Abstract
Cell wall is a complex biopolymer on the surface of all Gram-positive bacteria. During infection, cell wall is recognized by the innate immune receptor Toll-like receptor 2 causing intense inflammation and tissue damage. In animal models, cell wall traffics from the blood stream to many organs in the body, including brain, heart, placenta and fetus. This protocol describes how to prepare purified cell wall from Streptococcus pneumoniae, detect its distribution in animal tissues, and study the tissue response using the placenta and fetal brain as examples.
Keywords: Cell wall, Peptidoglycan, Bacterial inflammation, Neuroproliferation, Fetal neurogenesis, Placental trafficking, Toll like receptor 2 ligand, Streptococcus pneumonae
Background
Host response to infection involves recognition of many bacterial components including the cell wall (CW), a complex macromolecule that forms the surface of all Gram-positive bacteria. The CW of Gram-positive bacteria is formed by the covalent network of peptidoglycan and teichoic acid. Streptococcus pneumoniae, a leading cause of pneumonia, sepsis, and meningitis, has served as an important model organism for studying the innate immune response to Gram-positive bacterial infection including CW.
When upon Streptococcus pneumoniae (pneumococcal) infection CW components are released from bacteria during growth or antibiotic-induced death, it circulates in the blood stream and crosses cellular barriers, including the placenta and blood brain barrier. CW components have inflammatory activities equal to or greater than intact bacteria (Tuomanen et al., 1985a and 1985b). The CW can be viewed as the Gram- positive equivalent of endotoxin. The vast amount of CW pieces released during infection greatly stimulates the host inflammatory response by activating the innate immune receptor, Toll like receptor 2 (TLR2) (Yoshimura et al., 1999). Responses differ depending on the organ infected: the postnatal brain undergoes apoptosis, scarring predominates in heart, and the fetal brain escapes damage, showing striking neuroproliferation (Orihuela et al., 2006; Braun et al., 1999; Fillon et al., 2006; Humann et al., 2016).
This protocol describes how to prepare purified CW from Streptococcus pneumoniae (Tuomanen et al., 1985b) and follow its distribution in mice after intravenous injection, focusing on the placenta and fetal brain as examples (Humann et al., 2016). This model yields histopathologic sections of organs for study of the tissue response to CW components. Our model’s focus on pneumococcal CW derives from its well-described role in inflammation and injury in many organs, its extensive known chemistry and its recognition as a classic TLR2 pathogen associated molecular pattern.
Materials and Reagents
0.22 µm bottle top filter (Corning, catalog number: 431096)
Glass tubes (Thermo Fisher Scientific, Fisher Scientific, catalog number: 14-961-32)
1,000 ml centrifuge bottle (Thermo Fisher Scientific, Thermo Scientific™, catalog number: 3120-1000)
30 ml centrifuge bottle (Thermo Fisher Scientific, Thermo Scientific™, catalog number: 3119-0050)
Serological pipettes, 1 case of 5 ml, 10 ml, 25 ml
Microcentrifuge tubes (Eppendorf, catalog number: 022364111)
Aluminum foil (Thermo Fisher Scientific, Fisher Scientific, catalog number: 01-213-100)
25 gauge needles (BD, catalog number: 305122)
50 ml polypropylene tubes (SARSTEDT, catalog number: 62.547.254)
Petri dishes (Thermo Fisher Scientific, Fisher Scientific, catalog number: FB0875712)
Small electric razor to shave animals
Superfrost microslides (VWR, catalog number: 48311-703)
Premium cover glass (Thermo Fisher Scientific, Fisher Scientific, catalog number: 12-548-5P)
Magnetic stir bars
Inoculation loops (Thermo Fisher Scientific, Fisher Scientific, catalog number: 22-363-595)
1 ml syringe (BD, catalog number: 309628)
Acid washed 106 µm glass beads (Sigma-Aldrich, catalog number: G4649)
Plastic embedding molds for histology (Polysciences, catalog number: 18646D-1)
Streptococcus pneumoniae strains - CW is much easier to purify from strains that are unencapsulated such as R6 (ATCC, catalog number: BAA-255)
C57Bl6 mice, mixture of male and female (THE JACKSON LABORATORY, catalog number: 000664)
Water (Sigma-Aldrich, catalog number: W3500-1L)
Ultrapure water (example: Milli-Q, EMD Millipore)
Tryptic soy agar (TSA) (EMD Millipore, catalog number: 105458)
Sterile defibrinated sheep blood (i-Tek Medical Technologies, catalog number: 103-100-3)
Glycerol
-
Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: L4390)
Note: This product has been discontinued.
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 71382)
Magnesium sulfate (MgSO4) (Sigma-Aldrich, catalog number: 208094)
DNase I (Sigma-Aldrich, catalog number: DN25-10MG)
RNase A (Sigma-Aldrich, catalog number: R6513-10MG)
Calcium chloride dehydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C-3881)
Trypsin (AMRESCO, catalog number: M150)
α-amylase (Sigma-Aldrich, catalog number: A3176)
Lithium chloride (LiCl) (Sigma-Aldrich, catalog number: L9650)
Ethylenediaminetetraacetic acid disodium salt dehydrate (EDTA) (Sigma-Aldrich, catalog number: E5134)
Acetone (Thermo Fisher Scientific, Fisher Scientific, catalog number: A946-4)
Pierce™ LAL Chromogenic Endotoxin Quantitation Kit (Thermo Fisher Scientific, Thermo Scientific™, catalog number: 88282)
Fluorescein isothiocyanate isomer I (FITC) (Sigma-Aldrich, catalog number: F7250)
Dulbecco’s phosphate buffered saline (DPBS) (Mediatech, catalog number: 21-030- CV)
Paraformaldehyde, 16% w/v (Alfa Aesar, catalog number: 43368)
Sucrose (Sigma-Aldrich, catalog number: S9378)
Tissue Tek OCT compound (SAKURA FINETEK USA, catalog number: 4583)
Prolong Gold Antifade with DAPI (Thermo Fisher Scientific, Molecular Probes™, catalog number: P36931)
Trizma HCl (Sigma-Aldrich, catalog number: T5941)
Tris-base (Sigma-Aldrich, catalog number: 1070897600)
Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 320331-500ml)
Sodium carbonate (Na2CO3) (Sigma-Aldrich, catalog number: 451614)
Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S-6014)
Magnesium chloride hexahydrate (MgCl2) (Sigma-Aldrich, catalog number: M0250)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C1016)
MnSO4 monohydrate (Sigma-Aldrich, catalog number: M7634)
Glucose (Sigma-Aldrich, catalog number: G7528)
Adenosine (Oakwood Products, catalog number: 093333)
Uridine (EMD Millipore, catalog number: 6680)
Glutamine (Sigma-Aldrich, catalog number: G8540)
Nicotinic acid (Sigma-Aldrich, catalog number: N4126)
(B6) Pyridoxine HCL (Sigma-Aldrich, catalog number: P9755)
-
Ca-pantothenate (D-pantothenic acid) (Sigma-Aldrich, catalog number: P3161)
Note: This product has been discontinued.
Thiamine HCL (Sigma-Aldrich, catalog number: 5871-100GM)
Riboflavin (Sigma-Aldrich, catalog number: R4500)
Biotin (Sigma-Aldrich, catalog number: B4639)
10 N NaOH solution (Thermo Fisher Scientific, Fisher Scientific, catalog number: SS255-1)
Ferrous sulfate heptahydrate (FeSO4·7H2O) (Thermo Fisher Scientific, Fisher Scientific, catalog number: I146-500)
Copper(II) sulfate pentahydrate (CuSO4·5H2O) (Sigma-Aldrich, catalog number: C8027)
Zinc sulfate heptahydrate (ZnSO4·7H2O) (Sigma-Aldrich, catalog number: Z4750)
Manganese chloride tetrahydrate (MnCl2·4H2O) (Thermo Fisher Scientific, Fisher Scientific, catalog number: M87-500)
Pyruvic acid (Sigma-Aldrich, catalog number: 107360-25g)
Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655)
Potassium phosphate dibasic (K2HPO4) (Sigma-Aldrich, catalog number: P3786)
Yeast extract (BD, Bacto™, catalog number: 212750)
Sodium acetate anhydrous (Sigma-Aldrich, catalog number: S8625)
Casamino acids technical (BD, Bacto™, catalog number: 223120)
L-tryptophan (Sigma-Aldrich, catalog number: T8941)
L-cysteine HCL (Thermo Fisher Scientific, Fisher Scientific, catalog number: BP376-100)
Asparagine (Sigma-Aldrich, catalog number: A4284)
Choline chloride (Sigma-Aldrich, catalog number: C7527)
Dry ice
- Solutions used to treat CW during purification
- 50 mM Tris-HCl, pH 7.0 (see Recipes)
- 5% (v/v) SDS (see Recipes)
- 1 M NaCl (see Recipes)
- 100 mM Tris, pH 7.5 (see Recipes)
- 1 M MgSO4 (see Recipes)
- 1 M CaCl2 (see Recipes)
- 1% (v/v) SDS (see Recipes)
- 8 M LiCl (see Recipes)
- 100 mM EDTA (see Recipes)
- Carbonate buffer, pH 9.2 (see Recipes)
- Preparation of C+Y components
- ‘3 in 1’ salts (see Recipes)
- 20% glucose (see Recipes)
- 50% sucrose (see Recipes)
- Adenosine (2 mg/ml) (see Recipes)
- Uridine (2 mg/ml) (see Recipes)
- Glutamine (1 mg/ml) (see Recipes)
- Adams I (see Recipes)
- Adams II (see Recipes)
- 2% pyruvate (see Recipes)
- 1 M KH2PO4 (see Recipes)
- 1 M K2H PO4 (see Recipes)
- 5% yeast extract (see Recipes)
- Media preparation
- PreC media (see Recipes)
- Supplement (see Recipes)
- Adams III (see Recipes)
- 1 M potassium phosphate buffer (see Recipes)
- C+Y medium (see Recipes)
Equipment
37 °C CO2 incubator (Thermo Fisher Scientific, Thermo Scientific™, model: 3110)
Benchtop microcentrifuge (Eppendorf, model: 5417C)
1,000 ml Erlenmeyer flasks
4,000 ml Erlenmeyer flasks
Sorvall centrifuge RC 5C Plus and appropriate rotor for the centrifuge tubes or bottles
500 ml beaker
Stirring hotplate
Vortex mixer
Speed-vac (Savant, model: SC110A)
Water-bath sonicator (Thermo Fisher Scientific, Fisher Scientific, model: FS20)
Heating pad
Cryostat microtome (Microme, model: HM505E)
Zeiss LSM 510 NLO Meta confocal microscope
Spectrophotometer (Turner, Model: 340)
Spectra MAX340 plate reader to measure absorbance at 405 nm (Molecular Device, model: Spectra MAX340)
Software
Zen 2008 software package (Carl Zeiss MicroImaging, Inc.)
ImageJ (imagej.net/Particle_Analysis)
Graphpad Prism (Graphpad)
Procedure
A. Preparation of pneumococcal CW: peptidoglycan-teichoic acid complex
Notes:
Prepare all the solutions and do all washes with distilled water (use cell culture distilled water from Sigma [#W3500] with low endotoxin concentration).
Filter all the solutions before use (with filter 0.22 µm).
Preferably use new clean glassware and equipment to avoid any contamination with endotoxin (LPS).
Prior to large-scale growth, check pneumococcal stock on blood plate without antibiotics to confirm the absence of contamination.
The protocol described here uses C+Y medium (see Recipes) as a partially defined growth medium. Other commercially available media are also suitable but have more diverse contaminating components such as endotoxin that needs to be removed for achieving a pure preparation.
-
Preparation of stock of Streptococcus pneumoniae
CW can be purified from any pneumococcal strain. However, the procedure is most successful with unencapsulated strains such as R6 (Tuomanen et al., 1985b).- Streak strain on TSA agar (20 g TSA per 500 ml water, autoclave) containing 3% (v/v) sterile defibrinated sheep blood and incubate the plate at 37 °C, 5% CO2 for 16–18 h (Figure 1). The growth on blood agar allows for the visualization of hemolysis, which is characteristic for S. pneumonia.
- Scrape all the colonies off the agar plate with an inoculation loop, and inoculate into 10 ml C+Y medium in a test tube. Incubate the culture at 37 °C, 5% CO2. When the optical density of the culture at 620 nm (OD620) reaches 0.4, harvest the culture by centrifugation at 1,500 × g, 4 °C for 10 min in a microcentrifuge.
- Remove the culture supernatent carefully. Resuspend the bacterial pellet in 5 ml of freezing medium (2.5 ml C+Y medium and 2.5 ml 80% [v/v] glycerol), and make 1 ml aliquots. Store the stocks in cryovials at −80 °C. These stocks contain approximately 1 × 107 cfu/ml.
- Large scale cultivation of pneumococcus
- Prepare 10 L of C+Y medium (see Recipes) and aliquot in three 4,000 ml Erlenmeyer flasks (3,300 ml per flask). Incubate the medium at 37 °C, 5% CO2 overnight to confirm absence of contamination.
-
Inoculate 1 ml/flask of pneumococcal stock (107 cfu/ml) into the medium. Incubate the culture at 37 °C, 5% CO2 without shaking.Notes:
- To estimate bacterial growth in flasks, transfer one 10 ml aliquot (using sterile serological pipet) from flask into a sterile glass tube when the culture begins to appear turbid. Read the culture turbidity at 620 nm (OD620) of this transferred culture. Keep the bacterial culture in the glass tube, and incubate it in the incubator with the flask culture. Monitor the OD620 of the glass tube culture over time.
- OD620 in flask will be approximately 0.1 higher than OD620 in glass tube.
- When the OD620 in the flask reaches 0.7–0.8, remove the flask from the incubator and immerse it into ice for 15 min. Swirl the flask every 2 min to cool culture rapidly.
- Pour the cooled culture into pre-chilled 1,000 ml centrifuge bottles, and harvest the culture by centrifugation at 4 °C, 4,000×g for 10 min in the Sorvall centrifuge.
- Decant supernatant carefully by pouring. Pellets can be stored at −80 °C until use
-
Preliminary harvest and mechanical breakage of the bacterial cells
Note: The operational definition of crude CW is material that is preciptable by boiling in SDS.-
Resuspend each pellet with 10 ml/bottle ice cold 50 mM Tris-HCl, pH 7.0 (see Recipes).Note: Perform this step as quickly as possible to avoid bacterial autolysis.
- Boil 200 ml 5% (v/v) SDS (see Recipes) in a 500 ml beaker on a hotplate. Carefully monitor temperature control to prevent boiling over the beaker’s edge.
- Combine the resuspended bacterial pellets and add the bacterial suspension into the boiling SDS slowly.
- Boil the suspension for 15 min, and cool it to room temperature.
- Aliquot the boiled suspension into 30 ml centrifuge tubes. Centrifuge the suspension at room temperature, 12,000 × g for 10 min in the Sorvall centrifuge. Discard the supernatant carefully to avoid dislodging the pellet.
- Resuspend the pellet in 20 ml/tube 1 M NaCl (see Recipes). Centrifuge the suspension at room temperature, 12,000 × g for 10 min in the Sorvall centrifuge. Discard the supernatant gently and carefully. Repeat this step for another 2 times.
- Resuspend the pellet in 20 ml/tube water. Centrifuge the suspension at room temperature, 12,000 × g for 10 min in the Sorvall centrifuge. Discard the supernatant gently and carefully. Repeat this step for another 7 times to remove detergent.
- Resuspend the pellet in 2 ml water, and add equal volume of glass beads.
- Vortex the pellet-glass beads suspension at 4 °C, maximum speed for 16–18 h.
-
- Elimination of DNA, RNA, and proteins
- Harvest the broken CWs by centrifugation at room temperature, 5,000 × g for 10 min in the Sorvall centrifuge.
- Carefully transfer the supernatant (contains CW) into a fresh 30 ml centrifuge tube.
- Add 10 ml water to the pellet and vortex vigorously. Incubate the suspension at room temperature for 20 min to separate supernatant by sedimentation. Then, remove the supernatant carefully with a sterile serological pipette. Transfer the supernatant into the centrifuge tube in step A4b. Repeat this step at least 5 times until the supernatant appears clear.
- Centrifuge the CW containing supernatant at room temperature, 27,000 × g for 15 min in the Sorvall centrifuge.
- Discard the supernatant gently and carefully resuspend the pellet in 10 ml 100 mM Tris, pH 7.5 (see Recipes).
- Add 0.2 ml of 1 M MgSO4 (see Recipes) into the suspension to make a final concentration of 20 mM.
- Add 10 µl DNase I and 50 µl RNase A into the suspension at final concentration of 10 and 50 µg/ml respectively. Incubate the suspension at 37 °C for 2 h.
- Add 0.1 ml of 1 M CaCl2 (see Recipes) into the suspension to make a final concentration of 10 mM.
- Add 0.1 ml of trypsin (10 mg/ml) to make a final concentration of 100 µg/ml and 0.3 U of α-amylase.
- Incubate the suspension at 37 °C for 12–15 h.
- Removal of non-covalent adducts and contaminating endotoxin
- Resuspend the pellet in 10 ml 8 M LiCl (see Recipes), incubate at 37 °C for 15 min.
- Centrifuge the suspension at room temperature, 27,000 × g for 15 min in the Sorvall centrifuge. Discard the supernatant.
- Resuspend the pellet with 10 ml 100 mM EDTA (see Recipes), and incubate at 37 °C, for 15 min.
- Centrifuge the suspension at room temperature, 27,000 × g for 15 min. Discard the supernatant.
- Resuspend the pellet in 10 ml water, centrifuge the suspension at room temperature, 27,000 × g for 15 min in the Sorvall centrifuge. Discard the supernatant.
- Resuspend the pellet in 10 ml acetone, centrifuge the suspension at room temperature, 27,000 × g for 15 min in the Sorvall centrifuge. Discard the supernatant very carefully because the pellet is very loose.
- Resuspend the pellet in 10 ml water, centrifuge the suspension at room temperature, 27,000 × g for 15 min in the Sorvall centrifuge. Discard the supernatant. Repeat this step for another 5 times.
- Resuspend the pellet in 2 ml water.
- Test the CW suspension for endotoxin by Pierce™ LAL Chromogenic Endotoxin Quantitation Kit according to the manufacturer’s instruction.
- Record the weight of the microcentrifuge tubes, and aliquot the CW suspension into the tubes.
- Lyophilize the CW suspension in a Speed-vac.
- Record the weight of the tube containing lyophilized CW. Subtract the weight of the empty tube to obtain mass of CW. Reconstitute the dried CW by adding 348 µl H2O per 46 mg CW material, which results in CW stock equivalent to 106 cfu/µl. Store the CW stocks at 4 °C.
-
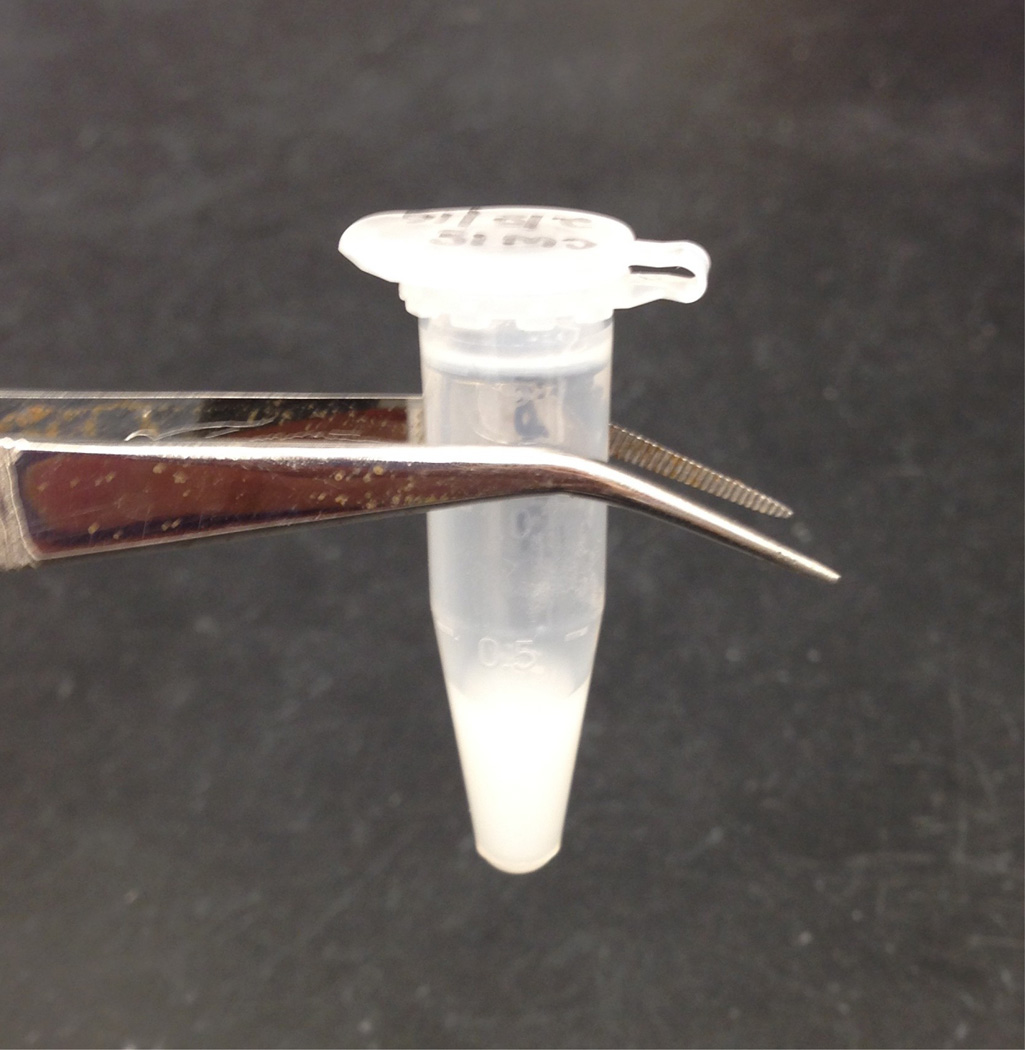
The composition of this CW material has been described (Holtje et al., 1975).Notes: The CW stock will have a ‘milky’ appearance (Figure 2).
- FITC labeling of CW
- Pipet the desired amount (100–200 µl depending on the number of mice) of CW from the CW stock into a fresh microcentrifuge tube.
- Sonicate the CW in a water-bath sonicator for 30 min.
- Resuspend the sonicated CW in 500 µl FITC solution (1 mg/ml, dissolved in sterile carbonate buffer; see Recipes). Protect the preparation from light by wrapping in aluminum foil. Incubate the suspension at room temperature for 1 h.
- Centrifuge the FITC-labeled CW at room temperature, 17,000 × g for 2 min in a microcentrifuge. Discard the supernatant, and resuspend in 1 ml DPBS. Repeat this step 2 times.
- Resuspend the FITC-labeled CW pellet in DPBS to a concentration of 1 × 106 bacterial equivalents/µl.
Figure 1. Overnight growth of unenapsulated strain R6 on TSA blood agar.
Pure culture of pneumococcus is the source of bacteria for seed stocks made in step A1b.
Figure 2. Final appearance of purified pneumococcal CW.
B. Detection of CW distribution in placenta and fetal tissues
All experiments should be performed in compliance with national and institutional guidelines (for example, National Institute of Health).
-
General description
To study CW distribution to the placenta and fetus in mice, FITC labeled CW is injected intravenously into gestating mice. Male and female mice are purchased and bred in the animal facility on-site. Healthy mice at a body weight of 15–20 g and no signs of dehydration or illness are anesthetized by isofluorane. The mice are kept under isofluorane throughout the duration of the procedure (~5–10 min). Mice recover from the 10 min procedure in ~15–20 min.
- CW injection of the mouse
- Personnel wear shoe covers, gown, hat, mask upon entering the surgical suite.
- Pregnancy is dated by ultrasound by a trained animal husbandry technician to target E10-E15 dams. Unless otherwise noted (such as knockout mice), C57Bl6 mice are used.
- In the surgical suite, the mouse is warmed on a heating pad 10–15 min before injection to dilate blood vessels. The mouse is then restrained with tail access.
- Bacterial equivalents of 2 × 107 of FITC CW (20 µl of the FITC CW preparation) is diluted into a total volume of 100 µl sterile PBS and injected into the tail vein. The injection is done using a 25 gauge needle.
- For recovery, the mice are moved to a recovery table and monitored by respiration rate, toe pinch for capillary refill time and a heating pad for maintaining body temp. They are monitored visually for at least 30 min post-operationally until they are moving freely in the cage to assure they can access water and food.
- Harvesting tissues at Embryonic Day E18–20
- Mice are euthanized and placenta and embryo heads are excised and fixed overnight at 4 °C in 4% paraformaldehyde (PFA) in a 50 ml conical tube.
- Drain the PFA and fill the tube with 30% sucrose. Tissue should initially float at the top of the tube.
- Keep tissue in sucrose at 4 °C for 3–5 days until tissue rests at or near the bottom of the tube.
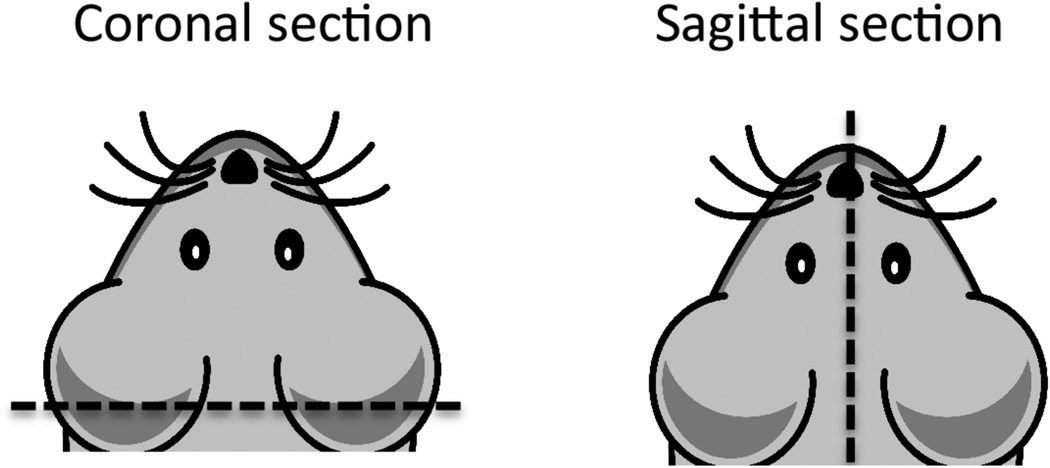
- Remove tissue one at a time to a clean 10 cm dish. Cut with a razor blade according to diagram in Figure 4 for either coronal or sagittal sectioning.
- Visualization of CW in the placenta and fetal tissues
- Place in embedding mold with cut surface on bottom of mold. Add OCT compound slowly to cover, avoiding bubbles.
- Place on dry ice to freeze for 30 min.
- Wrap each mold in aluminum foil and label. Store at −80 °C.
- Cut 20 µm sections with a cryostat microtome using superfrost slides, 3–5 serial sections per slide.
- Once dried, apply a thin layer of Prolong Gold anti-fade mounting medium with DAPI and carefully cover with a glass coverslip to avoid introducing air bubbles.
-
Allow slides to cure overnight at room temperature and analyze the following day with confocal microscope.Fluorescent images were acquired with an inverted confocal microscope (LSM510; Carl Zeiss MicroImaging, Inc.). Argon laser (excitation 488 nm) and a filter set to detect FITC emission (BP500 – 550) for imaging FITC; Chameleon laser (excitation 740 nm) and a filter set to detect DAPI emission (BP435 – 485) for imaging DAPI. Cells were observed using a 20x/0.75 Plan Neofluor objective with the confocal pinhole set to one Airy unit. Imaging parameters, such as gain and offset levels, and line averaging, are optimized to avoid oversaturation of pixels and to improve signal:noise ratio. Images were acquired using Zen 2008 software package (Carl Zeiss MicroImaging, Inc.). CW pieces per section were counted using the particle analysis tool in ImageJ (imagej.net/Particle_Analysis)
Figure 4. Cross sections of mouse brain.
For sectioning, make incision along dotted line. Coronal section is preferred to remove cerebellum and create flat surface for embedding the cortex.
Data analysis
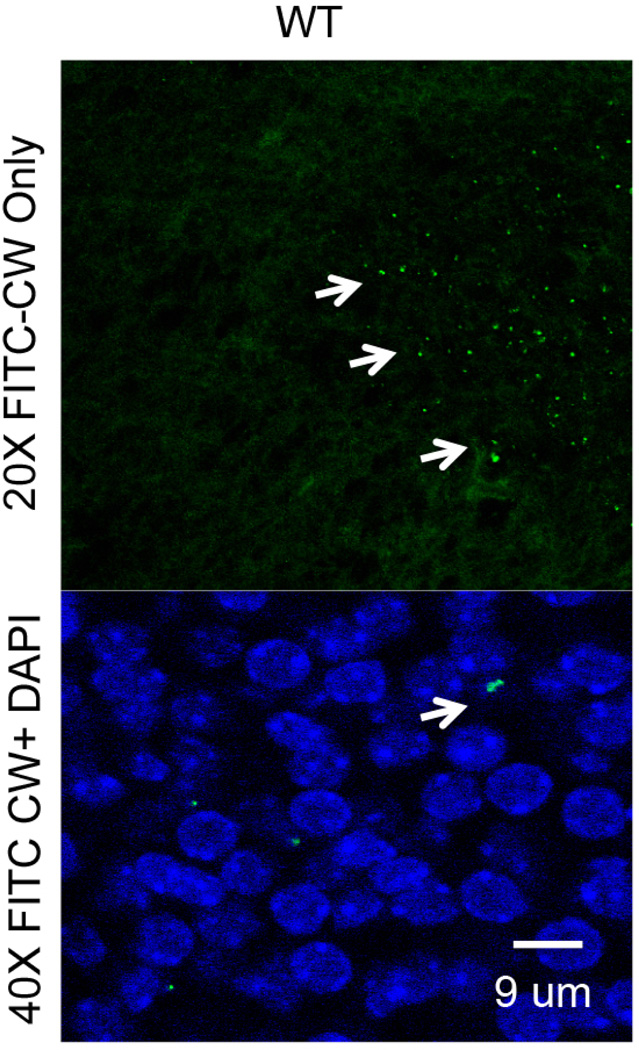
Three independent experiments are carried out for each treatment condition and the results are combined. Data are analyzed using Graphpad Prism (Graphpad) or any other statistical software with two-tailed t-test with Welch’s correction used for statistical analysis. For example, within 24 h of injection into the maternal circulation, CW crossed into the embryonic brain (Figure 5). A CW density of ~400 CW pieces/mm2 corresponds to 4 × 105 CW pieces per entire cortical plate indicating that ~2% of the inoculum into mother trafficked to the fetal brain.
Figure 5. FITC CW in the fetal brain or placenta is documented and quantified by microscopy.
Confocal images of CW distribution in placental and fetal brain tissue (arrows) are analyzed using ImageJ.
Recipes
A. Solutions used to treat CW during purification
50 mM Tris-HCl, pH 7.0 (7.88 g/L Trizma HCl)
5% (v/v) SDS (50 g/L)
1 M NaCl (58.44 g/L)
100 mM Tris, pH 7.5 (12.11 g/L Tris-base)
1 M MgSO4 (246.47 g/L)
1 M CaCl2 (147 g/L)
1% (v/v) SDS (10 g/L)
8 M LiCl (339.12 g/L)
100 mM EDTA (37.22 g/L)
50 mM carbonate buffer, pH 9.2 (5.5 ml 0.1 M Na2CO3, 34 ml 0.1 M NaHCO3, 60.5 ml Milli-Q H2O)
B. Preparation of C+Y pneumococcal growth medium components
-
‘3 in 1’ salts
50 g MgCl2·6H2O
0.25 g CaCl2 anhydrous
0.1 ml of 1 M MnSO4 solution
Dissolve in final volume of 500 ml with Milli-Q water
-
20% glucose
100 g/500 ml Milli-Q H2O (dissolve 100 g glucose in 500 ml Milli-Q H2O)
-
50% sucrose
50 g/100 ml Milli-Q H2O
-
Adenosine (2 mg/ml)
1 g/500 ml Milli-Q H2O
-
Uridine (2 mg/ml)
1 g/500 ml Milli-Q H2O
-
Glutamine (1 mg/ml)
0.5 g/500 ml Milli-Q H2O
-
Adams I
30 mg Nicotinic acid (Niacin)
35 mg (B6) pyridoxine HCL
120 mg Ca-pantothenate (D-pantothenic acid)
32 mg thiamine HCL
14 mg riboflavin
Dissolve in 200 ml final volume Milli-Q H2O
Add 0.06 ml of biotin (0.5 mg/ml stock, add 1 drop of 1 N NaOH to go into solution)
Store covered with foil
-
Adams II
50 mg FeSO4·7H2O
50 mg CuSO4·5H2O
50 mg ZnSO4·7H2O
20 mg MnCl2·4H2O
1 ml HCl
Add Milli-Q H2O up to 100 ml final volume
-
Prepare 2% pyruvate
2 g pyruvic acid
100 ml Milli-Q H2O
-
1 M KH2PO4
13.6 g/100 ml Milli-Q H2O
Autoclave at 121 °C for 15 min
-
1 M K2HPO4
174.2 g/1 L Milli-Q H2O
Autoclave at 121 °C for 15 min
-
5% yeast extract
5 g/100 ml Milli-Q H2O
Autoclave at 121 °C for 15 min
Notes:- Filter sterilize all components of C+Y and store at 4 °C.
- Shelf life for components of C+Y is 3 months.
C. Media preparation
-
Prepare PreC media
Dissolve the following in 800 ml Milli-Q water
4.83 g sodium acetate
20 g casamino acids
20 mg L-tryptophan
200 mg L-cysteine HCL
Adjust pH to 7.4–7.6 by adding 10 N NaOH (~14 drops with Pasteur pipet)
Stir well (30–60 min) at room temperature
Increase final volume to 4 L with Milli-Q water
Aliquot 400 ml into 10 × 500 ml Erlenmeyer flasks
Autoclave for 30 min at 125 °C
-
Prepare supplement
60 ml ‘3 in 1’ salts
120 ml 20% glucose
6 ml 50% sucrose
120 ml adenosine (2 mg/ml)
120 ml uridine (2 mg/ml)
Mix in a beaker and store in 200 ml portions
-
Prepare Adams III
800 mg asparagine
80 mg choline chloride
0.64 ml CaCl2 (1% solution)
64 ml Adams I
16 ml Adams II
360 ml Milli-Q H2O
Mix and store in foil covered flasks
-
Prepare 1 M potassium phosphate buffer, pH 8.0
26.5 ml of KH2PO4 (1 M)
473 ml of KH2PO4 (1 M)
-
Mix the following amounts of previously described components for C+Y
400 ml PreC
13 ml supplement
10 ml glutamine (1 mg/ml)
10 ml Adams III
5 ml 2% pyruvate
15 ml 1 M phosphate buffer
9 ml 5% yeast extract
Filter sterilize and store at 4 °C**
Warm to 37 °C before inoculating with bacteria
Note: **Shelf life for prepared C+Y is 1 month.
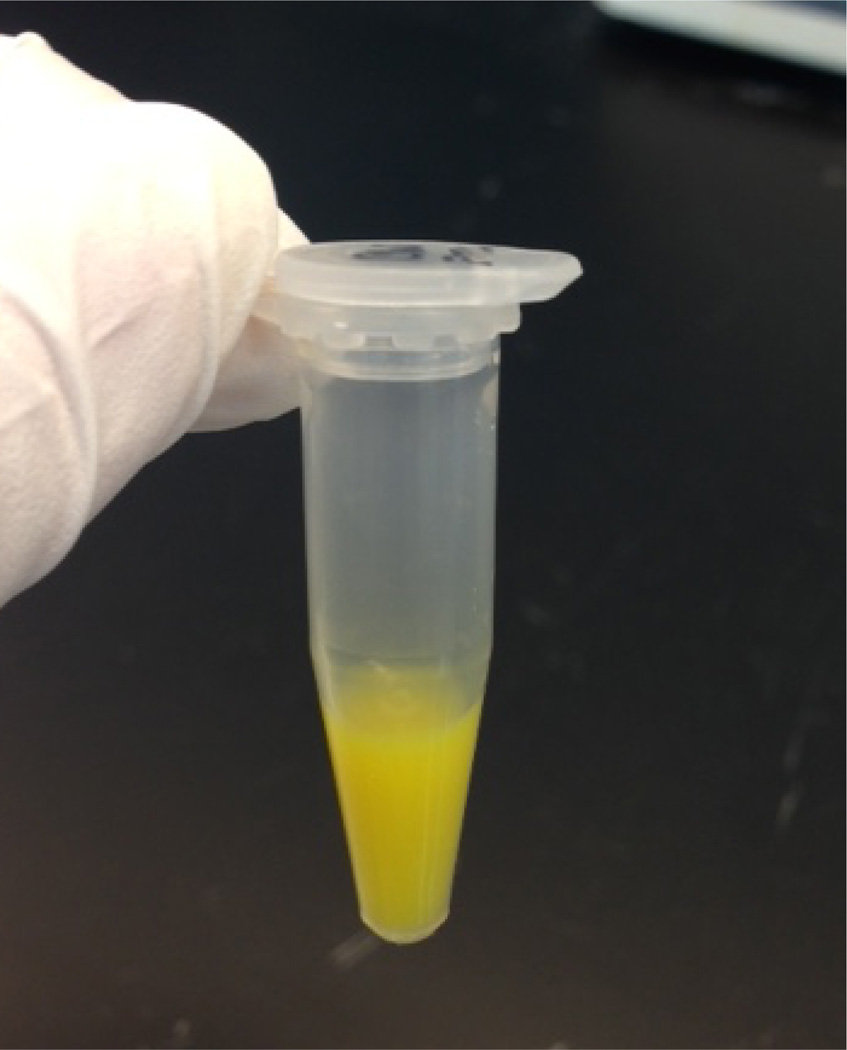
Figure 3. Final appearance of FITC labeled pneumococcal CW.
Acknowledgments
This work was supported by NIAID 27913 and ALSAC.
References
- 1.Braun JS, Novak R, Herzoq KH, Bodmer SM, Cleveland JL, Tuomanen EI. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat Med. 1999;5:298–302. doi: 10.1038/6514. [DOI] [PubMed] [Google Scholar]
- 2.Fillon S, Soulis K, Rajasekaran S, Benedict-Hamilton H, Radin JN, Orihuela CJ, Kasmi KC, Murti G, Kaushal D, Gaber MW, Weber JR, Murray PJ, Tuomanen EI. Platelet-activating factor receptor and innate immunity: uptake of gram-positive bacterial cell wall into host cells and cell-specific pathophysiology. J Immunol. 2006;177:6182–6191. doi: 10.4049/jimmunol.177.9.6182. [DOI] [PubMed] [Google Scholar]
- 3.Holtje JV, Tomasz A. Specific recognition of choline residues in the cell wall teichoic acid by the N-acetylmuramyl-L-alanine amidase of Pneumococcus. J Biol Chem. 1975;250(15):6072–6076. [PubMed] [Google Scholar]
- 4.Humann J, Mann B, Gao G, Moresco P, Ramahi J, Loh LN, Farr A, Hu Y, Durick Eder K, Fillon SA, Smeyne RJ, Tuomanen EI. Bacterial peptidoglycan traverses the placenta to induce fetal neuroproliferation and aberrant postnatal behavior. Cell Host Microbe. 2016;19(6):901. doi: 10.1016/j.chom.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Orihuela CJ, Fillon S, Smithsielicki SH, Kasmi KCE, Gao G, Soulis K, Patil A, Murray JP, Tuomanen IE. Cell wall-mediated neuronal damage in early sepsis. Infect Immun. 2006;74:3783–3789. doi: 10.1128/IAI.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985a;151(5):859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 7.Tuomanen E, Tomasz A, Hengstler B, Zak O. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J Infect Dis. 1985b;151(3):535–540. doi: 10.1093/infdis/151.3.535. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163(1):1–5. [PubMed] [Google Scholar]