Abstract
Quantifying metabolic derangements in pulmonary hypertension (PH) by plasma metabolomics could identify biomarkers useful for diagnosis and treatment. The objective of this paper is to test the hypotheses that circulating metabolites are differentially expressed in PH patients compared with controls and among different hemodynamic subtypes of PH associated with left heart disease. We studied patients enrolled in the CATHGEN biorepository with PH (right heart catheterization mPAP ≥ 25 mmHg; n = 280). Of these, 133 met criteria for postcapillary PH, 82 for combined precapillary and postcapillary PH (CpcPH), and 65 for precapillary PH. Targeted profiling of 63 metabolites (acylcarnitines, amino acids, and ketones) was performed using tandem flow injection mass spectrometry. Multivariable linear regression was used to determine differences in metabolite factors derived from a principal components analysis between PH cases, PH subtypes, and non-PH controls. In adjusted models, the metabolite factor loaded with long-chain acylcarnitines was higher in all PH cases versus non-PH controls (P = 0.00008), but did not discriminate between CpcPH and postcapillary PH (P = 0.56). In analyses of subtypes, CpcPH patients had lower levels of factors loaded with urea cycle amino acids and short chain acylcarnitines as compared to controls (P = 0.002 and P = 0.01, respectively) and as compared to postcapillary PH (P = 0.04 and P = 0.02, respectively). Compared to controls, PH was strongly associated with greater concentrations of long-chain acylcarnitines. Postcapillary PH and CpcPH were weakly associated with distinct metabolomic profiles. These findings suggest the presence of unique metabolic abnormalities in subtypes of PH and may reflect underlying pathophysiology.
Keywords: metabolomics, hemodynamics, pulmonary hypertension
Pulmonary hypertension (PH) associated with left-sided heart disease is the most common form of PH. Left ventricular systolic dysfunction, diastolic dysfunction, or valvular disease (Table 1) can all cause increased pulmonary arterial pressure from pulmonary venous congestion. While this disease results in increased morbidity and mortality for vulnerable patients,1 therapy has primarily focused on treating only the constituents of the left-sided heart disease. While pulmonary vasodilators effectively treat pulmonary arterial hypertension (PAH)—a rare disease of the pulmonary vasculature that results in PH, their safety and efficacy to treat patients with PH associated with left heart disease is largely unknown.
Table 1.
Definitions of hemodynamic subtypes of pulmonary hypertension. Definitions are based on Fang et al.2 and Galie et al.23
| Nomenclature | Description | Physiologic definition | Hemodynamic criteria |
|---|---|---|---|
| Pulmonary hypertension (PH) | Sustained elevation of mPAP at rest | Precapillary, postcapillary, mixed or high flow state | Mean PAP ≥ 25 mmHg |
| Precapillary PH | PH with “normal” left-sided filling pressure | Precapillary vasoconstriction, remodeling, thrombosis | Mean PAP ≥ 25 mmHg PCWP ≤ 15 mmHg |
| Passive or postcapillary PH | PH with elevated left-sided filling pressure | Postcapillary passive congestion | Mean PAP ≥ 25 mmHg PCWP > 15 mmHg PVR < 3 WU |
| Combined precapillary and postcapillary PH (CpcPH) | PH with elevated left-sided filling pressure and elevated pulmonary vascular resistance | Pre- and postcapillary (passive congestion with excessive arterial vasoconstriction ± vascular remodeling) | Mean PAP ≥ 25 mmHg PCWP > 15 mmHg PVR ≥ 3 WU |
Additionally, PH in left heart disease is further divided into two broad hemodynamic subtypes that are defined by different levels of precapillary (pulmonary arterial) and postcapillary (pulmonary venous) PH (Table 1). As a whole, PH is defined by a mean pulmonary artery pressure (mPAP) ≥ 25 mmHg.2 An abnormal pulmonary capillary wedge pressure differentiates PH in left heart disease (PCWP > 15 mmHg) from precapillary PH (PCWP ≤ 15 mmHg). In “passive” or postcapillary PH, elevated pressures result purely from postcapillary (pulmonary venous) congestion from elevated left side pressures, with no precapillary component of PH (pulmonary vascular resistance [PVR] of less than 3 Woods units [WU]). However, patients occasionally develop both precapillary and postcapillary PH, where the PCWP > 15 mmHg and PVR ≥ 3 WU. This combined precapillary and postcapillary PH—defined subsequently as “CpcPH”—can develop in the setting of prolonged or severe postcapillary pressure elevations.3
While postcapillary PH may occur in any pathology raising left atrial pressure, the prevalence and severity of CpcPH is highly variable. Factors leading to pulmonary vascular remodeling depend on individual genetic, biologic, and environmental influences. Pulmonary vascular remodeling in CpcPH exhibits features seen in PAH, including intimal fibrosis and medial hypertrophy.4 The management of patients with CpcPH is unclear:2 while CpcPH has features of PAH, it is unknown whether using therapies targeting pathogenic pathways in PAH is beneficial. Identifying reliable biomarkers that could distinguish between postcapillary PH and CpcPH could lead to earlier diagnosis and give insight to novel therapeutic pathways.
Abnormalities in energy metabolism are common features in experimental models of both right and left ventricular failure.5 In both failing left and right hearts, as mitochondrial metabolism is actively suppressed,6,7 a shift occurs from the highly efficient fatty acid oxidation to glucose metabolism and glycolysis. The resultant increase in lactate production and acidosis further impairs cardiac function. Advances in metabolomic profiling have enabled more comprehensive snapshots of dysregulated metabolism; in fact, metabolomic profiling has already been used to identify circulating markers of this impaired energy metabolism in coronary artery disease and heart failure.8–10 Similar profiling may increase understanding of PH and especially PH associated with left heart disease and provide long-sought biomarkers that provide earlier diagnosis of PH and discriminate CpcPH and postcapillary PH. Thus, we hypothesized that circulating metabolites reflecting mitochondrial energetics—including amino acids, acylcarnitines, and ketones—would discriminate PH from non-PH controls, subtypes of PH associated with left heart disease from non-PH controls, and between postcapillary PH and CpcPH in a discovery cohort.
Methods
Study sample
The CATHGEN biorepository consists of biological samples collected on 9300 sequential consenting individuals undergoing cardiac catheterization at Duke University Medical Center (Durham, NC, USA) between 2001 and 2010.11 The majority of the participants enrolled in the biorepository were undergoing a catheterization for the evaluation of ischemic cardiovascular disease. At the time of arterial access for cardiac catheterization, blood was obtained from the femoral artery, immediately processed to separate plasma, and frozen at −80℃ for later use. All patients had fasted at least 6 h prior to sample collection. The Duke Databank for Cardiovascular Disease provided data on demographics, medical history, angiographic findings, and longitudinal follow-up. For this study, for PH cases, we identified study participants who had undergone right heart catheterization which showed a mPAP ≥ 25 mmHg, and who also had targeted metabolomic profiling data available (n = 280). Of these, postcapillary PH was defined as PCWP > 15 mmHg, PVR < 3 WU; CpcPH as PCWP > 15 mmHg, PVR ≥ 3 WU; and precapillary PH as PCWP ≤ 15 mmHg (Table 1). A cohort of 280 participants matched to reflect a similar distribution of clinical variables—including age deciles, race, sex, left ventricular ejection fraction, presence of coronary artery disease—and without PH based on echocardiogram estimated RVSP < 35 mmHg served as controls. The protocol for CATHGEN was approved by the Duke Institutional Review Board and all participants provided written informed consent for future analysis of stored samples.
Metabolite profiling
As previously described, we used targeted, quantitative tandem flow injection mass spectrometry (MS/MS) to determine levels of 60 metabolites (45 acylcarnitines and 15 amino acids) and standard assays for measurement of ketones, β-hydroxybutyrate, and non-esterified fatty acids.12,13 For MS/MS analyses, plasma samples were spiked with a cocktail of heavy-isotope internal standards (Cambridge Isotope Laboratories, MA, USA; CDN Isotopes, Canada) and deproteinated with methanol. The aliquoted supernatant was dried and esterified with hot, acidic methanol (acylcarnities) or n-butanol (amino acids). Tandem MS/MS using a Waters TQD (Milford, MA, USA) was employed to quantitatively assess acylcarnitine and amino acid ester concentrations. Total ketones (KET), 3-hydroxybutyrate (HBUT), and total non-esterified fatty acids (NEFA) were measured on a Beckman DxC600 autoanalyzer (Brea, CA, USA) using reagents from Wako (Richmond, VA, USA). All assays were tested in random batch order by the Metabolomics Core Laboratory at the Duke Molecular Physiology Institute, Duke University, Durham, NC, USA. Laboratory personnel were blinded to the clinical status of patients.
Statistical analysis
Given the large number of metabolites, principal components analysis (PCA) with varimax rotation was used in the larger CATHGEN cohort with metabolomics data (n = 3899) to reduce the large number of co-linear metabolites into 14 uncorrelated components9,13 (Table 4). As participants in this study were taken from the larger PCA, scoring coefficients from the original analysis were used to calculate weighted factor scores for each individual in this PH substudy. Metabolites with an absolute factor load ≥0.4 are listed as composing a given factor. To test the significance of differences in metabolite factors between overall PH case/control status and between PH subtypes, we constructed generalized multivariable linear regression models for each factor, adjusted for relevant clinical variables (age, presence/absence of coronary artery disease, sex, race, diabetes, and left ventricular ejection fraction) and metabolite assay batch number. Factor 5 (ketone-related analytes) was also adjusted for preoperative heparin status, as heparin is known to affect β-hydroxybutyrate, ketone, fatty acid, and triglyceride levels.14 Separate regression models were used to test for mean metabolite factor levels between: (1) all PH participants and non-PH controls (primary analysis); (2) left heart disease-associated PH subgroups (postcapillary PH and CpcPH participants) each with non-PH controls (secondary analysis); and (3) postcapillary PH participants and CpcPH participants (secondary analysis). As these analyses were exploratory in nature and given co-linearity of the metabolites, we considered two-sided P values with significance defined as P ≤ 0.05. Although results are presented in the context of Bonferroni correction for multiple comparisons for the primary analysis, secondary analyses are presented without this adjustment. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Table 4.
Principal components analysis.
| Factor | Description | Metabolites within factor |
|---|---|---|
| 1 | Medium-chain acylcarnitines | C8, C10, C12, C14:1, C14, C16:2, C16:1, C14:2, C12:1, C10:1 |
| 2 | Long-chain dicarboxyl-acylcarnitines | C20:1-OH/C18:1-DC, C18-OH/C16-DC, C20-OH/C18-DC, C16-OH/C14-DC, C18:1-OH/C16:1-DC, C20, C12-OH/ C10-DC, C14-OH/C12-DC |
| 3 | Short-chain dicarboxyl-acylcarnitines | C5-DC, C6:1-DC/C8:1-OH, C8:1-DC, C6-DC, Ci4-DC/C4-DC, C10-OH/C8-DC, C12-OH/C10-DC, Cit |
| 4 | Long-chain acylcarnitines | C18:1, C18:2, C18, C16, C20:4, C16:1-OH/C14:1-DC |
| 5 | Ketone-related | KET, HBUT, C4-OH, C2, Ala |
| 6 | Medium-chain acylcarnitines | C10:3, C8:1, C10:2, C10:1 |
| 7 | Branched-chain amino acids | Phe, Tyr, Leu/Ile, Val, Met |
| 8 | Urea cycle amino acids | Gly, Met, Ser, Orn, Arg, C5:1, Pro |
| 9 | Short-chain acylcarnitines | C4/Ci4, C3, C5’s |
| 10 | Malonyl carnitine, Aspartate | C5-OH/C3-DC, Asx |
| 11 | Histidine, 3-Hydroxy-linoleylcarnitine, Arginine, Tiglyl-carnitine | His, C18:2-OH, Arg, C5:1 |
| 12 | Glutamate, Valine | Glx, Val |
| 13 | Alanine, Proline, Non-esterified Fatty acids | Ala, Pro, NEFA |
| 14 | Docosanoyl-carnitine | C22 |
Included in the table are 12 factors identified from PCA. Column 2 lists the overall description of the factor, column 3 the individual metabolites comprising that factor. ALA, alanine; ARG, arginine; ASX, aspartic acid/asparagine; CIT, citrulline; GLX, glutamine/glutamate; GLY, glycine; HBUT, beta-hydroxybutyrate; HIS, histidine; KET, ketones; LEU/ILE, leucine/isoleucine; MET, methionine; NEFA, non-esterified fatty acids; ORN, ornithine; PHE, phenylalanine; PRO, proline; SER, serine; TYR, tyrosine; VAL, valine.
Results
Baseline characteristics
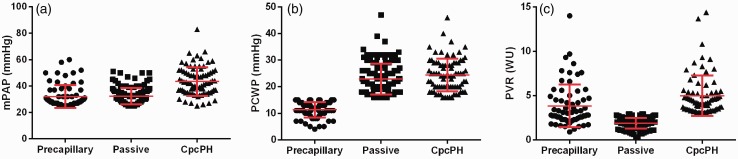
Of the 280 PH case participants, 133 met criteria for postcapillary PH, 82 for CpcPH, and 65 for precapillary PH. Table 2 contains the baseline characteristics of study participants. Overall, the mean age of PH cases was 61.4 ± 11.7 years, 44% were women, and 68% were Caucasian. Non-PH controls were well-matched on baseline co-morbidities. CpcPH patients were more likely to be women, have the lowest mean left ventricular ejection fraction, the highest percentage of diabetes, and a greater burden of coronary artery disease (Table 2). The hemodynamic differences among the PH subtypes reflected their clinical classification. Mean PAP and PVR was highest in the CpcPH subtype compared to precapillary PH and postcapillary PH subtypes (mean, 44 versus 30 versus 32 mmHg, mean PAP, respectively; mean, 4.8 versus 3.8 versus 1.9 WU, PVR, respectively) (Table 3, Fig. 1). In general, reflecting the CATHGEN biorepository focus on patients with coronary artery disease and left heart failure rather than PAH, precapillary PH patients had mild disease by hemodynamic criteria.
Table 2.
Baseline patient characteristics.
| All PH (n = 280) | Precapillary PH (n = 65) | Postcapillary PH (n = 133) | CpcPH (n = 82) | Non-PH controls (n = 280) | |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 61.4 (11.7) | 58.6 (11.0) | 62.0 (11.5) | 62.5 (12.3) | 61.2 (11.9) |
| Female (%) | 43.9 | 41.5 | 31.6 | 65.9 | 43.6 |
| Race | |||||
| Caucasian (%) | 67.5 | 61.5 | 70.7 | 67.1 | 67.5 |
| African American (%) | 26.4 | 26.1 | 23.3 | 31.7 | 27.9 |
| Ejection fraction (%), mean (SD) | 51.4 (17.1) | 58.8 (10.9) | 50.0 (17.4) | 47.8 (18.9) | 53.4 (14.7) |
| Diabetes mellitus (%) | 27.1 | 16.9 | 29.3 | 31.7 | 27.1 |
| Number of diseased coronary arteries (%) | |||||
| 0 | 65.4 | 73.9 | 61.7 | 64.6 | 61.2 |
| 1 | 9.3 | 12.3 | 9.8 | 6.1 | 11.8 |
| 2 | 11.1 | 4.6 | 15.0 | 9.8 | 9.3 |
| 3 | 14.3 | 9.2 | 13.5 | 19.5 | 17.1 |
Values are mean or percentages.
Table 3.
Baseline patient hemodynamics.
| Precapillary PH (n = 65) | Postcapillary PH (n = 133) | CpcPH (n = 82) | |
|---|---|---|---|
| Mean PAP (mmHg) | 30 (9) | 32 (6) | 44 (11) |
| PVR (Woods units) | 3.8 (2.4) | 1.9 (0.6) | 4.8 (2.3) |
| PCWP (mmHg) | 11 (3) | 22 (6) | 23 (7) |
| DPG (mmHg) | 10 (7) | 0 (5) | 5 (6) |
| TPG (mmHg) | 21 (10) | 10 (4) | 20 (8) |
| Cardiac output (L/min) | 5.8 (1.5) | 5.5 (1.6) | 4.3 (1.0) |
| Cardiac index (L/min/m2) | 3.0 (0.8) | 2.6 (0.6) | 2.3 (0.5) |
Values are mean (standard deviation).
Fig. 1.
Distributions of hemodynamics in PH subgroups for (a) mean pulmonary artery pressure (mPAP, mmHg), (b) pulmonary capillary wedge pressure (PCWP, mmHg), and (c) pulmonary vascular resistance (PVR, Woods units). Due to the cutoffs used to define precapillary, postcapillary, and CpcPH, there are skewed distributions of the PCWP and PVR.
Metabolomic profiling of PH cases versus no-PH controls
Using scoring coefficients from our prior analysis, PCA identified 14 metabolite factors reflecting underlying metabolic pathways.9,13 Table 4 contains the constituent individual small-molecule metabolites of the factors and the overall basic biologic descriptions of each factor based on the individual metabolites with the heaviest load on each factor. Table 5 reports mean metabolite factor levels by PH subtype. In multivariable models, factor 4 (long-chain acylcarnitines, nominal P = 0.00008) was significantly different between PH cases and PH controls after Bonferroni adjustment (P < 0.0035) with higher levels in PH cases. Six additional factors were nominally significantly different between PH cases and non-PH controls: factor 6 (medium-chain acylcarnitines, P = 0.009) levels were higher in PH cases and factors 8 (urea cycle amino acids, P = 0.007), factor 9 (C3-C5 acylcarnitines, P = 0.04), factor 11 (histidine, arginine, C18:2-OH and C5:1 acylcarnitines; P = 0.04), factor 12 (glutamate/glutamine, valine; P = 0.006), and factor 14 (C22 acylcarnitine, P = 0.004) were all lower in PH cases compared to controls.
Table 5.
Mean values for metabolite factor levels, stratified by PH subtype.
| Factor | Overall |
All PH vs. Control |
Precapillary PH vs. Control |
Precapillary vs. CpcPH |
Pre- vs. Postcapillary PH |
Postcapillary PH vs. Control |
CpcPH vs. Control |
Postcapillary PH vs. CpcPH |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 280) | All PH (n = 280) | Precapillary PH (n = 65) | Postcapillary PH (n = 133) | CpcPH (n = 82) | P value | P value | P value | P value | P value | P value | P value | |
| 1. Medium-chain acylcarnitines | 0.08 (1.4) | 0.27 (1.11) | 0.19 (0.79) | 0.31 (1.34) | 0.26 (0.90) | 0.10 | 0.49 | 0.89 | 0.52 | 0.10 | 0.45 | 0.35 |
| 2. Long-chain dicarboxyl- acylcarnitines | 0.02 (0.86) | 0.14 (1.37) | −0.07 (0.84) | 0.17 (1.77) | 0.25 (0.83) | 0.24 | 0.61 | 0.07 | 0.66 | 0.34 | 0.01* | 0.89 |
| 3. Short-chain dicarboxyl- acylcarnitines | −0.02 (0.79) | 0.02 (0.86) | −0.05 (0.88) | 0.03 (0.86) | 0.06 (0.86) | 0.52 | 0.78 | 0.68 | 0.77 | 0.82 | 0.47 | 0.56 |
| 4. Long-chain acylcarnitines | −0.01 (0.78) | 0.38 (1.4) | 0.34 (2.16) | 0.46 (1.15) | 0.28 (0.94) | 0.00008*† | 0.01* | 0.61 | 0.93 | 0.00001*† | 0.002*† | 0.57 |
| 5. Ketone-related | 0.19 (1.08) | 0.19 (1.1) | 0.21 (1.06) | 0.02 (1.11) | 0.43 (1.06) | 0.57 | 0.48 | 0.49 | 0.30 | 0.04* | 0.37 | 0.04* |
| 6. Medium-chain acylcarnitines | 0.04 (1.03) | 0.27 (1.01) | 0.15 (0.92) | 0.24 (0.96) | 0.38 (1.14) | 0.01* | 0.09 | 0.75 | 0.78 | 0.08 | 0.09 | 0.73 |
| 7. Branched-chain amino acids | −0.07 (0.91) | −0.04 (1.03) | −0.25 (0.99) | 0.05 (1.00) | −0.02 (1.11) | 0.84 | 0.14 | 0.49 | 0.18 | 0.54 | 0.41 | 0.89 |
| 8. Urea cycle amino acids | 0.05 (1.00) | −0.17 (1.00) | −0.17 (1) | −0.07 (1.06) | −0.33 (0.89) | 0.01* | 0.22 | 0.07 | 0.58 | 0.29 | 0.001*† | 0.04* |
| 9. Short-chain acylcarnitines | 0.16 (1.58) | −0.08 (1.07) | −0.42 (0.86) | 0.17 (1.2) | −0.20 (0.90) | 0.04* | 0.05 | 0.35 | 0.01* | 0.97 | 0.01* | 0.02* |
| 10. Malonyl carnitine, Aspartate | −0.11 (0.94) | −0.05 (0.95) | −0.17 (0.63) | 0.10 (1.09) | −0.19 (0.89) | 0.42 | 0.67 | 0.58 | 0.14 | 0.11 | 0.97 | 0.20 |
| 11. Histidine, 3-Hydroxy- linoleylcarnitine, Arginine, Tiglyl-carnitine | 0.07 (0.10) | −0.08 (0.98) | −0.12 (0.95) | −0.03 (1.01) | −0.14 (0.97) | 0.04* | 0.05 | 0.59 | 0.35 | 0.30 | 0.22 | 0.54 |
| 12. Glutamate, Valine | −0.06 (1.02) | −0.32 (1.13) | −0.28 (0.94) | −0.26 (1.16) | −0.44 (1.22) | 0.01* | 0.06 | 0.22 | 0.97 | 0.08 | 0.04* | 0.19 |
| 13. Alanine, Proline, Non-esterified fatty acids | −0.05 (0.97) | −0.02 (1.11) | −0.11 (0.96) | −0.08 (1.13) | 0.17 (1.17) | 0.67 | 0.67 | 0.51 | 0.84 | 0.80 | 0.11 | 0.26 |
| 14. Docosanoyl-carnitine | 0.02 (1.00) | −0.26 (0.85) | −0.24 (0.62) | −0.23 (0.97) | −0.33 (0.81) | 0.0004*† | 0.02* | 0.74 | 0.69 | 0.01* | 0.04* | 0.82 |
Values are mean (standard deviation) and are unitless.
*P value < 0.05 in comparison of listed categories after adjustment for age, race, sex, diabetes, ejection fraction, number of diseased coronary arteries, and batch number.
†Correction for multiple comparisons, with 14 components, is P < 0.0036.
Metabolomic profiling of PH-LHD subtypes
In comparing PH associated with left heart disease subtypes to no-PH controls, the most significant results revealed that both postcapillary PH and CpcPH groups individually continued to show higher mean levels of factor 4 than no-PH controls (long-chain acylcarnitines, P = 0.00001 and P = 0.002, respectively). Individuals with postcapillary PH also had lower mean levels of factor 5 (ketone-related metabolites, P = 0.04) and factor 14 (C22 acylcarnitine, P = 0.01). Individuals with CpcPH also demonstrated higher factor 2 levels (long-chain dicarboxyacylcarnitines, P = 0.01) and lower factor 8 (urea cycle amino acids, P = 0.005), factor 9 (C3-C5 acylcarnitines, P = 0.01), factor 12 (glycine and valine, P = 0.04), and factor 14 (C22 acylcarnitine, P = 0.04). Factor 4, however, was not significantly different between CpcPH and postcapillary PH patients. Instead, when compared to postcapillary PH patients, CpcPH patients exhibited higher factor 5 levels (ketone-related metabolites, P = 0.04), but lower factor 8 (urea cycle amino acids, P = 0.04) and factor 9 (C3-C5 acylcarnitines, P = 0.02) levels.
Discussion
In this study, we hypothesized that metabolite profiles reporting on mitochondrial energetics would differentiate PH from controls without PH, and different hemodynamic subtypes of PH associated with left heart disease from one another. Our most significant finding was for higher levels of circulating long-chain acylcarnitines (factor 4) in patients with PH as compared with controls without PH even after adjustment for many clinical variables, a finding that met rigorous adjustment for multiple comparisons overall and was significant in comparisons of each PH subtype with controls. These results suggest a shared mitochondrial dysfunction common to all types of PH. Our second most significant finding was for urea cycle metabolites (factor 8), with lower levels in PH patients than no-PH controls, and differentiating PH subtypes in left heart disease with CpcPH having the lowest levels overall.
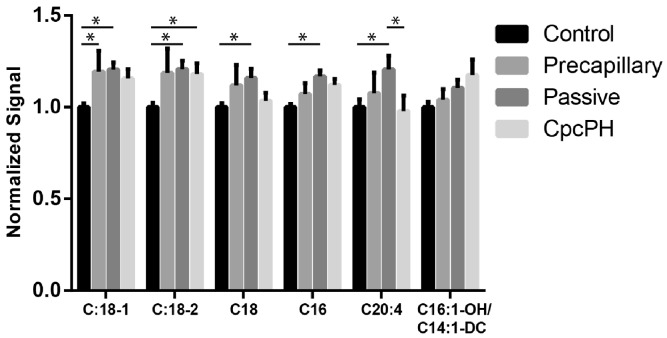
While the metabolomics changes that we found were limited to a discovery cohort, they are broadly consistent with other findings that have been described in the literature. The non-failing heart primarily utilizes long-chain fatty acids as an energy source, and an early step in their oxidation in the mitochondria is the formation of long-chain acylcarnitines.15 In our study, the increased levels of long-chain acylcarnitines (factor 4, Fig. 2) seen across the PH subtypes as compared with no-PH potentially reflects the inefficiencies in mitochondrial fuel metabolism previously described in both left and right ventricular failure. Recently, these abnormalities in fatty acid metabolism have been identified in the plasma and RV in patients with PAH.16 Similar abnormalities in long-chain acylcarnitine levels have been noted in left heart failure including heart failure with preserved ejection fraction and improve with ventricular assist device therapy.8,10 These findings are consistent with abnormalities in metabolic reprogramming in animal models of heart failure.17 This suggests that abnormalities in long-chain acylcarnitines reflect a common signature associated with myocardial mitochondrial dysfunction.
Fig. 2.
Normalized concentrations of long-chain acylcarnitines in PH hemodynamic subgroups. Shown are values normalized to control. *P < 0.05 by two-way ANOVA with Tukey test for multiple comparisons.
The other nominally significant results included results for factor 2, comprised of long-chain dicarboxylacylcarnitines, unique in being the only factor which was only different in CpcPH compared to controls. Dicarboxylated acylcarnitines are derivatives of mitochondrial lipid or amino acid oxidation and a product of omega-oxidation by microsomal cytochrome p450 enzymes in peroxisomes. We have previously described a strong association of similar dicarboxyl acylcarnitines with cardiovascular events in a cardiovascular disease cohort.21 The precise substrates and pathways that generate the dicarboxylated acylcarnitines and factors that foster their production require further investigation.
Urea cycle amino acids (factor 8) were lower in CpcPH compared to postcapillary PH patients as well as controls. This factor is strongly weighted by glycine and may be reporting on subsequent degradation to ammonia and entry into the urea cycle. Abnormalities in these metabolites has been noted in human pulmonary endothelium with BMPR2 mutations.18 Recent data have linked increased glycine levels and decreased urea cycle metabolites with a chromosomal locus and identified a strong association with decreased risk of atherosclerosis.19 Glycine may have cardioprotective properties against coronary arterial endothelial cell inflammation, further supporting the hypothesis that genetic susceptibility can lead to a differential response to cardiopulmonary vascular inflammation and stress.20
A number of other metabolic abnormalities have been reported in pulmonary vascular disease and right heart failure. In a recent study using an alternative targeted metabolomics platform, differences in indoleamine 2,3-dioxygenase-dependent tryptophan metabolites, tricarboxylic acid intermediates, purine metabolites, and arginine-nitric oxide metabolic pathway constituents were associated with right ventricular–pulmonary vascular dysfunction.22 In future studies, combining multiple biomarkers reflecting the many pathways dysregulated in pulmonary vascular disease—including the nitric oxide pathway, mitochondrial bioenergetics, and vasoactive mediators—could provide additional insight into left heart disease associated PH.
Our results simultaneously highlight metabolic pathways dysregulated in PH while identifying circulating, easily measurable biomarkers that distinguish PH from no-PH and PH subtypes associated with left heart disease. There are some limitations to our study, however. The cross-sectional design of our analysis allows only for an examination of associations, not causation. Given the exploratory nature of this small sample size and multiple comparisons made, we may be subject to both false-positive and false-negative findings. The use of PCA mitigates the number of multiple comparisons by reducing a large number of metabolites into unrelated groupings in an unbiased manner, and our primary finding for long-chain acylcarnitines survives adjustment for a conservative correction for multiple comparisons. While we adjusted for potential confounders such as age, race, diabetes, left ventricular ejection fraction, and coronary artery disease, there may be unmeasured confounders. Also, as a result of the inclusion criteria to the CATHGEN biorepository, patients with precapillary PH had very mild disease by hemodynamic criteria. Thus, our findings do not likely extend to those with severe PAH. Finally, while some results were nominally significant for factors with lower eigenvalues (e.g. factor 12 which was nominally significant in comparisons of PH to no-PH and CpcPH to no-PH) likely are false positives representing statistical noise of higher number factors that explain a much smaller proportion of variance in the dataset.
Conclusions
We observed unique metabolite profiles in PH compared to controls and in different hemodynamic subtypes of PH seen in left heart disease. These metabolites likely reflect changes in mitochondrial energetics associated with pulmonary vascular disease and heart failure, but could also report on global metabolic disturbances in this disease state.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This project was supported in part by grant number HL095987 from the National Institutes of Health (Bethesda, MD, USA). SR is supported by HL114643 and a Burroughs Wellcome Career Award for Medical Scientists (Durham, NC, USA).
References
- 1.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail 2013; 1: 290–299. [DOI] [PubMed] [Google Scholar]
- 2.Fang JC, DeMarco T, Givertz MM, et al. World Health Organization Pulmonary Hypertension group 2: pulmonary hypertension due to left heart disease in the adult–a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2012; 31: 913–933. [DOI] [PubMed] [Google Scholar]
- 3.Khush KK, Tasissa G, Butler J, et al. Effect of pulmonary hypertension on clinical outcomes in advanced heart failure: analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) database. Am Heart J 2009; 157: 1026–1034. [DOI] [PubMed] [Google Scholar]
- 4.Pietra GG, Capron F, Stewart S, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 2004; 43(12 Suppl S): 25S–32S. [DOI] [PubMed] [Google Scholar]
- 5.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res 2014; 115: 148–164. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer S. The failing heart–an engine out of fuel. N Engl J Med 2007; 356: 1140–1151. [DOI] [PubMed] [Google Scholar]
- 7.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 2014; 115: 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad T, Kelly JP, McGarrah RW, et al. Prognostic implications of long-chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J Am Coll Cardiol 2016; 67: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah SH, Bain JR, Muehlbauer MJ, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet 2010; 3: 207–214. [DOI] [PubMed] [Google Scholar]
- 10.Hunter WG, Kelly JP, McGarrah RW, 3rd, et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc 2016; 5: e003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraus WE, Granger CB, Sketch MH, Jr, et al. A guide for a cardiovascular genomics biorepository: the CATHGEN Experience. J Cardiovasc Transl Res 2015; 8: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah SH, Kraus WE, Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation 2012; 126: 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009; 9: 311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunner MP, Shah SH, Craig DM, et al. Effect of heparin administration on metabolomic profiles in samples obtained during cardiac catheterization. Circ Cardiovasc Genet 2011; 4: 695–700. [DOI] [PubMed] [Google Scholar]
- 15.Nickel A, Loffler J, Maack C. Myocardial energetics in heart failure. Basic Res Cardiol 2013; 108: 358. [DOI] [PubMed] [Google Scholar]
- 16.Brittain EL, Talati M, Fessel JP, et al. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation 2016; 133: 1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai L, Leone TC, Keller MP, et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail 2014; 7: 1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fessel JP, Hamid R, Wittmann BM, et al. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm Circ 2012; 2: 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartiala JA, Tang WH, Wang Z, et al. Genome-wide association study and targeted metabolomics identifies sex-specific association of CPS1 with coronary artery disease. Nat Commun 2016; 7: 10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa S, Ichiyama T, Sonaka I, et al. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin Exp Immunol 2012; 167: 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah SH, Sun JL, Stevens RD, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J 2012; 163: 844–850.e841. [DOI] [PubMed] [Google Scholar]
- 22.Lewis GD, Ngo D, Hemnes AR, et al. Metabolic profiling of right ventricular-pulmonary vascular function reveals circulating biomarkers of pulmonary hypertension. J Am Coll Cardiol 2016; 67: 174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]