haq, Takenaka, & Shiina, 2004). Because of the starch‐rich nature of sweet potato and its co‐products from processing industry, various fermentation routes have been used to develop value‐added products (Ray & Ward, 2006). Ray, Panda, Swain, and Sivakumar (2012) developed wine from anthocyanin‐rich sweet potato by fermentation of Saccharomyces cerevisiae. The resultant wine showed a 58.95% anti‐oxidant activity with a similar pH, tannin, and phenol profile of grape wine. Pagana et al. (2014) used organic waste from sweet potato processing for canning as raw material for production of lactic acid, using Lactobacillus rhamnosus with yield of 10 g/l of lactic acid production in 72 hr. Additionally, sweet potato and its waste has been used to produce antibiotic such as tetracycline (Yang and Yuan, 1989), citric acid, lactic acid and ethanol (Ray & Palaniswami, 2008; Wongkhalaung, 1995).
Food‐ and feed‐borne gastroenteritis are common among humans and animals. Some of the pathogens to blame for these outbreaks are Salmonella, E. coli, and Clostridium difficile to name a few major pathogens (Govaris, Solomakos, Pexara, & Chatzopoulou, 2010). These gastrointestinal disorders can be cured by use of viable microorganisms called “probiotics”. Some of the well‐known prokaryotic probiotic genera of microorganism are Lactobacillus, Bifidobacterium, Enterococcus, Oenoccoccus, Propionibacterium, Bacillus, and Clostridium butyricum (Gibson, 1998). Only a few eukaryotic microbes such as Saccharomyces cerevisiae and Saccharomyces boulardii are known to exhibit probiotic effect in humans and animals. They have been extensively studied for their ability in reducing gastrointestinal inflammation and other disorders (Hamedi et al., 2013; Hudson et al., 2014; Pothoulakis, 2009). Saccharomyces boulardii is known to act by providing receptor sites for binding some toxins produced by pathogens. Yeast glucans and mannans are also known to play a critical role in the toxin‐binding process. These actions are well mediated at different physiological conditions including anaerobic stress, low pH, and osmotic shocks (Klis, Boorsma, & DeGroot, 2006; Lesage & Bussey, 2006; Orlean, 2012); S. boulardii can induce immune response by increasing the IgA and IgG levels (Lauren et al. 2016; Matar et al., 2015; Kim et al., 2013; Buts, Bernasconi, Vaerman, & Dive, 1990; Qamar et al., 2001; Rodrigues et al., 2000); and S. boulardii proteases are known to inhibit Toxin A and Toxin B of Clostridium difficile, which are mainly responsible for diarrhea and colitis (Castagliuolo, Riegler, Valenick, LaMont, & Pothoulakis, 1999). Due to the probiotic nature of S. boulardii, attempts have been made to ferment various food products and agricultural produce to provide supplemental probiotic for humans as well as for animals. For example Fratianni et al. (2013) used berry juice as a medium to grow yeast and used alginate‐inulin‐xanthan gum microencapsulation for better delivery and enhancement of cell viability. They reported a healthy growth of 7.59 × 1010 CFU/ml viable cells when stored for a month at 4°C. Many other co‐products of food industry such as rice bran (Ryan et al., 2011), raw wheat (Nguyen & Herve, 1997) and , tomato juice (Fratianni et al., 2013) have been used for fermenting S. boulardii. With the abundant supply of sweet potato in southern United States, production of value‐added products provides an opportunity to diversify sweet potato uses. Keeping this in mind, sweet potato was used a feedstock for production of S. boulardii‐enriched fermented product. The enriched product can serve as potential probiotic in animal feed supplement to control gastrointestinal disorders and also have application in human health and nutrition. The overall objective of this study was to develop S. boulardii‐fermented sweet potato and characterize the viable cell counts over time and nutritional profiling of the end product (Table 1).
Table 1.
Viable cell counts at different time points
| Months | 4°C | 25°C |
|---|---|---|
| 0 | 7.9 × 1010 a | 8.5 × 1010 a |
| 4 | 8.0 × 1010 a | 7.3 × 1010 a |
| 8 | 7.9 × 1010 a | 6.9 × 1010 b |
| 12 | 7.7 × 1010 a | 5.9 × 1010 b |
Counts are average of two replicates.
2. Materials and Methods
2.1. Microbial culture
Lyophilized cultures of S. boulardii (MAY 796) were obtained from American Type Culture Collection (ATCC, Manassas, VA), revived on potato dextrose agar (PDA) and stored at 4°C for 10 days. After revival, cultures were inoculated into yeast extract malt extract broth and incubated at 30°C on an orbital shaker at 300 rpm for 5 days and stored in a deep freezer at −80°C.
2.2. Inoculum generation
About one vial (1 ml) from – 80°C storage was thawed and was inoculated into potato dextrose broth which was sterilized at 121°C for 30 min. Inoculum was grown at 30°C on an orbital shaker at 200 rpm for 48 hr.
2.3. Media preparation for high solid fermentation
“Beauregard” sweet potato was collected from Mound Bayou, MS, and was stored at room temperature at Alcorn State University Experiment Station, Lorman, MS. Selected sweet potatoes were washed thoroughly with running water to remove any surface debris, was chopped and placed in a drying chamber for 3 days at 28°C. Sweet potato flour was prepared by milling in an Udy bench top grinder. About 60 g of the finely ground sweet potato flour was added to 1000 ml flask with 300 ml of H2O. Other inorganic salts included were 1 g KH2PO4, 0.5 g MgSO4, and 0.5 g MnSO4 and ZnSO4 per liter. Samples were autoclaved at 121°C for 30 min. Upon cooling, flasks were inoculated with 10 ml of MAY 796. This production media was then placed in an incubator shaker at 300 rpm for 5 days at 30°C. Duplicate samples were prepared for statistical analysis.
2.4. Fermentation conditions
Flasks were inoculated and incubated at 30°C, 300 rpm for 5 days. Control flasks without inoculum were also maintained. Two replicates per treatment were employed. Samples were freeze dried for 48 h and stored at −80°C until further analyses.
2.5. Determination of viable cells of S. boulardii
Serial dilution technique was used to determine CFU which represents viable cells per gram of the final fermented samples. In brief, a serial dilution method was used and spread plate method of culturing yeast cells on PDA medium was carried out. Sample dilutions up to 10−12 were carried out. After incubation of plates at 30°C for 4 days, colonies from plates with the highest dilutions (where the colony numbers varied between 50 and 100) were counted and CFU/g of fermented samples was expressed.
2.6. Stability of the viable yeast cells
Samples were stored in Zip‐lock bags at 4° and 25°C (Room Temperature) and periodically (every 4 months) subjected for determination of viability, using serial dilution technique.
2.7. Nutritional profiling
Known quantity of freeze‐dried samples were weighed and sent to the Agricultural Experiment Station Chemical Laboratories (AESCL) at the University of Missouri‐Columbia for biochemical compositional analyses, using the ASOS methods as described in Nanjundaswamy and Vadlani (2011). Nutrition composition analyses included total amino acid profile, total fatty acid profile, crude fat and protein, crude fiber, % neutral detergent fiber (NDF), and % acid detergent fiber (ADF).
2.8. Statistical analyses
Data were analyzed by Statistical Analysis Software (SAS) version 9.2. Analysis of Variance was measured, using PROC ANOVA and Tukey test was used for pair‐wise comparisons. Significance was set at p < .05.
3. Results and Discussion
3.1. Viable cell count in fermented product
The end of fermentation viable cell count for S. boulardii was about 8.0 × 1010 CFU/g (Table 1). This cell viability count was similar to other studies (Fratianni et al., 2014; Torija, Rozès, Poblet, Guillamèn, & Mas, 2002). When the fermented product was stored at room temperature (25°C) and at 4°C, there was a decrease in the CFU over the next 12 months. The decrease in the CFU was not significant at 4°C (p = .8362), whereas the CFU decrease at room temperature was significant (p = .0032), which was about 44%. This information is very vital, since cell viability is critical for probiotic efficiency of the fermented‐product. Some of the earlier yeast cell stability studies conducted in liquid medium showed clear drop in the CFU over time due to the inherent metabolic stress, ethanol production, and decrease in membrane functionality (Casey, Magnus, & Ingledew, 1984; Nagodawithana, Castellano, & Steinkraus, 1974; Ough, 1966). It is plausible that the removal of water and reducing the water activity has a great impact on long‐term stability of the probiotic product. Also storage of the product at 4°C prevents any loss of viability of the probiotic.
3.2. Overall nutritional profile
The overall nutritional profile of fermented sweet potato by S. bouldardii is shown in Figure 1. Fermentation significantly (p ≤ .05) enhanced the % crude protein, total amino acid content, % crude fat, % crude fiber, % ADF, % NDF, and ash content compared to the control. Moisture was significantly greater (p ≤ .05) in the control than in the fermented sample. Actively growing yeast produced significant protein which contributed to the enhanced levels of total protein. A similar trend was observed in red yeast fermentation of dry distillers grain with solubles (DDGS), a co‐product of corn ethanol production (Nanjundaswamy & Vadlani, 2011). Endo‐ and exo‐proteases in the actively growing yeast can break down proteins into numerous amino acids, resulting in enhanced total amino acids (Sturley & Young, 2013). Neutral detergent fiber represents the total plant fiber or cell wall, including hemicellulose, cellulose, and lignin. Increased NDF results in higher digestible energy.
Figure 1.
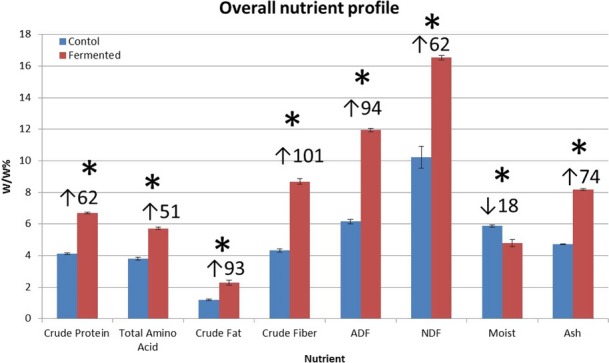
Nutrition composition. Means and standard errors are provided. *significantly different (p < .05) treatment from control. ↑% increase, ↓%decrease compared to control
3.3. Amino acid profile of control and fermented sweet potato
Compared to the control, fermented samples had significantly lower (p ≤ .05) Taurine, hydroxylysine, and tryptophan (Figure 2). For all other amino acids, fermented samples had significantly higher (p ≤ .05) content than control, except for glutamic acid which was greater than control but not statistically significant. Overall, total amino acid content was significantly greater (p ≤ .05) in fermented samples than control.
Figure 2.
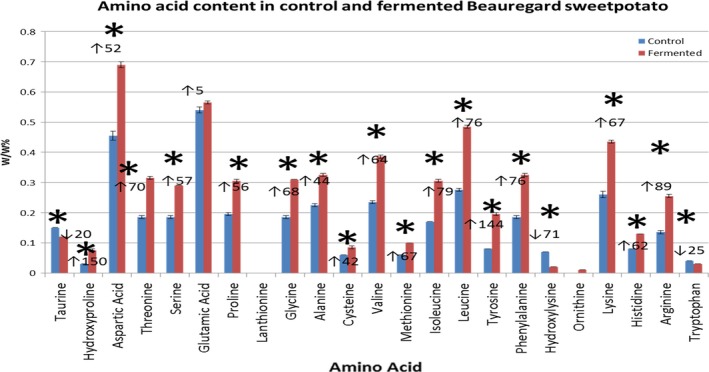
Amino acid Content. Means and standard errors are provided. *significantly different treatment from control (p < .05). ↑% increase, ↓%decrease compared to control
Some of the important amino acids such as lysine, leucine, glutamic acid, and aspartic acid levels were higher in fermented samples compared to control. These amino acids are of importance in animal feed. The lysine levels of the fermented sweet potato is comparable to DDGS (Stein et al. 2005; Pahm, Hoehler, Pedersen, Simon, & Stein, 2006; Pahm, Pedersen, & Stein, 2006; Stein, Pedersen, Gibson, & Boersma, 2006; Urriola et al., 2009).
3.4. Fatty acid profile of control and fermented sweet potato
Figure 3 represents fatty acid profile of fermented and control samples. At least 12 fatty acids were detected. Fermented samples had significantly higher (p ≤ .05) levels of myristic acid, stearic acid, linolenic acid, and DHA compared to the control. Of the remaining nine fatty acids, only six fatty acids were higher in content in fermented samples than control, but were not statistically significant; three fatty acids namely palmitic (16:0), stearidonic acid, and behnoic acid content were greater in control than fermented samples, but not statistically significant. A similar fatty acid profile was reported in wine from fermentation of different species of Saccharomyces under different temperatures (Torija et al., 2002). A very limited metabolic profiling has been reported with respect to fatty acid profile of S. boulardii. Ryan et al. (2011) provided insights into the metabolomics of rice bran‐fermented with S. boulardii. The S. boulardii fermentation undoubtedly changed the metabolomic profile for rice bran. GC‐MS profiling of the volatile fatty acid was positively influenced by fermentation. Fermented rice bran showed elevated levels of secondary metabolites such as ferulic acid.
Figure 3.
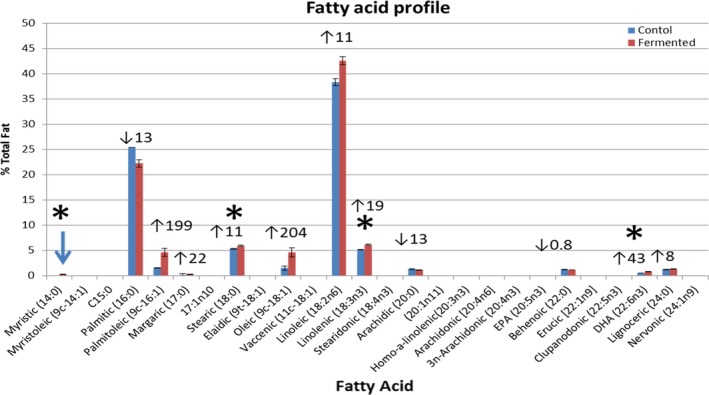
Fatty acid profile. Means and standard errors are provided. At p < .05, treatments were significantly different only for myristci acid, stearic acid, linolenic acid, and DHA. ↑% increase, ↓%decrease compared to control
4. Conclusions
High‐solid submerged fermentation of sweet potato with S. boulardii was successfully carried out with an end of fermentation viable cell count of 8.0 × 1010 CFU/g. The freeze‐dried fermented product showed good stability with respect to CFU at 4°C for 12 months. The overall nutritional profile of the fermented product was positively changed with increased total protein, total amino acid and NDF. S. boulardii value‐added sweet potato opens up new opportunities for value‐added probiotics for animal feed and human nutrition.
Conflict of Interest
None declared.
Acknowledgment
The authors acknowledge the USDA (US Department of Agriculture) Evans Allen for providing financial support.
Campbell C, Nanjundaswamy AK, Njiti V, Xia Q, Chukwuma F. Value‐added probiotic development by high‐solid fermentation of sweet potato with Saccharomyces boulardii . Food Sci Nutr. 2017;5:633–638. https://doi.org/10.1002/fsn3.441
References
- Buts, J. P. , Bernasconi, P. , Vaerman, J. P. , & Dive, C. (1990). Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii . Digestive Diseases and Sciences, 35, 251–256. [DOI] [PubMed] [Google Scholar]
- Casey, G. P. , Magnus, C. A. , & Ingledew, W. M. (1984). High gravity brewing: Effects of nutrition on yeast composition, fermentative ability and alcohol production. Applied and Environmental Microbiology., 48, 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagliuolo, I. , Riegler, M. F. , Valenick, L. J. , LaMont, T. , & Pothoulakis, C. (1999). Saccharomyces boulardii protease inhibits the effects of clostridium difficile toxins A and B in human colonic mucosa. Infection and immunity., 67, 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittaranjan, K. (2007). Genome mapping and molecular breeding in plants, pulses, sugar and tuber crops. Berlin Heidelberg, NY: Springer‐Verlag Press. [Google Scholar]
- Fratianni, F. , Cardinale, F. , Russo, I. , Iuliano, C. , Cucciniello, A. C. , Maione, M. , … Nazzaro, F. (2013). Fermentation of tomato juice with the probiotic yeast Saccharomyces cerevisiae boulardii In Robinson A., & Emerson D. (Eds.), Functional foods: Sources, biotechnology applications, and health challenges (pp. 143–152). New York, NY: Nova Science Publisher. [Google Scholar]
- Fratianni, F. , Cardinale, F. , Russo, I. , Iuliano, C. , Tremonte, P. , Coppola, R. , & Nazzaro, F. (2014). Ability of synbiotic encapsulated Saccharomyces cerevisiae boulardii to grow in berry juice and to survive under simulated gastrointestinal conditions. Journal of Microencapsulation, 31(3), 299–305. [DOI] [PubMed] [Google Scholar]
- Gibson, G. R. (1998). Dietary modulation of human gut microflora using prebiotics. British Journal of Nutrition, 80, 209–212. [PubMed] [Google Scholar]
- Govaris, A. , Solomakos, N. , Pexara, A. , & Chatzopoulou, P. (2010). The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. International Journal of Food Microbiology, 137, 175–180. [DOI] [PubMed] [Google Scholar]
- Hamedi, H. , Misaghi, A. , Modarressi, M. H. , Salehi, T. Z. , Khorasanizadeh, D. , & Khalaj, V. (2013). Generation of a uracil auxotroph strain of the probiotic yeast Saccharomyces boulardii as a host for the recombinant protein production. Avicenna Journal of Medical Biotechnology, 5, 29–34. [PMC free article] [PubMed] [Google Scholar]
- Hudson, L. E. , Fasken, M. B. , McDermott, C. D. , McBride, S. M. , Kuiper, E. G. , & Guiliano, D. B. (2014). Functional heterologous protein expression by genetically engineered probiotic yeast Saccharomyces boulardii . PLoS ONE, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karovičova, J. , Grief, G. , Kohajdova, Z. , & Hybenova, E. (2001). Vyuzitie multivariacnej analyzy pri hodoteni mliecne fermentovanych. Zeleninovych Stiav Bulletin. PV., 40, 119–131. [Google Scholar]
- Kim, D. , Pertea, G. , Trapnell, C. , Pimentel, H. , Kelley, R. , & Salzberg, S. L. (2013). TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biology, 14, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis, F. M. , Boorsma, A. , & DeGroot, P. W. J. (2006). Cell wall construction in Saccharomyces cerevisiae . Yeast, 23, 185–202. [DOI] [PubMed] [Google Scholar]
- Lauren, E. H. , McDermott, C. D. , Stewart, T. P. , Hudson, W. H. , Rios, D. , Fasken, M. B. , Corbett, A. H. , & Lamb, T. J. (2016). Characterization of the Probiotic Yeast Saccharomyces boulardii in the Healthy Mucosal Immune System. PLoS One, 11(4), e0153351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebot, V. (2009). Tropical root and tuber crops: Cassava, sweet potato, yams and aroids. Crop production science in horticulture. Wallingford, UK: CAB books, CABI. [Google Scholar]
- Lesage, G. , & Bussey, H. (2006). Cell Wall Assembly in Saccharomyces cerevisiae . Microbiology and Molecular Biology Reviews, 70, 317–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maifreni, M. , Marino, M. , & Conte, L. (2004). Lactic acid fermentation of Brassica rapa: Chemical and microbial evaluation of a typical Italian product (brovada). European Food Research and Technology, 218, 469–473. [Google Scholar]
- Matar, C. G. , Anthony, N. R. , O'Flaherty, B. M. , Jacobs, N. T. , Priyamvada, L. , & Engwerda, C. R. (2015). Gammaherpesvirus co‐infection with malaria suppresses anti‐parasitic humoral immunity. PLoS Pathogens, 11, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagodawithana, T. W. , Castellano, C. , & Steinkraus, K. H. (1974). Effect of dissolved oxygen, temperature, initial cell count and sugar concentration on the viability of Saccharomyces cerevisiae in rapid fermentations. Applied Microbiology., 28, 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjundaswamy, A. , & Vadlani, P. V. (2011). Fiber reduction and lipid enrichment in carotenoid‐enriched distillers dried grain with solubles produced by secondary fermentation of Phaffia rhodozyma and Sporobolomyces roseus . Journal of Agricultural and Food Chemistry, 58, 12744–12748. [DOI] [PubMed] [Google Scholar]
- Nguyen, T. H. , & Herve, M. (1997). Food ingredients obtained by fermentation with S. boulardii and foods containing them US Patent. US 5639496 A
- Orlean, P. (2012). Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics, 192, 775–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ough, C. S. (1966). Fermentation rates of grape juice. III. Effects of initial ethyl alcohol, pH and fermentation temperature. American Journal of Enology and Viticulture, 17, 74–78. [Google Scholar]
- Pagana, I. , Morawicki, R. , & Hager, J. T. (2014). Lactic acid production using waste generated from sweet potato processing. International J. Food Science and Technology, 49(2), 641–649. [Google Scholar]
- Pahm, A. A. , Hoehler, D. , Pedersen, C. , Simon, D. , & Stein, H. H. (2006). Amino acid digestibility and measurement of blocked lysine in five samples of distillers dried grains with solubles in growing pigs. Journal of Animal Science, 84, 285–289. [Google Scholar]
- Pahm, A. A. , Pedersen, C. , & Stein, H. H. (2006). Evaluation of reactive lysine (homoarginine) as an in vitro procedure to predict lysine digestibility of distillers dried grains with solubles by growing pigs. Journal of Animal Science, 84, 121–128. [Google Scholar]
- Pothoulakis, C. (2009). Review article: Anti‐inflammatory mechanisms of action of Saccharomyces boulardii . Alimentary Pharmacology & Therapeutics, 30, 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamar, A. , Aboudola, S. , Warny, M. , Michetti, P. , Pothoulakis, C. , & LaMont, J. T. (2001). Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to clostridium difficile toxin A in mice. Infection and Immunity, 69, 2762–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, R. , & Palaniswami, M. (2008). Bioprocessing for value additions In Palaniswami M., & Peter K. (Eds.), Horticulture science series: Tuber & root crops, Vol. 9 (pp. 381–395). New Delhi, India: New India Publishing Agency. [Google Scholar]
- Ray, R. C. , Panda, S. K. , Swain, M. R. , & Sivakumar, P. S. (2012). Proximate composition and sensory evaluation of anthocyanin‐rich. International Journal of Food Science and Technology., 47, 452–458. [Google Scholar]
- Ray, R. C. , & Tomlins, K. I. (2010). Sweet potato: Post harvest aspects in purple sweet potato (Ipomoea batatas L.) wine food, feed and industry. New York, NY: Nova Science Publishers Inc. [Google Scholar]
- Ray, R. , & Ward, O. (2006). Post‐harvest microbial biotechnology of topical root and tuber crops In Ray R., & Ward O. (Eds.), Microbial biotechnology in horticulture, Vol. 1 (pp. 345–396). Enfield, NH: Science Publishers. [Google Scholar]
- Rodrigues, A. , Cara, D. , Fretez, S. , Cunha, F. , Vieira, E. , & Nicoli, J. (2000). Saccharomyces boulardii stimulates sIgA production and the phagocytic system of gnotobiotic mice. Journal of Applied Microbiology, 89, 404–414. [DOI] [PubMed] [Google Scholar]
- Ryan, P. E. , Heuberger, A. , Weir, T. L. , Barnett, B. , Broekling, C. , & Prenni, E. (2011). Rice bran fermented with Saccharomyces boulardii generates novel metabolite profile with bioactivity. Journal of Agricultural and Food Chemistry, 59, 1862–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa‐Maria, M. C. , Yencho, C. G. , Haigler, C. H. , & Sosinski, B. (2011). Starch self‐ processing in transgenic sweet potato roots expressing hyperthermophillic α‐amylase. Biotechnology Processes, 27, 351–359. [DOI] [PubMed] [Google Scholar]
- Shivashankara, K. S. , Isobe, S. , Al‐Haq, M. J. , Takenaka, M. , & Shiina, T. (2004). Fruit antioxidant activity, ascorbic acid, total phenol, quercetin, and carotene of Irwin mango fruits stored at low temperature after high electric field pretreatment. Journal of Agricultural Food Chemistry, 52, 1281–1286. [DOI] [PubMed] [Google Scholar]
- Stein, H. H. , Pedersen, C. , & Boersma, M. G. (2005). Energy and nutrient digestibility in dried distillers grain with solubles. J. Anim. Sci., 83(2), 49–55.15583042 [Google Scholar]
- Stein, H. H. C. , Pedersen, M. , Gibson, L. , & Boersma, M. G. (2006). Amino acid and energy digestibility in ten samples of distillers dried grain with solubles by growing pigs. Journal of Animal Science, 84, 853–860. [DOI] [PubMed] [Google Scholar]
- Sturley, S. L. , & Young, T. W. (2013). Extracellular protease activity in a strain of Saccharomyces cerevisiae . Journal of the Institute of Brewing., 94, 23–27. [Google Scholar]
- Torija, M. J. , Rozès, N. , Poblet, M. , Guillamèn, J. M. , & Mas, A. (2002). Effects of fermentation temperature on the strain population of Saccharomyces cerevisiae . International Journal of Food Microbiology, 80, 47–53. [DOI] [PubMed] [Google Scholar]
- Urriola, P. E. , Hoehler, D. , Pedersen, C. , Stein, H. H. , Johnston, L. J. , & Shurson, G. C. (2009). Amino acid digestibility by growing pigs of distillers dried grain with solubles produced from corn, sorghum, or a corn‐sorghum blend. Journal of Animal Science, 87, 2574–2580. [DOI] [PubMed] [Google Scholar]
- USDA‐NASS (2015). https://www.nass.usda.gov
- Wongkhalaung, C. (1995). Lactic acid fermentation of sweet potato. Kasetsart Journal: Natural Sciences, 29, 521–526. [Google Scholar]
- Woolfe, J. A. (1992). Sweet potato: An untapped food resource. Cambridge, NY: Cambridge University Press. [Google Scholar]
- Yang, S. S. , & Ling, M. Y. (1989). Tetracycline production with sweet potato residue by solid state fermentation. Biotechnol Bioeng, 33(8), 1021–1028. [DOI] [PubMed] [Google Scholar]


