Abstract
Brugada phenocopy (BrP) refers to a group of clinical conditions that have etiologies distinct from Brugada syndrome (BrS). Although both demonstrate features of ST-segment elevation in the right precordial leads on the electrocardiogram (ECG), one must be distinguished from the other as their treatment options are different. We report a male patient who presented with recurrent syncope with a Brugada and a S1Q3T3 pattern on the ECG. Acute pulmonary embolism (APE) complicated by BrS was suspected. Twenty-four hours Holter monitoring did not demonstrate any evidence of ventricular arrhythmias. Computed tomography pulmonary angiogram confirmed the presence of an APE. He was treated with low molecular weight heparin and a repeat ECG taken the next day showed resolution of the Brugada and S1Q3T3 patterns. This case report illustrates that APE and BrS can present with similar clinical and electrocardiographic features of recurrent syncope and Brugada pattern, respectively.
INTRODUCTION
The term Brugada phenocopy (BrP) has been coined to describe a group of heterogeneous conditions that induce Brugada electrocardiogram (ECG) patterns under the following circumstances of metabolism conditions, mechanical compression, myocardial ischemia, pulmonary embolism, myocardial and pericardial disease, ECG modulations and miscellaneous conditions [1–3]. These conditions must be distinguished from true Brugada syndrome (BrS) as these are potential reversible causes. This case report illustrates that a patient can present with recurrent syncope associated with Brugada (ST-segment elevation in V1–V3) and S1Q3T3 patterns on the ECG. Co-existing BrS and acute pulmonary embolism (APE) were suspected. However, following prompt treatment of his PE, the patient recovered and the ECG Brugada and S1Q3T3 patterns disappeared.
CASE REPORT
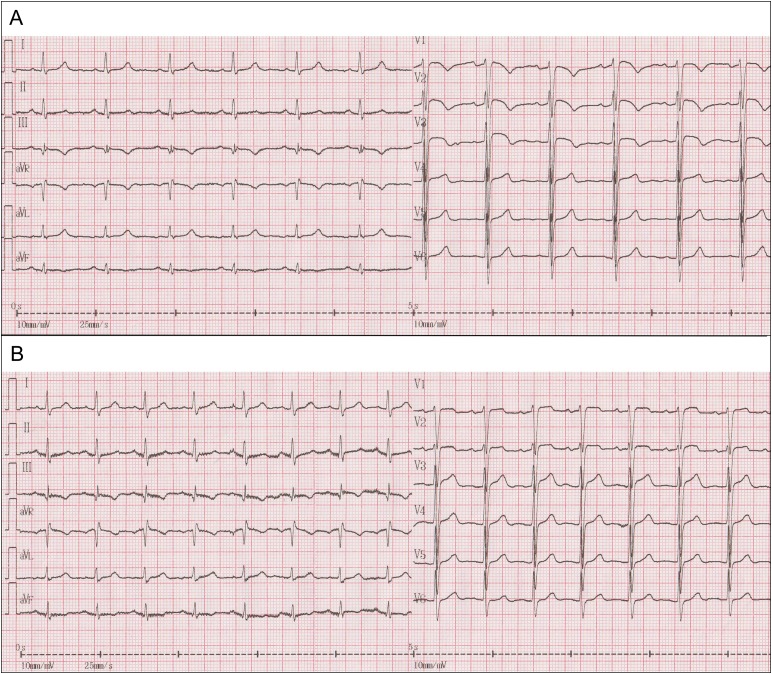
A 78-year-old male with a history of hypertension and varicose veins in lower limbs was admitted to the Department of Cardiology, Second Hospital of Tianjin Medical University due to episodes of recurrent syncope. He reported four episodes of syncope per day but denied chest pain, dyspnea, cough or hemoptysis. On arrival, his blood pressure was 130/90 mmHg and had a temperature of 36.7°C, Cardiac auscultation revealed a normal S1, loud and split S2 in the absence of murmurs. A 12-lead electrocardiogram revealed sinus rhythm, a S1Q3T3 pattern, ‘coved’ ST-segment elevation (STE) with T-wave inversion in the right precordial leads V1–V3 (Fig. 1A, trace). These findings were consistent of a Brugada pattern (Fig. 1A). Blood tests revealed raised levels of troponin (0.094 ng/ml, normal range: 0–0.02 ng/ml), brain natriuretic peptide (2286.0 pg/ml, normal range: 0–100 pg/ml) and D-dimer (10.0 mg/l, normal range: 0–0.5 mg/l). Blood gas revealed pO2 of 64 mmHg, pCO2 of 28.7 mmHg and pH of 7.47. Echocardiography revealed thickened left ventricular septum to 11.9 mm, enlarged right heart (anteroposterior diameter and supra-inferior diameter of right atrium: 49.3 and 54.6 mm; right ventricular diastolic diameter: 29.8 mm) that were accompanied by mild tricuspid regurgitation and increased pulmonary artery diameter of 27.3 mm. Systolic pulmonary artery pressure at rest by tricuspid regurgitation pressure gradient was estimated to be 51 mmHg (Supplementary Video 1). The mitral E/A ratio and left ventricular ejection fraction took values of as 0.6 and 56%, respectively. Twenty-four hours Holter monitoring did not demonstrate any evidence of ventricular arrhythmias. APE was highly suspected and this was confirmed by computed tomographic pulmonary angiogram (Fig. 2). Oxygen and low molecular weight heparin were promptly initiated. ECG performed the next day showed ongoing ST elevation in leads V1 and V2 but resolution of the coved ST segment and T-wave inversion, and therefore disappearance of the Type 1 pattern (Fig. 1B, trace). Eleven days after his admission, he recovered fully and was discharged home with warfarin.
Figure 1:
(A) A 12-lead ECG of a patient with APE showing sinus rhythm, a S1Q3T3 pattern, ‘coved’ STE with T-wave inversion in the right precordial leads V1–V3. (B) ECG trace showing there was ongoing ST elevation in leads V1 and V2; however, the coved ST segment and T-wave inversion were resolved and looked less like typical Type 1 Brugada pattern following treatment of the embolism
Figure 2:
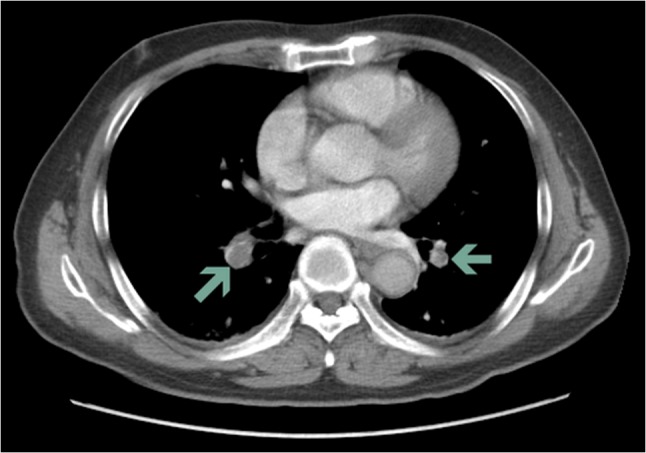
Computed tomographic pulmonary angiogram showing clots in the pulmonary artery
DISCUSSION
BrS is a cardiac ion channelopathy characterized on ECG by coved STE (≥2 mm) in the right precordial leads, which predisposes affected individuals to syncope, ventricular arrhythmias and sudden cardiac death [4–8]. BrPs are clinical entities characterized by ECG patterns similar or identical to those of BrS, which are caused by underlying disorders such as metabolic disturbances, mechanical compression, ischemia, myocardial and pericardial diseases [9–14]. BrP can be observed in patients with sub-massive to massive APE, with the latter being accompanied by hypotension and cardiogenic shock. In contrast to findings from previous case reports [15–19], our patient presented with recurrent syncope without complaints of chest pain, dyspnea or hypotension. To the best of our knowledge, this is the first case report regarding BrP in a case with APE with recurrent syncope. In BrS, ST segment changes can be intermittent. Therefore, it was possible, although less likely, that the patient suffered from a PE that precipitated a Type 1 Brugada pattern on the ECG. Moreover, BrP needs to be distinguished from BrS to avoid missed or incorrect diagnosis, which often leads to unnecessary medical tests and treatment.
Supplementary Material
SUPPLEMENTARY MATERIAL
Supplementary material is available at Oxford Medical Case Reports online.
CONFLICTS OF INTEREST STATEMENT
None declared.
FUNDING
G.T. is supported by the Croucher Foundation. The Foundation played no role in the preparation or content of this manuscript.
REFERENCES
- 1. Dendramis G. Brugada syndrome and Brugada phenocopy. The importance of a differential diagnosis. Int J Cardiol 2016;210:25–7. [DOI] [PubMed] [Google Scholar]
- 2. Gottschalk BH, Garcia-Niebla J, Anselm DD, Jaidka A, De Luna AB, Baranchuk A. New methodologies for measuring Brugada ECG patterns cannot differentiate the ECG pattern of Brugada syndrome from Brugada phenocopy. J Electrocardiol 2016;49:187–91. [DOI] [PubMed] [Google Scholar]
- 3. Enriquez A, Brugada J, Baranchuk A. Exercise-induced Brugada phenocopy. J Cardiovasc Electrophysiol 2016;27:360–1. [DOI] [PubMed] [Google Scholar]
- 4. Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005;111:659–70. [DOI] [PubMed] [Google Scholar]
- 5. Antzelevitch C, Yan GX. J-wave syndromes: Brugada and early repolarization syndromes. Heart Rhythm 2015;12:1852–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation 1999;100:1660–6. [DOI] [PubMed] [Google Scholar]
- 7. Letsas KP, Liu T, Shao Q, Korantzopoulos P, Giannopoulos G, Vlachos K, et al. Meta-analysis on risk stratification of asymptomatic individuals with the Brugada phenotype. Am J Cardiol 2015;116:98–103. [DOI] [PubMed] [Google Scholar]
- 8. Tse G, Liu T, Li KH, Laxton V, Chan YW, Keung W, et al. Electrophysiological mechanisms of Brugada syndrome: insights from pre-clinical and clinical studies. Front Physiol 2016;7:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Awad SF, Barbosa-Barros R, Belem Lde S, Cavalcante CP, Riera AR, Garcia-Niebla J, et al. Brugada phenocopy in a patient with pectus excavatum: systematic review of the ECG manifestations associated with pectus excavatum. Ann Noninvasive Electrocardiol 2013;18:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baranchuk A, Nguyen T, Ryu MH, Femenia F, Zareba W, Wilde AA, et al. Brugada phenocopy: new terminology and proposed classification. Ann Noninvasive Electrocardiol 2012;17:299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Genaro NR, Anselm DD, Cervino N, Estevez AO, Perona C, Villamil AM, et al. Brugada phenocopy clinical reproducibility demonstrated by recurrent hypokalemia. Ann Noninvasive Electrocardiol 2014;19:387–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gottschalk B, Anselm DD, Baranchuk A. Brugada phenocopy: morphological classification and importance of provocative testing. Ann Noninvasive Electrocardiol 2014;19:604–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rambod M, Elhanafi S, Mukherjee D. Response to: Brugada phenocopy: morphological classification and importance of provocative testing. Ann Noninvasive Electrocardiol 2014;19:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rambod M, Elhanafi S, Mukherjee D. Brugada phenocopy in concomitant ethanol and heroin overdose. Ann Noninvasive Electrocardiol 2015;20:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aksu U, Kalkan K, Gulcu O, Topcu S, Tanboga IH. Massive pulmonary embolism mimicking electrocardiographic pattern of Brugada syndrome. Am J Emerg Med 2015;34:933.e1–2. [DOI] [PubMed] [Google Scholar]
- 16. Anselm DD, Baranchuk A. Brugada Phenocopy in the context of pulmonary embolism. Int J Cardiol 2013;168:560. [DOI] [PubMed] [Google Scholar]
- 17. Cheng TO. Brugada syndrome vs. pulmonary embolism vs. paradoxical embolism--what are we to believe. Int J Cardiol 2004;94:119. [DOI] [PubMed] [Google Scholar]
- 18. Wynne J, Littmann L. Brugada electrocardiogram associated with pulmonary embolism. Int J Cardiol 2013;162:e32–3. [DOI] [PubMed] [Google Scholar]
- 19. Zhan ZQ, Wang CQ, Nikus KC, Perez-Riera AR, Baranchuk A. Brugada phenocopy in acute pulmonary embolism. Int J Cardiol 2014;177:e153–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.