Abstract
Rationale: Patients who progress to brain death after resuscitation from cardiac arrest have been hypothesized to represent an underused source of potential organ donors; however, there is a paucity of data regarding the viability of lung allografts after a period of cardiac arrest in the donor.
Objectives: To analyze postoperative complications and survival after lung transplant from brain-dead donors resuscitated after cardiac arrest.
Methods: The United Network for Organ Sharing database records donors with cardiac arrest occurring after brain death. Adult recipients of lung allografts from these arrest/resuscitation donors between 2005 and 2011 were compared with nonarrest donors. Propensity score matching was used to reduce the effect of confounding. Postoperative complications and overall survival were assessed using McNemar’s test for correlated binary proportions and Kaplan–Meier methods.
Measurements and Main Results: A total of 479 lung transplant recipients from arrest/resuscitation donors were 1:1 propensity matched from a cohort of 9,076 control subjects. Baseline characteristics in the 1:1-matched cohort were balanced. There was no significant difference in perioperative mortality, airway dehiscence, dialysis requirement, postoperative length of stay (P ≥ 0.38 for all), or overall survival (P = 0.52). A subanalysis of the donor arrest group demonstrated similar survival when stratified by resuscitation time quartile (P = 0.38).
Conclusions: There is no evidence of inferior outcomes after lung transplant from brain-dead donors who have had a period of cardiac arrest provided that good lung function is preserved and the donor is otherwise deemed acceptable for transplantation. Potential expansion of the donor pool to include cardiac arrest as the cause of brain death requires further study.
Keywords: lung transplantation, organ donation, heart arrest, cardiopulmonary resuscitation
At a Glance Commentary
Scientific Knowledge on the Subject
Patients who progress to brain death after resuscitation from cardiac arrest have been hypothesized to represent an underused source of potential organ donors; however, there is a paucity of data regarding the viability of lung allografts after a period of cardiac arrest in the donor.
What This Study Adds to the Field
The authors present the collective, national experience analyzing outcomes after lung transplantation from donors who had progressed to brain death after undergoing cardiac arrest and resuscitation. This study provides further guidance in donor selection and identifies potential implications for expanding the existing donor pool. In addition, clinicians involved in managing terminally ill patients after cardiac arrest should be aware of the organ donation prospects in this population.
More than 1,800 lung transplants were performed in the United States in 2011; however, more than 2,200 new lung transplant candidates were added to the waitlist during that same time, far surpassing the available donors (1). Not surprisingly, the waitlist death rate has also increased, with more than 150 pretransplant deaths per 1,000 waitlist years in 2010–2011 (1, 2). Given that less than 20% of eligible deaths result in lung donation (1, 3, 4), heightened awareness of acceptable donors is needed to improve the use of the currently available pool of deceased donors (5–7).
Adult patients who progress to brain death after resuscitation from cardiac arrest have been hypothesized to represent an underused source of potential organ donors (8–10). Approximately 450,000 Americans have a cardiac arrest each year (11), of which approximately 5–10% will be resuscitated with return of spontaneous circulation (12), and 8–16% of those (or approximately 1,800–7,200 patients) will result in brain death (13). Furthermore, care is withdrawn from many cardiac arrest patients before the evolution to brain death occurs (14, 15), which could further increase the estimated number of potential organ donors via this mechanism. At present, these donors are underrepresented in the organ donation pool, which is dominated by cerebrovascular cause of death and head trauma (1).
Brain-dead donors who have subsequently suffered cardiac arrest with successful resuscitation (referred to as “arrest/resuscitation donors”) represent a dilemma for solid-organ transplant teams given concerns for ischemic organ injury and the potential for impaired posttransplant graft function (9, 16–18). Cardiac arrest may occur before and directly contribute to brain death or occur after brain death (19). The current body of literature to guide clinical decisions in managing this donor population for lung transplant is limited. In a systematic review of solid organs transplanted from brain-dead patients due to cardiac arrest, these donor organs had acceptable recipient outcomes; however, only two lung transplant recipients were included (20). In the only study focused on lung transplant, a single-center analysis also showed acceptable outcomes but the sample size was limited to 22 recipients (21). Although reassuring that arrest/resuscitation donors may be acceptable for lung transplant, these studies are limited by single-center practices and small sample size, and thus increase the risk of a type II error. To date there has not been a multicenter or national study to evaluate postoperative complications and overall survival after lung transplant from these donors. Furthermore, there is a paucity of information in the literature regarding potential differences in the outcome profile based on the duration of resuscitation.
The United Network of Organ Sharing (UNOS) database records cardiac arrest occurring after brain death in lung donors, representing what is likely the best available source of information to evaluate the viability of lung transplantation after a period of cardiac arrest in the donor. Previous research from our institution has analyzed this donor population for purposes of heart transplantation, demonstrating equivalent outcomes (22). Interestingly, this study also demonstrated higher survival among donors with a brief period of cardiac arrest compared with nonarrest donors. This provides important insight into potentially broadening the acceptable donor pool for heart transplantation, and raises the possibility that similar advancements could be made in lung transplantation. Our objective was therefore to analyze the usage of arrest/resuscitation donors in a cohort of all U.S. lung transplantations since 2005, with a comparison of postoperative complications and overall survival to nonarrest donors. Second, we sought to evaluate the impact of resuscitation time on postoperative survival as well as differing levels of lung function parameters after resuscitation in these donors.
Methods
The Institutional Review Board at Duke University Medical Center (Durham, NC) approved this study.
Study Population
The Organ Procurement and Transplantation Network’s national computerized database of donor, waitlist, organ-matching, transplant, and posttransplant information as maintained by the UNOS was used for this analysis (referred to as the UNOS database) (23). Our study population included all U.S. adult lung transplantations as recorded in the UNOS database subsequent to the initiation of the lung allocation score (24) (effective May 4, 2005) through December 2011 to reflect current practice patterns for organ allocation and recipient demographics. Multiorgan transplants, pediatric recipients, and patients undergoing repeat transplantation were excluded. Patients were categorized on the basis of whether the donor had a “cardiac arrest since neurological event that led to declaration of brain death” (this is a yes/no field on the “Deceased Donor Registration Worksheet” [Office of Management and Budget approved form number 0915-0157]). Patients with unknown or missing donor arrest information were excluded.
Primary and Secondary Outcomes
The primary outcome variable was overall survival compared between recipients of lung allografts from arrest/resuscitation donors and control subjects. Secondary outcome measures included perioperative mortality, airway dehiscence before discharge, dialysis before discharge, and postoperative length of stay greater than 25 days (75th percentile).
Propensity Matching
Patients receiving a lung allograft from a brain-dead arrest/resuscitation donor were matched 1:1 with the control group on the basis of propensity score matching (25, 26). The propensity score is the probability of treatment assignment (in this case the use of an arrest/resuscitation donor) conditional on observed baseline characteristics to mimic a randomized controlled trial with respect to similarity in the distribution of observed baseline covariates between comparison groups (27). Of the various techniques using propensity scores (28), both the statistical literature (25, 26) as well as the lung transplant literature specific to the use of the UNOS database (29) advocate the use of propensity matching in situations in which a sufficiently large sample of control subjects is available relative to the size of the study group of interest, as is the case in the current analysis.
Covariates selected for the propensity model included variables that affect the exposure (use of an arrest/resuscitation donor) as well as variables that do not affect exposure but affect the primary outcome (survival) in accordance with previously established methodology advocating this approach (27, 29, 30). We therefore included baseline variables related to (1) recipient sociodemographic characteristics: age, sex, ethnicity, year of transplant, geographic region, functional status (need assistance with activities of daily living vs. not), employment status (working vs. not), and insurance carrier (private/self-pay, Medicaid, Medicare, other [Veterans’ Affairs, U.S. government, privately donated care, pending payment, free care, or foreign government payment]); (2) donor/waitlist characteristics: donor age, donor sex, donor ethnicity, donor cause of death, and waitlist days; and (3) recipient risk characteristics: primary diagnosis (obstructive disease, restrictive disease, cystic fibrosis or immunodeficiency, pulmonary vascular disease), cytomegalovirus status at transplant, ABO match level, human leukocyte antigen mismatch level, medical condition before transplant (hospitalized, intensive care unit, or neither), life support before transplant (intravenous inotropes, ventilator, intraaortic balloon pump, or ventricular assist device), ventilator at transplant, transplant type (single vs. double lung), diabetes, body mass index (kg/m2), serum creatinine level at transplant, preoperative 6-minute walk distance, and lung allocation score.
Consistent with previously established methods specific to addressing missing data in propensity score calculations (29, 31), a separate category for “unknown” was created for missing data for nominal variables. For continuous missing covariates, we added a binary variable for whether or not each variable was missing and imputed a value of 0 for missing values for each of these covariates. A forward, stepwise method was used for variable selection based on the minimum corrected Akaike information criterion (32). Optimal matching was performed without replacement (27). Balance in baseline characteristics between the matched cohorts was assessed using standardized difference, which is calculated by determining the difference in the mean between the two groups being compared and dividing this mean by the square root of the pooled variance, with modification to reflect proportions for categorical variables (33). A standardized difference less than 0.1 is generally accepted to indicate a negligible difference in the mean or prevalence of a covariate between treatment groups (27).
Primary Analysis
Baseline recipient and donor characteristics were described for the full study population and the 1:1-matched sample, stratified by the presence or absence of cardiac arrest in the donor, using medians and interquartile range (IQR) for continuous variables and proportions (frequency, percentage) for discrete variables. Comparisons for continuous variables in the full sample were made using Kruskal–Wallis tests, and unordered categorical variables were compared using the Pearson chi-squared test. Comparisons in the 1:1-matched sample were made using standardized difference, as described previously. The trend in use of arrest/resuscitation donors was assessed using the Cochran–Armitage trend test.
Overall survival rates for the original total sample were estimated using the product-limit (Kaplan–Meier) method and compared by the log-rank test. Survival estimates for the 1:1-matched cohort were also estimated by the Kaplan–Meier method and comparisons in this case were made using the test described by Klein and Moeschberger to account for the matched nature of the data (34). Comparisons of perioperative mortality and complications (secondary outcome measures listed previously) were made using McNemar’s test for correlated binary proportions, again to account for the matched nature of the data (35).
Secondary Analysis
For donors who underwent cardiac arrest, a secondary analysis of resuscitation time was performed for the subset of cases for whom this information was available (n = 428/479 [89.4%]). Survival curves were constructed by resuscitation time quartile using the Kaplan–Meier method and compared by the log-rank test.
To better assess the impact allograft quality in comparing arrest/resuscitation donors with control subjects, donor Po2 on 100% oxygen was stratified into quartiles and the 1:1-matched cohorts were separately compared for each quartile, using Kaplan–Meier methods and the Klein and Moeschberger test to account for the matched nature of the data as described previously. Note that only cases in which both members of the matched pair were in the same Po2 quartile were included.
For all statistical comparisons, P values less than or equal to 0.05 were considered significant. Statistical analyses were performed with JMP version 10.0 (SAS Institute Inc., Cary, NC).
Results
Arrest/Resuscitation Donor Characteristics
Of the 24,473 lung transplants included in the UNOS database, 13,694 transplants were outside the timeframe of our analysis and 1,224 transplants met exclusion criteria (Figure 1). This resulted in a study population of 9,555 lung transplants, of which 479 (5.0%) were from arrest/resuscitation donors. Of these 479 donors suffering cardiac arrest after the neurologic event leading to the declaration of brain death, the inciting cause of death was anoxia for 186 donors (39%) (information as to whether the anoxia was related to cardiac arrest was not available), head trauma for 156 (32%), cerebrovascular/stroke for 124 (26%), central nervous system tumor for 5 (1%), and “other” for 8 (2%). When compared with the entire cohort of donors without cardiac arrest, arrest/resuscitation donors were significantly younger (median 30 yr, IQR 21–44 vs. median 32, IQR 21–46; P = 0.041) with a higher incidence of cocaine use (14.1 vs. 11.1%; P = 0.039), higher terminal creatinine (median 1.0 mg/dl, IQR 0.8–1.6 vs. median 1.0, IQR 0.8–1.3; P < 0.001), and lower Po2 on inspired oxygen of 100% (median 405 mm Hg, IQR 214–488 vs. median 422, IQR 256–493; P < 0.001) (Table 1).
Figure 1.
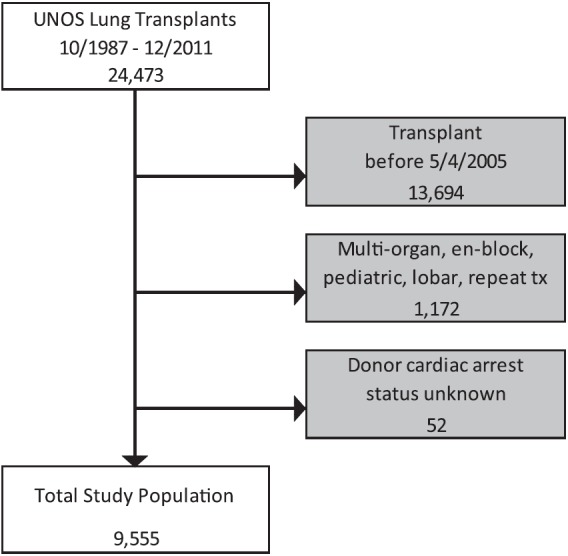
Study inclusion algorithm. tx = transplant; UNOS = United Network of Organ Sharing.
TABLE 1.
DONOR, RECIPIENT, AND TRANSPLANT CHARACTERISTICS
| Characteristic | Donor Arrest (n = 479) | No Donor Cardiac Arrest (Each
Compared against Donor Arrest Group) |
|||
|---|---|---|---|---|---|
| Full Sample (n = 9,076) | P Value | 1:1 Matched (n = 479) | Standardized Difference* | ||
| Donor characteristics | |||||
| Donor age | 30 (21, 44) | 32 (21, 46) | 0.041† | 33 (22, 45) | −0.088 |
| Donor diabetes | 35 (7.3%) | 561 (6.2%) | 0.32 | 31 (6.5%) | 0.032 |
| Donor smoking history | 62 (13.1%) | 1,105 (12.3%) | 0.62 | 55 (11.6%) | 0.046 |
| Donor cocaine use | 67 (14.1%) | 988 (11.1%) | 0.039† | 55 (11.6%) | 0.075 |
| Terminal creatinine, mg/dl | 1.0 (0.8, 1.6) | 1.0 (0.8, 1.3) | <0.001† | 1.0 (0.8, 1.4) | −0.011 |
| Donor BMI, kg/m2 | 25.1 (22.1, 28.7) | 24.7 (22.1, 28.0) | 0.05 | 24.7 (22.1, 27.7) | 0.089 |
| Po2 on 100% inspired oxygen, mm Hg | 405 (214, 488) | 422 (256, 493) | 0.001† | 421 (195, 492) | −0.021 |
| Recipient characteristics | |||||
| Age | 58 (47, 63) | 58 (49, 64) | 0.17 | 58 (47, 64) | 0.005 |
| Female sex | 200 (41.8%) | 3,742 (41.2%) | 0.82 | 200 (41.8%) | 0.000 |
| White ethnicity | 421 (87.9%) | 7,659 (84.4%) | 0.039† | 408 (85.2%) | 0.079 |
| Diagnosis | 0.029† | ||||
| Obstructive | 154 (32.2%) | 3,194 (35.2%) | 143 (29.9%) | 0.050 | |
| Restrictive | 229 (47.8%) | 4,458 (49.1%) | 231 (48.2%) | −0.008 | |
| CF or immunodeficiency | 71 (14.8%) | 1,135 (12.5%) | 75 (15.7%) | −0.025 | |
| Pulmonary vascular | 25 (5.2%) | 289 (3.2%) | 30 (6.3%) | −0.047 | |
| Diabetes | 87 (18.2%) | 1,600 (17.8%) | 0.79 | 91 (19.1%) | −0.023 |
| BMI (kg/m2) at transplant | 24.9 (21.1, 28.4) | 25.1 (21.3, 28.5) | 0.49 | 25.1 (21.2, 28.7) | −0.015 |
| Requiring ventilator at transplant | 34 (7.1%) | 510 (5.6%) | 0.17 | 24 (5.0%) | 0.088 |
| Pulmonary function | |||||
| FEV1, % predicted | 34 (20, 54) | 34 (20, 52) | 0.54 | 36 (21, 56) | −0.046 |
| FVC, % predicted | 46 (36, 59) | 46 (36, 59) | 0.88 | 46 (36, 61) | −0.061 |
| FEV1/FVC | 0.91 (0.47, 1.11) | 0.86 (0.46, 1.10) | 0.30 | 0.87 (0.49, 1.10) | −0.009 |
| Lung allocation score | 39.7 (34.7, 50.0) | 38.9 (34.3, 47.9) | 0.021† | 40.6 (34.7, 49.7) | 0.010 |
| Days on waitlist | 73 (23, 211) | 74 (22, 233) | 0.76 | 65 (21, 211) | 0.001 |
| Transplant characteristics | |||||
| Bilateral transplant | 330 (68.9%) | 5,868 (64.7%) | 0.06 | 329 (68.7%) | 0.004 |
| Sex mismatch | 142 (29.7%) | 2,833 (31.2%) | 0.47 | 164 (34.2%) | −0.097 |
| Donor/recipient race mismatch | 187 (39.0%) | 3,811 (42.0%) | 0.20 | 187 (39.0%) | 0.000 |
| CMV mismatch | 118/439 (26.9%) | 2,141/8,449 (25.3%) | 0.47 | 109/451 (24.2%) | 0.062 |
| Ischemic time, h | 5.2 (4.1, 6.3) | 5.0 (3.9, 6.0) | 0.030† | 5.2 (4.1, 6.3) | 0.018 |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; CMV = cytomegalovirus; Po2 = arterial partial pressure of oxygen.
Shown are the median (interquartile range) for nonparametric continuous variables; n (%) for categorical variables. If data are missing for more than 5% of the study population, the denominator is give for categorical variables and “n” is given for continuous variables. The Wilcoxon signed-rank test was done for continuous variables, and the Pearson chi-squared test was done for categorical variables. Note that matched-pair comparisons of balance using standardized differences are explained in text.
Standardized difference is the difference in mean between the two groups divided by the square root of the pooled variance, with modification to reflect proportions for categorical variables (33).
Statistical significance.
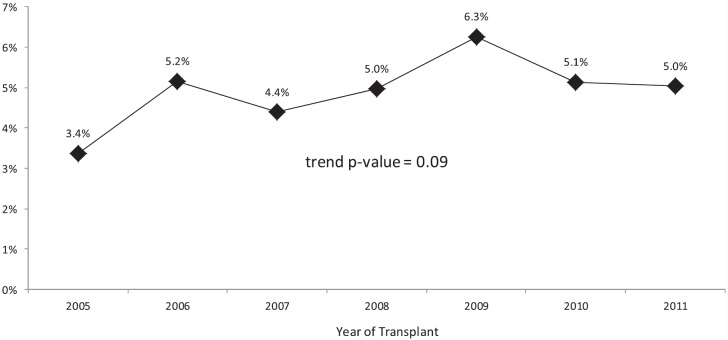
The use of arrest/resuscitation donors as a percentage of all lung transplants ranged from a low of 3.4% in 2005 to a high of 6.3% in 2009; however, overall there was no significant change in the trend of arrest/resuscitation donor use over time (P = 0.09) (Figure 2).
Figure 2.
Trend in usage of lung donors who have suffered cardiopulmonary arrest.
Recipient Characteristics
On comparison of the full sample, recipients of lung allografts from arrest/resuscitation donors had a higher likelihood of white ethnicity (87.9 vs. 84.4%; P = 0.039), a lower incidence of obstructive lung disease (32.2 vs. 35.3%; P = 0.029), and a higher lung allocation score (median 39.7, IQR 34.7–50.0 vs. median 38.9, IQR 34.3–47.9; P = 0.021) (Table 1). Allografts from arrest/resuscitation donors demonstrated longer ischemic time (median 5.2 h, IQR 4.1–6.3 vs. median 5.0, IQR 3.9–6.0; P = 0.030). After 1:1 propensity score matching, the baseline characteristics between groups were balanced with a standardized difference of less than 0.1 for all characteristics.
Primary Analysis
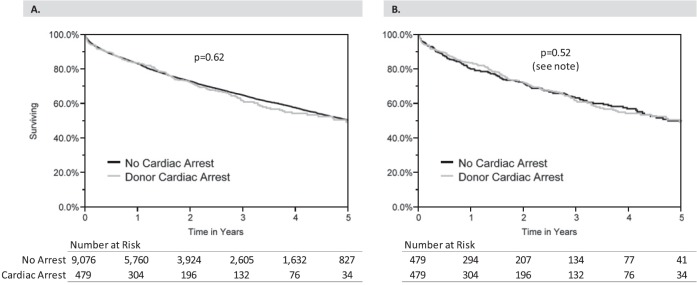
When comparing recipients of lung allografts from arrest/resuscitation donors with control subjects from the total original sample, there was no difference in unadjusted survival based on Kaplan–Meier analysis (median survival 4.96 vs. 5.10 yr for the control group; P = 0.62) (Figure 3A). In addition, there was no difference in recipient survival in the 1:1-matched cohort (median survival 4.96 vs. 5.04 yr for the control group; P = 0.52) (Figure 3B).
Figure 3.
Kaplan–Meier survival curves by donor cardiac arrest status. (A) Original total sample; (B) 1:1-matched cohort. Note: Statistical comparison for the original total sample was performed using the log-rank test, and for the 1:1-matched cohort was performed using the test described by Klein and Moeschberger to account for the matched nature of the data (34).
In comparing postoperative complications in the 1:1-matched cohort, there was no significant difference in 30- or 90-day mortality (P = 0.63 and P = 0.78, respectively), airway dehiscence (P = 1.00), postoperative dialysis requirement (P = 0.38), or postoperative length of stay greater than 25 days (P = 0.76) (Table 2).
TABLE 2.
COMPARISON OF PERIOPERATIVE MORTALITY AND COMPLICATIONS IN 1:1-MATCHED COHORT
| Complication | Comparison of Propensity-matched
Pairs |
|||||
|---|---|---|---|---|---|---|
| n Pairs | Both = No | Both = Yes | Donor Arrest = Yes Matched Control = No | Donor Arrest = No Matched Control = Yes | P Value* | |
| 30-Day mortality | 457 | 417 (91.2%) | 2 (0.4%) | 21 (4.6%) | 17 (3.7%) | 0.63 |
| 90-Day mortality | 447 | 386 (86.4%) | 6 (1.3%) | 29 (6.5%) | 26 (5.8%) | 0.78 |
| Airway dehiscence before discharge | 456 | 443 (97.1%) | 0 (0.0%) | 6 (1.3%) | 7 (1.5%) | 1.00 |
| Dialysis before discharge | 467 | 419 (89.7%) | 1 (0.2%) | 20 (4.3%) | 27 (5.8%) | 0.38 |
| Postoperative length of stay > 25 d | 462 | 246 (53.2%) | 43 (9.3%) | 84 (18.2%) | 89 (19.3%) | 0.76 |
Statistical comparisons were performed using McNemar’s test for correlated binary proportions.
Secondary Analysis
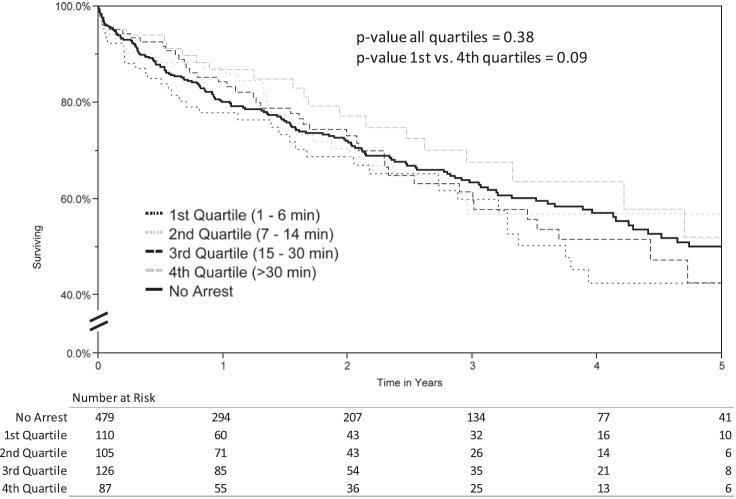
Information regarding the duration of resuscitation was available for 89.4% (428 of 479) of arrest/resuscitation donors (first quartile, 1–6 min; second quartile, 7–14 min; third quartile, 15–30 min; and fourth quartile, >30 min). When comparing Kaplan–Meier unadjusted recipient survival stratified by donor resuscitation time quartile, the longest recipient survival was observed in the fourth quartile (>30 min of donor resuscitation time); however, this did not meet statistical significance based on log-rank comparison (P = 0.38; Figure 4).
Figure 4.
Kaplan–Meier survival curves by resuscitation time quartile. Note: The “No Arrest” group is displayed for reference. The statistical comparison relates only to the resuscitation time quartile groups.
Across both groups in the 1:1-matched cohorts, donor Po2 on 100% inspired oxygen was distributed as follows: first quartile, less than 198 mm Hg; second quartile, 199–411 mm Hg; third quartile, 412–491 mm Hg; and fourth quartile, greater than 491 mm Hg. In 181 of the matched pairs both members were in the same Po2 quartile and therefore included in this subanalysis. In comparing propensity-matched subjects stratified by donor Po2, there was significantly increased survival in arrest/resuscitation donor lung recipients in the first Po2 quartile (P = 0.013) (Figure 5). Survival in the second, third, and fourth quartiles was similar (P = 0.09, P = 0.67, and P = 0.92, respectively).
Figure 5.
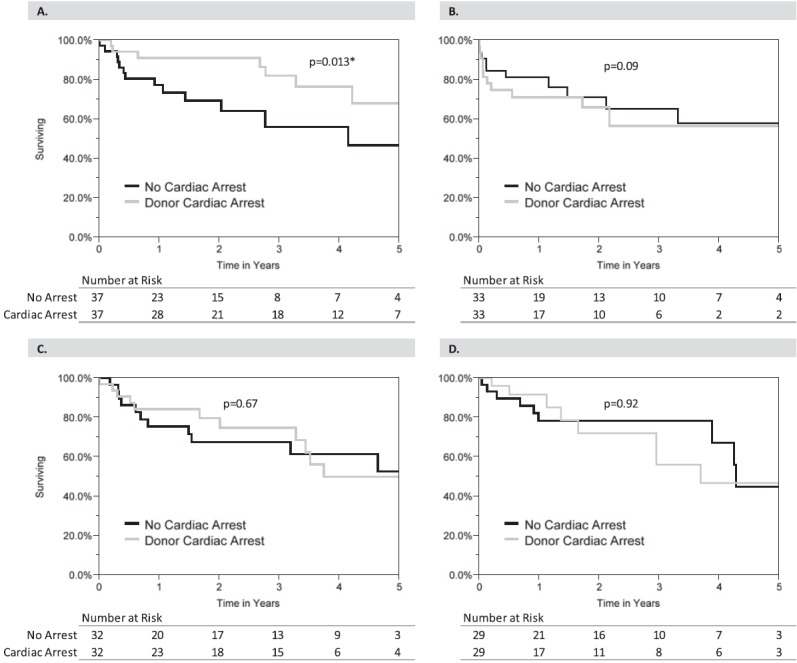
Survival comparison stratified by donor arterial oxygen pressure. (A) First quartile (Po2 ≤ 198 mm Hg). (B) Second quartile (Po2 199\x{2013}411 mm Hg). (C) Third quartile (Po2 412\x{2013}491 mm Hg). (D) Fourth quartile (>491 mm Hg). *Statistical significance. Statistical comparison was performed using the test described by Klein and Moeschberger to account for the matched nature of the data (34). Po2 = arterial oxygen pressure on 100% inspired oxygen.
Discussion
The availability of lung transplantation as an option to treat end-stage lung disease continues to be limited by the lack of sufficient donor organs to meet demand. In the current study, we report equivalent short- and long-term outcomes in lung transplant recipients receiving allografts from brain-dead donors who have subsequently suffered cardiopulmonary arrest and resuscitation with return of spontaneous circulation. These findings persisted regardless of the duration of resuscitation and also at varying levels of donor lung function as measured by arterial oxygen pressure on 100% inspired oxygen. This evidence provides further guidance in donor selection as well as potential implication for expanding the donor pool.
Our findings of equivalent short- and long-term survival with arrest/resuscitation donors are consistent with studies of other solid organ transplants using such donors. In heart transplantation, no evidence of inferior survival was demonstrated in two separate single-center reviews composed of 38 and 25 patients following transplant after cardiac arrest in the organ donor (17, 36). A national study of 930 cardiac arrest donors confirmed these findings of equivalent outcomes for heart transplant recipients (22). Similarly, a single-center review of 37 orthotopic liver transplants from arrest/resuscitation donors (37), as well as a separate review of 12 intestinal transplant recipients from such donors (38), both demonstrated equivalent outcomes when compared with control groups, each study concluding that cardiac arrest should not automatically exclude the use of an arrest/resuscitation graft for transplantation. Furthermore, a systematic review of brain-dead donors after cardiac arrest demonstrated equivalent survival rates for kidneys (n = 29), liver (n = 14), heart (n = 45), and intestine (n = 12) (20).
In the current study demonstrating the largest series of lung transplantation after donor arrest/resuscitation yet reported in the literature, our data confirm that cardiac arrest in the donor should not preclude lung donation, provided the allograft is otherwise deemed acceptable. It is important to note that the arrest/resuscitation donors used for lung transplant in our study demonstrated excellent lung function (median Po2 on 100% oxygen of >400 mm Hg) and therefore likely represent the subset of patients who arrested, were resuscitated, and continued to have good lung function. There may well be a large cohort of patients never made available for donation that were resuscitated and had poor lung function. However, the subanalysis stratified by donor Po2 quartile provides interesting information by demonstrating longer survival in the arrest/resuscitation group compared with propensity-matched control subjects with donor Po2 not exceeding 198 mm Hg. This may indicate that the upper stratum of lung function for arrest/resuscitation donors may not be necessary when compared with criteria for conventional donors, although caution should be taken when interpreting these data given the small sample sizes in the Po2 strata.
Successful use of lung allografts after cardiac arrest in the donor raises the possibility of further expanding the donor pool. Patients suffering out-of-hospital cardiac arrest have been hypothesized to represent an underused source of organ donors (8, 9). Many of these patients have care withdrawn at early stages, potentially precluding evolution to brain death (8, 14, 15). Moreover, concern persists regarding the suitability of such organs because of potential ischemic injury (20). Although our study population consists of patients suffering cardiac arrest after brain death (not before), this is nonetheless the strongest evidence yet reported in the literature to demonstrate that the insult to lung allografts after a period of cardiac arrest in the donor does not preclude successful lung transplantation when the donor is otherwise acceptable. These findings may serve as the foundation for advances in lung transplantation pertaining to organ donation from individuals who have suffered a cardiac arrest/resuscitation. Moreover, clinicians involved in the care of the critically ill patient subsequent to cardiac arrest may benefit from information regarding organ donation prospects to guide terminal management in accordance with the patient’s wishes should he or she have wanted to be an organ donor. In addition, advances in ex vivo lung perfusion technology may further expand the feasibility of lung transplantation from high-risk out-of-hospital cardiac arrest donors (39).
Interestingly, our study demonstrated numerically longer survival in the highest resuscitation time quartile (>30 min), when compared with other resuscitation time quartiles as well as when compared with nonarrest donors (Figure 4). Although this did not meet statistical significance (possibly related to sample size), these findings raise the possibility of ischemic preconditioning, as previously reported in the literature (40–45). This concept has also been hypothesized to play a role in the function of heart allografts from donors with a brief period of cardiac arrest (22). In animal models, ischemic preconditioning has been shown to ameliorate the effects of ischemia–reperfusion injury on lung parenchyma (46, 47). Proposed mechanisms include antiinflammatory response, altered cell energy metabolism, nitric oxide production, and involvement of nuclear transcription factors (48, 49). These proposed mechanisms may in part explain the observed recipient survival with arrest/resuscitation donors, further supporting viability of these organs for lung transplantation. An alternative explanation for these findings is that lungs from donors with prolonged cardiac arrest that recovered to otherwise meet criteria for transplantation may be naturally more resistant to ischemic injury and subsequent primary graft dysfunction. Further delineation of a potential impact of resuscitation time on outcomes would require further study.
Successful transplantation with donor lungs having recovered from a period of warm ischemic injury encourages further advancement in expanding the donor pool beyond brain-dead donors to include donation after determination of circulatory death (DCD) (50, 51). Between 2008 and 2011, only 0.8 to 1.9% of lung transplants in the United States were from DCD donors (1). Preliminary results with the use of such donors are encouraging (52–54); however, barriers to widespread adoption have included concerns over diminished quality of the organ as well as ethical considerations (6, 55). Although the physiological alterations observed with DCD organ procurement are likely different from those of a brain-dead arrest/resuscitation donor, the results of the current study nonetheless contribute to alleviating concerns about DCD organ quality and the viability of successful lung transplantation after a period of brief warm ischemic injury.
An important limitation to this analysis is that the number of potential donors excluded from organ procurement due to arrest/resuscitation events, as well as the number of out-of-hospital cardiac arrest patients for whom care is withdrawn before evolution to brain death, are not well captured. This precludes definitive quantification of the potential impact of more liberal inclusion of such donors into the organ allocation pool. Moreover, given that our data source only includes information for arrest/resuscitation donors used for transplant, we are unable to determine whether these donors are physiologically more functional than the overall arrest/resuscitation cohort, or what factors determine whether an arrest/resuscitation donor should be used.
Several other limitations should be considered when evaluating the results of the current study. Given the retrospective nature of this review, unmeasured confounders that impact postoperative outcomes and/or the decision to use or decline an arrest/resuscitation donor may exist. For arrest/resuscitation donors, the time between return of spontaneous circulation and organ harvest was unavailable for analysis, and this may directly impact willingness to use these organs. Moreover, our analysis is limited to donors suffering cardiac arrest after the index event causing brain death. We have not analyzed donors for whom brain death was the subsequent result of cardiac arrest.
Conclusions
Recipients of lung transplants from brain-dead arrest/resuscitation organ donors have equivalent outcomes when compared with the general UNOS cohort as well as matched control subjects in the UNOS database. Brain-dead donors subsequently suffering cardiac arrest should not be excluded from lung procurement when good lung function is preserved and the donor is otherwise deemed acceptable for transplantation. The potential effect of ischemic preconditioning on lung allograft function, as well as the potential expansion of the donor pool to include cardiac arrest as the cause of brain death, require further study.
Acknowledgments
Acknowledgment
By use of the UNOS database, this work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content herein is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Author Contributions: A.W.C., M.W., R.P., and A.A.O. each contributed to the conception and design, acquisition of data, analysis and interpretation of data, drafting, and revising the article. L.D.S., S.M.P., R.D.D., and M.G.H. each contributed to the conception and design, interpretation of data, and writing and/or revising the article critically for important intellectual content. All provided final approval.
Originally Published in Press as DOI: 10.1164/rccm.201303-0588OC on June 18, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Health Resources and Services Administration, U.S. Department of Health and Human ServicesOPTN (Organ Procurement and Transplantation Network) and SRTR (Scientific Registry of Transplant Recipients) annual data report: 2011. Rockville, MD: Department of Health and Human Services; 2012150–158. [Google Scholar]
- 2.Yusen RD, Shearon TH, Qian Y, Kotloff R, Barr ML, Sweet S, Dyke DB, Murray S. Lung transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1047–1068. doi: 10.1111/j.1600-6143.2010.03055.x. [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Wang Y, Fang X, Warnock M, Sakuma T, Hall TS, Matthay M. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet. 2002;360:619–620. doi: 10.1016/s0140-6736(02)09774-x. [DOI] [PubMed] [Google Scholar]
- 4.Venkateswaran RV, Patchell VB, Wilson IC, Mascaro JG, Thompson RD, Quinn DW, Stockley RA, Coote JH, Bonser RS. Early donor management increases the retrieval rate of lungs for transplantation. Ann Thorac Surg. 2008;85:278–286. doi: 10.1016/j.athoracsur.2007.07.092. discussion 286. [DOI] [PubMed] [Google Scholar]
- 5.Botha P. Extended donor criteria in lung transplantation. Curr Opin Organ Transplant. 2009;14:206–210. doi: 10.1097/mot.0b013e328326c834. [DOI] [PubMed] [Google Scholar]
- 6.McKellar SH, Durham LA, III, Scott JP, Cassivi SD. Successful lung transplant from donor after cardiac death: a potential solution to shortage of thoracic organs. Mayo Clinic Proc. 2010;85:150–152. doi: 10.4065/mcp.2009-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonser RS, Taylor R, Collett D, Thomas HL, Dark JH, Neuberger J Cardiothoracic Advisory Group to NHS Blood and Transplant and the Association of Lung Transplant Physicians (UK) Effect of donor smoking on survival after lung transplantation: a cohort study of a prospective registry. Lancet. 2012;380:747–755. doi: 10.1016/S0140-6736(12)60160-3. [DOI] [PubMed] [Google Scholar]
- 8.Adrie C, Haouache H, Saleh M, Memain N, Laurent I, Thuong M, Darques L, Guerrini P, Monchi M. An underrecognized source of organ donors: patients with brain death after successfully resuscitated cardiac arrest. Intensive Care Med. 2008;34:132–137. doi: 10.1007/s00134-007-0885-7. [DOI] [PubMed] [Google Scholar]
- 9.Raoof M, Joseph BA, Friese RS, Kulvatunyou N, O’Keeffe T, Tang A, Wynne J, Latifi R, Rhee P.Organ donation after traumatic cardiopulmonary arrest Am J Surg 2011202701–705., discussion 705–706. [DOI] [PubMed] [Google Scholar]
- 10.Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, et al. American Heart Association. Part 9: post-cardiac arrest care: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18, Suppl 3):S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 11.Young GB. Clinical practice: neurologic prognosis after cardiac arrest. N Engl J Med. 2009;361:605–611. doi: 10.1056/NEJMcp0903466. [DOI] [PubMed] [Google Scholar]
- 12.Chestnut JM, Kuklinski AA, Stephens SW, Wang HE. Cardiovascular collapse after return of spontaneous circulation in human out-of-hospital cardiopulmonary arrest. Emerg Med J. 2012;29:129–132. doi: 10.1136/emj.2010.108340. [DOI] [PubMed] [Google Scholar]
- 13.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 14.Zandbergen EGJ, Hijdra A, Koelman JHTM, Hart AAM, Vos PE, Verbeek MM, de Haan RJ PROPAC Study Group. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006;66:62–68. doi: 10.1212/01.wnl.0000191308.22233.88. [DOI] [PubMed] [Google Scholar]
- 15.Geocadin RG, Buitrago MM, Torbey MT, Chandra-Strobos N, Williams MA, Kaplan PW. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67:105–108. doi: 10.1212/01.wnl.0000223335.86166.b4. [DOI] [PubMed] [Google Scholar]
- 16.Naik PM, Angel LF. Special issues in the management and selection of the donor for lung transplantation. Semin Immunopathol. 2011;33:201–210. doi: 10.1007/s00281-011-0256-x. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Lázaro IJ, Almenar-Bonet L, Martínez-Dolz L, Buendía-Fuentes F, Agüero J, Navarro-Manchón J, Raso-Raso R, Salvador-Sanz A. Can we accept donors who have suffered a resuscitated cardiac arrest? Transplant Proc. 2010;42:3091–3092. doi: 10.1016/j.transproceed.2010.05.054. [DOI] [PubMed] [Google Scholar]
- 18.Fischer S, Maclean AA, Liu M, Cardella JA, Slutsky AS, Suga M, Moreira JF, Keshavjee S. Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia–reperfusion injury in lung transplantation. Am J Respir Crit Care Med. 2000;162:1932–1939. doi: 10.1164/ajrccm.162.5.9910064. [DOI] [PubMed] [Google Scholar]
- 19.Wilson DJ, Fisher A, Das K, Goerlitz F, Holland BK, De La Torre AN, Merchant A, Seguel J, Samanta AK, Koneru B. Donors with cardiac arrest: improved organ recovery but no preconditioning benefit in liver allografts. Transplantation. 2003;75:1683–1687. doi: 10.1097/01.TP.0000064542.63798.6B. [DOI] [PubMed] [Google Scholar]
- 20.Sandroni C, Adrie C, Cavallaro F, Marano C, Monchi M, Sanna T, Antonelli M. Are patients brain-dead after successful resuscitation from cardiac arrest suitable as organ donors? A systematic review. Resuscitation. 2010;81:1609–1614. doi: 10.1016/j.resuscitation.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 21.Pilarczyk K, Osswald BR, Pizanis N, Tsagakis K, Massoudy P, Heckmann J, Jakob HG, Kamler M. Use of donors who have suffered cardiopulmonary arrest and resuscitation in lung transplantation. Eur J Cardiothorac Surg. 2011;39:342–347. doi: 10.1016/j.ejcts.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Southerland KW, Castleberry AW, Williams JB, Daneshmand MA, Ali AA, Milano CA. Impact of donor cardiac arrest on heart transplantation. Surgery. 2013;154:312–319. doi: 10.1016/j.surg.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown RS, Belton AM, Martin JM, Simmons DD, Taylor GJ, Willard E. Evolution of quality at the Organ Center of the Organ Procurement and Transplantation Network/United Network for Organ Sharing. Prog Transplant. 2009;19:221–226. doi: 10.1177/152692480901900306. [DOI] [PubMed] [Google Scholar]
- 24.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 26.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 27.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Shuhaiber JH, Kim JB, Hur K, Gibbons RD. Survival of primary and repeat lung transplantation in the United States. Ann Thorac Surg. 2009;87:261–266. doi: 10.1016/j.athoracsur.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 30.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Agostino RB, Rubin DB. Estimating and using propensity scores with partially missing data. J Am Stat Assoc. 2000;95:749–759. [Google Scholar]
- 32.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 33.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 34.Klein JP, Moeschberger ML.Survival analysis: techniques for censored and truncated data, 2nd ed. New York: Springer; 2003 [Google Scholar]
- 35.Lyles RH, Williamson JM, Lin H-M, Heilig CM. Extending McNemar’s test: estimation and inference when paired binary outcome data are misclassified. Biometrics. 2005;61:287–294. doi: 10.1111/j.0006-341X.2005.040135.x. [DOI] [PubMed] [Google Scholar]
- 36.Ali AA, Lim E, Thanikachalam M, Sudarshan C, White P, Parameshwar J, Dhital K, Large SR. Cardiac arrest in the organ donor does not negatively influence recipient survival after heart transplantation. Eur J Cardiothorac Surg. 2007;31:929–933. doi: 10.1016/j.ejcts.2007.01.074. [DOI] [PubMed] [Google Scholar]
- 37.Totsuka E, Fung JJ, Urakami A, Moras N, Ishii T, Takahashi K, Narumi S, Hakamada K, Sasaki M. Influence of donor cardiopulmonary arrest in human liver transplantation: possible role of ischemic preconditioning. Hepatology. 2000;31:577–580. doi: 10.1002/hep.510310305. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto CS, Kaufman SS, Girlanda R, Little CM, Rekhtman Y, Raofi V, Laurin JM, Shetty K, Fennelly EM, Johnson LB, et al. Utilization of donors who have suffered cardiopulmonary arrest and resuscitation in intestinal transplantation. Transplantation. 2008;86:941–946. doi: 10.1097/TP.0b013e3181852f9a. [DOI] [PubMed] [Google Scholar]
- 39.Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, Sato M, Laratta J, Azad S, Madonik M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 40.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 41.Du ZY, Hicks M, Winlaw D, Spratt P, Macdonald P. Ischemic preconditioning enhances donor lung preservation in the rat. J Heart Lung Transplant. 1996;15:1258–1267. [PubMed] [Google Scholar]
- 42.Soncul H, Oz E, Kalaycioglu S. Role of ischemic preconditioning on ischemia–reperfusion injury of the lung. Chest. 1999;115:1672–1677. doi: 10.1378/chest.115.6.1672. [DOI] [PubMed] [Google Scholar]
- 43.Pasupathy S, Homer-Vanniasinkam S. Surgical implications of ischemic preconditioning. Arch Surg. 2005;140:405–409. doi: 10.1001/archsurg.140.4.405. discussion 410. [DOI] [PubMed] [Google Scholar]
- 44.Banga NR, Homer-Vanniasinkam S, Graham A, Al-Mukhtar A, White SA, Prasad KR. Ischaemic preconditioning in transplantation and major resection of the liver. Br J Surg. 2005;92:528–538. doi: 10.1002/bjs.5004. [DOI] [PubMed] [Google Scholar]
- 45.Mallick IH, Winslet MC, Seifalian AM. Ischemic preconditioning of small bowel mitigates the late phase of reperfusion injury: heme oxygenase mediates cytoprotection. Am J Surg. 2010;199:223–231. doi: 10.1016/j.amjsurg.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Friedrich I, Spillner J, Lu EX, Bartling B, Barnscheid M, Sablotzki A, Schade U, Reidemeister JC, Silber RE, Gunther A. Ischemic pre-conditioning of 5 minutes but not of 10 minutes improves lung function after warm ischemia in a canine model. J Heart Lung Transplant. 2001;20:985–995. doi: 10.1016/s1053-2498(01)00290-x. [DOI] [PubMed] [Google Scholar]
- 47.Ucar G, Topaloglu E, Kandilci HB, Gümüsel B. Effect of ischemic preconditioning on reactive oxygen species–mediated ischemia–reperfusion injury in the isolated perfused rat lung. Clin Biochem. 2005;38:681–684. doi: 10.1016/j.clinbiochem.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Jun N, Ke J, Gang C, Lin C, Jinsong L, Jianjun W. The protective effect of ischemic preconditioning associated with altered gene expression profiles in rat lung after reperfusion. J Surg Res. 2011;168:281–293. doi: 10.1016/j.jss.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 49.Peralta C, Serafin A, Fernández-Zabalegui L, Wu ZY, Roselló-Catafau J. Liver ischemic preconditioning: a new strategy for the prevention of ischemia–reperfusion injury. Transplant Proc. 2003;35:1800–1802. doi: 10.1016/s0041-1345(03)00571-2. [DOI] [PubMed] [Google Scholar]
- 50.Egan TM. Non–heart-beating donors in thoracic transplantation. J Heart Lung Transplant. 2004;23:3–10. doi: 10.1016/s1053-2498(02)00658-7. [DOI] [PubMed] [Google Scholar]
- 51.Dark JH. Lung transplantation from the non–heart beating donor. Transplantation. 2008;86:200–201. doi: 10.1097/TP.0b013e31817c87b6. [DOI] [PubMed] [Google Scholar]
- 52.De Oliveira NC, Osaki S, Maloney JD, Meyer KC, Kohmoto T, D’Alessandro AM, Love RB. Lung transplantation with donation after cardiac death donors: long-term follow-up in a single center. J Thorac Cardiovasc Surg. 2010;139:1306–1315. doi: 10.1016/j.jtcvs.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Mason DP, Brown CR, Murthy SC, Vakil N, Lyon C, Budev MM, Pettersson GB.Growing single-center experience with lung transplantation using donation after cardiac death Ann Thorac Surg 201294406–411., discussion 411–412 [DOI] [PubMed] [Google Scholar]
- 54.Levvey BJ, Harkess M, Hopkins P, Chambers D, Merry C, Glanville AR, Snell GI.Excellent clinical outcomes from a national donation-after-determination-of-cardiac-death lung transplant collaborative Am J Transplant 2012122406–2013.. [DOI] [PubMed] [Google Scholar]
- 55.Mason DP, Thuita L, Alster JM, Murthy SC, Budev MM, Mehta AC, Pettersson GB, Blackstone EH. Should lung transplantation be performed using donation after cardiac death? The United States experience. J Thorac Cardiovasc Surg. 2008;136:1061–1066. doi: 10.1016/j.jtcvs.2008.04.023. [DOI] [PubMed] [Google Scholar]