Abstract
Pulmonary arterial hypertension (PH) and chronic kidney disease (CKD) both profoundly impact patient outcomes, whether as primary disease states or as co-morbid conditions. PH is a common co-morbidity in CKD and vice versa. A growing body of literature describes the epidemiology of PH secondary to chronic kidney disease and end-stage renal disease (ESRD) (WHO group 5 PH). But, there are only limited data on the epidemiology of kidney disease in group 1 PH (pulmonary arterial hypertension [PAH]). The purpose of this review is to summarize the current data on epidemiology and discuss potential disease mechanisms and management implications of kidney dysfunction in PAH. Kidney dysfunction, determined by serum creatinine or estimated glomerular filtration rate, is a frequent co-morbidity in PAH and impaired kidney function is a strong and independent predictor of mortality. Potential mechanisms of PAH affecting the kidneys are increased venous congestion, decreased cardiac output, and neurohormonal activation. On a molecular level, increased TGF-β signaling and increased levels of circulating cytokines could have the potential to worsen kidney function. Nephrotoxicity does not seem to be a common side effect of PAH-targeted therapy. Treatment implications for kidney disease in PAH include glycemic control, lifestyle modification, and potentially Renin-Angiotensin-Aldosterone System (RAAS) blockade.
Keywords: pulmonary arterial hypertension, kidney disease, epidemiology, disease mechanisms
Introduction
Kidney dysfunction is a common co-morbidity and independent risk factor for poor outcome in a broad spectrum of cardiovascular disease, including left ventricular systolic and diastolic dysfunction.1–3 Pulmonary hypertension (PH) describes a cardiovascular syndrome affecting the right heart that is characterized by increased blood pressure in the pulmonary circulation (mean pulmonary artery pressure [mPAP] >25 mmHg), which can occur in the context of a large number of disease states.4 Currently, PH is divided into five different clinical groups according to the underlying pathophysiology, clinical presentation, and treatment strategy (Table 1).4 This classification, derived during the 5th World Symposium held in Nice, France, in 2013, provides the most commonly used framework to approach PH. A hemodynamically focused schema, tightly aligned with the Nice Classification, identifies patients who will benefit from drug therapy for pre-capillary PH. A hemodynamic classification rigorously divides according to hemodynamic conditions, using the pulmonary capillary bed to divide conditions into pre-capillary and post-capillary forms of PH.
Table 1.
Classification of pulmonary hypertension (adjusted from Simonneau et al.201).
| Group | Entities | |
|---|---|---|
| 1 | Pulmonary arterial hypertension | Idiopathic, Heritable, CTD, portopulmonary, CHD, others |
| 2 | Pulmonary hypertension due to left heart disease | Left ventricular systolic/diastolic dysfunction, valvular heart disease, others |
| 3 | Pulmonary hypertension due to lung disease or hypoxia | COPD, ILD, sleep-disordered breathing, others |
| 4 | Chronic thromboembolic pulmonary hypertension (CTEPH) | |
| 5 | Pulmonary hypertension with unclear mechanisms | CKD, hematologic disorders, others |
CHD: congenital heart disease; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CTD: connective tissue disease; ILD: interstitial lung disease.
Pre-capillary PH is defined by elevated pulmonary artery pressure (PAP) in the absence of increased pulmonary venous or left sided filling pressures. In contrast, post-capillary PH is defined by increased PAPs with evidence of elevated pulmonary venous and/or left-sided filling pressures. A combined pre- and post-capillary form of PH can also be present, defined as PH with an increased diastolic pressure gradient.5
There is evidence for an overlap between pre- and post-capillary PH and certain patients with post-capillary PH might benefit from pre-capillary PH-targeted therapy.6
Of course, the Nice and hemodynamic classification schemes are intimately related. For example, WHO Group 1 PH is a distinct group of pre-capillary PH, also called pulmonary arterial hypertension (PAH) and defined by a mPAP >25 mmHg in the presence of a pulmonary capillary wedge pressure ≤15 mmHg. PAH has a low incidence but typically high mortality rate. PAH occurs as an idiopathic disease entity or one associated with a co-existent condition such as a PAH-specific gene mutation, drug or toxin exposure, connective tissue disease (CTD), congenital heart disease, or other condition. WHO Group 2 captures PH due to left-heart disease, therefore describing a population of subjects with post-capillary PH; this group has the highest incidence and prevalence of PH. It was reported that around 60–80% of patients with left heart disease have PH.7 WHO Group 3 PH is associated with chronic hypoxemic pulmonary disease, which is most often pre-capillary in nature. Group 4 PH is due to chronic thromboembolic occlusion of the pulmonary arteries and oftentimes is associated with a history of pulmonary embolism or thrombophilic disorders; while post-capillary disease can exist, this too is more often pre-capillary in nature. Group 5 encompasses PH in the setting of a primary disease outside the lungs that affects the pulmonary circulation via multiple different and/or unknown mechanisms and may involve both pre- and/or post-capillary presentations. PH in the setting of CKD and ESRD belongs to this group. The majority of data about the epidemiology of kidney dysfunction comes from WHO Groups 2 and 5. Data about kidney dysfunction and potential disease mechanisms in PH group 1 (PAH) are more limited in scope.
CKD, or CKD stage 3, is defined as the presence of a reduced glomerular filtration rate (GFR) below 60 mL/min/1.73 m2 for three or more months. The definition of CKD also includes persistent albuminuria with a spot urine albumin to creatinine ratio of 30 mg/g or above for three or more month regardless of GFR. Additional criteria of CKD include the presence of urinary white or red cell casts for three or more month and renal imaging or biopsy abnormalities.8 In this review, we consider kidney dysfunction as CKD stage 2 or higher. The rationale for this definition comes from epidemiological data showing that mild kidney dysfunction (CKD stage 2) with an estimated GFR (eGFR) above 60 mL/min/1.73 m2 is independently associated with the risk for cardiovascular disease.9,10
This review will summarize the epidemiology of kidney dysfunction in PAH and its impact on outcome in this patient population. Potential disease mechanism evident in PAH that can impact kidney function will be discussed. Conversely, the impact of kidney dysfunction on PAH pathophysiology will be addressed. Potential effects of PAH targeted therapy on kidney function and potential treatment implications will be reviewed. The most evident overlapping molecular mediators contributing to PAH and kidney dysfunction will be reviewed. Finally, we discuss important treatment implications for PAH patients with evidence of kidney dysfunction.
Epidemiology and impact of kidney dysfunction on outcomes in PAH
The precise prevalence of kidney dysfunction in PAH has not been determined and may vary according to subtype. It is clear that the prevalence of kidney dysfunction in PAH is high, in the range of 4–36% (Table 2). This substantially exceeds the prevalence of kidney dysfunction in the general population, which was reported on the order of 0.6% in men and 0.3% in women.11,12 Patients with connective tissue disease-associated PAH (CTD-PAH) have the highest rates of kidney impairment, although kidney dysfunction is also a feature of other PAH forms. Older age, high systemic blood pressure, diabetes, and increased right atrial pressure seem to be associated with kidney impairment in PAH patients.
Table 2.
Prevalence of kidney dysfunction in Group 1 PAH and its impact on outcome.
| Studies | Year | Patients with RHC (n) | CKD stage III | Patient population | Patients on Diuretics | Impact of CKD on outcome |
|---|---|---|---|---|---|---|
| Benza (Reveal) | 2010 | 2716 | 4% | 86% prevalent cases; 47% IPAH; 2.9% FPAH; 11.8% CHD; 23.9% CTD; 13.5% SSc; 0.9% others | No data | Unadjusted: HR 3.3 (2.3–4.7); Adjusted*: HR 1.9 (1.3–2.7) |
| Shah et al. | 2008 | 500 | 12% | Prevalent cases: 47% IPAH; 11% CHD; 31% CTD; 11% others | 50% | Unadjusted for creatinine >1.4 mg/dl: HR 2.54 (1.7–3.7) |
| Chung (Reveal) | 2010 | 1251 | 3.9% IPAH; 6.9% CTD; 8.7% SSc | Prevalent and Incident | No data | No data |
| Campo | 2010 | 76 | 46% | Prevalent 100% SSc | 61% | Unadjusted: HR 2.6 (1.3–5.4) |
| Cogswell | 2013 | 126 | 19% | Prevalent 100% PAH | No data | No data |
| Dimopoulos | 2008 | 102 | 18% | Prevalent and Incident 100% CHD | 59% | Unadjusted: HR 3.3 (1.5–6.9)†; Adjusted‡: HR 2.3 (1.2–4.6)† |
| Navaneethan | 2014 | 552 | 36% | Prevalent 100% PAH | 68%† | Unadjusted: HR: 1.7 (1.4–1.9)†; Adjusted§: HR: 1.4 (1.1–1.7)† |
| Chakinala (Reveal) | 2016 | 2368 | 29% | Prevalent 45% IPAH 28% CTD 10% CHD 17% others | No data | Unadjusted: per 10% decline in GFR: HR 1.24 (1.1–1.4); Adjusted**: per 10% decline in GFR: HR 1.2 (1.00–1.33) |
| Leuchte | 2007 | 66 | 17% | Prevalent | No data | Unadjusted: HR 1.6 (1.1–2.4)† |
| O’Leary | 2016 | 840 | 37% | Prevalent and Incident | No data | Unadjusted: HR 1.4 (1.2–1.6)† |
Adjusted for age, gender, BMI, hemodynamics, WHO functional class, 6MWD.
Based on PAH and non-PAH cases.
Adjusted for hemodynamics and WHO functional class.
Adjusted for age, gender, diabetes, hemodynamics, hemoglobin, albumin, PH class.
Adjusted for baseline eGFR, change in 6MWD, change in WHO functional class.
CHD: congenital heart disease; CKD: chronic kidney disease; CTD: connective tissue disease; FPAH: familiar pulmonary arterial hypertension; HR: hazard ratio; IPAH: idiopathic pulmonary arterial hypertension; SSc: scleroderma.
Table 2 summarizes the available information related to the epidemiology of kidney dysfunction in PAH. The Registry to Evaluate Early And Long-term PAH disease management (REVEAL) evaluated 2716 patients with PAH, and found that 4 percent of REVEAL subjects had kidney dysfunction, although it was not clearly defined. Kidney dysfunction in this cohort was associated with a threefold increased risk for death in a univariate regression model and a twofold increase for poor outcome in a multivariate model, adjusted for PAH subgroup, BMI, six-minute walking distance (6MWD), demographic, hemodynamic, and functional variables.13 In a subgroup analysis from the REVEAL cohort, Chung et al. found that compared to idiopathic PAH (IPAH) patients, patients with CTD-PAH had a higher incidence of kidney dysfunction (7% versus 4%). Scleroderma-associated PAH (SSc-PAH) patients had the highest incidence (8.4%) among those with CTD-PAH. The differences in kidney function could not be explained by hemodynamic variables at the time of catheterization.14 Similarly, Tedford and Kane et al. confirmed the higher incidence of kidney dysfunction in CTD-PAH, compared to other PAH subgroups.15,16 None of these studies reported prevalence of pre-existing renal disease in patients with CTD-PAH.
Meanwhile, Navaneethan et al. reported a prevalence of kidney dysfunction defined as stage 3 (GFR < 60 mL/min/1.73 m2 measured on two occasions 90 days apart) in 47% of 552 PAH patients. This study identified factors associated with kidney dysfunction as older age, systemic hypertension, and higher right atrial pressure. PAH patients with kidney dysfunction at baseline or worsening kidney function had an increased risk of death even after adjusting for demographic and hemodynamic variables.17 In a cross-sectional study of patients referred for right heart catheterization, O’Leary et al. found that in 840 patients with PAH, the prevalence of kidney dysfunction was 37% and mortality increased in a graded fashion across CKD stages.18
Shah et al. investigated baseline serum creatinine levels in 578 PAH patients and found that 12% of patients had evidence of kidney dysfunction defined by a single eGFR below 60 mL/min/1.73 m2. Age, male gender, systemic hypertension, diabetes mellitus, CAD, worse right ventricular hemodynamics, and diuretic use were associated with worse kidney function.19
Chakinala investigated the impact of changes in kidney function in PAH patients on outcome using an updated REVEAL cohort including 2368 patients with serial GFR measurements. Patients with a decrease in GFR over time had worse outcomes compared to patients with a stable or increasing GFR, independent of the baseline eGFR, 6MWD, and WHO-FC.20
The variability in the prevalence of kidney dysfunction in PAH patients reported above has likely multiple reasons, including the lack of a consistent definition of impaired kidney function, differences in GFR estimation, different inclusion and exclusion criteria, diverse distribution of PAH subgroups, differences in co-morbidities associated with CKD, and lack of report of diuretic use.
In the initial REVEAL registry, for example, each single investigator determined CKD and it is unclear what criteria were used. Shah et al. used a single serum creatinine measurement at study entry and excluded patients with documented kidney insufficiency. Navaneethan et al. used two eGFRs <60 mL/min/1.73 m2 three months apart to define CKD.
The differences of GFR estimations (MDRD, CKD-EPI, Cockcroft) in PAH is likely minimal. Kaiser et al. applied all three formulas in 64 patients with PH (32 with PAH) and did not find significant differences in estimation of eGFR. The Cockcroft formula performed best in terms of prediction survival.21 Renal dysfunction is common in many CTDs and may account for the higher prevalence of kidney impairment found in CTD-PAH patients. Renal dysfunction was reported to range from 5% in SSc to 50% in systemic lupus erythematosus.22,23 Various mechanisms have been associated with kidney disease in CTDs, including tubule-interstitial nephritis, minimal change disease, and membranous nephropathy (reviewed in Kronbichler and Mayer24). Besides PAH subtypes, it is also important to note differences in co-morbidities like systemic hypertension and diabetes when interpreting study results of kidney disease in PAH.
The use of diuretic therapy is—as standard of care—highly prevalent in patients with PAH. Amount, combination, and duration of diuretic therapy can impact serum creatinine levels and GFR. Most studies do not report details of diuretics used. In those studies that do report the use of diuretics, the variance of patients on diuretics is high, in the range of 50–90%.19,25
In summary, even though there is a wide discrepancy in the prevalence kidney dysfunction in patients with PAH and only one study so far used the Kidney Dialysis Outcomes Quality Initiative (KDOQI) CKD definition of two separate GFR values at least three months apart, there is robust evidence of impaired kidney function in PAH. It seems that right ventricular hemodynamics, PAH subtype (especially CTD-PAH) and traditional risk factors like systemic hypertension, age, and diabetes are associated with kidney dysfunction in PAH patients. Kidney dysfunction has an important impact on survival in PAH and even mild impairment of kidney function seems to be an independent predictor of mortality.
Potential mechanisms of kidney dysfunction in PAH
Numerous potential mechanisms exist to explain the high prevalence of kidney dysfunction in PAH. Major contributors are the tight interplay between the heart and the kidney (cardiorenal syndrome [CRS]) and neurohormonal activation in the setting of right heart failure. As vasoactive substances, the majority of PAH-targeted therapy seems to have renal protective properties.
Cardiorenal syndrome and the right ventricle
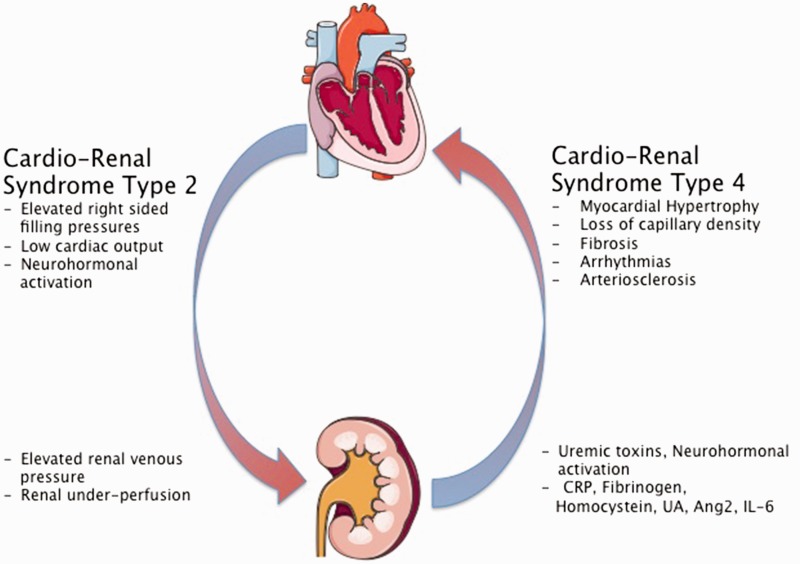
A common feature of kidney dysfunction in all forms of PH is CRS, a clinical state in which the treatment of heart or kidney failure is limited by worsening heart or kidney function. CRS describes the tight bidirectional relationship between these two organs (reviewed in Ronco et al.26).
CRS type 2 describes kidney dysfunction in the setting of chronic heart disease. Evidence for presence of CRS in PAH comes from the observation that there was a particularly strong correlation between right atrial pressure and kidney dysfunction that outperformed the association with cardiac index in PAH patients.19 The strong interaction of elevated right atrial pressures and kidney function was also observed in group 2 PH patients, suggesting the importance of venous congestion in kidney dysfunction.17,27,28 In one study there was a significant correlation between right atrial pressure and GFR in all groups of PH patients and an elevated right atrial pressure was in independent predictor of CKD.17 This is in line with the observation in patients with different forms of heart failure, where it was shown that elevated right-sided filling pressures were the most important predictor of worsening kidney function.29,30
CKD defined by a GFR <60 mL/min/1.73 m2 in patients with left-sided heart failure is common and in the range of 20–80%.3,31,32 Patients with kidney dysfunction and left heart disease are older and have a higher prevalence of systemic hypertension and diabetes.2,3 Apart from a higher rate of cardiovascular disease, kidney function in patients with left heart disease was shown to be independent of left ventricular ejection fraction or cardiac index, but linked to diastolic dysfunction and right atrial pressures.1,2,31,33 In patients with heart failure with reduced ejection fraction (HFrEF), the presence of PH was associated with worse kidney function. In patients undergoing right heart catheterization for evaluation of valvular heart disease, coronary artery disease, and heart failure, central venous pressure was independently associated with kidney function.34 The degree of kidney dysfunction pre- and postoperatively in patients undergoing aortic valve replacement was directly related to the degree of PH measured by right heart catheterization.35
Experimental evidence from isolated dog kidneys revealed that changes of venous pressure had a greater influence on the reduction of kidney blood flow and urine output then an equal reduction of arterial pressures.36
In summary, right ventricular hemodynamics, especially right atrial pressure, seem to play a major role in the development and progression of kidney dysfunction in PAH (Fig. 1).
Fig. 1.
Cardiorenal syndrome in PAH and CKD. CRP, C-reactive protein; UA, uric acid; Ang-2, angiopoietin 2; IL-6, Interleukin 6. Figures were produced using Servier Medical Art found on www.servier.com.
Neurohormonal activation
Right ventricular hemodynamics are of major prognostic importance in patients with PAH and have been linked to increased neurohormonal activation.37–41
Although it is uncertain whether increased neurohormonal activation is caused by right heart failure, sympathetic activation in PAH patients measured by postganglionic activity was shown to be related to the progression of right heart failure and to decrease after atrial septostomy.37,38 Sympathetic nerve fibers are located in the bifurcation of the main pulmonary artery in men.42,43 It was shown that sympathetic denervation of the pulmonary artery improved hemodynamics and functional capacity in patients with IPAH. Furthermore, pulmonary artery denervation was shown to decrease neurohormonal activity in preclinical models.44,45
The prognostic importance of increased Renin-Angiotensin-Aldosterone-System (RAAS) activity has been demonstrated in PAH for circulating levels of renin, angiotensin-1 and 2, vasopressin, and high prevalence of hyponatremia (as an indirect marker of RAAS activation).25,46,47 Polymorphisms in angiotensin converting enzyme (ACE) and angiotensin two receptor type 1 (ART1) have been found in patients with PAH and were associated with favorable right ventricular hemodynamics and later age of disease onset.48 Evidence for a pathobiological role of the RAAS system in PAH comes from studies demonstrating increased ACE activity, upregulated angiotensin receptor type 1 expression, and enhanced angiotensin II production in pulmonary vessels of PAH patients that was linked to hyperplasia of pulmonary arterial endothelial cells (ECs).46 Increased aldosterone expression in human pulmonary arterial ECs was linked to nitric oxide production and worsening of experimental PH via modulation of endothelin B (ET-B) receptor.41
Increased activity of the neurohormonal axis is a well-established phenomenon in patients with CKD.49,50 There is also evidence that diseased kidneys are the primary source of induction of neurohormonal and RAAS activity, independent of blood pressure, GFR, or volume status.49,51,52 Similarly to the effect on the pulmonary circulation, it was shown that infusion of angiotensin II in rats leads to structural remodeling of the renal vasculature.53,54 Overwhelming clinical evidence of the pathobiological importance of RAAS in CKD comes form studies that show significant reno- and cardiovascular protective effects of pharmacological RAAS blockade in CKD.55–58
In summary, there is evidence that a combination of PH and CKD leads to increased neurohormonal activation, which can be linked to worsening vascular remodeling of the pulmonary and renal circulation. Therefore, patients with PAH and concomitant kidney dysfunction are at higher risk of increased neurohormonal activation and poor outcome. Clinical trials to investigate safety and efficacy of neurohormonal inhibition in PAH are lacking.
PAH targeted therapy and CKD
The pathogenesis of CKD consists of hemodynamic changes, inflammation, and oxidative stress, leading to glomerulosclerosis and kidney atrophy.59
The mainstay of current PAH-targeted therapy consists of potent vasoactive substances like Endothelin receptor antagonists (ERAs), Phosphodiesterase type 5 Inhibitors (PDE5I), and Prostacyclin analogues and therefore can affect the highly vascularized kidneys.60,61
Sildenafil, the first PED5I studied in PAH, has multiple nephroprotective effects, including lowering systemic blood pressure, reducing tissue damage after ischemia-reperfusion injury, decreasing contrast and cyclosporine-induced kidney injury, and reduction of diabetes induced glomerulosclerosis (reviewed in Afsar et al.62).
The endothelin system is a family of peptides and G-protein coupled receptors with an important role for vascular tone, especially in the kidneys and lungs. Endothelins bind to type A and type B receptors. Type A receptor (ET-A) is preferentially expressed on smooth muscle cells (SMCs) and promotes vasoconstriction. Type B receptor (ET-B) is expressed on ECs and promotes vasodilation (reviewed in Kedzierski and Yanagisawa63). Circulating endothelin levels are increased in patients with PAH and CKD and the endothelin system plays important roles in both disease states.64,65 From a physiological point of view, ET-A blockade should be superior to dual endothelin blockade in CKD due to an antagonistic role of ET-B to ET-A mediated glomerular vasoconstriction. A small randomized controlled trial (RCT) could show that ETA receptor antagonism has renal-protective effects that were independent of blood pressure lowering effects and not present in patients receiving combined ETA/B receptor antagonism.66 In addition, preclinical data showed that ET-B blockade caused salt-sensitive hypertension and fluid retention,67,68 whereas ET-A blockade showed promising results in different animal models of CKD (reviewed in Neuhofer and Pittrow69). In summary, selective ET-A blockade could have superior renal protective effects, compared to dual ET-A/ET-B blockade in PAH patients.
Prostanoids are arachidonic acid metabolites that play an important role in vascular homeostasis and are among the first PAH-targeted therapies (reviewed in Mubarak70).
Several lines of evidence support renal protective effects of Iloprost in patients with CKD or kidney dysfunction. Iloprost was shown in a RCT to protect against contrast-induced nephropathy.71 In patients undergoing coronary artery bypass grafting, prophylactic Iloprost administration was associated with significantly improved urine output and decreased serum creatinine.72 Administration of Iloprost in patients with inoperable limb ischemia and kidney dysfunction (CKD stage 2) was associated with improvements in serum creatinine and eGFR.73 Similarly, long-term administration of Beraprost in patients with diabetic CKD was shown to decrease urinary albumin excretion.74 A recent RCT in patients with non-diabetic CKD showed a trend towards slower decline in renal function in patients on Beraprost compared to placebo.75
Riociguat
Riociguat is a novel treatment for PAH that stimulates the soluble guanylate cyclase in vascular SMCs. There is preclinical evidence that soluble guanylate cyclase stimulators have reno-protective effects in different animal models (reviewed in Stasch et al.76). There are no clinical data on the effects of soluble guanylate cyclase stimulators on kidney disease available as of now. Riociguat is renally cleared, but Riociguat has not been evaluated in patients’ ESRD (reviewed in Khaybullina et al.77).
In summary, most PAH-targeted therapy was shown to have nephroprotective potential in a pre-clinical or clinical setting. However, there are no dedicated clinical trials investigating the role of PAH-targeted therapy and its impact on kidney function. These trials are highly warranted.
Systemic impact of CKD in PAH
Regardless of the mechanism(s) involved in CKD in PAH patients, its impact may be significant. The presence of CKD is a key contributor to several pathophysiologic processes, which may amplify the deleterious effects of PAH.
Kidney dysfunction was shown to contribute to cardiac remodeling, EC dysfunction, and decreased clearance of toxins and cytokines leading to systemic inflammation.
Cardiorenal syndrome type 4
CRS type 4 is defined by reduced kidney function, increased blood pressure, volume overload, and circulating uremic toxins, leading to myocardial dysfunction characterized as myocardial hypertrophy, fibrosis, and loss of capillary density (reviewed in Larsen et al.78 and Cerasola et al.79). Chronic kidney insufficiency at any stage is associated with a significant increased risk for or progression of cardiovascular disease, heart failure, and atrial fibrillation.80–83 Recent reports provided evidence that patients with PAH also have toxic myocardial lipid accumulation and the prevalence of atrial fibrillation was reported to be four times higher in patients with PAH and inoperable chronic thromboembolic pulmonary hypertension (CTEPH) when compared to the general population.84–86 It is possible that in PAH the presence of kidney dysfunction contributes to adverse cardiac remodeling leading to right heart failure and atrial arrhythmias.
Systemic endothelial cell dysfunction, inflammation, and uremic toxins
Systemic vascular changes in ECs and SMCs are common in CKD including accelerated arteriosclerosis, increased vascular stiffness, and SMC transformation (reviewed in Moe and Chen87). EC dysfunction measured by flow-mediated vasodilation was shown to be present in the early stages of CKD and seemed to be independent from other co-morbidities like hypertension and diabetes.88 Similar observations were made in patients with PAH.89,90 It was further shown that patients with CKD have elevated levels of C-reactive protein (CRP), fibrinogen, and homocysteine, markers that are associated with cardiovascular disease and that are also elevated and of prognostic importance in PAH.91–94 Hyperuricemia is common in patients with PAH and was shown to be an independent predictor of survival.95,96 Nagaya et al. showed that uric acid (UA) levels correlated with kidney function, cardiac output, and higher doses of diuretics, respectively.95 It was further shown that UA levels decreased with the initiation of PAH targeted therapy.96,97 An ultrasound-based study showed that increasing UA levels could predict the development of PH in SLE patients.98 Voelkel et al. found a striking correlation between UA levels and right atrial pressure in PAH patients that could not be explained by impaired kidney or liver function alone.97 The authors therefore concluded that the ischemic right ventricle might be the site of excessive UA production.
Potential mechanisms by which UA could contribute to PAH include systemic inflammation,99–101 vasoconstriction,102 and vascular remodeling.103,104 Impaired kidney function could contribute to decreased clearance of UA and therefore promote unfavorable pulmonary vascular remodeling.
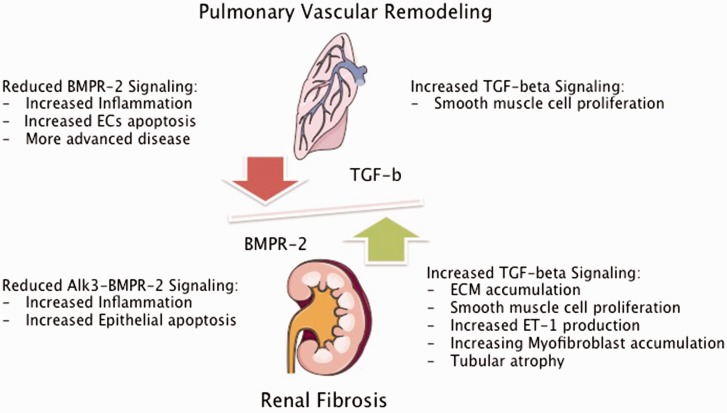
In summary, impaired kidney function could contribute to the progression of pulmonary vascular and/or right ventricular remodeling in PAH by induction or decreased clearance of inflammatory cytokines and uremic toxins, associated with vasoconstriction, EC dysfunction, and adverse cardiac remodeling (Fig. 2).
Fig. 2.
Impact of impaired TGF-β signaling in PAH and CKD. BMPR-2, bone morphogenetic protein 2; TGF-β, transforming growth factor beta; ECs, endothelial cells; Alk-3, activin receptor like kinase 3; ECM, extracellular matrix; ET-1, endothelin 1. Figures were produced using Servier Medical Art found on www.servier.com.
Molecular mediators
PAH and CKD are different pathologic and pathophysiologic entities. However, there is a significant overlap of multiple critical cell signaling pathways and circulating cytokines/chemokines that seem to play important roles in development and progression in both diseases. This overlap may reveal new insights into the factors that contribute to deleterious outcomes in PAH.
Transforming growth factor β (TGF-β) and bone morphogenetic protein (BMP) signaling
TGF-β and BMP signaling is fundamental for many important cellular processes. The proper functioning of the TGF-β/BMP axis is dependent on a highly abundant and overwhelmingly complex network of ligands, receptor assemblies, and downstream signaling pathways that depend on an extensive crosstalk with other signaling molecules (reviewed in Moustakas et al.105). Smad proteins are important downstream transducers of the TGF-β/BMP pathway. Cell-based studies have shown that TGF-β ligands predominantly activate Smad2/3, whereas BMP ligands seem to activate Smad1/5/8 (reviewed in Moustakas et al.105). The TGF-β/BMP axis is cell-type and context dependent. It is therefore difficult to draw parallels between TGF-β signaling in PAH and CKD, but there is evidence that the TGF network is a fundamental player in both pathologic states. Current concepts of disease development and progression propose that PAH is caused by an imbalance of TGF-β and BMP signaling. Loss of function mutations of the BMPR-2 (found in more than 70% of HPAH and about 20% of sporadic cases106) were associated with increased TGF-β signaling and more advanced disease, compared to patients without mutations in the BMPR-2 receptor.107 It was shown that patients with PAH have about four times higher levels of circulating TGF-β, when compared to healthy controls. Furthermore, PAH patients had a significant trans-pulmonary gradient of TGF-β levels that was not seen in controls, suggesting production of TGF-β in the PAH lung.108 There is also cumulative evidence that TGF-β and Smad2 expression is increased in the pulmonary vasculature of PAH patients, when compared to healthy control or emphysema lungs.109,110 In contrast to that, one study showed significant reduction of TGF-β receptors and activation of Smad2 in the MCT-induced PH rat model.111 It was shown that TGF-β induces proliferation of pulmonary arterial SMCs from patients with PAH when compared to pulmonary SMCs from control arteries.107,110 Furthermore, in pulmonary arterial SMCs with reduced BMPR2 expression, the TGF-β, Smad2 pathway was potentiated.112 Reduced BMPR-2 expression is associated with pulmonary vascular inflammation, ECs apoptosis in humans, and the development of PH in several animal models.113–118 In addition, it was shown that BMP-2 signaling induces differentiation, inhibits proliferation, and prevents post-injury hyperplasia of aortic SMCs.119,120
CKD is characterized by vascular rarefication and fibrosis. A variety of different pathologic processes contributing to CKD have been identified, including premature cell-cycle arrest, activation of myofibroblasts and fibrocytes, extension of extracellular matrix (ECM), and recruitment of various infiltrating immune cells to the site of injury (reviewed in Chawla et al.121).
There is growing evidence that increased TGF-β signaling also plays a key role in the progression of various kidney diseases (reviewed in Wang et al.122). Research in human and experimental kidney disease has established the critical role of Smad2 and Smad3 signaling in promoting renal fibrosis, the most common pathobiological pathway of progressive CKD.123–125 TGF-β overproduction was linked to various human kidney diseases including IgA nephropathy, lupus nephritis, and diabetic nephropathy.126,127
In several animal models, enhancement of the TGF-β pathway has been shown to promote renal fibrosis, due to renal vascular, glomerular, and tubular damage characterized by ECM accumulation and SMC hyperplasia.128,129 In contrast, inhibition of TGF-β was associated with decreased renal vascular and SMC hypertrophy.130,131 One study could demonstrate that reduction of kidney injury by TGF-β neutralization was characterized by significantly decreased endothelin 1 production, an important promoter of pulmonary vascular constriction and treatment target in PAH.131,132
In preclinical studies it was shown that BMP-7 was able to ameliorate TGF-β-induced renal fibrosis by decreasing the accumulation of myofibroblasts and tubular atrophy and to improve GFR.133,134 It was shown in a mouse model of nephrotoxic-induced kidney fibrosis, that deletion of a BMPR2 co-receptor, activin-like kinase 3 (Alk3) in tubular epithelial cells was associated with enhanced TGF-β–Smad 3 signaling and worsening of renal fibrosis characterized by increased interstitial inflammation and tubular epithelial apoptosis. Restoration of BMP signaling with a peptide agonist improved kidney fibrosis. This study therefore suggests a protective role of the Alk3-BMPR2 pathway in kidney injury.135 Even though this study received substantial criticism for the role of the BMP agonist,136 it underscores the importance of disturbed TGF-β/BMPR2 signaling in kidney fibrosis. It is possible that in PAH, decreased systemic BMPR2 signaling and increased systemic TGF-β levels, lead to increased activity of the TGF-β/Smad2/3 pathways and promotes significant proliferative stress in the kidneys, leading to expansion of ECM and SMC hyperplasia and therefore increased risk of the development of CKD (Fig. 2).
Angiopoietins
Angiopoietin (Ang)-1 and its antagonist Ang-2 are essential factors for vascular development and homeostasis. Both proteins bind to the Tie2 receptor, which is almost exclusively expressed on ECs. Ang-1 is believed to constitutively phosphorylate Tie2 to maintain vascular quiescence and structural integrity. Ang-2 can disrupt Ang-1/Tie2 signaling and destabilize EC homeostasis leading to an angiogenic response or vascular regression (reviewed in Fagiani and Christofori137). Several lines of evidence support a role of dysregulated Ang/Tie2 signaling in experimental and human PH/PAH associated with SMC proliferation and EC apoptosis.138–143 Even though the data are controversial, it was shown that circulating Ang-1 and Ang-2 were elevated in PAH. Ang-2 levels correlated with right ventricular hemodynamics, response to treatment, and outcome. Furthermore, levels of Ang-2 were highly expressed in areas of vascular remodeling.143
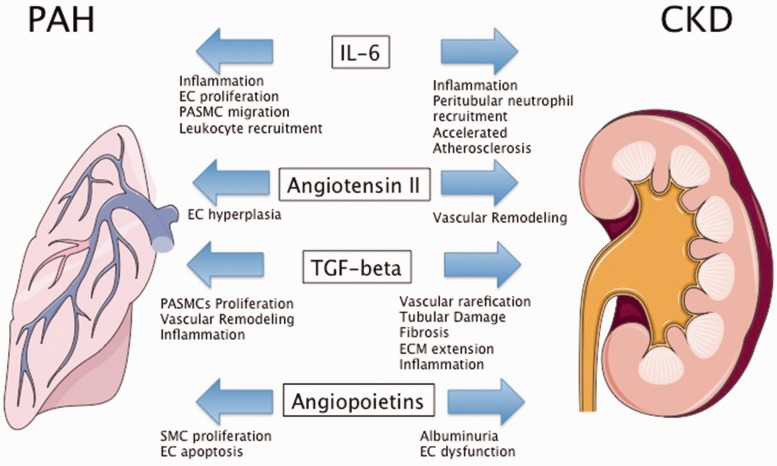
The Ang/Tie-2 system is also of significant importance in kidney development, homeostasis, and glomerular pathobiology (reviewed in Woolf et al.144). Ang-2 overexpression was shown to result in a significant increase in albuminuria, indicating EC dysfunction.145,146 Patients with CKD have higher circulating levels of Ang-2 compared to healthy controls that predict poor outcome in this patient population.147 In PAH, increased circulating levels of Ang-2 could disturb vascular homeostasis and promote pathology in pulmonary and renal vessels, resulting in disease worsening of pulmonary hypertension and progression of CKD (Fig. 3).
Fig. 3.
Molecular mediators of PAH and CKD. EC, endothelial cells; PASMC, pulmonary arterial smooth muscle cells; SMC, smooth muscle cell; ECM, extracellular matrix. Figures were produced using Servier Medical Art found on www.servier.com.
Asymmetric dimethylarginine
Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of nitric oxide synthesis and associated with systemic endothelial dysfunction (reviewed in Cooke148).
In PAH, increased levels of ADMA were shown to be associated with unfavorable pulmonary hemodynamics and worse outcome. ADMA is considered a uremic toxin that was shown to be elevated in patients with CKD and associated with cardiovascular events in this patient population.149,150 Elevated circulating levels of ADMA were directly linked to the development of renal fibrosis in mice characterized by EC dysfunction, ECM synthesis, and rarefaction of peritubular capillaries.151 Increased levels of ADMA could promote worsening PAH and CKD by depleting nitric oxide in the pulmonary and renal vasculature, leading to vascular constriction and remodeling.
Interleukin (IL-6)
Several pro-inflammatory cytokines were shown to be elevated in patients with PAH and animal data support a pathophysiological role of inflammation in pulmonary vascular remodeling. Similarly, pro-inflammatory cytokines were shown to play a pivotal role in the response to injury in the kidneys (reviewed in Shum et al.152). IL-6 is the most studied cytokine in PAH/PH and seems to play an important role in the pathogenesis of pulmonary vascular remodeling, associated with EC proliferation, pulmonary arterial SMC migration as well as pulmonary macrophage and lymphocyte recruitment (reviewed in Groth et al.153 and El Chami and Hassoun154). IL-6 is an acute-phase cytokine, with diverse biological function such as hypercoagulability, accelerated atherosclerosis, and associated with activation of lymphocytes and leukocyte recruitment (reviewed in Jones et al.155). Serum levels of IL-6 are significantly elevated in patients with PAH compared to normal controls and associated with outcome and mutations in BMPR2.156,157 Interestingly, IL-6 does not seem to correlate with hemodynamic parameters measured by right heart catheterization, which could suggests that IL-6 levels are not a reflection of right ventricular failure, but potentially systemically involved in pathogenesis of vascular remodeling in PAH.157 Animal data showed that IL-6 is expressed in lung tissue of rats with experimentally induced PH and dexamethasone decreased IL-6 production and attenuated PH.158 Subcutaneous injection of IL-6 causes mild PH in mice and worsens hypoxia-induced PH.159 Lung-specific IL-6 overexpressing mice showed profound vascular remodeling that resembled changes seen in human PAH (plexiform lesions).160 IL-6 was shown to be elevated and an important independent predictor of mortality in patients with CKD. IL-6 levels were associated with higher CKD stages, but did not correlate with GFR.161,162 Elevated levels of IL-6 have been linked to adverse cardiac remodeling and atrial fibrillation in patients with CKD.163,164 Experimental data provided evidence for a direct role of IL-6 in models of acute kidney injury. In mice, systemic levels of IL-6 rise during kidney injury and expression of IL-6 was increased in in tubular epithelial cells. Furthermore, IL-6 deficient mice were protected against mercury chloride-induced kidney damage and showed reduced peritubular neutrophil accumulation.165 Kidney dysfunction could contribute to elevated levels of IL-6 in PAH and worsen pulmonary and cardiac remodeling. Vice versa, elevated levels of IL-6 could contribute to worsening kidney function by inducing inflammation of the tubular epithelium (Fig. 3).
Treatment implications for CKD in PAH
There are no dedicated clinical trials or guidelines addressing best clinical management of kidney dysfunction in PAH patients. Given the high prevalence of kidney dysfunction in PAH and its substantial effects on patient outcome, management strategies to prevent kidney dysfunction and its progression are important. Studies to formulate management guidelines of kidney dysfunction in PAH are lacking. Findings from non-PAH patients with CKD can provide important insights into potential management strategies of kidney disease in PAH. These could include systemic blood pressure control, glucose and UA management, lifestyle modifications, as well as RAAS blockade.166
Blood pressure control
Lowering systemic blood pressure reduces the rate of CKD progression and international consensus guidelines recommend a systemic systolic blood pressure below 140 mmHg and a diastolic blood pressure below 90 mmHg in non-diabetic patients with CKD.167 Elevated systemic blood pressure is an increasingly recognized co-morbidity in PAH and was reported in recent PAH registries between 27% and 40%.168,169 Data from the CKD population without PH cannot be extrapolated to patients with PAH and there are no RCTs addressing blood pressure control in this vulnerable population and current guidelines do not support use of ACE inhibitors, angiotensin receptor blockers (ARBs), or beta-blockers.60,61 In addition, tight blood pressure control in patients with cardiovascular disease might be associated with increasing risk for cardiovascular events.170,171
RAAS blockade
ACE inhibitors and ARBs are standard treatment for patients with CKD and have been shown to reduce progression of CKD, CVD, and improve outcome.172–175
Aldosterone antagonists are considered as standard in heart failure management and associated with improved survival.176 Early trials from the 1980s with the ACE inhibitor Captopril in patients with PAH did not show any effect on pulmonary hemodynamics, but kidney function was not monitored.177,178 A recent study showed that aldosterone blockade with spironolactone in conjunction with Ambrisentan tends to improve clinical parameters like 6MWD, WHO-FC, and reduce circulating levels of NT-proBNP. However, given the small sample size, none of those parameters reached a clinical significant difference. In addition, there was no assessment of kidney function in this study.179
Glycemic control
Glycemic control in patients with diabetes and CKD has an important influence on microvascular complications, progression of CKD, and was shown to reduce albuminuria.180 In the UKPDS study, intensive glucose control (hemoglobin A1C < 7%) was associated with a significant reduction in progression of CKD in patients with type 2 diabetes mellitus.181 Given the high prevalence of hyperglycemia and insulin resistance in patients with PAH,182–184 attention to improved glycemic control could prevent progression of CKD and improve outcome in this patient group.
Protein intake
Data from the Nurses Health Study implies that in patients with mild kidney dysfunction (GFR above 55 but below 80 mL/min/1.73 m2) and high protein intake (>82 g/d) is associated with a threefold higher risk for a further decline in kidney function. Given the high prevalence of mild kidney dysfunction in PAH patients, an upper limit of daily protein intake warrants examination in this patient population.185
Salt intake
Accumulating evidence linked increased salt intake to worsening albuminuria and increased likelihood of worsening GFR.186 Salt restriction (1.15 g/d versus 4.6 g/day) was shown to enhance the beneficial effects of ACE inhibitors on kidney function in patients with non-diabetic CKD.187 Experimental evidence has linked increased salt intake with stimulation of the TGF-β pathway.188,189 Given the importance of the TGF-β/BMPR-2 axis in PAH and CKD (see above), excessive dietary salt intake could have an important impact on the progression of CKD in patients with PAH.
Hyperuricemia
Pharmacological reduction of UA levels has been linked to delayed progression of CKD and improved cardiovascular risk profile.190–192 However, current guidelines do not recommend the use of UA lowering agents in asymptomatic CKD patients.193 Given the high incidence of hyperuricemia in PAH, clinical trials would be warranted to investigate the usefulness of lowering UA levels for prevention of the progression of CKD and right ventricular failure in PAH.
Physical activity
Patients with CKD and reduced exercise capacity have increased mortality and poor quality of life.194,195 A RCT in CKD patients could show improved physical capacity, quality of life, and arterial stiffness in patients undergoing a supervised exercise program.196 Beneficial effects of regular exercise are thought to have positive effects on blood pressure, lipid profile, and glycemic control.196–200 Current guidelines recommend supervised exercise and physical rehabilitation in deconditioned patients with PH. RCTs have shown that supervised physical training improved exercise and functional capacity, cardiorespiratory function, and quality of life in PAH patients. Supervised physical activity could also prevent development or progression of CKD due to improved control of associated risk factors.
Summary
Kidney dysfunction is highly prevalent in PAH patients and numerous potential mechanisms exist to explain the interaction of PAH and kidney disease, including CRS and neurohormonal activation. The majority of PAH-targeted therapy seems to have potential nephroprotective effects. Kidney disease constitutes a significant risk for mortality in patients with PAH. Impaired kidney function could potentially contribute to progression of PAH by worsening pulmonary vascular and cardiac remodeling. Even though PAH and CKD are pathophysiologic distinct entities, there is a significant overlap of molecular mediators in both diseases. Potential management implications to reduce kidney disease in PAH include: systemic blood pressure, glucose and UA control, lifestyle modifications, and RAAS blockade.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Ahmed A, Rich MW, Sanders PW, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol 2007; 99: 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 3.Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006; 113: 671–678. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–41. [DOI] [PubMed] [Google Scholar]
- 5.Naeije R, Vachiery JL, Yerly P, et al. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013; 41: 217–223. [DOI] [PubMed] [Google Scholar]
- 6.Opitz CF, Hoeper MM, Gibbs JS, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol 2016; 68: 368–378. [DOI] [PubMed] [Google Scholar]
- 7.Guglin M, Khan H. Pulmonary hypertension in heart failure. J Card Fail 2010; 16: 461–474. [DOI] [PubMed] [Google Scholar]
- 8.Kdoqi KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007; 49: S12–154. [DOI] [PubMed] [Google Scholar]
- 9.Muntner P, He J, Hamm L, et al. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 2002; 13: 745–753. [DOI] [PubMed] [Google Scholar]
- 10.Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol 2003; 41: 47–55. [DOI] [PubMed] [Google Scholar]
- 11.Cirillo M, Laurenzi M, Mancini M, et al. Low glomerular filtration in the population: prevalence, associated disorders, and awareness. Kidney Int 2006; 70: 800–806. [DOI] [PubMed] [Google Scholar]
- 12.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 2005; 16: 180–188. [DOI] [PubMed] [Google Scholar]
- 13.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 14.Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest 2010; 138: 1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tedford RJ, Mudd JO, Girgis RE, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail 2013; 6: 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane GC, Maradit-Kremers H, Slusser JP, et al. Integration of clinical and hemodynamic parameters in the prediction of long-term survival in patients with pulmonary arterial hypertension. Chest 2011; 139: 1285–1293. [DOI] [PubMed] [Google Scholar]
- 17.Navaneethan SD, Wehbe E, Heresi GA, et al. Presence and outcomes of kidney disease in patients with pulmonary hypertension. Clin J Am Soc Nephrol 2014; 9: 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Leary JM, Assad T, Hemnes A, et al. Pulmonary hypertension and chronic kidney disease: invasive hemodynamic etiology and outcomes in a large electronic medical record-based cohort. J Am Coll Cardiol 2016; 67: 2048. [Google Scholar]
- 19.Shah SJ, Thenappan T, Rich S, et al. Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation 2008; 117: 2475–2483. [DOI] [PubMed] [Google Scholar]
- 20.Chakinala M. Predicting outcomes in pulmonary arterial hypertension based on estimated glomerular filtration rate. Paper presented at: American Thoracic Society Anual Meeting 2016; San Fransico, CA, USA.
- 21.Kaiser R, Seiler S, Held M, et al. Prognostic impact of renal function in precapillary pulmonary hypertension. J Intern Med 2014; 275: 116–126. [DOI] [PubMed] [Google Scholar]
- 22.Penn H, Howie AJ, Kingdon EJ, et al. Scleroderma renal crisis: patient characteristics and long-term outcomes. QJM 2007; 100: 485–494. [DOI] [PubMed] [Google Scholar]
- 23.Seshan SV, Jennette JC. Renal disease in systemic lupus erythematosus with emphasis on classification of lupus glomerulonephritis: advances and implications. Arch Pathol Lab Med 2009; 133: 233–248. [DOI] [PubMed] [Google Scholar]
- 24.Kronbichler A, Mayer G. Renal involvement in autoimmune connective tissue diseases. BMC Med 2013; 11: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forfia PR, Mathai SC, Fisher MR, et al. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2008; 177: 1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008; 52: 1527–1539. [DOI] [PubMed] [Google Scholar]
- 27.Thenappan T, Shah SJ, Gomberg-Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2011; 4: 257–265. [DOI] [PubMed] [Google Scholar]
- 28.Damman K, Navis G, Smilde TD, et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 2007; 9: 872–878. [DOI] [PubMed] [Google Scholar]
- 29.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damman K, van Deursen VM, Navis G, et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009; 53: 582–588. [DOI] [PubMed] [Google Scholar]
- 31.Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 2013; 34: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 32.Unger ED, Dubin RF, Deo R, et al. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2016; 18: 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 2008; 51: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 34.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail 2013; 1: 290–299. [DOI] [PubMed] [Google Scholar]
- 35.Zlotnick DM, Ouellette ML, Malenka DJ, et al. Effect of preoperative pulmonary hypertension on outcomes in patients with severe aortic stenosis following surgical aortic valve replacement. Am J Cardiol 2013; 112: 1635–1640. [DOI] [PubMed] [Google Scholar]
- 36.Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol 1931; 72: 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciarka A, Doan V, Velez-Roa S, et al. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am J Respir Crit Care Med 2010; 181: 1269–1275. [DOI] [PubMed] [Google Scholar]
- 38.Ciarka A, Vachiery JL, Houssiere A, et al. Atrial septostomy decreases sympathetic overactivity in pulmonary arterial hypertension. Chest 2007; 131: 1831–1837. [DOI] [PubMed] [Google Scholar]
- 39.Nootens M, Kaufmann E, Rector T, et al. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol 1995; 26: 1581–1585. [DOI] [PubMed] [Google Scholar]
- 40.Velez-Roa S, Ciarka A, Najem B, et al. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 2004; 110: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 41.Maron BA, Zhang YY, White K, et al. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation 2012; 126: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baylen BG, Emmanouilides GC, Juratsch CE, et al. Main pulmonary artery distention: a potential mechanism for acute pulmonary hypertension in the human newborn infant. J Pediatr 1980; 96: 540–544. [DOI] [PubMed] [Google Scholar]
- 43.Osorio J, Russek M. Reflex changes on the pulmonary and systemic pressures elicited by stimulation of baroreceptors in the pulmonary artery. Circ Res 1962; 10: 664–647. [DOI] [PubMed] [Google Scholar]
- 44.Liu C, Jiang XM, Zhang J, et al. Pulmonary artery denervation improves pulmonary arterial hypertension induced right ventricular dysfunction by modulating the local renin-angiotensin-aldosterone system. BMC Cardiovasc Disord 2016; 16: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothman AM, Arnold ND, Chang W, et al. Pulmonary artery denervation reduces pulmonary artery pressure and induces histological changes in an acute porcine model of pulmonary hypertension. Circ Cardiovasc Interv 2015; 8: e002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Man FS, Tu L, Handoko ML, et al. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nickel NP, Lichtinghagen R, Golpon H, et al. Circulating levels of copeptin predict outcome in patients with pulmonary arterial hypertension. Respir Res 2013; 14: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abraham WT, Raynolds MV, Badesch DB, et al. Angiotensin-converting enzyme DD genotype in patients with primary pulmonary hypertension: increased frequency and association with preserved haemodynamics. J Renin Angiotensin Aldosterone Syst 2003; 4: 27–30. [DOI] [PubMed] [Google Scholar]
- 49.Klein IH, Ligtenberg G, Neumann J, et al. Sympathetic nerve activity is inappropriately increased in chronic renal disease. J Am Soc Nephrol 2003; 14: 3239–3244. [DOI] [PubMed] [Google Scholar]
- 50.Hollenberg NK, Price DA, Fisher ND, et al. Glomerular hemodynamics and the renin-angiotensin system in patients with type 1 diabetes mellitus. Kidney Int 2003; 63: 172–178. [DOI] [PubMed] [Google Scholar]
- 51.Kim KE, Onesti G, Schwartz AB, et al. Hemodynamics of hypertension in chronic end-stage renal disease. Circulation 1972; 46: 456–464. [DOI] [PubMed] [Google Scholar]
- 52.Klein IH, Ligtenberg G, Oey PL, et al. Sympathetic activity is increased in polycystic kidney disease and is associated with hypertension. J Am Soc Nephrol 2001; 12: 2427–2433. [DOI] [PubMed] [Google Scholar]
- 53.Edgley AJ, Kett MM, Anderson WP. Evidence for renal vascular remodeling in angiotensin II-induced hypertension. J Hypertens 2003; 21: 1401–1406. [DOI] [PubMed] [Google Scholar]
- 54.Stevenson KM, Edgley AJ, Bergstrom G, et al. Angiotensin II infused intrarenally causes preglomerular vascular changes and hypertension. Hypertension 2000; 36: 839–844. [DOI] [PubMed] [Google Scholar]
- 55.Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349: 1857–1863. [PubMed] [Google Scholar]
- 56.Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 57.Rossing K, Schjoedt KJ, Smidt UM, et al. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care 2005; 28: 2106–2112. [DOI] [PubMed] [Google Scholar]
- 58.Chrysostomou A, Pedagogos E, MacGregor L, et al. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol 2006; 1: 256–262. [DOI] [PubMed] [Google Scholar]
- 59.Klahr S, Schreiner G, Ichikawa I. The progression of renal disease. N Engl J Med 1988; 318: 1657–1666. [DOI] [PubMed] [Google Scholar]
- 60.Galie N, Humbert M, Vachiery JL, et al. [2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension]. Kardiologia Polska 2015; 73: 1127–1206. [DOI] [PubMed] [Google Scholar]
- 61.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53: 1573–1619. [DOI] [PubMed] [Google Scholar]
- 62.Afsar B, Ortiz A, Covic A, et al. Phosphodiesterase type 5 inhibitors and kidney disease. Int Urol Nephrol 2015; 47: 1521–1528. [DOI] [PubMed] [Google Scholar]
- 63.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol 2001; 41: 851–876. [DOI] [PubMed] [Google Scholar]
- 64.Zanatta CM, Gerchman F, Burttet L, et al. Endothelin-1 levels and albuminuria in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2008; 80: 299–304. [DOI] [PubMed] [Google Scholar]
- 65.Stewart DJ, Levy RD, Cernacek P, et al. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med 1991; 114: 464–469. [DOI] [PubMed] [Google Scholar]
- 66.Goddard J, Johnston NR, Hand MF, et al. Endothelin-A receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure: a comparison of selective and combined endothelin receptor blockade. Circulation 2004; 109: 1186–1193. [DOI] [PubMed] [Google Scholar]
- 67.Gariepy CE, Ohuchi T, Williams SC, et al. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest 2000; 105: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsumura Y, Kuro T, Kobayashi Y, et al. Exaggerated vascular and renal pathology in endothelin-B receptor-deficient rats with deoxycorticosterone acetate-salt hypertension. Circulation 2000; 102: 2765–2773. [DOI] [PubMed] [Google Scholar]
- 69.Neuhofer W, Pittrow D. Endothelin receptor selectivity in chronic kidney disease: rationale and review of recent evidence. Eur J Clin Invest 2009; 39(Suppl. 2): 50–67. [DOI] [PubMed] [Google Scholar]
- 70.Mubarak KK. A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Respir Med 2010; 104: 9–21. [DOI] [PubMed] [Google Scholar]
- 71.Spargias K, Adreanides E, Demerouti E, et al. Iloprost prevents contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 2009; 120: 1793–1799. [DOI] [PubMed] [Google Scholar]
- 72.Darcin OT, Zor MH, Sahin V, et al. Effect of iloprost on renal function in patients undergoing coronary artery bypass grafting: a clinical study. Ann Thorac Cardiovasc Surg 2013; 19: 12–17. [DOI] [PubMed] [Google Scholar]
- 73.Ay Y, Kara I, Ay NK, et al. The effect of iloprost on renal function in patients with critical limb ischemia. Curr Ther Res Clin Exp 2013; 75: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Owada A, Suda S, Hata T. Effect of long-term administration of prostaglandin I(2) in incipient diabetic nephropathy. Nephron 2002; 92: 788–796. [DOI] [PubMed] [Google Scholar]
- 75.Koyama A, Fujita T, Gejyo F, et al. Orally active prostacyclin analogue beraprost sodium in patients with chronic kidney disease: a randomized, double-blind, placebo-controlled, phase II dose finding trial. BMC Nephrol 2015; 16: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011; 123: 2263–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khaybullina D, Patel A, Zerilli T. Riociguat (adempas): a novel agent for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. P T 2014; 39: 749–758. [PMC free article] [PubMed] [Google Scholar]
- 78.Larsen TR, Kinni V, Zaks J, et al. A lethal case of influenza and type 5 cardiorenal syndrome. Blood Purif 2013; 36: 112–115. [DOI] [PubMed] [Google Scholar]
- 79.Cerasola G, Nardi E, Palermo A, et al. Epidemiology and pathophysiology of left ventricular abnormalities in chronic kidney disease: a review. J Nephrol 2011; 24: 1–10. [DOI] [PubMed] [Google Scholar]
- 80.Briasoulis A, Bakris GL. Chronic kidney disease as a coronary artery disease risk equivalent. Curr Cardiol Rep 2013; 15: 340. [DOI] [PubMed] [Google Scholar]
- 81.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16: 489–495. [DOI] [PubMed] [Google Scholar]
- 82.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 83.Baber U, Howard VJ, Halperin JL, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol 2011; 4: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hemnes AR, Brittain EL, Trammell AW, et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 2014; 189: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brittain EL, Talati M, Fessel JP, et al. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation 2016; 133: 1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Talati MH, Brittain EL, Fessel JP, et al. Mechanisms of lipid accumulation in the bone morphogenic protein receptor 2 mutant right ventricle. Am J Respir Crit Care Med 2016; 194: 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol 2008; 19: 213–216. [DOI] [PubMed] [Google Scholar]
- 88.Caglar K, Yilmaz MI, Saglam M, et al. Serum fetuin-a concentration and endothelial dysfunction in chronic kidney disease. Nephron Clin Pract 2008; 108: c233–240. [DOI] [PubMed] [Google Scholar]
- 89.Friedman D, Szmuszkovicz J, Rabai M, et al. Systemic endothelial dysfunction in children with idiopathic pulmonary arterial hypertension correlates with disease severity. J Heart Lung Transplant 2012; 31: 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hughes R, Tong J, Oates C, et al. Evidence for systemic endothelial dysfunction in patients and first-order relatives with pulmonary arterial hypertension. Chest 2005; 128: 617S. [DOI] [PubMed] [Google Scholar]
- 91.Muntner P, Hamm LL, Kusek JW, et al. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Int Med 2004; 140: 9–17. [DOI] [PubMed] [Google Scholar]
- 92.Arroliga AC, Sandur S, Jacobsen DW, et al. Association between hyperhomocysteinemia and primary pulmonary hypertension. Respir Med 2003; 97: 825–829. [DOI] [PubMed] [Google Scholar]
- 93.Can MM, Tanboga IH, Demircan HC, et al. Enhanced hemostatic indices in patients with pulmonary arterial hypertension: an observational study. Thromb Res 2010; 126: 280–282. [DOI] [PubMed] [Google Scholar]
- 94.Quarck R, Nawrot T, Meyns B, et al. C-reactive protein: a new predictor of adverse outcome in pulmonary arterial hypertension. J Am Coll Cardiol 2009; 53: 1211–1218. [DOI] [PubMed] [Google Scholar]
- 95.Nagaya N, Uematsu M, Satoh T, et al. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am J Respir Crit Care Med 1999; 160: 487–492. [DOI] [PubMed] [Google Scholar]
- 96.Dhaun N, Vachiery JL, Benza RL, et al. Endothelin antagonism and uric acid levels in pulmonary arterial hypertension: clinical associations. J Heart Lung Transplant 2014; 33: 521–527. [DOI] [PubMed] [Google Scholar]
- 97.Voelkel MA, Wynne KM, Badesch DB, et al. Hyperuricemia in severe pulmonary hypertension. Chest 2000; 117: 19–24. [DOI] [PubMed] [Google Scholar]
- 98.Castillo-Martinez D, Marroquin-Fabian E, Lozada-Navarro AC, et al. Levels of uric acid may predict the future development of pulmonary hypertension in systemic lupus erythematosus: a seven-year follow-up study. Lupus 2016; 25: 61–66. [DOI] [PubMed] [Google Scholar]
- 99.Martinon F, Petrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440: 237–241. [DOI] [PubMed] [Google Scholar]
- 100.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 2003; 425: 516–521. [DOI] [PubMed] [Google Scholar]
- 101.Jasiewicz M, Knapp M, Waszkiewicz E, et al. Enhanced IL-6 trans-signaling in pulmonary arterial hypertension and its potential role in disease-related systemic damage. Cytokine 2015; 76: 187–192. [DOI] [PubMed] [Google Scholar]
- 102.Zharikov S, Krotova K, Hu H, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol 2008; 295: C1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corry DB, Eslami P, Yamamoto K, et al. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens 2008; 26: 269–275. [DOI] [PubMed] [Google Scholar]
- 104.Chao HH, Liu JC, Lin JW, et al. Uric acid stimulates endothelin-1 gene expression associated with NADPH oxidase in human aortic smooth muscle cells. Acta Pharmacol Sin 2008; 29: 1301–1312. [DOI] [PubMed] [Google Scholar]
- 105.Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci 2001; 114: 4359–4369. [DOI] [PubMed] [Google Scholar]
- 106.Soubrier F, Chung WK, Machado R, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 2013; 62: D13–21. [DOI] [PubMed] [Google Scholar]
- 107.Sztrymf B, Coulet F, Girerd B, et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med 2008; 177: 1377–1383. [DOI] [PubMed] [Google Scholar]
- 108.Selimovic N, Bergh CH, Andersson B, et al. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J 2009; 34: 662–668. [DOI] [PubMed] [Google Scholar]
- 109.Richter A, Yeager ME, Zaiman A, et al. Impaired transforming growth factor-beta signaling in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2004; 170: 1340–1348. [DOI] [PubMed] [Google Scholar]
- 110.Atkinson C, Stewart S, Imamura T, et al. Immunolocalisation of BMPR-II and TGF-ss type I and II receptors in primary plexogenic pulmonary hypertension. J Heart Lung Transplant 2001; 20: 149. [DOI] [PubMed] [Google Scholar]
- 111.Zakrzewicz A, Kouri FM, Nejman B, et al. The transforming growth factor-beta/Smad2,3 signalling axis is impaired in experimental pulmonary hypertension. Eur Respir J 2007; 29: 1094–1104. [DOI] [PubMed] [Google Scholar]
- 112.Ogo T, Chowdhury HM, Yang J, et al. Inhibition of overactive transforming growth factor-beta signaling by prostacyclin analogs in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2013; 48: 733–741. [DOI] [PubMed] [Google Scholar]
- 113.Graham BB, Chabon J, Gebreab L, et al. Transforming growth factor-beta signaling promotes pulmonary hypertension caused by Schistosoma mansoni. Circulation 2013; 128: 1354–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Long L, Crosby A, Yang X, et al. Altered bone morphogenetic protein and transforming growth factor-beta signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation 2009; 119: 566–576. [DOI] [PubMed] [Google Scholar]
- 115.Mata-Greenwood E, Meyrick B, Steinhorn RH, et al. Alterations in TGF-beta1 expression in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2003; 285: L209–221. [DOI] [PubMed] [Google Scholar]
- 116.Tanaka Y, Bernstein ML, Mecham RP, et al. Site-specific responses to monocrotaline-induced vascular injury: evidence for two distinct mechanisms of remodeling. Am J Respir Cell Mol Biol 1996; 15: 390–397. [DOI] [PubMed] [Google Scholar]
- 117.Soon E, Crosby A, Southwood M, et al. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production. A gateway to pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192: 859–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, et al. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res 2006; 98: 209–217. [DOI] [PubMed] [Google Scholar]
- 119.Willette RN, Gu JL, Lysko PG, et al. BMP-2 gene expression and effects on human vascular smooth muscle cells. J Vasc Res 1999; 36: 120–125. [DOI] [PubMed] [Google Scholar]
- 120.Nakaoka T, Gonda K, Ogita T, et al. Inhibition of rat vascular smooth muscle proliferation in vitro and in vivo by bone morphogenetic protein-2. J Clin Invest 1997; 100: 2824–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014; 371: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang W, Koka V, Lan HY. Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology 2005; 10: 48–56. [DOI] [PubMed] [Google Scholar]
- 123.Li JH, Huang XR, Zhu HJ, et al. Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB J 2004; 18: 176–8. [DOI] [PubMed] [Google Scholar]
- 124.Salimi K, Moser K, Zassler B, et al. Glial cell line-derived neurotrophic factor enhances survival of GM-CSF dependent rat GMIR1-microglial cells. Neurosci Res 2002; 43: 221–229. [DOI] [PubMed] [Google Scholar]
- 125.Eddy AA. Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl (2011) 2014; 4: 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamamoto T, Noble NA, Cohen AH, et al. Expression of transforming growth factor-beta isoforms in human glomerular diseases. Kidney Int 1996; 49: 461–469. [DOI] [PubMed] [Google Scholar]
- 127.Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol 2004; 15: S55–57. [DOI] [PubMed] [Google Scholar]
- 128.Mozes MM, Bottinger EP, Jacot TA, et al. Renal expression of fibrotic matrix proteins and of transforming growth factor-beta (TGF-beta) isoforms in TGF-beta transgenic mice. J Am Soc Nephrol 1999; 10: 271–280. [DOI] [PubMed] [Google Scholar]
- 129.Yamamoto T, Noble NA, Miller DE, et al. Sustained expression of TGF-beta 1 underlies development of progressive kidney fibrosis. Kidney Int 1994; 45: 916–927. [DOI] [PubMed] [Google Scholar]
- 130.Akagi Y, Isaka Y, Arai M, et al. Inhibition of TGF-beta 1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int 1996; 50: 148–155. [DOI] [PubMed] [Google Scholar]
- 131.Lavoie P, Robitaille G, Agharazii M, et al. Neutralization of transforming growth factor-beta attenuates hypertension and prevents renal injury in uremic rats. J Hypertens 2005; 23: 1895–1903. [DOI] [PubMed] [Google Scholar]
- 132.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002; 346: 896–903. [DOI] [PubMed] [Google Scholar]
- 133.Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 2003; 9: 964–968. [DOI] [PubMed] [Google Scholar]
- 134.Morrissey J, Hruska K, Guo G, et al. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol 2002; 13: S14–21. [PubMed] [Google Scholar]
- 135.Sugimoto H, LeBleu VS, Bosukonda D, et al. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat Med 2012; 18: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Whitman M, Rosen V, Brivanlou AH, et al. Regarding the mechanism of action of a proposed peptide agonist of the bone morphogenetic protein receptor activin-like kinase 3. Nat Med 2013; 19: 809–810. [DOI] [PubMed] [Google Scholar]
- 137.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett 2013; 328: 18–26. [DOI] [PubMed] [Google Scholar]
- 138.Chu D, Sullivan CC, Du L, et al. A new animal model for pulmonary hypertension based on the overexpression of a single gene, angiopoietin-1. Ann Thorac Surg 2004; 77: 449–456. [DOI] [PubMed] [Google Scholar]
- 139.Kido M, Du L, Sullivan CC, et al. Gene transfer of a TIE2 receptor antagonist prevents pulmonary hypertension in rodents. J Thorac Cardiovasc Surg 2005; 129: 268–276. [DOI] [PubMed] [Google Scholar]
- 140.Kugathasan L, Ray JB, Deng Y, et al. The angiopietin-1-Tie2 pathway prevents rather than promotes pulmonary arterial hypertension in transgenic mice. J Exp Med 2009; 206: 2221–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rondelet B, Kerbaul F, Van Beneden R, et al. Signaling molecules in overcirculation-induced pulmonary hypertension in piglets: effects of sildenafil therapy. Circulation 2004; 110: 2220–2225. [DOI] [PubMed] [Google Scholar]
- 142.Zhao YD, Campbell AI, Robb M, et al. Protective role of angiopoietin-1 in experimental pulmonary hypertension. Circ Res 2003; 92: 984–991. [DOI] [PubMed] [Google Scholar]
- 143.Kumpers P, Nickel N, Lukasz A, et al. Circulating angiopoietins in idiopathic pulmonary arterial hypertension. Eur Heart J 2010; 31: 2291–300. [DOI] [PubMed] [Google Scholar]
- 144.Woolf AS, Gnudi L, Long DA. Roles of angiopoietins in kidney development and disease. J Am Soc Nephrol 2009; 20: 239–244. [DOI] [PubMed] [Google Scholar]
- 145.Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 2003; 111: 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 2006; 354: 1387–1401. [DOI] [PubMed] [Google Scholar]
- 147.David S, John SG, Jefferies HJ, et al. Angiopoietin-2 levels predict mortality in CKD patients. Nephrol Dial Transplant 2012; 27: 1867–1872. [DOI] [PubMed] [Google Scholar]
- 148.Cooke JP. Asymmetrical dimethylarginine: the Uber marker? Circulation 2004; 109: 1813–1818. [DOI] [PubMed] [Google Scholar]
- 149.Fliser D, Kronenberg F, Kielstein JT, et al. Asymmetric dimethylarginine and progression of chronic kidney disease: the mild to moderate kidney disease study. J Am Soc Nephrol 2005; 16: 2456–2461. [DOI] [PubMed] [Google Scholar]
- 150.Young JM, Terrin N, Wang X, et al. Asymmetric dimethylarginine and mortality in stages 3 to 4 chronic kidney disease. Clin J Am Soc Nephrol 2009; 4: 1115–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mihout F, Shweke N, Bige N, et al. Asymmetric dimethylarginine (ADMA) induces chronic kidney disease through a mechanism involving collagen and TGF-beta1 synthesis. J Pathol 2011; 223: 37–45. [DOI] [PubMed] [Google Scholar]
- 152.Shum HP, Yan WW, Chan TM. Recent knowledge on the pathophysiology of septic acute kidney injury: A narrative review. J Crit Care 2016; 31: 82–89. [DOI] [PubMed] [Google Scholar]
- 153.Groth A, Vrugt B, Brock M, et al. Inflammatory cytokines in pulmonary hypertension. Respir Res 2014; 15: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.El Chami H, Hassoun PM. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Prog Cardiovasc Dis 2012; 55: 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jones SA, Richards PJ, Scheller J, et al. IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res 2005; 25: 241–253. [DOI] [PubMed] [Google Scholar]
- 156.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995; 151: 1628–1631. [DOI] [PubMed] [Google Scholar]
- 157.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010; 122: 920–927. [DOI] [PubMed] [Google Scholar]
- 158.Bhargava A, Kumar A, Yuan N, et al. Monocrotaline induces interleukin-6 mRNA expression in rat lungs. Heart Dis 1999; 1: 126–132. [PubMed] [Google Scholar]
- 159.Golembeski SM, West J, Tada Y, et al. Interleukin-6 causes mild pulmonary hypertension and augments hypoxia-induced pulmonary hypertension in mice. Chest 2005; 128: 572S–573S. [DOI] [PubMed] [Google Scholar]
- 160.Steiner MK, Syrkina OL, Kolliputi N, et al. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 2009; 104: 236–244. 28p following 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Barreto DV, Barreto FC, Liabeuf S, et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int 2010; 77: 550–556. [DOI] [PubMed] [Google Scholar]
- 162.Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 2004; 65: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 163.Gupta J, Mitra N, Kanetsky PA, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 2012; 7: 1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Amdur RL, Mukherjee M, Go A, et al. Interleukin-6 is a risk factor for atrial fibrillation in chronic kidney disease: findings from the CRIC Study. PLoS One 2016; 11: e0148189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 2008; 19: 1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Andrassy KM. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int 2013; 84: 622–623. [DOI] [PubMed] [Google Scholar]
- 167.Chapter 3: Blood pressure management in CKD ND patients without diabetes mellitus. Kidney Int Suppl (2011) 2012; 2: 357–362. [DOI] [PMC free article] [PubMed]
- 168.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010; 137: 376–387. [DOI] [PubMed] [Google Scholar]
- 169.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186: 790–796. [DOI] [PubMed] [Google Scholar]
- 170.Berl T, Hunsicker LG, Lewis JB, et al. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol 2005; 16: 2170–2179. [DOI] [PubMed] [Google Scholar]
- 171.Weiner DE, Tighiouart H, Levey AS, et al. Lowest systolic blood pressure is associated with stroke in stages 3 to 4 chronic kidney disease. J Am Soc Nephrol 2007; 18: 960–966. [DOI] [PubMed] [Google Scholar]
- 172.Brugts JJ, Boersma E, Chonchol M, et al. The cardioprotective effects of the angiotensin-converting enzyme inhibitor perindopril in patients with stable coronary artery disease are not modified by mild to moderate renal insufficiency: insights from the EUROPA trial. J Am Coll Cardiol 2007; 50: 2148–2155. [DOI] [PubMed] [Google Scholar]
- 173.Saruta T, Hayashi K, Ogihara T, et al. Effects of candesartan and amlodipine on cardiovascular events in hypertensive patients with chronic kidney disease: subanalysis of the CASE-J Study. Hypertens Res 2009; 32: 505–512. [DOI] [PubMed] [Google Scholar]
- 174.Solomon SD, Rice MM, Jablonski AK, et al. Renal function and effectiveness of angiotensin-converting enzyme inhibitor therapy in patients with chronic stable coronary disease in the Prevention of Events with ACE inhibition (PEACE) trial. Circulation 2006; 114: 26–31. [DOI] [PubMed] [Google Scholar]
- 175.Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000; 342: 145–153. [DOI] [PubMed] [Google Scholar]
- 176.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 177.Leier CV, Bambach D, Nelson S, et al. Captopril in primary pulmonary hypertension. Circulation 1983; 67: 155–161. [DOI] [PubMed] [Google Scholar]
- 178.Rich S, Martinez J, Lam W, et al. Captopril as treatment for patients with pulmonary hypertension. Problem of variability in assessing chronic drug treatment. Br Heart J 1982; 48: 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Maron BA, Waxman AB, Opotowsky AR, et al. Effectiveness of spironolactone plus ambrisentan for treatment of pulmonary arterial hypertension (from the [ARIES] study 1 and 2 trials). Am J Cardiol 2013; 112: 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis 2012; 60: 850–886. [DOI] [PubMed] [Google Scholar]
- 181.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 182.Zamanian RT, Hansmann G, Snook S, et al. Insulin resistance in pulmonary arterial hypertension. Eur Respir J 2009; 33: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pugh ME, Robbins IM, Rice TW, et al. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant 2011; 30: 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hansmann G, Wagner RA, Schellong S, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation 2007; 115: 1275–1284. [DOI] [PubMed] [Google Scholar]
- 185.Knight EL, Stampfer MJ, Hankinson SE, et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med 2003; 138: 460–467. [DOI] [PubMed] [Google Scholar]
- 186.Jones-Burton C, Mishra SI, Fink JC, et al. An in-depth review of the evidence linking dietary salt intake and progression of chronic kidney disease. Am J Nephrol 2006; 26: 268–275. [DOI] [PubMed] [Google Scholar]
- 187.Slagman MC, Waanders F, Hemmelder MH, et al. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ 2011; 343: d4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ying WZ, Sanders PW. Dietary salt modulates renal production of transforming growth factor-beta in rats. Am J Physiol 1998; 274: F635–641. [DOI] [PubMed] [Google Scholar]
- 189.Ying WZ, Sanders PW. Dietary salt increases endothelial nitric oxide synthase and TGF-beta1 in rat aortic endothelium. Am J Physiol 1999; 277: H1293–1298. [DOI] [PubMed] [Google Scholar]
- 190.Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 2010; 5: 1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Siu YP, Leung KT, Tong MK, et al. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 2006; 47: 51–59. [DOI] [PubMed] [Google Scholar]
- 192.Malaguarnera M, Vacante M, Russo C, et al. A single dose of rasburicase in elderly patients with hyperuricaemia reduces serum uric acid levels and improves renal function. Expert Opin Pharmacother 2009; 10: 737–742. [DOI] [PubMed] [Google Scholar]
- 193.Chapter 3: Management of progression and complications of CKD. Kidney Int Suppl (2011) 2013; 3: 73–90. [DOI] [PMC free article] [PubMed]
- 194.Beddhu S, Baird BC, Zitterkoph J, et al. Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol 2009; 4: 1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen JL, Lerner D, Ruthazer R, et al. Association of physical activity with mortality in chronic kidney disease. J Nephrol 2008; 21: 243–252. [PubMed] [Google Scholar]
- 196.Mustata S, Groeneveld S, Davidson W, et al. Effects of exercise training on physical impairment, arterial stiffness and health-related quality of life in patients with chronic kidney disease: a pilot study. Int Urol Nephrol 2011; 43: 1133–1141. [DOI] [PubMed] [Google Scholar]
- 197.Sato Y, Nagasaki M, Nakai N, et al. Physical exercise improves glucose metabolism in lifestyle-related diseases. Exp Biol Med 2003; 228: 1208–1212. [DOI] [PubMed] [Google Scholar]
- 198.Sigal RJ, Kenny GP, Boule NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 2007; 147: 357–369. [DOI] [PubMed] [Google Scholar]
- 199.Stewart KJ. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA 2002; 288: 1622–1631. [DOI] [PubMed] [Google Scholar]
- 200.Weinstein AA, Chin LM, Keyser RE, et al. Effect of aerobic exercise training on fatigue and physical activity in patients with pulmonary arterial hypertension. Respir Med 2013; 107: 778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Simonneau G, Gatzoulis MA, Adatia I, et al. [Updated clinical classification of pulmonary hypertension]. Turk Kardiyol Dern Ars 2014; 42(Suppl. 1): 45–54. [PubMed] [Google Scholar]