Abstract
The primary aim was to explore the safety and tolerability of inhaled treprostinil when used in patients with pulmonary hypertension (PH) with concomitant chronic obstructive pulmonary disease (COPD). Patients with a diagnosis of pre-capillary PH (defined as pulmonary artery mean pressure of ≥ 25 mmHg and pulmonary artery wedge pressure or left ventricular end diastolic pressure of ≤ 15 mmHg) who were being initiated on inhaled treprostinil and had concomitant COPD (defined as FEV1/FVC ratio ≤ 70% with FEV1 ≥ 40% predicted) were considered for inclusion in this pilot study. Assessments included adverse events, physical exam, World Health Organization (WHO) functional class, 6-minute walk test (6MWT), modified Borg dyspnea score, and concomitant medication. At baseline and week 16 St. George’s Respiratory Questionnaire (SGRQ), arterial blood gas (ABG), and pulmonary function test (PFT) were assessed. The median age was 65 years (age range, 56–80 years) and five patients (56%) were men. Among the nine patients, a majority had an increase in 6MWT from baseline to week 16 (median change, 19 m). Only three of the nine patients (33%) had an increase in A-a gradient at week 16 (median change, –7). There was no difference in any of the following: arterial blood gases, WHO functional class, 6MWT results, or SGRQ scores from baseline to week 16. There was a statistically significant decline in several of the PFT measures, including FEV1 (median change, –0.18 L; P = 0.004; median change, –7% of predicted; P = 0.016), FVC (median change, –0.23 L; P = 0.027), and diffusion capacity for carbon monoxide (DLCO) (median change, –5% of predicted; P = 0.023). The small number of patients limits firm conclusions; however, inhaled treprostinil did not seem to adversely impact oxygenation in the majority of the study patients with pre-capillary PH and COPD. While there may have an adverse impact on some pulmonary function parameters, the clinical significance is unclear.
Keywords: pulmonary hypertension, chronic obstructive pulmonary disease (COPD), inhaled treprostinil, safety, tolerability
Introduction
Pulmonary hypertension (PH) secondary to chronic obstructive pulmonary disease (COPD) is classified as diagnostic group 3, otherwise known as PH associated with lung diseases and/or hypoxemia.1 PH with COPD is defined by a pulmonary artery mean pressure (mPAP) ≥ 25 mmHg and severe when the mPAP is ≥ 35 mmHg. The presence of PH in COPD adversely impacts survival and exercise capacity.2 Reports of the prevalence of PH in stable COPD are variable, in the range of 20–91% depending on the definition of PH, the severity of COPD, and the method of measuring the PAP.3–8 A number of mechanisms have been implicated in the development of PH in COPD and include pulmonary vascular vasoconstriction, remodeling, endothelial dysfunction, and genetic predisposition.7,9 It has also been shown that a mPAP > 18 mmHg is associated with an increased risk of severe acute exacerbation in patients with moderate to severe COPD.10 During such an exacerbation, the systolic PAP may increase as much as 20 mmHg but returns to baseline after resolution.11,12
The adverse effect of PH on survival and exercise capacity, as well as the increased risk of severe acute exacerbations caused by PH, provide the rationale for treating PH in COPD. Hence, the goals of treatment are to improve exercise tolerance, quality of life, and survival as well as reduce exacerbations. While pulmonary vasodilators may improve hemodynamics, the benefit may be offset by an adverse effect on ventilation-perfusion matching with resultant hypoxemia at rest.13–15
The phenomenon of worsening gas exchange due to altered ventilation-perfusion matching has been observed with oral pulmonary vasodilators; however, there are limited data on inhaled pulmonary vasodilator therapy for PH in COPD. Delivery of the vasodilator to better ventilated lung units may help to avoid the undesired deterioration in gas exchange. Treprostinil sodium is a prostacyclin analogue, which is used to treat pulmonary arterial hypertension (PAH) and is available in intravenous, subcutaneous, oral, and inhaled formulations. To explore the safety and tolerability of inhaled treprostinil, we performed a pilot study of the impact on arterial gas tension, symptoms, exercise capacity, and lung function when used in patients with pre-capillary PH and COPD.
Methods
Patients with a diagnosis of pre-capillary PH (defined as a pulmonary artery mean pressure of ≥ 25 mmHg and pulmonary artery wedge pressure or left ventricular end diastolic pressure of ≤ 15 mmHg) who were being initiated on inhaled treprostinil and had concomitant COPD (defined as forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio ≤ 70% with FEV1 ≥ 40% predicted) were considered for inclusion in this prospective, multi-center, open-label pilot study. The criteria were established using guidance from previous major efficacy trials in group 1 PAH that included patients with COPD. Inclusion criteria also included age range of 18–80 years and a baseline 6-minute walk test (6MWT) ≥ 150 m with no change in COPD treatment for at least 30 days prior to enrollment. Patients were excluded if they were treated with an endothelial receptor antagonist, phosphodiesterase inhibitor, or parenteral prostanoid within 3 months prior to enrollment. No change in COPD medications was permitted unless necessitated by exacerbation.
After enrollment, patients were assessed at baseline and weeks 4, 8, 12, and 16. Assessments included adverse events, physical examination, World Health Organization (WHO) functional class (FC) for PAH, 6MWT, modified Borg dyspnea score, and concomitant medication. At baseline and week 16, the following assessments were performed: St. George’s Respiratory Questionnaire (SGRQ), arterial blood gas (ABG), and pulmonary function test (PFT).
The primary aim was to explore the impact of inhaled treprostinil sodium inhalation on gas exchange when used in PH with COPD patients by measuring ABG at both the beginning and end of study. Secondary aims included the effects of inhaled treprostinil sodium on COPD-related quality of life, 6MWT, PFT, WHO FC, and the modified Borg dyspnea score at the end of the 6MWT.
Sample size
Originally it was determined that a sample size of 20 patients would be reasonable for the exploratory aims of this pilot study. However, the study was stopped early owing to lower than expected enrollment.
Statistical analysis
Categorical variables were summarized using the number and percent while numeric variables were summarized by the sample median and range and/or mean and standard deviation (SD). The change from baseline to week 16 was explored using a Wilcoxon signed rank test. P values ≤ 0.05 were considered statistically significant without adjustment for multiple testing.
Results
Nine patients were included in this prospective single-arm pilot study. The median age was 65 years (age range, 56–80 years) and five (56%) were men. Patient demographics, co-morbidities, and right heart catheterization results are summarized in Table 1.
Table 1.
Patient demographics, co-morbidities, and results of the diagnostic right-heart catheterization.
| Characteristic | Summary (n = 9) |
|---|---|
| Sex | |
| Female | 4 (44%) |
| Male | 5 (56%) |
| Age (years) | 65 (56–80) |
| Race | |
| Black or African American | 2 (22%) |
| White or Caucasian | 7 (78%) |
| Weight (kg) | 75 (46–177) |
| Height (cm) | 170.2 (157.5–182.9) |
| BMI (kg/m2) | 23.1 (18.5–59.0) |
| Smoking status | |
| Never | 0 |
| Former | 9 (100%) |
| Current | 0 |
| Pack-years of smoking | 40 (10–48) |
| Right heart catheterization | |
| RAP (mmHg) | 10 (2–14) |
| PAS (mmHg) | 74 (36–93) |
| PAD (mmHg) | 30 (14–48) |
| PAM (mmHg) | 46 (26–58) |
| PAWP or LVEDP (mmHg) | 11 (7–18) |
| CO (L/min) | 3.9 (2.2–7.6) |
| CI (L/min/m2) | 2.4 (1.3–4.0) |
| PVR (dynes/s/cm-5) | 729 (211–1491) |
Data are reported as sample median (minimum–maximum) or n (%).
BMI: body mass index; CI: cardiac index; CO: cardiac output; CVP: central venous pressure; LVEDP: left ventricular end diastolic pressure; PAD: pulmonary artery diastolic; PAH: pulmonary arterial hypertension; PAM: pulmonary artery mean; PAS: pulmonary artery systolic; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; RAP: right atrial pressure.
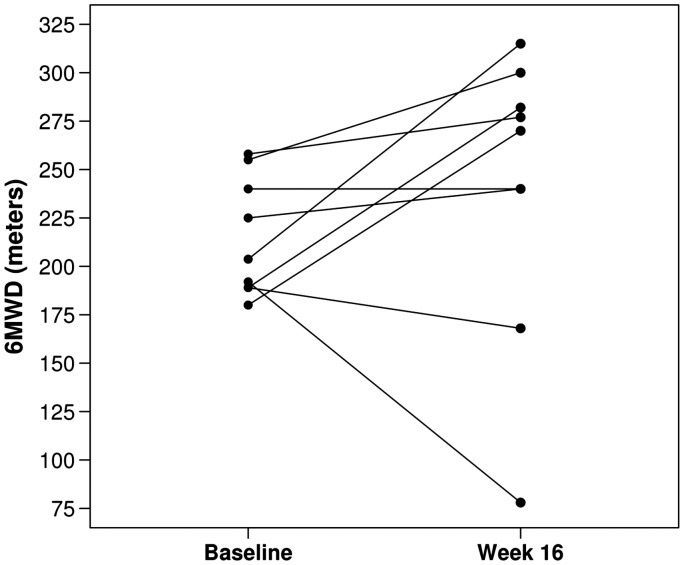
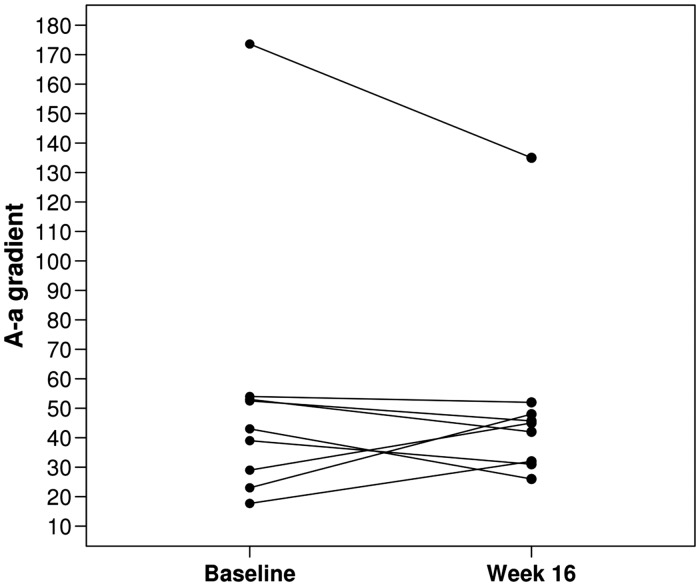
Among the nine patients, six (67%) had an increase in 6MWT from baseline to week 16 with an overall median change of 19 m (Fig. 1, Table 2). There was no overall difference in the A-a gradient from baseline to week 16 with only three of the nine patients (33%) experiencing an increase in A-a gradient from baseline to week 16 (median change, –7; Fig. 2, Table 2). There was no evidence of a significant difference in any of the following: ABG, WHO FC, 6MWT results, or SGRQ scores from baseline to week 16 (all P values ≥ 0.14; Table 2). There was a statistically significant decline in several of the PFT measures, including FEV1 (median change, –0.18 L; P = 0.004; median change, –7% of predicted; P = 0.016), FVC (median change, –0.23 L; P = 0.027), and diffusion capacity for carbon monoxide (DLCO) (median change, –5% of predicted; P = 0.023). None of the other PFT measures showed a statistically significant change from baseline to week 16 (all P values ≥ 0.074; Table 2).
Fig. 1.
6MWT at baseline and end of study.
Table 2.
Arterial blood gas (ABG), WHO functional class, 6-minute walk test (6MWT), pulmonary function testing (PFT), and quality of life at baseline and week 16 (n = 9).
| Variable | Baseline | Week 16 | Difference, Week 16–Baseline | P |
|---|---|---|---|---|
| ABG | ||||
| PaO2 (mmHg) | 0.64 | |||
| Median (range) | 58 (50–80) | 61 (46–79) | 0 (–24 – 29) | |
| PaCO2 (mmHg) | 0.44 | |||
| Median (range) | 35 (29–44) | 34 (32–62) | 2 (–4 – 23) | |
| Ph | 0.38 | |||
| Median (range) | 7.44 (7.39–7.51) | 7.42 (7.36–7.49) | –0.02 (–0.05 – 0.05) | |
| SaO2 % | 0.76 | |||
| Median (range) | 90.9 (86.7–95.6) | 91.7 (84.2–95.2) | –1.0 (–8.2 – 8.5) | |
| FiO2 (%) | 1.00 | |||
| Median (range) | 21 (21–36) | 21 (21–36) | 0 (0–0) | |
| A-a gradient | 0.73 | |||
| Median (range) | 43 (18–174) | 45 (26–135) | –7 (–39 – 25) | |
| WHO functional class | 1.00 | |||
| II | 0 | 1 (11%) | Decrease, 1 (11%) | |
| III | 9 (100%) | 7 (78%) | No change, 7 (78%) | |
| IV | 0 | 1 (11%) | Increase, 1 (11%) | |
| 6MWT | ||||
| Distance (m) | 0.38 | |||
| Median (range) | 204 (180–258) | 270 (78–315) | 19 (–114 – 111) | |
| Borg dyspnea scale | 0.67 | |||
| Median (range) | 3 (2–10) | 3 (1–10) | 0 (–7 – 6) | |
| PFT | ||||
| TLC (L) | 0.84 | |||
| Median (range) | 4.91 (3.64–6.37) | 5.07 (3.12–6.25) | –0.08 (–0.52 – 0.95) | |
| TLC (% of predicted) | 0.82 | |||
| Median (range) | 82 (79–102) | 88 (68–106) | –4 (–12 – 19) | |
| FEV1 (L) | 0.004 | |||
| Median (range) | 1.50 (0.97–2.45) | 1.32 (0.74–2.31) | –0.18 (–0.61 – –0.03) | |
| FEV1 (% of predicted) | 0.016 | |||
| Median (range) | 54 (41–79) | 54 (25–75) | –7 (–16 – 4) | |
| FVC (L) | 0.027 | |||
| Median (range) | 2.73 (1.45–4.26) | 2.46 (1.52–3.83) | –0.23 (–0.6 – 0.17) | |
| FVC (% of predicted) | 0.090 | |||
| Median (range) | 66 (56–95) | 68 (53–83) | –8 (–15 – 12) | |
| FEV1/FVC (%) | 0.16 | |||
| Median (range) | 67 (45–71) | 59 (33–71) | –4 (–16 – 3) | |
| RV/TLC (%) | 0.65 | |||
| Median (range) | 118 (29–167) | 115 (35.65–181) | 8 (–51.35 – 24) | |
| DLCO (mL/min/mmHg) | 0.074 | |||
| Median (range) | 8.60 (5.10–18.42) | 8.92 (5.20–14.01) | –1.20 (–4.93 – 1.18) | |
| DLCO (% of predicted) | 0.023 | |||
| Median (range) | 38 (28–63) | 32 (23–60) | –5 (–16 – 4) | |
| SGRQ | ||||
| Total score | 0.20 | |||
| Median (range) | 61 (48–73) | 64 (27–71) | –4 (–22 – 18) | |
| Symptoms score | 0.73 | |||
| Median (range) | 65 (32–81) | 56 (34–79) | 2 (–29 – 32) | |
| Activity score | 0.14 | |||
| Median (range) | 80 (64–93) | 73 (31–93) | –13 (–37 – 13) | |
| Impacts score | 0.57 | |||
| Median (range) | 52 (28–68) | 60 (17–72) | –2 (–322 – 22) |
P values result from Wilcoxon signed rank tests.
6MWT: 6-minute-walk test; A-a: Alveolar–arterial; DLCO: diffusion capacity; FEV1: forced expiratory volume in 1 s; FiO2: fraction of inspired oxygen; FVC: forced vital capacity; PaCO2: partial pressure of carbon dioxide; PFT: pulmonary function testing; PaO2: partial pressure of oxygen; RV: residual volume; SaO2: oxygen saturation; SGRQ: St. George’s Respiratory Questionnaire; TLC: total lung capacity; WHO: World Health Organization.
Fig. 2.
A-a gradient at baseline and end of study.
Concomitant COPD medications, WHO FC, and 6MWT results were obtained at baseline and every 4 weeks through the end of study. The data are summarized for each visit in Table 3. Although most of the patients maintained a stable FC, one patient declined to WHO FC 4 and one improved to WHO FC 2. Adverse events are summarized in Table 4 and are similar to reported data from previous trials in inhaled treprostinil.
Table 3.
WHO functional class, concomitant COPD medications, and 6MWT results at each study visit.
| Characteristic | Baseline (n = 9) | Week 4 (n = 9) | Week 8 (n = 9) | Week 12 (n = 8) | Week 16 (n = 9) |
|---|---|---|---|---|---|
| WHO functional class | |||||
| II | 0 | 0 | 0 | 1 (13%) | 1 (11%) |
| III | 9 (100%) | 9 (100%) | 8 (89%) | 7 (88%) | 7 (78%) |
| IV | 0 | 0 | 0 | 0 | 1 (11%) |
| Not reported | 0 | 0 | 1 (11%) | 0 | 0 |
| Concomitant PAH therapy | |||||
| Diuretic | 5 (56%) | 4 (44%) | 5 (56%) | 4 (50%) | 6 (67%) |
| Digoxin | 0 | 0 | 0 | 0 | 0 |
| Oxygen | 5 (56%) | 4 (44%) | 3 (33%) | 4 (50%) | 6 (67%) |
| Calcium channel blocker | 0 | 1 (11%) | 1 (11%) | 0 | 2 (22%) |
| Other | 8 (89%) | 7 (78%) | 7 (78%) | 5 (63%) | 7 (78%) |
| On oxygen | |||||
| No | 3 (33%) | 4 (44%) | 4 (44.4%) | 3 (38%) | 4 (44%) |
| Yes | 5 (56%) | 5 (56%) | 5 (55.6%) | 5 (63%) | 5 (56%) |
| Not reported | 1 (11%) | ||||
| Borg dyspnea scale | |||||
| Before 6MWT | 0.5 (0–5) | 0 (0–3) | 0 (0–3) | 1 (0–6) | 1 (0–7) |
| After 6MWT | 3 (2–10) | 3 (0–7) | 2 (0.5–9) | 3 (1–9) | 3 (1–10) |
| Oxygen saturation (%) | |||||
| Before 6MWT | 95 (92–98) | 95 (89–99) | 94 (88–99) | 95 (86–96) | 95 (79–98) |
| After 6MWT | 86 (82–95) | 84 (76–96) | 88 (77–95) | 84 (74–95) | 86 (71–98) |
| 6MWT distance (m) | 204 (180–258) | 231 (126–273) | 257 (135–360) | 230 (144–336) | 270 (78–315) |
Data for modified Borg dyspnea score (range 0–10) and oxygen saturation are reported as median (range) or n (%).
6MWT: 6-minute walk test; COPD: chronic obstructive pulmonary disease; WHO: World Health Organization.
Table 4.
Adverse events.
| Adverse events | Summary (n = 9) |
|---|---|
| Number of adverse events | |
| None | 1 (11%) |
| 1 | 0 |
| 2 | 1 (11%) |
| 3 | 3 (33%) |
| 4 or more | 4 (44%) |
| One of more of the following events | |
| Cough | 4 (44%) |
| Headache | 5 (56%) |
| Nausea | 1 (11%) |
| Dizziness | 0 |
| Flushing | 0 |
| Throat irritation | 3 (33%) |
| Pharyngeal discomfort | 0 |
| Diarrhea | 0 |
| O2 saturation < 88% | 0 |
| Symptomatic systemic hypotension | 0 |
| Clinically significant bleeding | 0 |
| Death | 0 |
| Other* | 7 (78%) |
| Highest degree of severity | |
| No adverse events | 1 (11%) |
| Mild | 0 |
| Moderate | 4 (44%) |
| Severe | 4 (44%) |
| Life-threatening | 0 |
| Fatal | 0 |
Other adverse events reported include: exacerbation of COPD (n = 2); shortness of breath (n = 2); decreased appetite or weight loss (n = 2); cold, rhinitis, productive cough, or pneumonia (n = 3); bloating (n = 1); blood in sputum (n = 1); urinary tract infection (n = 1); pulmonary embolism (n = 1); and suicidal ideation (n = 1).
Discussion
The current study is the first to date to comprehensively assess the impact of inhaled treprostinil on several parameters in patients with PH and COPD over the course of 16 weeks. Although the recruitment goals were not achieved, the results illustrate that a number of variables are important to consider while treating patients with PH and COPD. As a group, the A-a gradient remained stable which was the primary aim of the study. The result suggests that most patients with PH and COPD tolerate inhaled treprostinil from a gas exchange perspective. In addition, the WHO FC, SGRQ, and 6MWT were stable. In contrast, there was a statistically significant reduction in expiratory flows and volumes (FEV1 and FVC). Worsening underlying COPD and/or an adverse effect of the inhaled treprostinil are likely explanations. By comparison, a study by Dernaika et al. evaluated 10 men with FEV1 < 65% with arterial oxygen tension (Pa O2) 60–75 mmHg and PH (defined as systolic PAP > 35 mmHg plus RV dilatation and/or RV hypertrophy on echocardiography) before and after inhaling two doses of iloprost (2.5 µg). The treatment effect was examined by PFT, ABG, 6MWT, and ventilatory equivalents for O2 (VE/VO2) and CO2 (VE/VCO2) were performed at baseline, 30 min following each dose of iloprost, and 2 h after the second dose. Iloprost was associated with improved ventilation-perfusion matching and exercise tolerance reflected by an increase in 6MWT.16 Dernaika’s study was a short-term duration of only 2 h and therefore has limited comparative value to the current pilot.
Examining the patients on an individual basis is reasonable for a small pilot. The majority of patients maintained or improved their A-a gradient. Symptom burden by WHO FC remained unchanged in seven patients. A positive trend was seen in 6MWT as the majority of patients either maintained or improved, while two patients had worsening. One of the patients that experienced worsening of the 6MWT also had an exacerbation of COPD around the time of evaluation (patient 6).
The FEV1 in liters and percentage predicted was lower on inhaled treprostinil compare to baseline in all nine patients. The study design may have contributed as the PFT was done 1 h after treprostinil inhalation. Distinguishing an acute or chronic effect of inhaled treprostinil on airflow versus disease progression of COPD is impossible. Temporary airway irritation is suggested by the cough during administration and perhaps explains the stable SGRQ throughout the course of the study. There was also a trend for deterioration in absolute DLCO and a statistically positive result for worsening in DLCO as percentage predicted. The study was not designed to address why this occurred. The majority of the patients had a DLCO of 10 or less; therefore, small absolute changes translate into larger percentages. Regardless, the clinical impact of the DLCO seemed minimal to non-existent on both the PaO2 and A-a gradient. Overall, the impact of inhaled treprostinil on the disease-related quality of life was not significant but there was a decline seen in some patients while others reported improvement. The impact of inhaled treprostinil on PFT and SGRQ require further study in larger trials. All patients completed the study and the adverse event profile was in keeping with previously reported data in several trials. The detailed data on individual patients are shown in the supplemental tables. Nonetheless, the decline in expiratory flows and volumes and the one COPD exacerbation reinforce the safety concerns cannot be ignored in this setting.
There are a number of limitations to the study—in particular, the small sample size. The possibility of a type II error (i.e. false-negative finding) should be strongly considered. Also, a type I error (i.e. false-positive finding) cannot be ruled out given the number of statistical tests performed. Nonetheless, inhaled treprostinil may offer a potentially a safe and effective alternative to treat PH in this population. Larger studies are required to evaluate thoroughly inhaled treprostinil as a potential safe and effective therapy for patients with pre-capillary PH and COPD.
Conclusion
The small number of patients limits firm conclusions; however, inhaled treprostinil did not seem to impact oxygenation adversely in the majority of the study patients with concomitant PH and COPD. While there may have an adverse impact on some pulmonary function parameters, the clinical significance is unclear. The results in this small pilot merit further investigation in larger trials to determine if inhaled treprostinil is a potentially safe and effective option for patients with pre-capillary PH and COPD.
Conflict of interest
Charles D. Burger: Research Grant for Multi-center Trials: United Therapeutics, Actelion, and Gilead; Advisory Board: Actelion and Gilead; Consulting work: Gilead. Abubakr A. Bajwa: Research Grant for Multi-center Trials: United Therapeutics, Actelion, Gilead, Bayer, GSK, Intermune; Advisory Board: Actelion, Gilead, United Therapeutics, Bayer; Consulting work: United Therapeutics. Franck Rahaghi: Research Grant for Multi-center Trials: United Therapeutics, Lung Rx, Actelion, Gilead, Bayer, Reata, Bellerophon; Speaker: Actelion, Gilead, United Therapeutics, Bayer, Lung Rx; Consulting work: United Therapeutics, Actelion, Lung Rx, Bayer, Gilead
Funding
The study was funded by an investigator initiated grant from United Therapeutics.
References
- 1.Galie N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 2.Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest 1995; 107: 1193–1198. [DOI] [PubMed] [Google Scholar]
- 3.Burrows B, Kettel LJ, Niden AH, et al. Patterns of cardiovascular dysfunction in chronic obstructive lung disease. N Engl J Med 1972; 286: 912–918. [DOI] [PubMed] [Google Scholar]
- 4.Weitzenblum E, Sautegeau A, Ehrhart M, et al. Long-term course of pulmonary arterial pressure in chronic obstructive pulmonary disease. Am Rev Respir Dis 1984; 130: 993–998. [DOI] [PubMed] [Google Scholar]
- 5.Oswald-Mammosser M, Apprill M, Bachez P, et al. Pulmonary hemodynamics in chronic obstructive pulmonary disease of the emphysematous type. Respiration 1991; 58: 304–310. [DOI] [PubMed] [Google Scholar]
- 6.Scharf SM, Iqbal M, Keller C, et al. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med 2002; 166: 314–322. [DOI] [PubMed] [Google Scholar]
- 7.Burger CD. Pulmonary hypertension in COPD: A review and consideration of the role of arterial vasodilators. COPD 2009; 6: 137–144. [DOI] [PubMed] [Google Scholar]
- 8.Cuttica MJ, Kalhan R, Shlobin OA, et al. Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir Med 2010; 104: 1877–1882. [DOI] [PubMed] [Google Scholar]
- 9.Shujaat A, Bajwa AA, Cury JD. Pulmonary hypertension secondary to COPD. Pulm Med 2012; 2012: 203952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo K, Ge RL, Koizumi T, et al. Pulmonary artery remodeling modifies pulmonary hypertension during exercise in severe emphysema. Respir Physiol 2000; 120: 71–79. [DOI] [PubMed] [Google Scholar]
- 11.Abraham AS, Cole RB, Green ID, et al. Factors contributing to the reversible pulmonary hypertension of patients with acute respiratory failure studies by serial observations during recovery. Circ Res 1969; 24: 51–60. [DOI] [PubMed] [Google Scholar]
- 12.Weitzenblum E, Loiseau A, Hirth C, et al. Course of pulmonary hemodynamics in patients with chronic obstructive pulmonary disease. Chest 1979; 75: 656–662. [DOI] [PubMed] [Google Scholar]
- 13.Blanco I, Gimeno E, Munoz PA, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med 2010; 181: 270–278. [DOI] [PubMed] [Google Scholar]
- 14.Stolz D, Rasch H, Linka A, et al. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J 2008; 32: 619–628. [DOI] [PubMed] [Google Scholar]
- 15.Valerio G, Bracciale P, Grazia D’Agostino A. Effect of bosentan upon pulmonary hypertension in chronic obstructive pulmonary disease. Ther Adv Respir Dis 2009; 3: 15–21. [DOI] [PubMed] [Google Scholar]
- 16.Dernaika TA, Beavin M, Kinasewitz GT. Iloprost improves gas exchange and exercise tolerance in patients with pulmonary hypertension and chronic obstructive pulmonary disease. Respiration 2010; 79: 377–382. [DOI] [PubMed] [Google Scholar]