Abstract
In 2013, chikungunya virus (CHIKV) transmission was documented in the Western Hemisphere, and the virus has since spread throughout the Americas with more than 1.8 million people infected in more than 40 countries. CHIKV targets the joints, resulting in symmetric polyarthritis that clinically mimics rheumatoid arthritis and can endure for months to years. At present, no approved treatment is effective in preventing or controlling CHIKV infection or disease. We treated mice with eight different disease-modifying antirheumatic drugs and identified CLTA4-Ig (abatacept) and tofacitinib as candidate therapies based on their ability to decrease acute joint swelling. CTLA4-Ig reduced T cell accumulation in the joints of infected animals without affecting viral infection. Whereas monotherapy with CTLA4-Ig or a neutralizing anti-CHIKV human monoclonal antibody provided partial clinical improvement, therapy with both abolished swelling and markedly reduced levels of chemokines, proinflammatory cytokines, and infiltrating leukocytes. Thus, combination CTLA4-Ig and antiviral antibody therapy controls acute CHIKV infection and arthritis and may be a candidate for testing in humans.
INTRODUCTION
Chikungunya virus (CHIKV) is a mosquito-transmitted alphavirus that causes severe acute and chronic polyarthritis. CHIKV was first isolated in Tanzania in 1947 (1), but the virus has emerged rapidly over the last decade, causing outbreaks in the islands of the Indian Ocean, in Southern Europe, and in Southeast Asia (2). In 2013, CHIKV spread to the Western Hemisphere and, by the end of 2015, had infected more than 1.8 million people in North America, Central America, and South America. The acute symptoms of CHIKV infection include fever and rash, which typically resolve within a few days, and joint and muscle pain (3). CHIKV and other arthritogenic alphaviruses directly invade the synovium to cause inflammatory arthritis (4), which is characterized by articular swelling and prolonged morning stiffness (3). CHIKV-induced arthritis in humans can persist, with as many as 60% of individuals progressing to chronic disease that lasts from months to years (3, 5–7). Epidemiological projections suggest that there are currently about 400,000 individuals in the Western Hemisphere with chronic CHIKV arthritis (8).
Chronic CHIKV arthritis clinically is similar to seronegative rheumatoid arthritis (RA) (3, 9–11), an autoimmune disease characterized by symmetrical joint pain, swelling, and morning stiffness. Treatment with newer disease-modifying antirheumatic drugs (DMARDs) has been effective in preventing the bone erosions and deformities seen in patients with untreated RA. Whether chronic CHIKV arthritis causes erosive disease remains controversial, although there are reports of bone erosions in patients infected with CHIKV (9). Effective treatment of RA relies on early diagnosis, because erosions can occur within months of onset of the disease (12). Because CHIKV and RA exhibit significant clinical overlap, there is potential for confusing the diagnoses and inadvertently treating CHIKV arthritis with DMARD therapy (3).
Over the last 20 years, studies in patients with RA have demonstrated that oral and biological DMARDs prevent joint pain, swelling, and damage. Oral DMARDs include hydroxychloroquine, methotrexate, and sulfasalazine, whereas biological DMARDs include the anticytokine antibodies and Ig chimeras [for example, anti–tumor necrosis factor–α (TNF-α) and anti–interleukin-6 (IL-6) receptor]. Other biological DMARDs block T cell costimulation (CTLA4-Ig) or deplete B cells (anti-CD20). Among the newest drugs used to treat RA is tofacitinib, an oral DMARD that inhibits JAK (Janus kinase)/STAT (signal transducers and activators of transcription) signaling and broadly blunts cytokine responses (13, 14). However, many DMARDs, by virtue of their immunosuppressive properties, may predispose to serious microbial infections. Thus, there is a need to determine whether DMARDs are effective, benign, or deleterious in the treatment of CHIKV arthritis. The current standard of care for CHIKV arthritis is treatment with non-steroidal anti-inflammatory drugs (NSAIDs) (15), although these often do not ameliorate symptoms (3). One trial compared chloroquine (a DMARD) to meloxicam (an NSAID) and found no difference in efficacy (15), although a placebo group was not included in the trial design. Another open-label study examined a combination of hydroxychloroquine and methotrexate in the treatment of CHIKV arthritis and found that some patients partially responded to therapy, although ~50% of patients did not achieve significant improvement in disease score (16).
Subcutaneous inoculation of young wild-type (WT) immunocompetent C57BL/6 mice with pathogenic strains of CHIKV results in acute foot swelling, myositis, and arthritis (4, 17). In this model, swelling resolves within the first 2 weeks of infection (17, 18), although mild chronic disease can be observed histologically for weeks to months (4). This finding contrasts with the disease in humans, which are natural hosts and frequently have a protracted clinical disease course. Human patients with CHIKV arthritis have increased numbers of circulating, activated cytolytic CD8+ T cells, as do patients with untreated RA (3).
Gene expression signatures observed in mouse models of CHIKV arthritis and RA suggested overlapping contributions of T cell–associated pathways in these diseases (19). Mice lacking or depleted of CD4+Tcells have reduced foot swelling and arthritis during acute CHIKV infection, suggesting that CD4+ T cells contribute to the pathology of arthritis (20). Inflammatory monocytes also are thought to play an initiating role in CHIKV arthritis in mice, because inhibiting production of monocyte chemoattractant protein-1 (MCP-1) with bindarit (21) improved the disease. Finally, treatment of mice with anti-CHIKV monoclonal antibodies (mAbs) 1 day before infection prevents arthritis (22–24), but whether therapy after infection is effective has not been studied. Moreover, no previous study has tested the efficacy of clinically available DMARDs against CHIKV arthritis in mice.
Here, we examined the efficacy of several U.S. Food and Drug Administration (FDA)–approved RA therapies in a mouse model of CHIKV infection. We identified two DMARDs (CTLA4-Ig and tofacitinib) with efficacy during acute CHIKV arthritis. In particular, CTLA4-Ig, when paired with the neutralizing anti-CHIKV human mAb 4N12, was highly effective at reducing joint inflammation, periarticular swelling, migration of inflammatory leukocytes, and infection even when administered several days after virus inoculation. Thus, combination of anti-inflammatory and antibody-based antiviral therapy may serve as a model for treating humans with arthritis caused by CHIKV or other related viruses.
RESULTS
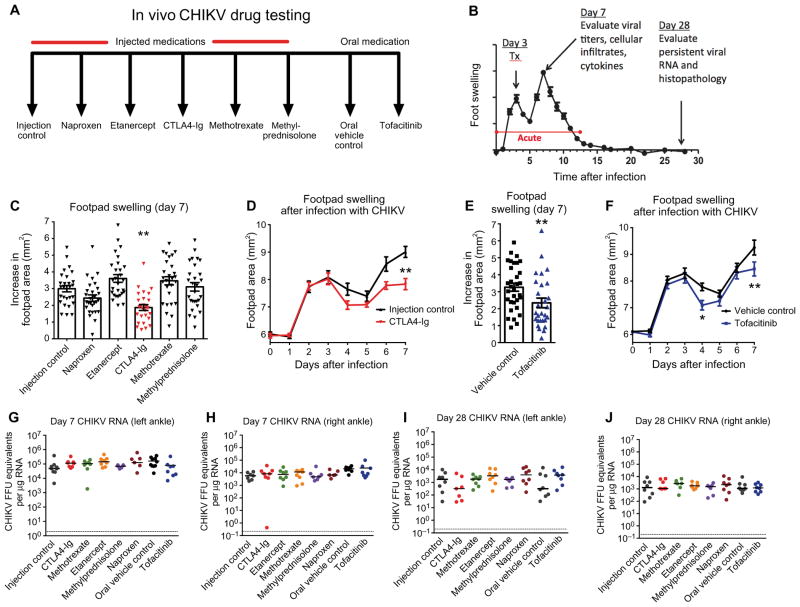
On the basis of previous data showing that immune cells and proinflammatory cytokines contribute to CHIKV arthritis in mice (20) and our study showing that CHIKV arthritis clinically can mimic seronegative RA (3), we hypothesized that some of the existing FDA-approved therapies for RA might ameliorate acute CHIKV arthritis. To test this idea, we performed a multiarm prospective study (Fig. 1A) by inoculating 224 4-week-old WT mice subcutaneously in the left rear foot with 103 focus-forming units (FFU) of a pathogenic clinical isolate of CHIKV (La Reunion, 2006). Mice were divided into groups of 28 animals that received one of the following treatments beginning on day 3 after infection: methylprednisolone, naproxen, methotrexate, etanercept (soluble human TNF-α receptor), CTLA4-Ig (abatacept), oral gavage vehicle control, or tofacitinib (JAK inhibitor). All of the biological agents that we used have been shown previously to have activity in mice (13, 25, 26). We chose day 3 after infection to initiate treatment, because this represents the time point of the first peak of clinically apparent foot swelling in the WTC57BL/6 mouse model of CHIKV infection (Fig. 1B). Animals were followed for clinical joint swelling in the ipsilateral foot and viral yield at different times after infection and treatment.
Fig. 1. Screen of candidate oral and biological DMARDs for treatment of acute CHIKV arthritis in mice.
Mice were inoculated with 103 FFU of CHIKV (La Reunion strain) via a subcutaneous route. (A) Schematic depicting the two controls [phosphate-buffered saline (PBS) injection control and oral vehicle control] and six treatment arms in our drug screen. Medications and doses were methylprednisolone (0.5 mg/kg) via intraperitoneal route daily from days 3 through 7, naproxen (10 mg/kg, intraperitoneally) daily from day 3 through 7, methotrexate (0.3 mg/kg, intraperitoneally) once weekly (day 3 and day 10) 2× doses, etanercept (300 μg, intraperitoneally) 1× dose on day 3, CTLA4-Ig (300 μg, intraperitoneally) 1× dose on day 3, and tofacitinib (50 mg/kg) oral gavage daily from day 3 through day 7. For subsequent studies of CTLA4-Ig, an isotype control antibody was used. (B) Experimental design included treatment on day 3 after infection and harvests on days 7 and 28 after infection for the indicated analyses including viral burden, histology, cytokine analysis, and flow cytometry of infiltrating leukocytes. Throughout the time course, foot swelling was measured using digital calipers. (C) Foot swelling (area in square millimeter) on day 7 compared to day 0 for all injected therapies. (D) Foot swelling over time for injection control and CTLA4-Ig–treated animals. (E) Foot swelling on day 7 compared to day 0 for tofacitinib and oral vehicle control. (F) Foot swelling over time for oral vehicle control and tofacitinib-treated animals. (G to J) Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of viral RNA levels in the left and right ankles at days 7 and 28 after infection for all treatment groups. Results in (C) to (J) are from at least two independent experiments with n = 28 per treatment group for measurement data from days 0 to 7 and n = 7 or 8 for viral burden analysis on days 7 and 28. Data represent the means (C to F) or median (G to J) ± SEM. *P < 0.05, **P < 0.005, ****P < 0.0001 [two-way analysis of variance (ANOVA) for analysis of swelling curves, Mann-Whitney for day 7 tofacitinib analysis, and Kruskal-Wallis with Dunn’s post hoc analysis for day 7 injected medication and viral burden analysis].
Treatment with CTLA4-Ig or tofacitinib at day 3 ameliorated foot swelling on day 7 after infection at the point of peak clinical disease [Fig. 1, C to F; 9.0 mm2 (injection control) versus 7.8 mm2 (CTLA4-Ig), P < 0.005 and 9.3 mm2 (oral control) versus 8.5 mm2 (tofacitinib), P < 0.005]. Other treatments had no significant effect at the dose tested in our experiments. To assess the systemic and local impact on viral burden of DMARD treatment, we evaluated CHIKV RNA levels on day 7 in serum and joint tissues. Remarkably, none of the therapies affected viral RNA levels in the right ankle or left ankle at day 7 or day 28 after infection compared to controls (Fig. 1, G to J; P > 0.1). Thus, CTLA4-Ig and tofacitinib ameliorate joint and periarticular inflammation in mice during the acute phase without substantively altering the viral burden in inflamed tissues. The beneficial effect of CTLA4-Ig, which blocks T cell activation (25), is consistent with data suggesting that CD4+ T cells contribute to immunopathology associated with CHIKV arthritis (20).
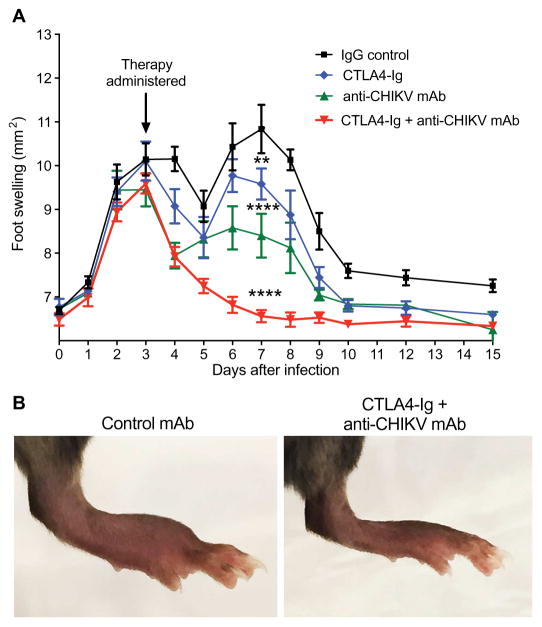
We next tested whether combination immunomodulatory and antiviral therapy might have greater beneficial effects. Potently neutralizing human anti-CHIKV mAbs previously were shown to protect against CHIKV-induced lethality in immunocompromised Ifnar1−/− mice, even when administered at late time points after infection (27). We administered either a single 600-μg dose of a control immunoglobulin G (IgG), 300 μg of CTLA4-Ig, 300 μg of anti-CHIKV mAb (4N12, a neutralizing anti-CHIKV human mAb), or 300 μg each of CTLA4-Ig + anti-CHIKV mAb (Fig. 2A) 3 days after CHIKV infection. Whereas either anti-CHIKV mAb or CTLA4-Ig partially reduced foot swelling at day 7, the combination completely abolished it (10.8 mm2 versus 6.6 mm2, P < 0.0001) relative to untreated or control IgG–treated animals (Fig. 2, A and B). Thus, anti-CHIKV mAb therapy initiated after disease onset can partially treat the acute clinical arthritis associated with CHIKV infection, and combination therapy with anti-CHIKV mAb and the immunomodulator CTLA4-Ig completely resolves clinical disease in mice within a few days of treatment.
Fig. 2. Combination therapy with CTLA4-Ig and an anti-CHIKV human mAb ameliorates acute CHIKV arthritis in mice.
Mice were inoculated with 103 FFU of CHIKV via a subcutaneous route. (A) Foot swelling (area in square millimeter) from day 0 through day 15 in mice receiving at day 3 a single intraperitoneal injection of 600 μg of isotype control antibody, 300 μg of CTLA4-Ig, 300 μg of anti-CHIKV mAb (4N12), or a combination of 300 μg of CTLA4-Ig and 300 μg of anti-CHIKV mAb. Data are pooled from two independent experiments (n = 15 to 19 animals per group). (B) Representative photographs of ipsilateral foot swelling in the control mAb or combination therapy (CTLA4-Ig + anti-CHIKV mAb) groups. Data represent the means ± SEM. **P < 0.005, ****P < 0.0001 (two-way ANOVA with multiple comparisons).
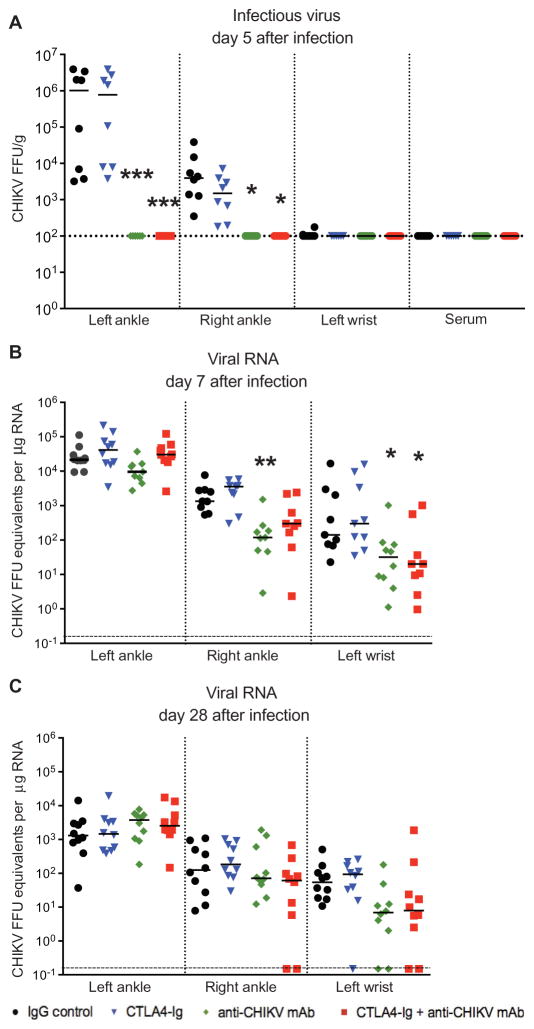
To confirm the activity of the anti-CHIKV mAb in this model, we assessed its effects on viral burden. Treatment with anti-CHIKV mAb (alone or in combination with CTLA4-Ig) at day 3 eliminated infectious virus in the joints of infected mice within 2 days, as we observed a ~10,000-fold reduction in the ipsilateral ankle (Fig. 3A; P < 0.0005) and a ~100-fold reduction in the contralateral ankle (Fig. 3A; P < 0.05). Viral burden in mice treated with only CTLA4-Ig was not reduced in either the ipsilateral or contralateral ankle (Fig. 3A). Although infectious CHIKV cannot be detected after day 7 in this mouse model [or during the chronic phase in humans (3, 4)], CHIKV RNA persists in joint tissues for months (28). Because persistent viral RNA is a pathogen-associated molecular pattern and may contribute to CHIKV arthritis (3, 28), we tested whether anti-CHIKV mAb treatment would reduce viral RNA levels. Although we did not observe a reduction in viral RNA levels in the ipsilateral foot joints (P > 0.1), other extremity joints exhibited a ~10-fold reduction in viral RNA on day 7 after infection, including the contralateral ankle and ipsilateral wrist (Fig. 3B; right ankle, P < 0.005; left wrist, P < 0.05). On day 28, there was a trend toward reduced RNA in distal joint tissues of animals treated with anti-CHIKV mAb, although this did not attain statistical significance (Fig. 3C; P = 0.2). Collectively, these results show that treatment with an anti-CHIKV mAb (alone or in combination with CTLA4-Ig) rapidly eliminates infectious virus within 2 days and reduces but fails to clear viral RNA in affected tissues.
Fig. 3. Viral burden in CHIKV-infected mice treated with CTLA4-Ig and anti-CHIKV mAb.
Mice were inoculated with 103 FFU of CHIKV via a subcutaneous route. Mice received at day 3 a single intraperitoneal injection of 600 μg of isotype control antibody, 300 μg of CTLA4-Ig, 300 μg of anti-CHIKV mAb, or a combination of 300 μg of CTLA4-Ig and 300 μg of anti-CHIKV mAb. (A) Infectious virus in joints and serum quantitated by focus-forming assay on day 5 after infection. (B and C) Real-time qRT-PCR analysis of viral RNA levels in the ipsilateral (left) and contralateral (right) ankles and left wrist at days 7 and 28 after infection. Results are pooled from two independent experiments with n = 10 per treatment group for viral RNA data and n = 8 for focus-forming assay data. Data represent the median ± SEM. *P < 0.05, **P < 0.005, ***P < 0.0005 (Kruskal-Wallis with Dunn’s post hoc analysis).
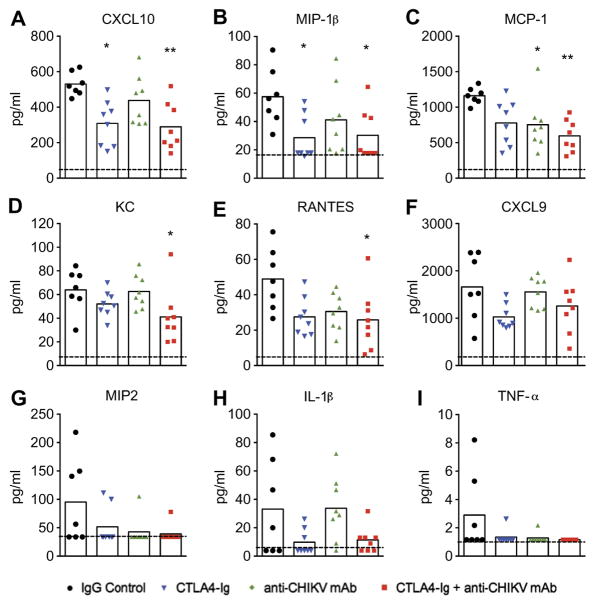
To begin to define the basis for reduced joint swelling associated with treatment, we analyzed the effects on local cytokine and chemokine production. Using a multiplexed assay, we measured cytokine and chemokine levels in the ipsilateral foot on day 7 after infection in animals that received either control mAb, CTLA4-Ig, anti-CHIKV mAb, or combination therapy with CTLA4-Ig and anti-CHIKV mAb. Monotherapy with CTLA4-Ig reduced levels of CXCL10 and macrophage inflammatory protein-1β (MIP-1β) (Fig. 4, A and B; P < 0.05), whereas treatment with anti-CHIKV mAb reduced levels of MCP-1 (Fig. 4C; P < 0.05). In comparison, combination therapy had more profound anti-inflammatory effects and resulted in decreased levels of many of the measured chemokines (for example, KC, CXCL10, MCP-1, MIP-1β, and RANTES; Fig. 4, A to I; P < 0.05) in the joint tissue.
Fig. 4. Cytokine and chemokine analysis in CHIKV-infected mice treated with CTLA4-Ig and anti-CHIKV mAb.
Mice were inoculated with 103 FFU of CHIKV via a subcutaneous route. Mice received at day 3 a single intraperitoneal injection of 600 μg of isotype control antibody, 300 μg of CTLA4-Ig, 300 μg of anti-CHIKV mAb, or a combination of 300 μg of CTLA4-Ig and 300 μg of anti-CHIKV mAb. (A to I) Cytokine and chemokine levels on day 7 in the ipsilateral foot of mice in each treatment group. Analytes included CXCL10 (A), MIP-1β (B), MCP-1 (C), KC (D), RANTES (E), CXCL9 (F), MIP2 (G), IL-1β (H), and TNF-α (I). Results are pooled from two independent experiments with n = 8 mice per treatment group. Data represent the means ± SEM. *P < 0.05, **P < 0.005 (Kruskal-Wallis with Dunn’s post hoc analysis).
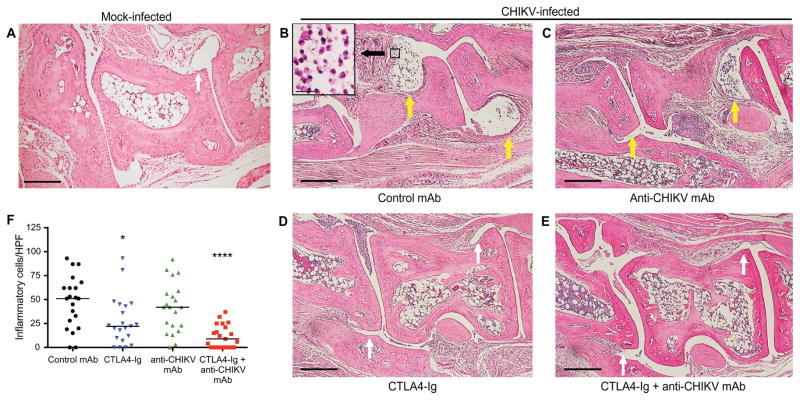
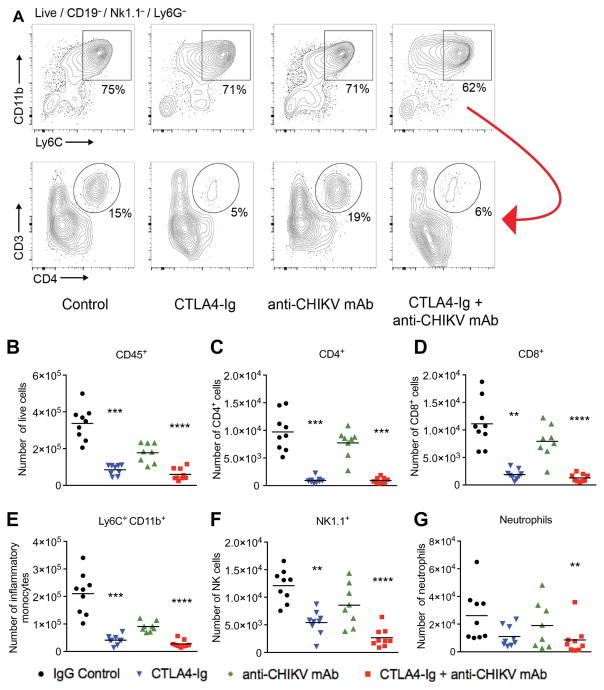
Histological analysis of joint tissues in the ipsilateral foot revealed reduced leukocyte infiltration into the midfoot joints of mice receiving CTLA4-Ig and combination therapy with CTLA4-Ig and anti-CHIKV mAb (Fig. 5, A to F; yellow arrows, moderate to severe synovitis; white arrows, absent or mild synovitis). We next quantitated the number of inflammatory cells per high-power field (HPF) in the synovial space of the midfoot. CTLA4-Ig and combination therapy with CTLA4-Ig and anti-CHIKV mAb resulted in decreased inflammatory cell infiltration into the synovial space (52 cells per HPF in the control group, 22 cells per HPF in CTLA4-Ig group, and 9 cells per HPF in the CTLA4-Ig + anti-CHIKV mAb group, P<0.05 and P < 0.0001, respectively). Histological analysis did not reveal evidence of bone erosion, proteoglycan loss, or effects on expression of receptor activator of nuclear factor κB ligand (RANKL) and osteoprotegerin (OPG) (fig. S1). Consistent with a role for CTLA4-Ig in modulating immune cell recruitment, administration of CTLA4-Ig 1 day before infection resulted in reduced swelling on day 7 but not on day 3 (fig. S2), a time point at which subcutaneous edema but not immune cell infiltration is observed. To quantitate differences in immune cell infiltration into the entire foot at day 7 after infection, we analyzed by flow cytometry how CTLA4-Ig and anti-CHIKV mAb treatments affected the total numbers of immune cells in the soft tissues of the ipsilateral foot, which included the skin, muscle, and joints. After treatment with CTLA4-Ig or a combination of CTLA4-Ig and anti-CHIKV mAb, we observed about a three- to fourfold reduction in the number of total CD45+ leukocytes (Fig. 6, A and B; P < 0.0005) with markedly reduced numbers of Ly6C+CD11b+ inflammatory monocytes, natural killer cells, and CD8+ T cells, and a nearly complete absence of CD4+ T cells (Fig. 6, B to G). By contrast, treatment with the anti-CHIKV mAb alone did not reduce the number of infiltrating CD45+ cells or individual leukocyte subsets into the infected foot (Fig. 6, A to G).
Fig. 5. Representative H&E staining of the ipsilateral midfoot joints in CHIKV-infected mice treated with CTLA4-Ig and anti-CHIKV mAb.
(A to F) Mice were inoculated with either PBS (A; mock) or 103 FFU of CHIKV (B to F) via a subcutaneous route. Animals were sacrificed, and histology of the ipsilateral foot was performed on day 7 after infection. CHIKV-infected mice received at day 3 a single intraperitoneal injection of 600 μg of isotype control antibody (B), 300 μg of anti-CHIKV mAb (C), 300 μg of CTLA4-Ig (D), or a combination of 300 μg of CTLA4-Ig and 300 μg of anti-CHIKV mAb (E). The number of inflammatory cells per HPF in the midfoot synovial space was quantitated in a blinded fashion (F). Results are representative of at least two independent experiments with n = 4 per treatment group and two sections assessed per foot. Scale bars, 200 μm. Yellow arrows, moderate to severe synovitis; white arrows, absent or mild synovitis *P < 0.05, ****P < 0.0001 (Kruskal-Wallis with Dunn’s post hoc analysis).
Fig. 6. Flow cytometry analysis of infiltrating leukocytes in the feet of mice on day 7 after infection with CHIKV.
Mice were inoculated with 103 FFU of CHIKV via a subcutaneous route. Mice received at day 3 a single intraperitoneal injection of 600 μg of isotype control antibody, 300 μg of CTLA4-Ig, 300 μg of anti-CHIKV mAb, or 300 μg of CTLA4-Ig and 300 μg of anti-CHIKV mAb. (A) Gating strategy showing subpopulations of live CD45+ cells, including the percentages of CD11b+Ly6C+ inflammatory monocytes, followed by CD3 and CD4 expression (lower panel) in the remaining Ly6C-negative cells (red arrow) isolated from the feet of mice from each treatment group. (B to G) Total number of isolated CD45+, CD4+, CD8+, Ly6C+CD11b+, NK1.1+, and Ly6G+ cells from the feet of CHIKV-infected mice in each treatment group. Results are pooled from two independent experiments with 4 to 5 mice per group in each experiment. Data represent the means ± SEM. **P < 0.005, ***P < 0.0005, ****P < 0.0001 (Kruskal-Wallis with Dunn’s post hoc analysis).
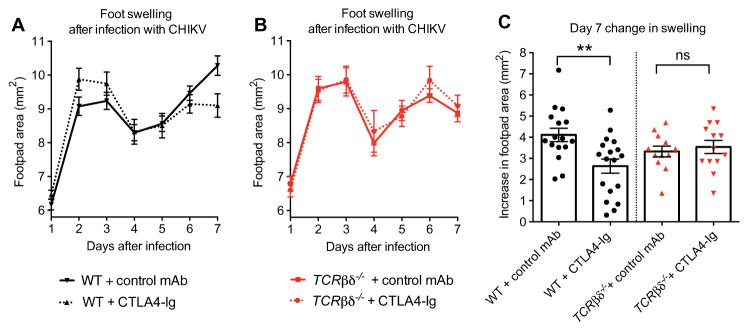
CTLA4-Ig blocks T cell costimulation, but it can also have immunomodulatory effects on antigen-presenting cells (APCs) (25, 29). Human CTLA4-Ig, which was used in our studies, binds murine B7.1 and B7.2 antigens and can modulate APC function (30, 31). We assessed APC activation on day 7 after infection but saw no difference in CD80, CD86, and class II major histocompatibility complex marker expression in APCs isolated from the spleen and feet of control and CTLA4-Ig–treated mice (fig. S3). To test whether CTLA4-Ig exerted its therapeutic benefits via T cells, we compared the response to CTLA4-Ig and a control mAb in CHIKV-infected WT and TCRβδ−/− mice, the latter of which lack both αβ and γδ T cells. As we observed a beneficial effect of CTLA4-Ig in WT animals (Fig. 7, A and C; P < 0.005) but not in TCRβδ−/− mice (Fig. 7, B and C; P > 0.9), CTLA4-Ig reduces swelling during acute CHIKV infection in part via its action on T cells.
Fig. 7. Clinical assessment of the response to CTLA4-Ig treatment in CHIKV-infected WT and TCR.
βδ−/− mice.
Mice were inoculated with 103 FFU of CHIKV via a subcutaneous route. (A and B) Foot swelling in WT (A) and TCRβδ−/− (B) mice from day 1 through 7 in mice receiving at day 3 a single intraperitoneal injection of 300 μg of isotype control antibody or 300 μg of CTLA4-Ig. (C) Increase in foot swelling (area in square millimeter) on day 7 after infection in control- and CTLA4-Ig–treated WT and TCRβδ−/− mice. ns, not significant. Measurements were conducted in a blinded fashion. Results are pooled from two or three independent experiments with total n = 16 to 18 per group for WT animals and n = 12 to 13 per group for TCRβδ−/− animals. Data represent the means ± SEM. **P < 0.005 (two-way ANOVA with multiple comparisons for swelling curves; Mann-Whitney test for day 7 swelling).
DISCUSSION
The worldwide emergence of CHIKV has created a need to identify treatments, as epidemiological estimates suggest there are millions suffering from acute arthritis and at least ~400,000 people in the Western Hemisphere suffering from chronic CHIKV arthritis (5, 8). We reasoned that established therapies used to treat other forms of inflammatory arthritis (for example, rheumatoid or psoriatic arthritis) also might mitigate CHIKV arthritis. Our experiments identified CTLA4-Ig and tofacitinib as candidate DMARD therapies based on efficacy against acute CHIKV arthritis in mice.
Immunopathology likely contributes to the pathogenesis of CHIKV arthritis. Previous studies using Rag2−/− animals suggested that the response depends on B and T cells, because Rag2−/− animals had no ipsilateral foot swelling (20). Leukocyte subsets infiltrating the peripheral joints of CHIKV-infected mice primarily consist of T cells, inflammatory monocytes, and macrophages (4). When we blocked T cell costimulation with CTLA4-Ig, we observed reduced infiltration of T cells and inflammatory monocytes into the joints of infected animals, although this was not sufficient to eliminate the clinical disease completely. Treatment of TCRβδ−/− mice with CTLA4-Ig had no effect on joint swelling, suggesting that CTLA4-Ig ameliorates clinical disease primarily via its action on T cells. These findings are consistent with previous studies suggesting that joint swelling in the foot of CD4−/− animals is reduced in severity but not completely controlled (20). Our findings suggest that other inhibitors of CD4+ T cell function also might limit musculoskeletal disease associated with CHIKV infection. Teo et al. (32) recently demonstrated that treatment with fingolimod, an agonist of the sphingosine 1-phosphate receptor, limited infiltration of CD4+ T cells into CHIKV-infected joints and adjacent muscle in mice, resulting in reduced joint swelling and muscle necrosis.
Previous studies showed that mouse and human antiviral antibodies could prevent CHIKV arthritis in mice when administered before infection (28) or protect against lethality in highly immunocompromised Ifnar1−/− mice (22, 27). We found that treatment of WT mice 3 days after CHIKV infection with an antiviral human mAb reduced but did not eliminate joint swelling, although infectious virus could not be detected in the joints within 2 days of therapy. Foot swelling likely depends on multiple factors including synovitis, myositis, and edema resulting from production of proinflammatory cytokines (4). The anti-CHIKV mAb eliminated infectious virus within 2 days of its administration and reduced foot swelling without altering leukocyte infiltration into joints of infected animals, suggesting that leukocyte recruitment is not the only factor that affects disease severity in this model. Local cytokine production in specific compartments (for example, serum, muscles, or joints) independently may affect the virus-induced swelling or edema. Future histological studies may define better the precise mechanism by which neutralization of infectious CHIKV by antiviral antibodies ameliorates foot swelling.
The type I interferon (IFN) response is important in controlling CHIKV infection. Ifnar1−/− mice lacking type I IFN signaling are highly vulnerable to disseminated infection with CHIKV and succumb within days due to high levels of virus in the brain and spinal cord (33). Remarkably, treatment with tofacitinib, which blocks JAK/STAT signaling that is downstream of Ifnar1 and other cytokine receptors, reduced clinical disease without ostensible effects on morbidity or mortality. This result may be related to the pharmacodynamics of tofacitinib including its short half-life (13). Tofacitinib therapy may blunt cytokine production and/or leukocyte infiltration without enhancement of CHIKV replication. Nevertheless, because tofacitinib is known to enhance the risk of some viral infections, including varicella zoster virus (34), future combination therapy studies with tofacitinib and an antiviral mAb may be warranted.
Anecdotal reports in humans have suggested that methotrexate may be effective for treatment of CHIKV arthritis (16, 35). In our studies, a low dose of methotrexate did not provide benefit against acute arthritis. It remains possible that methotrexate would show greater benefit if higher doses were used. Mouse models of Ross River virus arthritis previously revealed exacerbation of disease after treatment with etanercept (36), whereas an uncontrolled study in humans suggested that blockade of TNF-α provided some benefit in 13 patients who were diagnosed with a “chronic rheumatologic disease” after acute CHIKV infection (35). However, it is important to note that some patients infected with CHIKV may develop rheumatologic disease sporadically or coincidentally after CHIKV infection. In a controlled study of mice infected with CHIKV, we did not find a benefit of etanercept therapy with acute CHIKV arthritis, but we were unable to assess chronic disease because of the histopathological absence of frank arthritis during the chronic phase. In light of our animal model results and the anecdotal nature of previous human studies, it seems that rigorous, blinded, placebo-controlled studies in human patients are necessary to define which DMARD therapies may be safe and optimal in patients with chronic CHIKV arthritis.
CHIKV and related arthritogenic alphaviruses directly invade the joints and leave persistent viral RNA in the joints and surrounding tissues even in the absence of detectable infectious virus (28). In previous studies, we found that most CHIKV-infected control C57BL/6 mice did not exhibit histopathological evidence of chronic arthritis 1 month after infection, although there was mild chronic tenosynovitis and myositis in some animals (4). Because we could readily detect viral RNA in the joints at day 28, our studies suggest that persistent viral RNA may not be sufficient to cause clinically apparent chronic arthritis in mice and that other factors must contribute to pathogenesis. Thus, it remains to be determined whether our combination treatment with an antiviral antibody and CTLA4-Ig has beneficial effects in chronic CHIKV arthritis. Other animal models with more severe chronic disease (for example, nonhuman primates) may be required to address these questions.
The beneficial effects of CTLA4-Ig and antiviral antibody therapy on CHIKV arthritis must be interpreted with caution because there are limitations with the mouse model. Unlike humans, mice are not natural hosts for CHIKV and therefore do not develop the severe, debilitating arthritis that is commonly observed in humans. Because CHIKV causes less severe disease in mice, it remains possible that immunomodulatory therapies, including CTLA4-Ig, may have no or even deleterious effects in humans.
The concept of combination antiviral and immunomodulatory therapy is a unique approach for the treatment of infectious diseases. The near-complete effectiveness of combination therapy in our mouse model of acute CHIKV arthritis has implications for treatment of other viral infections in which both virus- and immune-mediated pathology result in morbidity and mortality (for example, influenza and severe acute respiratory syndrome coronavirus). Given the large number of clinically available biological and small molecular DMARDs, this work may provide greater impetus for studies that test combination antiviral and immunomodulatory therapies for the treatment of infectious diseases.
MATERIALS AND METHODS
Study design
We initiated this study to determine whether anti-inflammatory and antiviral therapy control acute CHIKV arthritis in mice. Our initial observation was that treatment with antiviral antibody reduced infectious viral burden in the ipsilateral joint and that therapy with CTLA4-Ig diminished recruitment of T cells and inflammatory monocytes as well as the accumulation of proinflammatory cytokines. Subsequent histological analysis confirmed these findings. We measured viral titers and RNA in tissues, immunologic parameters (including influx of specific cell subtypes into the joints and surrounding tissues), and cytokine levels. Sample sizes and end points were selected on the basis of our extensive experience with these systems. Mice were age- and sex-matched between groups. Histological analysis was performed in a blinded fashion. Initial footpad measurements were performed by three individuals in an unblinded fashion. However, the measurements after CTLA4-Ig treatment were reproduced by a fourth individual who performed a blinded assessment. Investigators were not blinded when conducting virus tissue burden analysis, cytokine measurements, or flow cytometry analysis. All primary data are in the Supplementary Materials (table S1).
Mouse experiments
All animal experiments were performed in accordance and with approval of Washington University Institutional Animal Care and Use Committee guidelines, and all mouse infection studies were performed in an animal biosafety level 3 laboratory. All experiments were performed with 4-week-old C57BL/6 mice or with TCRβδ−/− mice that were obtained commercially (The Jackson Laboratories).
Virus infection studies
A recombinant LR2006 OPY1 strain of CHIKV was provided by S. Higgs (Kansas State University) and generated from in vitro transcribed cDNA, as previously described (37). At 4 weeks of age, mice were inoculated in the left rear footpad with 103 FFU of the LR2006 OPY1 strain of CHIKV in a volume of 10 μl. Infected mice were monitored daily for foot swelling with digital calipers for 28 days. At the termination of experiments, mice were sedated with a ketamin-exylazene cocktail and euthanized, and perfused via intracardiac injection with PBS. Tissues (injected left ankle, contralateral ankle, and ipsilateral wrist) were harvested and snap-frozen on dry ice and stored at −80°C until processing for RNA isolation. For serum analysis, blood was collected at the time of sacrifice and centrifuged for 10 min at 10,000 g and stored at −80°C. In some experiments, serum and joint tissues were isolated from mice on day 5 after infection for subsequent analysis by focus-forming assay.
Tissue viral burden analysis
Focus-forming assays were performed as previously described (22). Briefly, tissue homogenates or serum were incubated for 90 min on a monolayer of Vero cells in 96-well plates, and then cells were overlaid with 1% (w/v) methylcellulose in modified Eagle media supplemented with 4% fetal bovine serum(FBS). Plates were harvested 18 to 24 hours later and fixed with 1% paraformaldehyde (PFA) in PBS. The plates were incubated sequentially with chimeric CHK-9 (500 ng/ml) (22) and horseradish peroxidase–conjugated goat anti-human IgG in PBS supplemented with 0.1% saponin and 0.1% bovine serum albumin. CHIKV-infected foci were visualized using TrueBlue peroxidase substrate (KPL) and quantitated on an ImmunoSpot 5.0.37 macro-analyzer (Cellular Technologies Ltd).
Real-time qRT-PCR analysis
RNA was extracted from tissue using the RNeasy Mini Kit (Qiagen). The quantity of CHIKV RNA was determined by qRT-PCR using the TaqMan RNA-to-CT 1-Step Kit (Applied Biosystems) with an E1-specific primer/probe set (38). Two microliters of the isolated RNA was analyzed by qRT-PCR and compared to a standard curve generated from RNA isolated from a CHIKV stock to determine FFU equivalents.
Therapeutic agents
4N12 is a human IgG1 mAb that neutralizes CHIKV infection and has been described previously (27). Antibody was purified from hybridoma supernatants by protein G affinity chromatography. Methotrexate, CTLA4-Ig (abatacept), and etanercept were obtained from the Center for Advanced Medicine Rheumatology Clinic (St. Louis, MO). Naproxen sodium and methylprednisolone acetate were purchased from Sigma. The isotype control antibody [humanized anti–West Nile virus antibody (E16)] was produced in Chinese hamster ovary cells and purified by protein G affinity chromatography (39).
Histopathological analysis
Infected mice were sacrificed and perfused by intracardiac injection of 4% PFA at the indicated days after infection. The infected ankle/foot tissue was dissected and fixed in 4% PFA/PBS for 48 hours, followed by decalcification in 14% acid-free EDTA for 10 to 14 days. Decalcified tissues were embedded in paraffin, and 5-μm sections were stained with hematoxylin and eosin (H&E) and evaluated by light microscopy. Embedding, sectioning, and staining were performed by the Musculoskeletal Histology and Morphometry Core at Washington University School of Medicine. All samples were visualized using a Nikon Eclipse microscope equipped with a QICAM 12-bit camera (QImaging) and processed with QCapture software using the same exposure times.
Cytokine/chemokine analysis
Ankles were harvested from euthanized infected mice at day 7 and collected in 500-μl PBS and homogenized using a MagNA Lyser (Roche). Cytokine levels were measured using Luminex technology with a Bio-Plex Pro mouse cytokine 13-plex assay (Millipore).
Immune cell analysis
Mice were sacrificed 7 days after inoculation and perfused with PBS. The inoculated foot was disarticulated at the ankle without fracturing the bone. Cutaneous and subcutaneous tissue were everted but still attached to the distal foot and digits during digestion. Tissues were incubated for 2 hours at 37°C in digestion buffer [RPMI, type I collagenase (2.5 mg/ml) (Sigma), DNase I (10 mg/ml) (Sigma), 15 mM Hepes, 10% FBS]. Digested tissues were passed through a 70-μm cell strainer. The number of viable cells was quantified by trypan blue staining. Isolated cells were incubated with anti-mouse CD16/CD32 (Clone 93; BioLegend) for 10 min at 4°C and then surface-stained in PBS containing 5% FBS for 30 min at 4°C with the following antibodies: anti–CD3e-FITC (fluorescein isothiocyanate) (eBioscience), anti–CD4-PE (phycoerythrin) (BD Biosciences), anti–CD8a-PerCP/Cy5.5 (BioLegend), anti–NK1.1-PE/Cy7 (BioLegend), anti–CD45-Brilliant Violet 605 (BioLegend), anti–Ly6C-Brilliant Violet 421 (BD Biosciences), anti–Ly6G–Alexa Fluor 700 (BioLegend), and anti–CD19-APC/Cy7 (BioLegend). Cells were washed and incubated at room temperature for 30 min in Foxp3 Fixation/Permeabilization buffer (eBioscience). The fixed cells were washed with Permeabilization buffer (eBioscience) and stained in Permeabilization buffer overnight at 4°C with anti–Foxp3–Alexa Fluor 647 (BioLegend). Cells were run on a LSR II (Becton Dickinson) flow cytometer and analyzed using BD FACSDiva and FlowJo software.
Statistical analysis
All data were analyzed with GraphPad Prism software. For viral burden analysis, cytokine measurements, and numbers of infiltrating leukocytes, data were analyzed by the Mann-Whitney test, ANOVA, or Kruskal-Wallis test with a Dunn’s post hoc analysis. P < 0.05 indicated statistically significant differences.
Acknowledgments
We thank G. Sapparapu, P. Matta, H. King, and R. Lampley (Vanderbilt University) for technical support.
Funding: This work was supported by grants from the NIH (R01 AI073755 and R01 AI104972 to M.S.D., and R01 AI114816 to J.E.C. and M.S.D.) and the Rheumatology Research Foundation (Innovative Research Grant to D.J.L.). J.J.M. was supported by a Rheumatology Research Foundation Scientist Development Award. A.R.Y. was supported by a NIGMS Cellular, Biochemical, and Molecular (CMB) Sciences Predoctoral Research Training Grant (GM: 007 067). Histological sections were prepared by the Washington University Musculoskeletal Research Center (NIH P30 AR057235).
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/9/375/eaah3438/DC1
Fig. S1. RANKL and OPG expression in CHIKV-infected joints.
Fig. S2. Foot swelling with CTLA4-Ig therapy administered before or during CHIKV infection.
Fig. S3. Flow cytometry analysis of APC activation in the spleen and ankles of mice on day 7 after infection with CHIKV.
Table S1. Primary data
Author contributions: Study concept and design: J.J.M., D.J.L., and M.S.D. Data acquisition: J.J.M. and A.M.S. (drug administration, all combination therapy experiments, RNA isolation, qRT-PCR analysis, tissue harvests, cytokine assays, histological sample acquisition, and flow cytometry), L.E.C. (infections, tissue harvests, histological analysis, flow cytometry, and cytokine assays), R.M.S. (drug screen foot measurements), J.P.H. (flow cytometry and TCRβδ−/− experiments), J.M.R. (flow cytometry), and S.P., A.R.Y., and K.M. (sample acquisition/harvesting). Analysis and interpretation of data: J.J.M., L.E.C., J.P.H., A.M.S., J.M.R., D.J.L., and M.S.D. Provided critical reagents: J.E.C. Initial drafting of the manuscript: J.J.M. and M.S.D. Critical revision of the manuscript: J.J.M., L.E.C., J.E.C., D.J.L., and M.S.D.
Competing interests: M.S.D. is a consultant for InBios, Visterra, Sanofi, and Takeda Pharmaceuticals; is on the Scientific Advisory Boards of Moderna and OraGene; and is a recipient of research grants from Moderna, Sanofi, and Visterra. J.E.C. is a consultant for Sanofi; is on the Scientific Advisory Boards of PaxVax, CompuVax, GigaGen, Meissa Vaccines, and Rensavir; is a co-inventor of the 4N12 antibody for which a patent application has been filed; and is a recipient of research grants from Moderna and Sanofi. All other authors declare that they have no competing interests.
Data and materials availability: The data sets, materials, and analysis generated during the current study are available from the corresponding author on reasonable request.
REFERENCES AND NOTES
- 1.Ross RW. The Newala epidemic: III. The virus: Isolation, pathogenic properties and relationship to the epidemic. J Hyg. 1956;54:177–191. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen LR, Powers AM. Chikungunya: Epidemiology. F1000Res. 2016;5:F1000. doi: 10.12688/f1000research.7171.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miner JJ, Aw Yeang HX, Fox JM, Taffner S, Malkova ON, Oh ST, Kim AHJ, Diamond MS, Lenschow DJ, Yokoyama WM. Brief report: Chikungunya viral arthritis in the United States: A mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol. 2015;67:1214–1220. doi: 10.1002/art.39027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison TE, Oko L, Montgomery SA, Whitmore AC, Lotstein AR, Gunn BM, Elmore SA, Heise MT. A mouse model of chikungunya virus–induced musculoskeletal inflammatory disease: Evidence of arthritis, tenosynovitis, myositis, and persistence. Am J Pathol. 2011;178:32–40. doi: 10.1016/j.ajpath.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Morales AJ, Gil-Restrepo AF, Ramírez-Jaramillo V, Montoya-Arias CP, Acevedo-Mendoza WF, Bedoya-Arias JE, Chica-Quintero LA, Murillo-García DR, García-Robledo JE, Castrillón-Spitia JD, Londoño JJ, Bedoya-Rendón HD, Cárdenas-Pérez JJ, Cardona-Ospina JA, Lagos-Grisales GJ. Post-chikungunya chronic inflammatory rheumatism: Results from a retrospective follow-up study of 283 adult and child cases in La Virginia, Risaralda, Colombia. F1000Res. 2016;5:360. doi: 10.12688/f1000research.8235.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, Arvin-Berod C, Paganin F. Persistent arthralgia associated with chikungunya virus: A study of 88 adult patients on reunion island. Clin Infect Dis. 2008;47:469–475. doi: 10.1086/590003. [DOI] [PubMed] [Google Scholar]
- 7.Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, Ledrans M, Pierre V. Post-epidemic chikungunya disease on reunion island: Course of rheumatic manifestations and associated factors over a 15-month period. PLOS Negl Trop Dis. 2009;3:e389. doi: 10.1371/journal.pntd.0000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Morales AJ, Cardona-Ospina JA, Villamil-Gómez W, Paniz-Mondolfi AE. How many patients with post-chikungunya chronic inflammatory rheumatism can we expect in the new endemic areas of Latin America? Rheumatol Int. 2015;35:2091–2094. doi: 10.1007/s00296-015-3302-5. [DOI] [PubMed] [Google Scholar]
- 9.Manimunda SP, Vijayachari P, Uppoor R, Sugunan AP, Singh SS, Rai SK, Sudeep AB, Muruganandam N, Chaitanya IK, Guruprasad DR. Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Trans R Soc Trop Med Hyg. 2010;104:392–399. doi: 10.1016/j.trstmh.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Fourie ED, Morrison JG. Rheumatoid arthritic syndrome after chikungunya fever. S Afr Med J. 1979;56:130–132. [PubMed] [Google Scholar]
- 11.Bouquillard E, Combe B. Rheumatoid arthritis after Chikungunya fever: A prospective follow-up study of 21 cases. Ann Rheum Dis. 2009;68:1505–1506. doi: 10.1136/ard.2008.097626. [DOI] [PubMed] [Google Scholar]
- 12.Larsen A, Thoen J. Hand radiography of 200 patients with rheumatoid arthritis repeated after an interval of one year. Scand J Rheumatol. 1987;16:395–401. doi: 10.3109/03009748709165409. [DOI] [PubMed] [Google Scholar]
- 13.Dowty ME, Jesson MI, Ghosh S, Lee J, Meyer DM, Krishnaswami S, Kishore N. Preclinical to clinical translation of tofacitinib, a Janus kinase inhibitor, in rheumatoid arthritis. J Pharmacol Exp Ther. 2014;348:165–173. doi: 10.1124/jpet.113.209304. [DOI] [PubMed] [Google Scholar]
- 14.Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, Gruben D, Wallenstein GV, Zwillich SH, Kanik KS. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 15.Chopra A, Saluja M, Venugopalan A. Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheumatol. 2014;66:319–326. doi: 10.1002/art.38221. [DOI] [PubMed] [Google Scholar]
- 16.Pandya S. Methotrexate and hydroxychloroquine combination therapy in chronic chikungunya arthritis: A 16 week study. Indian J Rheumatol. 2008;3:93–97. [Google Scholar]
- 17.Gardner J, Anraku I, Le TT, Larcher T, Major L, Roques P, Schroder WA, Higgs S, Suhrbier A. Chikungunya virus arthritis in adult wild-type mice. J Virol. 2010;84:8021–8032. doi: 10.1128/JVI.02603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goupil BA, McNulty MA, Martin MJ, McCracken MK, Christofferson RC, Mores CN. Novel lesions of bones and joints associated with chikungunya virus infection in two mouse models of disease: New insights into disease pathogenesis. PLOS ONE. 2016;11:e0155243. doi: 10.1371/journal.pone.0155243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakaya HI, Gardner J, Poo YS, Major L, Pulendran B, Suhrbier A. Gene profiling of Chikungunya virus arthritis in a mouse model reveals significant overlap with rheumatoid arthritis. Arthritis Rheum. 2012;64:3553–3563. doi: 10.1002/art.34631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teo TH, Lum FM, Claser C, Lulla V, Lulla A, Merits A, Rénia L, Ng LFP. A pathogenic role for CD4+ T cells during Chikungunya virus infection in mice. J Immunol. 2013;190:259–269. doi: 10.4049/jimmunol.1202177. [DOI] [PubMed] [Google Scholar]
- 21.Rulli NE, Rolph MS, Srikiatkhachorn A, Anantapreecha S, Guglielmotti A, Mahalingam S. Protection from arthritis and myositis in a mouse model of acute Chikungunya virus disease by bindarit, an inhibitor of monocyte chemotactic protein-1 synthesis. J Infect Dis. 2011;204:1026–1030. doi: 10.1093/infdis/jir470. [DOI] [PubMed] [Google Scholar]
- 22.Pal P, Dowd KA, Brien JD, Edeling MA, Gorlatov S, Johnson S, Lee I, Akahata W, Nabel GJ, Richter MKS, Smit JM, Fremont DH, Pierson TC, Heise MT, Diamond MS. Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLOS Pathog. 2013;9:e1003312. doi: 10.1371/journal.ppat.1003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goh LYH, Hobson-Peters J, Prow NA, Gardner J, Bielefeldt-Ohmann H, Pyke AT, Suhrbier A, Hall RA. Neutralizing monoclonal antibodies to the E2 protein of chikungunya virus protects against disease in a mouse model. Clin Immunol. 2013;149:487–497. doi: 10.1016/j.clim.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Selvarajah S, Sexton NR, Kahle KM, Fong RH, Mattia KA, Gardner J, Lu K, Liss NM, Salvador B, Tucker DF, Barnes T, Mabila M, Zhou X, Rossini G, Rucker JB, Sanders DA, Suhrbier A, Sambri V, Michault A, Muench MO, Doranz BJ, Simmons G. A neutralizing monoclonal antibody targeting the acid-sensitive region in Chikungunya virus E2 protects from disease. PLOS Negl Trop Dis. 2013;7:e2423. doi: 10.1371/journal.pntd.0002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 26.Liu R, Bal HS, Desta T, Behl Y, Graves DT. Tumor necrosis factor-α mediates diabetes-enhanced apoptosis of matrix-producing cells and impairs diabetic healing. Am J Pathol. 2006;168:757–764. doi: 10.2353/ajpath.2006.050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SA, Silva LA, Fox JM, Flyak AI, Kose N, Sapparapu G, Khomandiak S, Ashbrook AW, Kahle KM, Fong RH, Swayne S, Doranz BJ, McGee CE, Heise MT, Pal P, Brien JD, Austin SK, Diamond MS, Dermody TS, Crowe JE., Jr Isolation and characterization of broad and ultrapotent human monoclonal antibodies with therapeutic activity against chikungunya virus. Cell Host Microbe. 2015;18:86–95. doi: 10.1016/j.chom.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawman DW, Stoermer KA, Montgomery SA, Pal P, Oko L, Diamond MS, Morrison TE. Chronic joint disease caused by persistent Chikungunya virus infection is controlled by the adaptive immune response. J Virol. 2013;87:13878–13888. doi: 10.1128/JVI.02666-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutolo M, Soldano S, Montagna P, Sulli A, Seriolo B, Villaggio B, Triolo P, Clerico P, Felli L, Brizzolara R. CTLA4-Ig interacts with cultured synovial macrophages from rheumatoid arthritis patients and downregulates cytokine production. Arthritis Res Ther. 2009;11:R176. doi: 10.1186/ar2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Jones B, Brady W, Janeway CA, Jr, Linley PS. Co-stimulation of murine CD4 T cell growth: Cooperation between B7 and heat-stable antigen. Eur J Immunol. 1992;22:2855–2859. doi: 10.1002/eji.1830221115. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Guo Y, Liu Y. A major costimulatory molecule on antigen-presenting cells, CTLA4 ligand A, is distinct from B7. J Exp Med. 1993;178:1789–1793. doi: 10.1084/jem.178.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teo TH, Chan YH, Lee WWL, Lum FM, Amrun SN, Her Z, Rajarethinam R, Merits A, Rotzschke O, Renia L, Ng LFP. Fingolimod treatment abrogates chikungunya virus–induced arthralgia. Sci Transl Med. 2017;9:eaal1333. doi: 10.1126/scitranslmed.aal1333. [DOI] [PubMed] [Google Scholar]
- 33.Couderc T, Chrétien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Desprès P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. A mouse model for Chikungunya: Young age and inefficient type-i interferon signaling are risk factors for severe disease. PLOS Pathog. 2008;4:e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, Koncz T, Krishnaswami S, Wallenstein GV, Zang C, Zwillich SH, van Vollenhoven RF. ORAL Start Investigators, Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 35.Blettery M, Brunier L, Polomat K, Moinet F, Deligny C, Arfi S, Jean-Baptiste G, De Bandt M. Management of chronic post-Chikungunya rheumatic disease: The martinican experience. Arthritis Rheumatol. 2016;68:2817–2824. doi: 10.1002/art.39775. [DOI] [PubMed] [Google Scholar]
- 36.Zaid A, Rulli NE, Rolph MS, Suhrbier A, Mahalingam S. Disease exacerbation by etanercept in a mouse model of alphaviral arthritis and myositis. Arthritis Rheum. 2011;63:488–491. doi: 10.1002/art.30112. [DOI] [PubMed] [Google Scholar]
- 37.Vanlandingham DL, Tsetsarkin K, Hong C, Klingler K, McElroy KL, Lehane MJ, Higgs S. Development and characterization of a double subgenomic chikungunya virus infectious clone to express heterologous genes in Aedes aegypti mosqutioes. Insect Biochem Mol Biol. 2005;35:1162–1170. doi: 10.1016/j.ibmb.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Bellini R, Medici A, Calzolari M, Bonilauri P, Cavrini F, Sambri V, Angelini P, Dottori M. Impact of Chikungunya virus on Aedes albopictus females and possibility of vertical transmission using the actors of the 2007 outbreak in Italy. PLOS ONE. 2012;7:e28360. doi: 10.1371/journal.pone.0028360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremont DH, Diamond MS. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]