Abstract
Lingual fatty acid receptors (i.e. CD36) mediate the orosensory perception of fat/fatty acids and may contribute to the susceptibility to develop obesity. The current study tested the hypothesis that fat/fatty acid preference in obesity-prone (OP, Osborne-Mendel) and obesity-resistant (OR, S5B/Pl) rats is mediated by nutritional status and lingual CD36. To determine if nutritional status affected linoleic acid (LA) preference in OP and OR rats, rats were either fasted overnight or fed a high fat diet (60% kcal from fat). In OR rats, fasting increased the preference for higher concentrations of LA (1.0%), while consumption of a high fat diet decreased LA preference. In OP rats, fasting increased the preference for lower concentrations of LA (0.25%), however high fat diet consumption did not alter LA preference. To determine if lingual CD36 mediated the effects of an overnight fast on LA preference, the expression of lingual CD36 mRNA was assessed and the effect of lingual application of CD36 siRNA on LA preference was determined. Fasting increased lingual CD36 mRNA expression in OR rats, but failed to alter lingual CD36 mRNA in OP rats. Following an overnight fast, application of lingual CD36 siRNA led to a decrease in LA preference in OR, but not OP rats. Lingual application of CD36 siRNA was also used to determine if lingual CD36 mediated the intake and preference for a high fat diet in OP and OR rats. CD36 siRNA decreased the preference and intake of high fat diet in OR rats, but not OP rats. The results from this study suggest that the dysregulation of lingual CD36 in OP rats is a potential factor leading to increased fat intake and fat preference and an enhanced susceptibility to develop obesity.
Keywords: obesity-prone, obesity-resistant, fat preference, fat taste, CD36
1. Introduction
The prevalence of obesity has been linked to an increase in the consumption of energy dense, palatable foods, particularly those high in fat [1]. The intake of highly palatable foods is a complex phenomenon that involves the processing of orosensory information by the central nervous system [2–6]. Therefore, the role of lingual fat sensors on dietary fat consumption has recently been investigated [2, 7–30]. Numerous studies report reduced orosensory fat perception (i.e. hyposensitivity) in obese people [31–42]. A potential mechanism for this hyposensitivity is the lingual CD36 receptor, which is located primarily on the circumvallate papillae (CV) of the tongue, and is considered the fat taste receptor. CD36 knock-out mice exhibit reduced fat preference, food intake, body weight and adiposity [7, 15, 17, 27, 43–45]. An increase in lingual CD36 mRNA expression has been reported in obesity-prone, Osborne-Mendel rats, but not obesity-resistant, S5B/Pl rats consuming a high fat diet (HFD) and lingual application of CD36 siRNA reduces the preference for the preferred concentration of linoleic acid (LA) in these models [11, 25]. In rats that are neither obesity-prone or obesity-resistant, long-term consumption of HFD decreases lingual CD36 mRNA and protein levels [30]. In obese people, a common variant in the CD36 gene (i.e. SNP rs1761667-A allele) has been associated with hyposensitive orosensory fat perception [37, 41, 46]. These studies support a role for lingual CD36 as a mediator of the orosensory perception of dietary fat and suggest that a dysregulation of CD36 may predispose an individual to developing obesity.
Though obesity is associated with the overconsumption of high fat foods [1, 47, 48], differences in the susceptibility to develop HFD-induced obesity exist [18, 22, 46, 48–51]. The orosensory perception of fat is a prospective contributor to individual differences in the susceptibility to becoming obese [11, 22, 25, 46, 49, 50, 52]. Models that differ in their susceptibility to develop obesity, provide a valuable tool to study the dysregulation of hedonic and homeostatic systems regulating energy intake. These models allow for the isolation of mediators of obesity and the discovery of factors which control obesity-resistance, making these models necessary for the development of strategies to combat diet-induced obesity. The obesity-prone Osborne-Mendel (OP) and obesity-resistant S5B/Pl (OR) rats have been utilized to investigate behavioral, physiological and neurochemical responses to HFD [53–61]. OP rats gain more weight and more adiposity when eating a HFD, than OR rats when consuming the same HFD. The effects of various compounds on the intake and preference for a HFD have been examined in these strains, as well as the effects of HFD on alterations in hypothalamic neurochemistry, circulating hormone levels and intestinal gene expression [53, 57, 60–65].
The goal of the current series of experiments was to test the hypothesis that fat/fatty acid preference in OP and OR rats was mediated by nutritional status and lingual CD36. The first experiment assessed the effects of either an overnight fast or HFD consumption on LA preference in OP and OR rats. The second experiment assessed lingual CD36 mRNA expression following an overnight fast in OP and OR rats and whether lingual application of CD36 siRNA decreased LA preference following an overnight fast. The final experiment investigated the role of lingual CD36 on HFD preference in OP and OR rats following the lingual application of CD36 siRNA.
2. Methods
2.1 Animals
The male obesity-prone Osborne-Mendel (OP) and obesity-resistant S5B/Pl (OR) rats (8–9 weeks old) used in these studies were bred in the AAALAC approved Pennington Biomedical Research Center vivarium. All rats were individually housed on a 12h/12h light/dark cycle (lights on at 0700) with food and water available ad libitum, unless otherwise described. All procedures were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee.
2.2 Effects of fasting on linoleic acid preference
To assess the effects of fasting on linoleic acid (LA) preference, OP and OR rats were either fasted overnight (16h) (OP n=8; OR n=7) or maintained on ad libitum standard laboratory chow (Laboratory Rodent Diet 5001, 13% kilocalories from fat, 30% kilocalories from protein, 57% kilocalories from carbohydrates; LabDiet) (OP n=8; OR n=8) and then given access to increasing concentrations of LA. For LA preference testing, all rats were given access to two water bottles, one bottle containing LA (in 0.3% Xanthan gum and deionized water) and one bottle containing 0.3% Xanthan gum in deionized water, as previously described [11, 27]. Bottle position was counterbalanced between rats to control for side preferences and consumption was measured by weighing each bottle (g). Following access to the LA, rats received deionized water in both bottles for a 72h washout period. LA preference was assessed for increasing concentrations of LA (0.0025, 0.025, 0.25, 0.5, 1.0 w/w). Testing was repeated until rats had access to all concentrations of LA. Preference for each concentration of LA was calculated by the formula (LA solution consumed (g)/(LA solution consumed (g) + Xanthan gum solution consumed (g))*100. In this experiment, the fasted group had access to LA for 1h and the ad libitum chow fed group had access to LA for 48h. For the ad libitum fed group, at 24h, solutions were replaced with fresh solutions and bottle position was reversed to control for side preferences. The average LA preference for the first 24h and the second 24h was determined and used as overall LA preference. A LA preference of 50% is indicative of no preference between the solutions and suggests that the rats are unable to significantly distinguish the LA from the Xanthan gum solution and is therefore an indicator of orosensory perception.
2.3 Effects of high fat diet consumption on linoleic acid preference
To assess the effects of high fat diet (HFD) consumption on LA preference, OP (n=5) and OR (n=5) rats were given ad libitum access to a HFD (56% kilocalories from fat, 24% kilocalories from protein, 20% kilocalories from carbohydrates, Research Diets #D01080902) [25] for 2 weeks or maintained on a standard chow diet prior to LA preference testing. Increasing concentrations of LA were presented for 48h. LA concentrations and preference assessment were described in Section 2.2.
2.4 Role of lingual CD36 on fasting-induced alterations in linoleic acid preference
To investigate the effects of fasting on lingual CD36 mRNA expression, OP and OR rats were either fed ad libitum standard chow (OP n=6; OR n=6), fasted overnight (16h) (OP n=9; OR n=6) or fasted overnight (16h) and refed for 2h (OP n=7; OR n=7). The circumvallate papillae of the tongue (CV) was harvested immediately following sacrifice. For this procedure, the tongue was removed, cleaned and the epithelial layer of the tongue, encompassing the CV, was excised using a sterile scalpel blade [11, 25]. Samples were immediately frozen on dry ice and stored at −80°C until further processing.
In a separate group of OP (n=5) and OR (n=5) rats, RNA interference techniques were used to decrease the gene expression of lingual CD36 as previously described [11]. To determine if lingual CD36 regulated fasting-induced increases in LA preference, preference was assessed following an overnight fast (16h) and access to LA began three hours following the 5th lingual CD36 siRNA application. A concentration of 0.25% LA was chosen because fasted OP rats exhibited a higher preference for this concentration. As described in Section 2.2, OP and OR rats were given access to LA and the control solution for 1h, intake (g) was measured and LA preference was determined. Four hours following access to LA, the CV was harvested for assessment of lingual CD36 mRNA expression using Real Time PCR.
2.4.1 Real Time Polymerase Chain Reaction (PCR)
RNA was isolated from the CV using Tri-Reagent (Molecular Research Ctr, Cincinnati, OH USA) and RNeasy Minikit procedures (Qiagen, Valencia, CA USA) and based on previous experiments [11, 25]. CV were homogenized in Tri-Reagent using a motorized tissue homogenizer, chloroform was added to the lysate, and the mixture was centrifuged (12,000xg) in phase lock tubes to separate RNA. Ethanol (70%) was added to the upper aqueous phase, applied to column and filtered by centrifugation (8000xg). Following multiple washes, the samples were subjected to an elution step using RNAase-free water. Reverse transcription was conducted using the High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, Foster City, CA, USA). For RT, 2.0μg of RNA from each sample was added to random primers (10x), dNTP (25x), MultiScribe Reverse Transcriptase (50U/μl) and RT buffer (10x) and incubated in a thermal cycler (MyCycler Thermal Cycler, BioRad, Hercules, CA) for 10 min at 27°C, then for 120 min at 37°C. Primers were designed using Primer Express (Applied Biosystems, Foster City, CA, USA). The following primers were used for CD36: 5′-GAGGTCCTTACACATACAGAGTTCGTT-3′ and 5′-ACAGACAGTGAAGGCTCAAAGATG -3′ and Cyclophilin: 5′-CCCACCGTGTTCTTCGACAT -3′ AND 5′-CTGTCTTTGGAACTTTGTCCTGCAA -3′. For Real Time PCR, SYBR Green 2x Master Mix), forward and reverse primers (10μM), and RT product (10ng) were added to 384 well plates (ABI Prism 7900 Sequence Detection System, Applied Biosystems). The cycling parameters consisted of an initial 2 min incubation at 50 °C, followed by 10 min at 95 °C, then 15 sec at 95 °C, and a 1 min annealing/extension step at 60 °C (40 cycles). The quantity of CD36 mRNA levels were based on a standard curve and normalized to cyclophilin levels.
2.4.2 Lingual CD36 siRNA application
CD36 siRNA was commercially designed and processed for in vivo use (Dharmacon, Inc./Thermo Fisher Scientific, Chicago, IL). Additionally, a non-targeting sequence (siGENOME Non-Targeting siRNA) was used as a control. siRNA was prepared as suggested by manufacturer prior to use. For application of siRNA, rats were anesthetized with isoflurane (1.5–3% in oxygen) between 0900–1000h. Once anesthetized, the rats were placed on their side, their mouth was opened and large forceps were placed in their mouths, just behind their teeth in order to keep the mouth open during siRNA application. The tongue was gently extended forward until the CV was visualized. For application of siRNA, 5μl of siRNA was slowly pipetted onto the most caudal ¼ region of the tongue in a circular pattern. The mouth remained open for 3–5 min to allow for absorption of the siRNA. Following this procedure, rats were returned to their home cage. This process was repeated for 5 consecutive days at the same time of day as previously described [11].
2.5 Effects of lingual CD36 siRNA on high fat diet preference
RNA interference was used to investigate the effects of decreased lingual CD36 mRNA on HFD preference and HFD intake in OP and OR rats (OP/siRNA n=8; OP/CTRL n=7; OR/siRNA n=8; OR/CTRL n=7). OP and OR rats were habituated to a non-pelleted HFD (56% kilocalories from fat) and a non-pelleted low fat diet (LFD, 10% kilocalories from fat, 24% kilocalories from protein, 66% kilocalories from carbohydrates, Research Diets #D01080901) in food jars for 2 weeks prior to siRNA application [25, 66]. Jar position was counterbalanced daily to discourage the development of place preferences. Lingual CD36 siRNA application was conducted as described in Section 2.4.2. Food intake (jar weight and spillage) was measured daily during CD36 siRNA application and for 6 days following CD36 siRNA application. A subset of CV samples were assessed for siRNA-induced decreases in lingual CD36 protein expression as previously described [11].
2.5.1 Protein isolation and Western Blot
Excised CV (n=2/group) were incubated on ice in RIPA buffer (Sigma-Aldrich, St. Louis, MO) containing 1:100 protease inhibitor (Sigma-Aldrich), and 1:100 phosphatase inhibitor (Sigma-Aldrich) for protein isolation. Protein concentrations were assessed using a BCA protein assay kit (Pierce/Thermo Fisher Scientific, Rockford, IL). For the Western Blot, equal amounts of protein (25 ug) were separated on a 10% Tris-Hepes-SDS premade gel (Pierce/Thermo Fisher Scientific) and transferred to a PVDF membrane (Amersham, Amersham, UK) as indicated by the manufacturer. The membrane was then blocked in TBS (20 mM Tris-Base, 150 mM NaCl, pH 7.6) containing 5% nonfat dry milk overnight at 4°C. On Day 2, the membrane was incubated for 1h with primary antibody for CD36 (1:500; Abcam) and were normalized to β-actin (1:1000; Abcam). The membrane was washed with TBS containing 5% nonfat dry milk and three times with TBS containing .05% Tween-20 for 15min. Following washing, the membrane was incubated for 45min with horseradish peroxidase-conjugated anti-rabbit antiserum (1:10,000, Abcam). Immunoreactivity was visualized using ECL Western blotting detection reagents (Amersham). Images were obtained using exposure to chemiluminescence film (Amersham). The specific taste bud marker, α-gustducin (1:500, Santa Cruz) was systematically assessed in this study to validate the purity of the circumvallate papillae preparation (data not shown). Bands were quantified using ImageJ densitometry.
2.6 Statistical Analyses
In the first and second experiments (Sections 2.2 & 2.3), data was analyzed to determine the effects of nutritional status on the preference for LA across multiple concentrations using a repeated measures ANOVA. In the third experiment (Section 2.4), lingual CD36 mRNA expression was analyzed using a one-way ANOVA across nutritional status, a repeated measures ANOVA was used to assess the effects of lingual CD36 siRNA on LA preference over time and Student’s t-test was used to determine if CD36 siRNA altered lingual CD36 mRNA levels. A repeated measures ANOVA was used to determine if lingual CD36 siRNA altered HFD preference, total food intake (kcal), HFD intake (kcal) and LFD intake (kcal) across time in the fourth experiment (Section 2.5). In this experiment, lingual CD36 protein levels were assessed using a Student’s t-test. Based on previous demonstrations of inherent and diet-induced differences between OP and OR rats, and the goals of the current study, OP and OR rats were not directly compared. A Bonferonni post-hoc test was used to determine differences at single time points and LA concentrations. A significance level of p<.05 was used for all tests.
3. Results
3.1 Effects of fasting on linoleic acid preference
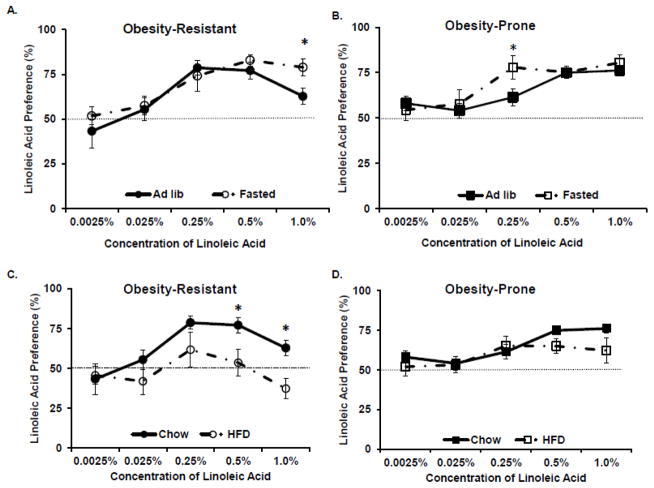
OP and OR rats underwent an overnight fast or received chow ad libitum and LA preference was determined. A repeated measures ANOVA revealed a significant effect of LA concentration in OP (F=11.4, p<.01) and OR (F=11.9, p<.01) rats, suggesting that LA preference was affected by LA concentration (See Figures 1A, 1B). Post-hoc tests indicated that fasting increased 0.25% LA preference in OP rats, compared to ad libitum chow fed OP rats. In OR rats, fasting significantly increased the preference for 1.0% LA acid, compared to the ad libitum fed OR rats, suggesting that the preference for higher concentrations of LA was potentiated by fasting.
Figure 1.
Linoleic acid preference was determined following an overnight fast or high fat diet consumption A. Fasting increased preference for 1.0% LA in Obesity-Resistant rats. B. Fasting increased the preference for 0.25% LA in Obesity-Prone rats. C. Consumption of high fat diet decreased the preference for 0.5% and 1.0% LA in Obesity-Resistant Rats. D. High fat diet intake did not affect LA preference in Obesity-Prone rats. Data is shown as mean ± SEM, n=8–10 rats/group, *p<.05 vs. chow fed.
3.2 Effects of high fat diet consumption on linoleic acid preference
OP and OR rats were fed a HFD or maintained on a chow diet for 2 weeks prior to measuring the preference for increasing concentrations of LA (See Figures 1C, 1D). A repeated measures ANOVA across all concentrations of LA indicated that OP rats significantly altered their preference for LA as the concentration of LA increased (F=4.9. p<.01). However, the preference for LA preference was not affected by HFD intake. In OR rats, a significant interaction between LA concentration and HFD intake was detected (F = 2.8, p<.05). The consumption of HFD decreased the preference for higher concentrations of LA (0.5% and 1.0%) in OR rats. The preference for 1.0% LA was below 50% (37.2 + 6.2%, mean ± SEM) in OR rats consuming HFD, suggesting an aversion to the highest concentration of LA tested.
3.3 Role of lingual CD36 on fasting-induced alterations in linoleic acid preference
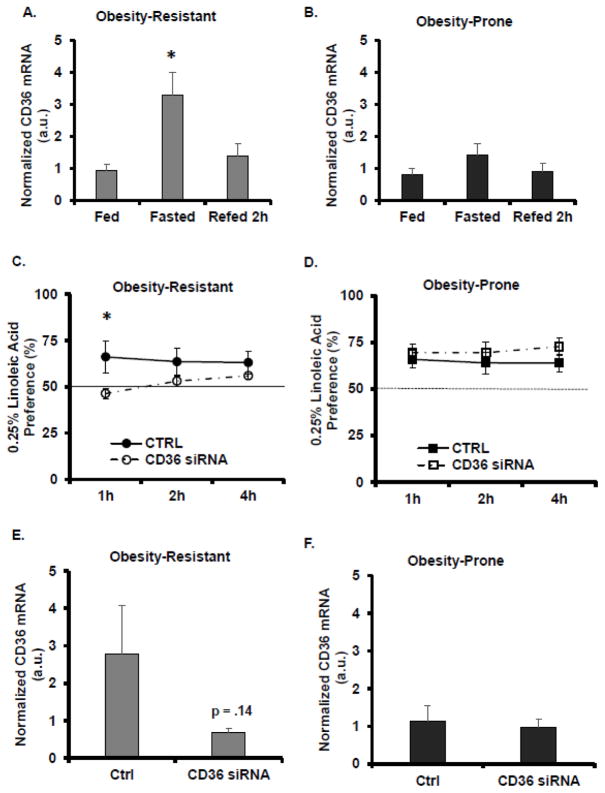
The effects of an overnight fast and refeeding on lingual CD36 mRNA were measured in OP and OR rats (See Figures 2A, 2B). Lingual CD36 mRNA expression in OP rats was not affected by the overnight fast. However, fasting increased lingual CD36 mRNA levels in OR rats and refeeding attenuated this increase (F=6.5, p<.01). To determine the functional significance of lingual CD36 in fasting-induced alterations in LA preference, lingual CD36 siRNA was applied to the CV and preference for 0.25% LA was determined at 1h, 2h and 4h in OP and OR rats (See Figures 2C, 2D). Application of lingual CD36 siRNA did not affect 0.25% LA preference in OP rats at any time point. In OR rats, an interaction was detected between time and CD36 siRNA (F = 7.4, p<.01). Lingual CD36 siRNA application in OR rats decreased 0.25% LA preference at 1h. Following the LA preference test, lingual CD36 mRNA was measured to verify a siRNA-induced decrease in CD36 expression (See Figures 2E, 2F). Application of CD36 siRNA did not decrease lingual CD36 mRNA in OP rats, however, a moderate decrease in lingual CD36 mRNA in the OR rat was detected. Since siRNA application in OR rats decreased CD36 mRNA expression by 75.2 ± .04% (p = .14) (mean ± SEM), the lack of statistical significance is likely due to the variability in the OR control group.
Figure 2.
The role of lingual CD36 on linoleic acid preference was assessed. A. Fasting-induced increases in lingual CD36 mRNA expression were attenuated by refeeding in Obesity-Resistant rats. B. In Obesity-Prone rats, fasting did not alter lingual CD36 mRNA expression. C. Lingual application of CD36 siRNA decreased LA preference in Obesity-Resistant rats. D. In Obesity-Prone rats, lingual application of CD36 siRNA did not alter LA preference. E. CD36 siRNA application led to a moderate decrease in lingual CD36 mRNA expression in Obesity-Resistant rats. F. Lingual application of CD36 siRNA did not decrease CD36 mRNA levels in Obesity-Prone rats. Data is shown as mean ± SEM, n=6–9 for A, B; n=5 C-F, * p<.05.
3.4 Effects of lingual CD36 siRNA on high fat diet preference
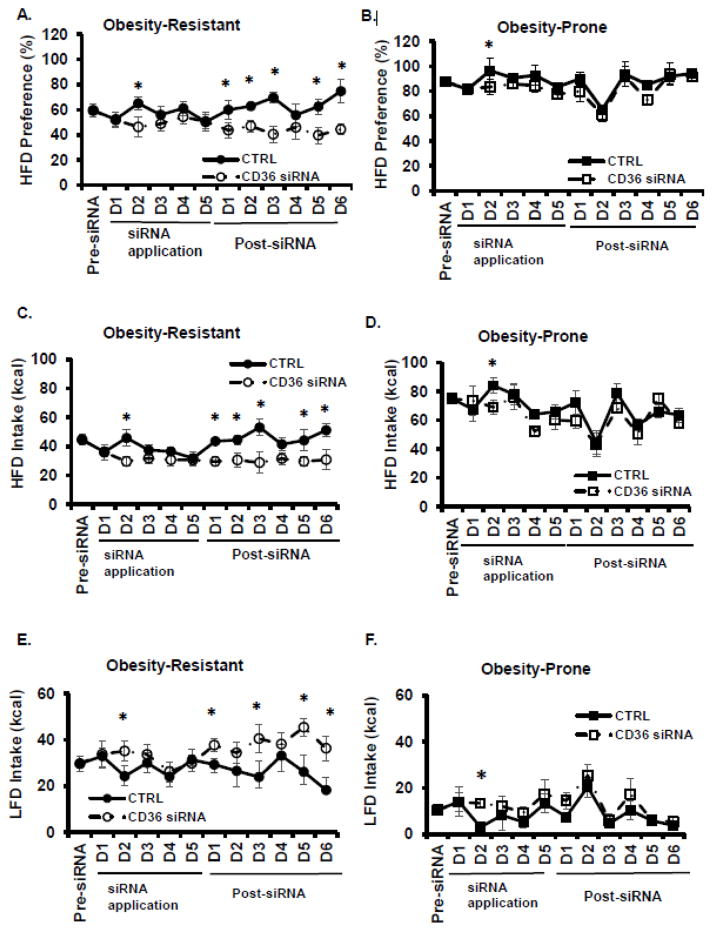
RNA interference was used to determine if lingual CD36 mediated HFD preference. Daily intake of LFD and HFD was assessed and total food intake and HFD preference were determined for the 5 days of siRNA application and for 6 days post-siRNA application. In OR rats, a significant day x siRNA interaction was detected for HFD preference (F=2.2, p<.05, See Figure 3A). CD36 siRNA decreased HFD preference in OR rats on siRNA application day 2 and post-siRNA days 1, 2, 3, 5, and 6. In OP rats, a main effect for day was detected (F=4.3, p<.01, See Figure 3B). CD36 siRNA decreased HFD preference on day 2 of siRNA application in OP rats. In OR rats, a significant interaction between days and siRNA was detected for HFD intake (F=1.9, p<.05, See Figure 3C). Lingual CD36 siRNA decreased HFD intake on siRNA application day 2 and post-siRNA days 1, 2, 3, 5, and 6. In OP rats, HFD intake differed across days (F=4.4, p<.01, See Figure 3D) and CD36 siRNA decreased HFD intake on day 2 of siRNA application. An interaction between days and siRNA were detected for LFD intake in OR rats (F= .9, p<.05, See Figure 3E). CD36 siRNA increased LFD intake in OR rats at day 2 of siRNA application and at post-siRNA days 1, 3, 5, and 6. In OP rats, LFD intake differed across days (F=3.9, p<.01, See Figure 3F) and was decreased by CD36 siRNA only on siRNA application day 2. Total food intake differed across days in OR (F=3.0, p<.01, data not shown) and OP rats (F=5.5, p<.01; data not shown), but was not affected by CD36 siRNA. Lingual protein expression of CD36 was assessed 6 days post-siRNA application. CD36 siRNA reduced lingual CD36 protein by 19.4 + 0.1% in OR rats and 31.0 + 0.2% in OP rats, but these values did not reach statistical significance (data not shown).
Figure 3.
The role of lingual CD36 on high fat diet preference was assessed. A. Lingual application of CD36 siRNA decreased HFD preference in Obesity-Resistant rats. B. Lingual application of CD36 siRNA produced a transient decrease in HFD preference in Obesity-Prone rats. C. HFD intake (kcal) was decreased by CD36 siRNA in Obesity-Resistant rats. D. HFD intake was transiently decreased by CD36 siRNA in Obesity-Prone rats. E. LFD intake (kcal) was increased in by CD36 siRNA in Obesity-Resistant rats. F. LFD intake was transiently increased by CD36 siRNA in Obesity-Prone rats. Data is shown as mean ± SEM, n=7–8, * p<.05 vs. control siRNA.
4. Discussion
The intake of energy dense, highly palatable foods has been linked to the increased prevalence of obesity throughout the world. Recent studies have shown that the orosensory perception of dietary fat is an important factor in mediating fat intake [2, 7–14, 16–22, 24–30, 67]. Differences in the orosensory fat perception may also influence the susceptibility to develop diet-induced obesity [11, 22, 25, 46, 49, 50, 52, 68]. The current study examined differences in the orosensory perception of fat/fatty acids in models that are either prone to developing obesity (OP) or resistant to developing obesity (OR). These specific models (Osborne-Mendel and S5B/Pl) have been well characterized and previous studies have reported differences in fat intake and preference, HFD-induced expression of lingual CD36, and LA preference thresholds [11, 25, 57]. OP rats consume more HFD and have a higher preference for HFD than OR rats. The lingual fatty acid receptor, CD36, may mediate intake and preference for HFD in OP and OR rats, since HFD intake for 3 and 14 days increases lingual CD36 mRNA in OP rats, but not in OR rats, suggesting differences in the response to HFD [25]. Furthermore, OP rats prefer higher concentrations of LA than OR rats and the preference threshold for LA is higher in OP rats [11]. Lingual application of CD36 siRNA was sufficient to eliminate the preference for the “preferred” concentration of LA in both OP and OR rats [11].
The goal of the current series of experiments was to test the hypothesis that fat/fatty acid preference in OP and OR rats was mediated by nutritional status and lingual CD36. The first experiment assessed the effects of an overnight fast or HFD consumption on LA preference in OP and OR rats. Fasting was expected to increase the preference for LA and consumption of a HFD was expected to decrease the preference for LA. As hypothesized, in OR rats, an overnight fast increased the preference for the highest concentration of LA tested (1.0%, See Figure 1A). However, in OP rats, an overnight fast increased the preference for 0.25% LA, which resulted in similar preference thresholds for 0.25%, 0.5% and 1.0% LA (See Figure 1B). Interestingly, fasting did not increase the preference for 1.0% LA in OP rats, which may be due to a ceiling effect, since LA preference did not exceed 82% preference in OP or OR rats, irrespective of LA concentration, in the current study or in a previously published study [11]. Also, as hypothesized, consumption of HFD decreased the preference for the higher concentrations of LA (See Figure 1C) in OR rats. The preference for the highest concentration of LA tested was less than 50%, suggesting that the OR rats avoided that concentration of LA when consuming the HFD. In OP rats, HFD intake did not alter LA preference at any concentration (See Figure 1D). These results were not due to higher intake of HFD in OR rats, since the average intake of HFD during access to 1.0% LA was higher in OP rats (15.9 ± 0.3g) compared to OR rats (14.2 ± 0.5g). Though the preference for LA was not directly assessed between the fasted and HFD fed groups, the OR rats exhibited a significant adaptation to nutritional status (1.0% LA preference, ad lib chow: 62.8±4.7%, fasted: 78.9±4.8%, HFD: 37.2±6.2%), while the OP rats do not exhibit a significant adaption to nutritional status (1.0% LA preference, ad lib chow: 76.2±2.8%; fasted: 80.5±7.3%, HFD: 62.2±7.7%). Future studies should investigate the relationship between fasting and HFD intake on LA preference thresholds. In this study, OR rats were susceptible to changes in nutritional status and exhibited a homeostatic adaptation, while OP rats were not able to adapt to nutritional status and alter LA preference. This lack of compensation for nutritional status in OP rats may play a role in their increased fat intake and fat preference.
Previous studies have reported fasting-induced increases in lingual CD36 mRNA expression [21], which may mediated increased fat intake and preference. The second experiment was designed to test the hypothesis that lingual CD36 mediates fasting-induced LA preference in OP and OR rats. Fasting-induced changes in lingual CD36 mRNA expression were assessed and the effects of experimentally reduced expression of lingual CD36 on LA preference were determined. Following an overnight fast, CD36 mRNA levels were increased in OR rats, but not in OP rats (See Figure 2A). Lingual application of CD36 siRNA, which has previously been shown to reduce lingual CD36 mRNA and protein expression [11], decreased LA preference in OR rats, but not OP rats. These results suggest a decrease in the orosensory perception of LA following application of lingual CD36 siRNA in OR rats. The transient effects of CD36 siRNA on LA preference may be due to the post-ingestive effects of LA consumption or confounded by LA-induced changes in lingual CD36 expression. CD36 siRNA reduced lingual CD36 mRNA levels by 75% in OR rats, however this was not statistically significant (See Figure 2E). These results may have been influenced by the consumption of LA, which increased the variability in the control group. In OP rats, fasting did not increase lingual CD36 mRNA expression, and though fasting increased the preference for 0.25% LA in OP rats, lingual CD36 siRNA application did not decrease LA preference and did not alter lingual CD36 mRNA expression. In a separate group of ad libitum, chow fed rats a similar expression pattern was detected in which lingual CD36 siRNA application produced a 64.8% decrease in CD36 mRNA in OR rats without altering CD36 mRNA expression in OP rats (data not shown). Taken together, these results suggest that lingual CD36 mediates LA preference in OR rats, but not in OP rats and that dysregulation of lingual CD36 in OP rats may account, at least in part, for their increased fat preference and decreased compensation for altered nutritional status.
An increased preference for dietary fat and an increased consumption of fat intake is related to the increased rates of obesity and are a defining characteristic of this OP model. Therefore, the goal of the third experiment was to assess the functional role of lingual CD36 on fat preference in OP and OR rats using a two diet choice test. HFD and LFD intake were measured during lingual CD36 siRNA application (5 days) and for 6 days following siRNA application. Interestingly, a transient effect of lingual CD36 siRNA on HFD preference, HFD intake and LFD intake was detected in both OP and OR rats on the 2nd day of CD36 siRNA application. In OP rats, there were no other effects of CD36 siRNA on HFD preference, HFD intake or LFD intake (See Figures 3B, 3D, 3E). OR rats were responsive to lingual CD36 siRNA application and a CD36 siRNA-induced decrease in HFD preference and HFD intake was detected in 5 of the 6 post-applications days (See Figures 3A, 3C). LFD intake was increased in 4 of the 6 post-siRNA application days in OR rats. These data suggest that OR rats are responsive to changes in lingual CD36 and that CD36 mediates the orosensory perception of dietary fat in OR rats. As seen with LA preference, OP rats are not responsive to changes in lingual CD36, suggesting a dysregulation in orosensory perception by CD36 which may contribute to their susceptibility to develop obesity.
The overall goal of the current study was to examine the role of lingual CD36 and nutritional status on fat/fatty acid preference in OP and OR rats. The results from this study suggest that rats that are prone to developing obesity (OP) rats were hypo-responsive to changes in nutritional status and were unable to adapt to either fasting or HFD intake by altering LA preference. Rats that are resistant to developing obesity (OR) were responsive to changes in nutritional status and adjusted their LA preference to reflect a homeostatic adaptation. The hypo-responsiveness to nutritional status in OP rats may contribute to the enhanced susceptibility to developing obesity in this strain. Lingual CD36 is considered the primary fat taste receptor and mediates fat intake. Fasting led to an increase in lingual CD36 mRNA in OR rats, which was attenuated by refeeding. Additionally, CD36 siRNA decreased LA preference in fasted OR rats, suggesting that the adaptation to nutritional status is mediated by lingual CD36 in OR rats. Lingual CD36 mRNA expression was not affected by fasting in OP rats and when CD36 siRNA was applied, LA preference was not affected. Furthermore, lingual application of CD36 siRNA produced a robust decrease in HFD intake and preference in OR rats, but not in OP rats. Taken together, these data suggest that OP rats do not alter their fat preference based on nutritional status or changes in lingual CD36 levels. Since reducing lingual CD36 in OR rats is sufficient to reduce fat preference and intake in OR rats, we hypothesize that a dysregulation of lingual CD36 in OP is a potential factor leading to increased fat intake, fat preference, weight gain and the susceptibility to develop obesity.
Highlights.
Nutritional status affects linoleic acid preference in OR rats.
Lingual CD36 mRNA levels are not increased by fasting in OP rats.
Application of CD36 siRNA reduces linoleic acid preference in OR rats.
Application of CD36 siRNA decreased fat intake and preference in OR rats.
Fat preference in OP rats is not affected by nutritional status or lingual CD36.
Acknowledgments
The authors would like to thank Elias Bench for his technical assistance with these studies. This research was supported by LSUHSC-NO to SDP. This work was supported in part by P20RR021945 from the National Center for Research Resources and NIH Center Grant P30DK072476 to Pennington Biomedical Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lissner L, Heitmann BL. Dietary fat and obesity: evidence from epidemiology. Eur J Clin Nutr. 1995;49(2):79–90. [PubMed] [Google Scholar]
- 2.Besnard P, Passilly-Degrace P, Khan NA. Taste of Fat: A Sixth Taste Modality? Physiol Rev. 2016;96(1):151–76. doi: 10.1152/physrev.00002.2015. [DOI] [PubMed] [Google Scholar]
- 3.de Araujo IE, Lin T, Veldhuizen MG, Small DM. Metabolic regulation of brain response to food cues. Curr Biol. 2013;23(10):878–83. doi: 10.1016/j.cub.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun X, Veldhuizen MG, Wray AE, de Araujo IE, Sherwin RS, Sinha R, Small DM. The neural signature of satiation is associated with ghrelin response and triglyceride metabolism. Physiol Behav. 2014;136:63–73. doi: 10.1016/j.physbeh.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tellez LA, Han W, Zhang X, Ferreira TL, Perez IO, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE. Separate circuitries encode the hedonic and nutritional values of sugar. Nat Neurosci. 2016;19(3):465–70. doi: 10.1038/nn.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220(4595):431–3. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 7.Abumrad NA. CD36 may determine our desire for dietary fats. J Clin Invest. 2005;115(11):2965–2967. doi: 10.1172/JCI26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calder PC, Deckelbaum RJ. CD36:taste the difference? Current Opinion in Clinical Nutrition and Metabolic Care. 2006;9:77–78. doi: 10.1097/01.mco.0000214562.14074.fa. [DOI] [PubMed] [Google Scholar]
- 9.Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30(25):8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chale’-Rush A, Burgess JR, Mattes RD. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chemical Senses. 2007;32:423–431. doi: 10.1093/chemse/bjm007. [DOI] [PubMed] [Google Scholar]
- 11.Chen CSY, Bench EM, Allerton TD, Schreiber AL, Arceneaux KP, Primeaux SD. Preference for linoleic acid in obesity-prone and obesity-resistant rats is attenuated by the reduction of CD36 on the tongue. Am J Physiol Regulatory Integrative Comp Physiol. 2013;305:R1346–R1355. doi: 10.1152/ajpregu.00582.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degrace-Passilly P, Besnard P. CD36 and taste of fat. Curr Opin Clin Nutr Metab Care. 2012;15:107–111. doi: 10.1097/MCO.0b013e32834ff19c. [DOI] [PubMed] [Google Scholar]
- 13.Dramane G, Abdoul-Azize S, Hichami A, Vogtle T, Akpona S, Chouabe C, Sadou H, Nieswandt B, Besnard P, Khan NA. STIM1 regulates calcium signaling in taste bud cells and preference for fat in mice. J Clin Invest. 2012;122(6):2267–2282. doi: 10.1172/JCI59953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Yassimi A, Hichami A, Besnard P, Khan NA. Linoleic acid induces calcium signaling, Src kinase phosphorylation and neurotransmitter release in mouse CD36-positive gustatory cells. J Biol Chem. 2008;283(19):12949–12959. doi: 10.1074/jbc.M707478200. [DOI] [PubMed] [Google Scholar]
- 15.Gaillard D, Laugerette F, Darcel N, El-Yassimi A, Passilly-Degrace P, Hichami A, Khan NA, Montmayeur JP, Besnard P. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22(5):1458–1468. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- 16.Gilbertson TA, Fontenot TD, Liu L, Zhang H, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol. 1997;272:C1203–C1210. doi: 10.1152/ajpcell.1997.272.4.C1203. [DOI] [PubMed] [Google Scholar]
- 17.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115(11):3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Hansen DR, Kim I, Gilbertson TA. Expression and characterization of delayed rectifying K+ channels in anterior rat taste buds. Am J Physiol Cell Physiol. 2005;289(4):C868–C880. doi: 10.1152/ajpcell.00115.2005. [DOI] [PubMed] [Google Scholar]
- 19.Liu P, Shah BP, Croasdell S, Gilbertson TA. Transient receptor potential channel type M5 is essential for fat taste. J Neurosci. 2011;31(23):8634–42. doi: 10.1523/JNEUROSCI.6273-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin C, Passilly-Degrace P, Chevrot M, Ancel D, Sparks SM, Drucker DJ, Besnard P. Lipid-mediated release of GLP-1 by mosue taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. J Lipid Res. 2012;53:2256–2265. doi: 10.1194/jlr.M025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin C, Passilly-Degrace P, Merlin JF, Chevrot M, Besnard P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLos One. 2011;6(8):e24014. doi: 10.1371/journal.pone.0024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattes RD. Fat taste and lipid metabolism in humans. Physiol Behav. 2005;86:691–697. doi: 10.1016/j.physbeh.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 23.Mizushige T, Inoue K, Fushiki T. Why is fat so tasty? Chemical reception of fatty acid on tongue. J Nutr Sci Vitaminol (Tokyo) 2007;53(1):1–4. doi: 10.3177/jnsv.53.1. [DOI] [PubMed] [Google Scholar]
- 24.Ong HH, Tan YN, Say YH. Fatty acid translocase gene CD36 rs1527483 variant influences oral fat perception in Malaysian subjects. Physiol Behav. 2016 doi: 10.1016/j.physbeh.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Primeaux SD, Braymer HD, Bray GA. CD36 mRNA in the gastrointestinal tract is differentially regulated by dietary fat intake in obesity-prone and obesity-resistant rats. Dig Dis Sci. 2013;58(2):369–370. doi: 10.1007/s10620-012-2364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Primeaux SD, Tzeng TH, Allerton TD, Chiang MC, Cosentino G, Dubin RL, Varughese A, Moore R, Geiselman PJ, Greenway FL, Uwaifo GI. Differences in short-term food preferences following vertical sleeve gastrectomy and Roux-en-Y gastric bypass surgery. Obes Res Clin Pract. 2015;9(6):628–32. doi: 10.1016/j.orcp.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regulatory Integrative Comp Physiol. 2007;293(5):R1823–R1832. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- 28.Simons PJ, Boon L. Lingual CD36 and obesity: a matter of fat taste? Acta Histochem. 2011;113(7):765–7. doi: 10.1016/j.acthis.2010.10.004. author reply 768–9. [DOI] [PubMed] [Google Scholar]
- 29.Tucker RM, Mattes RD. Are free fatty acids effective taste stimuli in humans? J Food Sci. 2012;77(3):S148–S150. doi: 10.1111/j.1750-3841.2011.02518.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XJ, Zhou LH, Ban X, Liu DX, Jiang W, Liu XM. Decreased expression of CD36 in circumvallate taste buds of high-fat diet induced obese rats. Acta Histochem. 2011;113(6):663–7. doi: 10.1016/j.acthis.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Asano M, Hong G, Matsuyama Y, Wang W, Izumi S, Izumi M, Toda T, Kudo TA. Association of Oral Fat Sensitivity with Body Mass Index, Taste Preference, and Eating Habits in Healthy Japanese Young Adults. Tohoku J Exp Med. 2016;238(2):93–103. doi: 10.1620/tjem.238.93. [DOI] [PubMed] [Google Scholar]
- 32.Chevrot M, Passilly-Degrace P, Ancel D, Bernard A, Enderli G, Gomes M, Robin I, Issanchou S, Vergès B, Nicklaus S, Besnard P. Obesity interferes with the orosensory detection of long-chain fatty acids in humans. Am J Clin Nutr. 2014;99(5):975–83. doi: 10.3945/ajcn.113.077198. [DOI] [PubMed] [Google Scholar]
- 33.Cvijanovic N, Feinle-Bisset C, Young RL, Little TJ. Oral and intestinal sweet and fat tasting: impact of receptor polymorphisms and dietary modulation for metabolic disease. Nutr Rev. 2015;73(5):318–34. doi: 10.1093/nutrit/nuu026. [DOI] [PubMed] [Google Scholar]
- 34.Hayes JE, Duffy VB. Oral sensory phenotype identifies level of sugar and fat required for maximal liking. Physiol Behav. 2008;95:77–87. doi: 10.1016/j.physbeh.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller KL. Genetic influences on oral fat perception and preference. J Food Sci. 2012;77(3):S143–S147. doi: 10.1111/j.1750-3841.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu D, Archer N, Duesing K, Hannan G, Keast R. Mechanism of fat taste perception: Association with diet and obesity. Prog Lipid Res. 2016;63:41–9. doi: 10.1016/j.plipres.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Mrizak I, Šerý O, Plesnik J, Arfa A, Fekih M, Bouslema A, Zaouali M, Tabka Z, Khan NA. The A allele of cluster of differentiation 36 (CD36) SNP 1761667 associates with decreased lipid taste perception in obese Tunisian women. Br J Nutr. 2015;113(8):1330–7. doi: 10.1017/S0007114515000343. [DOI] [PubMed] [Google Scholar]
- 38.Newman LP, Bolhuis DP, Torres SJ, Keast RS. Dietary fat restriction increases fat taste sensitivity in people with obesity. Obesity (Silver Spring) 2016;24(2):328–34. doi: 10.1002/oby.21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Primeaux SD, de Silva T, Tzeng TH, Chiang MC, Hsia DS. Recent advances in the modification of taste and food preferences following bariatric surgery. Rev Endocr Metab Disord. 2016 doi: 10.1007/s11154-016-9365-0. [DOI] [PubMed] [Google Scholar]
- 40.Proserpio C, Laureati M, Bertoli S, Battezzati A, Pagliarini E. Determinants of Obesity in Italian Adults: The Role of Taste Sensitivity, Food Liking, and Food Neophobia. Chem Senses. 2016;41(2):169–76. doi: 10.1093/chemse/bjv072. [DOI] [PubMed] [Google Scholar]
- 41.Sayed A, Šerý O, Plesnik J, Daoudi H, Rouabah A, Rouabah L, Khan NA. CD36 AA genotype is associated with decreased lipid taste perception in young obese, but not lean, children. Int J Obes (Lond) 2015;39(6):920–4. doi: 10.1038/ijo.2015.20. [DOI] [PubMed] [Google Scholar]
- 42.Stewart JE, Feinle-Bisset C, Golding M, Delahunty C, Clifton PM, Keast RS. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr. 2010;104(1):145–152. doi: 10.1017/S0007114510000267. [DOI] [PubMed] [Google Scholar]
- 43.Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an importnt role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274(27):19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 44.Febbraio M, Guy E, Coburn C, Knapp FF, Beets AL, Abumrad NA, Silverstein RL. The impact of overexpression and deficiency of fatty acid traslocase (FAT)/CD36. Mol Cell Biochem. 2002;239(1–2):193–197. [PubMed] [Google Scholar]
- 45.Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, Eckel R, Abumrad NA. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes. 2007;56(7):1872–1880. doi: 10.2337/db06-1699. [DOI] [PubMed] [Google Scholar]
- 46.Pepino MY, Love-Gregory L, Klein S, Abumrad NA. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res. 2012;53:561–566. doi: 10.1194/jlr.M021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr. 1998;68(6):1157–1173. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- 48.Bray GA, Paeratakul S, Popkin BM. Dietary fat and obesity: a review of animal, clinical and epidemiological studies. Physiol Behav. 2004;83(4):549–555. doi: 10.1016/j.physbeh.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 49.Little TJ, Feinle-Bisset C. Oral and gastrointestinal sensing of dietary fat and appetite regulation in humans: modification by diet and obesity. Frontiers in Neurosci. 2010;4(178) doi: 10.3389/fnins.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilbertson TA, Liu L, York DA, Bray GA. Dietary fat preferences are inversely correlated with peripheral gustatory fatty acid sensitivity. Ann N Y Acad Sci. 1998;855:165–168. doi: 10.1111/j.1749-6632.1998.tb10560.x. [DOI] [PubMed] [Google Scholar]
- 51.Schemmel R, Mickelsen O, Gill JL. Dietary obesity in rats: Body weight and body fat accretion in seven strains of rats. 1970:1041–1048. doi: 10.1093/jn/100.9.1041. [DOI] [PubMed] [Google Scholar]
- 52.Gilbertson TA, Liu L, Kim I, Burks KA, Hansen DR. Fatty acid responses in taste cells from obesity-prone and -resistant rats. Physiol Behav. 2005;86:681–690. doi: 10.1016/j.physbeh.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 53.Barnes MJ, Holmes G, Primeaux SD, York DA, Bray GA. Increased expression of mu opioid receptors in animals susceptible to diet-induced obesity. Peptides. 2006;27(12):3292–3298. doi: 10.1016/j.peptides.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Madiehe AM, Schaffhauser AO, Braymer DH, Bray GA, York DA. Differential expression of leptin receptor in high- and low-fat-fed Osborne-Mendel and S5B/Pl rats. Obes Res. 2000;8(6):467–474. doi: 10.1038/oby.2000.58. [DOI] [PubMed] [Google Scholar]
- 55.Pittman DW, Smith KR, Crawley ME, Corbin CH, Hansen DR, Watson KJ, Gilbertson TA. Orosensory detection of fatty acids by obesity-prone and obesity-resistant rats: strain and sex differences. Chem Senses. 2008;33(5):449–60. doi: 10.1093/chemse/bjn012. [DOI] [PubMed] [Google Scholar]
- 56.Primeaux SD, Barnes MJ, Bray GA. Olfactory bulbectomy increases food intake and hypothalamic neuropeptide Y in obesity-prone but not obesity-resistant rats. Behav Brain Res. 2007;180(2):190–6. doi: 10.1016/j.bbr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Primeaux SD, Barnes MJ, Braymer HD, Bray GA. Sensitivity to the satiating effects of Exendin 4 is decreased in obesity-prone Osborne-Mendel rats compared to obesity-resistant S5B/Pl rats. Int J Obes. 2010;34(9):1427–1433. doi: 10.1038/ijo.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White CL, Kashima K, Bray GA, York DA. Effect of serotonin 1-A agonist on food intake of Osborne-Mendel and S5B/P1 rats. Physiol Behav. 2000;68:715–722. doi: 10.1016/s0031-9384(99)00243-7. [DOI] [PubMed] [Google Scholar]
- 59.White CL, Ishii Y, Mendoza T, Upton N, Stasi LP, Bray GA, York DA. Effect of a selective OX1R antagonist on food intake and body weight in two strains of rats that differ in susceptibility to dietary-induced obesity. Peptides. 2005;26(11):2331–2338. doi: 10.1016/j.peptides.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 60.White CL, Ishihara Y, York DA, Bray GA. Effect of meta-chlorophenylpiperazine and cholecystokinin on food intake of Osborne-Mendel and S5B/P1 rats. Obesity. 2007;15(3):624–631. doi: 10.1038/oby.2007.579. [DOI] [PubMed] [Google Scholar]
- 61.White CL, Braymer HD, York DA, Bray GA. Effect of a high or low ambient perinatal temperature on adult obesity in Osborne-Mendel and S5B/Pl rats. Am J Physiol Regulatory Integrative Comp Physiol. 2005;288(5):R1376–R1384. doi: 10.1152/ajpregu.00162.2004. [DOI] [PubMed] [Google Scholar]
- 62.Ishihara Y, White CL, Kageyama H, Kageyama A, York DA, Bray GA. Effects of diet and time of the day on serum and CSF leptin levels in Osborne-Mendel and S5B/Pl rats. Obes Res. 2004;12(7):1067–1076. doi: 10.1038/oby.2004.134. [DOI] [PubMed] [Google Scholar]
- 63.Ookuma K, Barton C, York DA, Bray GA. Differential response to kappa-opioidergic agents in dietary fat selection between Osborne-Mendel and S5B/P1 rats. Peptides. 1998;19(1):141–147. doi: 10.1016/s0196-9781(97)00255-6. [DOI] [PubMed] [Google Scholar]
- 64.Schaffhauser AO, Madiehe AM, Braymer HD, Bray GA, York DA. Effects of a high-fat diet and strain on hypothalamic gene expression in rats. Obes Res. 2002;10(11):1188–1196. doi: 10.1038/oby.2002.161. [DOI] [PubMed] [Google Scholar]
- 65.Schemmel RA, Teague RJ, Bray GA. Obesity in Osborne-Mendel and S5B/Pl rats: effects of sucrose solutions, castration, and treatment with estadiol or insulin. 1982:R347–R353. doi: 10.1152/ajpregu.1982.243.3.R347. [DOI] [PubMed] [Google Scholar]
- 66.Primeaux SD, York DA, Bray GA. Neuropeptide Y administration into the amygdala alters high fat food intake. Peptides. 2006;27(7):1644–1651. doi: 10.1016/j.peptides.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Gaillard D, Passilly-Degrace P, Besnard P. Molecular mechanisms of fat preference and overeating. Ann NY Acad Sci. 2008;1141:163–175. doi: 10.1196/annals.1441.028. [DOI] [PubMed] [Google Scholar]
- 68.Pittman DW, Hansen DR, Gilbertson TA. High-Fat Diet Alters the Orosensory Sensitivity to Fatty Acids in Obesity-Resistant but not Obesity-Prone Rats. J Mol Genet Med. 2015;9(2) doi: 10.4172/1747-0862.1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]