Abstract
Background
Optimal pancreatic β-cell function is essential for the regulation of glucose homeostasis in both humans and animals and its impairment leads to the development of diabetes. Type 2 diabetes is a polygenic disease aggravated by environmental factors such as low physical activity or a hypercaloric high-fat diet.
Results
Free fatty acids represent an important factor linking excess fat mass to type 2 diabetes. Several studies have shown that chronically elevated free fatty acids have a negative effect on β-cell function leading to elevated insulin secretion basally but with an impaired response to glucose. The transcription factors PPARα, PPARγ and SREBP-1c respond to changing fat concentrations in tissues, thereby coordinating the genomic response to altered metabolic conditions to promote either fat storage or catabolism. These transcription factors have been identified in β-cells and it appears that each may exert influence on β-cell function in health and disease.
Conclusion
The role of the PPARs and SREBP-1c as potential mediators of lipotoxicity is an emerging area of interest.
Introduction
Fatty acids are physiologically important both structurally, as components of phospholipids and glycolipids, as well as functionally, as fuel molecules. Metabolites of fatty acids, such as leukotrienes or prostaglandins, act as potent mediators in many biological processes. Fatty acids provide energy [1,2], particularly in the fasted state (Figure 1), but abnormalities in the metabolism of fatty acids can contribute to the pathogenesis of obesity and type 2 diabetes.
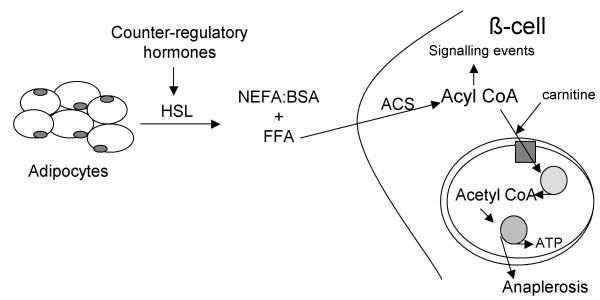
Figure 1.
Schematic diagram of fatty acid metabolism in the fasted state. Counter-regulatory hormones such as catecholamines act on adipocytes to increase lipolysis via hormone-sensitive lipase (HSL). Circulating FFA enter the cell and are converted to acyl CoAs, catalyzed by acyl CoA synthase (ACS). Acyl CoA enter the mitochondria via carnitine palmitoyl transferase-I (solid square) and enter the β-oxidation cycle (stippled circle) to produce acetyl CoA that is then available for further metabolism in the TCA cycle, leading to increased ATP and substrates for anaplerosis. In the β-cell, acyl CoA also participate as signalling molecules to promote insulin secretion (see text).
Type 2 diabetes and free fatty acids
Diabetes affects 6 % of the adult population and, with a growth rate of 6% per year, it is estimated that 200 to 300 million people worldwide will be afflicted by the end of this decade [3]. Type 1 diabetes, which accounts for < 10 % of all cases of diabetes [4], results from autoimmune-mediated destruction of pancreatic β-cells. The destruction may occur over months to years and can result in complete loss of the endogenous insulin supply and therefore results in exogenous insulin dependency.
Type 2 diabetes, which accounts for 90 to 95 % of diabetes cases worldwide, is a heterogeneous disorder and its prevalence is rising. Type 2 diabetes is accompanied by chronic insulin resistance and a progressive decline in β-cell function [5]. Obesity is a major risk factor for the development of type 2 diabetes [6] and is believed to confer increased risk through obesity-associated insulin resistance [7]. Type 2 diabetes is often associated with hypertriglyceridemia or increased circulating concentrations of free fatty acids (FFA) [8]. Therefore, type 2 diabetes can be considered a lipid disorder as well as a disease of glucose intolerance [9].
Metabolism of fatty acids in the beta cell and insulin secretion
Fatty acids, not glucose, are the major endogenous energy source for unstimulated islets [10]. This is consistent with the observation that although islets contain little glycogen, they maintain high rates of oxygen consumption in the absence of glucose [11]. Stimulation of islets by glucose diminishes fatty acid oxidation and increases total respiration [12]. Thus, rising post-prandial plasma glucose shifts the β-cells from fatty acids to glucose as an oxidative fuel. However, plasma concentrations of other nutrients such as FFA and amino acids can modulate the process of glucose-induced insulin secretion [9]. The plasma levels of nutrient metabolites vary with dietary composition. Thus, feeding behavior plays an important role in the control of islet β-cell function [13,14].
Short-term (2–6 hours) elevation of the plasma FFA concentration in human subjects [15] and animals [16,17] enhances while an acute decrease inhibits glucose-stimulated insulin secretion [15,18]. Following lipid infusion or ingestion of a mixed meal, the plasma FFA concentration rises and FFA diffuse into the β-cells [19]. Within the cytosol, fatty acids are converted to their fatty acyl CoA derivatives (Figure 1), which in turn augment insulin secretion via different signalling mechanisms: increased formation of phosphatidic acid and diacylglycerol, which directly and indirectly (through activation of protein kinase C) enhance exocytosis of insulin stored within secretory granules; stimulation of endoplasmic reticulum Ca2+-adenosine triphosphatase, leading to an increase in intracellular calcium concentration and augmentation of insulin secretion; and closure of the K+- ATP channel with resultant depolarization of the β-cell membrane, which causes an increase in intracellular Ca2+ and stimulation of exocytosis of insulin-containing granules [21]. In addition to being oxidized, glucose can be metabolized through anaplerotic processes to increase malonyl CoA concentrations in the β-cell. Malonyl CoA inhibits CPT-I, thus impairing the transport of fatty acyl CoAs into the mitochondria where they would be oxidized [20,21]. The fact that de novo fatty acid synthesis in the β-cell is very low [22] indicates that malonyl-CoA is used as a switch compound, not as a precursor or effector molecule like long chain fatty acyl-CoA. The cytosolic concentration of long chain fatty acyl-CoA is controlled by feedback inhibition of acyl-CoA synthetase, and is buffered by fatty acid and long chain fatty acyl-CoA binding proteins [23]. The total concentration of long chain fatty acyl-CoA in livers of fed and fasted rats, is about 95 and 220 nmol/g dry weight, respectively [24], however quantification of cytosolic long chain fatty acyl-CoA in other tissues has yet to be done.
In contrast to the acute effect of elevated plasma FFA to enhance insulin secretion, longer-term (> 48 h) exposure results in an impaired β-cell response to glucose both in vitro and in vivo in animals [25,26] and humans [27-31]. The inhibitory effect of chronically elevated plasma FFA is more prominent in individuals with a genetic predisposition to develop type 2 diabetes [32], thus a reduction in the plasma FFA concentration in type 2 diabetes improves insulin secretion [32,33]. The term lipotoxicity describes the deleterious effect of chronic FFA elevation on insulin secretion from the pancreatic β-cell [34]. In the Zucker diabetic fatty rat, chronically increased plasma FFA levels lead initially to a physiological impairment in insulin secretion. With time, β-cell mass is reduced by more than 50 % [26]. Within the β-cell, elevated fatty acyl CoAs increase the formation of ceramide, a sphingolipid. Ceramide, in turn, augments the formation of the inducible isoform of nitric oxide, which is toxic to the β-cell [35]. Incubation of human islets with FFA or ceramide has been shown to cause β-cell apoptosis [36].
Transcriptional regulation of free fatty acid metabolism
Free fatty acid metabolism responds to varying metabolic states partially by induction of enzymes that promote either catabolic or anabolic processes. There are two major classes of transcriptional regulators of enzymes involved in fatty acid metabolism, the peroxisome proliferator-activated receptors (PPARs) and the sterol regulatory element binding proteins (SREBPs), which both exist in several isoforms. In general, PPARγ and SREBP-1c regulate processes involved in lipogenesis whereas lipolytic enzymes are induced by PPARα [37].
Peroxisome proliferator-activated receptors
The PPARs form a subfamily in the nuclear receptor superfamily. PPARs, like other nuclear receptors, regulate gene expression in response to specific ligands through their actions as transcription factors. Peroxisomes contain PPAR-regulated enzymes involved in fatty acid β-oxidation [38]. Genetic deficiencies in peroxisome biogenesis in the human cause an accumulation of long chain fatty acids in cells [39]. So far, three isoforms encoded by separate genes and designated PPARα, PPARδ and PPARγ have been identified [40].
PPARα
PPARα was the first member of this nuclear receptor subclass to be described. PPARα is expressed in numerous metabolically active tissues including liver, kidney, heart, skeletal muscle, brown fat [41-43], and also in monocytes [44], vascular endothelium [45] and vascular smooth muscle cells [46].
PPARα plays an important role in the regulation of cellular uptake, activation and β-oxidation of fatty acids. The natural, preferentially-binding ligands of PPARα are long chain unsaturated fatty acids including arachidonic acid, linoleic acid, and oleic acid but saturated fatty acids like palmitic acid can also act as ligands [47]. In hepatocytes and other tissues where it has been studied, ligand-activated PPARα binds to peroxisome proliferator response elements (PPRE) of DNA (Figure 2) and up-regulates transcription of genes involved in lipid catabolism and lipoprotein metabolism (Table 1) [48,49]. Consequently PPARα serves as a long chain fatty acid sensor that leads to autoregulation of long chain fatty acid metabolism mainly in the liver and heart and to a lesser extent in muscle, thus decreasing tissue content of lipids and minimizing lipotoxicity as circulating levels fluctuate [50]. Activation of PPARα also induces hepatic proliferation, hepatomegaly and hepatocarcinogensis in animal [51] but not human liver [52]. Obesity is a major risk factor in the development of type 2 diabetes and PPARα may affect body weight through regulation of fatty acid catabolism or expending energy [53]. PPARα ligands (such as fibrate drugs) could therefore improve insulin sensitivity by reducing lipid accumulation in tissues [54].
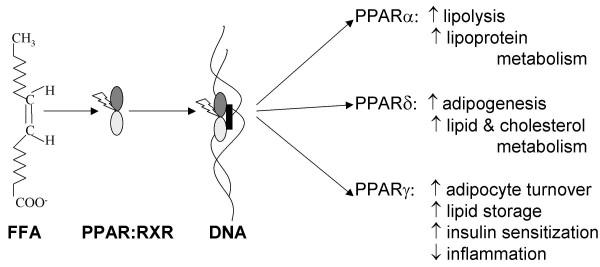
Figure 2.
Overview of PPAR activation and effects. FFA (eg. oleic acid) interact with PPAR, which dimerize with retinoid X receptor (RXR) and translocate to the nucleus where the complex interacts with PPRE to activate gene transcription. The general effects of transcriptional activation of PPARα, PPARδ and PPARγ are shown on the right of the figure.
Table 1.
Selected hepatic PPARα regulated genes with at least one functional peroxisome proliferator receptor element (PPRE) identified within the promoter sequence
| Gene | Function | Species | References |
| Acyl CoA binding protein | fatty acyl-CoA ester transport | rat | 127 |
| Acyl CoA oxidase | peroxisomal β-oxidation | rat, human | 128-130 |
| Apolipoprotein-AI and AII | plasma HDL metabolism | human, mouse, rat | 131-134 |
| Apolipoprotein-AV | plasma triglyceride metabolism | human | 134 |
| Apolipoprotein-CIII | plasma HDL metabolism | rat | 135 |
| Bifunctional enzyme | peroxisomal β-oxidation | rat | 136 |
| Carnitine palmitoyl transferase-I and -II | mitochondrial β-oxidation | human, mouse, rat, hamster | 132, 137-139 |
| Cytochrome P450 enzymes | fatty acid and cholesterol metabolism | rat, mouse, human | 130, 141-145 |
| Δ6- and Δ5-desaturase | desaturation of fatty acyl-CoA | mouse | 146 |
| Fatty acid binding protein | fatty acid binding/transport | mouse | 147 |
| Fatty acid transport protein and translocase | fatty acid transport | mouse | 148, 149 |
| Lipoprotein lipase | triglyceride clearance | mouse | 148, 149 |
| Liver X receptor α | cholesterol metabolism | mouse | 150, 151 |
| Long-chain acyl-CoA synthetase | fatty acid activation | human, mouse | 139, 152 |
| Malic enzyme | fatty acid synthesis | mouse, rat | 153, 154 |
| Mitochondrial HMG-CoA synthase | ketogenesis | rat, human | 152, 155 |
| Medium-chain acyl-CoA dehydrogenase | mitochondrial β-oxidation | mouse | 138, 139 |
| Phospholipid transfer protein | HDL metabolism | human | 156 |
| Stearoyl-CoA desaturase-1 | desaturation of fatty acyl CoA | mouse | 157 |
| Superoxide dismutase | free radical metabolism | rat | 158 |
| Thiolase B | mitochondrial β-oxidation | rat | 159 |
| Transferrin | iron transport | human | 160 |
| Very long- and long-chain acyl CoA dehydrogenases | mitochondrial β-oxidation | mouse | 139 |
Abbreviations: HDL, high density lipoprotein; HMG-CoA, hydroxymethylglutaryl-Coenzyme A
PPARδ
PPARδ was initially reported as PPARβ in Xenopus laevis [49]. Subsequently, the receptor was cloned in the human [55] as well as in rodents [56] and was named PPARδ. PPARδ is expressed in a wide range of tissues and cells with the highest levels of expression found in digestive tract, heart, kidney, liver, adipose and brain [57]. Saturated and unsaturated fatty acids are natural ligands for PPARδ [58,59]. PPARδ is implicated in adipocyte differentiation, which is induced by long-chain fatty acids [60]. In skeletal muscle, activation of PPARδ results in induction of proteins involved in lipid catabolism, cholesterol efflux and respiratory coupling in skeletal muscle independent from the effects of PPARα and PPARγ agonists [61].
PPARγ
PPARγ stimulates fatty acid storage in adipose tissue by increasing both the storage capacity and the fatty acid flux into adipocytes. PPARγ is expressed in many cell types, including epithelial cells, B and T cells, macrophages, endothelial cells, smooth muscle cells [62,63] and predominantly in adipose tissue where it is necessary for the differentiation of adipocytes [64]. There are 2 splice variant of the isoform called PPARγ1 and γ2; the expression distribution of PPARγ2 is more limited than that of PPARγ1 [65].
The natural ligands of PPARγ are several unsaturated fatty acids such as oleate, linoleate, eicosapentaenoic and arachidonic acids [53]. Members of the thiazolidinedione (TZD) family, which are known as antidiabetic compounds, are synthetic ligands of PPARγ [54]. In adipocytes, PPARγ increases the expression of numerous genes involved in lipid metabolism and uptake [66,67]. Activation of PPARγ also induces adipocyte apoptosis, which is restricted primarily to large fully differentiated adipocytes [68]. This pro-apoptotic effect of PPARγ activation on large adipocytes, coupled with its capacity to enhance differentiation of adipocytes de novo, favours the formation of small adipocytes that tend to replace the large adipocytes normally constituting white adipose tissue [68].
PPARγ also negatively regulates transcription of several genes that impair insulin action, including tumor necrosis factor-α (TNFα) and leptin, proinflammatory cytokines produced by adipocytes and associated with insulin resistance [69-72]. Thus, the TZD drugs lower hyperglycemia, hyperinsulinemia and hypertriglyceridemia by indirectly enhancing the sensitivity of tissues to insulin, especially in skeletal muscle. However, the function of PPARγ is not restricted to adipogenesis and insulin sensitization. In peripheral monocytes and macrophages, PPARγ agonists inhibit the production of inflammatory cytokines [73] and induce differentiation and apoptosis in various cancer cells [74,75].
Peroxisome proliferator-activated receptors and β-cell function
Both PPARα and PPARγ have been detected in pancreatic β-cells [76,77]. One caveat that complicates interpretation of some of the work described below is that PPARα and PPARγ agonists have effects on β-cell function independent of their interaction with the transcription factors. Thus, both fibrates and TZD can alter ATP-dependent K channel activity and rapidly (within 10 minutes) increase insulin secretion [78]. In addition, the generalized metabolic effects of these compounds may mean that effects observed in vitro on isolated islets may not apply to the in vivo situation. Therefore, the mode of delivery of the agents (directly onto islets versus in diets) and the time frame of study are important considerations.
In pancreatic islets, exposure to long chain fatty acids (mixed unsaturated and saturated) induces PPARα expression [76] whereas high glucose in vitro or hyperglycemia in vivo suppresses expression [79,80]. Artificial ligands of PPARα such as WY14643 and clofibrate also induce PPARα expression in rat islets [76,81,82]. Similar to hepatocytes, this leads to up-regulation of enzymes favouring lipolysis, including acyl-CoA oxidase [76,81], pyruvate dehydrogenase-4 [82] and CPT-I [76,81].
The question arises as to the role of PPARα in the physiological regulation of insulin secretion. Its induction by long chain fatty acids and its ability to augment the insulin response to low glucose [81] suggests that it may play a role in sustaining β-cell secretory capacity during normal, cyclical periods of fasting. Thus, when glucose is low, PPARα will be induced, favouring β-oxidation of lipids to maintain β-cell ATP at a maintenance level. Moreover, the ability of β-cells to oxidize lipids is a critical for resumption of glucose-stimulated insulin secretion at the end of the fasting period [17]. However, when glucose is elevated above basal, PPARα will be reduced, allowing efficient glucose metabolism-dependent insulin secretion while inhibiting fatty acid oxidation. Overall, the effect of oscillating PPARα activity inversely with glucose concentration may help to maintain glucose responsiveness of the β-cell [83]. Four-to-six-hour fasted PPARα KO mice had normal circulating insulin [84,85] and their islets had normal glucose sensitivity [84] whereas 24 hour fasted mice had a 3-fold increase in circulating insulin [85]. The longer-term fast would allow for greater adaptation to occur; higher fasting insulin may reflect hepatic insulin resistance rather than altered β-cell function.
In addition to these postulated mechanisms of PPARα control over β-cell glucose and lipid metabolism, it has also been proposed that amino acid metabolism might be affected. In the liver, an increase in PPARα is associated with a decrease in amino acid catabolism [86]. Because glutamine metabolites are potential signaling molecules in the β-cell [87], PPARα induction under conditions of low glucose could impair glucose-stimulated insulin secretion via its effects on glutamine catabolism [83]. This hypothesis has yet to be proven.
In pathophysiological conditions involving deranged glucose and lipid metabolism, altered expression of PPARα may be important in the β-cell's lack of glucose responsiveness. In Zucker diabetic fatty rat islets, despite chronic hyperlipidemia, expression of PPARα, acyl-CoA oxidase and CPT-I mRNA is reduced [88]. It has thus been proposed that glucose is the dominant regulator of PPARα in the β-cell and that its suppression is a component of glucolipoxicity [89].
Glucolipotoxicity is a state in which β-cells are exposed to elevated plasma concentrations of both glucose and FFA, as is the case in insulin resistance. Several signalling pathways of the β-cell may be affected by altered PPARα expression and the overall outcome is predicted to depend upon whether fat or glucose has the dominant effect. In cases where glucose is elevated relative to lipid, a chronic reduction in PPARα would be expected to decrease the lipid oxidizing capacity of the β-cell [88,89], eliminating a detoxification route for fat metabolites [89]. Accumulation of lipids, for example as triglyceride within the β-cell, is associated with impaired glucose-stimulated insulin secretion, increased ceramide formation and apoptosis [88]. When lipid is chronically elevated relative to carbohydrate, induction of PPARα presumably would cause strong up-regulation of fat oxidizing genes but also UCP2 (see below), which would suppress glucose-stimulated insulin secretion. The implication of these hypotheses is that either too much or too little PPARα would impair β-cell function. Evidence in the literature supports this contention when in vitro models are employed. Notably, culture of islets or INS-1 cells with high glucose (6–20 mM) for 48 hours strongly suppresses PPARα protein expression by 80%. As predicted, fatty acid oxidation and glucose-stimulated insulin secretion are attenuated, while islet triglyceride and lipid esterification are increased [79]. Conversely, induction of endogenous β-cell PPARα (with clofibrate) leads to an increase in CPT-I expression and fatty acid oxidation, resulting in blunted basal and glucose-stimulated insulin secretion [90]. However, the situation is less clear when experiments are performed in vivo, leading to the conclusion that activation of PPARα in tissues other than β-cells causes indirect effects on insulin secretion secondary to changes in peripheral insulin sensitivity [83]. Thus, type 2 diabetic mice given dietary WY14,643, a PPARα agonist, have normalized serum lipids, glucose and insulin. PPARα activation also improves glucose-stimulated insulin secretion, reduces β-cell proliferation and β-cell mass compared with untreated controls [91]. Similarly, fenofibrate-treated obese diabetes-prone OLETF rats retain β-cell mass and have lower islet triglyceride content and fatty oxidation than untreated animals [92]. In both cases, the effects on β-cells are likely secondary to the observed weight loss and increase in insulin sensitivity of peripheral tissues.
Chronic induction of PPARα may influence also insulin secretion indirectly because PPRE have been found in the promoter region of uncoupling protein-2 (UCP2) [93]. In general, uncoupling proteins (numbered 1–3 in order of their discovery) decrease metabolic efficiency by dissociating ATP synthesis from substrate oxidation in the mitochondrion by promoting translocation of protons from the inter-membrane space, across the inner mitochondrial membrane to the matrix [94]. Therefore, circumstances that limit mitochondrial proton gradient formation, such as up-regulation of UCP2 expression and activity, are predicted to limit insulin secretion. A study specifically examining the role of PPARα by use of the ligand clofibrate demonstrated induction UCP2 in islets [90]. In liver, stimulation of PPARα (or PPARδ when PPARα was absent) caused induction of UCP2 [95]. UCP2 expression inversely correlates with β-cell ATP and glucose-stimulated insulin secretion [96-99]. The significance of these findings is that up-regulation of UCP2 expression suppresses glucose-stimulated insulin secretion and is implicated as a potential contributor to lipotoxic effects mediated by PPARα in β-cells.
PPARγ may also be an important transcriptional regulator of both normal and abnormal metabolism in pancreatic β-cells. In hyperglycemic, pancreatectomized rats the expression of PPARγ mRNA is increased [80] but others found that high fat but not high glucose up-regulates PPARγ protein expression in vitro [100]. In adipocytes, PPARγ alters the expression of fat metabolizing enzymes to increase FFA uptake into storage while simultaneously preventing the release of FFA [66,67]. However, in the β-cell some actions of PPARγ seem to mimic those of PPARα. Thus, one of the earliest demonstrations in islets of direct activation of PPARγ showed that TZD caused mobilization of triglyceride and increased FFA oxidation in Zucker diabetic fatty rats [101], resulting in improved insulin secretion [101,102]. This observation has been reinforced in more recent work. Induction of PPARγ by three different methods enhances expression of genes that participate in fatty acid oxidation [103]. Glucose-stimulated insulin secretion is enhanced by both PPARα and -γ agonists in db/db mice [104]. Consistent with this, mice with a partial global knockdown of PPARγ (PPARγ+/-) on a high fat diet had blunted glucose-stimulated insulin secretion in isolated islets that was associated with an islet-specific accumulation of triglyceride [105] even though insulin resistance was partially prevented [106].
The TZD increase glucokinase and GLUT2 expression and activity via interaction with PPRE in the respective gene promoters [107,108]. A PPARα-agonist also induced GLUT2 expression in islets but the effect on glucokinase was not documented [109]. Improved glucose metabolism, however, has not been a consistent outcome of PPARγ induction [103]. Nonetheless, overexpression of PPARγ in a β-cell line is detrimental to glucose-stimulated insulin secretion and proinsulin synthesis, with PPARγ agonists causing a further negative effect [110]. Since PPARγ was not detected in control cells, it is unclear whether these results are physiologically relevant to primary β-cell function. Interestingly, in rodent islets PPARα is expressed at higher levels than PPARγ [76], while in human islets the situation is reversed [111]; the functions regulated by PPARα in rodents may be more pertinent to PPARγ in human β-cells.
PPARγ activation also regulates some β-cell functions that have not been ascribed to PPARα. PPARγ activation by TZD may relieve oxidative stress in β-cells of diabetic animals [112], leading to preservation of β-cell mass [104,112-114] and partial improvement in glucose-stimulated insulin secretion from isolated islets [112]. The potential anti-oxidative or anti-inflammatory effects of TZD in islets of type 2 diabetes models are interesting in light of reports that TZD reduce diabetes incidence in non-obese diabetic (NOD) mice [115] and more generalized inflammatory/immune responses in a variety of tissues [116]. Moreover, PPARγ appears to be a critical determinant of β-cell expansion in response to a high fat diet [117]. However, despite these studies showing that PPARγ exists in β-cells and that its activation can regulate gene expression and cell function, Rosen et al. [117] recently showed that the TZD's antidiabetic effects are still fully present in mice in which PPARγ has been specifically eliminated only in β-cells. Thus, the dominant effects of dietary TZD on insulin secretion are likely indirect, a consequence of improved lipemia and glycemia.
Sterol regulatory element binding protein
The family of SREBPs governs transcriptional activation of a large number of genes involved in regulation of lipid metabolism, including lipogenesis, cholesterol transport and synthesis [118]. Of interest is the high expression of SREBP-1c in liver and adipose tissue [119], and its detection in pancreatic β-cells [120]. The primary function of SREBP-1c is to regulate transcription of genes involved in lipogenesis, such as acetyl-CoA carboxylase, fatty acid synthase and steroyl-CoA desaturase [119] and enzymes of glycolysis [118,119]. In the liver SREBP-1c appears to mediate the transcription of most insulin-responsive genes and in turn its expression, and possibly its activation, are induced by insulin [119]. Thus, SREBP-1c activity is enhanced during periods of dietary plenty; when glucose is abundant and insulin is stimulated. The outcome of SREBP-1c activation is to promote fat-sparing, leading to an increased synthesis of saturated and monounsaturated fatty acids, triglycerides and phospholipids, as well as enhanced glucose utilization via the glycolytic pathway [119]. Elevation of SREBP-1c in obesity characterized by hyperinsulinemia may therefore explain the onset of fatty liver.
SREBP-1c appears to have a similar function in lipogenesis in pancreatic β-cells as in hepatocytes, but the effects on glycolytic enzymes have received little attention. Notably, blockade of SREBP-1c expression attenuates the glucose-induced increase in acetyl-CoA carboxylase activity seen in control β-cells [121] whereas an increase in SREBP-1c induces lipogenic enzymes, triglyceride accumulation and UCP2 expression [105,122-124]. The outcome of elevated SREBP-1c is a decrease in glucose metabolism and glucose-stimulated insulin secretion in all cases. Consistent with these studies utilizing molecular manipulation of SREBP-1c expression, studies of Zucker diabetic fatty rats demonstrate increased SREBP-1c levels in islets [120]. SREBP-1c has also been implicated as a regulator of apoptosis in β-cells [122]; thus the loss of β-cell mass seen in obese-diabetic models might be related to events triggered by this transcription factor. Indeed, β-cell apoptosis might be under control of both PPARγ and SREBP-1c because TZD has been reported to block the increase in SREBP-1c in diabetic fatty rats [120]; this implies that PPARγ regulates SREBP-1c. Conversely, other groups have evidence that SREBP-1c can up-regulate PPARγ mRNA expression [103,123] ; thus, the relationship between these two factors is not yet clear. The UCP2 promoter has a sterol response element [124] so the negative effects of SREBP-1c on insulin secretion might be caused by its induction of UCP2. However, reducing UCP2 expression by means of a small interfering RNA only partially restored glucose-stimulated insulin secretion in SREBP-1c-overexpressing cells. Likewise, activation of the AMP-activated kinase partially rescued the phenotype of the cells with SREBP-1c induction [125]. Certainly, SREBP-1c is implicated as a key contributor to lipotoxicity, as proposed elsewhere [89,126] but further research is required to fully understand its role in regulating insulin secretion in health and diabetes.
Conclusions
FFA exert dual effects on insulin secretion, dependent on the duration of exposure. Acute exposure to FFA increase glucose-stimulated insulin secretion whereas chronic exposure attenuate glucose sensitivity of pancreatic β-cells. The coordinated control of these processes by lipid-sensing transcription factors and its relevance to β-cell dysfunction in type 2 diabetes mellitus is increasingly a subject of investigation.
PPARs (especially PPARα and PPARγ) are involved in the long-term regulation of lipid metabolism and their activity is modulated by endogenous lipid-derived ligands. PPAR agonists have positive effects on glucose homeostasis and lipid metabolism and can reduce cardiovascular events in obese-diabetic patients. PPARα is a fasting lipid oxidation-glucose sparing regulator whereas PPARγ is post-prandial lipid storing-glucose utilizing regulator. In islets, however, both PPARα and -γ appear to have some functions more consistent with PPARα, particularly induction of lipid oxidizing enzymes, which is potentially particularly important for maintaining basal insulin secretion. Growing evidence suggests that PPARγ is a regulator of β-cell proliferation and that PPARγ agonist-mediated anti-oxidative effects may also contribute to anti-diabetic activity.
SREBP-1c up-regulates lipogenic enzymes in β-cells as it does in liver. Its chronic induction in islets of obese-diabetic rodents may therefore contribute to lipotoxicity by promoting triglyceride accumulation and removing fatty-acid derived signalling factors from the cellular pool. SREBP-1c and PPAR functions appear to be closely linked through cross-talk between the pathways that control their own expression, and may function in concert to affect not only fatty acid metabolism but also glucose metabolism, β-cell proliferation and apoptosis.
Drugs given orally to activate PPARs can improve insulin sensitivity of peripheral tissues and generally appear to enhance β-cell function secondary to their insulin-sensitizing effects. However, it remains possible that specific effects on β-cells are also important contributors to the positive metabolic effects of PPAR agonists in type 2 diabetes treatment.
Declaration of Competing Interests
The author(s) declare that they have no competing interests.
Authors Contributions
ZF-H and CBC contributed equally to the writing of this review.
Acknowledgments
Acknowledgments
Research by the authors' group is supported by the Canadian Institutes for Health Research and the Canadian Diabetes Association. CBC holds a Levesque Research Chair in Nutrisciences and Health at the University of Prince Edward Island. The authors thank MB Wheeler and MC Saleh for reading the manuscript and for their helpful comments.
Contributor Information
Zahra Fatehi-Hassanabad, Email: zfatehi@upei.ca.
Catherine B Chan, Email: cchan@upei.ca.
References
- Kutchai HC. Digestion and absorption. In: Berne RM, Levy MN, editor. In Principles of Physiology. Toronto: Mosby Company; 1990. pp. 410–414. [Google Scholar]
- Lehninger AL, Nelson DL, Cox MM, editor. In Principles of Biochemistry. 2. New York: Worth Publishers Inc; 1997. Oxidation of fatty acids; pp. 479–497. [Google Scholar]
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . National Diabetes Fact Sheet: National estimate and general information on diabetes in the United States, 2000. Atlanta: US Department of Health and Human Services; 2003. [Google Scholar]
- Defronzo RA. The triumvirate: beta cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Burke JP, Williams K, Gaskill SP, Hazuda HP, Haffner SM, Stern MP. Rapid rise in the incidence of type 2 diabetes from 1987 to 1996: results from the San Antonio Heart Study. Arch Intern Med. 1999;159:1450–1456. doi: 10.1001/archinte.159.13.1450. [DOI] [PubMed] [Google Scholar]
- Ludvik B, Nolan JJ, Baloga J, Sacks D, Olefsky J. Effect of obesity on insulin resistance in normal subjects and patients with NIDDM. Diabetes. 1995;44:1121–1125. doi: 10.2337/diab.44.9.1121. [DOI] [PubMed] [Google Scholar]
- Fraze E, Donner CC, Swislocki AL, Chiou YA, Chen YD, Reaven GM. Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. J Clin Endocrinol Metab. 1985;61:807–811. doi: 10.1210/jcem-61-5-807. [DOI] [PubMed] [Google Scholar]
- McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- Malaisse WJ, Best L, Kawazu S, Malaisse-Lagae F, Sener A. The stimulus-secretion coupling of glucose-induced insulin release: fuel metabolism in islet deprived of exogenous nutrient. Arch Biochem Biophys. 1983;224:102–110. doi: 10.1016/0003-9861(83)90193-5. [DOI] [PubMed] [Google Scholar]
- Hellerstrom C. Effects of carbohydrates on the oxygen consumption of isolated pancreatic islets of mice. Endocrinology. 1967;81:105–112. doi: 10.1210/endo-81-1-105. [DOI] [PubMed] [Google Scholar]
- Vara E, Tamarit-Rodriguez J. Glucose stimulation of insulin secretion in islets of fed and starved rats and its dependence on lipid metabolism. Metab Clin Exp. 1986;35:266–271. doi: 10.1016/0026-0495(86)90212-x. [DOI] [PubMed] [Google Scholar]
- Malaisse WJ. Insulin secretion: Multifactorial regulation for a single process of release. Diabetologia. 1973;9:167–173. doi: 10.1007/BF01219778. [DOI] [PubMed] [Google Scholar]
- Carpinelli AR, Curi R, Malaisse WJ. Long-term regulation of pancreatic B-cell responsiveness to D-glucose by food availability, feeding schedule and diet composition. Physiol Behav. 1992;52:1193–1196. doi: 10.1016/0031-9384(92)90481-G. [DOI] [PubMed] [Google Scholar]
- Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic β cell signal transduction. Annu Rev Biochem. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- Warnotte C, Gilon P, Nenquin M, Henquin JC. Mechanisms of the stimulation of insulin release by saturated fatty acids: a study of palmitate effects in mouse β-cells. Diabetes. 1994;43:703–711. doi: 10.2337/diab.43.5.703. [DOI] [PubMed] [Google Scholar]
- Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB, Chen S, McGarry JD. Essentially of circulating fatty acids for glucose-stimulated insulin secretion in fasted rats. J Clin Invest. 1996;97:2728–2735. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999;42:128–138. doi: 10.1007/s001250051130. [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Civelek VN, Kamp F, Tornheim K, Corkey BE. Changes in internal pH caused by movement of fatty acids into and out of clonal pancreatic β-cells (HIT) J Biol Chem. 1994;269:20852–20856. [PubMed] [Google Scholar]
- Matschinsky FM. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- McGarry JD. Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- Berne C. The metabolism of lipid in mouse pancreatic islets. The biosynthesis of triacylglycerols and phospholipids. Biochem J. 1975;152:667–673. doi: 10.1042/bj1520667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan JG, Hamilton JA. Interactions of acyl-coenzyme A with phosphatidylcholine bilayers and serum albumin. Biochemistry. 1992;31:557–567. doi: 10.1021/bi00117a037. [DOI] [PubMed] [Google Scholar]
- Corkey B. Analysis of acyl-coenzyme A esters in biological samples. Methods Enzymol. 1988;166:55–70. doi: 10.1016/s0076-6879(88)66011-3. [DOI] [PubMed] [Google Scholar]
- Mason TM, Goh T, Tchipashvili V, Sandhu H, Gupta N, Lewis GF, Giacca A. Prolonged elevation of plasma free fatty acids desensitizes the insulin secretory response to glucose in vivo in rats. Diabetes. 1999;48:524–530. doi: 10.2337/diabetes.48.3.524. [DOI] [PubMed] [Google Scholar]
- Unger RH. How obesity causes diabetes in Zucker diabetic fatty rats. Trends Endocrinol Metab. 1997;7:276–282. doi: 10.1016/S1043-2760(97)00094-5. [DOI] [PubMed] [Google Scholar]
- Carpentier A, Mittelman SD, Lamarche B, Bergman RN, Giacca A, Lewis GF. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am J Physiol. 1999;276:E1055–E1066. doi: 10.1152/ajpendo.1999.276.6.E1055. [DOI] [PubMed] [Google Scholar]
- Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF. Prolonged elevation of plasma free fatty acids impairs pancreatic B-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes. 2000;49:399–408. doi: 10.2337/diabetes.49.3.399. [DOI] [PubMed] [Google Scholar]
- Kashyap SR, Belfort R, Berria R, Suraamornkul S, Pratipranawatr T, Finlayson J, Barrentine A, Bajaj M, Mandarino L, DeFronzo R, Cusi K. Discordant effects of a chronic physiological increase in plasma FFA on insulin signaling in healthy subjects with or without a family history of type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E537–E546. doi: 10.1152/ajpendo.00541.2003. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Gambardella A. Opposite effects of short and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia. 1995;38:1295–1299. doi: 10.1007/BF00401761. [DOI] [PubMed] [Google Scholar]
- Stefan N, Fritsche A, Haring H, Stumvoll M. Effect of experimental elevation of free fatty acids on insulin secretion and insulin sensitivity in healthy carriers of the Pro12 Ala polymorphism of the peroxisome proliferator-activated receptor γ2 gene. Diabetes. 2001;50:1143–1148. doi: 10.2337/diabetes.50.5.1143. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Tagliamonte MR, Rizzo MR, Gualdiero P, Saccomanno F, Gambardella A, Giugliano D, D'Onofrio F, Howard BV. Lowering fatty acids potentiates acute insulin response in first degree relatives of people with type II diabetes. Diabetologia. 1998;41:1127–1132. doi: 10.1007/s001250051041. [DOI] [PubMed] [Google Scholar]
- Qvigstad E, Mostad IL, Bjerve KS, Grill VE. Acute lowering of circulating fatty acids improves insulin secretion in a subset of type 2 diabetes subjects. AM J Physiol Endocrinol Metab. 2003;284:E129–E137. doi: 10.1152/ajpendo.00114.2002. [DOI] [PubMed] [Google Scholar]
- Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid induced β-cell apoptosis: a link between diabetes and obesity. Proc Natl Acad Sci USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: Evidence that cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51:1437–1442. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- Kersten S. Effects of fatty acids on gene expression: role of peroxisome proliferator-activated receptor α, liver X receptor α and sterol regulatory element-binding protein-1 c. Proc Nutr Soc. 2002;61:371–374. doi: 10.1079/PNS2002169. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Chu R. Peroxisome proliferator-induced pleiotropic responses: Pursuit of a phenomenon. Ann NY Acad Sci. 1996;804:176–201. doi: 10.1111/j.1749-6632.1996.tb18616.x. [DOI] [PubMed] [Google Scholar]
- Goldfischer SL. Peroxisomal diseases. Prog Clin Biol Res. 1988;282:117–137. [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.M106424200. [DOI] [PubMed] [Google Scholar]
- Sher T, Yi H-F, McBride OW, Gonzalez FJ. cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry. 1993;32:5598–5604. doi: 10.1021/bi00072a015. [DOI] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator activated receptor (PPARs): tissue distribution of PPAR-α, -β and -γ in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/en.137.1.354. [DOI] [PubMed] [Google Scholar]
- Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, Staels B, Auwerx J, Laville M, Vidal H. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor α in humans. Diabetes. 1997;46:1319–1327. doi: 10.2337/diab.46.8.1319. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- Inoue I, Shino K, Noji S, Awata T, Katayama S. Expression of peroxisome proliferator-activated receptor α in primary cultures of human vascular endothelial cells. Biochem Biophys Res Commun. 1998;246:370–374. doi: 10.1006/bbrc.1998.8622. [DOI] [PubMed] [Google Scholar]
- Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf J, Tedgui A. Activation of human aortic smooth-muscle cells is inhibited by PPARα but not PPARγ activators. Nature. 1998;393:790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- Gottlicher M, Widmark E, Li Q, Gustafsson JA. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci USA. 1992;89:4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. J Biol Chem. 1997;272:28210–28217. doi: 10.1074/jbc.272.45.28210. [DOI] [PubMed] [Google Scholar]
- Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- Kersten S. Peroxisome proliferator activated receptors and obesity. Eur J Pharmacol. 2002;440:223–234. doi: 10.1016/S0014-2999(02)01431-0. [DOI] [PubMed] [Google Scholar]
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by some peroxisome proliferators. Nature. 1990;347:645–649. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Cattley RC, DeLuca J, Elcombe C, Fenner-Crisp P, Lake BG, Marsman DS, Pastoor TA, Popp JA, Robinson DE, Schwetz B, Tugwood J, Wahli W. Do peroxisome proliferating compounds pose a hepatocarcinogenic hazard to humans? Regul Toxicol Pharmacol. 1998;27:47–60. doi: 10.1006/rtph.1997.1163. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/er.20.5.649. [DOI] [PubMed] [Google Scholar]
- Verges B. Clinical interest of PPAR ligands. Particular benefit in type 2 diabetes and metabolic syndrome. Diabetes Metab. 2004;30:7–12. doi: 10.1016/s1262-3636(07)70083-6. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Endo N, Rutledge SJ, Vogel R, Shinar D, Rodan GA. Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acid. Mol Endocrinol. 1992;6:1634–1641. doi: 10.1210/me.6.10.1634. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, Desvergne B. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology. 2001;142:4195–4202. doi: 10.1210/en.142.10.4195. [DOI] [PubMed] [Google Scholar]
- Bastie C, Holst D, Gaillard D, Jehl-Pietri C, Grimaldi PA. Expression of peroxisome proliferator-activated receptor PPARδ promotes induction of PPARγ and adipocyte differentiation in 3T3C2 fibroblasts. J Biol Chem. 1999;274:21920–21925. doi: 10.1074/jbc.274.31.21920. [DOI] [PubMed] [Google Scholar]
- Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403. doi: 10.1016/S1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- Gaw A, Packard CJ, Shepherd J. Fibrates. Handb Exp Pharmacol. 1994;109:325–48. [Google Scholar]
- Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. The peroxisome proliferator-activated receptor β/δ agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol. 2003;17:2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- Law RE, Goetze S, Xi XP, Jackson S, Kawano Y, Demer L, Fishbein MC, Meehan WP, Hsueh WA. Expression and function of PPARγ in rat and human vascular smooth muscle cells. Circulation. 2000;101:1311–1318. doi: 10.1161/01.cir.101.11.1311. [DOI] [PubMed] [Google Scholar]
- Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ. The nuclear receptor PPARγ and immunoregulation. PPARγ mediates inhibition of helper T cell responses. J Immunol. 2000;164:1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/S1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97:2553–61. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Curr Opin Genet Dev. 1995;5:571–576. doi: 10.1016/0959-437X(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPAR α and PPAR γ activators. J Biol Chem. 1997;272:28210–28217. doi: 10.1074/jbc.272.45.28210. [DOI] [PubMed] [Google Scholar]
- Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y, Kadowaki T. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998;101:1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Walczak R, Tontonoz P. PPARadigms and PPARadoxes: expanding roles for PPAR γ in the control of lipid metabolism. J Lipid Res. 2002;43:177–186. [PubMed] [Google Scholar]
- Kallen CB, Lazar MA. Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 1996;93:5793–5796. doi: 10.1073/pnas.93.12.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos P, Lefebvre AM, Miller SG, Guerre-Millo M, Wong KM, Saladin R, Hamann LG, Staels B, Briggs MR, Auwerx J. Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor γ. J Clin Invest. 1996;98:1004–1009. doi: 10.1172/JCI118860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPARγ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D, Koeffler HP. Ligands for peroxisome proliferator-activated receptorγ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci USA. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Pighetti GM, Lerner MR, Wu X, Lightfoot SA, Brackett DJ, Darcy K, Hollingsworth AB. Expression of peroxisome proliferator-activated receptor mRNA in normal and tumorigenic mammary glands. Biochem Biophys Res Commun. 1998;253:813–817. doi: 10.1006/bbrc.1998.9858. [DOI] [PubMed] [Google Scholar]
- Zhou Y-T, Shimabukuro M, Wang M-Y, Lee Y, Higa M, Milburn JL, Newgard CB, Unger RH. Role of peroxisome proliferator-activated receptor α in disease of pancreatic β cells. Proc Natl Acad Sci USA. 1998;95:8898–8903. doi: 10.1073/pnas.95.15.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Paltou F, Kerr-Conte J, Gyr V, Vandewalle B, Desreumaux P, Auwerx J, Schoonjans K, Lefebvre J. Expression of peroxisome proliferator-activated receptor γ in normal human pancreatic islet cells. Diabetologia. 2000;43:1165–1169. doi: 10.1007/s001250051508. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Shimizu H, Ikeda M, Okada S, Kakei M, Matsumoto S, Mori M. Fenofibrate, troglitazone, and 15-deoxy-Δ12, 14-prostaglandin J2 close KATP channels and induce insulin secretion. J Pharmacol Exp Ther. 2004;310:1273–1280. doi: 10.1124/jpet.104.067249. [DOI] [PubMed] [Google Scholar]
- Roduit R, Morin J, Masse F, Segall L, Roche E, Newgard CB, Assimacopoulos-Jeannet F, Prentki M. Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-α gene in the pancreatic β-cell. J Biol Chem. 2000;275:35799–35806. doi: 10.1074/jbc.M006001200. [DOI] [PubMed] [Google Scholar]
- Laybutt R, Hasenkamp W, Groff A, Grey S, Jonas JC, Kaneto H, Sharma A, Bonner-Weir S, Weir G. Beta-cell adaptation to hyperglycemia. Diabetes. 2001;50:S180–S181. doi: 10.2337/diabetes.50.2007.s180. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Tajiri Y, Sako Y, Hashimoto T, Umeda F, Nawata H. Effects of free fatty acids on beta-cell functions: a possible involvement of peroxisome proliferator-activated receptors alpha or pancreatic/duodenal homeobox. Metabolism. 2001;50:613–618. doi: 10.1053/meta.2001.22565. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Bulmer K, Augustine D, Holness MJ. Selective modification of pyruvate dehydrogenase kinase isoform expression in rat pancreatic islets elicited by starvation and activation of peroxisome proliferator-activated receptor-α : implications for glucose-stimulated insulin secretion. Diabetes. 2001;50:2729–2736. doi: 10.2337/diabetes.50.12.2729. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Holness MJ. Potential role of peroxisome proliferator-activated receptor-α in the modulation of glucose-stimulated insulin secretion. Diabetes. 2004;53:S71–S81. doi: 10.2337/diabetes.53.2007.s71. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M, Rouault C, Poulain P, Andre J, Poitout V, Peters JM, Gonzalez FJ, Fruchart JC, Reach G, Staels B. PPAR-α-null mice are protected from high-fat diet-induced insulin resistance. Diabetes. 2001;50:2809–2814. doi: 10.2337/diabetes.50.12.2809. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Bulmer K, Gibbons GF, Knight BL, Holness MJ. Peroxisome-proliferator-activated receptor-α (PPARα) deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Biochem J. 2002;364:361–368. doi: 10.1042/BJ20011699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S, Mandard S, Escher P, Gonzalez FJ, Tafuri S, Desvergne B, Wahli W. The peroxisome proliferator-activated receptor alpha regulates amino acid metabolism. FASEB J. 2001;15:1971–1978. doi: 10.1096/fj.01-0147com. [DOI] [PubMed] [Google Scholar]
- Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 1999;402:685–689. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- Prentki M, Joly E, El-Assaad W, Roduit R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity. Role in β-cell adaptation and failure in the etiology of diabetes. Diabetes. 2002;51:S405–S413. doi: 10.2337/diabetes.51.2007.s405. [DOI] [PubMed] [Google Scholar]
- Tordjman K, Standley KN, Bernal-Mizrachi C, Leone TC, Coleman T, Kelly DP, Semenkovich CF. PPARα suppresses insulin secretion and induces UCP2 in insulinoma cells. J Lipid Res. 2002;43:936–943. [PubMed] [Google Scholar]
- Kim H, Haluzik M, Asghar Z, Yau D, Joseph JW, Fernandez AM, Reitman ML, Yakar S, Stannard B, Heron-Milhavet L, Wheeler MB, LeRoith D. Peroxisome proliferator-activated receptor-α agonist treatment in a transgenic model of type 2 diabetes reverses the lipotoxic state and improves glucose homeostasis. Diabetes. 2003;52:1770–1778. doi: 10.2337/diabetes.52.7.1770. [DOI] [PubMed] [Google Scholar]
- Koh EH, Kim M-S, Park J-Y, Kim HS, Youn J-Y, Park H-S, Youn JH, Lee K-U. Peroxisome proliferator-activated receptor (PPAR)-α activation prevents diabetes in OLETF rats. Comparison with PPAR-γ activation. Diabetes. 2003;52:2331–2337. doi: 10.2337/diabetes.52.9.2331. [DOI] [PubMed] [Google Scholar]
- Kelly LJ, Vicario PP, Thompson GM, Candelore MR, Doebber TW, Ventre J, Wu MS, Meurer R, Forrest MJ, Conner MW, Cascieri MA, Molle DE. Peroxisome proliferator-acitvated receptors γ and α mediate in vivo regulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression. Endocrinology. 1998;139:4920–4927. doi: 10.1210/en.139.12.4920. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Hesselink M. UCP2 and UCP3 in muscle controlling body metabolism. J Exp Biol. 2002;205:2275–85. doi: 10.1242/jeb.205.15.2275. [DOI] [PubMed] [Google Scholar]
- Grav HJ, Tronstad KJ, Gudbrandsen OA, Berge K, Fladmark KE, Martinsen TC, Waldum H, Wergedahl H, Berge RK. Changed energy state and increased mitochondrial beta-oxidation rate in liver of rats associated with lowered proton electrochemical potential and stimulated uncoupling protein 2 (UCP-2) expression: evidence for peroxisome proliferator-activated receptor-alpha independent induction of UCP-2 expression. J Biol Chem. 2003;278:30525–33. doi: 10.1074/jbc.M303382200. [DOI] [PubMed] [Google Scholar]
- Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, Tsushima RG, Pennefather PS, Salapatek AM, Wheeler MB. Increased uncoupling protein-2 levels in beta cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes. 2001;50:1302–1310. doi: 10.2337/diabetes.50.6.1302. [DOI] [PubMed] [Google Scholar]
- Hong Y, Fink BD, Dillon JS, Sivitz W. Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Endocrinology. 2001;142:249–56. doi: 10.1210/en.142.1.249. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction and type 2 diabetes. Cell. 2001;105:745–55. doi: 10.1016/S0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, Wheeler MB. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high fat diet. Diabetes. 2002;51:3211–3219. doi: 10.2337/diabetes.51.11.3211. [DOI] [PubMed] [Google Scholar]
- Patane G, Anello M, Piro S, Vigneri R, Purrello F, Rabuazzo AM. Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-γ inhibition. Diabetes. 2002;51:2749–2756. doi: 10.2337/diabetes.51.9.2749. [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Zhou Y-T, Lee Y, Unger RH. Induction of uncoupling protein-2 mRNA by troglitazone in the pancreatic islets of Zucker diabetic fatty rats. Biochem Biophys Res Commun. 1997;237:359–361. doi: 10.1006/bbrc.1997.7140. [DOI] [PubMed] [Google Scholar]
- Sreenan S, Sturis J, Pugh W, Burant CF, Polonsky KS. Prevention of hyperglycemia in the Zucker diabetic fatty rat by treatment with metformin or troglitazone. Am J Physiol. 1996;271:E742–E747. doi: 10.1152/ajpendo.1996.271.4.E742. [DOI] [PubMed] [Google Scholar]
- Parton LE, Diraison F, Neill SE, Ghosh SK, Rubino MA, Bisi JE, Briscoe CP, Rutter GA. Impact of PPARγ overexpression and activation on pancreatic islet gene expression profile analyzed with oligonucleotide microarrays. Am J Physiol Endocrinol Metab. 2004;287:E390–E404. doi: 10.1152/ajpendo.00016.2004. [DOI] [PubMed] [Google Scholar]
- Yajima K, Hirose H, Fujita H, Seto Y, Fujita H, Ukeda K, Miyashita K, Kawai T, Yamamoto Y, Ogawa T, Yamada T, Saruta T. Combination therapy with PPARγ and PPARα agonists increases glucose-stimulated insulin secretion in db/db mice. Am J Physiol Endocrinol Metab. 2003;284:E966–E791. doi: 10.1152/ajpendo.00149.2002. [DOI] [PubMed] [Google Scholar]
- Eto K, Yamashita T, Matsui J, Terauchi Y, Noda M, Kadowaki T. Genetic manipulation of fatty acid metabolism in β-cells are associated with dysregulated insulin secretion. Diabetes. 2002;51:S414–S420. doi: 10.2337/diabetes.51.2007.s414. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Nagai R, Tobe K, Kimura S, Kadowaki T. PPAR-γ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/S1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- Kim HI, Cha JY, Kim SY, Kim JW, Roh KJ, Seong JK, Lee NT, Choi KY, Kim KS, Ahn YH. Peroxisomal proliferator-activated receptor-gamma upregulates glucokinase gene expression in beta-cells. Diabetes. 2002;51:676–685. doi: 10.2337/diabetes.51.3.676. [DOI] [PubMed] [Google Scholar]
- Kim HI, Kim JW, Kim SH, Cha JY, Kim AS, Ahn YH. Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter. Diabetes. 2000;49:1517–1524. doi: 10.2337/diabetes.49.9.1517. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gleichmann H. Glut2 in pancreatic islets: crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes. 1998;47:50–56. doi: 10.2337/diab.47.1.50. [DOI] [PubMed] [Google Scholar]
- Nakamichi Y, Kikuta T, Ito E, Ohara-Imaizumi M, Nishiwaki C, Ishida H, Nagamatsu S. PPAR-γ overexpression suppresses glucose-induced proinsulin biosynthesis and insulin release synergistically with pioglitazone in MIN6 cells. Biochem Biophys Res Commun. 2003;306:832–836. doi: 10.1016/S0006-291X(03)01045-3. [DOI] [PubMed] [Google Scholar]
- Lupi R, Del Guerra S, Marselli L, Bugliani M, Boggi U, Mosca F, Marchetti P, Del Prato S. Rosiglitazone prevents the impairment of human islet function induced by fatty acids: evidence for a role of PPARγ2 in the modulation of insulin secretion. Am J Physiol Endocrinol Metab. 2003;286:E560–E567. doi: 10.1152/ajpendo.00561.2002. [DOI] [PubMed] [Google Scholar]
- Ishida H, Takizawa M, Ozawa S, Nakamichi Y, Yamaguchi S, Katsuta H, Tanaka T, Maruyama M, Katahira H, Yoshimoto K, Itagaki E, Nagamatsu S. Pioglitazone improves insulin secretory capacity and prevents the loss of β-cell mass in obese diabetic db/db mice: possible protection of β-cells from oxidative stress. Metabolism. 2004;53:488–494. doi: 10.1016/j.metabol.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Diani AR, Sawada G, Wyse B, Marray FT, Khan M. Pioglitazone preserves pancreatic islet structure and insulin secretory funciton in three murine models of type 2 diabetes. Am J Physiol Endocrinol Metab. 2003;286:E116–E122. doi: 10.1152/ajpendo.00331.2003. [DOI] [PubMed] [Google Scholar]
- Higa M, Zhou Y-T, Ravazzola M, Baetens D, Orci L, Unger RH. Troglitazone prevents mitochondrial alterations, β cell destruction and diabetes in obese prediabetic rats. Proc Natl Acad Sci USA. 1999;96:11513–11518. doi: 10.1073/pnas.96.20.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales PE, Liddi R, Giorgini AE, Signore A, Procaccini E, Batchelor K, Pozzilli P. Troglitazone prevents insulin dependent diabetes in the non-obese diabetic mouse. Eur J Pharmacol. 1998;357:221–225. doi: 10.1016/S0014-2999(98)00574-3. [DOI] [PubMed] [Google Scholar]
- Pershadsingh HA. Peroxisome proliferator-activated receptor-γ: therapeutic target for diseases beyond diabetes: quo vadis? Expert Opin Investig Drugs. 2004;13:215–228. doi: 10.1517/eoid.13.3.215.27349. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu C-H, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, Spiegelman BM. Targeted elimination of peroxisome proliferator-activated receptor γ in β cells leads to abnormalities in islet mass without compromising glucose homeostasis. Mol Cell Biol. 2003;23:7222–7229. doi: 10.1128/MCB.23.20.7222-7229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foufelle F, Ferre P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem J. 2002;366:377–391. doi: 10.1042/BJ20020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI200215593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuma T, Lee Y, Higa M, Wang Z, Pan W, Shimomura I, Unger RH. Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc Natl Acad Sci USA. 2000;97:8536–8541. doi: 10.1073/pnas.97.15.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreolas C, da Silva Xavier G, Diraison F, Zhao C, Varadi A, Lopez-Casillas F, Ferre P, Foufelle F, Rutter GA. Stimulation of acetyl-CoA carboxylase gene expression by glucose requires insulin release and sterol regulatory element binding protein 1c in pancreatic MIN6 beta-cells. Diabetes. 2002;51:2536–2545. doi: 10.2337/diabetes.51.8.2536. [DOI] [PubMed] [Google Scholar]
- Wang H, Maechler P, Antinozzi PA, Herrero L, Hagenfeldt-Johansson KA, Bjorklund A, Wollheim CB. The transcription factor SREBP-1c is instrumental in the development of beta-cell dysfunction. J Biol Chem. 2003;278:16622–16629. doi: 10.1074/jbc.M212488200. [DOI] [PubMed] [Google Scholar]
- Diraison F, Parton L, Ferre P, Foufelle F, Briscoe CP, Leclerc I, Rutter GA. Over-expression of sterol-regulatory-element-binding protein-1c (SREBP1c) in rat pancreatic islets induces lipogenesis and decreases glucose-stimulated insulin release: modulation by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) Biochem J. 2004;378:769–778. doi: 10.1042/BJ20031277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev AV, Robidoux J, Bai X, Cao W, Floering LM, Daniel KW, Collins S. Regulation of the uncoupling protein-2 gene in INS-1 β-cells by oleic acid. J Biol Chem. 2002;277:42639–42644. doi: 10.1074/jbc.M208645200. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Eto K, Okazaki Y, Yamashita S, Yamauchi T, Sekine N, Nagai R, Noda M, Kadowaki T. Role of uncoupling protein-2 up-regulation and triglyceride accumulation in impaired glucose-stimulated insulin secretion in a β-cell lipotoxicity model overexpressing sterol regulatory element binding protein-1c. Endocrinology. 2004;145:3566–3577. doi: 10.1210/en.2003-1602. [DOI] [PubMed] [Google Scholar]
- Poitout V. β-cell lipotoxicity: burning fat into heat? Endocrinology. 2004;145:3563–3565. doi: 10.1210/en.2004-0479. [DOI] [PubMed] [Google Scholar]
- Elholm M, Bjerking G, Knudsen J, Kristiansen K, Mandrup S. Regulatory elements in the promoter region of the rat gene encoding the acyl-CoA-binding protein. Gene. 1996;173:233–238. doi: 10.1016/0378-1119(96)00213-2. [DOI] [PubMed] [Google Scholar]
- Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARα-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- Varanasi U, Chu R, Huang Q, Castellon R, Yeldandi AV, Reddy JK. Identification of a peroxisome proliferator-responsive element upstream of the human peroxisomal fatty acyl coenzyme A oxidase gene. J Biol Chem. 1996;271:2147–2155. doi: 10.1074/jbc.271.4.2147. [DOI] [PubMed] [Google Scholar]
- Karam WG, Ghanayem BI. Induction of replication DNA synthesis and PPARα-dependent gene transcription by Wy-14643 in primary rat hepatocyte and non-parenchymal cell co-cultures. Carcinogenesis. 1997;18:2077–2083. doi: 10.1093/carcin/18.11.2077. [DOI] [PubMed] [Google Scholar]
- Berthou L, Duverger N, Emmanuel F, Langouet S, Auwerx J, Guillouzo A, Fruchart JC, Rubin E, Denefle P, Staels B, Branellec D. Opposite regulation of human versus mouse apolipoprotein A-I by fibrates in human apolipoprotein A-I transgenic mice. J Clin Invest. 1996;97:2408–2416. doi: 10.1172/JCI118687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspe E, Madsen L, Lefebvre AM, Leitersdorf I, Gelman L, Peinado-Onsurbe J, Dallongeville J, Fruchart JC, Berge R, Staels B. Modulation of rat liver apolipoprotein gene expression and serum lipid levels by tetradecylthioacetic acid (TTA) via PPARα activation. J Lipid Res. 1999;40:2099–2110. [PubMed] [Google Scholar]
- Vu-Dac N, Schoonjans K, Kosykh V, Dallongeville J, Fruchart JC, Staels B, Auwerx J. Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. J Clin Invest. 1995;96:741–750. doi: 10.1172/JCI118118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur X, Coste H, Rodriguez JC. The human apolipoprotein AV gene is regulated by PPAR α and contains a novel FXR response element. J Biol Chem. 2003;278:25468–25480. doi: 10.1074/jbc.M301302200. [DOI] [PubMed] [Google Scholar]
- Hertz R, Bishara-Shieban J, Bar-Tana J. Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. J Biol Chem. 1995;270:13470–13475. doi: 10.1074/jbc.270.22.13470. [DOI] [PubMed] [Google Scholar]
- Nicolas-Frances V, Dasari VK, Abruzzi E, Osumi T, Latruffe N. The peroxisome proliferator response element (PPRE) present at positions-681/-669 in the rat liver 3-ketoacyl-CoA thiolase B gene functionally interacts differently with PPARα and HNF-4. Biochem Biophys Res Commun. 2000;269:347–351. doi: 10.1006/bbrc.2000.2249. [DOI] [PubMed] [Google Scholar]
- Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor α in the cellular fasting response: the PPARα-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci USA. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajuima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α. J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- Minnich A, Tian N, Byan L, Bilder G. A potent PPARα agonist stimulates mitochondrial fatty acid β-oxidation in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E270–E279. doi: 10.1152/ajpendo.2001.280.2.E270. [DOI] [PubMed] [Google Scholar]
- Kroetz DL, Yook P, Costet P, Bianchi P, Pineau T. Peroxisome proliferator-activated receptor α controls the hepatic CYP4A induction adaptive response to starvation and diabetes. J Biol Chem. 1998;273:31581–31589. doi: 10.1074/jbc.273.47.31581. [DOI] [PubMed] [Google Scholar]
- Aldridge TC, Tugwood JD, Green S. Identification and characterization of DNA elements implicated in the regulation of CYP4A1 transcription. Biochem J. 1995;306:473–479. doi: 10.1042/bj3060473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Cao WQ, Kashireddy P, Meyer K, Jia Y, Hughes DE, Tan Y, Feng J, Yeldandi AV, Rao MS, Costa RH, Gonzalez FJ, Reddy JK. Human peroxisome proliferator-activated receptor α supports the induction of peroxisome proliferation in PPARα-deficient mouse liver. J Biol Chem. 2001;276:42485–42491. doi: 10.1074/jbc.M106480200. [DOI] [PubMed] [Google Scholar]
- Patel DD, Knight BL, Soutar AK, Gibbons GF, Wade DP. The effect of peroxisome-proliferator-activated receptor-α on the activity of the cholesterol 7 α-hydroxylase gene. Biochem J. 2000;351:747–753. doi: 10.1042/0264-6021:3510747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema SK, Agellon LB. The murine and human cholesterol 7 α-hydroxylase gene promoters are differentially responsive to regulation by fatty acids mediated via peroxisome proliferator-activated receptor α. J Biol Chem. 2000;275:12530–12536. doi: 10.1074/jbc.275.17.12530. [DOI] [PubMed] [Google Scholar]
- Guillou H, Martin P, Jan S, D'Andrea S, Roulet A, Catheline D, Rioux V, Pineaut T, Legrand P. Comparative effect of fenofibrate on hepatic desaturases in wild-type and peroxisome proliferator-activated receptor α-deficient mice. Lipids. 2002;37:981–989. doi: 10.1007/s11745-006-0990-3. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Ellinghaus P, Fobker M, Seedorf U, Assmann G, Borchers T, Spener F. Phytanic acid is ligand and transcriptional activator of muring liver fatty acid binding protein. J Lipid Res. 1999;40:708–714. [PubMed] [Google Scholar]
- Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor α and γ activators in a tissue- and inducer-specific manner. J Biol Chem. 1998;273:16710–16714. doi: 10.1074/jbc.273.27.16710. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Kuromitsu J, Tanaka I. Microarray analysis of gene expression changes in mouse liver induced by peroxisome proliferator-activated receptor α agonists. Biochem Biophys Res Commun. 2002;290:1114–1122. doi: 10.1006/bbrc.2001.6319. [DOI] [PubMed] [Google Scholar]
- Kok T, Wolters H, Bloks W, Havinga R, Jansen PL, Staels B, Kuipers F. Induction of hepatic ABC transporter expression is part of the PPARα-mediated fasting response in the mouse. Gastroenterology. 2003;124:160–171. doi: 10.1053/gast.2003.50007. [DOI] [PubMed] [Google Scholar]
- Tobin KA, Steineger HH, Alberti S, Spydevold O, Auwerx J, Gustafsson JA, Nebb HI. Cross talk between fatty acid and cholesterol metabolism mediated by liver X receptor-α. Mol Endocrinol. 2000;14:741–752. doi: 10.1210/me.14.5.741. [DOI] [PubMed] [Google Scholar]
- Hsu MH, Savas U, Griffin KJ, Johnson EF. Identification of peroxisome proliferator-responsive human genes by elevated expression of the peroxisome proliferator-activated receptor α in HepG2 cells. J Biol Chem. 2001;276:27950–27958. doi: 10.1074/jbc.M100258200. [DOI] [PubMed] [Google Scholar]
- Shin M, Ohnishi M, Iguchi S, Sano K, Umezawa C. Peroxisome-proliferator regulates key enzymes of the tryptophan-NAD+ pathway. Toxicol Appl Pharmacol. 1999;158:71–80. doi: 10.1006/taap.1999.8683. [DOI] [PubMed] [Google Scholar]
- Wan YJ, Cai Y, Lungo W, Fu P, Locker J, French S, Sucov HM. Peroxisome proliferator-activated receptor a-mediated pathways are altered in hepatocyte-specific retinoid X receptor a-deficient mice. J Biol Chem. 2000;275:28285–28290. doi: 10.1074/jbc.M000934200. [DOI] [PubMed] [Google Scholar]
- Hegardt FG. Transcriptional regulation of mitochondrial HMG-CoA synthase in the control of ketogenesis. Biochimie. 1998;80:803–806. doi: 10.1016/S0300-9084(00)88874-4. [DOI] [PubMed] [Google Scholar]
- Bouly M, Masson D, Gross B, Jiang XC, Fievet C, Castro G, Tall AR, Fruchart JC, Staels B, Lagrost L, Luc G. Induction of the phospholipid transfer protein gene accounts for the high density lipoprotein enlargement in mice treated with fenofibrate. J Biol Chem. 2001;276:25841–25847. doi: 10.1074/jbc.M101160200. [DOI] [PubMed] [Google Scholar]
- Miller CW, Ntambi JM. Peroxisome proliferators induce mouse liver stearoyl-CoA desaturase 1 gene expression. Proc Natl Acad Sci USA. 1996;93:9443–9448. doi: 10.1073/pnas.93.18.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HY, Chang MS, Rho HM. Induction of the rat Cu/Zu superoxide dismutase gene through the peroxisome proliferator-responsive element by arachidonic acid. Gene. 1999;234:87–91. doi: 10.1016/S0378-1119(99)00176-6. [DOI] [PubMed] [Google Scholar]
- Latruffe N, Nicolas-Frances V, Dasari VK, Osumi T. Studies on regulation of the peroxisomal beta-oxidation at the 3-ketothiolase step. Dissection of the rat liver thiolase B gene promoter. Adv Exp Med Biol. 1999;466:253–259. doi: 10.1007/0-306-46818-2_30. [DOI] [PubMed] [Google Scholar]
- Hertz R, Seckbach M, Zakin MM, Bar-Tana J. Transcriptional suppression of the transferrin gene by hypolipidemic peroxisome proliferators. J Biol Chem. 1996;271:218–224. doi: 10.1074/jbc.271.1.218. [DOI] [PubMed] [Google Scholar]