Abstract
Alterations in the nitric oxide (NO) pathway play a major role in pulmonary arterial hypertension (PAH). L-arginine (LA) and tetrahydrobiopterin (BH4) are main substrates in the production of NO, which mediates pulmonary vasodilation. Administration of either LA or BH4 decrease pulmonary artery pressure (PAP). A combined administration of both may have synergistic effects in the therapy of PAH. In a telemetrically monitored model of unilateral pneumonectomy and monocrotaline-induced PAH, male Sprague-Dawley rats received either LA (300 mg/kg; n = 15), BH4 (20 mg/kg; n = 15), the combination of LA and BH4 (300 mg/kg, 20 mg/kg; n = 15), or vehicle (control group; n = 10) from day 28 after monocrotaline induction. Therapy was orally administered once daily over consecutive 14 days. LA, BH4, or both equally lowered PAP, increased pulmonary vascular elasticity, restored spontaneous locomotoric activity, prevented body weight loss and palliated small vessel disease of severely pulmonary hypertensive rats. BH4 substitution lowered asymmetric dimethylarginine levels sustainably at 60 min after administration and downregulated endothelial NO synthase mRNA expression. No significant survival, macro- and histomorphologic or hemodynamic differences were found between therapy groups at the end of the study period. Administration of LA and BH4 both mediated a decrease of mean PAP, attenuated right ventricular hypertrophy and small vessel disease in monocrotaline-induced pulmonary hypertensive rats, though a combined administration of both substances did not reveal any synergistic therapy effects in our animal model.
Keywords: pulmonary arterial hypertension (PAH), animal model, monocrotaline, combination therapy, L-arginine, tetrahydrobiopterin
Pulmonary arterial hypertension (PAH) is a fatal disease leading to a significant shortening of life. Constriction and occlusion of the pulmonary arterial bed pose an increased afterload on the right ventricle, thereby leading to right heart failure and death.
Besides prostacyclines and endothelin receptor blockers, nitric oxide (NO) is a potent modulator of pulmonary hemodynamics. Intermittently administered, inhaled NO effectively lowers pulmonary artery pressures (PAP).1 In addition, pulmonary vasoreactivity response to NO predicts long-term survival.2 Further downstream, the NO pathway is modified by phosphodiesterase-5 inhibitors or by the novel substance riociguat that interacts with the soluble guanylate cyclase.3 These and several other publications suggest a pivotal role of NO alteration in the pathogenesis of PAH. Upregulation and maintenance of the endogenous NO production in the pulmonary vasculature have therefore been a target of extensive experimental research.4
Endogenous production of vasoactive NO is mainly determined by endothelial NO synthase (eNOS), a dynamically regulated isoform of the mammalian NO synthase family. Dimerization of two eNOS molecules couples the heme domains, which is essential for the electron transfer with reduction of molecular oxygen and oxidation of the bound substrate L-arginine (LA). This reaction results in the liberation of NO and L-citrullin.5 Binding of NO to the heme group leads to 400-fold activation of the soluble guanylate cyclase and catalyses the synthesis of cyclic guanosin monophosphate that consequently accomplishes relaxation of pulmonary arterial smooth muscle cells. 6,7
Due to its limited half-life, NO administration needs to be administered continuously in order to maintain its beneficial effects on the pulmonary hypertensive vasculature.8 Therefore, the application of inhaled NO is limited to the acute setting, e.g. acute right heart failure.
LA is a semi-essential basic amino acid that contains four nitrogen molecules and serves as a substrate for eNOS. In human PAH, decreased levels of LA were detected.9 Administration of LA alone has been reported to restore vascular endothelial NO production and to subsequently decrease PAP in rodents and in humans.10,11 By contrast to the previous assumption that eNOS enzyme is constitutively active, it has been recently demonstrated that eNOS activity highly depends on the intracellular presence of tetrahydrobiopterin (BH4).12 Binding of BH4 to eNOS increases stability of the active eNOS dimer and significantly increases enzymatic turnover of LA. BH4 deficiency leads to uncoupling of the dimeric eNOS molecule and enhances generation of superoxide anion that in turn reacts with and neutralizes NO before it is able to accomplish its vasodilatory effects.13 This mechanism occurs independently from enzyme saturation with the substrate LA.14 Therefore, optimal BH4 levels are fundamentally important for a proper function of eNOS in pulmonary endothelial cells. The therapeutic potential of BH4 in cardiovascular disease has been shown in several experimental studies, where BH4 ameliorated endothelial dysfunction and thus plays a major role in regulation of the vascular tone in human vasculature.15,16
Asymmetric dimethylarginine (ADMA) is a methylated LA that was identified as an independent risk factor for cardiovascular mortality.17 It inhibits eNOS function and mediates vasoconstriction. Boger et al. reported that in patients with high ADMA levels LA supplementation was necessary to enhance statin-mediated eNOS function.18 In a human cohort of PAH patients, ADMA levels were reduced after oral administration of a phosphodiesterase-5 inhibitor.19
To the best of our knowledge, combined administration of LA and BH4 with the purpose to treat PAH has never been tested before. In animal models of renal failure and hind-leg ischemia it has been successfully applied.20,21 In a translational approach we tested if LA and BH4 have synergistic effects in lowering the mean PAP in pulmonary hypertensive rats.
Methods
Animal model and pulmonary artery pressure measurement
All experiments conducted in this study were approved by the Federal Ministry of Science, Research and Economy (6609/196-II/10 b/2008). A total of 62 male Sprague-Dawley rats, weighing 350–500 g, underwent left-sided unilateral pneumonectomy and simultaneous implantation of a telemetry catheter into the common pulmonary artery trunk. This technique was developed and refined by our group (manuscript under review).
Animals were kept at room temperature of 22℃ and 12:12 h day-night cycle. They were provided tap water and common rat chow (ssniff-RMH, Soest, Germany) ad libitum. Seven days after surgical intervention, PAH was induced by subcutaneous administration of 60 mg/kg monocrotaline (MCT; Sigma, Vienna, Austria).
Therapy
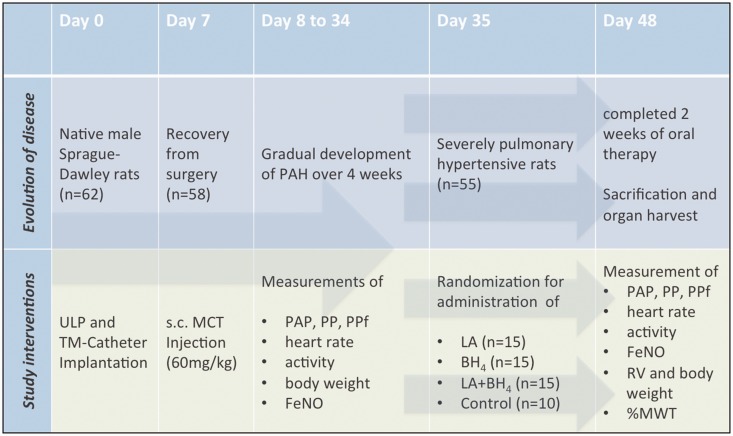
Twenty-eight days after PAH induction, equating day 35 of the study protocol, animals were randomly assigned to one of three treatment arms: LA group (300 mg/kg; n = 15; Sigma, Vienna, Austria), BH4 group (20 mg/kg; n = 15; AOP orphan, Vienna, Austria), combination group (LA + BH4; 300 mg/kg LA and 20 mg/kg BH4, n = 15) or the control (C) group (vehicle; n = 10; Fig. 1). Study drug doses were chosen according to previous publications.11,13,22
Fig. 1.
Experimental design. ULP, unilateral pneumonectomy; TM, telemetry; s.c., subcutaneous; MCT, monocrotaline; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressures; PP, pulse pressure; PPf, fractional pulse pressure; FeNO, fraction of exhaled nitric oxide; RV, right ventricle; LA, L-arginine; BH4, tetrahydrobiopterin.
Therapy was administered once daily over 14 consecutive days by oral gavaging in short inhalation narcosis with isoflurane 2%. The rats were held in an upright position until awake to prevent regurgitation or aspiration.
Plasma LA and ADMA levels were measured with high performance liquid chromatography (HPLC) analysis from EDTA blood samples obtained before, as well as 30 and 60 min after treatment on day 35. Withdrawal of 0.5 mL blood was performed via the vena lingualis. Blood samples were centrifuged (1000 rpm/5 min), followed by separation and storage of plasma at –20℃. HPLC analysis was performed as described by Teerlink et al.23
After 14 days of oral therapy, euthanasia was performed according to American Veterinary Medical Association guidelines.24 For this purpose, animals were anesthetized with ketamine (100 mg/kg) and xylazine (4 mg/kg). Animals received an intracardiac bolus injection of 10 mL (0.5 g) thiopental.
Hemodynamic studies
Recording of hemodynamic data was carried out with a DSI PA-C40 pressure catheter and the DSI 4.2 ART software system (Datascience International, Boston, MA, USA). Segments of 5 min measurement of systolic (sPAP), diastolic (dPAP), mean pulmonary arterial pressure (mPAP), pulse pressure (PP), heart rate (HR), and spontaneous locomotoric activity (SLA), expressed by a numerical value (unit) for the distance the transmitter travels by movement of the rat during a 5 min measurement period, were recorded every hour. The fractional pulse pressure (PPf) was calculated as PP (mmHg) × 1/mPAP (mmHg) as previously described.25
eNOS expression and NO exhalation
Fraction of exhaled NO (FeNO) was measured before initiation of therapy and after 14 days of oral therapy. Normal values for healthy rats and a standardized extrapolation method were used as previously developed by our group.26 Briefly, animals were placed in a 3.9 L NO-free whole-body plethysmography chamber for unrestrained animals (PLY 3213 Buxco Research Systems, Wilmington, NC, USA) and inflow/outflow accesses were closed with a three-way stopcock. After 5 min, the air was evacuated from the box and NO concentration was measured by use of a chemiluminescence apparatus (CLD66, Ecophysics, Duernten, Switzerland).
In addition, eNOS mRNA expression in pulmonary hypertensive right lungs was determined and compared with the healthy left lung that had been removed prior to MCT administration. mRNA was isolated from nitrogen-snap-frozen tissue samples (RNeasy Mini Kit, Qiagen, Hilden, Germany) and mRNA expression of eNOS was quantified in RT-PCR using an eNOS primer (Qiagen, Hilden Germany).
eNOS western blot protein quantification
Lung tissue was lysed (RIPA buffer; 50 mM Tris-HCl, pH 8.0, with 150 mM sodium chloride, 1.0% Igepal CA-630 (NP-40), 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate) and protein concentration was measured with bicinchoninic assay (BCA, Sigma-Aldrich, St. Louis, MO, USA). Equal amounts of total protein (20 µg) were separated by SDS-PAGE on a Bio-Rad Criterion cell using Criterion precast gels (Bio-Rad, Hercules, CA, USA). Proteins were electroblotted onto polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with 5% bovine serum albumin (BSA) (Sigma-Aldrich) and incubated with an antibody against eNOS (1:300, Abcam, Cambridge, UK) in 1% BSA overnight at 4℃. After incubation with the secondary, peroxidase-coupled antibody (Polyclonal Rabbit Anti-Mouse Ig/HRP P0260; DakoCytomation, Carpinteria, CA, USA) detection was accomplished by using enhanced chemiluminescence solution (Western Lightning reagent; Perkin Elmer, Boston, MA, USA) and a ChemiDoc XRS chemiluminescence detection system (Bio-Rad). Densitometric eNOS quantification was accomplished using NIH ImageJ software (1.47, Bethesda, MD, USA). Results were normalized to the respective GAPDH bands.
Structural studies and histological analyses
Post-mortem the RV/LV + S ratio ([right/left + septum] ventricular weight) and RV/body weight ratio ([right ventricular/body] weight) were used as indicators for right ventricular hypertrophy. After removal of atrial appendages, harvested hearts were dissected into the right ventricular wall and left ventricle plus septum and weighed on an electronic precision scale (Ohaus CS 200, Pine Brook, Canada).
Morphology of pulmonary artery vessels was assessed by trichrome stains. Formaldehyde-fixed samples were paraffin-embedded with an automatic embedding device (TissueTek AutoTEC, Sakura Finetek, OH, US), cut into 2 µm sections with a microtome (RM 2255, Leica Microsystems, Wetzlar, Germany) and mounted on cover slips. Trichrome staining was done as described by Garvey et al.27 External diameters of small (<50 µm) and medium-sized pulmonary vessels (51–100 µm) and intimal and medial thickness were assessed in three specimens per animal with cell digital image analysis (Olympus Soft Imaging Solutions, Muenster, Germany). The percentage of intima and media thickness compared to outer vessel diameter (%MWT) ([intimal + medial thickness × 2] / external diameter) × 100 was calculated as described by Sahara et al.28
eNOS expression was visualized by immunohistochemistry incubation with eNOS primary antibody (ab5589, abcam, Cambridge, UK) and a secondary antibody kit (Histostain SP, Invitrogen, Carlsbad, CA, USA). The percentage of positively stained tissue in four peripheral fields of view per lung were assessed with a statistical model of color detection by two independent observers using digital image analysis software (NIH ImageJ 1.47, Bethesda, MD, USA) as published by Shu et al.29
Statistical analysis
This was an exploratory study, therefore no sample size calculations were made in advance. Results were statistically compared using the SPSS19 Statistic software (IBM, Armonk, NY, USA). Survival was compared with the log-rank test. For comparison between two groups the unpaired t-test, for more than two groups, the univariate ANOVA with Tukey post-hoc analysis was applied. A P value < 0.05 was considered significant. For graphical illustration Excel 2010 software (Microsoft, Redmond, WA, USA) was used.
Results
Animal data
A total of 62 rats underwent surgery and were equipped with a telemetry catheter. Animals that died at the time of surgery (n = 4) or prior to initiation of medical therapy due to infection (n = 1) or early right heart failure (n = 2) were not included in the analysis. On day 35, study animals were randomly assigned to one of the three treatment arms: LA (n = 15), BH4 (n = 15), LA + BH4 (n = 15), or the untreated control group C (n = 10). One rat in the control group died on day 41 before completion of the study protocol.
Bioavailability of L-arginine and ADMA levels
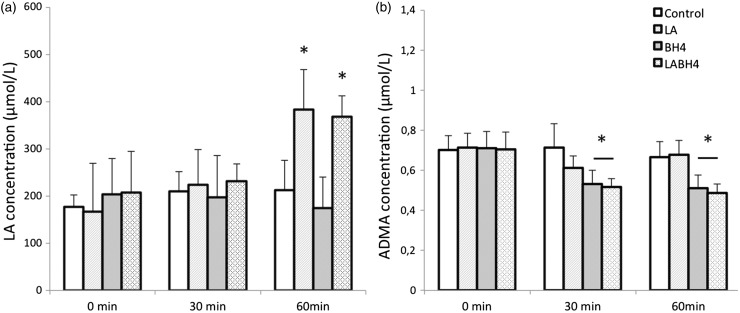
Sixty minutes after oral therapy administration of LA on day 35, HPLC analysis showed a significant increase in LA plasma concentration to a maximum of 383 ± 113 µmol/L in the LA and 368 ± 88 µmol/L in the LA + BH4 group compared to the control group (212 ± 127 µmol/L) or the BH4 group (175 ± 130 µmol/L, P < 0.001, Fig. 2a). With respect to ADMA levels, a sustained decrease of ADMA in the BH4 group (0.51 ± 0.11 µmol/L) as well as in the LA + BH4 group (0.48 ± 0.09 µmol/L) was encountered, compared with the control and the LA groups (LA, 0.67 ± 0.13 µmol/L; C, 0.66 ± 0.15 µmol/L) (P < 0.001; Fig. 2b).
Fig. 2.
HPLC plasma concentrations before, 30 min, and 60 min after therapy administration on day 35. (a) In the LA and LA + BH4 groups, the LA concentration increased significantly at 60 min compared to the BH4 and control groups (P = 0.01). (b) Concentration of ADMA is markedly reduced at 30 and 60 min after therapy administration in the BH4 and LA + BH4 groups (P < 0.001). All data are presented as mean + SEM.
Body weight
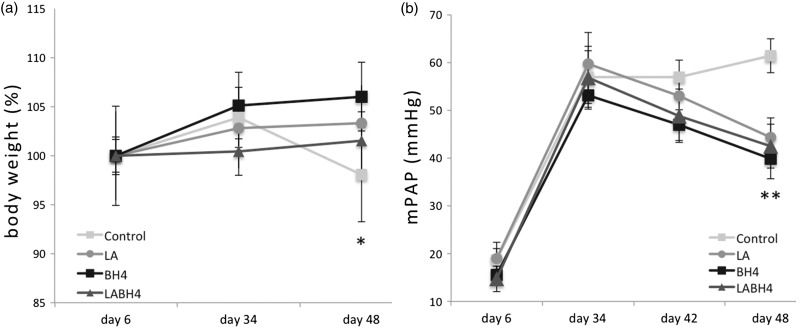
In the LA + BH4 group, mean body weight before therapy was significantly lower in comparison with the LA, BH4, and control groups (P = 0.031). Thus, we calculated the weight difference between day 35 and day 48. Rats that received LA, BH4, or LA + BH4 therapy gained 2.2 ± 26, 4.2 ± 22, and 4.3 ± 24 g of body weight, respectively (P = 0.964). During the same time, rats in the control group experienced a significant weight loss of 18 ± 29 g, equivalent to 6% of their body weight (P = 0.017, for untreated rats compared with all treated rats; Fig. 3a).
Fig. 3.
Evolution of body weight and mean PAP. (a) Body weight. Animals in the control group experienced a significant weight loss towards the end of the study compared to animals that received treatment (P = 0.017). Body weight measures on day 34 and day 48 are related to body weight at baseline and are exhibited in %. (b) mPAP. At the end of oral treatment (day 48) mPAP values were significantly lower in all treatment groups compared to control rats (P = 0.001). All data are presented as mean + SEM. Control, control group (n = 10); LA, L-arginine group (n = 15); BH4, tetrahydrobiopterin group (n = 15); LA + BH4, combined treatment group (n = 15). Day 6: baseline, day before induction of pulmonary arterial hypertension with monocrotaline; Day 34: start of oral therapy; Day 42: 1 week of oral therapy; Day 48: 2 weeks of oral therapy.
Pulmonary hemodynamics
With respect to hemodynamic parameters, including sPAP (P = 0.606), mPAP (P = 0.541; Fig. 3b), dPAP (P = 0.476) and PPf (P = 0.233), no statistically significant difference could be found between the treatment groups at follow-up. However, rats in the control group had significantly higher pulmonary pressures (sPAP, 83 ± 14 mmHg; mPAP, 61 ± 13 mmHg; dPAP, 44 ± 13 mmHg; P < 0.001; P < 0.001, and P = 0.033, respectively) and a reduced PPf (0.64 ± 0.2, P = 0.029) as compared to treated animals.
Spontaneous locomotoric activity and heart rate
At the end of treatment, SLA in all therapy groups had increased (LA, 3.7 ± 1.7 units; BH4, 3.5 ± 0.7 units; LA + BH4, 3.4 ± 1.1 units), but did not differ between groups (P = 0.797). Control rats were significantly less active with a mean SLA of 2.3 ± 1.0 units (P = 0.037; Table 1). However, with respect to heart rate, no statistically significant between-group differences could be encountered at the end of the treatment period with 326 ± 49 beats per minute (bpm) for LA + BH4, 319 ± 51 bpm for LA, 318 ± 50 bpm for BH4, and 343 ± 52 bpm for control rats (P = 0.224).
Table 1.
Survival, hemodynamic, macro- and histomorphologic parameters of treatment and control groups.
| Variable | Group |
|||||
|---|---|---|---|---|---|---|
| Control (n = 9) | P value #1 | LA + BH4 (n = 15) | BH4 (n = 15) | LA (n = 15) | P value #2 | |
| Survival (%) | 90% | 0.212* | 100% | 100% | 100% | * |
| Spap (mmHg) | 83 ± 14 | < 0.001 | 63 ± 13 | 60 ± 13 | 64 ± 14 | 0.696 |
| Mpap (mmHg) | 61 ± 13 | < 0.001 | 42 ± 13 | 39 ± 12 | 44 ± 12 | 0.541 |
| Dpap (mmHg) | 44 ± 13 | 0.003 | 28 ± 14 | 25 ± 12 | 31 ± 14 | 0.476 |
| PPf (%) | 0.64 ± 0.2 | 0.029 | 0.84 ± 0.2 | 0.90 ± 0.2 | 0.85 ± 0.3 | 0.233 |
| Heart rate (bpm) | 343 ± 52 | 0.224 | 326 ± 49 | 318 ± 50 | 319 ± 52 | 0.893 |
| Activity (unit) | 2.3 ± 1.0 | 0.037 | 3.4 ± 1.1 | 3.5 ± 0.7 | 3.7 ± 1.7 | 0.797 |
| FeNO (ppb/h) | 27 ± 10.7 | 0.168 | 29.3 ± 12.6 | 16.35 ± 5.3 | 23.6 ± 10.1 | 0.104 |
| RV/LV + S | 0.69 ± 0.2 | 0.078 | 0.58 ± 0.1 | 0.57 ± 0.1 | 0.64 ± 0.1 | 0.129 |
| RV/body weight × 100 | 0.18 ± 0.05 | < 0.001 | 0.14 ± 0.04 | 0.13 ± 0.03 | 0.12 ± 0.02 | 0.165 |
| %MWT small | 60 ± 16 | 0.034 | 48 ± 11 | 43 ± 16 | 49 ± 12 | 0.417 |
| %MWT medium | 48 ± 23 | 0.386 | 43 ± 9 | 38 ± 8 | 41 ± 16 | 0.497 |
| eNOS + % | 0.29 ± 0.3 | 0.382 | 0.39 ± 0.6 | 0.21 ± 0.3 | 0.37 ± 0.2 | 0.254 |
| eNOS mRNA (RQ) | 0.27 log10 | 0.033 † | –0.76 log10 | –1.03 log10 | –0.15 log10 | 0.280 |
Values are given as mean and standard deviation. P value #1: univariate ANOVA analysis with post-hoc Tukey-test comparing controls with treatment groups. P value #2: univariate ANOVA analysis for comparison between treatment groups.
For survival comparison the log-rank test was applied.
For Control versus BH4 and LA + BH4.
LA + BH4, L-arginine + tetrahydrobiopterin; LA, L-arginine; BH4, tetrahydrobiopterin; ANOVA, univariate analysis of variance; sPAP, systolic pulmonary artery pressure (PAP); dPAP, diastolic PAP; mPAP, mean PAP; PPf, fractional pulse pressure; FeNO, fraction of exhaled nitric oxide in parts per billion/hour (ppb/h); RV, right ventricle; LV + S, left ventricle + septum; %MWT, percentage of medial wall thickness: ([intimal + medial thickness × 2] / external diameter) × 100 for small (<50 µm) and medium (51–100 µm) pulmonary arteries; eNOS + %, percentage of endothelial nitric oxide synthase (eNOS)-positive stained lung tissue per field of view; RQ, relative quantity, the amount of eNOS mRNA in diseased lungs normalized to the amount of eNOS mRNA in non-diseased lungs.
Heart and lung structure
Compared with healthy controls (0.25 ± 0.01; internal data), RV/LV + S ratio was significantly increased in all rats with pulmonary hypertension (P < 0.001). Compared with untreated pulmonary hypertensive control rats, a trend towards a reduced RV/LV + S ratio was observed in all groups that received therapy (P = 0.078; Table 1). As normalized for the body weight of the rats we saw a significantly reduced RV/body weight ratio in all three therapy groups compared to untreated control rats (P < 0.001). In line with hemodynamic findings, there were no statistically significant differences in RV/LV + S or RV/body weight ratio between the three therapy groups (P = 0.129 and 0.165).
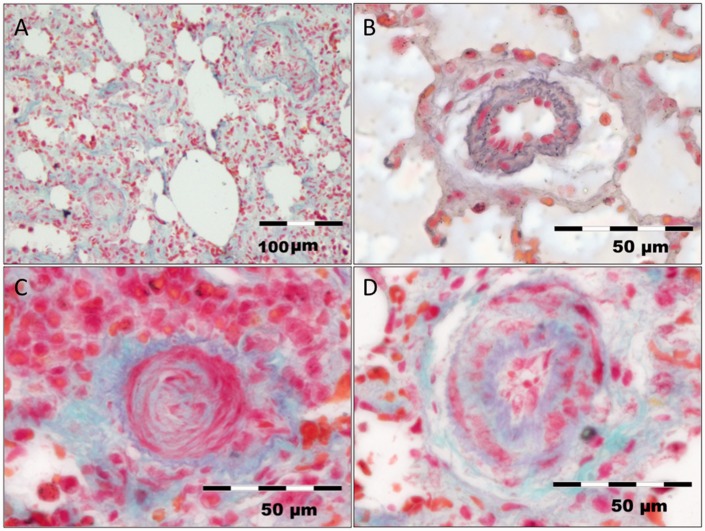
Trichrome-stained lung tissues showed severely narrowed pulmonary arterial vessels in right-sided PH lungs compared to left-sided healthy lungs (P < 0.001, Fig. 4a and b). This effect was most pronounced in small diameter vessels, which in part were totally occluded (Fig. 4c). LA, BH4, or LA + BH4 reduced the %MWT in small pulmonary arterioles compared to controls (P = 0.034; Fig. 4d), whereas in medium-sized vessels no treatment effect could be shown (P = 0.386). With regard to the administered therapies, no relevant between-group differences for small or medium-sized vessels were found (P = 0.417 and 0.497, respectively; Table 1).
Fig. 4.
Trichrome stains of representative lung samples. (a) Overview picture of hypertensive lung tissue with severe arterial remodeling and incipient fibrosis. (b) Pulmonary artery from a healthy left lung with normal intimal and medial wall thickness. (c) Right-sided untreated control lung with total occlusion of a small pulmonary artery. (d) Diminished intimal and medial wall thickness in a pulmonary artery from a rat that received LA + BH4 therapy.
NO and eNOS
NO exhalation studies showed a FeNO of 18.4 ± 5 ppb/h at baseline. After 2 weeks of therapy, FeNO levels did not show any significant differences between therapy and control groups (Table 1).
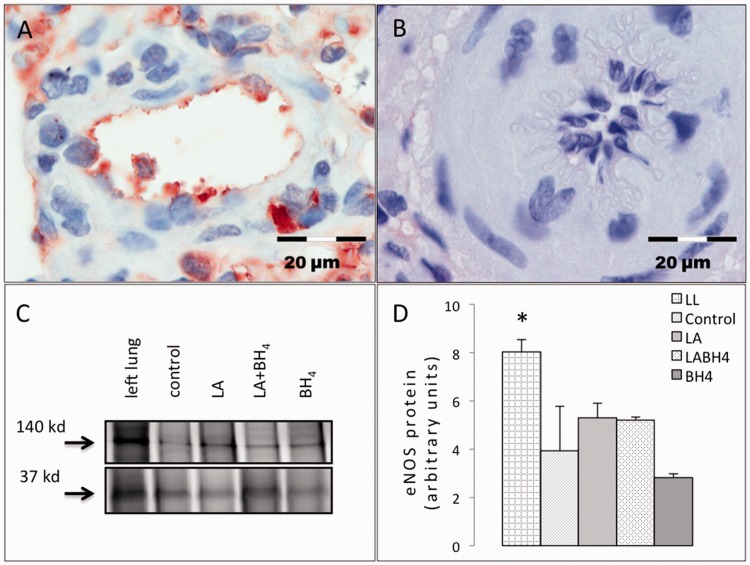
Immunohistochemical analysis revealed overall depletion of eNOS-staining from the endothelium of pulmonary hypertensive right lungs as compared to healthy left lungs (LA, 0.37 ± 0.24%; BH4, 0.21 ± 0.34%; LA + BH4, 0.39 ± 0.6% vs. healthy left control lungs, 2.24 ± 4.6%; P = 0.023; Fig. 5a and b). No difference was found between untreated controls and respective therapy groups (P = 0.382). This was also reflected in western blot studies that exhibited eNOS-depletion in diseased lungs compared to healthy controls (P = 0.041; Fig. 5c). Decreased eNOS-levels did not differ between the LA, BH4, or combined therapy groups (P = 0.328, Fig. 5d).
Fig. 5.
eNOS protein in pulmonary hypertensive lungs. While healthy lungs show abundant eNOS protein expression, a depletion is noticed in diseased lungs, irrespective of treatment. (a) eNOS-positive immunostained tissue from a healthy left lung. (b) eNOS depletion in immunostained lung tissue from a rat that received combination therapy. (c) Representative western blot shows an eNOS-positive band at 140 kD. GAPDH (37 kD) indicates equal protein amounts for all samples. (d) eNOS quantification by densitometry was normalized to GAPDH and confirms eNOS depletion in diseased lungs as compared to healthy tissue (P = 0.041). No restoration of eNOS expression is achieved by treatment. All data are presented as mean + SEM. LL, healthy left lung (n = 3); Control, control group (n = 3); LA, L-arginine group (n = 3); BH4, tetrahydrobiopterin group (n = 5); LA + BH4, combined treatment group (n = 5); GAPDH, glycerinaldehyd 3-phosphate dehydrogenase.
eNOS mRNA expression was significantly upregulated by 0.27log10 in diseased right lungs of non-treated control rats as compared to healthy left lungs. Administration of either BH4 alone or BH4 combined with LA significantly lowered eNOS gene expression by –1.03log10 and –0.76log10 (P = 0.033, Table 1). In the LA group, eNOS expression remained unaltered (RQ –0.15log10) as compared to healthy left lungs (P = 0.98).
Discussion
In the present study, we hypothesized that combined oral administration of LA and BH4 increases the enzymatic turnover of eNOS and thereby lowers PAP more effectively than either substance alone. To that end, we used an established rat model of severe pulmonary hypertension. While we could demonstrate that administration of LA or BH4 had beneficial effects on the pulmonary vasculature, no synergistic effects were encountered when the two substances were combined.
Beneficial effects of LA or BH4 on the pulmonary vasculature are in line with previous studies. For LA, a series of studies in experimental models and in humans have demonstrated not only a reduction of mean PAP but also attenuation of the typical histomorphological changes of pulmonary vessels as well as a regression of pre-existing right ventricular hypertrophy.30,31 In humans, Mehta et al. observed that short-term infusion of LA had an immediate effect on pulmonary vascular resistance and mPAP in pulmonary hypertensive patients but not in healthy individuals or patients with heart failure.10 Similar effects could be achieved in an experimental rodent model when LA was administered intraperitoneally.30 Moreover, less invasive oral LA administration via supplemented drinking water was similarly effective with respect to lowering PAP.31 In the present study, we could show that orally administered LA reached the systemic circulation and led to a significant decrease of PAP within 2 weeks of daily administration. Furthermore, it prevented body weight loss and increased SLA in severely pulmonary hypertensive rats.
For BH4, only data from hypoxic rats but not from MCT-induced PH are at hand.32 Francis et al. reported that BH4, given in a dose of 100 mg/kg, which corresponds to a fivefold higher dose than was administered here, increased cGMP levels, reversed pulmonary hypertension, and reduced right ventricular hypertrophy.
Although accurate measurements of arterial wall thickness are difficult,33 in our study BH4 administration lowered the %MWT in the small vasculature and effectively reduced the RV/body weight ratio. Although the observed effect was more pronounced than in the LA group, it failed to reach statistical significance at this dose and was also not apparent in the group receiving combined treatments. We cannot exclude that a higher dose may have had a measurable beneficial effect.
In humans, given in a dose of 5 mg/kg, BH4 improved the 6-minute walking distance.22 In line with this, BH4 led to a significant reduction of PAP and improved SLA in our experimental setting.
Overall, a combined administration of LA and BH4 did not reveal any further advantages or synergistic effects in our study. By contrast, Ahmed et al. did find a beneficial synergistic effect of LA and naringenin, a flavanone and antioxidant, in classically MCT-induced pulmonary hypertensive rats.34 Naringenin further augmented the therapeutic effect of baseline LA therapy. In addition to differences in the timing of drug combination—up-front in our study versus sequential in the cited study—a major difference seems to lie in the choice of animal models. We chose to start our medical therapy at a very late stage of PH, because this approach is more likely to reflect conditions in human disease. Clinical symptoms develop rather late in the course of PAH, when the pulmonary vascular resistance is already markedly increased and medical therapies should be able to exert their effects despite a severely altered pulmonary endothelium. It is well known that MCT poses an inflammatory stimulus on the lung vasculature. Manifest structural changes of the right ventricle and pulmonary vasculature develop not before day 21 of MCT application.35 Initiation of a drug therapy simultaneously with or shortly after MCT induction would blunt the inflammatory stimulus of MCT and result in milder remodeling of the pulmonary vasculature and less severe hemodynamic alterations. In our model, however, the full picture of advanced pulmonary vascular disease, including intimal thickening and medial hypertrophy was manifest before randomization to respective treatment arms. While it can be stated that a synergistic effect of the combination therapy occurs in blunting the inflammatory response to MCT, thereby preventing the development of pulmonary vascular disease, our experiments do not disclose a synergistic effect of combination therapy in the treatment of severe PH.
PAH control rats showed an upregulation of eNOS mRNA expression and concurrent depletion of eNOS antigen from the pulmonary endothelium. This is in accordance with prior publications which reported that greater shear stress in the hypertensive lungs may lead to eNOS upregulation.36 LA and even more pronounced BH4 led to a downregulation of eNOS mRNA, while endothelial eNOS protein in immunhistochemistry and western blot studies was depleted in all groups. This might indicate that the metabolic turnover of the remaining eNOS is enhanced by therapy administration. We did not perform eNOS activity analysis. However, data from Yan et al. show a higher eNOS activity and improved peripheral perfusion after simultaneous administration of LA, BH4 and ascorbic acid in a rat model of hind limb ischemia.20 A series of measurable treatment effects in our model, including a reduced PAP, suggest increased eNOS activity.
Taken together, our data suggest that the metabolic turnover rate of eNOS in the endothelium of a severely hypertensive pulmonary vasculature can be improved by the application of LA or BH4, but cannot be enhanced by a combined administration of both substances. The primary hypothesis of the present study, i.e. a pronounced synergistic effect of a combined administration of LA and BH4, could not be confirmed in a rat model of advanced PAH. Although both LA and BH4 significantly lowered PAP, our results do neither encourage a combined administration of LA and BH4 in PAH patients nor an initiation of a respective clinical trial.
Acknowledgments
The authors thank the nurses of the animal facility and Veronika Seidl for her excellent technical support in the histology and PCR lab.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
The project has been funded by the grant L-513 from the Austrian Science Fund (FWF) and a research fund from the Austrian Society for Cardiology.
References
- 1.Barst RJ, Channick R, Ivy D, et al. Clinical perspectives with long-term pulsed inhaled nitric oxide for the treatment of pulmonary arterial hypertension. Pulm Circ 2012; 2: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhotra R, Hess D, Lewis GD, et al. Vasoreactivity to inhaled nitric oxide with oxygen predicts long-term survival in pulmonary arterial hypertension. Pulm Circ 2011; 1: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schermuly RT, Janssen W, Weissmann N, et al. Riociguat for the treatment of pulmonary hypertension. Expert Opin Investig Drugs 2011; 20: 567–576. [DOI] [PubMed] [Google Scholar]
- 4.Sparacino-Watkins CE, Lai YC, Gladwin MT. Nitrate-nitrite-nitric oxide pathway in pulmonary arterial hypertension therapeutics. Circulation 2012; 125: 2824–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.List BM, Klösch B, Völker C, et al. Characterization of bovine endothelial nitric oxide synthase as a homodimer with down-regulated uncoupled NADPH oxidase activity: Tetrahydrobiopterin binding kinetics and role of haem in dimerization. Biochem J 1997; 323: 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993; 329: 2002–2012. [DOI] [PubMed] [Google Scholar]
- 7.Stasch J-P, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011; 123: 2263–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin LJ. Primary pulmonary hypertension. N Engl J Med 1997; 336: 111–117. [DOI] [PubMed] [Google Scholar]
- 9.Beyer J, Kolditz M, Ewert R, et al. L-arginine plasma levels and severity of idiopathic pulmonary arterial hypertension. Vasa 2008; 37: 61–67. [DOI] [PubMed] [Google Scholar]
- 10.Mehta S, Stewart DJ, Langleben D, et al. Short-term pulmonary vasodilation with L-arginine in pulmonary hypertension. Circulation 1995; 92: 1539–1545. [DOI] [PubMed] [Google Scholar]
- 11.Eddahibi S, Adnot S, Carville C, et al. L-arginine restores endothelium-dependent relaxation in pulmonary circulation of chronically hypoxic rats. Am J Physiol 1992; 263: L194–L200. [DOI] [PubMed] [Google Scholar]
- 12.Gross SS, Levi R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J Biol Chem 1992; 267: 25722–25729. [PubMed] [Google Scholar]
- 13.Shinozaki K, Nishio Y, Okamura T, et al. Oral administration of tetrahydrobiopterin prevents endothelial dysfunction and vascular oxidative stress in the aortas of insulin-resistant rats. Circ Res 2000; 87: 566–573. [DOI] [PubMed] [Google Scholar]
- 14.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 2004; 24: 413–420. [DOI] [PubMed] [Google Scholar]
- 15.Cunnington C, Channon KM. Tetrahydrobiopterin: Pleiotropic roles in cardiovascular pathophysiology. Heart 2010; 96: 1872–1877. [DOI] [PubMed] [Google Scholar]
- 16.Rochette L, Lorin J, Zeller M, et al. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol Ther 2013; 140: 239–257. [DOI] [PubMed] [Google Scholar]
- 17.Sibal L, Agarwal SC, Home PD, et al. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev 2010; 6: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böger GI, Rudolph TK, Maas R, et al. Asymmetric dimethylarginine determines the improvement of endothelium-dependent vasodilation by simvastatin. Effect of combination with oral L-arginine. J Am Coll Cardiol 2007; 49: 2274–2282. [DOI] [PubMed] [Google Scholar]
- 19.Henrohn D, Sandqvist A, Egeröd H, et al. Changes in plasma levels of asymmetric dimethylarginine, symmetric dimethylarginine, and arginine after a single dose of vardenafil in patients with pulmonary hypertension. Vascul Pharmacol 2015; 73: 71–77. [DOI] [PubMed] [Google Scholar]
- 20.Yan J, Tie G, Messina LM. Tetrahydrobiopterin, L-arginine and vitamin C act synergistically to decrease oxidative stress, increase nitric oxide and improve blood flow after induction of hindlimb ischemia in the rat. Mol Med 2012; 18: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamizu K, Shinozaki K, Ayajiki K, et al. Oral administration of both tetrahydrobiopterin and L-arginine prevents endothelial dysfunction in rats with chronic renal failure. J Cardiovasc Pharmacol 2007; 49: 131–139. [DOI] [PubMed] [Google Scholar]
- 22.Robbins IM, Hemnes AR, Gibbs JS, et al. Safety of sapropterin dihydrochloride (6r-bh4) in patients with pulmonary hypertension. Exp Lung Res 2011; 37: 26–34. [DOI] [PubMed] [Google Scholar]
- 23.Teerlink T, Nijveldt RJ, de Jong S, et al. Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography. Anal Biochem 2002; 303: 131–137. [DOI] [PubMed] [Google Scholar]
- 24.Leary S, Underwood W, Anthony R, et al. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. Schaumburg, IL: The American Veterinary Medical Assocation, 1931.
- 25.Nakayama Y, Nakanishi N, Sugimachi M, et al. Characteristics of pulmonary artery pressure waveform for differential diagnosis of chronic pulmonary thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol 1997; 29: 1311–1316. [DOI] [PubMed] [Google Scholar]
- 26.Strobl M, Schreiber C, Panzenböck A, et al. Exhaled nitric oxide measurement to monitor pulmonary hypertension in a pneumonectomy-monocrotaline rat model. Am J Physiol Lung Cell Mol Physiol 2013; 305: L485–L490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garvey W, Fathi A, Bigelow F, et al. A combined elastic, fibrin and collagen stain. Stain Technol 1987; 62: 365–368. [DOI] [PubMed] [Google Scholar]
- 28.Sahara M, Sata M, Morita T, et al. Nicorandil attenuates monocrotaline-induced vascular endothelial damage and pulmonary arterial hypertension. PLoS One 2012; 7: e33367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shu J, Dolman GE, Duan J, et al. Statistical colour models: An automated digital image analysis method for quantification of histological biomarkers. Biomed Eng Online 2016; 15: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitani Y, Maruyama K, Sakurai M. Prolonged administration of L-arginine ameliorates chronic pulmonary hypertension and pulmonary vascular remodeling in rats. Circulation 1997; 96: 689–697. [PubMed] [Google Scholar]
- 31.Sasaki S, Asano M, Ukai T, et al. Nitric oxide formation and plasma L-arginine levels in pulmonary hypertensive rats. Respir Med 2004; 98: 205–212. [DOI] [PubMed] [Google Scholar]
- 32.Francis BN, Hale A, Channon KM, et al. Effects of tetrahydrobiopterin oral treatment in hypoxia-induced pulmonary hypertension in rat. Pulm Circ 2014; 4: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyvelin JM, Howell K, Nichol A, et al. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 2005; 97: 185–191. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed LA, Obaid AAZ, Zaki HF, et al. Naringenin adds to the protective effect of l-arginine in monocrotaline-induced pulmonary hypertension in rats: Favorable modulation of oxidative stress, inflammation and nitric oxide. Eur J Pharm Sci 2014; 62: 161–170. [DOI] [PubMed] [Google Scholar]
- 35.Okada K, Tanaka Y, Bernstein M, et al. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol 1997; 151: 1019–1025. [PMC free article] [PubMed] [Google Scholar]
- 36.Resta TC, Chicoine LG, Omdahl JL, et al. Maintained upregulation of pulmonary eNOS gene and protein expression during recovery from chronic hypoxia. Am J Physiol 1999; 276: H699–H708. [DOI] [PubMed] [Google Scholar]