Abstract
Pulmonary arterial hypertension (PAH) is rare in children and few data are available in a pediatric general population. This study aims to calculate the annual incidence and prevalence of PAH and to describe these children in a large US population of patients aged under 18 years. Using the US MarketScan claims database we identified 695 children with PAH in 2010–2013. We calculated annual incidence rates and prevalence overall, by age and PAH type (idiopathic and non-idiopathic) using Byar’s method. We also described characteristics, co-morbidities, treatment patterns, and diagnostic procedures for these children. In 2010–2013, the annual incidence rates of PAH per 1,000,000 children-years was in the range of 4.8–8.1; 0.5–0.9 for idiopathic PAH and 4.3–7.3 for non-idiopathic PAH. The annual prevalence of PAH was in the range of 25.7–32.6 per 1,000,000 children; 4.4–6.0 for idiopathic PAH and 21.3–27.0 for non-idiopathic PAH. Incidence rates and prevalence were highest in children under age 2 years. Around 36% of affected children were born prematurely. Most (75%) had some type of congenital heart defect and 13% had Down’s syndrome. Most patients received PAH monotherapy (83%), while 13% received dual therapy. Phosphodiesterase type 5 inhibitors were the most commonly used treatments. Around 92% had at least one echocardiogram and 37% a right heart catheterization. PAH is very rare in children especially in the absence of etiological factors such as congenital heart defects. A large proportion of diagnoses in children seem to be based on echocardiography rather than right heart catheterization.
Keywords: incidence, prevalence, population-based, cohort
Introduction
Pulmonary hypertension (PH), characterized by abnormal elevation of mean pulmonary artery pressure equal to or above 25 mmHg, is often associated with diverse cardiac, pulmonary, and systemic diseases, and causes significant morbidity and mortality in children.1,2 Pulmonary arterial hypertension (PAH), formerly referred to as primary pulmonary hypertension, encompasses Group 1 in the Dana point classification of PH.3–5 PAH accounts for a majority (88%) of pediatric PH cases,6 and the main etiological subtypes of pediatric PAH, besides persistent pulmonary hypertension of the newborn (PPHN), are idiopathic PAH and PAH associated with congenital heart defects (CHD).7 Over the past few decades, advances in understanding basic pulmonary vascular biology have led to the development of several novel therapies, which have expanded therapeutic options and improved survival and quality of life for children with PAH.8 However, long-term outcomes for children with severe PAH remain poor.1 Currently, pediatric PAH is treated following guidelines mostly based on strategies developed for the adult population. In the absence of specific pediatric therapeutic and diagnostic evidence, there is general acceptance of adult-based evidence among pediatricians.9 However, it has been reported that extrapolating all results from adult PAH patients to children may not be completely appropriate and thus specific studies in pediatric populations are needed.10,11
Despite the serious nature of PAH, its true incidence and prevalence in the pediatric population remain uncertain. To date, only a few European and North-American registry-based studies have been published and they estimated the incidence and prevalence of PAH to be 0.5–2.2 cases per million children-years and 2–16 cases per million children, respectively.12–14
Although registry-based studies provide useful information on the clinical management of patients, data often lack generalizability. We identified a population-based source of data, US commercially insured patients, from which to calculate the annual incidence rates and prevalence of PAH and to describe characteristics, co-morbidities, treatments, and diagnostic procedures used in a population of children aged under 18 years with PAH in 2010–2013. These data should provide useful information to guide future clinical management of pediatric PAH patients.
Methods
The data were derived from a Boston University held copy of the MarketScan Commercial Claims and Encounters Database (CCE) of Truven Health Analytics, a large US-based claims database containing data from 2007 through 2013 on over 50 million patients from over 150 large employers geographically distributed throughout the US that covers employees and their dependent family members. It has been reported that there is reasonable agreement on age, sex, and census region between the CCE database and the Current Population Survey respondents aged <65 years, who participated in employer-sponsored private insurance.15 The database contains basic demographic and enrollment data, and information on paid claims for pharmaceuticals, medical services (with diagnoses recorded), and inpatient and outpatient procedures. Diagnoses are coded using the ICD-9-CM system. Procedures are coded using the Current Procedural Terminology, Fourth Edition system and the Healthcare Common Procedure Coding system. Drug prescriptions are coded using the National Drug Code. This study was approved by the Boston University Medical Center Institutional Review Board.
Study population
The study population comprised all patients aged under 18 years at any time during the years 2010–2013 in the CCE data.
Study outcome
Among the study population we identified all patients aged under 18 years who had at least one primary or secondary diagnosis claim for primary pulmonary hypertension (ICD-9-CM: 416.0), other chronic pulmonary heart diseases (ICD-9-CM: 416.8), or PPHN (ICD-9-CM: 747.83). Among these, we defined PAH cases as those who had ≥ 1 prescription(s) for PH treatment, including phosphodiesterase type-5 (PDE 5) inhibitors, endothelin receptor antagonists, calcium channel blockers (CCBs), prostanoids, and riociguat. The index date was the earliest claim for PH in the study period. To minimize misclassification of PAH, we excluded patients with claims for conditions listed in Groups 2, 3, 4, and 5 of the Dana Point classification before the index date in the absence of one of the following: CHD; chromosomal anomalies or syndromes (such as Down’s syndrome, velocardiofacial syndrome, etc.); connective tissue diseases; HIV infection; portal hypertension; schistosomiasis; or chronic hemolytic anemia.5 See Supplementary Table 1 for all codes.
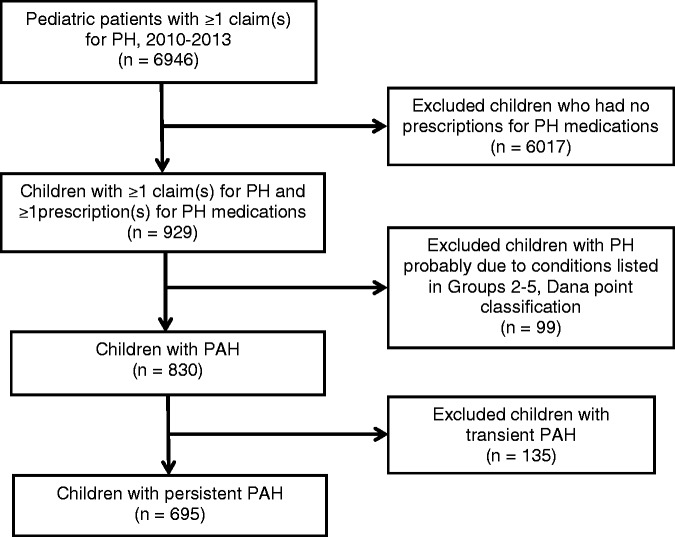
We classified each PAH patient into two groups: (1) transient PAH; or (2) persistent PAH; based on the following algorithm: transient PAH cases were those diagnosed early in life (usually aged less than 1 year) that resolved after treating the underlying cause. These included cases with claims for PPHN only and cases whose last claim for PH or PPHN occurred before the patient was 180 days old (based on estimated birth date; Appendix I and Supplementary Table 2). The persistent PAH category included all remaining cases, i.e. cases who had claims for PH after 180 days of age. We then restricted the analysis to cases with persistent PAH because cases with transient PAH had different natural history, outcome, and treatment patterns that usually resolved during the neonatal period. See Fig. 1 for a description of the case selection process.
Fig. 1.
Selection of pediatric pulmonary arterial hypertension patients.
Persistent PAH cases were further classified as non-idiopathic if they had claims for CHD, chromosomal anomalies or syndromes, or claims for connective tissue diseases, HIV infection, portal hypertension, schistosomiasis, or chronic hemolytic anemia recorded any time before or on the date of the first-ever claim for PH; otherwise, they were considered to be idiopathic PAH cases. We also identified incident PAH cases. These were cases with no claims for PH before 1 January 2010, no PH treatment before the index date, and who had been registered for at least 1 year in the database before the index date if they were aged 1 year or older.
Statistical analysis
We calculated annual incidence rates based on the number of incident persistent PAH cases over the person years of follow-up in the database for people aged under 18 years in each calendar year using Byar’s method. Person time was accrued from the day of patient registration or 1 January 2010 (whichever came later) until they met our definition for PAH, reached age 18 years, left the database, or the end of data collection (December 2013), whichever was earliest. For incidence analyses we restricted the study population to persons who had been registered for at least 1 year in total in the database except for patients who were aged less than 1 year, in which case we accrued person-time since registration date. To calculate annual prevalence, we estimated the number of patients aged under 18 years in the database in each year during 2010–2013. A person contributed to the denominator of each year from the date they entered the database on or after 1 January 2010 until they reached 18 years, end of data collection, or they left the database, whichever came first. A person was included in the numerator of the appropriate calendar year in which the first claim for PH—treatment or diagnosis—was recorded, and for each year of follow-up in which another PH diagnosis or treatment was recorded. Both incidence and prevalence were stratified by age (<1, 1 - < 2, 2 - < 6, 6 - < 12, 12 - < 18 years) and idiopathic versus non-idiopathic PAH.
Based on PH-targeted treatments recorded in the study period, we described treatment patterns by grouping them into mono-, dual, or triple therapy. The treatments considered for the analysis were: PDE-5 inhibitors; endothelin receptor antagonists; CCBs; and prostanoids (riociguat was not found in the study population). Users with monotherapy were those who either received one type of PH treatment only during the study period or who switched from one type only to another type. Users with dual or triple therapy were those who concomitantly received two or three types of PH treatment at some point during the study period. We also described the diagnostic procedures recorded within 30 days before, on, or any time after the index date. Diagnostic procedures of interest are those described in the 2015 ESC/ERS guidelines for diagnosis and treatment of PH.16 Specifically, chest radiograph (X-ray), electrocardiogram, echocardiography, chest computed tomography (CT) with and without contrast, and right heart catheterization were considered critical diagnostic procedures.17 All relevant codes are listed in Supplementary Table 3. Finally, we collected information on different co-morbidities as listed in Supplementary Table 1. A patient was considered to have a co-morbidity if they had at least two claims for that co-morbidity at least 1 day apart.
Since transient PAH included those whose last claim for PH or PPHN occurred before the patient was aged 180 days, and persistent PAH category included cases with claims for PPHN before 180 days of age followed by claims for PH after 180 days of age, we conducted a sensitivity analysis to use a 365-day cutoff instead of a 180-day cutoff for the PAH grouping and then repeated the main analyses.
All analyses were conducted using SAS statistical software version 9.3 (SAS Institute, Cary, NC, USA).
Results
There were between 10 and 12.6 million patients aged under 18 years in the CCE data in any given year between 2010 and 2013 among whom we identified 830 children with PAH: 135 with transient PAH and 695 with persistent PAH (including 219 incident cases) (Table 1). See Fig. 1 for the selection process. Of these persistent PAH cases, 35% had their first diagnosis before they were aged 1 year, around half were boys (53.2%), and 36% were born prematurely (<37 weeks).
Table 1.
Distribution of basic characteristics of persistent PAH cases.
| Persistent PAH n = 695 (%) | |
|---|---|
| Age at first diagnosis of PAH | |
| <180 days | 144 (20.7) |
| 180 - < 365 days | 100 (14.4) |
| 1 - < 2 years | 73 (10.5) |
| 2 - < 6 years | 133 (19.1) |
| 6 - < 12 years | 110 (15.8) |
| 12 - < 18 years | 135 (19.4) |
| Age at last diagnosis of PAH | |
| <180 days | 0 |
| 180 - < 365 days | 88 (12.7) |
| 1 - < 2 years | 108 (15.5) |
| 2 - < 6 years | 213 (30.6) |
| 6 - < 12 years | 121 (17.4) |
| 12 - < 18 years | 165 (23.7) |
| Sex | |
| Male | 370 (53.2) |
| Female | 325 (46.8) |
| Gestational age | |
| Preterm | |
| ≤32 weeks | 112 (16.1) |
| >32 weeks | 89 (12.8) |
| Unspecified weeks | 49 (7.1) |
| Full term | 445 (64.0) |
| Year of first-recorded diagnosis of PAH | |
| Before 2010 | 115 (16.5) |
| 2010 | 157 (22.6) |
| 2011 | 189 (27.2) |
| 2012 | 138 (19.9) |
| 2013 | 96 (13.8) |
| Length of medical history before first diagnosis of PAH | |
| <180 days | 472 (67.9) |
| 180 - < 365 days | 72 (10.4) |
| 1 - < 2 years | 75 (10.8) |
| ≥ 2 years | 76 (10.9) |
| Length of follow-up after first diagnosis of PAH | |
| <180 days | 103 (14.8) |
| 180 - < 365 days | 131 (18.8) |
| 1 - < 2 years | 193 (27.8) |
| ≥ 2 years | 268 (38.6) |
The annual incidence rates of persistent PAH were in the range of 4.8–8.1 per million children-years and prevalence of 25.7–32.6 per million children across the study period, and were higher in children aged under 2 years and then dropped markedly. The incidence and prevalence of idiopathic PAH were 0.5–0.9 per million children-years and 4.4–6.0 per million children, respectively (Tables 2 and 3).
Table 2.
Annual incidence of pediatric persistent PAH, US MarketScan Data, 2010–2013.
| Cases (n) | Children- years | Incidence per 1,000,000 children-years (95% CI) | |
|---|---|---|---|
| 2010 | |||
| Total | 54 | 8,595,453 | 6.3 (4.7–8.2) |
| Age (years) | |||
| <1 | 30 | 273,872 | 109.5 (73.9–156.4) |
| 1 - < 2 | 18 | 429,984 | 41.9 (24.8–66.2) |
| 2 - < 6 | 2 | 1,832,981 | 1.1 (0.1–3.9) |
| 6 - < 12 | 1 | 2,931,260 | 0.3 (0–1.9) |
| 12 - < 18 | 3 | 3,127,356 | 1.0 (0.2–2.8) |
| Type of PAH | |||
| Idiopathic | 4 | 8,595,453 | 0.5 (0.1–1.2) |
| Non-idiopathic | 50 | 8,595,453 | 5.8 (4.3–7.7) |
| 2011 | |||
| Total | 79 | 9,699,243 | 8.1 (6.5–10.2) |
| Age (years) | |||
| <1 | 31 | 314,670 | 98.5 (66.9–139.8) |
| 1 - < 2 | 24 | 482,674 | 49.7 (31.9–74.0) |
| 2 - < 6 | 7 | 2,065,065 | 3.4 (1.4–7.0) |
| 6 - < 12 | 7 | 3,296,302 | 2.1 (0.9–4.4) |
| 12 - < 18 | 10 | 3,540,531 | 2.8 (1.4–5.2) |
| Type of PAH | |||
| Idiopathic | 8 | 9,699,243 | 0.8 (0.4–1.6) |
| Non-idiopathic | 71 | 9,699,243 | 7.3 (5.7–9.2) |
| 2012 | |||
| Total | 57 | 9,134,430 | 6.2 (4.7–8.1) |
| Age (years) | |||
| <1 | 25 | 307,789 | 81.2 (52.6–119.9) |
| 1 - < 2 | 19 | 477,784 | 39.8 (23.9–62.1) |
| 2 - < 6 | 2 | 1,957,535 | 1.0 (0.1–3.7) |
| 6 - < 12 | 4 | 3,065,386 | 1.3 (0.4–3.3) |
| 12 - < 18 | 7 | 3,325,936 | 2.1 (0.8–4.3) |
| Type of PAH | |||
| Idiopathic | 8 | 9,134,430 | 0.9 (0.4–1.7) |
| Non-idiopathic | 49 | 9,134,430 | 5.4 (4.0–7.1) |
| 2013 | |||
| Total | 29 | 5,990,915 | 4.8 (3.2–7.0) |
| Age (years) | |||
| <1 | 11 | 232,034 | 47.4 (23.6–84.8) |
| 1 - < 2 | 12 | 349,614 | 34.3 (17.7–60.0) |
| 2 - < 6 | 1 | 1,296,013 | 0.8 (0.01–4.3) |
| 6 - < 12 | 1 | 1,952,385 | 0.5 (0.01–2.9) |
| 12 - < 18 | 4 | 2,160,870 | 1.9 (0.5–4.7) |
| Type of PAH | |||
| Idiopathic | 3 | 5,990,915 | 0.5 (0.1–1.5) |
| Non-idiopathic | 26 | 5,990,915 | 4.3 (2.8–6.4) |
Table 3.
Annual prevalence of pediatric persistent PAH, US MarketScan Data, 2010–2013.
| Cases (n) | Patients (n) | Prevalence per 1,000,000 (95% CI) | |
|---|---|---|---|
| 2010 | |||
| Total | 285 | 11,086,365 | 25.7 (22.8–28.9) |
| Age (years) | |||
| <1 | 32 | 430,980 | 74.3 (50.8–104.8) |
| 1 - < 2 | 56 | 513,019 | 109.2 (82.5–141.8) |
| 2 - < 6 | 77 | 2,253,524 | 34.2 (27.0–42.7) |
| 6 - < 12 | 50 | 3,745,948 | 13.4 (9.9–17.6) |
| 12 - < 18 | 70 | 4,142,894 | 16.9 (13.2–21.4) |
| Type of PAH | |||
| Idiopathic | 49 | 11,086,365 | 4.4 (3.3–5.8) |
| Non-idiopathic | 236 | 11,086,365 | 21.3 (18.7–24.2) |
| 2011 | |||
| Total | 411 | 12,598,795 | 32.6 (29.5–35.9) |
| Age (years) | |||
| <1 | 35 | 496,225 | 70.5 (49.1–98.1) |
| 1 - < 2 | 73 | 579,038 | 126.1 (98.8–158.5) |
| 2 - < 6 | 135 | 2,567,183 | 52.6 (44.1–62.2) |
| 6 - < 12 | 78 | 4,270,476 | 18.3 (14.4–22.8) |
| 12 - < 18 | 90 | 4,685,873 | 19.2 (15.4–23.6) |
| Type of PAH | |||
| Idiopathic | 76 | 12,598,795 | 6.0 (4.8–7.6) |
| Non-idiopathic | 335 | 12,598,795 | 26.6 (23.8–29.6) |
| 2012 | |||
| Total | 400 | 12,572,116 | 31.8 (28.8–35.1) |
| Age (years) | |||
| <1 | 29 | 495,912 | 58.5 (39.2–84.0) |
| 1 - < 2 | 50 | 582,243 | 85.9 (63.7–113.2) |
| 2 - < 6 | 138 | 2,540,212 | 54.3 (45.6–64.2) |
| 6 - < 12 | 87 | 4,259,774 | 20.4 (16.4–25.2) |
| 12 - < 18 | 96 | 4,693,975 | 20.5 (16.6–25.0) |
| Type of PAH | |||
| Idiopathic | 61 | 12,572,116 | 4.9 (3.7–6.2) |
| Non-idiopathic | 339 | 12,572,116 | 27.0 (24.2–30.0) |
| 2013 | |||
| Total | 287 | 10,175,994 | 28.2 (25.0–31.7) |
| Age (years) | |||
| <1 | 12 | 403,757 | 29.7 (15.3–51.9) |
| 1 - < 2 | 41 | 473,785 | 86.5 (62.1–117.4) |
| 2 - < 6 | 96 | 2,025,510 | 47.4 (38.4–57.9) |
| 6 - < 12 | 63 | 3,457,836 | 18.2 (14.0–23.3) |
| 12 - < 18 | 75 | 3,815,106 | 19.7 (15.5–24.6) |
| Type of PAH | |||
| Idiopathic | 45 | 10,175,994 | 4.4 (3.2–5.9) |
| Non-idiopathic | 242 | 10,175,994 | 23.8 (20.9–27.0) |
Of the persistent PAH cases, 83% received monotherapy, 13% received dual therapy, and 3% received triple therapy (Table 4). Note that two children had concomitant use of four drug types. The majority of treatments received were PDE-5 inhibitors; CCBs were the second most commonly prescribed drugs.
Table 4.
Distribution of drug treatment among persistent PAH.
| Persistent PAH n = 695 (%) | |
|---|---|
| PAH treatment before 2010 | 40 (5.8) |
| PAH treatment in 2010 or after | 655 (94.2) |
| Monotherapy among children with PAH treatment in 2010 or after | |
| One drug category during follow-up | |
| Total | 528 (80.6) |
| PDE-5 inhibitors | 449 (68.5) |
| Endothelin receptor antagonists | 9 (1.4) |
| CCBs | 59 (9.0) |
| Prostanoids | 11 (1.7) |
| Two drug categories during follow-up (one mono switched to another mono therapy) | |
| Total | 18 (2.7) |
| PDE-5 inhibitors to CCBs | 8 (1.2) |
| PDE-5 inhibitors to endothelin receptor antagonists | 3 (0.5) |
| PDE-5 inhibitors to prostanoids | 2 (0.3) |
| CCBs to PDE-5 inhibitors | 3 (0.5) |
| CCBs to endothelin receptor antagonists | 1 (0.2) |
| Endothelin receptor antagonists to PDE-5 inhibitors | 1 (0.2) |
| Concomitant dual therapy among children with PAH treatment in 2010 or after | |
| Total | 86 (13.1) |
| PDE-5 inhibitors + endothelin receptor antagonists | 49 (7.5) |
| PDE-5 inhibitors + CCBs | 14 (2.1) |
| PDE-5 inhibitors + prostanoids | 18 (2.7) |
| Endothelin receptor antagonists + prostanoids | 2 (0.3) |
| Others* | 3 (0.5) |
| Concomitant triple therapy among children with PAH treatment in 2010 or after | |
| Total | 21 (3.2) |
| PDE-5 inhibitors + endothelin receptor antagonists + CCBs | 2 (0.3) |
| PDE-5 inhibitors + endothelin receptor antagonists + prostanoids | 18 (2.7) |
| Endothelin receptor antagonists + CCBs + prostanoids | 1 (0.2) |
| Concomitant quad therapy among children with PAH treatment in 2010 or after | |
| PDE-5 inhibitors + endothelin receptor antagonists + CCBs + prostanoids | 2 (0.3) |
Switched from one dual therapy to another.
Medical procedures used to diagnose PAH are described in Table 5. Around 93% of children had received either an echocardiography or a right heart catheterization just before, on, or after the PAH diagnosis. Table 5 shows more details.
Table 5.
Distribution of medical procedures claimed in the 30 days before, on, or after diagnosis among persistent PAH.
| Procedure | Persistent PAH n = 695 (%) |
||
|---|---|---|---|
| Within 30 days before, on, or any time after index date | |||
| Before and on | After | Total* | |
| Critical procedures | |||
| At least one critical procedure | 524 (75.4) | 644 (92.7) | 675 (97.1) |
| Chest radiograph | 346 (49.8) | 529 (76.1) | 558 (80.3) |
| Chest CT | 10 (1.4) | 56 (8.1) | 63 (9.1) |
| Chest CT with contrast | 28 (4.0) | 98 (14.1) | 112 (16.1) |
| Electrocardiogram | 258 (37.1) | 513 (73.8) | 559 (80.4) |
| Echocardiography | 408 (58.7) | 589 (84.7) | 641 (92.2) |
| Right heart catheterization | 63 (9.1) | 228 (32.8) | 254 (36.5) |
| Echocardiography or right heart catheterization | 421 (60.6) | 589 (84.7) | 643 (92.5) |
| Other procedures | |||
| Pulmonary function tests | 26 (3.7) | 138 (19.9) | 144 (20.7) |
| Arterial blood gases analysis | 57 (8.2) | 214 (30.8) | 234 (33.7) |
| Ventilation/perfusion lung scan | 6 (0.9) | 36 (5.2) | 38 (5.5) |
| Pulmonary angiography | 3 (0.4) | 8 (1.2) | 9 (1.3) |
| Cardiac magnetic resonance imaging | 7 (1.0) | 28 (4.0) | 34 (4.9) |
Total means the number of children with at least one procedure either within 30 days before or any time after the index date.
Co-morbidities of the 695 persistent PAH children are provided in Table 6. Around 75% of children had a CHD diagnosis in their record at some time and most of these were “bulbus cordis anomalies and anomalies of cardiac septal closure” and “other congenital anomalies of the heart.” In addition, 27% had some kind of congenital syndrome such as Down’s syndrome and 20% had some kind of congenital musculoskeletal or respiratory system anomaly. Other co-morbidities recorded in the 90 days prior to the index date are also noted in Table 6. Note that one child could have more than one type of co-morbidity.
Table 6.
Distribution of co-morbidities of persistent PAH.
| Persistent PAH | |
|---|---|
| n = 695 (%) | |
| Congenital heart defects recorded any time in medical records | |
| At least one congenital heart defect | 522 (75.1) |
| Bulbus cordis anomalies and anomalies of cardiac septal closure | 428 (61.6) |
| Other congenital anomalies of heart | 347 (49.9) |
| Patent ductus arteriosus | 167 (24.0) |
| Anomalies of pulmonary artery | 72 (10.4) |
| Coarctation of aorta | 61 (8.8) |
| Anomalies of great veins | 47 (6.8) |
| Other anomalies of aorta | 37 (5.3) |
| Syndromes recorded any time in medical records | |
| At least one syndrome | 189 (27.2) |
| Down | 87 (12.5) |
| Other and unspecified congenital anomalies* | 52 (7.5) |
| Various chromosomal anomalies† | 40 (5.8) |
| Digeorge | 22 (3.2) |
| Congenital hydrocephalus | 13 (1.9) |
| Velocardiofacial | 7 (1.0) |
| Prune belly | 2 (0.3) |
| Ehlers-Danlos | 1 (0.1) |
| Congenital musculoskeletal deformities or congenital anomalies of respiratory system | |
| At least one condition below | 136 (19.6) |
| Other congenital anomalies of larynx, trachea, and bronchus | 70 (10.1) |
| Congenital agenesis hypoplasia and dysplasia of lung | 50 (7.2) |
| Congenital anomalies of diaphragm | 33 (4.7) |
| Congenital anomalies of lung | 23 (3.3) |
| Congenital musculoskeletal deformities of spine | 10 (1.4) |
| Congenital cystic lung | 4 (0.6) |
| Other genetic co-morbidities recorded any time in medical records | |
| Cystic fibrosis | 2 (0.3) |
| Gaucher disease | 2 (0.3) |
| Von Willebrand’s disease | 2 (0.3) |
| Alpha-1-antitrypsin deficiency | 1 (0.1) |
| Neurofibromatosis | 1 (0.1) |
| Co-morbidities recorded any time before or on the first diagnosis of PAH | |
| Valvular disease | 96 (13.8) |
| With congenital heart diseases | 93 (13.4) |
| Without congenital heart diseases | 3 (0.4) |
| Heart failure | 84 (12.1) |
| With congenital heart diseases | 81 (11.7) |
| Without congenital heart diseases | 3 (0.4) |
| Sleep-disordered breathing | 42 (6.0) |
| Chronic obstructive pulmonary disease | 41 (5.9) |
| Cardiomyopathy | 35 (5.0) |
| With congenital heart diseases | 34 (4.9) |
| Without congenital heart diseases | 1 (0.1) |
| Thyroid disorders | 19 (2.7) |
| Scoliosis and kyphoscoliosis | 13 (1.9) |
| Chronic hemolytic anemia | 11 (1.6) |
| Portal hypertension | 10 (1.4) |
| Infantile cerebral palsy | 8 (1.2) |
| Connective tissue diseases | 6 (0.9) |
| Interstitial lung disease | 5 (0.7) |
| Myeloproliferative disorders‡ | 4 (0.6) |
| Vasculitis | 3 (0.4) |
| Fibrosing mediastinitis | 3 (0.4) |
| Obesity hypoventilation syndrome | 1 (0.1) |
| Lung cancer | 1 (0.1) |
| Glycogen storage disease | 1 (0.1) |
| Kidney cancer | 1 (0.1) |
| Co-morbidities recorded within 90 days before index date | |
| Respiratory failure | 135 (19.4) |
| Pulmonary collapse | 113 (16.3) |
| Pneumonia | 79 (11.4) |
| Respiratory distress syndrome | 49 (7.1) |
| Respiratory failure of newborn | 30 (4.3) |
| Pneumothorax | 11 (1.6) |
| Post-inflammatory pulmonary fibrosis | 11 (1.6) |
| Pulmonary embolism | 10 (1.4) |
| Chronic renal failure | 4 (0.6) |
Includes Patau's syndrome, Edward's syndrome, Cri-du-chat syndrome, other microdeletions, other autosomal deletions, balanced autosomal translocation in normal individual, other conditions due to autosomal anomalies, gonadal dysgenesis, Klinefelter's syndrome, other conditions due to sex chromosome anomalies, other conditions due to chromosome anomalies, conditions due to anomaly of unspecified chromosome.
Includes Prader-willi syndrome, Marfan syndrome, fragile X syndrome, other specified congenital anomalies.
Includes polycythemia vera, essential thrombocytosis, primary or idiopathic myelofibrosis, chronic myelogenous leukemia.
Discussion
Although PAH is very rare in children and especially rare in the absence of etiological factors such as CHD, the annual incidence rates and prevalence of PAH in this US commercially insured pediatric population were higher than the estimates reported in registry-based studies from European countries. This study represents the first large, pediatric, population-based, epidemiological study using a highly specific definition of PAH. The PAH patients were identified from over 10 million children (aged < 18 years) distributed across the US and represented in the MarketScan Database.
In this pediatric population, the annual incidence rate of PAH was in the range of 5–8 per million children-years in 2010–2013. The prevalence of PAH was in the range of 26–33 per million children. The incidence rate and prevalence for idiopathic PAH were even lower: 0.5–0.9 per million children-years and 4.4–6.0 per million children, respectively. Although the incidence and prevalence of PAH are higher than those reported in previous studies from Europe, our estimates of idiopathic PAH were similar to those reported. A multicenter registry-based study conducted in France estimated a prevalence of 3.7 cases per million children for PAH and a prevalence of 2.2 cases for idiopathic PAH.12 However, this study excluded PAH cases caused by CHD. Similarly, the annual incidence and prevalence in the UK was 0.48 and 2.1 cases of idiopathic PAH per million children, respectively.13 Using the Dutch national registry data, van Loon et al. reported an annual incidence and point prevalence of 0.7 and 4.4 cases of idiopathic PAH and 2.2 and 15.6 cases for PAH-CHD per million children, respectively.14
A few registry-based epidemiological studies have described the clinical features or treatment patterns for pediatric PAH. The TOPP registry study, an international registry for pediatric PH, found that PAH was present in 317 (88%) out of 362 children with PH, and, among those with PAH, 182 (57%) were idiopathic or familial and 115 (36%) were associated with CHD. In addition, 13% of children with PH had chromosomal anomalies (mainly Down’s syndrome) and 13% were premature (gestation < 37 weeks).6 Data from 216 US pediatric patients from the REVEAL registry showed approximately half of the patients had idiopathic PAH and 35% had PAH associated with CHD. At enrollment, monotherapy with prostanoids, PDE-5 inhibitors or endothelin receptor antagonists was given to 87 (41%) of the overall PAH patients, dual therapy was given to 63 (30%), and triple therapy was given to 28 (13%). In total, 121 (56%) of the patients included in this analysis were treated with PDE-5 inhibitors alone or in combination.18 In the French registry study, 50 children with PAH were followed, of whom 70% were idiopathic or familial and 18% had a history of premature birth (gestation < 36 weeks). Treatment changed markedly during follow-up with an increase in the proportion of patients treated with sildenafil (6% at enrollment and 34% at the last assessment). Treatment patterns also changed from monotherapy to dual and triple therapy. Bosentan was the most frequently prescribed drug at start and end of follow-up within this population.12 In the UK, Moledina et al. identified 64 children with idiopathic PAH: 41 treatment-naïve at start; and 23 who were already receiving treatment. Around 57% of those initially treated with monotherapy progressed to dual therapy after a median of 23 months and seven progressed to triple therapy.13 In the Netherlands, data from two national registries showed that among 154 progressive PAH pediatric patients, 23% were idiopathic and 72% were associated with CHD. PAH drugs were prescribed to 53 (34%) of all progressive PAH patients, of whom 37 received one drug, 12 received two drugs, and four received three drugs.14
In our study, most PAH cases (75%) had some type of CHD, 13% had Down’s syndrome, and only 18% were idiopathic. These results were similar to the Dutch registry report,14 but not other registry studies.6,12,18 Since there was no information available on the etiological nature of PAH, we cannot rule out the possibility that our etiological classification of PAH cases, based on the co-morbidity profile, resulted in some misclassification of non-idiopathic cases. It is important to note that 82% of cases were associated with other conditions in our study, mainly CHD and chromosomal anomalies or syndromes. Together with idiopathic PAH, PAH associated with CHD is the most common form of PAH in pediatric patients. In addition, CHD is frequently associated with complex syndromes like Down’s syndrome. In a recent analysis of patients with PAH and CHD, 45% had concurrent syndromes, mostly Down’s syndrome (30/60 children), or other associated complex syndromes like Noonan or DiGeorge.19 Both CHD and/or other complex syndromes or diseases can cause PAH and increase the need for specialist care in pediatric patients, often from the first day of life, or even prenatally. In addition, the prognosis in these complex patients may be aggravated by their co-morbidities. Thus, (multi)specialist care in these patients is expected and management of PAH may be only one of several problems that need attention. If repair of the CHD occurred in children whose PAH was diagnosed early, these PAH-CHD cases may not have been captured in registry-based studies. In addition, 36% of our study population was born prematurely, which is a higher proportion as compared to the TOPP registry study (13%) and the French registry study (18%).6,12 Differences in the study source population and in the methods for PAH case ascertainment may explain the discrepancies between the US and European analyses. We did not exclude patients born prematurely from the PAH case definition because, while prematurity often causes chronic lung disease (CLD), which is the most frequent cause of PH in Group 3 of Dana Point Classification, only a small proportion of premature babies are born early enough to develop severe CLD and associated PH. To assess the extent of prematurity we provided information on gestational age in Table 1: 16% of children were known to be born before or at 32 weeks of gestation. It is acknowledged that the number of hospitalizations due to CLD associated with PH have increased in the US, likely explained by the improved survival of very low and extremely low birthweight infants.20 However, those who had CLD due to prematurity would likely have received a diagnosis of CLD and thus been excluded from the case definition in the absence of an etiological cause for PAH.
We also found that most PAH cases were treated with monotherapy only during follow-up and a smaller proportion of children were treated with dual or triple therapy compared with previous studies. Drug treatments included predominantly use of PDE-5 inhibitors (80%) and CCBs (9%), both in monotherapy. It is difficult to compare these results directly with previous findings from registry-based studies which included a number of patients who were treatment-naïve at enrollment, while PH treatment was part of the definition of PAH in our study. However, treatment patterns at the end of follow-up in most European and US registries show a similar distribution of treatments with a predominance of PDE-5 inhibitors; though in the French registry, endothelin receptor antagonists were the most commonly prescribed drugs. In addition, there was more polytherapy in the UK registry as compared to our study, which may be related to differences in severity of PAH between the two study populations. It was expected that PDE-5 inhibitors would be the most commonly used treatments in the study period due to its recent commercialization. The PDE-5 inhibitor sildenafil was first approved for use in adults in June 2005 by the US Food and Drug Administration. The intravenous formulation was approved in November 2009, also for adults. The pediatric studies and experience with this drug have led the medical community to often use PDE-5 inhibitors as the base for PAH treatment (alone or in combination). The findings of our research indicate a scarcity of treatment options for this population and the need for alternative treatment combinations that could be more tailored to individual patient needs.
Only 37% of PAH patients in our study had a record for right heart catheterization, whereas nearly all, 92%, had echocardiography, which is a non-invasive, though less accurate, technique for diagnosing PAH. The rate of right heart catheterization found in our study is lower than in some registry-based studies (e.g. 71% in the Dutch Registry14), but is consistent with the general notion that most children with PH do not undergo a complete evaluation.14,21 Right heart catheterization in children has a higher risk of adverse outcomes than in adults,21 in particular among critically ill preterm babies already at risk for adverse outcomes. This could be the reason for avoiding right heart catheterization in some patients. Clinical and echocardiographic assessment may be the diagnostic approach with the best benefit–risk profile in these high-risk patients.22 Among study PAH patients who were known to be born before or at 32 weeks of gestation, only 19% had a record for right heart catheterization, which lends support to this possibility. Other reasons could include lack of consent by parents or patients. If true, this observation suggests that there is a need for non-invasive, safe diagnostic procedures at least as accurate as right heart catheterization to improve the rate of diagnosis and precise clinical evaluation of PAH. Furthermore, right heart catheterization is also used to assess the response to pulmonary vasodilators before starting CCBs, thus it is possible that patients did not have the opportunity to receive guideline-recommended CCBs as a first therapy because no acute vasoreactivity testing was performed in the majority of patients. Moreover, CCBs are contraindicated in children who have not undergone or are non-responsive to acute vasoreactivity testing.1 In our study, only around 33% of the CCB users had a record for right heart catheterization. The reported proportion of children with idiopathic PAH who received CCBs as monotherapy (and were acute responders) has been quite variable: 24% in a Dutch cohort,23 11% in the French registry,12 6% in the Spanish registry,24 and 9% in the UK registry.13 In the Reveal registry,25 20% (43/211) of all PAH patients received CCBs as monotherapy (21/211) or in combination (22/211), and 42 of 43 patients had undergone acute vasoreactivity testing. Twenty-two (52%) were responders, according to the adapted pediatric definition. These patients were treated with CCBs and had a better prognosis. In our analysis, 9% of PAH patients received CCBs in monotherapy, 2.1% in dual combination therapy, 0.5% in triple combination therapy, and 0.3% in quadruple combination therapy; 11.9% of patients in total. Despite this, because a majority of PAH patients (68.5%) were prevalent cases, i.e. they may not have been in the database when the condition first occurred, we cannot rule out the possibility that we missed some right heart catheterization procedures if they occurred when the diagnosis of PAH was first made or the PAH-targeted treatment was initiated.
This study has some limitations to be considered when interpreting the results. First, our analysis is based on a claims database that, because of its administrative character, may have included incorrect diagnosis codes. However, we identified PAH patients based on diagnostic codes and PAH-targeted treatment, likely increasing the validity of our case definition and reducing potential inclusion of non-PAH cases (e.g. those with only rule-out diagnoses that were later not confirmed and consequently did not result in treatment). Second, the CCE database provides year of birth (not month and day) for confidentiality reasons. Although we estimated actual birthdate by searching for the first ICD-9-CM birth code, there is a possibility that we misclassified the age at the first/last diagnosis of PH or PPHN for some cases, especially in very young children. To examine the potential impact of age misclassification, we conducted secondary analyses to use 365 days of age instead of 180 days of age as the cutoff for the PAH grouping. The results were similar to the primary findings: incidence rates of 2–6 cases per million children-years and prevalence of 23.4–30.4 cases per million children. Third, there is no access to original clinical records in the CCE database, thus validation of PAH was limited to electronic record review. In a later step, we excluded those cases who had an etiological cause compatible with groups 2–5 of the Dana Point classification and none compatible with PAH to minimize misclassification. Still, some of the PAH patients have a concomitant underlying condition compatible with other PH groups (Table 6) but the causal relationship between these conditions and the PH diagnosis could not be disentangled. Therefore, although some misclassification of PAH may have occurred, we believe it was minimal given that in clinical practice almost 90% of diagnosed PH cases among children are in fact PAH.14 We did not have information on the point of service or the specialty of the treating physician, which could have provided additional confidence in a PAH diagnosis. We also did not have complete information on surgery or catheter treatment among those who had CHD, particularly among prevalent cases aged 4 years or older since such treatments could have occurred very early in their life and thus not have been captured in the CCE database. When we classified PAH cases into idiopathic and non-idiopathic, we relied on their co-morbidity profile to identify conditions listed in the Dana Point group 1 and chromosomal anomalies or syndromes (such as Down’s syndrome). Cases of idiopathic PAH, by definition, did not have conditions such as neurofibromatosis, chronic pulmonary disease, etc., because these patients were excluded at an earlier stage in the study. Nevertheless, it is likely that some PAH and idiopathic PAH cases were misclassified. We did not have information on gene mutations to distinguish heritable PAH from idiopathic PAH thus there may be some heritable PAH cases included in the idiopathic group. Additionally, patients in the US change health insurance plans frequently so long-term follow-up in the CCE database was limited. Consequently, we did not always have clinical data at the time that the original PH/PPHN diagnosis was made. To minimize the impact of this difficulty we restricted the study population to patients who had been registered for at least 1 year if they were aged 1 year or older, hence ensuring a follow-up of at least 1 year among PAH cases before the first diagnosis was recorded. This selection is likely non-biased and thus the population at risk in the CCE database is still fairly representative of the overall US population. Fourth, the CCE database does not record drugs prescribed in hospitals. Therefore, it is possible that we missed some drug treatments, especially for severe cases who were likely to be hospitalized. Fifth, while the CCE database is geographically largely representative of the US, it does represent commercially insured patients and their families and thus not all social groups are equally covered, e.g. families on social welfare and Medicaid are not represented. However, there is no evidence for differences in pediatric PAH occurrence across different socioeconomic groups. Finally, there were more boys than girls in each year of the study (difference in the range of 4.29–4.46%). This is consistent with data from the U.S. Census Bureau statistics which also reported a difference (4.5%) between boys and girls aged ≤19 years in 2010.26
In conclusion, this analysis of a large, population-based cohort of pediatric patients using claims data indicates that the incidence and prevalence of PAH among US children is low and most affected patients have CHD and/or chromosomal anomalies/syndromes. PDE-5 inhibitors are the most commonly used therapy as monotherapy or in combination with other drugs. The results suggest that many patients do not undergo the recommended right heart catheter diagnostic evaluation. Further research is needed in the field of pediatric PAH to provide the medical community with a better understanding of this condition for better management and improved outcomes in these patients. New non-invasive diagnostic methods or algorithms and new evidence-based treatment options should be the focus of future pediatric PAH research. Further epidemiological research will benefit from accurate and validated case ascertainment methods.
Appendix I. Birthdate estimation
Although MarketScan data only provide year of birth, for confidentiality reasons, we tried to identify the actual birthdates for each child by searching their record for ICD-9-CM birth codes (see Supplementary Table 2 below) for those whose year of birth was 2007 or later (date of MarketScan data available at BU). If the date of the first recorded birth code was the date of their first medical activity (including claims, procedures, or drugs) and the recorded year of the first birth code matched the year of birth provided in MarketScan date, we assumed the date of the first birth code was the actual birthdate. For other patients who were born in 2007 or later and did not have a birth code, we assumed their birthdate was January 1 in the year of their birth if they had their first medical activity recorded before July 1 in that year, otherwise, their birthdate was considered to be July 1 in the year of their birth. For the remaining patients who were born before 2007, their birthdates were considered to be July 1 in the year of their birth.
Conflict of interest
LL and SJ received significant funding from Bayer Pharma AG to conduct the study. SB, GH, AM, and DV are employees of Bayer Pharma AG.
Funding
This study was sponsored by Bayer Pharma AG.
References
- 1.Abman SH, Hansmann G, Archer SL, et al. American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society. Pediatric Pulmonary Hypertension: Guidelines from the American Heart Association and American Thoracic Society. Circulation 2015; 132: 2037–2099. [DOI] [PubMed] [Google Scholar]
- 2.Hopper RK, Abman SH, Ivy DD. Persistent challenges in pediatric pulmonary hypertension. Chest 2016; 150: 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonneau G, Galiè N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004; 43: S5–S12. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009; 54: S43–S54. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 6.Berger RM, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet 2012; 379: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivy DD, Abman SH, Barst RJ, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol 2013; 62: D117–D126. [DOI] [PubMed] [Google Scholar]
- 8.Abman SH. Pulmonary hypertension in children: a historical overview. Pediatr Crit Care Med 2010; 11: S4–S9. [DOI] [PubMed] [Google Scholar]
- 9.Barst RJ. Children deserve the same rights we do: the need for paediatric pulmonary arterial hypertension clinical drug development. Heart 2010; 96: 1337–1338. [DOI] [PubMed] [Google Scholar]
- 10.Barst RJ, Ertel SI, Beghetti M, et al. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J 2011; 37: 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansmann G, Hoeper MM. Registries for paediatric pulmonary hypertension. Eur Respir J 2013; 42: 580–583. [DOI] [PubMed] [Google Scholar]
- 12.Fraisse A, Jais X, Schleich JM, et al. Characteristics and prospective 2-year follow-up of children with pulmonary arterial hypertension in France. Arch Cardiovasc Dis 2010; 103: 66–74. [DOI] [PubMed] [Google Scholar]
- 13.Moledina S, Hislop AA, Foster H, et al. Childhood idiopathic pulmonary arterial hypertension: a national cohort study. Heart 2010; 96: 1401–1406. [DOI] [PubMed] [Google Scholar]
- 14.van Loon RL, Roofthooft MT, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation 2011; 124: 1755–1764. [DOI] [PubMed] [Google Scholar]
- 15.Pickens G, Moldwin E and Marder W. Healthcare spending index for employer-sponsored insurance: methodology and baseline results. Available at: http://truvenhealth.com/Portals/0/Assets/HealthInsights/TRU_15667_0415_HSI_ESI_WP.pdf (accessed 15 September 2015).
- 16.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 17.Rosenzweig EB, Feinstein JA, Humpl T, et al. Pulmonary arterial hypertension in children: Diagnostic work-up and challenges. Prog Pediatr Cardiol 2009; 27: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barst RJ, McGoon MD, Elliott CG, et al. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation 2012; 125: 113–122. [DOI] [PubMed] [Google Scholar]
- 19.Zijlstra WM, Douwes JM, Ploegstra MJ, et al. Clinical classification in pediatric pulmonary arterial hypertension associated with congenital heart disease. Pulm Circ 2016; 6: 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank DB, Crystal MA, Morales DLS, et al. Trends in pediatric pulmonary hypertension–related hospitalizations in the United States from 2000–2009. Pulm Circ 2015; 5: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beghetti M, Schulze-Neick I, Berger RMF, et al. Haemodynamic characterisation and heart catheterisation complications in children with pulmonary hypertension: Insights from the Global TOPP Registry (tracking outcomes and practice in paediatric pulmonary hypertension). Int J Cardiol 2016; 203: 325–330. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan U, Rosenzweig EB. Pulmonary hypertension in chronic lung disease of infancy. Curr Opin Pediatr 2015; 27: 177–183. [DOI] [PubMed] [Google Scholar]
- 23.van Loon RL, Roofthooft MT, Delhaas T, et al. Outcome of pediatric patients with pulmonary arterial hypertension in the era of new medical therapies. Am J Cardiol 2010; 106: 117–124. [DOI] [PubMed] [Google Scholar]
- 24.del Cerro Marín MJ, Sabate Rotés A, Rodriguez Ogando A, et al. Assessing pulmonary hypertensive vascular disease in childhood: data from the Spanish registry. Am J Respir Crit Care Med 2014; 190: 1421–1429. [DOI] [PubMed] [Google Scholar]
- 25.Barst RJ, McGoon DC, Elliot CG, et al. Survival in childhood pulmonary arterial hypertension insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management. Circulation 2012; 125: 113–122. [DOI] [PubMed] [Google Scholar]
- 26.Howden LM and Meyer JA. Age and sex composition: 2010. Available at: http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf (accessed 1 December 2015).