Abstract
Patients with idiopathic pulmonary arterial hypertension (IPAH) and a reduced diffusion capacity of the lung for carbon monoxide (DLCO) have a worse survival compared to IPAH patients with a preserved DLCO. Whether this poor survival can be explained by unresponsiveness to pulmonary hypertension (PH)-specific vasodilatory therapy is unknown. Therefore, the aim of this study was to evaluate the hemodynamic and cardiac response to PH-specific vasodilatory therapy in patients with IPAH and a reduced DLCO. Retrospectively, we studied treatment naïve hereditary and IPAH patients diagnosed between January 1990 and May 2015 at the VU University Medical Center. After exclusion of participants without available baseline DLCO measurement or right heart catheterization data and participants carrying a BMPR2 mutation, 166 participants could be included in this study. Subsequently, hemodynamics, cardiac function, exercise capacity, and oxygenation at baseline and after PH-specific vasodilatory therapy were compared between IPAH patients with a preserved DLCO (DLCO >62%), IPAH patients with a moderately reduced DLCO (DLCO 43–62%), and IPAH patients with a severely reduced DLCO (DLCO <43%). Baseline hemodynamics and right ventricular function were not different between groups. Baseline oxygenation was worse in patients with IPAH and a severely reduced DLCO. Hemodynamics and cardiac function improved in all groups after PH-specific vasodilatory therapy without worsening of oxygenation at rest or during exercise. Patients with IPAH and a severely reduced DLCO show a similar response to PH-specific vasodilatory therapy in terms of hemodynamics, cardiac function, and exercise capacity as patients with IPAH and a moderately reduced or preserved DLCO.
Keywords: diffusion capacity of the lung for carbon monoxide (DLCO), oxygenation, pulmonary arterial hypertension (PAH), right ventricular (RV) function
Introduction
Patients with pulmonary hypertension (PH) tend to have a mildly reduced pulmonary diffusion capacity for carbon monoxide (DLCO) compared to healthy participants.1 A severely reduced DLCO is most often seen in PH related to connective tissue disease, lung parenchymal disease, or in pulmonary veno-occlusive disease, but also in a subset of patients with idiopathic pulmonary arterial hypertension (IPAH) without signs of these underlying conditions.2–5 Recent studies revealed that IPAH patients with a low DLCO have a worse survival.6 Although it is yet unknown what causes the difference in survival, it has been argued that compared to other IPAH patients, IPAH patients with a severely reduced DLCO may have a distinct type of pulmonary vasculopathy that is less responsive to PAH-specific therapy.7,8
Therefore, the aim of this study was to compare the response to PAH-specific vasodilatory therapy in terms of hemodynamics, cardiac function, exercise capacity, and oxygenation between IPAH patients with different degrees of DLCO impairment.
Methods
Patient selection
We retrospectively studied treatment naïve hereditary and IPAH patients who were diagnosed between January 1990 and May 2015 at the VU University Medical Center. Part of this cohort was described in the study of Trip et al.5 A diagnosis of hereditary and IPAH was established by a multidisciplinary PH team, after rigorous clinical evaluation according to the ERS/ESC guideline (9).
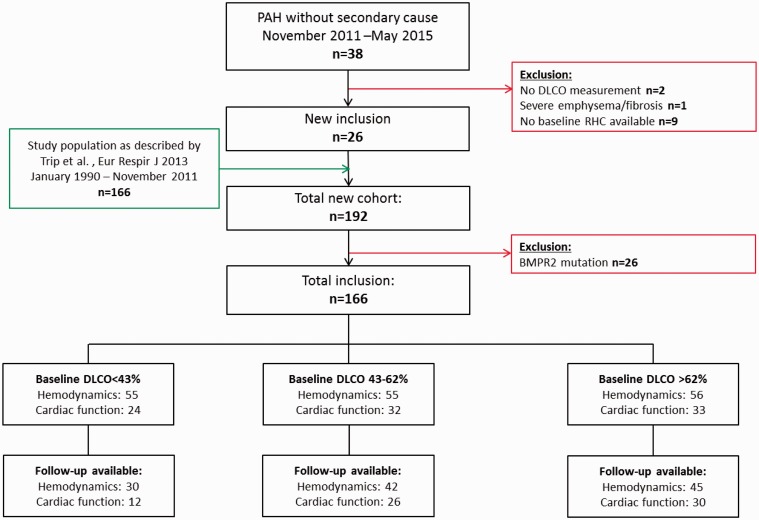
Participants without available baseline DLCO measurement, with severe emphysema or pulmonary fibrosis on high resolution computed tomography (HRCT) (5) were excluded from this study. Furthermore, to avoid clouding of the results, patients carrying a BMPR2 mutation were excluded from this study as recent studies showed the reduced life expectancy but preserved DLCO status in these participants.4,10 In total, 166 patients were included in this study (Fig. 1).
Fig. 1.
Flow chart. PAH, pulmonary arterial hypertension; RHC, right heart catheterization.
The cohort was divided into three groups using tertiles, leading to one group with a severely reduced DLCO (<43%), one group with a moderately reduced DLCO (43–62%), and one group with a preserved DLCO (>62%).
Right heart catheterization
Hemodynamics were assessed with a balloon-tipped, flow-directed 7.5-F triple lumen Swan-Ganz catheter (Edwards Lifesciences LLC, Irvine, CA, USA). Cardiac output measurements were performed using thermodilution or the direct Fick method.
Cardiac function and volumes
Right ventricular (RV) function and volumes were assessed using cardiac magnetic resonance imaging (CMRI). All scans were performed on a Siemens 1.5-T Sonata or Avanto scanner (Siemens Medical Solutions, Erlangen, Germany). Image acquisition and post-processing was done as described previously.11 Left and RV volumes were indexed to body surface area (BSA). Stroke volume (SV) and ejection fraction (EF) were calculated according to the following formulas, in which EDV = end-diastolic volume and ESV = end-systolic volume: SV = EDV–ESV and EF = (EDV–ESV)/EDV.
Six-minute walking test (6MWT)
6MWTs were performed according to the ATS guidelines.12,13 The distance walked (in meters) and the arterial oxygen saturation at rest and during exercise were measured at baseline and during follow-up.
Treatment response
Treatment responses were assessed by the baseline-to-follow-up responses in hemodynamics, cardiac function, and 6MWD. Time between baseline and follow-up was 1.9 ± 1.6 years in the DLCO < 43% group, 1.6 ± 1.2 years in the DLCO 43–62% group, and 2.1 ± 2.2 years in the DLCO >62% group (P = 0.860).
Statistical analysis
Data are presented as mean ± standard deviation (SD) unless stated otherwise. Comparisons of baseline hemodynamics, cardiac function, and the change in hemodynamic and cardiac function after PAH-specific therapies between the DLCO <43%, DLCO 43–62%, and the DLCO >62% groups were performed using one-way ANOVA with Bonferroni post-hoc corrections and Kruskal–Wallis tests with Dunn’s multiple comparisons post-hoc test as appropriate.
Kaplan–Meier analyses were performed to test for survival differences between the DLCO <43%, DLCO 43–62%, and the DLCO >62% groups in the entire cohort and the cohort in which a MRI at follow-up was present. Kaplan–Meier analysis was also performed to test for survival differences between the participants with and without a CMRI at follow-up in the DLCO <43% group. Subsequently, multivariable cox regression analyses were performed to correct the association between DLCO and survival for age differences.
Statistical analyses were performed using SPSS for Windows version 20 (IBM Corp., Armonk, NY, USA) and GraphPad Prism for Windows version 6 (GraphPad Software, Inc., San Diego, CA, USA). P values <0.05 were considered statistically significant.
Results
Characteristics of the DLCO <43%, DLCO 43–62%, and DLCO >62% patients are summarized in Table 1. A detailed characterization of the majority of patients was already given in our previous study.5
Table 1.
Baseline characteristics.
| DLCO <43% | DLCO 43–62% | DLCO >62% | P value | |
|---|---|---|---|---|
| General characteristics | ||||
| Age at diagnosis (years) | 65 ± 13 | 53 ± 18* | 48 ± 14† | <0.0001 |
| Male (%) | 58 | 18 | 27 | <0.0001 |
| 6MWT | ||||
| 6MWD (m) | 286 ± 136 | 366 ± 119* | 416 ± 134† | <0.0001 |
| 6MWD (% predicted) | 56 ± 23 | 70 ± 19* | 71 ± 23† | <0.01 |
| SaO2-rest (%) | 91 ± 4 | 94 ± 3* | 95 ± 2† | <0.0001 |
| SaO2-exercise (%) | 79 ± 7 | 89 ± 6* | 89 ± 6† | <0.0001 |
| ΔSaO2 (%) | −11 (−16 – 6) | −4 (−8 – 2)* | −5 (−10 – 2)† | <0.0001 |
| Laboratory tests | ||||
| NT-proBNP (ng·L) | 1004 (304–2487) | 802 (194–2888) | 555 (156–1887) | 0.45 |
| PCO2 (mmHg) | 30 ± 6 | 33 ± 7 | 33 ± 6 | 0.19 |
| PO2 (mmHg) | 61 ± 15 | 68 ± 11 | 72 ± 13† | <0.05 |
| SaO2 (%) | 91 ± 5 | 94 ± 3* | 94 ± 3† | <0.01 |
| Baseline hemodynamics | ||||
| HR (beats/min) | 80 ± 17 | 78 ± 15 | 80 ± 13 | 0.84 |
| mPAP (mmHg) | 48 ± 12 | 51 ± 14 | 52 ± 15 | 0.33 |
| mRAP (mmHg) | 7 (4–9) | 7 (4–11) | 8 (5–11) | 0.67 |
| PAWP (mmHg) | 10 ± 3 | 9 ± 4 | 8 ± 4 | 0.27 |
| PVR (dynes·s·cm–5) | 706 (540–1000) | 858 (476–1041) | 569 (441–961) | 0.56 |
| CI (L/min/m2) | 2.3 ± 0.7 | 2.5 ± 1.0 | 2.7 ± 0.9 | 0.18 |
| SvO2 (%) | 61 ± 9 | 63 ± 9 | 67 ± 9† | <0.01 |
| Baseline cardiac function and volumes | ||||
| LV EDVI (mL/m2) | 40 ± 10 | 41 ± 11 | 48 ± 13† | <0.05 |
| LV ESVI (mL/m2) | 15 ± 6 | 14 ± 6 | 18 ± 7‡ | <0.05 |
| LV EF (%) | 63 ± 10 | 66 ± 10 | 62 ± 10 | 0.21 |
| RV EDVI (mL/m2) | 76 ± 27 | 76 ± 17 | 88 ± 21 | 0.06 |
| RV ESVI (mL/m2) | 54 ± 26 | 50 ± 18 | 59 ± 23 | 0.31 |
| RV EF (%) | 33 ± 12 | 36 ± 12 | 35 ± 12 | 0.67 |
DLCO <43% significantly different compared to DLCO 43–62%.
DLCO <43% significantly different compared to DLCO >62%.
DLCO 43–62% significantly different compared to DLCO >62%.
6MWD, six minute walking distance; SaO2, arterial oxygen saturation; HR, heart rate; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance; CI, cardiac index; SvO2, mixed venous oxygen saturation; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVEF, left ventricular ejection fraction; RVEDVI, right ventricular end-diastolic volume index; RVESVI, right ventricular end-systolic volume index; RVEF, right ventricular ejection fraction.
Baseline measurements
Mixed venous oxygen saturation (SvO2), left ventricular end-diastolic volume index (LVEDVI), and left ventricular end-systolic volume index (LVESVI) were significantly lower in the DLCO <42% group compared to the DLCO >62% group. Apart from these, baseline hemodynamics and cardiac function were not different between the groups.
Exercise capacity, assessed by the 6MWT, was significantly lower in the DLCO <42% group compared to the moderately reduced and preserved DLCO group, as well as the arterial oxygen tension and saturation at rest. Furthermore, the DLCO <42% group showed a larger drop in arterial oxygen saturation during exercise (Table 1).
Treatment response
As can be appreciated from Table 2, the DLCO <42% group received more double-therapy, more prostacyclin monotherapy, and less endothelin receptor antagonist or phosphodiesterase type 5 inhibitor monotherapy. Interestingly, a significantly higher percentage of patients switched from monotherapy to combination therapy in the severely reduced DLCO group (Table 3).
Table 2.
PAH-specific medication during follow-up.
| DLCO <43% | DLCO 43–62% | DLCO >62% | P value | |
|---|---|---|---|---|
| Mono ERA/PDE5i (%) | 26.7 | 41.9 | 52.8 | <0.001 |
| Mono PGI2 (%) | 26.7 | 7.0 | 11.1 | <0.001 |
| Double: ERA+PDE5i (%) | 40.0 | 37.2 | 22.2 | <0.05 |
| Double: PGI2 +ERA/PDE5i (%) | 5.7 | 2.3 | 0 | <0.05 |
| Triple (%) | 0 | 2.3 | 8.3 | <0.01 |
| Calcium antagonist (%) | 0 | 9.3 | 5.6 | <0.01 |
ERA, endothelin receptor antagonist; PGI2, prostacyclin; PDE5i, phosphodiesterase type 5 inhibitor.
Table 3.
Treatment changes during follow-up (changes after an unsatisfactory response to previous treatment, hemodynamic, or clinical worsening).
| DLCO <43% | DLCO 43–62% | DLCO >62% | P value | |
|---|---|---|---|---|
| Monotherapy to combination therapy (%) | 32.0 | 20.0 | 11.1 | <0.01 |
| Monotherapy to triple therapy (%) | 0 | 0 | 2.8 | 0.05 |
| Combination therapy to triple therapy (%) | 0 | 2.5 | 2.8 | 0.22 |
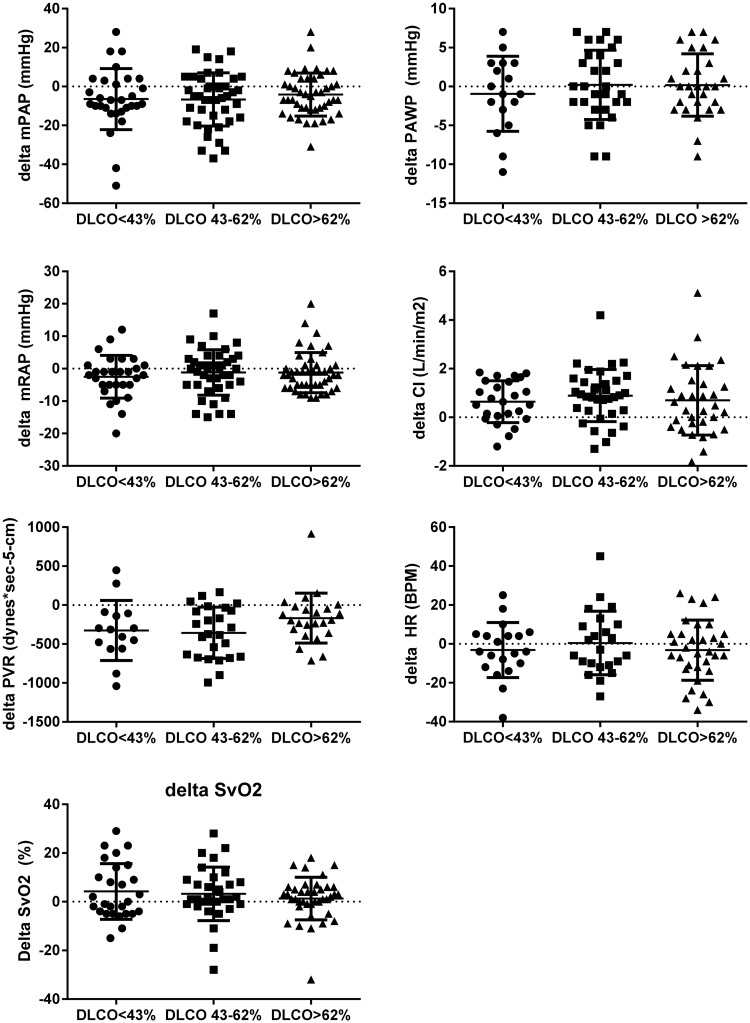
All groups showed a decrease in mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR), an increase in cardiac index (CI), and no change in pulmonary arterial wedge pressure (PAWP) and heart rate (HR) from baseline to follow-up (Fig. 2).
Fig. 2.
Hemodynamic treatment response. Data are presented as mean ± SEM. mPAP, mean pulmonary artery pressure; PAWP, pulmonary arterial wedge pressure; mRAP, mean right atrial pressure; PVR, pulmonary vascular resistance; CI, cardiac index; HR, heart rate; ns, non-significant.
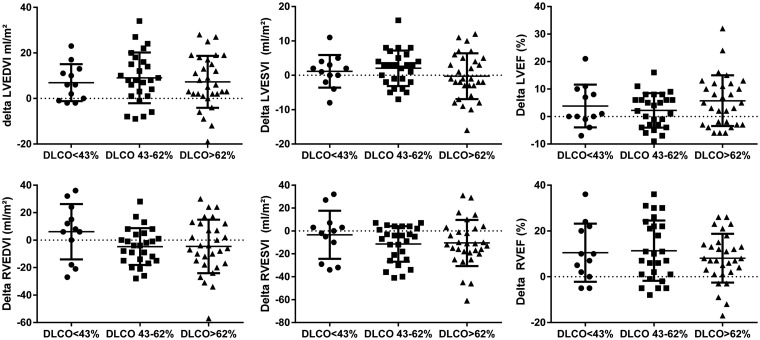
Cardiac responses are depicted in Fig. 3. Both groups showed no change in LVESVI. Delta LVEDVI and delta left ventricular ejection fraction (LVEF) did not differ between the three groups. Delta RVEDVI, RVESVI, and RVEF were similar between the severely reduced DLCO group, the moderately reduced group, and the group with a preserved DLCO.
Fig. 3.
Cardiac response to treatment. Data are presented as mean ± SEM. LVEDV, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVEF, left ventricular ejection fraction; RVEDVI, right ventricular end-diastolic volume index; RVESVI, right ventricular end-systolic volume index; RVEF, right ventricular ejection fraction.
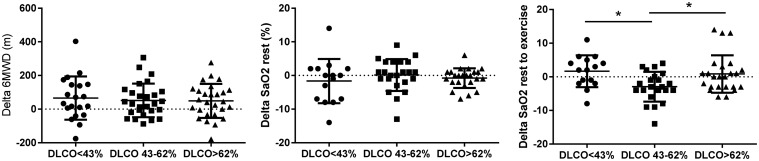
6MWD increased in all three groups after PH-specific vasodilatory therapy. Arterial oxygen saturation did not change from baseline to follow-up, while the moderately reduced DLCO group had a lower arterial oxygen saturation after exercise compared to the severely reduced DLCO and preserved DLCO group (Fig. 4).
Fig. 4.
Treatment response in oxygenation and 6MWD. Data are presented as mean ± SEM. 6MWD, 6 minute walking distance; SaO2 rest, arterial oxygen saturation in rest; SaO2 rest-ex, change in arterial oxygen saturation during exercise. *P < 0.05.
Survival analyses
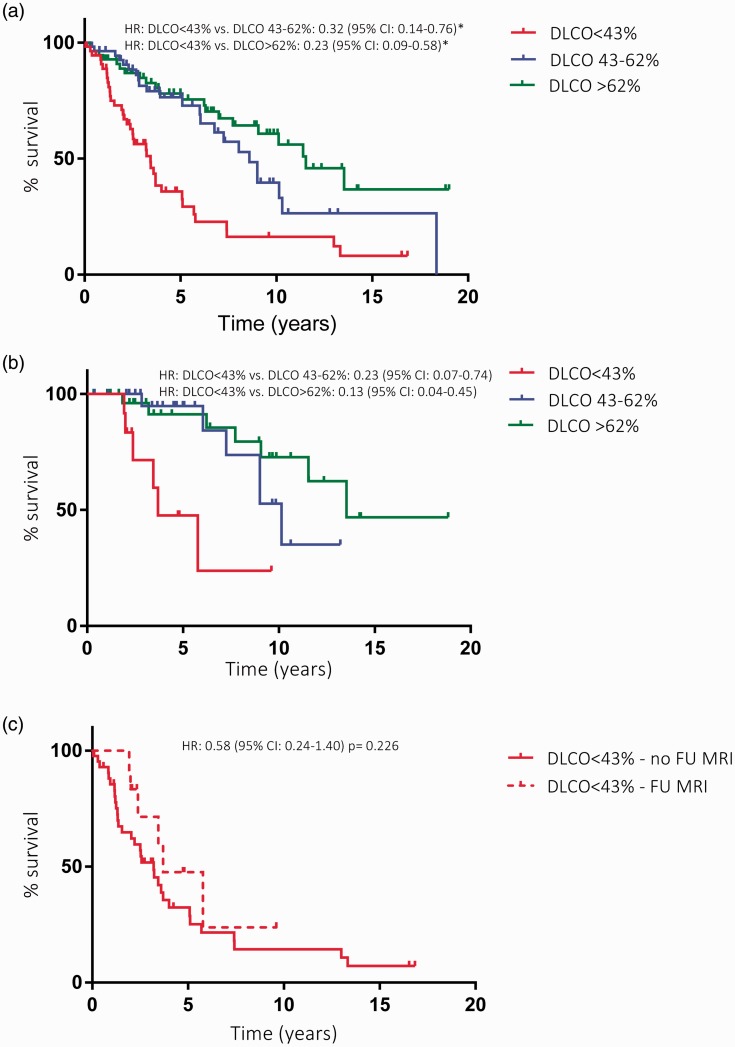
LVEDVI and LVESVI proved to be confounders to the survival analysis. LVEDVI and age-corrected survival was worse for patients with a DLCO <43% (Fig. 5a). These survival differences between DLCO <43% and DLCO 43–62% and DLCO >62% were also present in the selected cohort with a CMRI available at follow-up (Fig. 5b). No difference in survival was seen between patients with and without a CMRI at follow-up in the DLCO <43% group (Fig. 5c).
Fig. 5.
Survival analyses. (a) Difference in survival between the DLCO < 42%, DLCO 43–62%, and DLCO ≥63% groups in the total cohort (n = 166). The DLCO <42% group showed a worse survival than the moderately reduced DLCO and preserved DLCO groups. (b) Difference in survival between the severely reduced DLCO group, the moderately reduced DLCO group, and the preserved DLCO group in the cohort in which a CMRI at follow-up was available (n = 68). Also in this selected cohort, the DLCO <42% group showed a worse survival compared to other two groups. (c) Difference in survival between the group with and without a CMRI at follow-up in the DLCO <42% group (n = 42). No difference in survival was found between the group with and without a CMRI at follow-up in the DLCO <42% group. *Adjusted for age and left ventricular end diastolic volume index.
Discussion
In the present study, we evaluated the effects of PH-specific vasodilatory therapy in IPAH patients with a severely reduced DLCO. It is known that IPAH patients with a low DLCO have a worse survival compared to IPAH patients with a preserved DLCO.6 In addition, it has been shown that IPAH patients with a low DLCO have more coronary artery disease, a higher tobacco exposure, a higher body mass index, are older, have worse pulmonary function tests, and show more mild abnormalities on HRCTs compared to IPAH patients with a preserved DLCO.5 Although it seems that the IPAH patients with a low DLCO share some risk factors with group 2 and group 3 PH, normal PAWP pressures, spirometry, and HRCT excluded left heart conditions and lung disease as a cause of PH. In addition, HRCTs of the IPAH patients with a low DLCO showed no signs of PVOD.14,15 The question arises whether the low DLCO group has a different pulmonary vasculopathy compared to IPAH patients with a preserved DLCO.7,8 The answer to this question remains elusive and requires further investigation. As a first step, we analyzed the treatment response in this patient cohort.
Remarkably, we observed a significant improvement in hemodynamics, right ventricular (RV) function and exercise capacity upon PH-specific vasodilatory therapy without an impact on oxygen saturation in this cohort as in comparison to the IPAH patients with a moderately reduced or preserved DLCO.
Fewer follow-up data were available in the DLCO <43% group compared to the DLCO 43–62% and DLCO >62% groups, which could have led to a selection bias and subsequent overestimation of the treatment effect in the DLCO <43% group. However, no survival difference existed in the DLCO <43% group between the participants with and without available follow-up data. This to some extent suggests that the participants in the DLCO <43% group with follow-up data are representative for the total DLCO <43% group. Furthermore, survival differences between the DLCO <43% and the moderately reduced and preserved DLCO groups continued to exist when only the participants with follow-up data were entered in the survival analysis further arguing against the presence of an important selection bias. At follow-up, the DLCO <43% group received more combination therapy compared to the DLCO ≥43% groups. This may have confounded our results.
Based on the pulmonary vascular response on treatment, there is no reason to withhold PAH- specific treatment from patients with IPAH and a severely reduced DLCO. The similarities in hemodynamic and cardiac treatment responses between IPAH patients with a severely reduced DLCO and IPAH patients with a preserved DLCO suggests that the poor survival in the low DLCO group is not explained by unresponsiveness of the pulmonary vasculature to current PAH-specific medications. Survival differences may be partially explained by the fact that the DLCO <42% group was older.16 Cox proportional hazard analyses showed that age was a confounder for the differences in survival between groups; however, survival differences remained after adjusting for age. As such, the question remains why survival in this subgroup of patients with IPAH and a severely reduced DLCO is so poor.6
Conclusions
Patients with IPAH and a severely reduced DLCO show a similar response to PH-specific vasodilatory therapy as patients with IPAH and a moderately or preserved DLCO in terms of hemodynamics, RV function, exercise capacity, and oxygenation.
Supplementary Material
Conflict of interest
A. Vonk Noordegraaf reports receiving lecture fees from Actelion, Bayer, GlaxoSmithKline, Lilly, and Pfizer, industry advisory board from Actelion and Bayer, and serving on steering committees for Actelion, Bayer, and Pfizer.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Take home message
Patients with idiopathic pulmonary arterial hypertension (IPAH) and a severely reduced diffusion capacity of the lung for carbon monoxide (DLCO) show a similar response to pulmonary hypertension (PH)-specific vasodilatory therapy in terms of hemodynamics, cardiac function, and exercise capacity as patients with IPAH and a preserved DLCO.
References
- 1.Sun XG, Hansen JE, Oudiz RJ, et al. Pulmonary function in primary pulmonary hypertension. J Am Coll Cardiol 2003; 41: 1028–1035. [DOI] [PubMed] [Google Scholar]
- 2.Allanore Y, Borderie D, Avouac J, et al. High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Arthritis Rheum 2008; 58: 284–291. [DOI] [PubMed] [Google Scholar]
- 3.Montani D, Achouh L, Dorfmuller P, et al. Pulmonary veno-occlusive disease: clinical, functional, radiologic, and hemodynamic characteristics and outcome of 24 cases confirmed by histology. Medicine 2008; 87: 220–233. [DOI] [PubMed] [Google Scholar]
- 4.Trip P, Girerd B, Bogaard HJ, et al. Diffusion capacity and BMPR2 mutations in pulmonary arterial hypertension. Eur Respir J 2014; 43: 1195–1198. [DOI] [PubMed] [Google Scholar]
- 5.Trip P, Nossent EJ, de Man FS, et al. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J 2013; 42: 1575–1585. [DOI] [PubMed] [Google Scholar]
- 6.Chandra S, Shah SJ, Thenappan T, et al. Carbon monoxide diffusing capacity and mortality in pulmonary arterial hypertension. J Heart Lung Transplant 2010; 29: 181–187. [DOI] [PubMed] [Google Scholar]
- 7.Hoeper MM, Simon RGJ. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev 2014; 23: 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souza R, Fernandes CJ, Hoeper MM. Carbon monoxide diffusing capacity and the complexity of diagnosis in pulmonary arterial hypertension. Eur Respir J 2014; 43: 963–965. [DOI] [PubMed] [Google Scholar]
- 9.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 10.Evans JD, Girerd B, Montani D, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med 2016; 4: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2511–2519. [DOI] [PubMed] [Google Scholar]
- 12.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117. [DOI] [PubMed]
- 13.Erratum: ATS Statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2016; 193: 1185. [DOI] [PubMed]
- 14.Resten A, Maitre S, Capron F, et al. [Pulmonary hypertension: CT findings in pulmonary veno-occlusive disease]. J Radiol 2003; 84: 1739–1745. [PubMed] [Google Scholar]
- 15.Resten A, Maitre S, Humbert M, et al. Pulmonary hypertension: CT of the chest in pulmonary venoocclusive disease. AJR Am J Roentgenol 2004; 183: 65–70. [DOI] [PubMed] [Google Scholar]
- 16.Hoeper MM, Huscher D, Ghofrani HA, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol 2013; 168: 871–880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.