Abstract
Background
Patients having mutations of Pax6 bear phenotypes that match age-associated neurological disorders. Mutations affect most cellular functions such as cell division, growth, differentiation, and cell death in brain, eyes, pituitary, pineal, and pancreas. The progressive reduction in the level of Pax6 during aging has also been observed. However, information about downstream targets of Pax6 in brain is unclear. Therefore, it is presumed that age-dependent alterations of Pax6 may also affect cascades of promoter sequence recognition in brain during aging.
Purpose
This study is aimed at studying the interaction of Pax6 with DNA sequence elements to explore alteration in gene targets and transcription networks of Pax6 in brain during aging.
Methods
Chromatin immunoprecipitation with anti-Pax6 using tissue extracts of brain from newborn, young, adult, and old mice was done. Pulled DNA from brain was analysed by gene-specific polymerase chain reaction (PCR). Amplified PCR products were sequenced and analyzed.
Results
Age-associated alterations in binding to genetic sequence elements by Pax6 were observed. Promoter analysis predicts genes involved in neuronal survival (Bdnf, Sparc), specificity of astrocyte (S100β, Gfap), cell-proliferation (Pcna), inflammation and immune response (interferon-γ, tumour necrosis factor-α), management of oxidative stress (Sod, Cat), and hypoxia (Ldh).
Conclusion
The Pax6 either directly or indirectly binds to promoter sequences of genes essential for immunological surveillance and energy metabolism in brain that alters during aging.
Keywords: Pax6, Chromatin immunoprecipitation, Brain, Brain aging
Introduction
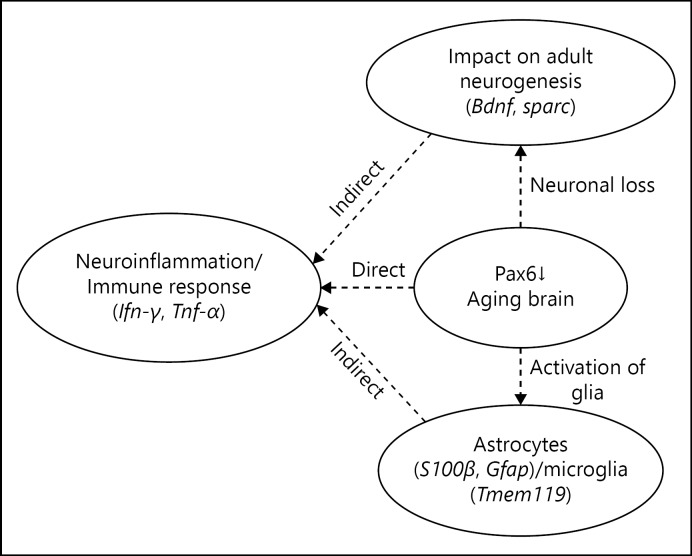
The brain is considered as an immune-privileged organ; however, the presence of lymphatic vessels in the meningeal compartment of the brain supports the involvement of peripheral lymphocytes in mediating brain immunity [1]. Mostly microglia in brain maintain homeostasis by secreting neurotrophic factors, but in case of infection or inflammation, they secrete pro-inflammatory cytokines (interferon [Ifn]-γ, tumour necrosis factor [Tnf]-α), ROS, or NO that induce neuronal damage, infiltration of lymphocytes, and secretion of anti-inflammatory cytokines transforming growth factor (TGF)-β and interleukin-10 [2]. During aging, several genetic switches alter, including paired box 6 genes or protein, Pax6. The Pax6 has been considered a critical regulator because its mutations lead to cognitive impairments, cerebellar ataxia, absence of olfactory system and endocrine pancreas, anophthalmia and nasal hypoplasia [3], pineal hypoplasia [4], congenital hypopituitarism [5], nystagmus, impaired auditory processing and verbal working memory, autism, and mental retardation [6, 7, 8, 9]. The reduced size of the corpus callosum and anterior commissure, abnormalities of the cerebral cortex and cerebellum, and absence of the pineal gland [7, 10, 11, 12, 13] have also been observed due to the mutation in Pax6. The age-associated neuronal loss, glial activation [14, 15], alterations in astrocytes and microglia [16], infiltration of T-cells, reduced neurogenesis [17], and Pax6-positive cells in brain [18] have also been reported. The Pax6-dependent regulation of astrocyte-such as glial development [19] and functions of Pax6 as a dual transcriptional activator and repressor [20] have also been described recently. Since the Pax6 has been found co-localised and interacting with p53, TGF-β, Smad, Sparc, and Bdnf in brain, it is presumed that Pax6 may interact with genes of microglia (Tmem119), astrocytes (S100β, Gfap), pro-inflammatory cytokines (Ifn-γ, Tnf-α), and anti-inflammatory cytokines (Tgf-β) to regulate cascades of genes involved in brain-specific immunity either directly or indirectly (Fig. 1). Therefore, it has been intended to explore interactions of Pax6 with sequence elements in the brain of aging mice.
Fig. 1.
Reduced level of Pax6, neural loss, glial activation, neuroinflamation or activation of glia, and neuronal loss in brain during aging may directly or indirectly affect neuroinflammation and immune response.
Methods
Maintenance of Animals
Albino mice of AKR strain were used for the experiments. Mice were maintained at 25 ± 2°C. They were fed upon standard mice feed in pellet form. Tap water was supplied. The life span of both male and female mice is 75 ± 5 weeks. Mice were maintained as per the approved guidelines of Institutional Animal Ethical Committee. The male mice were used for experiments. Their age was postnatal (P0), day 0; young (Y), 4 ± 2 weeks; adult (A), 15 ± 4 weeks; and old (O), 70 ± 5 weeks. The experiment was carried out 4 times (n = 4) using the brain of one mice in each age group per experiment.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) was done using the basic method [21], with some modifications. The lysates of brain (neonate, young, adult, old) was prepared in 30 mM Tris-Cl (pH 7.4), containing 1 mM EDTA, 250 mM sucrose, and 50 mM mannitol. Cross linking and chromatin preparation from lysate was done by 1% formaldehyde. To stop the cross-linking reaction, 125 mM glycine was used. Nuclear extract was collected by centrifugation at 10,000 g for 10 min. Nuclear lysis was performed in ChIP lysis buffer containing 0.1% sodium deoxycholate, 1% triton x-100, 1 mM EDTA (pH 8.0), 140 mM NaCl, 50 mM HEPES-KOH (pH 7.5) followed by sonication. The supernatant after sonication containing chromatin was incubated for 4 h at 4°C for immunoprecipitation with anti-Pax6 antibody (sc-11357, Santa-Cruz Biotech, USA). In negative control, anti-human IgG (HPO-1, Genei) was used. After centrifugation at 10,000 g, reverse linking was performed by adding 120 mM NaCl and incubation at 65°C for 1 h. DNA obtained through immunoprecipitation was purified through phenol: chloroform purification method. The pulled DNA was checked on 1% agarose gel. The input DNA was obtained by reverse linking the chromatin prepared without antibody incubation as described above.
Polymerase Chain Reaction Using Pulled DNA
The pattern of genes in pulled DNA was analysed using gene-specific primers for Pcna, S100β, Gfap, Bdnf, Sparc, Sod, Cat, Ldh, Ifn-γ, Tnf-α, Tmem119 (Table 1). The polymerase chain reaction (PCR)-amplified products were evaluated on 1% agarose gel.
Table 1.
List primer pairs for identification of genes after interaction of ChIP with anti-Pax6
| Forward primer | (5′–3′) | Reverse primer | (5′–3′) |
|---|---|---|---|
| Pax6F | GCATGCAGAACAGTCACAGCGGAG | Pax6R | CTGTTGCTTTTCGCTAGCCAGGTT |
| Tmem119F | TCACCCAGAGCTGGTTCCATA | Tmem119R | GAGGTGTCCAGGCCTTCTTC |
| IFN-γF | AGGAAGCGGAAAAGGAGTCG | IFN-γR | GGGTCACTGCAGCTCTGAAT |
| S100βF | GAGGAGCACAGCCACACTTA | S100βR | CATTCCCCTCCTCTGTCCT |
| GfapF | ACATCGAGATCGCCACCTAC | GfapR | TCACATCACCACGTCCTTGT |
| PcnaF | GCACGTATATGCCGAGACCT | PcnaR | CAGTGGAGTGGCTTTTGTGA |
| SparcF | CAAAGTGTCCACACCACCAC | SparcR | CATACGGCACAACCACACTC |
| TNF-αF | ACGTGGAACTGGCAGAAGAG | TNF-αR | GGGGCTCTGAGGAGTAGACA |
| LdhF | CGGCTCAACCTGGT | LdhR | TAGGCACTGTCCACCAC |
| SodF | TGGGGACAATACACAAGGCTGT | SodR | TTTCCACCTTTGCCCAAGTCA |
| CatF | CCTCCTCGTTCAGGATGTGGTT | CatR | CGAGGGTCACGAA CTGTGTCAG |
| BdnfF | CCGAGGTTCGGCTCACACCG | BdnfR | GCCCCTGCAGCCTTCCTTGG |
Sequencing and Annotation of Sequencing
Sequences were analysed using Blat (http://genome.ucsc.edu/cgi-bin/hgBlat) and annotation using neural network promoter prediction (http://www.fruitfly.org/seq_tools/promoter.html). Predicted promoter sequences were analysed using Blastn (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and predicted genes using uniprot (http://www.uniprot.org/) and GeneCards (http://www.genecards.org/).
Results
Pax6 Shows Differential Binding to Promoter Sequence Elements in Brain
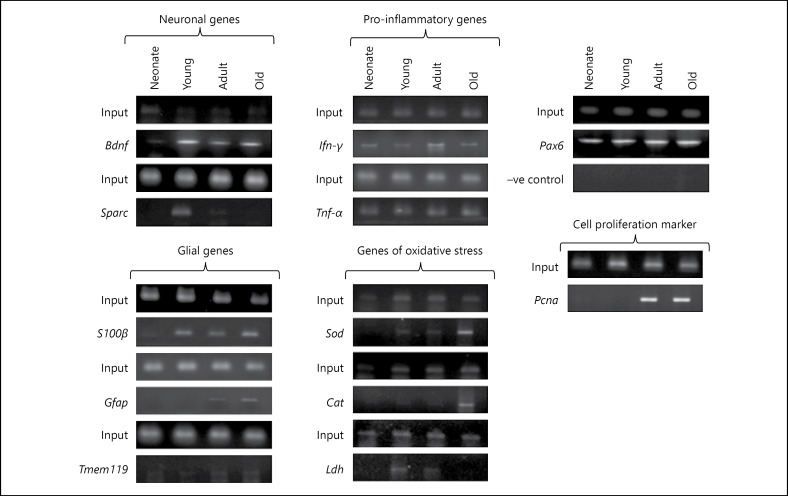
The results suggest age-dependent alterations in binding of Pax6 with genetic sequence elements of Bdnf, Sparc, Pcna, Ifn-γ, Tnf-α,Sod, Cat, Ldh, Tmem119, S100β, and Gfap (Fig. 2). The interaction of Pax6 with genetic sequence elements of Sod was found from young to old brain, whereas Cat was observed in old brain and Ldh in young and adult brain. The interaction of Pax6 with sequences of Bdnf was observed in the brain of young to old age group mice, while Sparc was observed in young and adult brain, which seems to be strongly associated with neuronal health. The Pax6 was observed interacting with sequences S100β from young to old age whereas Gfap was recognised only in adult and old brain. The S100β is known to play a neuroprotective role in nanomolar concentration and leads to neurodegeneration when found in micromolar concentration. The GFAP being an astrocyte activation marker indicates Pax6-mediated activation of glial cells during aging. The interaction of Pax6 with sequences of Ifn-γ and Tnf-α from neonate to old, and microglia-specific gene Tmem119 was observed from young to old indicating association of Pax6 with brain-specific immunity and immunological surveillance in brain.
Fig. 2.
It shows the status of marker genes amplified from DNA obtained through the ChIP with anti-Pax6.
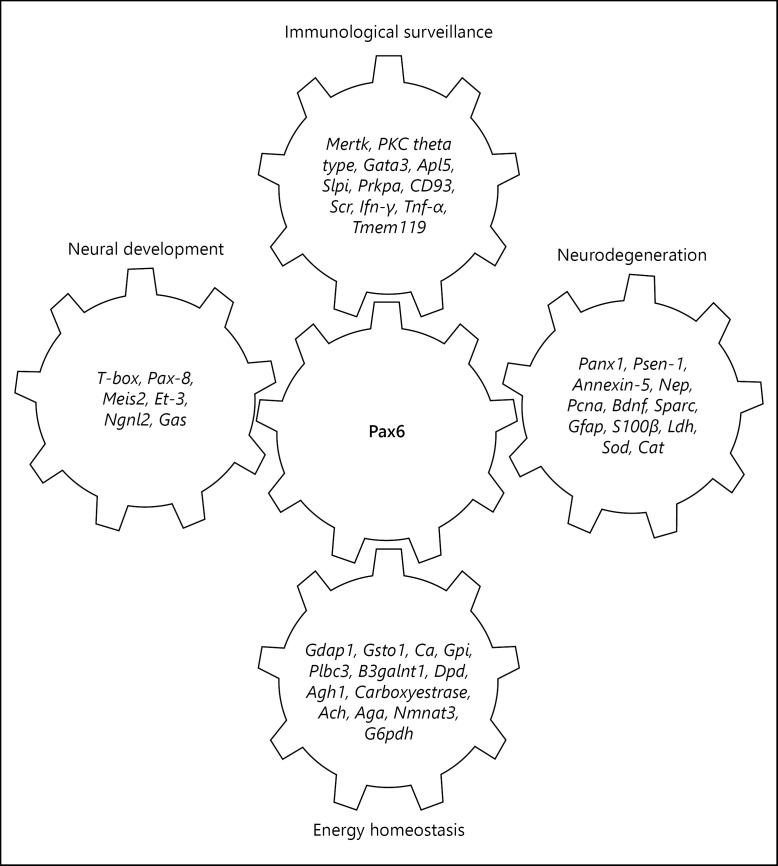
Promoter Sequence Analysis in silico Indicates Genes Associated with Energy Homeostasis and Neurodegeneration
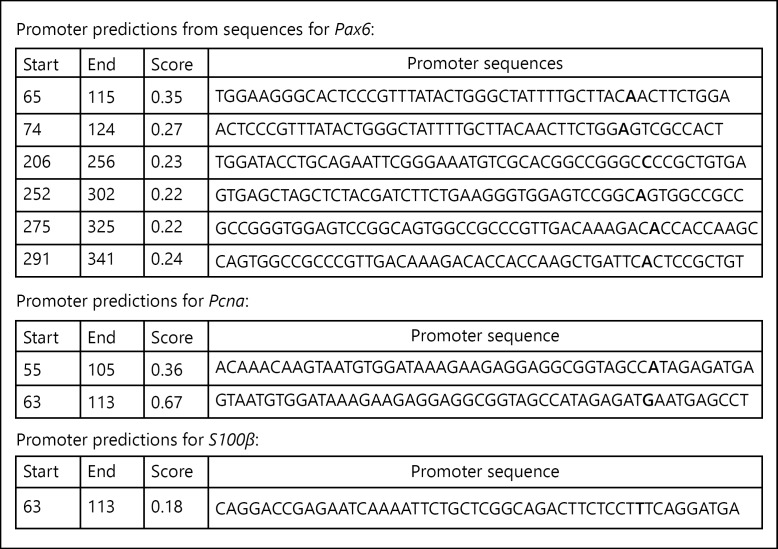
Sequencing of PCR products of Pax6 (KT3142180), Pcna (KT 314220), and S100β (KT 314222) and analysis indicates the presence of promoters (Fig. 3) in each sequence. Genomic BLAST suggests its presence on 5′-upstream and 3′-downstream of various genes of diverse biological functions including energy homeostasis, metabolic activity, and immunity (Fig. 4). These promoters are found to be present on the 5′-upstream of tyrosine protein kinase Mer which transduces signals from the extracellular matrix into the cytoplasm. It has been associated with macrophage clearance of apoptotic cells, inhibition of TLRs-mediated innate immune response by activating STAT1, which selectively induces the production of suppressors of cytokine signalling SOCS1 and SOCS3. The protein kinase C theta type mediates non-redundant functions in TCR signalling, including T-cell activation, proliferation, survival by mediating activation of multiple transcription factors such as NF-κB, Jun, and NFATC1. The retinoic acid receptor and retinoid X receptor alpha downregulates TGF-β1 promoter and plays a major role in axon regeneration, during aging, cellular response to insulin, and inflammatory responses. The oxidative stress responsive serine rich protein maintains homeostasis by regulating the immune system, cellular response to hydrogen peroxide, and protein metabolism. The GATA3, transactivation enhances T cell receptor alpha and delta genes. It is required for Th2 differentiation process following immune and inflammatory responses. The complement component, C1q receptor (CD93), mediates the enhancement of phagocytosis in monocytes and macrophages. The neuronal proto-oncogene tyrosine protein kinase (Src) responds to ROS, TGF-β stimulus, and regulates innate immunity. The anti-leukoproteinase (Slpi) modulates the innate immune response after bacterial infection. It regulates the activation of NF-κB and inflammatory response.
Fig. 3.
It shows the promoter sequence analysis of genes amplified from DNA obtained through ChIP with anti-Pax6.
Fig. 4.
Age-dependent alterations in binding of Pax6 to the promoter sequence elements of genes involved in immunological surveillance, energy homeostasis, neural development, and neurodegeneration in brain.
These promoters are also found on Presenilin-1 (Psen1) that regulates amyloid precursor protein (APP), processing through their effects on gamma-secretase, an enzyme that cleaves APP, Annexin A5, favourable markers of AD, restricted to glial cells, Pannexin-1, activates innate immune cells of choroid plexus and involves in cell-cell signalling, synaptic plasticity, neprilysin, abeta degrading endopeptidase clears brain amyloid protein. These promoter sequences also match to upstream of genes, which regulate metabolic activities and maintain energy homeostasis such as glutathione S-transferase omega-1, carbonic anhydrase, glucose-6-phosphate isomerase, phospholipase C β3, beta-1,3-N-acetylgalactosaminyltransferase 1, dihydropyrimidine dehydrogenase, alcohol dehydrogenase 1, carboxylestrase, acylcarnitine hydrolase, Aspartylglucosaminidase, nicotinamide nucleotide adenylyltransferase 3, glucose-6-phosphate dehydrogenase (Fig. 4).
Conclusion
ChIP with anti-Pax6 antibody indicates age-dependent alterations in binding of Pax6 to the regulatory sequence elements of genes involved in immunological surveillance and energy homeostasis in brain during aging. Interaction of Pax6 with the regulatory element of Psen1 also regulates the cleavage of APP and management of Alzheimer's disease. Thus, Pax6 appears as a grid to regulate cascades of genes associated with energy homeostasis, neurodegeneration, and immunological surveillance in brain.
Authors Contribution
R.M. planned experiments, mentored the progress of experiments, analysed results, and revised draft of the manuscript. S.K.M. worked on planned experiments, compiled and analysed data, and prepared draft of the manuscript.
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgement
Partial financial support from CSIR (37(1521)/12/EMR-II dated 03-04-2012) and ICMR (54/2/CFP/GER/2011-NCD-II dated 04-10-2012) are gratefully acknowledged. This article complies with the International Committee of Medical Journal editor's uniform requirements for manuscript.
References
- 1.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.London A, Cohen M, Schwartz M. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front Cell Neurosci. 2013;7:34. doi: 10.3389/fncel.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- 4.Hanish AE, Butman JA, Thomas F, Yao J, Han JC. Pineal hypoplasia, reduced melatonin and sleep disturbance in patients with PAX6 haploinsufficiency. J Sleep Res. 2016;25:16–22. doi: 10.1111/jsr.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takagi M, Nagasaki K, Fujiwara I, Ishii T, Amano N, Asakura Y. Heterozygous defects in PAX6 gene and congenital hypopituitarism. Eur J Endocrinol. 2015;172:37–45. doi: 10.1530/EJE-14-0255. [DOI] [PubMed] [Google Scholar]
- 6.Malandrini A, Mari F, Palmeri S, Gambelli S, Berti G, Bruttini M. PAX6 mutation in a family with aniridia, congenital ptosis, and mental retardation. Clin Genet. 2001;60:151–154. doi: 10.1034/j.1399-0004.2001.600210.x. [DOI] [PubMed] [Google Scholar]
- 7.Bamiou DE, Musiek FE, Sisodiya SM, Free SL, Davies RA, Moore A, van Heyningen V, Luxon LM. Deficient auditory interhemispheric transfer in patients with PAX6 mutations. Ann Neurol. 2004;56:503–509. doi: 10.1002/ana.20227. [DOI] [PubMed] [Google Scholar]
- 8.Hingorani M, Williamson KA, Moore AT, van Heyningen V. Detailed ophthalmologic evaluation of 43 individuals with PAX6 mutations. Invest Ophthalmol Vis Sci. 2009;50:2581–2590. doi: 10.1167/iovs.08-2827. [DOI] [PubMed] [Google Scholar]
- 9.Maekawa M, Iwayama Y, Nakamura K, Sato M, Toyota T, Ohnishi T, Yamada K, Miyachi T, Tsujii M, Hattori E, Maekawa N, Osumi N, Mori N, Yoshikawa T. A novel missense mutation (Leu46Val) of PAX6 found in an autistic patient. Neurosci Lett. 2009;462:267–271. doi: 10.1016/j.neulet.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Sisodiya SM, Free SL, Williamson KA, Mitchell TN, Willis C, Stevens JM, Kendall BE, Shorvon SD, Hanson IM, Moore AT, van Heyningen V. PAX6 haploinsufficiency causes cerebral malformation and olfactory dysfunction in humans. Nat Genet. 2001;28:214–216. doi: 10.1038/90042. [DOI] [PubMed] [Google Scholar]
- 11.Free SL, Mitchell TN, Williamson KA, Churchill AJ, Shorvon SD, Moore AT, van Heyningen V, Sisodiya SM. Quantitative MR image analysis in subjects with defects in the PAX6 gene. Neuroimage. 2003;20:2281–2290. doi: 10.1016/j.neuroimage.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell TN, Free SL, Williamson KA, Stevens JM, Churchill AJ, Hanson IM, Shorvon SD, Moore AT, van Heyningen V, Sisodiya SM. Polymicrogyria and absence of pineal gland due to PAX6 mutation. Ann Neurol. 2003;53:658–663. doi: 10.1002/ana.10576. [DOI] [PubMed] [Google Scholar]
- 13.Ellison-Wright Z, Heyman I, Frampton I, Rubia K, Chitnis X, Ellison-Wright I, Williams SC, Suckling J, Simmons A, Bullmore E. Heterozygous PAX6 mutation, adult brain structure and fronto-striato-thalamic function in a human family. Eur J Neurosci. 2004;19:1505–1512. doi: 10.1111/j.1460-9568.2004.03236.x. [DOI] [PubMed] [Google Scholar]
- 14.West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- 15.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–429. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 16.Jyothi HJ, Vidyadhara DJ, Mahadevan A, Philip M, Parmar SK, Manohari SG, Shankar SK, Raju TR, Alladi PA. Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta. Neurobiol Aging. 2015;36:3321–3333. doi: 10.1016/j.neurobiolaging.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Huang Z, Ha G, Petitto J. Reversal of neuronal atrophy: role of cellular immunity in neuroplasticity and aging. J Neurol Disord. 2014;2 doi: 10.4172/2329-6895.1000170. pii:1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tripathi R, Mishra R. Aging-associated modulation in the expression of Pax6 in mouse brain. Cell Mol Neurobiol. 2012;32:209–218. doi: 10.1007/s10571-011-9749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki T, Takayama R, Sato M. Eyeless/Pax6 controls the production of glial cells in the visual center of Drosophila melanogaster. Dev Biol. 2016;409:343–353. doi: 10.1016/j.ydbio.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Cvekl A, Callaerts P. PAX6: 25th anniversary and more to learn. Exp Eye Res. 2016 doi: 10.1016/j.exer.2016.04.017. pii:S00144835(16)30090-2. [DOI] [PubMed] [Google Scholar]
- 21.Huang HS, Matevossian A, Jiang Y, Akbarian S. Chromatin immunoprecipitation in postmortem brain. J Neurosci Methods. 2006;156:284–292. doi: 10.1016/j.jneumeth.2006.02.018. [DOI] [PubMed] [Google Scholar]