Abstract
Development of the pulmonary circulation is a complex process with a spatial pattern that is tightly controlled. This process is vulnerable for disruption by various events in the prenatal and early postnatal periods. Disruption of normal pulmonary vascular development leads to abnormal structure and function of the lung vasculature, causing neonatal pulmonary vascular diseases. Premature babies are especially at risk of the development of these diseases, including persistent pulmonary hypertension and bronchopulmonary dysplasia. Reactive oxygen species play a key role in the pathogenesis of neonatal pulmonary vascular diseases and can be caused by hyperoxia, mechanical ventilation, hypoxia, and inflammation. Besides the well-established short-term consequences, exposure of the developing lung to injurious stimuli in the perinatal period, including oxidative stress, may also contribute to the development of pulmonary vascular diseases later in life, through so-called “fetal or perinatal programming.” Because of these long-term consequences, it is important to develop a follow-up program tailored to adolescent survivors of neonatal pulmonary vascular diseases, aimed at early detection of adult pulmonary vascular diseases, and thereby opening the possibility of early intervention and interfering with disease progression. This review focuses on pathophysiologic events in the perinatal period that have been shown to disrupt human normal pulmonary vascular development, leading to neonatal pulmonary vascular diseases that can extend even into adulthood. This knowledge may be particularly important for ex-premature adults who are at risk of the long-term consequences of pulmonary vascular diseases, thereby contributing disproportionately to the burden of adult cardiovascular disease in the future.
Keywords: pulmonary circulation, lung development, pulmonary vascular disease, oxygen, oxidative stress
The development of the pulmonary vasculature is a highly complex process, in which temporal and spatial expression of multiple morphogens, transcription factors, and growth factors regulates the different stages of development.1–3 This complex process of normal lung development, with its tight regulation of the expression of numerous regulators, can be disrupted at multiple levels and in various stages of development. Such disruption of normal development of the pulmonary vasculature plays a pivotal role in the pathogenesis of several neonatal pulmonary vascular diseases, including persistent pulmonary hypertension of the newborn (PPHN) and bronchopulmonary dysplasia (BPD). Understanding the response of the developing lung to injury, and its repair mechanisms, is of great importance for elucidating pathogenic processes.
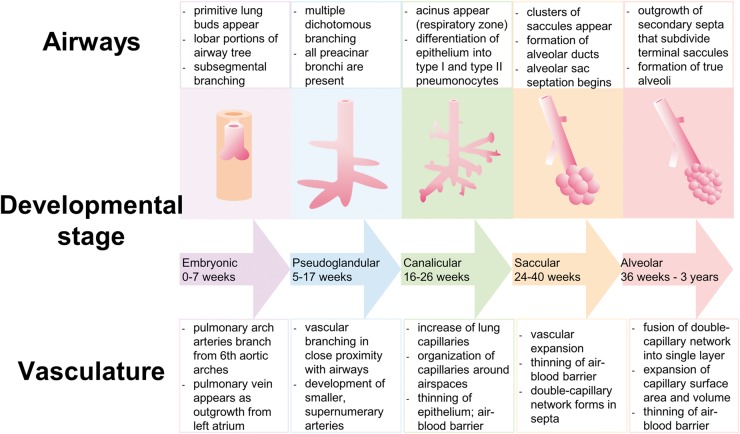
The development of the lung starts with the appearance of a primitive lung bud, which splits to form the left and the right lung. In the human embryo, at day 34 of gestation, each lung bud is already supplied by a pulmonary artery extending from the outflow tract of the heart, which is connected to a primary capillary plexus and traced back via a vein to the prospective left atrium.2,4,5 Lung development continues in five different stages (embryonic, pseudoglandular, canalicular, saccular, and alveolar). The alveolar stage, when the gas-exchanging surface area develops, starts at week 36 of gestation and continues after birth, even up to the third year of life (Fig. 1).2,5,6
Fig. 1.
Diagram illustrating normal lung development: airway structure and pulmonary vasculature.
The development of the pulmonary vasculature is closely related to that of the airways, as they develop at the same time and follow the same spatial pattern (Fig. 1). Growing evidence exists that tissue interactions of lung mesenchyme, epithelium, and endothelium are critical for both branching of the airways and growth and differentiation of the pulmonary vasculature.4,5,7,8
The formation of the pulmonary vasculature is governed by two principal mechanisms: vasculogenesis and angiogenesis. Vasculogenesis is the process by which angioblasts or endothelial progenitor cells differentiate to vascular endothelial cells and form blood vessels de novo. In angiogenesis, new blood vessels arise by direct extension of pre-existing vessels.8–10 The relative contribution of vasculogenesis and angiogenesis to vessel formation in the developing lung is still incompletely understood, but three hypotheses have been forwarded.10 One hypothesis is that the proximal vasculature develops through angiogenesis, while the distal vessels are formed by vasculogenesis. In the pseudoglandular phase, these two structures then fuse through a lytic process.11 The second hypothesis proposes that new arteries are derived from a continuous expansion and coalescence of the primary capillary plexus around the terminal airways, and thus principally from vasculogenesis.4 The third hypothesis proposes that lung vascular development occurs through distal angiogenesis, through the formation of new capillaries from pre-existing vessels at the periphery of the lung as the lung bud grows.12
Pulmonary vascular development is regulated by interplay between many different factors. Although a lot of knowledge about the pulmonary vasculature development is obtained through experiments in rodents, we will focus on vascular endothelial growth factor (VEGF) and transforming growth factor-beta (TGF-β), because their roles have also been confirmed in human samples. Among all different regulators of lung vascular development, VEGF is a frequently studied and crucial regulator of normal pulmonary vascular development. VEGF induces angiogenesis and is a key player in the regulation of vasculogenesis.13 VEGF exerts its effect by binding to two trans-membrane tyrosine-kinase receptors, VEGFR-1 (Flt-1) and VEGFR-2 (Flk-1), which are strongly expressed in endothelial cells. In addition to its mandatory role in the development of the pulmonary vasculature, VEGF also plays an important role in epithelial branching morphogenesis and alveolar development.14,15 VEGF expression is regulated by hypoxia-inducible factors (HIF)-1 and 2, which are transcriptional complexes responding to changes in oxygen levels. Normal lung development takes place in the relatively hypoxic environment of the uterus. This “Everest in utero” stabilizes the HIF complex, leading to transcription of hypoxic responsive target genes, such as VEGF, thereby stimulating epithelial branching and vascular development.16–18
Another important growth factor in normal pulmonary vascular development is transforming growth factor-beta (TGF-β). The exact role of TGF-β signaling in lung development is not known yet. However, it appears to play a key role in epithelial–mesenchymal interactions as well as endothelial–mesenchymal interactions. Thus, tightly regulated temporal and spatial TGF-β signaling is necessary for both normal branching morphogenesis and pulmonary vascular development.19–21 A role for TGF-β signaling in pulmonary vascular development is further underlined by the observation that mutations in the bone-morphogenetic protein receptor II (BMP-RII), which lead to a disturbed balance between TGF-β -and BMP-signaling, as well as in endoglin, a co-receptor of the TGF-β receptors, are associated with pulmonary arterial hypertension.22,23
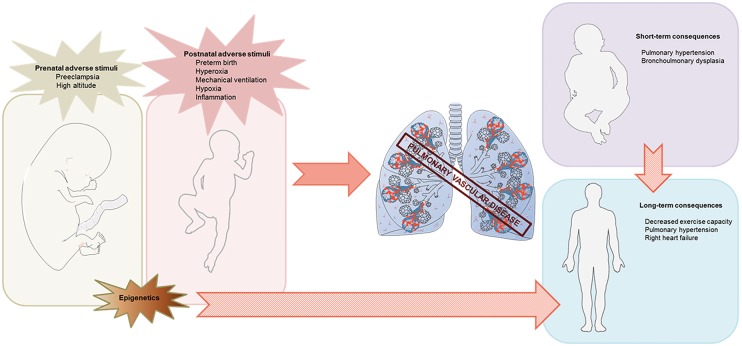
In this review, we will focus on several pathophysiologic insults in the prenatal and early postnatal periods, which can disrupt normal pulmonary vascular development and, consequently, lead to a variety of neonatal pulmonary vascular diseases that can extend even into adulthood (Fig. 2). Most of these insults cause oxidative injury of the pulmonary vasculature, which will therefore be discussed in more detail. Babies that are born prematurely are particularly vulnerable to disruptions in the pulmonary vascular development. As improved neonatal care has dramatically improved the survival of these babies, there is a growing cohort of young adults that were born preterm. Knowledge about pathophysiological processes in the developing lung may be particularly important for these ex-premature adults who are at higher risk for the long-term consequences of pulmonary vascular diseases, thereby contributing disproportionately to the future burden of adult cardiovascular disease.24,25
Fig. 2.
Schematic illustrating perinatal adverse stimuli contributing to pulmonary vascular disease that can extend even into adulthood.
Oxidative injury in early life
Reactive oxygen species (ROS) are oxygen-derived metabolites and can be subdivided into free radicals and oxidants. Examples of free radicals, defined as atoms or molecules that contain unpaired electrons, are superoxide, nitric oxide, and hydroxyl- and peroxyl-radical. Oxidants, such as hydrogen peroxide, peroxynitrite, and lipid peroxide, do not contain an unpaired electron and are therefore not free radicals, but are highly reactive oxygenated molecules that can easily lead to free radical reactions.26–30 Because of their highly reactive nature, ROS can react with various intracellular proteins and alter their structure and function.
Small amounts of ROS are continuously produced in the human body, mainly during ATP production in the mitochondria, and have been shown to play a role in many signaling processes.29–34 However, in order to prevent deleterious effects of ROS and to maintain proper cellular function, the amount of ROS needs to be carefully controlled. Protection of the cells against oxidative damage is ensured by cellular antioxidants, which include endogenous antioxidants such as superoxide dismutase, catalase, glutathione peroxidase, and several metal-binding proteins (transferrin, ferritin, and albumin). Furthermore, there are exogenous antioxidants present in food or dietary supplements like vitamins C and E.29–34
Oxidative stress occurs when antioxidant defense mechanisms are insufficient to cope with ROS production, either through increased production of ROS or through insufficient presence of antioxidants. Oxidative stress causes degradation of lipids, protein damage, and even DNA damage.27,28,30,35–38 At birth, all newborns are exposed to a sudden increase in oxygen tension compared to the hypoxic environment in utero, leading to increased ROS production. Also, several perinatal adverse events induce a high exposure to ROS, as described below in more detail. Neonates, and especially those who are born premature, are particularly vulnerable to oxygen toxicity, as their levels of antioxidant enzymes are inadequate and unable to protect the rapidly growing tissues, including the developing lung, from oxidative injury.28,29,38–40 The endothelial cells and the alveolar type II cells especially are extremely susceptible to oxidative injury. Activation of transcription factors and pathways by oxidative stress lead to inactivation of surfactant, cellular dysfunction, and impaired cell survival.3,39–42 Thus oxidative injury plays a critical role in the pathogenesis and pathophysiology of neonatal pulmonary vascular disease such as BPD.43,44
Prenatal adverse stimuli
Placental hypoxia
Normal perfusion and function of the placenta is essential for the fetus, as the placenta is ultimately responsible for oxygen and nutrient supply to the fetus. A reduction in the placental perfusion, and consequently placental hypoxia, is associated with pre-eclampsia and intrauterine growth retardation (IUGR).45,46
Pre-eclampsia is the most common maternal complication of pregnancy, characterized by hypertension, edema, and proteinuria. It is a frequent cause of IUGR and premature birth, which are both risk factors for neonatal pulmonary vascular diseases.47,48 Central to the pathogenesis of pre-eclampsia is placental hypoperfusion and/or inflammation, resulting in oxidative stress and the release of vasoactive factors by the diseased and hypoxic placenta.
It has been shown that ROS are elevated and antioxidant levels are decreased in the maternal circulation in pre-eclampsia.27,49–51 Studies on the oxidative stress in infants born to mothers with pre-eclampsia have reported conflicting outcomes. Some papers show an increased antioxidant status, probably protecting the fetus against the maternal oxidative stress.52–54 Others report elevated levels of ROS and decreased levels of antioxidants in neonates of pre-eclamptic mothers, contributing to “oxygen radical disease of neonatology,” including BPD.42,55,56 The increased maternal oxidative stress and ensuing endothelial dysfunction are thought to aggravate to placental hypoperfusion and impact on the release of vasoactive factors by the diseased and hypoxic placenta.57,58
Placental hypoxia induces an imbalance between pro-angiogenic and anti-angiogenic factors. Thus, there is an increased placental production of soluble fms-like tyrosine kinase-1 (sFlt-1 or soluble VEGF-receptor 1) and soluble endoglin (sEng).46,59,60 Elevated levels of sFlt-1 scavenge free VEGF, thereby inhibiting VEGF signaling.46,59–62 Similarly, elevated levels of sEng interfere with the TGF-β pathway. sFlt-1 and sEng are thought to act synergistically in the development of the maternal complications of pre-eclampsia.59 However, both sFlt-1 and sEng are also present in amniotic fluid and may thereby affect the developing fetus. Indeed, elevated levels of sEng in amniotic fluid during preterm labor were associated with development of BPD in the infants.63 In contrast, amniotic sFlt-1 levels during mid-term gestation in humans were not predictive for the development of pulmonary vascular disease in their infants,62 although it has recently been shown that elevated levels of intra-amniotic sFlt-1 lead to reduced VEGF signaling in the developing rat lung, resulting in impaired pulmonary vascular growth and alveolarization in newborn rat-pups.61 Altogether, this suggests that prenatal exposure to high sEng and sFlt-1 levels may compromise normal fetal lung development and may be responsible for the increased risk of pulmonary vascular disease in preterm born neonates of pre-eclamptic mothers.14,61
High altitude
It is well-known that living at high altitudes poses a major challenge to the human body. In adults, residing at high altitudes can cause altitude-specific disorders, such as acute and chronic mountain sickness, high-altitude pulmonary edema, and symptomatic high-altitude pulmonary hypertension. These conditions can be considered a direct result of exposure to hypobaric hypoxia.64,65
In pregnant high-altitude residents, maternal exposure to hypoxia can negatively influence the oxygen delivery to the fetus and thereby hamper the development of the fetus. For example, it has been shown that pre-eclampsia is more common at high altitudes than at low altitudes. Also, hypoxia is a key factor responsible for lower birth weights and IUGR in newborns at high altitudes, independently of the presence of pre-eclampsia.64,66,67 This may, at least in part, be due to altered function of the placenta due to hypoxia. Indeed, it has been shown that HIF-1 expression is increased in placentas from high-altitude residents and that these placentas contain more TGF-β3 as well as VEGF and sFlt-1,68,69 which may spillover into the fetal circulation and may impact on the developing pulmonary vasculature.
Because oxygen plays a crucial role in the perinatal period, the perinatal cardiopulmonary transition proceeds more slowly in babies that are born under conditions of high-altitude hypoxia. It has been shown that such infants have lower arterial oxygen saturations and that the physiologically rapid fall in pulmonary artery pressure after birth does not occur. This can ultimately lead in adult life to symptomatic high-altitude pulmonary hypertension, which is the condition of pulmonary hypertension accompanied by muscularization of the pulmonary arteries due to hypoxic vasoconstriction and vascular remodeling. In terminal stages of the disease, this results in right heart failure.70,71 Another sign of disrupted perinatal transition in these infants is the persistence of the fetal vascular connections (ductus arteriosus and foramen ovale).71
An important observation is that the incidence of BPD is also increased at higher altitudes, the mechanism of which is currently unknown. It has been hypothesized that the exposure of the preterm infant to the hypoxic environment causes injury to the lung in a critical stage of development. Moreover, IUGR can play a role in the higher risk of BPD as well.72
In summary, although it is well-established that neonatal pulmonary vascular disease is more common in high-altitude pregnancies, the effect of high-altitude residence during gestation on the prenatal pulmonary vascular development and the exact underlying molecular and/or biochemical mechanisms are still incompletely understood in humans. Animals studies, however, have implicated a higher vasoconstrictor reactivity of the pulmonary small arteries.73–75
Postnatal adverse stimuli
Hyperoxia
The development and maturation of the fetal organs normally takes place under hypoxic conditions in the uterus. Preterm birth leads to premature transition of the pulmonary circulation from the hypoxic fetal environment to a relative hyperoxic postnatal environment (air). For adequate functioning of the body’s tissues and organs, in particular the brain, intestines, and kidneys, sufficient oxygenation of these tissues is required. Although the optimal systemic oxygen saturation in preterm infants is currently unknown, the consensus is that systemically circulating oxygen saturation levels need to be targeted above 85% to fulfill the oxygen demands of the body. Because of the incomplete lung development in these infants, with simplified alveolar structure and thick alveolar septae, oxygen diffusion is hampered. To compensate for these diffusion abnormalities, often high levels of supplemental oxygen are required to increase alveolar oxygen tension and the diffusion gradient in order to achieve the targeted intravascular oxygen saturation levels. This high level of oxygen supplementation further augments the already existing (relative) hyperoxic state postnatally. The rapid alteration in oxygen concentration at birth results in changes in oxygen sensitive molecular mechanisms.3,41,76,77 Under normoxic and/or hyperoxic conditions rapid proteasomal degradation of the HIF-1α subunit occurs and thus binding to the promotor regions of target genes is hampered.16–18,41 This leads to impaired VEGF expression, resulting in disrupted angiogenesis and alveolarization. So, (relative) hyperoxia induces vascular arrest, leading to pulmonary vascular diseases.
Relative hyperoxia also increases generation of ROS and induces oxidative stress, an important contributor to the development of neonatal pulmonary vascular disease. As outlined above, preterm infants are more prone to oxidative injury, due to lower levels of antioxidants including vitamin E, transferrin, and superoxide dismutase, and higher levels of free iron leading to the production of hydroxyl radical.28,29,39,78,79
Mechanical ventilation
Mechanical ventilation is essential and life-saving in the treatment of severely premature infants. Yet mechanical ventilation can also provoke ventilator-induced lung injury (VILI). The main mechanism resulting in VILI is over-distension of the lung, thereby over-stretching of the distal epithelium and capillary endothelium, which increases microvascular permeability, inhibits surfactant production and leads to the release of cytokines into the alveolar space and the systemic circulation.3,80–83 The type and duration of mechanical ventilation as well as the volume and pressure that is used are contributing factors to the development of VILI.81 Furthermore, the developmental stage of the lung, and thus gestational age at birth, is an important determinant of VILI. The lung in the alveolar stage can expand extensively without any stretch injury, while the more immature saccular lung has less surface area to expand and is more injury prone to stretch.83
Mechanical ventilation does not only directly injure the neonatal lung through overdistension, it also results in alterations in angiogenesis-related factors. Thus, VEGF-1 and its receptor flt-1 as well as angiopoietin 1 and its receptor Tie2 are downregulated while the TGF-β co-receptor endoglin is upregulated, in lungs of infants that were mechanically ventilated.84,85 The imbalance in angiogenic factors likely contributed to dysmorphic angiogenesis and altered alveolarization observed in mechanically ventilated lungs.84,85
Hypoxia
In addition to periods of hyperoxia, due to premature perinatal transition (relative hyperoxia) and the need of supplemental oxygen, premature babies are exposed to chronic or intermittent hypoxia. Hypoxia can be caused by different mechanisms. Often, hypoxia is the result of immature lungs or occurs in the setting of apnea of prematurity. It can also be caused by inadequate ventilation of the preterm infant.41,77 In infants born extremely premature, studies suggest the persistence of intrapulmonary arteriovenous shunts, which are physiologically present in the fetus and normally regress in the early neonatal phase. Beside the immaturity of the lung in premature born babies, a high pulmonary vascular resistance can also prevent the regression of intrapulmonary arteriovenous shunts. These shunts bypass the alveolar capillary gas exchange units and therefore cause hypoxemia in the neonate.14,86,87
In contrast to the vessels of the systemic circulation that dilate in response to hypoxia, the pulmonary vasculature constricts. This so called hypoxic pulmonary vasoconstriction (HPV) is an important physiologic mechanism to ensure ventilation-perfusion matching by preventing blood flow to areas of the lung that are not well-ventilated, thereby optimizing systemic oxygenation. The exact mechanisms underlying HPV remain incompletely understood, but animal studies show a critical role of ROS in HPV.88–91 The “redox theory” states that precapillary pulmonary arterial smooth muscle (PASM) cells are the oxygen-sensing cells as well as the effector cells.91–95 Mitochondria in the PASM cells senses a drop in alveolar O2 and respond by creating a signal that alters opening of redox-sensitive potassium and calcium channels, thereby increasing vascular tone.91–95 However, it is not yet resolved if increased or reduced levels of ROS during hypoxia underlie the signal transduction of HPV.92–95 During general hypoxia, as seen in premature infants, generalized pulmonary vasoconstriction occurs, which results in an increase in pulmonary vascular resistance and hence pulmonary hypertension. When sustained, hypoxic vasoconstriction produces vascular remodeling of the pulmonary vascular bed and, ultimately, leads to right heart failure.92,93
Besides hypoxic vasoconstriction, it is well-known from animal studies that hypoxia interferes with the physiological process of alveolarization. In healthy newborns, a large part of this process takes place after birth in a normoxic environment (ambient air, 21% oxygen).2,5,6 Postnatal exposure to hypoxia has been shown to impair alveolarization, resulting in alveolar simplification with fewer and larger alveoli. Since the airway and vascular development and maturation are closely related, impaired alveolarization also impairs the vascular maturation in the alveolar wall. Both perturbed signaling of HIF-1α, VEGF, as well as TGF-β, mediate these disruptions.41,77,96
Inflammation
Postnatal exposure to intermittent hypoxia and hyperoxia induces oxidative stress, which—in premature infants—is insufficiently reduced due to immature anti-oxidant mechanisms. As a result of direct cellular injury, oxidation of DNA, induction of cytokines, and recruitment of neutrophils and macrophages to the lung, oxidative stress induces pulmonary inflammation. In addition to oxygen-free radicals, mechanical ventilation also triggers pulmonary inflammation. Vice versa, oxygen radicals are rapidly released by immune cells with the oxidative burst, a crucial reaction in the immune system.42 Infiltration of inflammatory cells in the immature lung, and the release of ROS, results in endothelial and epithelial cell injury. Interestingly, the pro-inflammatory cytokine IL-8 is increased and the anti-inflammatory cytokine IL-10 is decreased in serum of preterm infants that subsequently developed BPD,97–99 suggesting that indeed a balance between pro-inflammatory and anti-inflammatory cytokines is required for normal lung development. The exact role of oxygen tension in perinatal inflammation is currently unknown and should be the topic of future investigation.41,100–102
Consequences
Short-term consequences
Both premature birth—with incomplete vascular growth, immature vascular function, and decreased host defenses—as well as exposure to injurious stimuli after birth, contribute to an abnormal development of the lung circulation. Failure of normal lung development will lead to neonatal pulmonary vascular disease due to an altered function of the pulmonary vessels (with an increased vasomotor tone), as well as an altered structure of the pulmonary vasculature, i.e. vascular remodeling (including smooth muscle cell proliferation).10,41,103,104
Both these functional and structural changes elevate pulmonary vascular resistance by narrowing vessel diameter and by decreasing vascular compliance, leading to pulmonary hypertension.10,14,41,103–106 Furthermore, disruption of normal pulmonary vascular development leads to an arrest in the development of the airways. BPD, a common complication of preterm birth, is recognized as a consequence of disrupted lung development. It is characterized by an arrest in vascular and alveolar growth, which leads to decreased and enlarged alveoli and a decrease in number of capillaries as compared to a normal lung.15,107–110 Besides morbidity and mortality in the neonatal period, BPD is associated with a variety of long-term health problems including reduced lung function, cognitive impairments, cardiovascular dysfunction, and exercise intolerance.111
Long-term consequences
The “developmental origins of health and disease” (DOHaD) concept112 has gained a great deal of attention in recent years, especially in pediatrics because of the dramatically increased survival of premature babies. Since approximately 10% of births are preterm, a growing cohort of prematurely born survivors reaches adolescence.113–115 While the majority of research in this field has focused on the developmental origins of metabolic disease, now there is growing evidence that disruption of normal pulmonary vascular development in the perinatal period contributes to the development of (pulmonary) vascular disease in adulthood. In the late 1990s, it was already shown that a transient perinatal insult to the pulmonary circulation increases the risk of developing pulmonary hypertension.116 It has also been shown that pulmonary artery pressure is elevated in offspring of mothers with pre-eclampsia, demonstrating that placental hypoxia causes pulmonary vascular dysfunction.117 Underlying mechanisms of this so-called “fetal or perinatal programming” are currently unknown, although oxidative stress has been proposed to play a key role. As described above, many perinatal insults are associated with oxidative injury. Reactive molecules can cause epigenetic changes by inducing DNA methylation and histone modification. Modulation of epigenetic modifications during this sensitive developmental period will alter organogenesis and organ function, thereby producing long-term programmed consequences.118–121 In view of the growing awareness of the long-term consequences of neonatal pulmonary vascular disease, it will become clinically more important to routinely screen high-risk (ex-premature) patients.
An important diagnostic tool in this patient population is exercise testing. By placing the cardiopulmonary system under stress with exercise testing, subtle dynamic abnormalities that are not apparent during conventional static tests may be revealed. Studies evaluating exercise capacity in long-term survivors of prematurity have reported highly variable results, with some research groups reporting no evidence of exercise limitation,122–127 while other investigators demonstrated significantly impaired exercise performance in former preterms.128–138
Exercise capacity is a resultant of pulmonary function and cardiovascular performance.139,140 Until now, most studies concerning long-term health outcomes of (extremely) premature infants have focused on respiratory outcomes. Indeed it is well-known that pulmonary function in childhood and adolescence is impaired in these patients, and this is even more pronounced in survivors of neonatal pulmonary vascular diseases like BPD.110,113,141–144
Less is known about cardiovascular function in survivors of neonatal pulmonary vascular disease. Exposure of the immature pulmonary vasculature to injurious stimuli after birth can potentially result in remodeling of the pulmonary vascular bed, in endothelial dysfunction, pulmonary hypertension, and, finally, right ventricular failure. A recent cardiac magnetic resonance imaging (MRI) study demonstrated that young adults, born preterm, have smaller right ventricular lumen size and greater mass, resulting in right ventricular dysfunction.145 Although less pronounced, adverse changes have also been shown in the left ventricle.146 These alteration in cardiac function and structure may increase the risk for cardiovascular events later in life, thereby contributing disproportionately to the burden of adult cardiovascular disease in the future.115,141,145,146
Clinical implications
Oxygen therapy is a cornerstone in the treatment of premature infants and is crucial for their survival. However, as outlined above, too much oxygen, or hyperoxia, causes injury and damage to several tissues including the lung. Paradoxically, hypoxia also interrupts normal lung vascular development. Therefore, both hyperoxia and hypoxia, together or independently, can lead to pulmonary vascular disease. Consequently, there is uncertainty about the optimal target of oxygen saturation in (prematurely born) neonates. A recently published systematic review and meta-analysis concluded that infants (born <28 weeks of gestation) cared for with a liberal saturation target (SpO2 91–95%) had significantly lower mortality before hospital discharge than infants cared for with a restricted oxygen target (SpO2 85–89%), although the quality of evidence for this estimate of effect was low. No significant difference was found for the incidence of BPD.147–149
In view of the growing cohort of adult survivors of prematurity and/or neonatal vascular disease, more research into the long-term consequences of perinatal pulmonary vascular events is imperative. Since this is a relatively new patient population, there is a lack of consensus for the follow-up of high-risk (formerly premature) patients. The American Heart Association and American Thoracic Society have made a guideline for diagnosis, evaluation, and monitoring of pediatric patients with pulmonary hypertension.150 They recommend monitoring of children with pulmonary hypertension (or neonatal pulmonary vascular disease) provided by comprehensive, multidisciplinary team of pulmonologists, cardiologists, neonatologists, anesthesiologists, and experienced nurses. Children with chronic diffuse lung disease should be evaluated for concomitant cardiovascular disease or pulmonary hypertension by echocardiogram every 3–6 months. The 6-min walk distance (6MWD) test or cardiopulmonary exercise test (CPET) can be useful to monitor exercise tolerance. MRI can be performed to assess right ventricular function and structure.150 These diagnostic tools could be very useful in the development of a follow-up program that might facilitate an optimal transition of the patient from the pediatric to the adult setting to ensure strong continuity of care to optimize clinical outcome. It is a future challenge to evolve a follow-up program for (neonatal) pulmonary vascular disease that ensures early detection of health problems in these patients, thereby diminishing the cardiovascular morbidity and mortality and improving the quality of life. Furthermore, the development of an exercise training program tailored for prematurely born adolescents, who may be at higher risk for early-onset adult diseases, should be considered. Increasing the level of regular physical activity has beneficial effects on overall health and plays an important role in the prevention of diseases in the long term. Establishing early, adequate levels of fitness and activity should therefore be a cornerstone in the follow-up of formerly premature adults.
Conclusions
Both antenatal and postnatal injurious stimuli can disrupt the normal lung vascular development, potentially leading to neonatal pulmonary vascular diseases entities such as BPD and pulmonary hypertension. These diseases not only contribute to morbidity and mortality in the neonatal period, but have also been shown to significantly increase the risk for a variety of health problems later in life. Pulmonary vascular disease can lead to endothelial dysfunction, vascular remodeling, and ultimately to cardiac dysfunction (right ventricular hypertrophy and failure).
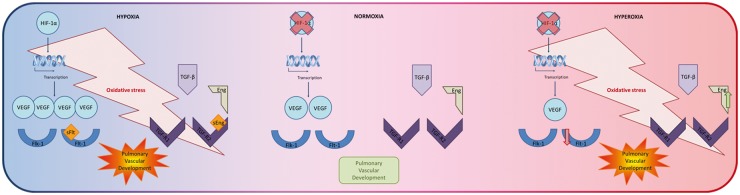
Oxygen tension is a key player in the pathogenesis of neonatal pulmonary vascular diseases. Both antenatal and postnatal exposure to either hypoxia and/or hyperoxia contribute to disruption of the normal development of the pulmonary vascular bed (Fig. 3). Despite decades of research focusing on the role of oxygen in pulmonary vascular development, the exact pathophysiologic mechanisms remain incompletely understood. Furthermore, it is important to study the optimal targeted oxygen saturation limits in much more detail, in order to improve clinical practice at the neonatal intensive care unit. Future endeavors should also include the development of a follow-up program tailored towards prematurely born adolescent survivors and/or survivors of (transient) neonatal pulmonary vascular diseases. Such a program may prove a key step in the early diagnosis and treatment of long-term vascular diseases to reduce morbidity and mortality in adult life, which is necessary in order to improve the health of the growing cohort of prematurely born survivors of neonatal pulmonary vascular disease and to reduce the burden of adult cardiopulmonary morbidity and mortality.
Fig. 3.
Summary figure depicting the receptors, ligands, and signaling pathways of relevance for pulmonary vascular development during perinatal hypoxia and hyperoxia as compared to normoxia.
Acknowledgments
The authors gratefully acknowledge the support from the Netherlands CardioVascular Research Initiative; the Dutch Heart Foundation, the Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development and the Royal Netherlands Academy of Science. CVON (2012-08), Phaedra and the Sophia Foundation for Medical Research, The Netherlands (Grant S13-12, 2012).
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work was supported in part by the Sophia Foundation for Medical Research, The Netherlands, Grant S13-12, 2012.
References
- 1.Galambos C, deMello DE. Molecular mechanisms of pulmonary vascular development. Pediatr Dev Pathol 2007; 10: 1–17. [DOI] [PubMed] [Google Scholar]
- 2.Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev 2010; 90: 1291–1335. [DOI] [PubMed] [Google Scholar]
- 3.Stenmark KR, Abman SH. Lung vascular development: Implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol 2005; 67: 623–661. [DOI] [PubMed] [Google Scholar]
- 4.Hall SM, Hislop AA, Pierce CM, et al. Prenatal origins of human intrapulmonary arteries: Formation and smooth muscle maturation. Am J Respir Cell Mol Biol 2000; 23: 194–203. [DOI] [PubMed] [Google Scholar]
- 5.Hislop AA. Airway and blood vessel interaction during lung development. J Anat 2002; 201: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merkus PJ, ten Have-Opbroek AA, Quanjer PH. Human lung growth: A review. Pediatr Pulmonol 1996; 21: 383–397. [DOI] [PubMed] [Google Scholar]
- 7.Gebb SA, Shannon JM. Tissue interactions mediate early events in pulmonary vasculogenesis. Dev Dyn 2000; 217: 159–169. [DOI] [PubMed] [Google Scholar]
- 8.van Tuyl M, Liu J, Wang J, et al. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol 2005; 288: L167–178. [DOI] [PubMed] [Google Scholar]
- 9.Risau W. Mechanisms of angiogenesis. Nature 1997; 386: 671–674. [DOI] [PubMed] [Google Scholar]
- 10.Abman SH, Baker C, Gien J, et al. The Robyn Barst Memorial Lecture: Differences between the fetal, newborn, and adult pulmonary circulations: Relevance for age-specific therapies (2013 Grover Conference series). Pulm Circ 2014; 4: 424–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deMello DE, Sawyer D, Galvin N, et al. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol 1997; 16: 568–581. [DOI] [PubMed] [Google Scholar]
- 12.Parera MC, van Dooren M, van Kempen M, et al. Distal angiogenesis: A new concept for lung vascular morphogenesis. Am J Physiol Lung Cell Mol Physiol 2005; 288: L141–149. [DOI] [PubMed] [Google Scholar]
- 13.Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999; 13: 9–22. [PubMed] [Google Scholar]
- 14.Baker CD, Abman SH, Mourani PM. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Pediatr Allergy Immunol Pulmonol 2014; 27: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thebaud B, Abman SH. Bronchopulmonary dysplasia: Where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med 2007; 175: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groenman F, Rutter M, Caniggia I, et al. Hypoxia-inducible factors in the first trimester human lung. J Histochem Cytochem 2007; 55: 355–363. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell PH. Hypoxia-inducible factor as a physiological regulator. Exp Physiol 2005; 90: 791–797. [DOI] [PubMed] [Google Scholar]
- 18.Rajatapiti P, de Rooij JD, Beurskens LW, et al. Effect of oxygen on the expression of hypoxia-inducible factors in human fetal lung explants. Neonatology 2010; 97: 346–354. [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi H, Sugiura T, Streisand JB, et al. TGF-beta-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol 2007; 293: L151–161. [DOI] [PubMed] [Google Scholar]
- 20.Southwood M, Jeffery TK, Yang X, et al. Regulation of bone morphogenetic protein signalling in human pulmonary vascular development. J Pathol 2008; 214: 85–95. [DOI] [PubMed] [Google Scholar]
- 21.Zeng X, Gray M, Stahlman MT, et al. TGF-beta1 perturbs vascular development and inhibits epithelial differentiation in fetal lung in vivo. Dev Dyn 2001; 221: 289–301. [DOI] [PubMed] [Google Scholar]
- 22.Brenner L, Chung WK. Clinical and molecular genetic features of hereditary pulmonary arterial hypertension. Compr Physiol 2011; 1: 1721–1728. [DOI] [PubMed] [Google Scholar]
- 23.Machado RD, Eickelberg O, Elliott CG, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: S32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: A registry study. Lancet 2012; 379: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Loon RL, Roofthooft MT, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: Epidemiology and characterization during the period 1991 to 2005. Circulation 2011; 124: 1755–1764. [DOI] [PubMed] [Google Scholar]
- 26.Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal 2007; 19: 1807–1819. [DOI] [PubMed] [Google Scholar]
- 27.Gitto E, Pellegrino S, Gitto P, et al. Oxidative stress of the newborn in the pre- and postnatal period and the clinical utility of melatonin. J Pineal Res 2009; 46: 128–139. [DOI] [PubMed] [Google Scholar]
- 28.O’Donovan DJ, Fernandes CJ. Free radicals and diseases in premature infants. Antioxid Redox Signal 2004; 6: 169–176. [DOI] [PubMed] [Google Scholar]
- 29.Ozsurekci Y, Aykac K. Oxidative stress related diseases in newborns. Oxid Med Cell Longev 2016; 2016: 2768365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taverne YJ, Bogers AJ, Duncker DJ, et al. Reactive oxygen species and the cardiovascular system. Oxid Med Cell Longev 2013; 2013: 862423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem 2015; 97: 55–74. [DOI] [PubMed] [Google Scholar]
- 32.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci 2008; 4: 89–96. [PMC free article] [PubMed] [Google Scholar]
- 33.Mates JM, Perez-Gomez C, Nunez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem 1999; 32: 595–603. [DOI] [PubMed] [Google Scholar]
- 34.Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000; 153: 83–104. [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B. Free radicals, proteins and DNA: Oxidative damage versus redox regulation. Biochem Soc Trans 1996; 24: 1023–1027. [DOI] [PubMed] [Google Scholar]
- 36.McCord JM, Fridovich I. The biology and pathology of oxygen radicals. Ann Intern Med 1978; 89: 122–127. [DOI] [PubMed] [Google Scholar]
- 37.Sarker AH, Watanabe S, Seki S, et al. Oxygen radical-induced single-strand DNA breaks and repair of the damage in a cell-free system. Mutat Res 1995; 337: 85–95. [DOI] [PubMed] [Google Scholar]
- 38.Saugstad OD. Mechanisms of tissue injury by oxygen radicals: Implications for neonatal disease. Acta Paediatr 1996; 85: 1–4. [DOI] [PubMed] [Google Scholar]
- 39.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol 2003; 8: 39–49. [DOI] [PubMed] [Google Scholar]
- 40.Saugstad OD. Oxygen and oxidative stress in bronchopulmonary dysplasia. J Perinat Med 2010; 38: 571–577. [DOI] [PubMed] [Google Scholar]
- 41.Vogel ER, Britt RD, Jr, Trinidad MC, et al. Perinatal oxygen in the developing lung. Can J Physiol Pharmacol 2015; 93: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dennery PA. Role of redox in fetal development and neonatal diseases. Antioxid Redox Signal 2004; 6: 147–153. [DOI] [PubMed] [Google Scholar]
- 43.Saugstad OD. The oxygen radical disease in neonatology. Indian J Pediatr 1989; 56: 585–593. [DOI] [PubMed] [Google Scholar]
- 44.Saugstad OD. Update on oxygen radical disease in neonatology. Curr Opin Obstet Gynecol 2001; 13: 147–153. [DOI] [PubMed] [Google Scholar]
- 45.van Patot MC, Ebensperger G, Gassmann M, et al. The hypoxic placenta. High Alt Med Biol 2012; 13: 176–184. [DOI] [PubMed] [Google Scholar]
- 46.Shah DA, Khalil RA. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem Pharmacol 2015; 95: 211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen AR, Barnes CM, Folkman J, et al. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr 2010; 156: 532–536. [DOI] [PubMed] [Google Scholar]
- 48.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 2005; 365: 785–799. [DOI] [PubMed] [Google Scholar]
- 49.Gupta S, Agarwal A, Sharma RK. The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet Gynecol Surv 2005; 60: 807–816. [DOI] [PubMed] [Google Scholar]
- 50.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med 1999; 222: 222–235. [DOI] [PubMed] [Google Scholar]
- 51.Vanderlelie J, Venardos K, Clifton VL, et al. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta 2005; 26: 53–58. [DOI] [PubMed] [Google Scholar]
- 52.Altunhan H, Annagur A, Kurban S, et al. Total oxidant, antioxidant, and paraoxonase levels in babies born to pre-eclamptic mothers. J Obstet Gynaecol Res 2013; 39: 898–904. [DOI] [PubMed] [Google Scholar]
- 53.Braekke K, Harsem NK, Staff AC. Oxidative stress and antioxidant status in fetal circulation in preeclampsia. Pediatr Res 2006; 60: 560–564. [DOI] [PubMed] [Google Scholar]
- 54.Tastekin A, Ors R, Demircan B, et al. Oxidative stress in infants born to preeclamptic mothers. Pediatr Int 2005; 47: 658–662. [DOI] [PubMed] [Google Scholar]
- 55.Namdev S, Bhat V, Adhisivam B, et al. Oxidative stress and antioxidant status among neonates born to mothers with pre-eclampsia and their early outcome. J Matern Fetal Neonatal Med 2014; 27: 1481–1484. [DOI] [PubMed] [Google Scholar]
- 56.Negi R, Pande D, Karki K, et al. Association of oxidative DNA damage, protein oxidation and antioxidant function with oxidative stress induced cellular injury in pre-eclamptic/eclamptic mothers during fetal circulation. Chem Biol Interact 2014; 208: 77–83. [DOI] [PubMed] [Google Scholar]
- 57.Aydin S, Benian A, Madazli R, et al. Plasma malondialdehyde, superoxide dismutase, sE-selectin, fibronectin, endothelin-1 and nitric oxide levels in women with preeclampsia. Eur J Obstet Gynecol Reprod Biol 2004; 113: 21–25. [DOI] [PubMed] [Google Scholar]
- 58.Sharma JB, Sharma A, Bahadur A, et al. Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. Int J Gynaecol Obstet 2006; 94: 23–27. [DOI] [PubMed] [Google Scholar]
- 59.Foidart JM, Schaaps JP, Chantraine F, et al. Dysregulation of anti-angiogenic agents (sFlt-1, PLGF, and sEndoglin) in preeclampsia–a step forward but not the definitive answer. J Reprod Immunol 2009; 82: 106–111. [DOI] [PubMed] [Google Scholar]
- 60.Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res 2008; 75: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang JR, Karumanchi SA, Seedorf G, et al. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: Linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2012; 302: L36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park CW, Park JS, Shim SS, et al. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol 2005; 193: 984–989. [DOI] [PubMed] [Google Scholar]
- 63.Kim SK, Romero R, Savasan ZA, et al. Endoglin in amniotic fluid as a risk factor for the subsequent development of bronchopulmonary dysplasia. Am J Reprod Immunol 2013; 69: 105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore LG. Altitude-aggravated illness: Examples from pregnancy and prenatal life. Ann Emerg Med 1987; 16: 965–973. [DOI] [PubMed] [Google Scholar]
- 65.Wilkins MR, Ghofrani HA, Weissmann N, et al. Pathophysiology and treatment of high-altitude pulmonary vascular disease. Circulation 2015; 131: 582–590. [DOI] [PubMed] [Google Scholar]
- 66.Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: Independent or interactive effects? Am J Public Health 1997; 87: 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mortola JP, Frappell PB, Aguero L, et al. Birth weight and altitude: A study in Peruvian communities. J Pediatr 2000; 136: 324–329. [DOI] [PubMed] [Google Scholar]
- 68.Nevo O, Soleymanlou N, Wu Y, et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol 2006; 291: R1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zamudio S, Wu Y, Ietta F, et al. Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J Pathol 2007; 170: 2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niermeyer S. Cardiopulmonary transition in the high altitude infant. High Alt Med Biol 2003; 4: 225–239. [DOI] [PubMed] [Google Scholar]
- 71.Niermeyer S, Andrade Mollinedo P, Huicho L. Child health and living at high altitude. Arch Dis Child 2009; 94: 806–811. [DOI] [PubMed] [Google Scholar]
- 72.Lee SK, Ye XY, Singhal N, et al. Higher altitude and risk of bronchopulmonary dysplasia among preterm infants. Am J Perinatol 2013; 30: 601–606. [DOI] [PubMed] [Google Scholar]
- 73.Herrera EA, Pulgar VM, Riquelme RA, et al. High-altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am J Physiol Regul Integr Comp Physiol 2007; 292: R2234–2240. [DOI] [PubMed] [Google Scholar]
- 74.Herrera EA, Riquelme RA, Ebensperger G, et al. Long-term exposure to high-altitude chronic hypoxia during gestation induces neonatal pulmonary hypertension at sea level. Am J Physiol Regul Integr Comp Physiol 2010; 299: R1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Llanos AJ, Ebensperger G, Herrera EA, et al. Fetal and postnatal pulmonary circulation in the Alto Andino. Placenta 2011; 32: S100–103. [DOI] [PubMed] [Google Scholar]
- 76.Hilgendorff A, Reiss I, Ehrhardt H, et al. Chronic lung disease in the preterm infant. Lessons learned from animal models. Am J Respir Cell Mol Biol 2014; 50: 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramani M, Bradley WE, Dell’Italia LJ, et al. Early exposure to hyperoxia or hypoxia adversely impacts cardio-pulmonary development. Am J Respir Cell Mol Biol 2015; 52: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saugstad OD. Oxidative stress in the newborn–a 30-year perspective. Biol Neonate 2005; 88: 228–236. [DOI] [PubMed] [Google Scholar]
- 79.Rogers S, Witz G, Anwar M, et al. Antioxidant capacity and oxygen radical diseases in the preterm newborn. Arch Pediatr Adolesc Med 2000; 154: 544–548. [DOI] [PubMed] [Google Scholar]
- 80.Plotz FB, Slutsky AS, van Vught AJ, et al. Ventilator-induced lung injury and multiple system organ failure: A critical review of facts and hypotheses. Intensive Care Med 2004; 30: 1865–1872. [DOI] [PubMed] [Google Scholar]
- 81.Terragni P, Ranieri VM, Brazzi L. Novel approaches to minimize ventilator-induced lung injury. Curr Opin Crit Care 2015; 21: 20–25. [DOI] [PubMed] [Google Scholar]
- 82.Vlahakis NE, Schroeder MA, Limper AH, et al. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol 1999; 277: L167–173. [DOI] [PubMed] [Google Scholar]
- 83.Jobe AH, Hillman N, Polglase G, et al. Injury and inflammation from resuscitation of the preterm infant. Neonatology 2008; 94: 190–196. [DOI] [PubMed] [Google Scholar]
- 84.De Paepe ME, Patel C, Tsai A, et al. Endoglin (CD105) up-regulation in pulmonary microvasculature of ventilated preterm infants. Am J Respir Crit Care Med 2008; 178: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Paepe ME, Greco D, Mao Q. Angiogenesis-related gene expression profiling in ventilated preterm human lungs. Exp Lung Res 2010; 36: 399–410. [DOI] [PubMed] [Google Scholar]
- 86.Galambos C, Sims-Lucas S, Abman SH. Histologic evidence of intrapulmonary anastomoses by three-dimensional reconstruction in severe bronchopulmonary dysplasia. Ann Am Thorac Soc 2013; 10: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lovering AT, Riemer RK, Thebaud B. Intrapulmonary arteriovenous anastomoses. Physiological, pathophysiological, or both? Ann Am Thorac Soc 2013; 10: 504–508. [DOI] [PubMed] [Google Scholar]
- 88.Archer SL, Nelson DP, Weir EK. Simultaneous measurement of O2 radicals and pulmonary vascular reactivity in rat lung. J Appl Physiol 1989; 67: 1903–1911. [DOI] [PubMed] [Google Scholar]
- 89.Archer SL, Souil E, Dinh-Xuan AT, et al. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest 1998; 101: 2319–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Archer SL, Weir EK, Reeve HL, et al. Molecular identification of O2 sensors and O2-sensitive potassium channels in the pulmonary circulation. Adv Exp Med Biol 2000; 475: 219–240. [DOI] [PubMed] [Google Scholar]
- 91.Michelakis ED, Thebaud B, Weir EK, et al. Hypoxic pulmonary vasoconstriction: Redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol 2004; 37: 1119–1136. [DOI] [PubMed] [Google Scholar]
- 92.Fuchs B, Sommer N, Dietrich A, et al. Redox signaling and reactive oxygen species in hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol 2010; 174: 282–291. [DOI] [PubMed] [Google Scholar]
- 93.Sommer N, Dietrich A, Schermuly RT, et al. Regulation of hypoxic pulmonary vasoconstriction: Basic mechanisms. Eur Respir J 2008; 32: 1639–1651. [DOI] [PubMed] [Google Scholar]
- 94.Archer S, Michelakis E. The mechanism(s) of hypoxic pulmonary vasoconstriction: Potassium channels, redox O(2) sensors, and controversies. News Physiol Sci 2002; 17: 131–137. [DOI] [PubMed] [Google Scholar]
- 95.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol 2005; 98: 390–403. [DOI] [PubMed] [Google Scholar]
- 96.Berger J, Bhandari V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am J Physiol Lung Cell Mol Physiol 2014; 307: L936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rocha G, Proenca E, Guedes A, et al. Cord blood levels of IL-6, IL-8 and IL-10 may be early predictors of bronchopulmonary dysplasia in preterm newborns small for gestational age. Dis Markers 2012; 33: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McGowan EC, Kostadinov S, McLean K, et al. Placental IL-10 dysregulation and association with bronchopulmonary dysplasia risk. Pediatr Res 2009; 66: 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koksal N, Kayik B, Cetinkaya M, et al. Value of serum and bronchoalveolar fluid lavage pro- and anti-inflammatory cytokine levels for predicting bronchopulmonary dysplasia in premature infants. Eur Cytokine Netw 2012; 23: 29–35. [DOI] [PubMed] [Google Scholar]
- 100.Bhandari V, Elias JA. Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free Radic Biol Med 2006; 41: 4–18. [DOI] [PubMed] [Google Scholar]
- 101.Chess PR, D’Angio CT, Pryhuber GS, et al. Pathogenesis of bronchopulmonary dysplasia. Semin Perinatol 2006; 30: 171–178. [DOI] [PubMed] [Google Scholar]
- 102.Kallapur SG, Jobe AH. Contribution of inflammation to lung injury and development. Arch Dis Child Fetal Neonatal Ed 2006; 91: F132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abman SH. Pulmonary hypertension in children: A historical overview. Pediatr Crit Care Med 2010; 11: S4–9. [DOI] [PubMed] [Google Scholar]
- 104.Robbins IM, Moore TM, Blaisdell CJ, et al. Improving outcomes for pulmonary vascular disease. Am J Respir Crit Care Med 2012; 185: 1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhat R, Salas AA, Foster C, et al. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 2012; 129: e682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mirza H, Ziegler J, Ford S, et al. Pulmonary hypertension in preterm infants: Prevalence and association with bronchopulmonary dysplasia. J Pediatr 2014; 165: 909–914. [DOI] [PubMed] [Google Scholar]
- 107.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol 2003; 8: 73–81. [DOI] [PubMed] [Google Scholar]
- 108.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr 2011; 23: 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163: 1723–1729. [DOI] [PubMed] [Google Scholar]
- 110.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007; 357: 1946–1955. [DOI] [PubMed] [Google Scholar]
- 111.Gough A, Spence D, Linden M, et al. General and respiratory health outcomes in adult survivors of bronchopulmonary dysplasia: A systematic review. Chest 2012; 141: 1554–1567. [DOI] [PubMed] [Google Scholar]
- 112.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl 2004; 93: 26–33. [DOI] [PubMed] [Google Scholar]
- 113.Bolton CE, Bush A, Hurst JR, et al. Lung consequences in adults born prematurely. Thorax 2015; 70: 574–580. [DOI] [PubMed] [Google Scholar]
- 114.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008; 371: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lewandowski AJ, Leeson P. Preeclampsia, prematurity and cardiovascular health in adult life. Early Hum Dev 2014; 90: 725–729. [DOI] [PubMed] [Google Scholar]
- 116.Sartori C, Allemann Y, Trueb L, et al. Augmented vasoreactivity in adult life associated with perinatal vascular insult. Lancet 1999; 353: 2205–2207. [DOI] [PubMed] [Google Scholar]
- 117.Jayet PY, Rimoldi SF, Stuber T, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation 2010; 122: 488–494. [DOI] [PubMed] [Google Scholar]
- 118.Thompson LP, Al-Hasan Y. Impact of oxidative stress in fetal programming. J Pregnancy 2012; 2012: 582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harding R, Maritz G. Maternal and fetal origins of lung disease in adulthood. Semin Fetal Neonatal Med 2012; 17: 67–72. [DOI] [PubMed] [Google Scholar]
- 120.Joss-Moore LA, Albertine KH, Lane RH. Epigenetics and the developmental origins of lung disease. Mol Genet Metab 2011; 104: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perrone S, Santacroce A, Picardi A, et al. Fetal programming and early identification of newborns at high risk of free radical-mediated diseases. World J Clin Pediatr 2016; 5: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Andreasson B, Lindroth M, Mortensson W, et al. Lung function eight years after neonatal ventilation. Arch Dis Child 1989; 64: 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clemm H, Roksund O, Thorsen E, et al. Aerobic capacity and exercise performance in young people born extremely preterm. Pediatrics 2012; 129: e97–e105. [DOI] [PubMed] [Google Scholar]
- 124.Jacob SV, Lands LC, Coates AL, et al. Exercise ability in survivors of severe bronchopulmonary dysplasia. Am J Respir Crit Care Med 1997; 155: 1925–1929. [DOI] [PubMed] [Google Scholar]
- 125.Kriemler S, Keller H, Saigal S, et al. Aerobic and lung performance in premature children with and without chronic lung disease of prematurity. Clin J Sport Med 2005; 15: 349–355. [DOI] [PubMed] [Google Scholar]
- 126.Narang I. Review series: What goes around, comes around: childhood influences on later lung health? Long-term follow-up of infants with lung disease of prematurity. Chron Respir Dis 2010; 7: 259–269. [DOI] [PubMed] [Google Scholar]
- 127.Zavorsky GS, Kryder JR, Jacob SV, et al. Exercise capacity of children with pediatric lung disease. Clin Invest Med 2009; 32: E302. [DOI] [PubMed] [Google Scholar]
- 128.Bader D, Ramos AD, Lew CD, et al. Childhood sequelae of infant lung disease: Exercise and pulmonary function abnormalities after bronchopulmonary dysplasia. J Pediatr 1987; 110: 693–699. [DOI] [PubMed] [Google Scholar]
- 129.Kaplan E, Bar-Yishay E, Prais D, et al. Encouraging pulmonary outcome for surviving, neurologically intact, extremely premature infants in the postsurfactant era. Chest 2012; 142: 725–733. [DOI] [PubMed] [Google Scholar]
- 130.Kilbride HW, Gelatt MC, Sabath RJ. Pulmonary function and exercise capacity for ELBW survivors in preadolescence: Effect of neonatal chronic lung disease. J Pediatr 2003; 143: 488–493. [DOI] [PubMed] [Google Scholar]
- 131.Mitchell SH, Teague WG, Robinson A. Reduced gas transfer at rest and during exercise in school-age survivors of bronchopulmonary dysplasia. Am J Respir Crit Care Med 1998; 157: 1406–1412. [DOI] [PubMed] [Google Scholar]
- 132.Parat S, Moriette G, Delaperche MF, et al. Long-term pulmonary functional outcome of bronchopulmonary dysplasia and premature birth. Pediatr Pulmonol 1995; 20: 289–296. [DOI] [PubMed] [Google Scholar]
- 133.Rogers M, Fay TB, Whitfield MF, et al. Aerobic capacity, strength, flexibility, and activity level in unimpaired extremely low birth weight (<or = 800 g) survivors at 17 years of age compared with term-born control subjects. Pediatrics 2005; 116: e58–65. [DOI] [PubMed] [Google Scholar]
- 134.Santuz P, Baraldi E, Zaramella P, et al. Factors limiting exercise performance in long-term survivors of bronchopulmonary dysplasia. Am J Respir Crit Care Med 1995; 152: 1284–1289. [DOI] [PubMed] [Google Scholar]
- 135.Smith LJ, van Asperen PP, McKay KO, et al. Reduced exercise capacity in children born very preterm. Pediatrics 2008; 122: e287–293. [DOI] [PubMed] [Google Scholar]
- 136.Tsopanoglou SP, Davidson J, Goulart AL, et al. Functional capacity during exercise in very-low-birth-weight premature children. Pediatr Pulmonol 2014; 49: 91–98. [DOI] [PubMed] [Google Scholar]
- 137.Vrijlandt EJ, Gerritsen J, Boezen HM, et al. Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med 2006; 173: 890–896. [DOI] [PubMed] [Google Scholar]
- 138.Welsh L, Kirkby J, Lum S, et al. The EPICure study: Maximal exercise and physical activity in school children born extremely preterm. Thorax 2010; 65: 165–172. [DOI] [PubMed] [Google Scholar]
- 139.Baraldi E, Carraro S. Exercise testing and chronic lung diseases in children. Paediatr Respir Rev 2006; 7: S196–198. [DOI] [PubMed] [Google Scholar]
- 140.Teoh OH, Trachsel D, Mei-Zahav M, et al. Exercise testing in children with lung diseases. Paediatr Respir Rev 2009; 10: 99–104. [DOI] [PubMed] [Google Scholar]
- 141.Doyle LW, Anderson PJ. Adult outcome of extremely preterm infants. Pediatrics 2010; 126: 342–351. [DOI] [PubMed] [Google Scholar]
- 142.Greenough A. Late respiratory outcomes after preterm birth. Early Hum Dev 2007; 83: 785–788. [DOI] [PubMed] [Google Scholar]
- 143.Greenough A. Long-term pulmonary outcome in the preterm infant. Neonatology 2008; 93: 324–327. [DOI] [PubMed] [Google Scholar]
- 144.Pike KC, Lucas JS. Respiratory consequences of late preterm birth. Paediatr Respir Rev 2015; 16: 182–188. [DOI] [PubMed] [Google Scholar]
- 145.Lewandowski AJ, Bradlow WM, Augustine D, et al. Right ventricular systolic dysfunction in young adults born preterm. Circulation 2013; 128: 713–720. [DOI] [PubMed] [Google Scholar]
- 146.Lewandowski AJ, Augustine D, Lamata P, et al. Preterm heart in adult life: Cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 2013; 127: 197–206. [DOI] [PubMed] [Google Scholar]
- 147.Khadawardi E, Al Hazzani F. Oxygen Saturation and Outcomes in Preterm Infants The BOOST II United Kingdom, Australia, and New Zealand Collaborative Groups. J Clin Neonatol 2013; 2: 73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Manja V, Lakshminrusimha S, Cook DJ. Oxygen saturation target range for extremely preterm infants: A systematic review and meta-analysis. JAMA Pediatr 2015; 169: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schmidt B, Whyte RK, Roberts RS. Trade-off between lower or higher oxygen saturations for extremely preterm infants: The first benefits of oxygen saturation targeting (BOOST) II trial reports its primary outcome. J Pediatr 2014; 165: 6–8. [DOI] [PubMed] [Google Scholar]
- 150.Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: Guidelines from the American Heart Association and American Thoracic Society. Circulation 2015; 132: 2037–2099. [DOI] [PubMed] [Google Scholar]